User login
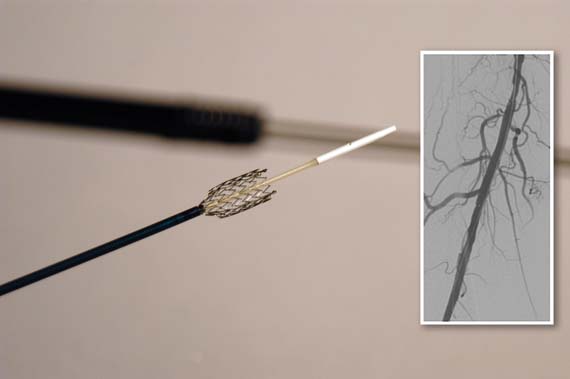
The Food and Drug Administration has approved a new paclitaxel-eluting stent indciated for the treatment of peripheral artery disease.
The Zilver PTX Drug-Eluting Peripheral Stent, which is manufactured by Cook Medical of Bloomington, Ind., is the first drug-eluting stent to win approval for this indication.
The approval was based on findings from both a randomized controlled trial and on a registry study, which together comprised more than 1,200 patients.
According to an FDA press statement, the studies indicate that treatment with the stent "is at least as safe as treatment with percutaneous transluminal angioplasty (PTA) and significantly more effective."
The randomized trial included a total of 479 patients who had a single stenotic lesion less than 140 mm in one or both of the femoropopliteal arteries.
The patients were randomized to the paclitaxel-eluting stent or to PTA. If the transluminal procedure failed, then the patients received either the paclitaxel-eluting stent or a bare-metal stent.
After 12 months, 83% of the arteries treated with the drug-eluting stent were still open, compared with 33% of those in the PTA group.
In those patients who had the stent placed after a failed PTA, 90% of arteries were open, compared with 73% in those who got the bare-metal stent.
In October 2011, the FDA's Circulatory System Devices Panel voted 11 to 0 that the benefits of the Zilver PTX stent outweighed its risks as a treatment for patients with symptomatic atherosclerotic stenosis of the femoropopliteal arteries on the basis of that trial.
This past October, the 3-year results of the study were presented at the Vascular Interventional Advances 2012 meeting in Las Vegas. The results showed that there was a 70.7% primary patency seen for the paclitaxel-eluting stent, compared with 49.1% for PTA and bare-metal stents.
The registry study followed 767 patients for 24 months. These patients had a maximum of four stents placed; the stents could be utilized to treat a single lesion or to treat multiple lesions.
At 12 months, the fracture rate was 1.5%; fractures were not associated with any clinical problems. The rate of stent thrombosis was 2.8% at 12 months and 3.5% at 24 months.
"The clinical study demonstrated that the [the paclitaxel-eluting stent] is more effective than the use of balloon angioplasty for the treatment of symptomatic peripheral artery disease in above-the-knee femoropopliteal artery," Christy Foreman, director of the Office of Device Evaluation at the FDA's Center for Devices and Radiological Health, said in the statement.
"This approval expands the treatment options for patients suffering from symptomatic peripheral artery disease," she added.
In both studies, the most common major adverse event was restenosis requiring additional treatment to reestablish adequate flow in the artery.
The device is contraindicated in patients with stenoses that cannot be dilated to permit passage of the catheter or proper placement of the stent.
It is also contraindicated in patients who cannot receive recommended drug therapy due to bleeding disorders, or women who are pregnant, breastfeeding, or planning to become pregnant in the next 5 years.
The FDA will now require the manufacturer to conduct a 5-year postapproval study of 900 patients to further evaluate the stent's safety and efficacy.
The banging noise you hear emanating from your radiologic imaging area is not the MRI machine, but nails being pounded into the coffin of the fem-pop bypass graft operation. Incremental but significant progress has been made in treating SFA occlusive disease over the last decade from POBA to tools that facilitate crossing/reentry of CTOs to bare-metal/covered stenting to DES. The Zilver PTX trial and registry have resulted in commercial approval of a long enough, large enough self-expanding stent to be useful in peripheral interventions.
| Dr. Brian Rubin |
Treated lesions included ISRs as well as primary atherosclerotic lesions, and data from both trials showed near-identical 12 and 24 month results, with Zilver PTX resulting in primary patency rates 15-20% higher than results with bare-metal stenting alone. As of mid-November 2012, the complete FDA 57-page PDF of both studies is available online and should be required reading While most surgeons would claim their 12 month fem-AK pop bypass patency to be better than the 83% primary patency reported for Zilver PTX, multiple recent published reports suggest otherwise.
A number of important issues remain unresolved including: 1. how much will each stent cost? (the grapevine has it that the pricing will be surprisingly low) 2. am I really limited to a maximum of 14 cm treated length per leg? (probably not since paclitaxel levels peaked at less than 30 minutes, were gone within a few hours and were very low anyway 3. what antiplatelet therapy is required? (still to be determined- ASA alone is probably adequate) and 4. how do I treat smaller diameter vessels? (Zilver PTX is only approved in 6-8 mm diameters). Although 3-year data have recently been reported, the long-term outcomes after DES remain unknown at this time.
While fem-pop bypass may still find limited utility and needs to remain in the surgical armamentarium, the commercial approval of Zilver PTX stents has nibbled away again at the difference in outcomes between endovascular and open surgical therapy for SFA occlusive disease.
If other studies including long-term outcome reports confirm these initial salutary results, the fem-pop graft will soon follow the utilization curve of open aortic surgery and become an endangered operation.
Dr. Brian Rubin is a professor of the department of surgery at the Washington University School of Medicine, St. Louis.
The banging noise you hear emanating from your radiologic imaging area is not the MRI machine, but nails being pounded into the coffin of the fem-pop bypass graft operation. Incremental but significant progress has been made in treating SFA occlusive disease over the last decade from POBA to tools that facilitate crossing/reentry of CTOs to bare-metal/covered stenting to DES. The Zilver PTX trial and registry have resulted in commercial approval of a long enough, large enough self-expanding stent to be useful in peripheral interventions.
| Dr. Brian Rubin |
Treated lesions included ISRs as well as primary atherosclerotic lesions, and data from both trials showed near-identical 12 and 24 month results, with Zilver PTX resulting in primary patency rates 15-20% higher than results with bare-metal stenting alone. As of mid-November 2012, the complete FDA 57-page PDF of both studies is available online and should be required reading While most surgeons would claim their 12 month fem-AK pop bypass patency to be better than the 83% primary patency reported for Zilver PTX, multiple recent published reports suggest otherwise.
A number of important issues remain unresolved including: 1. how much will each stent cost? (the grapevine has it that the pricing will be surprisingly low) 2. am I really limited to a maximum of 14 cm treated length per leg? (probably not since paclitaxel levels peaked at less than 30 minutes, were gone within a few hours and were very low anyway 3. what antiplatelet therapy is required? (still to be determined- ASA alone is probably adequate) and 4. how do I treat smaller diameter vessels? (Zilver PTX is only approved in 6-8 mm diameters). Although 3-year data have recently been reported, the long-term outcomes after DES remain unknown at this time.
While fem-pop bypass may still find limited utility and needs to remain in the surgical armamentarium, the commercial approval of Zilver PTX stents has nibbled away again at the difference in outcomes between endovascular and open surgical therapy for SFA occlusive disease.
If other studies including long-term outcome reports confirm these initial salutary results, the fem-pop graft will soon follow the utilization curve of open aortic surgery and become an endangered operation.
Dr. Brian Rubin is a professor of the department of surgery at the Washington University School of Medicine, St. Louis.
The banging noise you hear emanating from your radiologic imaging area is not the MRI machine, but nails being pounded into the coffin of the fem-pop bypass graft operation. Incremental but significant progress has been made in treating SFA occlusive disease over the last decade from POBA to tools that facilitate crossing/reentry of CTOs to bare-metal/covered stenting to DES. The Zilver PTX trial and registry have resulted in commercial approval of a long enough, large enough self-expanding stent to be useful in peripheral interventions.
| Dr. Brian Rubin |
Treated lesions included ISRs as well as primary atherosclerotic lesions, and data from both trials showed near-identical 12 and 24 month results, with Zilver PTX resulting in primary patency rates 15-20% higher than results with bare-metal stenting alone. As of mid-November 2012, the complete FDA 57-page PDF of both studies is available online and should be required reading While most surgeons would claim their 12 month fem-AK pop bypass patency to be better than the 83% primary patency reported for Zilver PTX, multiple recent published reports suggest otherwise.
A number of important issues remain unresolved including: 1. how much will each stent cost? (the grapevine has it that the pricing will be surprisingly low) 2. am I really limited to a maximum of 14 cm treated length per leg? (probably not since paclitaxel levels peaked at less than 30 minutes, were gone within a few hours and were very low anyway 3. what antiplatelet therapy is required? (still to be determined- ASA alone is probably adequate) and 4. how do I treat smaller diameter vessels? (Zilver PTX is only approved in 6-8 mm diameters). Although 3-year data have recently been reported, the long-term outcomes after DES remain unknown at this time.
While fem-pop bypass may still find limited utility and needs to remain in the surgical armamentarium, the commercial approval of Zilver PTX stents has nibbled away again at the difference in outcomes between endovascular and open surgical therapy for SFA occlusive disease.
If other studies including long-term outcome reports confirm these initial salutary results, the fem-pop graft will soon follow the utilization curve of open aortic surgery and become an endangered operation.
Dr. Brian Rubin is a professor of the department of surgery at the Washington University School of Medicine, St. Louis.
The Food and Drug Administration has approved a new paclitaxel-eluting stent indciated for the treatment of peripheral artery disease.
The Zilver PTX Drug-Eluting Peripheral Stent, which is manufactured by Cook Medical of Bloomington, Ind., is the first drug-eluting stent to win approval for this indication.
The approval was based on findings from both a randomized controlled trial and on a registry study, which together comprised more than 1,200 patients.
According to an FDA press statement, the studies indicate that treatment with the stent "is at least as safe as treatment with percutaneous transluminal angioplasty (PTA) and significantly more effective."
The randomized trial included a total of 479 patients who had a single stenotic lesion less than 140 mm in one or both of the femoropopliteal arteries.
The patients were randomized to the paclitaxel-eluting stent or to PTA. If the transluminal procedure failed, then the patients received either the paclitaxel-eluting stent or a bare-metal stent.
After 12 months, 83% of the arteries treated with the drug-eluting stent were still open, compared with 33% of those in the PTA group.
In those patients who had the stent placed after a failed PTA, 90% of arteries were open, compared with 73% in those who got the bare-metal stent.
In October 2011, the FDA's Circulatory System Devices Panel voted 11 to 0 that the benefits of the Zilver PTX stent outweighed its risks as a treatment for patients with symptomatic atherosclerotic stenosis of the femoropopliteal arteries on the basis of that trial.
This past October, the 3-year results of the study were presented at the Vascular Interventional Advances 2012 meeting in Las Vegas. The results showed that there was a 70.7% primary patency seen for the paclitaxel-eluting stent, compared with 49.1% for PTA and bare-metal stents.
The registry study followed 767 patients for 24 months. These patients had a maximum of four stents placed; the stents could be utilized to treat a single lesion or to treat multiple lesions.
At 12 months, the fracture rate was 1.5%; fractures were not associated with any clinical problems. The rate of stent thrombosis was 2.8% at 12 months and 3.5% at 24 months.
"The clinical study demonstrated that the [the paclitaxel-eluting stent] is more effective than the use of balloon angioplasty for the treatment of symptomatic peripheral artery disease in above-the-knee femoropopliteal artery," Christy Foreman, director of the Office of Device Evaluation at the FDA's Center for Devices and Radiological Health, said in the statement.
"This approval expands the treatment options for patients suffering from symptomatic peripheral artery disease," she added.
In both studies, the most common major adverse event was restenosis requiring additional treatment to reestablish adequate flow in the artery.
The device is contraindicated in patients with stenoses that cannot be dilated to permit passage of the catheter or proper placement of the stent.
It is also contraindicated in patients who cannot receive recommended drug therapy due to bleeding disorders, or women who are pregnant, breastfeeding, or planning to become pregnant in the next 5 years.
The FDA will now require the manufacturer to conduct a 5-year postapproval study of 900 patients to further evaluate the stent's safety and efficacy.
The Food and Drug Administration has approved a new paclitaxel-eluting stent indciated for the treatment of peripheral artery disease.
The Zilver PTX Drug-Eluting Peripheral Stent, which is manufactured by Cook Medical of Bloomington, Ind., is the first drug-eluting stent to win approval for this indication.
The approval was based on findings from both a randomized controlled trial and on a registry study, which together comprised more than 1,200 patients.
According to an FDA press statement, the studies indicate that treatment with the stent "is at least as safe as treatment with percutaneous transluminal angioplasty (PTA) and significantly more effective."
The randomized trial included a total of 479 patients who had a single stenotic lesion less than 140 mm in one or both of the femoropopliteal arteries.
The patients were randomized to the paclitaxel-eluting stent or to PTA. If the transluminal procedure failed, then the patients received either the paclitaxel-eluting stent or a bare-metal stent.
After 12 months, 83% of the arteries treated with the drug-eluting stent were still open, compared with 33% of those in the PTA group.
In those patients who had the stent placed after a failed PTA, 90% of arteries were open, compared with 73% in those who got the bare-metal stent.
In October 2011, the FDA's Circulatory System Devices Panel voted 11 to 0 that the benefits of the Zilver PTX stent outweighed its risks as a treatment for patients with symptomatic atherosclerotic stenosis of the femoropopliteal arteries on the basis of that trial.
This past October, the 3-year results of the study were presented at the Vascular Interventional Advances 2012 meeting in Las Vegas. The results showed that there was a 70.7% primary patency seen for the paclitaxel-eluting stent, compared with 49.1% for PTA and bare-metal stents.
The registry study followed 767 patients for 24 months. These patients had a maximum of four stents placed; the stents could be utilized to treat a single lesion or to treat multiple lesions.
At 12 months, the fracture rate was 1.5%; fractures were not associated with any clinical problems. The rate of stent thrombosis was 2.8% at 12 months and 3.5% at 24 months.
"The clinical study demonstrated that the [the paclitaxel-eluting stent] is more effective than the use of balloon angioplasty for the treatment of symptomatic peripheral artery disease in above-the-knee femoropopliteal artery," Christy Foreman, director of the Office of Device Evaluation at the FDA's Center for Devices and Radiological Health, said in the statement.
"This approval expands the treatment options for patients suffering from symptomatic peripheral artery disease," she added.
In both studies, the most common major adverse event was restenosis requiring additional treatment to reestablish adequate flow in the artery.
The device is contraindicated in patients with stenoses that cannot be dilated to permit passage of the catheter or proper placement of the stent.
It is also contraindicated in patients who cannot receive recommended drug therapy due to bleeding disorders, or women who are pregnant, breastfeeding, or planning to become pregnant in the next 5 years.
The FDA will now require the manufacturer to conduct a 5-year postapproval study of 900 patients to further evaluate the stent's safety and efficacy.