User login
Pathophysiology of type 2 diabetes mellitus: potential role of incretin-based therapies
Glucose-lowering effects of incretin-based therapies
Safety, tolerability, and nonglycemic effects of incretin-based therapies
- The pathophysiology of type 2 diabetes mellitus (T2DM) is multifactorial and includes mechanisms beyond insulin resistance and pancreatic β-cell dysfunction
- The differential actions of glucagon-like peptide (GLP)-1 agonists and dipeptidyl peptidase (DPP)-4 inhibitors include:
- The additional actions of GLP-1 agonists on pathophysiologic mechanisms include:
The authors received editorial assistance from the Primary Care Education Consortium and WriteHealth, LLC in the development of this activity and honoraria from the Primary Care Education Consortium. They have disclosed that Dr Campbell is on the advisory board for Daiichi-Sankyo and the speakers bureau for Eli Lilly and Co; Dr Cobble is on the advisory board for Abbott Laboratories, AstraZeneca, and Eli Lilly and Co and speakers bureau for Abbott Laboratories, AstraZeneca/Bristol Myers Squibb, Eli Lilly and Co, GlaxoSmithKline, and Novo Nordisk Inc; Dr Reid is on the advisory board and speakers bureau for Amylin Pharmaceuticals, Medtronic, Novo Nordisk Inc, and sanofi-aventis; and Dr Shomali is on the advisory board for Novo Nordisk Inc and speakers bureau for Amylin Pharmaceuticals, Eli Lilly and Co, sanofi-aventis, and Takeda Pharmaceuticals.
Introduction
The pathophysiology of T2DM and the incretin system have been described in considerable detail in previous Journal of Family Practice supplements.1,2 In this article, we will briefly review the multiple pathophysiologic mechanisms known to be involved in T2DM, with a focus on the incretin system, to gain a better understanding of the disease progression affecting our 3 patients presented in the supplement introduction.
The central roles of insulin resistance and pancreatic β-cell dysfunction (insulin deficiency) in the pathogenesis of T2DM have been recognized for decades. But other causes of T2DM have been identified, including:
- Altered glucose release and disposal
- Altered glucagon secretion
- Impaired incretin response
- Rapid gastric emptying
- Impaired satiety
While there is a link between insulin resistance and pancreatic β-cell failure,3 the extent to which other causes interact is only beginning to be recognized. With a better understanding of the multifactorial pathogenesis of T2DM, however, has come additional treatment options and the opportunity for a more individualized and complementary approach to treatment.
Insulin resistance and pancreatic β-cell dysfunction
T2DM is a progressive disease characterized by worsening hyperglycemia,4 caused in part by insulin resistance and deterioration in pancreatic β-cell function. Insulin resistance appears to result from a complex interaction between visceral fat and the immune system that results in a state of chronic inflammation. Proinflammatory proteins secreted primarily from visceral fat block the action of insulin in adipocytes.5 Elevated levels of free fatty acids, which are common in obesity, further promote insulin resistance.6
Pancreatic β-cell dysfunction occurs progressively over a decade or more until β-cells are no longer capable of secreting sufficient insulin. Data from several studies suggest that, on average, 50% to 80% of β-cell function has been lost at the time of diagnosis of T2DM.7-9 The extent of pancreatic β-cell dysfunction is important for 2 reasons. First, medications that act by stimulating the β-cell to secrete insulin lose their effectiveness over time, as shown in the UK Prospective Diabetes Study (UKPDS)7 and, more recently, in A Diabetes Outcome Progression Trial (ADOPT).10 Second, since only 20% to 50% of pancreatic β-cell function remains at the time of diagnosis, preserving β-cell function would likely be beneficial for long-term glycemic control.
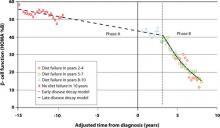
But there is no time to waste following diagnosis of T2DM. The Belfast Diet Study,8 a 10-year prospective clinical trial of 432 newly diagnosed persons with T2DM treated with intensive lifestyle management, suggested that β-cell decline occurs in 2 phases. Beginning well before diagnosis, the annual rate of decline in β-cell function during the first phase, ≥15 years prediagnosis, is about 2%, while during the second phase, beginning about 3 years postdiagnosis, the annual rate of decline in β-cell function accelerates to 18% (FIGURE 1). This second phase appears to result from a combination of steadily increasing β-cell death rate and decreasing replication rate.8
Case 2: A 47-year-old man diagnosed with T2DM several years ago who has experienced edema and weight gain on pioglitazone
Case 3: A 68-year-old woman with longstanding T2DM who has failed dual oral therapy and has microvascular complications
FIGURE 1 Phases of decline in pancreatic β-cell function8
HOMA %B, homeostasis model of assessment–percent β-cell function.
Bagust A, Beale S. Deteriorating beta-cell function in type 2 diabetes: a long-term model. QJM. 2003;96(4):281-288, by permission of Oxford University Press.
Considering insulin resistance and pancreatic β-cell dysfunction in our 3 cases
- Newly diagnosed with T2DM, the patient in Case 1 probably has 20% to 50% of his β-cell function remaining; preservation of β-cell function would be desirable, especially before he reaches the second phase of decline in β-cell function, about 3 years after diagnosis
- Diagnosed with T2DM about 2.5 years ago and not previously treated with a secretagogue, the patient in Case 2 has some β-cell function remaining; however, he is likely close to entering the second phase of steep decline in β-cell function observed in the Belfast Diet Study; in addition, insulin resistance related to his obesity must be addressed, as his obesity serves to stimulate pancreatic β-cells to secrete more insulin
- The Case 3 patient, diagnosed with T2DM about 5 years ago, is probably in the second phase of steep decline in β-cell function and has limited β-cell function remaining; furthermore, she has failed dual oral therapy that included almost 2 years of treatment with glyburide
The incretin system and GLP-1
As discussed in greater detail in a previous Journal of Family Practice supplement,1 the incretin system is integrally involved in glucose homeostasis. Glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 are the 2 most important incretin hormones secreted in response to food ingestion. Patients with T2DM are resistant to GIP, which contributes to the impaired incretin response. In animal models and humans, GLP-1, the most clinically important incretin hormone, has been shown to:
- Increase insulin biosynthesis in a glucose-dependent manner through direct activation of receptors on islet β-cells and via the vagus nerve11-13
- Inhibit glucagon secretion in a glucose-dependent manner through direct activation of receptors on islet α-cells12,14,15
- Slow gastric emptying16
- Promote satiety17,18
- Promote proliferation, increase differentiation, and prolong survival of β-cells19-21
- Improve myocardial function22
The actions of endogenous GLP-1 are short-lived, as GLP-1 undergoes rapid degradation by the enzyme DPP-4. To overcome this rapid degradation, 2 treatment approaches have been taken. Injectable GLP-1 agonists, which are resistant to the enzymatic action of DPP-4, have been developed. Oral DPP-4 inhibitors, which allow for prolonged physiologic actions of endogenous GLP-1, also have been developed. Because of the pharmacologic levels of GLP-1 activity achieved, GLP-1 agonists have better glucose-lowering efficacy and also promote weight loss compared with DPP-4 inhibitors.23-26
Impaired incretin effect in T2DM
Until recently, it was thought that secretion of GLP-1 in response to ingestion of a meal in people with T2DM was significantly impaired compared with that in healthy controls (P<.001).27,28 Recent investigation, however, found that the GLP-1 levels in patients with T2DM did not decrease after oral ingestion of glucose or a mixed meal. The secretion of GIP, on the other hand, increased following oral ingestion of a mixed meal but not of glucose.29 These observations suggest that the unaltered secretion of GLP-1 in people with T2DM may be insufficient to make up for the diminished insulinotropic activity of GIP in people with T2DM. Consequently, pharmacologic levels of GLP-1 would be necessary to restore the insulinotropic actions of the incretin system.30
Incretin effects on the pancreatic β-cell and insulin resistance
Animal studies have indicated that GLP-1 has the ability to preserve β-cell function by suppressing β-cell apoptosis and stimulating neogenesis and proliferation.31,32 Several trials in people with T2DM have shown that administration of a GLP-1 agonist (exenatide or liraglutide) for up to 52 weeks either as monotherapy or added on to existing therapy results in significant improvement in pancreatic β-cell function.33-39 One trial involving patients whose treatment with metformin had not provided glycemic control showed a significant increase in first- and second-phase glucose-stimulated C-peptide secretion, both markers of β-cell function, with exenatide (1.53- and 2.85-fold, respectively; P<.0001) compared with insulin glargine.34 A 26-week trial found that the addition of exenatide or liraglutide to metformin, a sulfonylurea, or both resulted in improvement in β-cell function, as determined by the homeostasis model of assessment– β-cell function (HOMA-B); the improvement was significantly greater with liraglutide than with exenatide (32% vs 3%; P<.0001).37 The greater improvement in β-cell function may be a reflection of the greater lowering of fasting plasma glucose with liraglutide than with exenatide.
Clinical trials with DPP-4 inhibitors (saxagliptin or sitagliptin) also have shown significant improvement in β-cell function (up to 16%; P<.05) over 24 weeks.40-43 Generally, however, there are fewer data regarding effects on pancreatic β-cell function for the DPP-4 inhibitors than for the GLP-1 agonists.44
Treatment with a GLP-1 agonist or DPP-4 inhibitor also appears to improve insulin resistance and sensitivity.34,41,42,45 A 52-week trial found a significant reduction in insulin resistance with liraglutide 1.2 mg or 1.8 mg once daily (–0.65% and –1.35%, respectively) compared with a 0.85% increase with glimepiride (P=.0249 and P=.0011 vs liraglutide 1.2 mg and 1.8 mg, respectively).45 Another trial found comparable improvement in insulin sensitivity following 52 weeks of treatment with exenatide or insulin glargine (0.9 vs 1.1 mg/min-1/kg-1, respectively; P=.49).34
These preclinical and clinical data regarding pancreatic β-cell function must be viewed as preliminary, and require further investigation. If confirmed, the ability to alter the natural progression of β-cell loss in T2DM and/or to reduce insulin resistance would be of significant clinical value.
Incretin effects on other causes of T2DM
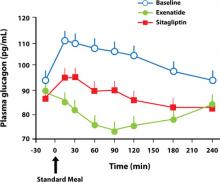
Both the GLP-1 agonists and the DPP-4 inhibitors affect other mechanisms involved in the pathogenesis of T2DM. Several trials have shown a significant reduction in fasting glucagon secretion with GLP-1 agonist37 or DPP-4 inhibitor treatment.43,46,47 The addition of exenatide or liraglutide to metformin, a sulfonylurea, or both for 26 weeks resulted in a reduction in fasting glucagon secretion of 12.3 and 19.4 ng/L, respectively (P=.1436), after 26 weeks.37 Addition of saxagliptin to a submaximal dose of a sulfonylurea resulted in a reduction of glucagon secretion of 0.8 ng/L compared with an increase of 4.5 ng/L with uptitration of the sulfonylurea alone for 24 weeks. A 2-week crossover trial comparing exenatide with sitagliptin showed that compared with sitagliptin, exenatide reduced postprandial glucagon by 12% (P=.0011)47 (FIGURE 2); in addition, exenatide slowed the gastric emptying rate by 44% (P<.0001), with a commensurate decrease in total caloric intake of 134 kcal with exenatide vs an increase of 130 kcal with sitagliptin (P=.0227).47
FIGURE 2 Reduction in glucagon secretion with exenatide or sitagliptin47
Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Current Medical Research and Opinion. 2008, reprinted with permission of Taylor & Francis Group.
Summary
The multifactorial nature of the pathogenesis of T2DM provides an opportunity to combine treatments that act upon different mechanisms. In addition to improving insulin resistance and pancreatic β-cell dysfunction, the GLP-1 agonists and DPP-4 inhibitors improve the impaired incretin response, as well as increase insulin secretion and reduce glucagon secretion, both in a glucose-dependent manner. As a result of these multiple actions, the GLP-1 agonists and DPP-4 inhibitors lower both fasting and postprandial glucose levels. The effects of GLP-1 agonists tend to be greater, probably because they produce pharmacologic levels of GLP-1 compared to physiologic levels with the DPP-4 inhibitors. Another difference is that unlike the DPP-4 inhibitors, the GLP-1 agonists also slow gastric emptying and promote satiety.
1. Holst JJ, LaSalle JR. An overview of incretin hormones. J Fam Prac. 2008;57(suppl):S4-S9.
2. Peterson GE. A checklist approach to selecting the optimal treatment regimen for a patient with type 2 diabetes. J Fam Pract. 2009;58(suppl):S21-S25.
3. Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(suppl 3):S16-S21.
4. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
5. Lin Y, Sun Z. Current views on type 2 diabetes. J Endocrinol. 2010;204:1-11.
6. Aronne LJ, Isoldi KK. Overweight and obesity: key components of cardiometabolic risk. Clin Cornerstone. 2007;8:29-37.
7. United Kingdom Prospective Diabetes Study Group. UK Prospective Diabetes Study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249-1258.
8. Bagust A, Beale S. Deteriorating beta-cell function in type 2 diabetes: a long-term model. QJM. 2003;96:281-288.
9. DeFronzo R, Banerji MA, Bray G, et al. Reduced insulin secretion/insulin resistance (disposition) index is the primary determinant of glucose intolerance in the pre-diabetic state: results from ACT NOW. Paper presented at: American Diabetes Association 68th Scientific Session; June 6-10, 2008; San Francisco, CA.
10. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
11. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153-165.
12. Naslund E, Bogefors J, Skogar S, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;277:R910-R916.
13. Fehmann HC, Habener JF. Insulinotropic hormone glucagon-like peptide-I(7-37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology. 1992;130:159-166.
14. Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300-1304.
15. Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239-1246.
16. Delgado-Aros S, Kim DY, Burton DD, et al. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G424-G431.
17. Gutzwiller JP, Goke B, Drewe J, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81-86.
18. Gutzwiller JP, Drewe J, Goke B, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541-R1544.
19. Xu G, Stoffers DA, Habener JF, et al. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270-2276.
20. Zhou J, Wang X, Pineyro MA, et al. Glucagon-like peptide 1 and exendin-4 convert pancreatic AR42J cells into glucagon- and insulin-producing cells. Diabetes. 1999;48:2358-2366.
21. Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17:161-171.
22. Girard J. The incretins: from the concept to their use in the treatment of type 2 diabetes. Part A: incretins: concept and physiological functions. Diabetes Metab. 2008;34:550-559.
23. Vilsboll T, Krarup T, Madsbad S, et al. Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia. 2002;45:1111-1119.
24. Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301-307.
25. Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824-830.
26. Deacon CF, Hughes TE, Holst JJ. Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig. Diabetes. 1998;47:764-769.
27. Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199-E206.
28. Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717-3723.
29. Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678-687.
30. Nauck MA. Unraveling the science of incretin biology. Am J Med. 2009;122(suppl):S3-S10.
31. Bulotta A, Hui H, Anastasi E, et al. Cultured pancreatic ductal cells undergo cell cycle re-distribution and beta-cell-like differentiation in response to glucagon-like peptide-1. J Mol Endocrinol. 2002;29:347-360.
32. Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149-5158.
33. Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448-1460.
34. Bunck MC, Diamant M, Corner A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32:762-768.
35. Mari A, Degn K, Brock B, et al. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on beta-cell function in normal living conditions. Diabetes Care. 2007;30:2032-2033.
36. Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26:268-278.
37. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47.
38. Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84-90.
39. Vilsboll T, Brock B, Perrild H, et al. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with type 2 diabetes mellitus. Diabet Med. 2008;25:152-156.
40. Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733-745.
41. Brazg R, Xu L, Dalla MC, et al. Effect of adding sitagliptin, a dipeptidyl peptidase-4 inhibitor, to metformin on 24-h glycaemic control and beta-cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:186-193.
42. Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638-2643.
43. DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes on metformin alone. Diabetes Care. 2009;32:1649-1655.
44. Meier JJ, Nauck MA. Is the diminished incretin effect in type 2 diabetes just an epi-phenomenon of impaired beta-cell function? Diabetes. 2010;59:1117-1125.
45. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473-481.
46. Chacra AR, Tan GH, Apanovitch A, et al. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract. 2009;63:1395-1406.
47. DeFronzo RA, Okerson T, Viswanathan P, et al. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943-2952.
Pathophysiology of type 2 diabetes mellitus: potential role of incretin-based therapies
Glucose-lowering effects of incretin-based therapies
Safety, tolerability, and nonglycemic effects of incretin-based therapies
- The pathophysiology of type 2 diabetes mellitus (T2DM) is multifactorial and includes mechanisms beyond insulin resistance and pancreatic β-cell dysfunction
- The differential actions of glucagon-like peptide (GLP)-1 agonists and dipeptidyl peptidase (DPP)-4 inhibitors include:
- The additional actions of GLP-1 agonists on pathophysiologic mechanisms include:
The authors received editorial assistance from the Primary Care Education Consortium and WriteHealth, LLC in the development of this activity and honoraria from the Primary Care Education Consortium. They have disclosed that Dr Campbell is on the advisory board for Daiichi-Sankyo and the speakers bureau for Eli Lilly and Co; Dr Cobble is on the advisory board for Abbott Laboratories, AstraZeneca, and Eli Lilly and Co and speakers bureau for Abbott Laboratories, AstraZeneca/Bristol Myers Squibb, Eli Lilly and Co, GlaxoSmithKline, and Novo Nordisk Inc; Dr Reid is on the advisory board and speakers bureau for Amylin Pharmaceuticals, Medtronic, Novo Nordisk Inc, and sanofi-aventis; and Dr Shomali is on the advisory board for Novo Nordisk Inc and speakers bureau for Amylin Pharmaceuticals, Eli Lilly and Co, sanofi-aventis, and Takeda Pharmaceuticals.
Introduction
The pathophysiology of T2DM and the incretin system have been described in considerable detail in previous Journal of Family Practice supplements.1,2 In this article, we will briefly review the multiple pathophysiologic mechanisms known to be involved in T2DM, with a focus on the incretin system, to gain a better understanding of the disease progression affecting our 3 patients presented in the supplement introduction.
The central roles of insulin resistance and pancreatic β-cell dysfunction (insulin deficiency) in the pathogenesis of T2DM have been recognized for decades. But other causes of T2DM have been identified, including:
- Altered glucose release and disposal
- Altered glucagon secretion
- Impaired incretin response
- Rapid gastric emptying
- Impaired satiety
While there is a link between insulin resistance and pancreatic β-cell failure,3 the extent to which other causes interact is only beginning to be recognized. With a better understanding of the multifactorial pathogenesis of T2DM, however, has come additional treatment options and the opportunity for a more individualized and complementary approach to treatment.
Insulin resistance and pancreatic β-cell dysfunction
T2DM is a progressive disease characterized by worsening hyperglycemia,4 caused in part by insulin resistance and deterioration in pancreatic β-cell function. Insulin resistance appears to result from a complex interaction between visceral fat and the immune system that results in a state of chronic inflammation. Proinflammatory proteins secreted primarily from visceral fat block the action of insulin in adipocytes.5 Elevated levels of free fatty acids, which are common in obesity, further promote insulin resistance.6
Pancreatic β-cell dysfunction occurs progressively over a decade or more until β-cells are no longer capable of secreting sufficient insulin. Data from several studies suggest that, on average, 50% to 80% of β-cell function has been lost at the time of diagnosis of T2DM.7-9 The extent of pancreatic β-cell dysfunction is important for 2 reasons. First, medications that act by stimulating the β-cell to secrete insulin lose their effectiveness over time, as shown in the UK Prospective Diabetes Study (UKPDS)7 and, more recently, in A Diabetes Outcome Progression Trial (ADOPT).10 Second, since only 20% to 50% of pancreatic β-cell function remains at the time of diagnosis, preserving β-cell function would likely be beneficial for long-term glycemic control.
But there is no time to waste following diagnosis of T2DM. The Belfast Diet Study,8 a 10-year prospective clinical trial of 432 newly diagnosed persons with T2DM treated with intensive lifestyle management, suggested that β-cell decline occurs in 2 phases. Beginning well before diagnosis, the annual rate of decline in β-cell function during the first phase, ≥15 years prediagnosis, is about 2%, while during the second phase, beginning about 3 years postdiagnosis, the annual rate of decline in β-cell function accelerates to 18% (FIGURE 1). This second phase appears to result from a combination of steadily increasing β-cell death rate and decreasing replication rate.8
Case 2: A 47-year-old man diagnosed with T2DM several years ago who has experienced edema and weight gain on pioglitazone
Case 3: A 68-year-old woman with longstanding T2DM who has failed dual oral therapy and has microvascular complications
FIGURE 1 Phases of decline in pancreatic β-cell function8
HOMA %B, homeostasis model of assessment–percent β-cell function.
Bagust A, Beale S. Deteriorating beta-cell function in type 2 diabetes: a long-term model. QJM. 2003;96(4):281-288, by permission of Oxford University Press.
Considering insulin resistance and pancreatic β-cell dysfunction in our 3 cases
- Newly diagnosed with T2DM, the patient in Case 1 probably has 20% to 50% of his β-cell function remaining; preservation of β-cell function would be desirable, especially before he reaches the second phase of decline in β-cell function, about 3 years after diagnosis
- Diagnosed with T2DM about 2.5 years ago and not previously treated with a secretagogue, the patient in Case 2 has some β-cell function remaining; however, he is likely close to entering the second phase of steep decline in β-cell function observed in the Belfast Diet Study; in addition, insulin resistance related to his obesity must be addressed, as his obesity serves to stimulate pancreatic β-cells to secrete more insulin
- The Case 3 patient, diagnosed with T2DM about 5 years ago, is probably in the second phase of steep decline in β-cell function and has limited β-cell function remaining; furthermore, she has failed dual oral therapy that included almost 2 years of treatment with glyburide
The incretin system and GLP-1
As discussed in greater detail in a previous Journal of Family Practice supplement,1 the incretin system is integrally involved in glucose homeostasis. Glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 are the 2 most important incretin hormones secreted in response to food ingestion. Patients with T2DM are resistant to GIP, which contributes to the impaired incretin response. In animal models and humans, GLP-1, the most clinically important incretin hormone, has been shown to:
- Increase insulin biosynthesis in a glucose-dependent manner through direct activation of receptors on islet β-cells and via the vagus nerve11-13
- Inhibit glucagon secretion in a glucose-dependent manner through direct activation of receptors on islet α-cells12,14,15
- Slow gastric emptying16
- Promote satiety17,18
- Promote proliferation, increase differentiation, and prolong survival of β-cells19-21
- Improve myocardial function22
The actions of endogenous GLP-1 are short-lived, as GLP-1 undergoes rapid degradation by the enzyme DPP-4. To overcome this rapid degradation, 2 treatment approaches have been taken. Injectable GLP-1 agonists, which are resistant to the enzymatic action of DPP-4, have been developed. Oral DPP-4 inhibitors, which allow for prolonged physiologic actions of endogenous GLP-1, also have been developed. Because of the pharmacologic levels of GLP-1 activity achieved, GLP-1 agonists have better glucose-lowering efficacy and also promote weight loss compared with DPP-4 inhibitors.23-26
Impaired incretin effect in T2DM
Until recently, it was thought that secretion of GLP-1 in response to ingestion of a meal in people with T2DM was significantly impaired compared with that in healthy controls (P<.001).27,28 Recent investigation, however, found that the GLP-1 levels in patients with T2DM did not decrease after oral ingestion of glucose or a mixed meal. The secretion of GIP, on the other hand, increased following oral ingestion of a mixed meal but not of glucose.29 These observations suggest that the unaltered secretion of GLP-1 in people with T2DM may be insufficient to make up for the diminished insulinotropic activity of GIP in people with T2DM. Consequently, pharmacologic levels of GLP-1 would be necessary to restore the insulinotropic actions of the incretin system.30
Incretin effects on the pancreatic β-cell and insulin resistance
Animal studies have indicated that GLP-1 has the ability to preserve β-cell function by suppressing β-cell apoptosis and stimulating neogenesis and proliferation.31,32 Several trials in people with T2DM have shown that administration of a GLP-1 agonist (exenatide or liraglutide) for up to 52 weeks either as monotherapy or added on to existing therapy results in significant improvement in pancreatic β-cell function.33-39 One trial involving patients whose treatment with metformin had not provided glycemic control showed a significant increase in first- and second-phase glucose-stimulated C-peptide secretion, both markers of β-cell function, with exenatide (1.53- and 2.85-fold, respectively; P<.0001) compared with insulin glargine.34 A 26-week trial found that the addition of exenatide or liraglutide to metformin, a sulfonylurea, or both resulted in improvement in β-cell function, as determined by the homeostasis model of assessment– β-cell function (HOMA-B); the improvement was significantly greater with liraglutide than with exenatide (32% vs 3%; P<.0001).37 The greater improvement in β-cell function may be a reflection of the greater lowering of fasting plasma glucose with liraglutide than with exenatide.
Clinical trials with DPP-4 inhibitors (saxagliptin or sitagliptin) also have shown significant improvement in β-cell function (up to 16%; P<.05) over 24 weeks.40-43 Generally, however, there are fewer data regarding effects on pancreatic β-cell function for the DPP-4 inhibitors than for the GLP-1 agonists.44
Treatment with a GLP-1 agonist or DPP-4 inhibitor also appears to improve insulin resistance and sensitivity.34,41,42,45 A 52-week trial found a significant reduction in insulin resistance with liraglutide 1.2 mg or 1.8 mg once daily (–0.65% and –1.35%, respectively) compared with a 0.85% increase with glimepiride (P=.0249 and P=.0011 vs liraglutide 1.2 mg and 1.8 mg, respectively).45 Another trial found comparable improvement in insulin sensitivity following 52 weeks of treatment with exenatide or insulin glargine (0.9 vs 1.1 mg/min-1/kg-1, respectively; P=.49).34
These preclinical and clinical data regarding pancreatic β-cell function must be viewed as preliminary, and require further investigation. If confirmed, the ability to alter the natural progression of β-cell loss in T2DM and/or to reduce insulin resistance would be of significant clinical value.
Incretin effects on other causes of T2DM
Both the GLP-1 agonists and the DPP-4 inhibitors affect other mechanisms involved in the pathogenesis of T2DM. Several trials have shown a significant reduction in fasting glucagon secretion with GLP-1 agonist37 or DPP-4 inhibitor treatment.43,46,47 The addition of exenatide or liraglutide to metformin, a sulfonylurea, or both for 26 weeks resulted in a reduction in fasting glucagon secretion of 12.3 and 19.4 ng/L, respectively (P=.1436), after 26 weeks.37 Addition of saxagliptin to a submaximal dose of a sulfonylurea resulted in a reduction of glucagon secretion of 0.8 ng/L compared with an increase of 4.5 ng/L with uptitration of the sulfonylurea alone for 24 weeks. A 2-week crossover trial comparing exenatide with sitagliptin showed that compared with sitagliptin, exenatide reduced postprandial glucagon by 12% (P=.0011)47 (FIGURE 2); in addition, exenatide slowed the gastric emptying rate by 44% (P<.0001), with a commensurate decrease in total caloric intake of 134 kcal with exenatide vs an increase of 130 kcal with sitagliptin (P=.0227).47
FIGURE 2 Reduction in glucagon secretion with exenatide or sitagliptin47
Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Current Medical Research and Opinion. 2008, reprinted with permission of Taylor & Francis Group.
Summary
The multifactorial nature of the pathogenesis of T2DM provides an opportunity to combine treatments that act upon different mechanisms. In addition to improving insulin resistance and pancreatic β-cell dysfunction, the GLP-1 agonists and DPP-4 inhibitors improve the impaired incretin response, as well as increase insulin secretion and reduce glucagon secretion, both in a glucose-dependent manner. As a result of these multiple actions, the GLP-1 agonists and DPP-4 inhibitors lower both fasting and postprandial glucose levels. The effects of GLP-1 agonists tend to be greater, probably because they produce pharmacologic levels of GLP-1 compared to physiologic levels with the DPP-4 inhibitors. Another difference is that unlike the DPP-4 inhibitors, the GLP-1 agonists also slow gastric emptying and promote satiety.
Pathophysiology of type 2 diabetes mellitus: potential role of incretin-based therapies
Glucose-lowering effects of incretin-based therapies
Safety, tolerability, and nonglycemic effects of incretin-based therapies
- The pathophysiology of type 2 diabetes mellitus (T2DM) is multifactorial and includes mechanisms beyond insulin resistance and pancreatic β-cell dysfunction
- The differential actions of glucagon-like peptide (GLP)-1 agonists and dipeptidyl peptidase (DPP)-4 inhibitors include:
- The additional actions of GLP-1 agonists on pathophysiologic mechanisms include:
The authors received editorial assistance from the Primary Care Education Consortium and WriteHealth, LLC in the development of this activity and honoraria from the Primary Care Education Consortium. They have disclosed that Dr Campbell is on the advisory board for Daiichi-Sankyo and the speakers bureau for Eli Lilly and Co; Dr Cobble is on the advisory board for Abbott Laboratories, AstraZeneca, and Eli Lilly and Co and speakers bureau for Abbott Laboratories, AstraZeneca/Bristol Myers Squibb, Eli Lilly and Co, GlaxoSmithKline, and Novo Nordisk Inc; Dr Reid is on the advisory board and speakers bureau for Amylin Pharmaceuticals, Medtronic, Novo Nordisk Inc, and sanofi-aventis; and Dr Shomali is on the advisory board for Novo Nordisk Inc and speakers bureau for Amylin Pharmaceuticals, Eli Lilly and Co, sanofi-aventis, and Takeda Pharmaceuticals.
Introduction
The pathophysiology of T2DM and the incretin system have been described in considerable detail in previous Journal of Family Practice supplements.1,2 In this article, we will briefly review the multiple pathophysiologic mechanisms known to be involved in T2DM, with a focus on the incretin system, to gain a better understanding of the disease progression affecting our 3 patients presented in the supplement introduction.
The central roles of insulin resistance and pancreatic β-cell dysfunction (insulin deficiency) in the pathogenesis of T2DM have been recognized for decades. But other causes of T2DM have been identified, including:
- Altered glucose release and disposal
- Altered glucagon secretion
- Impaired incretin response
- Rapid gastric emptying
- Impaired satiety
While there is a link between insulin resistance and pancreatic β-cell failure,3 the extent to which other causes interact is only beginning to be recognized. With a better understanding of the multifactorial pathogenesis of T2DM, however, has come additional treatment options and the opportunity for a more individualized and complementary approach to treatment.
Insulin resistance and pancreatic β-cell dysfunction
T2DM is a progressive disease characterized by worsening hyperglycemia,4 caused in part by insulin resistance and deterioration in pancreatic β-cell function. Insulin resistance appears to result from a complex interaction between visceral fat and the immune system that results in a state of chronic inflammation. Proinflammatory proteins secreted primarily from visceral fat block the action of insulin in adipocytes.5 Elevated levels of free fatty acids, which are common in obesity, further promote insulin resistance.6
Pancreatic β-cell dysfunction occurs progressively over a decade or more until β-cells are no longer capable of secreting sufficient insulin. Data from several studies suggest that, on average, 50% to 80% of β-cell function has been lost at the time of diagnosis of T2DM.7-9 The extent of pancreatic β-cell dysfunction is important for 2 reasons. First, medications that act by stimulating the β-cell to secrete insulin lose their effectiveness over time, as shown in the UK Prospective Diabetes Study (UKPDS)7 and, more recently, in A Diabetes Outcome Progression Trial (ADOPT).10 Second, since only 20% to 50% of pancreatic β-cell function remains at the time of diagnosis, preserving β-cell function would likely be beneficial for long-term glycemic control.
But there is no time to waste following diagnosis of T2DM. The Belfast Diet Study,8 a 10-year prospective clinical trial of 432 newly diagnosed persons with T2DM treated with intensive lifestyle management, suggested that β-cell decline occurs in 2 phases. Beginning well before diagnosis, the annual rate of decline in β-cell function during the first phase, ≥15 years prediagnosis, is about 2%, while during the second phase, beginning about 3 years postdiagnosis, the annual rate of decline in β-cell function accelerates to 18% (FIGURE 1). This second phase appears to result from a combination of steadily increasing β-cell death rate and decreasing replication rate.8
Case 2: A 47-year-old man diagnosed with T2DM several years ago who has experienced edema and weight gain on pioglitazone
Case 3: A 68-year-old woman with longstanding T2DM who has failed dual oral therapy and has microvascular complications
FIGURE 1 Phases of decline in pancreatic β-cell function8
HOMA %B, homeostasis model of assessment–percent β-cell function.
Bagust A, Beale S. Deteriorating beta-cell function in type 2 diabetes: a long-term model. QJM. 2003;96(4):281-288, by permission of Oxford University Press.
Considering insulin resistance and pancreatic β-cell dysfunction in our 3 cases
- Newly diagnosed with T2DM, the patient in Case 1 probably has 20% to 50% of his β-cell function remaining; preservation of β-cell function would be desirable, especially before he reaches the second phase of decline in β-cell function, about 3 years after diagnosis
- Diagnosed with T2DM about 2.5 years ago and not previously treated with a secretagogue, the patient in Case 2 has some β-cell function remaining; however, he is likely close to entering the second phase of steep decline in β-cell function observed in the Belfast Diet Study; in addition, insulin resistance related to his obesity must be addressed, as his obesity serves to stimulate pancreatic β-cells to secrete more insulin
- The Case 3 patient, diagnosed with T2DM about 5 years ago, is probably in the second phase of steep decline in β-cell function and has limited β-cell function remaining; furthermore, she has failed dual oral therapy that included almost 2 years of treatment with glyburide
The incretin system and GLP-1
As discussed in greater detail in a previous Journal of Family Practice supplement,1 the incretin system is integrally involved in glucose homeostasis. Glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 are the 2 most important incretin hormones secreted in response to food ingestion. Patients with T2DM are resistant to GIP, which contributes to the impaired incretin response. In animal models and humans, GLP-1, the most clinically important incretin hormone, has been shown to:
- Increase insulin biosynthesis in a glucose-dependent manner through direct activation of receptors on islet β-cells and via the vagus nerve11-13
- Inhibit glucagon secretion in a glucose-dependent manner through direct activation of receptors on islet α-cells12,14,15
- Slow gastric emptying16
- Promote satiety17,18
- Promote proliferation, increase differentiation, and prolong survival of β-cells19-21
- Improve myocardial function22
The actions of endogenous GLP-1 are short-lived, as GLP-1 undergoes rapid degradation by the enzyme DPP-4. To overcome this rapid degradation, 2 treatment approaches have been taken. Injectable GLP-1 agonists, which are resistant to the enzymatic action of DPP-4, have been developed. Oral DPP-4 inhibitors, which allow for prolonged physiologic actions of endogenous GLP-1, also have been developed. Because of the pharmacologic levels of GLP-1 activity achieved, GLP-1 agonists have better glucose-lowering efficacy and also promote weight loss compared with DPP-4 inhibitors.23-26
Impaired incretin effect in T2DM
Until recently, it was thought that secretion of GLP-1 in response to ingestion of a meal in people with T2DM was significantly impaired compared with that in healthy controls (P<.001).27,28 Recent investigation, however, found that the GLP-1 levels in patients with T2DM did not decrease after oral ingestion of glucose or a mixed meal. The secretion of GIP, on the other hand, increased following oral ingestion of a mixed meal but not of glucose.29 These observations suggest that the unaltered secretion of GLP-1 in people with T2DM may be insufficient to make up for the diminished insulinotropic activity of GIP in people with T2DM. Consequently, pharmacologic levels of GLP-1 would be necessary to restore the insulinotropic actions of the incretin system.30
Incretin effects on the pancreatic β-cell and insulin resistance
Animal studies have indicated that GLP-1 has the ability to preserve β-cell function by suppressing β-cell apoptosis and stimulating neogenesis and proliferation.31,32 Several trials in people with T2DM have shown that administration of a GLP-1 agonist (exenatide or liraglutide) for up to 52 weeks either as monotherapy or added on to existing therapy results in significant improvement in pancreatic β-cell function.33-39 One trial involving patients whose treatment with metformin had not provided glycemic control showed a significant increase in first- and second-phase glucose-stimulated C-peptide secretion, both markers of β-cell function, with exenatide (1.53- and 2.85-fold, respectively; P<.0001) compared with insulin glargine.34 A 26-week trial found that the addition of exenatide or liraglutide to metformin, a sulfonylurea, or both resulted in improvement in β-cell function, as determined by the homeostasis model of assessment– β-cell function (HOMA-B); the improvement was significantly greater with liraglutide than with exenatide (32% vs 3%; P<.0001).37 The greater improvement in β-cell function may be a reflection of the greater lowering of fasting plasma glucose with liraglutide than with exenatide.
Clinical trials with DPP-4 inhibitors (saxagliptin or sitagliptin) also have shown significant improvement in β-cell function (up to 16%; P<.05) over 24 weeks.40-43 Generally, however, there are fewer data regarding effects on pancreatic β-cell function for the DPP-4 inhibitors than for the GLP-1 agonists.44
Treatment with a GLP-1 agonist or DPP-4 inhibitor also appears to improve insulin resistance and sensitivity.34,41,42,45 A 52-week trial found a significant reduction in insulin resistance with liraglutide 1.2 mg or 1.8 mg once daily (–0.65% and –1.35%, respectively) compared with a 0.85% increase with glimepiride (P=.0249 and P=.0011 vs liraglutide 1.2 mg and 1.8 mg, respectively).45 Another trial found comparable improvement in insulin sensitivity following 52 weeks of treatment with exenatide or insulin glargine (0.9 vs 1.1 mg/min-1/kg-1, respectively; P=.49).34
These preclinical and clinical data regarding pancreatic β-cell function must be viewed as preliminary, and require further investigation. If confirmed, the ability to alter the natural progression of β-cell loss in T2DM and/or to reduce insulin resistance would be of significant clinical value.
Incretin effects on other causes of T2DM
Both the GLP-1 agonists and the DPP-4 inhibitors affect other mechanisms involved in the pathogenesis of T2DM. Several trials have shown a significant reduction in fasting glucagon secretion with GLP-1 agonist37 or DPP-4 inhibitor treatment.43,46,47 The addition of exenatide or liraglutide to metformin, a sulfonylurea, or both for 26 weeks resulted in a reduction in fasting glucagon secretion of 12.3 and 19.4 ng/L, respectively (P=.1436), after 26 weeks.37 Addition of saxagliptin to a submaximal dose of a sulfonylurea resulted in a reduction of glucagon secretion of 0.8 ng/L compared with an increase of 4.5 ng/L with uptitration of the sulfonylurea alone for 24 weeks. A 2-week crossover trial comparing exenatide with sitagliptin showed that compared with sitagliptin, exenatide reduced postprandial glucagon by 12% (P=.0011)47 (FIGURE 2); in addition, exenatide slowed the gastric emptying rate by 44% (P<.0001), with a commensurate decrease in total caloric intake of 134 kcal with exenatide vs an increase of 130 kcal with sitagliptin (P=.0227).47
FIGURE 2 Reduction in glucagon secretion with exenatide or sitagliptin47
Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Current Medical Research and Opinion. 2008, reprinted with permission of Taylor & Francis Group.
Summary
The multifactorial nature of the pathogenesis of T2DM provides an opportunity to combine treatments that act upon different mechanisms. In addition to improving insulin resistance and pancreatic β-cell dysfunction, the GLP-1 agonists and DPP-4 inhibitors improve the impaired incretin response, as well as increase insulin secretion and reduce glucagon secretion, both in a glucose-dependent manner. As a result of these multiple actions, the GLP-1 agonists and DPP-4 inhibitors lower both fasting and postprandial glucose levels. The effects of GLP-1 agonists tend to be greater, probably because they produce pharmacologic levels of GLP-1 compared to physiologic levels with the DPP-4 inhibitors. Another difference is that unlike the DPP-4 inhibitors, the GLP-1 agonists also slow gastric emptying and promote satiety.
1. Holst JJ, LaSalle JR. An overview of incretin hormones. J Fam Prac. 2008;57(suppl):S4-S9.
2. Peterson GE. A checklist approach to selecting the optimal treatment regimen for a patient with type 2 diabetes. J Fam Pract. 2009;58(suppl):S21-S25.
3. Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(suppl 3):S16-S21.
4. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
5. Lin Y, Sun Z. Current views on type 2 diabetes. J Endocrinol. 2010;204:1-11.
6. Aronne LJ, Isoldi KK. Overweight and obesity: key components of cardiometabolic risk. Clin Cornerstone. 2007;8:29-37.
7. United Kingdom Prospective Diabetes Study Group. UK Prospective Diabetes Study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249-1258.
8. Bagust A, Beale S. Deteriorating beta-cell function in type 2 diabetes: a long-term model. QJM. 2003;96:281-288.
9. DeFronzo R, Banerji MA, Bray G, et al. Reduced insulin secretion/insulin resistance (disposition) index is the primary determinant of glucose intolerance in the pre-diabetic state: results from ACT NOW. Paper presented at: American Diabetes Association 68th Scientific Session; June 6-10, 2008; San Francisco, CA.
10. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
11. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153-165.
12. Naslund E, Bogefors J, Skogar S, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;277:R910-R916.
13. Fehmann HC, Habener JF. Insulinotropic hormone glucagon-like peptide-I(7-37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology. 1992;130:159-166.
14. Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300-1304.
15. Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239-1246.
16. Delgado-Aros S, Kim DY, Burton DD, et al. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G424-G431.
17. Gutzwiller JP, Goke B, Drewe J, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81-86.
18. Gutzwiller JP, Drewe J, Goke B, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541-R1544.
19. Xu G, Stoffers DA, Habener JF, et al. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270-2276.
20. Zhou J, Wang X, Pineyro MA, et al. Glucagon-like peptide 1 and exendin-4 convert pancreatic AR42J cells into glucagon- and insulin-producing cells. Diabetes. 1999;48:2358-2366.
21. Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17:161-171.
22. Girard J. The incretins: from the concept to their use in the treatment of type 2 diabetes. Part A: incretins: concept and physiological functions. Diabetes Metab. 2008;34:550-559.
23. Vilsboll T, Krarup T, Madsbad S, et al. Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia. 2002;45:1111-1119.
24. Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301-307.
25. Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824-830.
26. Deacon CF, Hughes TE, Holst JJ. Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig. Diabetes. 1998;47:764-769.
27. Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199-E206.
28. Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717-3723.
29. Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678-687.
30. Nauck MA. Unraveling the science of incretin biology. Am J Med. 2009;122(suppl):S3-S10.
31. Bulotta A, Hui H, Anastasi E, et al. Cultured pancreatic ductal cells undergo cell cycle re-distribution and beta-cell-like differentiation in response to glucagon-like peptide-1. J Mol Endocrinol. 2002;29:347-360.
32. Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149-5158.
33. Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448-1460.
34. Bunck MC, Diamant M, Corner A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32:762-768.
35. Mari A, Degn K, Brock B, et al. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on beta-cell function in normal living conditions. Diabetes Care. 2007;30:2032-2033.
36. Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26:268-278.
37. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47.
38. Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84-90.
39. Vilsboll T, Brock B, Perrild H, et al. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with type 2 diabetes mellitus. Diabet Med. 2008;25:152-156.
40. Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733-745.
41. Brazg R, Xu L, Dalla MC, et al. Effect of adding sitagliptin, a dipeptidyl peptidase-4 inhibitor, to metformin on 24-h glycaemic control and beta-cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:186-193.
42. Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638-2643.
43. DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes on metformin alone. Diabetes Care. 2009;32:1649-1655.
44. Meier JJ, Nauck MA. Is the diminished incretin effect in type 2 diabetes just an epi-phenomenon of impaired beta-cell function? Diabetes. 2010;59:1117-1125.
45. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473-481.
46. Chacra AR, Tan GH, Apanovitch A, et al. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract. 2009;63:1395-1406.
47. DeFronzo RA, Okerson T, Viswanathan P, et al. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943-2952.
1. Holst JJ, LaSalle JR. An overview of incretin hormones. J Fam Prac. 2008;57(suppl):S4-S9.
2. Peterson GE. A checklist approach to selecting the optimal treatment regimen for a patient with type 2 diabetes. J Fam Pract. 2009;58(suppl):S21-S25.
3. Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004;53(suppl 3):S16-S21.
4. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
5. Lin Y, Sun Z. Current views on type 2 diabetes. J Endocrinol. 2010;204:1-11.
6. Aronne LJ, Isoldi KK. Overweight and obesity: key components of cardiometabolic risk. Clin Cornerstone. 2007;8:29-37.
7. United Kingdom Prospective Diabetes Study Group. UK Prospective Diabetes Study 16. Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249-1258.
8. Bagust A, Beale S. Deteriorating beta-cell function in type 2 diabetes: a long-term model. QJM. 2003;96:281-288.
9. DeFronzo R, Banerji MA, Bray G, et al. Reduced insulin secretion/insulin resistance (disposition) index is the primary determinant of glucose intolerance in the pre-diabetic state: results from ACT NOW. Paper presented at: American Diabetes Association 68th Scientific Session; June 6-10, 2008; San Francisco, CA.
10. Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427-2443.
11. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153-165.
12. Naslund E, Bogefors J, Skogar S, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;277:R910-R916.
13. Fehmann HC, Habener JF. Insulinotropic hormone glucagon-like peptide-I(7-37) stimulation of proinsulin gene expression and proinsulin biosynthesis in insulinoma beta TC-1 cells. Endocrinology. 1992;130:159-166.
14. Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300-1304.
15. Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239-1246.
16. Delgado-Aros S, Kim DY, Burton DD, et al. Effect of GLP-1 on gastric volume, emptying, maximum volume ingested, and postprandial symptoms in humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G424-G431.
17. Gutzwiller JP, Goke B, Drewe J, et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut. 1999;44:81-86.
18. Gutzwiller JP, Drewe J, Goke B, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541-R1544.
19. Xu G, Stoffers DA, Habener JF, et al. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270-2276.
20. Zhou J, Wang X, Pineyro MA, et al. Glucagon-like peptide 1 and exendin-4 convert pancreatic AR42J cells into glucagon- and insulin-producing cells. Diabetes. 1999;48:2358-2366.
21. Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17:161-171.
22. Girard J. The incretins: from the concept to their use in the treatment of type 2 diabetes. Part A: incretins: concept and physiological functions. Diabetes Metab. 2008;34:550-559.
23. Vilsboll T, Krarup T, Madsbad S, et al. Defective amplification of the late phase insulin response to glucose by GIP in obese type II diabetic patients. Diabetologia. 2002;45:1111-1119.
24. Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301-307.
25. Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824-830.
26. Deacon CF, Hughes TE, Holst JJ. Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig. Diabetes. 1998;47:764-769.
27. Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199-E206.
28. Toft-Nielsen MB, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717-3723.
29. Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes. 2008;57:678-687.
30. Nauck MA. Unraveling the science of incretin biology. Am J Med. 2009;122(suppl):S3-S10.
31. Bulotta A, Hui H, Anastasi E, et al. Cultured pancreatic ductal cells undergo cell cycle re-distribution and beta-cell-like differentiation in response to glucagon-like peptide-1. J Mol Endocrinol. 2002;29:347-360.
32. Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149-5158.
33. Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448-1460.
34. Bunck MC, Diamant M, Corner A, et al. One-year treatment with exenatide improves beta-cell function, compared with insulin glargine, in metformin-treated type 2 diabetic patients: a randomized, controlled trial. Diabetes Care. 2009;32:762-768.
35. Mari A, Degn K, Brock B, et al. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on beta-cell function in normal living conditions. Diabetes Care. 2007;30:2032-2033.
36. Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26:268-278.
37. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47.
38. Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84-90.
39. Vilsboll T, Brock B, Perrild H, et al. Liraglutide, a once-daily human GLP-1 analogue, improves pancreatic B-cell function and arginine-stimulated insulin secretion during hyperglycaemia in patients with type 2 diabetes mellitus. Diabet Med. 2008;25:152-156.
40. Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733-745.
41. Brazg R, Xu L, Dalla MC, et al. Effect of adding sitagliptin, a dipeptidyl peptidase-4 inhibitor, to metformin on 24-h glycaemic control and beta-cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:186-193.
42. Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638-2643.
43. DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes on metformin alone. Diabetes Care. 2009;32:1649-1655.
44. Meier JJ, Nauck MA. Is the diminished incretin effect in type 2 diabetes just an epi-phenomenon of impaired beta-cell function? Diabetes. 2010;59:1117-1125.
45. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473-481.
46. Chacra AR, Tan GH, Apanovitch A, et al. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract. 2009;63:1395-1406.
47. DeFronzo RA, Okerson T, Viswanathan P, et al. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943-2952.