User login
Statin therapy has been shown to substantially reduce adverse events associated with low-density-lipoprotein cholesterol (LDL-C) and cardiovascular disease (CVD). Statins alone are often not adequate to achieve treatment goals, and residual CVD risk remains high. Combination therapies of statins with ezetimibe and resins to further lower LDL-C, fibrates and omega 3 fatty acids to lower triglycerides, and niacin to lower both and raise high-density-liproprotein cholesterol are available, but additional risk reduction has not been consistently demonstrated in clinical trials.
The link between atherogenic lipoproteins and CVD is strong, and the need to develop therapies in addition to statins to substantially and safely reduce LDL-C is a priority. The association of reduced proprotein convertase subtilisin/kexin type 9 (PCSK9) activity with reduced LDL-C and CVD events has led to the rapid development and approval of monoclonal antibody therapies to inhibit PCSK9.
In this review, we discuss trials of these therapies that have shown durable reductions in LDL-C of more than 50%, with acceptable tolerability. Now that PCSK9 inhibitors are approved by the US Food and Drug Administration (FDA), extended data are needed as to long-term tolerability, safety, and efficacy of these agents and, most importantly, demonstration of additional reduction in CVD events.
A CASE FOR ADDITIONAL THERAPIES
CVD is the leading cause of morbidity and death in the United States, responsible for one in four deaths. Hyperlipidemia and, specifically, elevated LDL-C have been found to be important drivers of atherosclerosis and, in turn, adverse cardiovascular (CV) events. Likewise, numerous observational and clinical trials have shown that reducing LDL-C, particularly with statins, decreases CVD events.1–4 More aggressive lowering with higher doses or more intensive statin therapy further reduces rates of adverse outcomes.3,4 In addition, the pleiotropic effects of statins imply that not all of their benefits are derived from LDL-C lowering alone.5 Consequently, it is now standard practice to use statins at the highest tolerable dose to reach target LDL-C levels and prevent CV events in high-risk patients with CVD or multiple coronary artery disease risk factors, regardless of the LDL-C levels.6,7
The American College of Cardiology (ACC) and the American Heart Association released cholesterol guidelines in 2013 that recommend a risk-based approach for statin therapy rather than targeting specific LDL-C levels.6 Although this evidence-based approach may better conform to clinical trials, the debate that lower LDL-C targets will further prevent CVD continues.
Indeed, it appears that lower is better, as demonstrated by the IMPROVE-IT trial.8 Although the control group receiving simvastatin monotherapy had low LDL-C levels (mean, 69.9 mg/dL; 1.8 mmol/L), the experimental group receiving simvastatin plus ezetimibe achieved even lower levels (mean, 53.2 mg/dL;1.4 mmol/L) after 1 year of therapy and had a significantly lower composite primary end point of CV death, major coronary event, or nonfatal stroke at 7 years (34.7% for simvastatin monotherapy vs 32.7% for combined therapy).9 Furthermore, the event-rate reduction with the addition of ezetimibe was the same as the average predicted by the Cholesterol Treatment Trialists’ meta-analysis: an LDL-C reduction of 1 mmol/L (38.6 mg/dL) yields a 23% risk reduction in major coronary events over 5 years.10 Although only a modest absolute reduction in outcomes, it supports the notion that further reduction of LDL-C levels by more potent therapies may offer greater benefit.
There is strong evidence that statin therapy reduces the risk of developing CVD in patients with or without a previous atherosclerotic event; however, residual CVD risk remains even for those on therapy. A contributing factor to this residual risk is that many statin-treated patients have insufficient response or intolerance and do not achieve adequate LDL-C reductions.
There are three clinically important patient populations who are inadequately managed with current therapies and remain at high risk of subsequent CV events; these are patients who would benefit from additional therapies.
1. Patients with familial hypercholesterolemia (FH). This is the most common genetic disorder in the world, yet it is frequently undiagnosed and untreated. Due to high baseline cholesterol levels, achieving LDL-C treatment goals is challenging.
- The prevalence may be closer to 1:200 to 1:250 rather than the often quoted 1:500.11
- Fewer than 12% of patients with heterzygous FH achieve the LDL-C goal of < 100 mg/dL with maximal statin treatment alone or with a second agent.12
2. Patients with hyperlipidemia not due to FH who are at elevated CV risk and undertreated. In US and European surveys, between 50% and 60% of patients receiving statins with or without other therapies failed to reach LDL-C reduction goals.13
- Variation in response to statin treatment between individuals may be considerable.
- Poor adherence to statin therapy is common.
3. Patients with side effects to statins, particularly muscle symptoms that prevent statin use or substantially limit the dose.
- Although the incidence of myopathy is low (< 0.1%) and rhabdomyolysis is even less common, observational studies suggest that 10% to 20% of patients may limit statin use due to muscle-associated complaints including muscle aching, cramps, or weakness.14
- Side effects may be dose-dependent, limiting the use of the high-intensity statin doses that are frequently necessary to achieve LDL-C goals.
Consequently, there is great interest in developing therapies beyond statins that may further reduce CV events. However, treatments other than ezetimibe for further management of hyperlipidemia and risk reduction have failed to demonstrate consistent benefit when added to statin therapy.15–19 The largest studies were with niacin and fibrates. Unfortunately, most trials demonstrated no overall outcomes benefit or only benefits in subgroup analyses, leaving the door open to other pharmacologic interventions.
Studies with the cholesterol ester transfer protein (CETP) inhibitor torcetrapib, in combination with statin therapy, actually demonstrated an overall increase in all-cause mortality in the treatment group.20 Two large outcome trials of the CETP inhibitors dalcetrapib and evacetrapib were stopped after interim analysis predicted no benefit. Although drugs such as lomitapide (a microsomal triglyceride transfer protein inhibitor) and mipomersen (an antisense oligonucleotide inhibitor of ApoB-100 synthesis) can lower LDL-C by reducing ApoB synthesis,21 they are approved only in the small population of individuals with homozygous FH and liver toxicity and side effects are a concern.
Accordingly, current cholesterol management guidelines continue to offer LDL-C as the main target of lipid-modifying therapy, with statins as the primary treatment choice. The desire to build on statin therapy to prevent further progression of atherosclerosis and clinical CVD has encouraged continued focus on strategies to lower LDL-C to even greater extents.
Fortunately for practitioners, for the first time since lovastatin was approved in 1987, there is a new therapy approved by the FDA that significantly lowers LDL-C and, potentially, improves CV outcomes—the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. This review will focus on the PCSK9 inhibitors, a novel therapeutic class that reduces LDL-C through increased hepatic clearance. These drugs are rapidly emerging as an ideal adjunctive therapy to statins for patients at the highest risk and as a highly efficacious alternate therapy in patients intolerant of statins.
PCSK9 INHIBITORS: DISCOVERY, MECHANISM, AND THERAPEUTIC INTERVENTIONS
Two PCSK9 inhibitors have received FDA approval: alirocumab (Praluent) and evolocumab (Repatha). Among new molecular entities for clinical use, PCSK9 inhibitor therapies had one of the shortest durations from discovery to development and approval.
Mutations in the PCSK9 gene associated with autosomal dominant hypercholesterolemia were first identified in 2003 in a French family.22 The PCSK9 protein is now known to be a secreted enzymatic serine protease that is primarily synthesized in the liver and binds to the LDL receptor (LDL-R)/LDL-C complex on the surface of hepatocytes, marking the receptor for lysosomal degradation rather than recycling to the cell surface. Thus, it reduces the quantity of LDL-R that is available to remove LDL-C from circulation.23 As a result, higher levels of PCSK9 are associated with higher levels of plasma LDL-C.
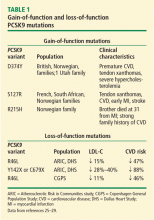
The clinical importance of PCSK9 in regulating LDL-C is supported by observed mutations and polymorphisms. Gain-of-function mutations that increase the activity of PCSK9 have been shown to be associated with elevated LDL-C, premature CVD, and myocardial infarction (MI).24 Conversely, loss-of-function mutations (heterozygotes found in 1% to 3% of the population) result in decreased activity of PCSK9, lower LDL-C, and lower incidence of CVD (Table 1).25–29 These observations, combined with data showing that homozygote loss-of-function individuals with very low LDL-C were generally very healthy, sparked interest in developing inhibition of PCSK9 activity as a therapeutic strategy for hyperlipidemia.
Multiple pharmacologic developments are aimed at inhibiting PCSK9, with many compounds in clinical trials. The approaches include gene silencing with loss-of-function mutations, synthetic peptides, oral small molecules, and monoclonal antibodies. Gene silencing was first observed in 2007 when administration of antisense oligonucleotides targeted to selectively inhibit PCSK9 mRNA was found to up-regulate LDL-R, thereby decreasing serum levels of LDL-C.30
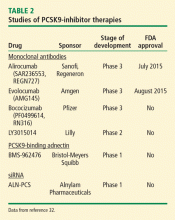
The first study to establish the role of synthetic peptides in PCSK9 inhibition was performed in 2008. In this study, the epidermal growth factor-like A synthetic peptide blocked the interaction between PCSK9 and LDL-R, thereby decreasing the degradation of LDL-R and preserving LDL uptake.31 Although studies are limited, synthetic peptides remain an area of great interest given their promising effects on lipid metabolism. Recently, a synthetic PCSK9-binding adnectin derived from the human fibronectin known as BMS-962476, had favorable results in a phase 1 clinical trial. An RNA interference molecule, subcutaneous ALN-PSC, inhibits PCSK9 gene expression by causing destruction of messenger RNA, thus inhibiting PCSK9 synthesis (Table 2).32
PCSK9 INHIBITORS: CLINICAL TRIALS
Subcutaneously administered monoclonal antibodies targeting PCSK9 currently are the only PCSK9 inhibitors FDA-approved for clinical use. The first study to demonstrate efficacy in enhancing uptake of serum LDL-C was performed in 2009.33 Multiple phase 1 and 2 studies soon followed, demonstrating acceptable safety and 50% to 70% reductions in LDL-C at upper-dose titrations.34 Additionally, there were significant reductions in total cholesterol, ApoB, triglycerides, and lipoprotein(a).
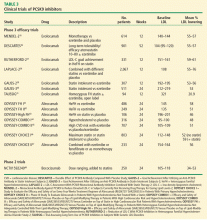
These early developments paved the way for larger phase 3 trials (Table 3).35–48 The PCSK9 inhibitors evolocumab and alirocumab have been shown in multiple phase 3 clinical trials to achieve a consistent dose-dependent 50% to 60% reduction in LDL-C across a broad range of CVD risk, pretreatment LDL-C levels, and background therapy: monotherapy (MENDEL-2, ODYSSEY COMBO I),35,44 added to statin therapy (LAPLACE-2, ODYSSEY CHOICE I),38,46 and in individuals with heterozygous FH (RUTHERFORD-2, ODYSSEY-FH).37,42 Trials with bococizumab are under way.
The GAUSS-2 clinical trial (Goal Achievement after Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects-2) demonstrated similar efficacy in reducing LDL-C in patients with clinically assessed statin intolerance due to muscle-related adverse symptoms.39 In GAUSS-3, patients were first identified as being statin intolerant secondary to muscle-associated symptoms based on a randomized, crossover trial of atorvastatin vs placebo.40 The 43% of participants who experienced intolerable muscle-related symptoms on the statin but not on placebo were then randomized to evolocumab vs ezetimibe. Results showed significant reduction in LDL-C in the evolocumab group (52.8%) compared with the ezetimibe group (16.7%). Additionally, among patients with muscle symptoms on statin therapy, PCSK9 therapy was discontinued for muscle symptoms in only 0.7% of evolocumab recipients and 6.8% of ezetimibe recipients.
Overall, the PCSK9 inhibitors are generally well tolerated with injection site reactions being the most common side effect. A meta-analysis published in 2015 of 25 trials including more than 12,000 patients treated with evolocumab and alirocumab reported no significant difference in adverse events or safety outcomes vs placebo or ezetimibe.49 Antidrug binding or neutralizing antibody production to these agents, thus far, has not been shown to be an issue. Additional analyses have not indicated an adverse effect on gonadal hormone levels or increased incidence of new-onset diabetes.
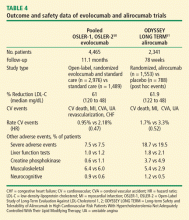
Two studies published in 2015 offer insight into longer term durability and safety as well as potential CVD outcome benefit (Table 4)50,51:
- OSLER-1 and 2: Open-Label Study of Long-Term Evaluation against LDL-Cholesterol (OSLER) trials—evolocumab trial;50
- ODYSSEY long term: Long-Term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy—alirocumab trial.51
The OSLER trials reported durable LDL-C reductions of 61% and the ODYSSEY trial reported a LDL-C reduction of 62%.50,51 In both studies, the overall occurrence of adverse events was similar to placebo, but both reported a higher rate of neurocognitive effects in the active treatment groups (evolocumab 0.9% vs 0.3% for standard therapy; alirocumab 1.2% vs 0.5% for placebo). It must be noted that although the absolute rate of neurocognitive adverse events is low, it is unclear if these events were related to the drugs themselves or to extreme lowering of LDL-C. Nevertheless, the FDA has raised concerns about neurocognitive events. A sub-study of the ongoing FOURIER trial with evolocumab—EBBINGHAUS—is expected to address this concern.
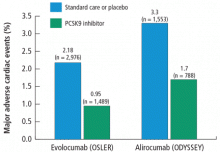
In addition, analyses of CV events showed that the PCSK9 inhibitors effectively cut the CV rate in half in both studies (Figure 1).50,51 In the OSLER trials,50 evolocumab recipients had 53% reduction in major CV events (0.95% vs 2.18% in the standard therapy group; P = .003). In ODYSSEY,51 alirocumab recipients had a 48% reduction in major CV events (1.7% vs 3.3% for placebo; P = .02). Furthermore, a 2015 meta-analysis of 24 phase 2 and 3 trials reported a statistically significant 55% reduction in all-cause mortality and 50% reduction in CV mortality with PCSK9 inhibitors.52
For many reasons including short length of follow-up, study design, and small numbers of outcome events, the OSLER and ODYSSEY studies, although enticing, are exploratory and hypothesis-generating only and results need to be interpreted with caution. Nevertheless, they have set the stage for ongoing prospective randomized outcome trials that are studying the CV effects and tolerability of PCSK9 inhibitors over a longer time frame. These include the following trials.
- The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) is an ongoing trial with the primary end point of CV death, MI, hospitalization for unstable angina, stroke, or coronary revascularization in high-risk patients receiving evolocumab or placebo.53
- The ODYSSEY trial is examining the effect of alirocumab vs placebo on the composite primary endpoint of coronary heart disease death, non-fatal MI, fatal and nonfatal ischemic stroke, and unstable angina requiring hospitalization in patients who have had an acute coronary syndrome event during the previous 4 to 52 weeks.54
- The Evaluation of Bococizumab in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE) trials are investigating the effect of bococizumab, a third PCSK9 “humanized” monoclonal antibody, vs placebo in reducing death, MI, stroke, or unstable angina in patients at high-risk of CVD who are receiving standard lipid-lowering therapy with LDL-C > 70 mg/dL (1.8 mmol/L) (SPIRE-1) or > 100 mg/dL (2.6 mmol/L) (SPIRE-2).55,56
Because these outcome trials are attempting to enroll more than 70,000 patients and are event driven, it is difficult to predict when they will be completed (Table 5).53–56 However, recent estimates indicate completion of at least one trial by the end of 2016 or early 2017, with interim analyses of others expected at that time. It is hoped that they will answer the all-important question of whether PCSK9 inhibitors are associated with further CV event reduction benefit.
CURRENT FDA INDICATIONS AND GUIDELINES
The two PCSK9 inhibitors approved by the FDA—alirocumab (subcutaneous 75 mg every 2 weeks up titrated to 150 mg) and evolocumab (subcutaneous 140 mg every 2 weeks or 420 mg every 4 weeks)—are both indicated for use with statins in patients with heterozygous FH or known atherosclerotic CVD who require further reduction in LDL-C levels despite lifestyle interventions and use of maximally tolerated statins. Evolocumab has also been approved for use in patients with homozygous FH.
Although PCSK9 inhibitors are not specifically approved for patients unable to tolerate statins, the results of GAUSS-3, which documented that statin intolerance is a real, definable entity and very responsive to PCSK9 inhibition, makes these drugs promising agents for patients intolerant of statins and, thus, unable to benefit from high-intensity stain therapy.
In April 2016, the ACC released a clinical consensus update to their 2013 cholesterol guidelines, which is their first recommendation specifically addressing the use of non-statin therapies, including the newer PCSK9 inhibitors.57 For high-risk patients with clinical atherosclerotic CVD or LDL-C > 190 and failure to achieve at least a 50% reduction in LDL-C on maximally tolerated statin, non-statins may be considered. Ezetimibe, given its safety and tolerability, should be the first additional medication added. Bile acid sequestrants may be used as a second-line therapy if ezetimibe is not tolerated and triglycerides are not elevated. If therapy goals are not met on maximally tolerated statin and ezetimibe, either approved PCSK9 inhibitor can be added or used to replace ezetimibe. The document also specifies that given the lack of long-term safety and efficacy data on the PCSK9 inhibitors, they are not recommended for use in primary prevention patients in the absence of FH.
CONCLUSION
Although statin therapy has been shown to substantially reduce LDL-C and CVD adverse events, there remains a high rate of inadequate goal achievement and residual CVD risk in patients receiving statins. Combination therapies with ezetimibe and resins to further lower LDL-C, fibrates and omega 3 fatty acids to lower triglycerides, and niacin to lower both and raise high-density-liproprotein cholesterol are available, even though additional CV risk reduction is minimal or elusive when these drugs are added to statin therapy.
The link between atherogenic lipoproteins and CVD is strong, and the need to develop therapies in addition to statins to substantially and safely reduce LDL-C remains a priority. The association of reduced PCSK9 activity with reduced LDL-C and CV events has led to rapid development and approval of monoclonal antibody therapies to inhibit PCSK9. In trials, these therapies have shown substantial and durable reductions in LDL-C of more than 50%, with acceptable tolerability. Now that PCSK9 inhibitors are approved by the FDA, extended data about long-term tolerability, safety, and efficacy and, most importantly, demonstration of additional reduction in CVD events are needed. It is hoped that the long-term ongoing trials will provide these data.
For the immediate future, statin therapy will continue to be the cornerstone of lipid and CVD risk management based on their low generic cost, proven CVD risk reduction, and clinicians’ comfort with their use. However, the reliable efficacy of PCSK9 inhibitors and the fact that statin therapy itself increases PCSK9 activity makes the addition of PCSK9 inhibitors to statins an attractive approach in high-risk patients failing to reach LDL-C treatment goals.
Although current indications are limited, there are patients at high CVD risk who would be appropriate candidates for these therapies. These include patients with the following:
- FH with lifetime burden of elevated LDL-C and associated low likelihood of achieving optimal LDL-C control on current available therapies
- Complete or partial statin intolerance with high-intensity statin dosing limited by side effects
- High CV risk who are not at LDL-C goal on current therapies.
Now that the first therapies are available, practitioners can expect newer approaches to tackle PCSK9-mediated LDL-C reduction. Bococizumab is lagging in phase 3 trials, but the SPIRE program is moving forward with special population studies expected to conclude in 2016 and simultaneous long-term outcomes trials. Other PCSK9 inhibitors being investigated include agents with more durable effect requiring less frequent injections, RNA-interference therapies, vaccinations, antisense therapies, and oral formulations.
The PCSK9 inhibitors hold promise as an adjunct to statin therapy. Their eventual clinical role will depend on a balance between substantial LDL-C reductions, long-term safety, tolerability, and reduction in CVD events vs the cost (estimated at $14,000 a year), access from payers, acceptance of injectable therapies, and magnitude of incremental benefit when added to current therapies. Nevertheless, initial clinical trial data are encouraging and these drugs may be an important addition to the therapeutic armamentarium against CVD.
- Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4,444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Sacks FM, Pfeiffer MA, Moye LA, et al; Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial Investigators. N Engl J Med 1996; 335:1001–1009.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al; Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Lipid Research Clinics Program. The Lipid Research Clinics Coronary Primary Prevention Trial results (reduction in incidence of coronary heart disease). JAMA 1984; 251:351–364.
- Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004; 109(suppl 1):III39–III43.
- Stone N, Robinson J, Lichtenstein A, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Jacobson TA, Ito MK, Maki KC, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1—full report. J Clin Lipidol 2015; 9:129–169.
- Jarcho JA, Keaney JF Jr. Proof that lower is better–LDL cholesterol and IMPROVE-IT. N Engl J Med 2015; 372:2448–2450.
- Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372:2387–2397.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- de Ferranti S, Rodday AM, Mendelson M, et al. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation 2016; 133:1067–1072.
- Perez de Isla L, Alonso R, Watts GF, et al; SAFEHEART investigators. Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-year SAFEHEART registry follow-up. J Am Coll Cardiol 2016; 67:1278–1285.
- Unni SK, Quek RGW, Biskupiak J, et al. Assessment of statin therapy, LDL-C levels, and cardiovascular events among high-risk patients in the United States. J Clin Lipidol 2016; 10:63–71.
- Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheum 2010; 22:644–650.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Keech A, Simes RJ, Barter P, et al; FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomized controlled trial. Lancet 2005; 366:1849–1861.
- Kaur N, Pandey A, Negi H, et al. Effect of HDL-raising drugs on cardiovascular outcomes: a systematic review and meta-regression. PLoS One 2014; 9:e94585.
- Barter PJ, Caulfield M, Eriksson M, et al; ILLUMINATE investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Rader D, Kastelein J. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation 2014; 129:1022–1032.
- Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003; 34:154–156.
- Verbeek R, Stoekenbroek RM, Hovingh GK. PCSK9 inhibitors: novel therapeutic agents for the treatment of hypercholesterolemia. Eur J of Pharm 2015; 763(Pt A):38–47.
- Steinberg D, Witztum JL. Inhibition of PCSK9: a powerful weapon for achieving ideal LDL cholesterol levels. Proc Natl Acad Sci USA 2009; 106:9546–9547.
- Abifadel M, Rabès J-P, Devillers M, et al. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum Mutat 2009; 30:520–529.
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272.
- Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybærg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease. J Am Coll Cardiol 2010; 55:2833–2842.
- Mortensen MB, Afzal S, Nordestgaard BG, Falk E. The high-density lipoprotein-adjusted SCORE model worsens SCORE-based risk classification in a contemporary population of 30,824 Europeans: the Copenhagen General Population Study. Eur Heart J 2015; 36:2446–2453.
- Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004; 93:1473–1480.
- Graham MJ, Lemonidis KM, Whipple CP, et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res 2007; 48:763–767.
- Shan L, Pang L, Zhang R, Murgolo NJ, Lan H, Hedrick JA. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem Biophys Res Comm 2008; 375:69–73.
- Stein EA, Raal F. Reduction of low-density lipoprotein cholesterol by monoclonal antibody inhibition of PCSK9. Annu Rev Med 2014; 65:417–431.
- Duff CJ, Scott MJ, Kirby IT, Hutchinson SE, Martin SL, Hooper NM. Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor. Biochem J 2009; 419:577–584.
- Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Eng J Med 2012; 366:1108–1118.
- Koren MJ, Lundqvist P, Bolognese M, et al; MENDEL-2 Investigators. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol 2014; 63:2531–2540.
- Blom DJ, Hala T, Bolognese M, et al; DESCARTES investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370:1809-1819.
- Raal FJ, Stein EA, Dufour R, et al; RUTHERFORD-2 Investigators. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2014; 385:331–340.
- Robinson JG, Nedergaard BS, Rogers WJ, et al; LAPLA C-2 Investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014; 311:1870–1882.
- Stroes E, Colquhoun D, Sullivan D, et al; GAUSS-2 Investigators. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014; 63:2541–2548.
- Nissen SE, Stroes E, Dent-Acosta RE, et al; GAUSS-3 Investigators. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance, the GAUSS-3 randomized clinical trial. JAMA 2016; 315:1580–1590.
- Trial assessing long term use of PCSK9 inhibition in subjects with genetic LDL disorders (TAUSSIG). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT-1624142. Updated June 25, 2015. Accessed October 23, 2016.
- Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015; 36:2996–3003.
- Efficacy and safety of alirocumab (SAR236553/REGN727) versus placebo on top of lipid-modifying therapy in patients with heterozygous familial hypercholesterolemia; the ODYSSEY HIGH FH trial. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01617655. Updated September 27, 2016. Accessed October 23, 2016.
- Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am Heart J 2015; 169:906–915.
- Efficacy and Safety of Alirocumab (SAR236553/REGN727) Versus Ezetimibe on Top of Statin in High Cardiovascular Risk Patients With Hypercholesterolemia (ODYSSEY COMBO II). U.S. National Institutes of Health website. Updated June 23, 2016. https://clinicaltrials.gov/ct2/show/NCT01644188. Accessed October 23, 2016.
- Roth EM, Moriarty P, Bergeron J, et al; ODYSSEY CHOICE I investigators. A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add-on to statin: ODYSSEY CHOICE I. Atherosclerosis 2016, doi: 10.1016/j.atherosclerosis.2016.08.043.
- Phase III Study To Evaluate Alirocumab in Patients With Hypercholesterolemia Not Treated With a Statin (ODYSSEY CHOICE II). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT02023879. Updated November 2, 2015. Accessed October 23, 2016.
- Monthly and twice monthly subcutaneous dosing of PF-04950615 (RN316) in hypercholesterolemic subjects on a statin. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/results?term=NCT01592240. Updated October 14, 2014. Accessed October 23, 2016.
- Zhang XL, Zhu QQ, Zhu L, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med 2015; 13:123.
- Sabatine MS, Giugliano RP, Wiviott SD, et al; OSLER Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1500–1509.
- Robinson JG, Farnier M, Krempf M, et al; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1489–1499.
- Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann Intern Med 2015; 163:40–51.
- Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01764633. Updated July 26, 2016. Accessed October 23, 2016.
- ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01663402. Updated October 23, 2016. Accessed September 13, 2016.
- The Evaluation of Bococizumab (PF-04950615;RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-1). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01975376. Updated September 22, 2016. Accessed October 23, 2016.
- The Evaluation of Bococizumab (PF-04950615; RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-2). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01975389. Updated July 26, 2016. Accessed October 23, 2016.
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al; Writing Committee. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 2016; 68:92–125.
Statin therapy has been shown to substantially reduce adverse events associated with low-density-lipoprotein cholesterol (LDL-C) and cardiovascular disease (CVD). Statins alone are often not adequate to achieve treatment goals, and residual CVD risk remains high. Combination therapies of statins with ezetimibe and resins to further lower LDL-C, fibrates and omega 3 fatty acids to lower triglycerides, and niacin to lower both and raise high-density-liproprotein cholesterol are available, but additional risk reduction has not been consistently demonstrated in clinical trials.
The link between atherogenic lipoproteins and CVD is strong, and the need to develop therapies in addition to statins to substantially and safely reduce LDL-C is a priority. The association of reduced proprotein convertase subtilisin/kexin type 9 (PCSK9) activity with reduced LDL-C and CVD events has led to the rapid development and approval of monoclonal antibody therapies to inhibit PCSK9.
In this review, we discuss trials of these therapies that have shown durable reductions in LDL-C of more than 50%, with acceptable tolerability. Now that PCSK9 inhibitors are approved by the US Food and Drug Administration (FDA), extended data are needed as to long-term tolerability, safety, and efficacy of these agents and, most importantly, demonstration of additional reduction in CVD events.
A CASE FOR ADDITIONAL THERAPIES
CVD is the leading cause of morbidity and death in the United States, responsible for one in four deaths. Hyperlipidemia and, specifically, elevated LDL-C have been found to be important drivers of atherosclerosis and, in turn, adverse cardiovascular (CV) events. Likewise, numerous observational and clinical trials have shown that reducing LDL-C, particularly with statins, decreases CVD events.1–4 More aggressive lowering with higher doses or more intensive statin therapy further reduces rates of adverse outcomes.3,4 In addition, the pleiotropic effects of statins imply that not all of their benefits are derived from LDL-C lowering alone.5 Consequently, it is now standard practice to use statins at the highest tolerable dose to reach target LDL-C levels and prevent CV events in high-risk patients with CVD or multiple coronary artery disease risk factors, regardless of the LDL-C levels.6,7
The American College of Cardiology (ACC) and the American Heart Association released cholesterol guidelines in 2013 that recommend a risk-based approach for statin therapy rather than targeting specific LDL-C levels.6 Although this evidence-based approach may better conform to clinical trials, the debate that lower LDL-C targets will further prevent CVD continues.
Indeed, it appears that lower is better, as demonstrated by the IMPROVE-IT trial.8 Although the control group receiving simvastatin monotherapy had low LDL-C levels (mean, 69.9 mg/dL; 1.8 mmol/L), the experimental group receiving simvastatin plus ezetimibe achieved even lower levels (mean, 53.2 mg/dL;1.4 mmol/L) after 1 year of therapy and had a significantly lower composite primary end point of CV death, major coronary event, or nonfatal stroke at 7 years (34.7% for simvastatin monotherapy vs 32.7% for combined therapy).9 Furthermore, the event-rate reduction with the addition of ezetimibe was the same as the average predicted by the Cholesterol Treatment Trialists’ meta-analysis: an LDL-C reduction of 1 mmol/L (38.6 mg/dL) yields a 23% risk reduction in major coronary events over 5 years.10 Although only a modest absolute reduction in outcomes, it supports the notion that further reduction of LDL-C levels by more potent therapies may offer greater benefit.
There is strong evidence that statin therapy reduces the risk of developing CVD in patients with or without a previous atherosclerotic event; however, residual CVD risk remains even for those on therapy. A contributing factor to this residual risk is that many statin-treated patients have insufficient response or intolerance and do not achieve adequate LDL-C reductions.
There are three clinically important patient populations who are inadequately managed with current therapies and remain at high risk of subsequent CV events; these are patients who would benefit from additional therapies.
1. Patients with familial hypercholesterolemia (FH). This is the most common genetic disorder in the world, yet it is frequently undiagnosed and untreated. Due to high baseline cholesterol levels, achieving LDL-C treatment goals is challenging.
- The prevalence may be closer to 1:200 to 1:250 rather than the often quoted 1:500.11
- Fewer than 12% of patients with heterzygous FH achieve the LDL-C goal of < 100 mg/dL with maximal statin treatment alone or with a second agent.12
2. Patients with hyperlipidemia not due to FH who are at elevated CV risk and undertreated. In US and European surveys, between 50% and 60% of patients receiving statins with or without other therapies failed to reach LDL-C reduction goals.13
- Variation in response to statin treatment between individuals may be considerable.
- Poor adherence to statin therapy is common.
3. Patients with side effects to statins, particularly muscle symptoms that prevent statin use or substantially limit the dose.
- Although the incidence of myopathy is low (< 0.1%) and rhabdomyolysis is even less common, observational studies suggest that 10% to 20% of patients may limit statin use due to muscle-associated complaints including muscle aching, cramps, or weakness.14
- Side effects may be dose-dependent, limiting the use of the high-intensity statin doses that are frequently necessary to achieve LDL-C goals.
Consequently, there is great interest in developing therapies beyond statins that may further reduce CV events. However, treatments other than ezetimibe for further management of hyperlipidemia and risk reduction have failed to demonstrate consistent benefit when added to statin therapy.15–19 The largest studies were with niacin and fibrates. Unfortunately, most trials demonstrated no overall outcomes benefit or only benefits in subgroup analyses, leaving the door open to other pharmacologic interventions.
Studies with the cholesterol ester transfer protein (CETP) inhibitor torcetrapib, in combination with statin therapy, actually demonstrated an overall increase in all-cause mortality in the treatment group.20 Two large outcome trials of the CETP inhibitors dalcetrapib and evacetrapib were stopped after interim analysis predicted no benefit. Although drugs such as lomitapide (a microsomal triglyceride transfer protein inhibitor) and mipomersen (an antisense oligonucleotide inhibitor of ApoB-100 synthesis) can lower LDL-C by reducing ApoB synthesis,21 they are approved only in the small population of individuals with homozygous FH and liver toxicity and side effects are a concern.
Accordingly, current cholesterol management guidelines continue to offer LDL-C as the main target of lipid-modifying therapy, with statins as the primary treatment choice. The desire to build on statin therapy to prevent further progression of atherosclerosis and clinical CVD has encouraged continued focus on strategies to lower LDL-C to even greater extents.
Fortunately for practitioners, for the first time since lovastatin was approved in 1987, there is a new therapy approved by the FDA that significantly lowers LDL-C and, potentially, improves CV outcomes—the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. This review will focus on the PCSK9 inhibitors, a novel therapeutic class that reduces LDL-C through increased hepatic clearance. These drugs are rapidly emerging as an ideal adjunctive therapy to statins for patients at the highest risk and as a highly efficacious alternate therapy in patients intolerant of statins.
PCSK9 INHIBITORS: DISCOVERY, MECHANISM, AND THERAPEUTIC INTERVENTIONS
Two PCSK9 inhibitors have received FDA approval: alirocumab (Praluent) and evolocumab (Repatha). Among new molecular entities for clinical use, PCSK9 inhibitor therapies had one of the shortest durations from discovery to development and approval.
Mutations in the PCSK9 gene associated with autosomal dominant hypercholesterolemia were first identified in 2003 in a French family.22 The PCSK9 protein is now known to be a secreted enzymatic serine protease that is primarily synthesized in the liver and binds to the LDL receptor (LDL-R)/LDL-C complex on the surface of hepatocytes, marking the receptor for lysosomal degradation rather than recycling to the cell surface. Thus, it reduces the quantity of LDL-R that is available to remove LDL-C from circulation.23 As a result, higher levels of PCSK9 are associated with higher levels of plasma LDL-C.
The clinical importance of PCSK9 in regulating LDL-C is supported by observed mutations and polymorphisms. Gain-of-function mutations that increase the activity of PCSK9 have been shown to be associated with elevated LDL-C, premature CVD, and myocardial infarction (MI).24 Conversely, loss-of-function mutations (heterozygotes found in 1% to 3% of the population) result in decreased activity of PCSK9, lower LDL-C, and lower incidence of CVD (Table 1).25–29 These observations, combined with data showing that homozygote loss-of-function individuals with very low LDL-C were generally very healthy, sparked interest in developing inhibition of PCSK9 activity as a therapeutic strategy for hyperlipidemia.
Multiple pharmacologic developments are aimed at inhibiting PCSK9, with many compounds in clinical trials. The approaches include gene silencing with loss-of-function mutations, synthetic peptides, oral small molecules, and monoclonal antibodies. Gene silencing was first observed in 2007 when administration of antisense oligonucleotides targeted to selectively inhibit PCSK9 mRNA was found to up-regulate LDL-R, thereby decreasing serum levels of LDL-C.30
The first study to establish the role of synthetic peptides in PCSK9 inhibition was performed in 2008. In this study, the epidermal growth factor-like A synthetic peptide blocked the interaction between PCSK9 and LDL-R, thereby decreasing the degradation of LDL-R and preserving LDL uptake.31 Although studies are limited, synthetic peptides remain an area of great interest given their promising effects on lipid metabolism. Recently, a synthetic PCSK9-binding adnectin derived from the human fibronectin known as BMS-962476, had favorable results in a phase 1 clinical trial. An RNA interference molecule, subcutaneous ALN-PSC, inhibits PCSK9 gene expression by causing destruction of messenger RNA, thus inhibiting PCSK9 synthesis (Table 2).32
PCSK9 INHIBITORS: CLINICAL TRIALS
Subcutaneously administered monoclonal antibodies targeting PCSK9 currently are the only PCSK9 inhibitors FDA-approved for clinical use. The first study to demonstrate efficacy in enhancing uptake of serum LDL-C was performed in 2009.33 Multiple phase 1 and 2 studies soon followed, demonstrating acceptable safety and 50% to 70% reductions in LDL-C at upper-dose titrations.34 Additionally, there were significant reductions in total cholesterol, ApoB, triglycerides, and lipoprotein(a).
These early developments paved the way for larger phase 3 trials (Table 3).35–48 The PCSK9 inhibitors evolocumab and alirocumab have been shown in multiple phase 3 clinical trials to achieve a consistent dose-dependent 50% to 60% reduction in LDL-C across a broad range of CVD risk, pretreatment LDL-C levels, and background therapy: monotherapy (MENDEL-2, ODYSSEY COMBO I),35,44 added to statin therapy (LAPLACE-2, ODYSSEY CHOICE I),38,46 and in individuals with heterozygous FH (RUTHERFORD-2, ODYSSEY-FH).37,42 Trials with bococizumab are under way.
The GAUSS-2 clinical trial (Goal Achievement after Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects-2) demonstrated similar efficacy in reducing LDL-C in patients with clinically assessed statin intolerance due to muscle-related adverse symptoms.39 In GAUSS-3, patients were first identified as being statin intolerant secondary to muscle-associated symptoms based on a randomized, crossover trial of atorvastatin vs placebo.40 The 43% of participants who experienced intolerable muscle-related symptoms on the statin but not on placebo were then randomized to evolocumab vs ezetimibe. Results showed significant reduction in LDL-C in the evolocumab group (52.8%) compared with the ezetimibe group (16.7%). Additionally, among patients with muscle symptoms on statin therapy, PCSK9 therapy was discontinued for muscle symptoms in only 0.7% of evolocumab recipients and 6.8% of ezetimibe recipients.
Overall, the PCSK9 inhibitors are generally well tolerated with injection site reactions being the most common side effect. A meta-analysis published in 2015 of 25 trials including more than 12,000 patients treated with evolocumab and alirocumab reported no significant difference in adverse events or safety outcomes vs placebo or ezetimibe.49 Antidrug binding or neutralizing antibody production to these agents, thus far, has not been shown to be an issue. Additional analyses have not indicated an adverse effect on gonadal hormone levels or increased incidence of new-onset diabetes.
Two studies published in 2015 offer insight into longer term durability and safety as well as potential CVD outcome benefit (Table 4)50,51:
- OSLER-1 and 2: Open-Label Study of Long-Term Evaluation against LDL-Cholesterol (OSLER) trials—evolocumab trial;50
- ODYSSEY long term: Long-Term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy—alirocumab trial.51
The OSLER trials reported durable LDL-C reductions of 61% and the ODYSSEY trial reported a LDL-C reduction of 62%.50,51 In both studies, the overall occurrence of adverse events was similar to placebo, but both reported a higher rate of neurocognitive effects in the active treatment groups (evolocumab 0.9% vs 0.3% for standard therapy; alirocumab 1.2% vs 0.5% for placebo). It must be noted that although the absolute rate of neurocognitive adverse events is low, it is unclear if these events were related to the drugs themselves or to extreme lowering of LDL-C. Nevertheless, the FDA has raised concerns about neurocognitive events. A sub-study of the ongoing FOURIER trial with evolocumab—EBBINGHAUS—is expected to address this concern.
In addition, analyses of CV events showed that the PCSK9 inhibitors effectively cut the CV rate in half in both studies (Figure 1).50,51 In the OSLER trials,50 evolocumab recipients had 53% reduction in major CV events (0.95% vs 2.18% in the standard therapy group; P = .003). In ODYSSEY,51 alirocumab recipients had a 48% reduction in major CV events (1.7% vs 3.3% for placebo; P = .02). Furthermore, a 2015 meta-analysis of 24 phase 2 and 3 trials reported a statistically significant 55% reduction in all-cause mortality and 50% reduction in CV mortality with PCSK9 inhibitors.52
For many reasons including short length of follow-up, study design, and small numbers of outcome events, the OSLER and ODYSSEY studies, although enticing, are exploratory and hypothesis-generating only and results need to be interpreted with caution. Nevertheless, they have set the stage for ongoing prospective randomized outcome trials that are studying the CV effects and tolerability of PCSK9 inhibitors over a longer time frame. These include the following trials.
- The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) is an ongoing trial with the primary end point of CV death, MI, hospitalization for unstable angina, stroke, or coronary revascularization in high-risk patients receiving evolocumab or placebo.53
- The ODYSSEY trial is examining the effect of alirocumab vs placebo on the composite primary endpoint of coronary heart disease death, non-fatal MI, fatal and nonfatal ischemic stroke, and unstable angina requiring hospitalization in patients who have had an acute coronary syndrome event during the previous 4 to 52 weeks.54
- The Evaluation of Bococizumab in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE) trials are investigating the effect of bococizumab, a third PCSK9 “humanized” monoclonal antibody, vs placebo in reducing death, MI, stroke, or unstable angina in patients at high-risk of CVD who are receiving standard lipid-lowering therapy with LDL-C > 70 mg/dL (1.8 mmol/L) (SPIRE-1) or > 100 mg/dL (2.6 mmol/L) (SPIRE-2).55,56
Because these outcome trials are attempting to enroll more than 70,000 patients and are event driven, it is difficult to predict when they will be completed (Table 5).53–56 However, recent estimates indicate completion of at least one trial by the end of 2016 or early 2017, with interim analyses of others expected at that time. It is hoped that they will answer the all-important question of whether PCSK9 inhibitors are associated with further CV event reduction benefit.
CURRENT FDA INDICATIONS AND GUIDELINES
The two PCSK9 inhibitors approved by the FDA—alirocumab (subcutaneous 75 mg every 2 weeks up titrated to 150 mg) and evolocumab (subcutaneous 140 mg every 2 weeks or 420 mg every 4 weeks)—are both indicated for use with statins in patients with heterozygous FH or known atherosclerotic CVD who require further reduction in LDL-C levels despite lifestyle interventions and use of maximally tolerated statins. Evolocumab has also been approved for use in patients with homozygous FH.
Although PCSK9 inhibitors are not specifically approved for patients unable to tolerate statins, the results of GAUSS-3, which documented that statin intolerance is a real, definable entity and very responsive to PCSK9 inhibition, makes these drugs promising agents for patients intolerant of statins and, thus, unable to benefit from high-intensity stain therapy.
In April 2016, the ACC released a clinical consensus update to their 2013 cholesterol guidelines, which is their first recommendation specifically addressing the use of non-statin therapies, including the newer PCSK9 inhibitors.57 For high-risk patients with clinical atherosclerotic CVD or LDL-C > 190 and failure to achieve at least a 50% reduction in LDL-C on maximally tolerated statin, non-statins may be considered. Ezetimibe, given its safety and tolerability, should be the first additional medication added. Bile acid sequestrants may be used as a second-line therapy if ezetimibe is not tolerated and triglycerides are not elevated. If therapy goals are not met on maximally tolerated statin and ezetimibe, either approved PCSK9 inhibitor can be added or used to replace ezetimibe. The document also specifies that given the lack of long-term safety and efficacy data on the PCSK9 inhibitors, they are not recommended for use in primary prevention patients in the absence of FH.
CONCLUSION
Although statin therapy has been shown to substantially reduce LDL-C and CVD adverse events, there remains a high rate of inadequate goal achievement and residual CVD risk in patients receiving statins. Combination therapies with ezetimibe and resins to further lower LDL-C, fibrates and omega 3 fatty acids to lower triglycerides, and niacin to lower both and raise high-density-liproprotein cholesterol are available, even though additional CV risk reduction is minimal or elusive when these drugs are added to statin therapy.
The link between atherogenic lipoproteins and CVD is strong, and the need to develop therapies in addition to statins to substantially and safely reduce LDL-C remains a priority. The association of reduced PCSK9 activity with reduced LDL-C and CV events has led to rapid development and approval of monoclonal antibody therapies to inhibit PCSK9. In trials, these therapies have shown substantial and durable reductions in LDL-C of more than 50%, with acceptable tolerability. Now that PCSK9 inhibitors are approved by the FDA, extended data about long-term tolerability, safety, and efficacy and, most importantly, demonstration of additional reduction in CVD events are needed. It is hoped that the long-term ongoing trials will provide these data.
For the immediate future, statin therapy will continue to be the cornerstone of lipid and CVD risk management based on their low generic cost, proven CVD risk reduction, and clinicians’ comfort with their use. However, the reliable efficacy of PCSK9 inhibitors and the fact that statin therapy itself increases PCSK9 activity makes the addition of PCSK9 inhibitors to statins an attractive approach in high-risk patients failing to reach LDL-C treatment goals.
Although current indications are limited, there are patients at high CVD risk who would be appropriate candidates for these therapies. These include patients with the following:
- FH with lifetime burden of elevated LDL-C and associated low likelihood of achieving optimal LDL-C control on current available therapies
- Complete or partial statin intolerance with high-intensity statin dosing limited by side effects
- High CV risk who are not at LDL-C goal on current therapies.
Now that the first therapies are available, practitioners can expect newer approaches to tackle PCSK9-mediated LDL-C reduction. Bococizumab is lagging in phase 3 trials, but the SPIRE program is moving forward with special population studies expected to conclude in 2016 and simultaneous long-term outcomes trials. Other PCSK9 inhibitors being investigated include agents with more durable effect requiring less frequent injections, RNA-interference therapies, vaccinations, antisense therapies, and oral formulations.
The PCSK9 inhibitors hold promise as an adjunct to statin therapy. Their eventual clinical role will depend on a balance between substantial LDL-C reductions, long-term safety, tolerability, and reduction in CVD events vs the cost (estimated at $14,000 a year), access from payers, acceptance of injectable therapies, and magnitude of incremental benefit when added to current therapies. Nevertheless, initial clinical trial data are encouraging and these drugs may be an important addition to the therapeutic armamentarium against CVD.
Statin therapy has been shown to substantially reduce adverse events associated with low-density-lipoprotein cholesterol (LDL-C) and cardiovascular disease (CVD). Statins alone are often not adequate to achieve treatment goals, and residual CVD risk remains high. Combination therapies of statins with ezetimibe and resins to further lower LDL-C, fibrates and omega 3 fatty acids to lower triglycerides, and niacin to lower both and raise high-density-liproprotein cholesterol are available, but additional risk reduction has not been consistently demonstrated in clinical trials.
The link between atherogenic lipoproteins and CVD is strong, and the need to develop therapies in addition to statins to substantially and safely reduce LDL-C is a priority. The association of reduced proprotein convertase subtilisin/kexin type 9 (PCSK9) activity with reduced LDL-C and CVD events has led to the rapid development and approval of monoclonal antibody therapies to inhibit PCSK9.
In this review, we discuss trials of these therapies that have shown durable reductions in LDL-C of more than 50%, with acceptable tolerability. Now that PCSK9 inhibitors are approved by the US Food and Drug Administration (FDA), extended data are needed as to long-term tolerability, safety, and efficacy of these agents and, most importantly, demonstration of additional reduction in CVD events.
A CASE FOR ADDITIONAL THERAPIES
CVD is the leading cause of morbidity and death in the United States, responsible for one in four deaths. Hyperlipidemia and, specifically, elevated LDL-C have been found to be important drivers of atherosclerosis and, in turn, adverse cardiovascular (CV) events. Likewise, numerous observational and clinical trials have shown that reducing LDL-C, particularly with statins, decreases CVD events.1–4 More aggressive lowering with higher doses or more intensive statin therapy further reduces rates of adverse outcomes.3,4 In addition, the pleiotropic effects of statins imply that not all of their benefits are derived from LDL-C lowering alone.5 Consequently, it is now standard practice to use statins at the highest tolerable dose to reach target LDL-C levels and prevent CV events in high-risk patients with CVD or multiple coronary artery disease risk factors, regardless of the LDL-C levels.6,7
The American College of Cardiology (ACC) and the American Heart Association released cholesterol guidelines in 2013 that recommend a risk-based approach for statin therapy rather than targeting specific LDL-C levels.6 Although this evidence-based approach may better conform to clinical trials, the debate that lower LDL-C targets will further prevent CVD continues.
Indeed, it appears that lower is better, as demonstrated by the IMPROVE-IT trial.8 Although the control group receiving simvastatin monotherapy had low LDL-C levels (mean, 69.9 mg/dL; 1.8 mmol/L), the experimental group receiving simvastatin plus ezetimibe achieved even lower levels (mean, 53.2 mg/dL;1.4 mmol/L) after 1 year of therapy and had a significantly lower composite primary end point of CV death, major coronary event, or nonfatal stroke at 7 years (34.7% for simvastatin monotherapy vs 32.7% for combined therapy).9 Furthermore, the event-rate reduction with the addition of ezetimibe was the same as the average predicted by the Cholesterol Treatment Trialists’ meta-analysis: an LDL-C reduction of 1 mmol/L (38.6 mg/dL) yields a 23% risk reduction in major coronary events over 5 years.10 Although only a modest absolute reduction in outcomes, it supports the notion that further reduction of LDL-C levels by more potent therapies may offer greater benefit.
There is strong evidence that statin therapy reduces the risk of developing CVD in patients with or without a previous atherosclerotic event; however, residual CVD risk remains even for those on therapy. A contributing factor to this residual risk is that many statin-treated patients have insufficient response or intolerance and do not achieve adequate LDL-C reductions.
There are three clinically important patient populations who are inadequately managed with current therapies and remain at high risk of subsequent CV events; these are patients who would benefit from additional therapies.
1. Patients with familial hypercholesterolemia (FH). This is the most common genetic disorder in the world, yet it is frequently undiagnosed and untreated. Due to high baseline cholesterol levels, achieving LDL-C treatment goals is challenging.
- The prevalence may be closer to 1:200 to 1:250 rather than the often quoted 1:500.11
- Fewer than 12% of patients with heterzygous FH achieve the LDL-C goal of < 100 mg/dL with maximal statin treatment alone or with a second agent.12
2. Patients with hyperlipidemia not due to FH who are at elevated CV risk and undertreated. In US and European surveys, between 50% and 60% of patients receiving statins with or without other therapies failed to reach LDL-C reduction goals.13
- Variation in response to statin treatment between individuals may be considerable.
- Poor adherence to statin therapy is common.
3. Patients with side effects to statins, particularly muscle symptoms that prevent statin use or substantially limit the dose.
- Although the incidence of myopathy is low (< 0.1%) and rhabdomyolysis is even less common, observational studies suggest that 10% to 20% of patients may limit statin use due to muscle-associated complaints including muscle aching, cramps, or weakness.14
- Side effects may be dose-dependent, limiting the use of the high-intensity statin doses that are frequently necessary to achieve LDL-C goals.
Consequently, there is great interest in developing therapies beyond statins that may further reduce CV events. However, treatments other than ezetimibe for further management of hyperlipidemia and risk reduction have failed to demonstrate consistent benefit when added to statin therapy.15–19 The largest studies were with niacin and fibrates. Unfortunately, most trials demonstrated no overall outcomes benefit or only benefits in subgroup analyses, leaving the door open to other pharmacologic interventions.
Studies with the cholesterol ester transfer protein (CETP) inhibitor torcetrapib, in combination with statin therapy, actually demonstrated an overall increase in all-cause mortality in the treatment group.20 Two large outcome trials of the CETP inhibitors dalcetrapib and evacetrapib were stopped after interim analysis predicted no benefit. Although drugs such as lomitapide (a microsomal triglyceride transfer protein inhibitor) and mipomersen (an antisense oligonucleotide inhibitor of ApoB-100 synthesis) can lower LDL-C by reducing ApoB synthesis,21 they are approved only in the small population of individuals with homozygous FH and liver toxicity and side effects are a concern.
Accordingly, current cholesterol management guidelines continue to offer LDL-C as the main target of lipid-modifying therapy, with statins as the primary treatment choice. The desire to build on statin therapy to prevent further progression of atherosclerosis and clinical CVD has encouraged continued focus on strategies to lower LDL-C to even greater extents.
Fortunately for practitioners, for the first time since lovastatin was approved in 1987, there is a new therapy approved by the FDA that significantly lowers LDL-C and, potentially, improves CV outcomes—the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. This review will focus on the PCSK9 inhibitors, a novel therapeutic class that reduces LDL-C through increased hepatic clearance. These drugs are rapidly emerging as an ideal adjunctive therapy to statins for patients at the highest risk and as a highly efficacious alternate therapy in patients intolerant of statins.
PCSK9 INHIBITORS: DISCOVERY, MECHANISM, AND THERAPEUTIC INTERVENTIONS
Two PCSK9 inhibitors have received FDA approval: alirocumab (Praluent) and evolocumab (Repatha). Among new molecular entities for clinical use, PCSK9 inhibitor therapies had one of the shortest durations from discovery to development and approval.
Mutations in the PCSK9 gene associated with autosomal dominant hypercholesterolemia were first identified in 2003 in a French family.22 The PCSK9 protein is now known to be a secreted enzymatic serine protease that is primarily synthesized in the liver and binds to the LDL receptor (LDL-R)/LDL-C complex on the surface of hepatocytes, marking the receptor for lysosomal degradation rather than recycling to the cell surface. Thus, it reduces the quantity of LDL-R that is available to remove LDL-C from circulation.23 As a result, higher levels of PCSK9 are associated with higher levels of plasma LDL-C.
The clinical importance of PCSK9 in regulating LDL-C is supported by observed mutations and polymorphisms. Gain-of-function mutations that increase the activity of PCSK9 have been shown to be associated with elevated LDL-C, premature CVD, and myocardial infarction (MI).24 Conversely, loss-of-function mutations (heterozygotes found in 1% to 3% of the population) result in decreased activity of PCSK9, lower LDL-C, and lower incidence of CVD (Table 1).25–29 These observations, combined with data showing that homozygote loss-of-function individuals with very low LDL-C were generally very healthy, sparked interest in developing inhibition of PCSK9 activity as a therapeutic strategy for hyperlipidemia.
Multiple pharmacologic developments are aimed at inhibiting PCSK9, with many compounds in clinical trials. The approaches include gene silencing with loss-of-function mutations, synthetic peptides, oral small molecules, and monoclonal antibodies. Gene silencing was first observed in 2007 when administration of antisense oligonucleotides targeted to selectively inhibit PCSK9 mRNA was found to up-regulate LDL-R, thereby decreasing serum levels of LDL-C.30
The first study to establish the role of synthetic peptides in PCSK9 inhibition was performed in 2008. In this study, the epidermal growth factor-like A synthetic peptide blocked the interaction between PCSK9 and LDL-R, thereby decreasing the degradation of LDL-R and preserving LDL uptake.31 Although studies are limited, synthetic peptides remain an area of great interest given their promising effects on lipid metabolism. Recently, a synthetic PCSK9-binding adnectin derived from the human fibronectin known as BMS-962476, had favorable results in a phase 1 clinical trial. An RNA interference molecule, subcutaneous ALN-PSC, inhibits PCSK9 gene expression by causing destruction of messenger RNA, thus inhibiting PCSK9 synthesis (Table 2).32
PCSK9 INHIBITORS: CLINICAL TRIALS
Subcutaneously administered monoclonal antibodies targeting PCSK9 currently are the only PCSK9 inhibitors FDA-approved for clinical use. The first study to demonstrate efficacy in enhancing uptake of serum LDL-C was performed in 2009.33 Multiple phase 1 and 2 studies soon followed, demonstrating acceptable safety and 50% to 70% reductions in LDL-C at upper-dose titrations.34 Additionally, there were significant reductions in total cholesterol, ApoB, triglycerides, and lipoprotein(a).
These early developments paved the way for larger phase 3 trials (Table 3).35–48 The PCSK9 inhibitors evolocumab and alirocumab have been shown in multiple phase 3 clinical trials to achieve a consistent dose-dependent 50% to 60% reduction in LDL-C across a broad range of CVD risk, pretreatment LDL-C levels, and background therapy: monotherapy (MENDEL-2, ODYSSEY COMBO I),35,44 added to statin therapy (LAPLACE-2, ODYSSEY CHOICE I),38,46 and in individuals with heterozygous FH (RUTHERFORD-2, ODYSSEY-FH).37,42 Trials with bococizumab are under way.
The GAUSS-2 clinical trial (Goal Achievement after Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects-2) demonstrated similar efficacy in reducing LDL-C in patients with clinically assessed statin intolerance due to muscle-related adverse symptoms.39 In GAUSS-3, patients were first identified as being statin intolerant secondary to muscle-associated symptoms based on a randomized, crossover trial of atorvastatin vs placebo.40 The 43% of participants who experienced intolerable muscle-related symptoms on the statin but not on placebo were then randomized to evolocumab vs ezetimibe. Results showed significant reduction in LDL-C in the evolocumab group (52.8%) compared with the ezetimibe group (16.7%). Additionally, among patients with muscle symptoms on statin therapy, PCSK9 therapy was discontinued for muscle symptoms in only 0.7% of evolocumab recipients and 6.8% of ezetimibe recipients.
Overall, the PCSK9 inhibitors are generally well tolerated with injection site reactions being the most common side effect. A meta-analysis published in 2015 of 25 trials including more than 12,000 patients treated with evolocumab and alirocumab reported no significant difference in adverse events or safety outcomes vs placebo or ezetimibe.49 Antidrug binding or neutralizing antibody production to these agents, thus far, has not been shown to be an issue. Additional analyses have not indicated an adverse effect on gonadal hormone levels or increased incidence of new-onset diabetes.
Two studies published in 2015 offer insight into longer term durability and safety as well as potential CVD outcome benefit (Table 4)50,51:
- OSLER-1 and 2: Open-Label Study of Long-Term Evaluation against LDL-Cholesterol (OSLER) trials—evolocumab trial;50
- ODYSSEY long term: Long-Term Safety and Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy—alirocumab trial.51
The OSLER trials reported durable LDL-C reductions of 61% and the ODYSSEY trial reported a LDL-C reduction of 62%.50,51 In both studies, the overall occurrence of adverse events was similar to placebo, but both reported a higher rate of neurocognitive effects in the active treatment groups (evolocumab 0.9% vs 0.3% for standard therapy; alirocumab 1.2% vs 0.5% for placebo). It must be noted that although the absolute rate of neurocognitive adverse events is low, it is unclear if these events were related to the drugs themselves or to extreme lowering of LDL-C. Nevertheless, the FDA has raised concerns about neurocognitive events. A sub-study of the ongoing FOURIER trial with evolocumab—EBBINGHAUS—is expected to address this concern.
In addition, analyses of CV events showed that the PCSK9 inhibitors effectively cut the CV rate in half in both studies (Figure 1).50,51 In the OSLER trials,50 evolocumab recipients had 53% reduction in major CV events (0.95% vs 2.18% in the standard therapy group; P = .003). In ODYSSEY,51 alirocumab recipients had a 48% reduction in major CV events (1.7% vs 3.3% for placebo; P = .02). Furthermore, a 2015 meta-analysis of 24 phase 2 and 3 trials reported a statistically significant 55% reduction in all-cause mortality and 50% reduction in CV mortality with PCSK9 inhibitors.52
For many reasons including short length of follow-up, study design, and small numbers of outcome events, the OSLER and ODYSSEY studies, although enticing, are exploratory and hypothesis-generating only and results need to be interpreted with caution. Nevertheless, they have set the stage for ongoing prospective randomized outcome trials that are studying the CV effects and tolerability of PCSK9 inhibitors over a longer time frame. These include the following trials.
- The Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER) is an ongoing trial with the primary end point of CV death, MI, hospitalization for unstable angina, stroke, or coronary revascularization in high-risk patients receiving evolocumab or placebo.53
- The ODYSSEY trial is examining the effect of alirocumab vs placebo on the composite primary endpoint of coronary heart disease death, non-fatal MI, fatal and nonfatal ischemic stroke, and unstable angina requiring hospitalization in patients who have had an acute coronary syndrome event during the previous 4 to 52 weeks.54
- The Evaluation of Bococizumab in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE) trials are investigating the effect of bococizumab, a third PCSK9 “humanized” monoclonal antibody, vs placebo in reducing death, MI, stroke, or unstable angina in patients at high-risk of CVD who are receiving standard lipid-lowering therapy with LDL-C > 70 mg/dL (1.8 mmol/L) (SPIRE-1) or > 100 mg/dL (2.6 mmol/L) (SPIRE-2).55,56
Because these outcome trials are attempting to enroll more than 70,000 patients and are event driven, it is difficult to predict when they will be completed (Table 5).53–56 However, recent estimates indicate completion of at least one trial by the end of 2016 or early 2017, with interim analyses of others expected at that time. It is hoped that they will answer the all-important question of whether PCSK9 inhibitors are associated with further CV event reduction benefit.
CURRENT FDA INDICATIONS AND GUIDELINES
The two PCSK9 inhibitors approved by the FDA—alirocumab (subcutaneous 75 mg every 2 weeks up titrated to 150 mg) and evolocumab (subcutaneous 140 mg every 2 weeks or 420 mg every 4 weeks)—are both indicated for use with statins in patients with heterozygous FH or known atherosclerotic CVD who require further reduction in LDL-C levels despite lifestyle interventions and use of maximally tolerated statins. Evolocumab has also been approved for use in patients with homozygous FH.
Although PCSK9 inhibitors are not specifically approved for patients unable to tolerate statins, the results of GAUSS-3, which documented that statin intolerance is a real, definable entity and very responsive to PCSK9 inhibition, makes these drugs promising agents for patients intolerant of statins and, thus, unable to benefit from high-intensity stain therapy.
In April 2016, the ACC released a clinical consensus update to their 2013 cholesterol guidelines, which is their first recommendation specifically addressing the use of non-statin therapies, including the newer PCSK9 inhibitors.57 For high-risk patients with clinical atherosclerotic CVD or LDL-C > 190 and failure to achieve at least a 50% reduction in LDL-C on maximally tolerated statin, non-statins may be considered. Ezetimibe, given its safety and tolerability, should be the first additional medication added. Bile acid sequestrants may be used as a second-line therapy if ezetimibe is not tolerated and triglycerides are not elevated. If therapy goals are not met on maximally tolerated statin and ezetimibe, either approved PCSK9 inhibitor can be added or used to replace ezetimibe. The document also specifies that given the lack of long-term safety and efficacy data on the PCSK9 inhibitors, they are not recommended for use in primary prevention patients in the absence of FH.
CONCLUSION
Although statin therapy has been shown to substantially reduce LDL-C and CVD adverse events, there remains a high rate of inadequate goal achievement and residual CVD risk in patients receiving statins. Combination therapies with ezetimibe and resins to further lower LDL-C, fibrates and omega 3 fatty acids to lower triglycerides, and niacin to lower both and raise high-density-liproprotein cholesterol are available, even though additional CV risk reduction is minimal or elusive when these drugs are added to statin therapy.
The link between atherogenic lipoproteins and CVD is strong, and the need to develop therapies in addition to statins to substantially and safely reduce LDL-C remains a priority. The association of reduced PCSK9 activity with reduced LDL-C and CV events has led to rapid development and approval of monoclonal antibody therapies to inhibit PCSK9. In trials, these therapies have shown substantial and durable reductions in LDL-C of more than 50%, with acceptable tolerability. Now that PCSK9 inhibitors are approved by the FDA, extended data about long-term tolerability, safety, and efficacy and, most importantly, demonstration of additional reduction in CVD events are needed. It is hoped that the long-term ongoing trials will provide these data.
For the immediate future, statin therapy will continue to be the cornerstone of lipid and CVD risk management based on their low generic cost, proven CVD risk reduction, and clinicians’ comfort with their use. However, the reliable efficacy of PCSK9 inhibitors and the fact that statin therapy itself increases PCSK9 activity makes the addition of PCSK9 inhibitors to statins an attractive approach in high-risk patients failing to reach LDL-C treatment goals.
Although current indications are limited, there are patients at high CVD risk who would be appropriate candidates for these therapies. These include patients with the following:
- FH with lifetime burden of elevated LDL-C and associated low likelihood of achieving optimal LDL-C control on current available therapies
- Complete or partial statin intolerance with high-intensity statin dosing limited by side effects
- High CV risk who are not at LDL-C goal on current therapies.
Now that the first therapies are available, practitioners can expect newer approaches to tackle PCSK9-mediated LDL-C reduction. Bococizumab is lagging in phase 3 trials, but the SPIRE program is moving forward with special population studies expected to conclude in 2016 and simultaneous long-term outcomes trials. Other PCSK9 inhibitors being investigated include agents with more durable effect requiring less frequent injections, RNA-interference therapies, vaccinations, antisense therapies, and oral formulations.
The PCSK9 inhibitors hold promise as an adjunct to statin therapy. Their eventual clinical role will depend on a balance between substantial LDL-C reductions, long-term safety, tolerability, and reduction in CVD events vs the cost (estimated at $14,000 a year), access from payers, acceptance of injectable therapies, and magnitude of incremental benefit when added to current therapies. Nevertheless, initial clinical trial data are encouraging and these drugs may be an important addition to the therapeutic armamentarium against CVD.
- Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4,444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Sacks FM, Pfeiffer MA, Moye LA, et al; Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial Investigators. N Engl J Med 1996; 335:1001–1009.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al; Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Lipid Research Clinics Program. The Lipid Research Clinics Coronary Primary Prevention Trial results (reduction in incidence of coronary heart disease). JAMA 1984; 251:351–364.
- Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004; 109(suppl 1):III39–III43.
- Stone N, Robinson J, Lichtenstein A, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Jacobson TA, Ito MK, Maki KC, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1—full report. J Clin Lipidol 2015; 9:129–169.
- Jarcho JA, Keaney JF Jr. Proof that lower is better–LDL cholesterol and IMPROVE-IT. N Engl J Med 2015; 372:2448–2450.
- Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372:2387–2397.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- de Ferranti S, Rodday AM, Mendelson M, et al. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation 2016; 133:1067–1072.
- Perez de Isla L, Alonso R, Watts GF, et al; SAFEHEART investigators. Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-year SAFEHEART registry follow-up. J Am Coll Cardiol 2016; 67:1278–1285.
- Unni SK, Quek RGW, Biskupiak J, et al. Assessment of statin therapy, LDL-C levels, and cardiovascular events among high-risk patients in the United States. J Clin Lipidol 2016; 10:63–71.
- Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheum 2010; 22:644–650.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Keech A, Simes RJ, Barter P, et al; FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomized controlled trial. Lancet 2005; 366:1849–1861.
- Kaur N, Pandey A, Negi H, et al. Effect of HDL-raising drugs on cardiovascular outcomes: a systematic review and meta-regression. PLoS One 2014; 9:e94585.
- Barter PJ, Caulfield M, Eriksson M, et al; ILLUMINATE investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Rader D, Kastelein J. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation 2014; 129:1022–1032.
- Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003; 34:154–156.
- Verbeek R, Stoekenbroek RM, Hovingh GK. PCSK9 inhibitors: novel therapeutic agents for the treatment of hypercholesterolemia. Eur J of Pharm 2015; 763(Pt A):38–47.
- Steinberg D, Witztum JL. Inhibition of PCSK9: a powerful weapon for achieving ideal LDL cholesterol levels. Proc Natl Acad Sci USA 2009; 106:9546–9547.
- Abifadel M, Rabès J-P, Devillers M, et al. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum Mutat 2009; 30:520–529.
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272.
- Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybærg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease. J Am Coll Cardiol 2010; 55:2833–2842.
- Mortensen MB, Afzal S, Nordestgaard BG, Falk E. The high-density lipoprotein-adjusted SCORE model worsens SCORE-based risk classification in a contemporary population of 30,824 Europeans: the Copenhagen General Population Study. Eur Heart J 2015; 36:2446–2453.
- Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004; 93:1473–1480.
- Graham MJ, Lemonidis KM, Whipple CP, et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res 2007; 48:763–767.
- Shan L, Pang L, Zhang R, Murgolo NJ, Lan H, Hedrick JA. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem Biophys Res Comm 2008; 375:69–73.
- Stein EA, Raal F. Reduction of low-density lipoprotein cholesterol by monoclonal antibody inhibition of PCSK9. Annu Rev Med 2014; 65:417–431.
- Duff CJ, Scott MJ, Kirby IT, Hutchinson SE, Martin SL, Hooper NM. Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor. Biochem J 2009; 419:577–584.
- Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Eng J Med 2012; 366:1108–1118.
- Koren MJ, Lundqvist P, Bolognese M, et al; MENDEL-2 Investigators. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol 2014; 63:2531–2540.
- Blom DJ, Hala T, Bolognese M, et al; DESCARTES investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370:1809-1819.
- Raal FJ, Stein EA, Dufour R, et al; RUTHERFORD-2 Investigators. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2014; 385:331–340.
- Robinson JG, Nedergaard BS, Rogers WJ, et al; LAPLA C-2 Investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014; 311:1870–1882.
- Stroes E, Colquhoun D, Sullivan D, et al; GAUSS-2 Investigators. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014; 63:2541–2548.
- Nissen SE, Stroes E, Dent-Acosta RE, et al; GAUSS-3 Investigators. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance, the GAUSS-3 randomized clinical trial. JAMA 2016; 315:1580–1590.
- Trial assessing long term use of PCSK9 inhibition in subjects with genetic LDL disorders (TAUSSIG). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT-1624142. Updated June 25, 2015. Accessed October 23, 2016.
- Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015; 36:2996–3003.
- Efficacy and safety of alirocumab (SAR236553/REGN727) versus placebo on top of lipid-modifying therapy in patients with heterozygous familial hypercholesterolemia; the ODYSSEY HIGH FH trial. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01617655. Updated September 27, 2016. Accessed October 23, 2016.
- Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am Heart J 2015; 169:906–915.
- Efficacy and Safety of Alirocumab (SAR236553/REGN727) Versus Ezetimibe on Top of Statin in High Cardiovascular Risk Patients With Hypercholesterolemia (ODYSSEY COMBO II). U.S. National Institutes of Health website. Updated June 23, 2016. https://clinicaltrials.gov/ct2/show/NCT01644188. Accessed October 23, 2016.
- Roth EM, Moriarty P, Bergeron J, et al; ODYSSEY CHOICE I investigators. A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add-on to statin: ODYSSEY CHOICE I. Atherosclerosis 2016, doi: 10.1016/j.atherosclerosis.2016.08.043.
- Phase III Study To Evaluate Alirocumab in Patients With Hypercholesterolemia Not Treated With a Statin (ODYSSEY CHOICE II). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT02023879. Updated November 2, 2015. Accessed October 23, 2016.
- Monthly and twice monthly subcutaneous dosing of PF-04950615 (RN316) in hypercholesterolemic subjects on a statin. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/results?term=NCT01592240. Updated October 14, 2014. Accessed October 23, 2016.
- Zhang XL, Zhu QQ, Zhu L, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med 2015; 13:123.
- Sabatine MS, Giugliano RP, Wiviott SD, et al; OSLER Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1500–1509.
- Robinson JG, Farnier M, Krempf M, et al; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1489–1499.
- Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann Intern Med 2015; 163:40–51.
- Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01764633. Updated July 26, 2016. Accessed October 23, 2016.
- ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01663402. Updated October 23, 2016. Accessed September 13, 2016.
- The Evaluation of Bococizumab (PF-04950615;RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-1). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01975376. Updated September 22, 2016. Accessed October 23, 2016.
- The Evaluation of Bococizumab (PF-04950615; RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-2). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01975389. Updated July 26, 2016. Accessed October 23, 2016.
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al; Writing Committee. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 2016; 68:92–125.
- Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4,444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Sacks FM, Pfeiffer MA, Moye LA, et al; Cholesterol and Recurrent Events Trial Investigators. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial Investigators. N Engl J Med 1996; 335:1001–1009.
- Schwartz GG, Olsson AG, Ezekowitz MD, et al; Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–1718.
- Lipid Research Clinics Program. The Lipid Research Clinics Coronary Primary Prevention Trial results (reduction in incidence of coronary heart disease). JAMA 1984; 251:351–364.
- Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation 2004; 109(suppl 1):III39–III43.
- Stone N, Robinson J, Lichtenstein A, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(suppl 2):S1–S45.
- Jacobson TA, Ito MK, Maki KC, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1—full report. J Clin Lipidol 2015; 9:129–169.
- Jarcho JA, Keaney JF Jr. Proof that lower is better–LDL cholesterol and IMPROVE-IT. N Engl J Med 2015; 372:2448–2450.
- Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372:2387–2397.
- Baigent C, Keech A, Kearney PM, et al; Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005; 366:1267–1278.
- de Ferranti S, Rodday AM, Mendelson M, et al. Prevalence of familial hypercholesterolemia in the 1999 to 2012 United States National Health and Nutrition Examination Surveys (NHANES). Circulation 2016; 133:1067–1072.
- Perez de Isla L, Alonso R, Watts GF, et al; SAFEHEART investigators. Attainment of LDL-cholesterol treatment goals in patients with familial hypercholesterolemia: 5-year SAFEHEART registry follow-up. J Am Coll Cardiol 2016; 67:1278–1285.
- Unni SK, Quek RGW, Biskupiak J, et al. Assessment of statin therapy, LDL-C levels, and cardiovascular events among high-risk patients in the United States. J Clin Lipidol 2016; 10:63–71.
- Mammen AL, Amato AA. Statin myopathy: a review of recent progress. Curr Opin Rheum 2010; 22:644–650.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010; 362:1563–1574.
- Keech A, Simes RJ, Barter P, et al; FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomized controlled trial. Lancet 2005; 366:1849–1861.
- Kaur N, Pandey A, Negi H, et al. Effect of HDL-raising drugs on cardiovascular outcomes: a systematic review and meta-regression. PLoS One 2014; 9:e94585.
- Barter PJ, Caulfield M, Eriksson M, et al; ILLUMINATE investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med 2007; 357:2109–2122.
- Rader D, Kastelein J. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation 2014; 129:1022–1032.
- Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet 2003; 34:154–156.
- Verbeek R, Stoekenbroek RM, Hovingh GK. PCSK9 inhibitors: novel therapeutic agents for the treatment of hypercholesterolemia. Eur J of Pharm 2015; 763(Pt A):38–47.
- Steinberg D, Witztum JL. Inhibition of PCSK9: a powerful weapon for achieving ideal LDL cholesterol levels. Proc Natl Acad Sci USA 2009; 106:9546–9547.
- Abifadel M, Rabès J-P, Devillers M, et al. Mutations and polymorphisms in the proprotein convertase subtilisin kexin 9 (PCSK9) gene in cholesterol metabolism and disease. Hum Mutat 2009; 30:520–529.
- Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med 2006; 354:1264–1272.
- Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybærg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease. J Am Coll Cardiol 2010; 55:2833–2842.
- Mortensen MB, Afzal S, Nordestgaard BG, Falk E. The high-density lipoprotein-adjusted SCORE model worsens SCORE-based risk classification in a contemporary population of 30,824 Europeans: the Copenhagen General Population Study. Eur Heart J 2015; 36:2446–2453.
- Victor RG, Haley RW, Willett DL, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 2004; 93:1473–1480.
- Graham MJ, Lemonidis KM, Whipple CP, et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res 2007; 48:763–767.
- Shan L, Pang L, Zhang R, Murgolo NJ, Lan H, Hedrick JA. PCSK9 binds to multiple receptors and can be functionally inhibited by an EGF-A peptide. Biochem Biophys Res Comm 2008; 375:69–73.
- Stein EA, Raal F. Reduction of low-density lipoprotein cholesterol by monoclonal antibody inhibition of PCSK9. Annu Rev Med 2014; 65:417–431.
- Duff CJ, Scott MJ, Kirby IT, Hutchinson SE, Martin SL, Hooper NM. Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor. Biochem J 2009; 419:577–584.
- Stein EA, Mellis S, Yancopoulos GD, et al. Effect of a monoclonal antibody to PCSK9 on LDL cholesterol. N Eng J Med 2012; 366:1108–1118.
- Koren MJ, Lundqvist P, Bolognese M, et al; MENDEL-2 Investigators. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol 2014; 63:2531–2540.
- Blom DJ, Hala T, Bolognese M, et al; DESCARTES investigators. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med 2014; 370:1809-1819.
- Raal FJ, Stein EA, Dufour R, et al; RUTHERFORD-2 Investigators. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2014; 385:331–340.
- Robinson JG, Nedergaard BS, Rogers WJ, et al; LAPLA C-2 Investigators. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014; 311:1870–1882.
- Stroes E, Colquhoun D, Sullivan D, et al; GAUSS-2 Investigators. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol 2014; 63:2541–2548.
- Nissen SE, Stroes E, Dent-Acosta RE, et al; GAUSS-3 Investigators. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance, the GAUSS-3 randomized clinical trial. JAMA 2016; 315:1580–1590.
- Trial assessing long term use of PCSK9 inhibition in subjects with genetic LDL disorders (TAUSSIG). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT-1624142. Updated June 25, 2015. Accessed October 23, 2016.
- Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015; 36:2996–3003.
- Efficacy and safety of alirocumab (SAR236553/REGN727) versus placebo on top of lipid-modifying therapy in patients with heterozygous familial hypercholesterolemia; the ODYSSEY HIGH FH trial. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01617655. Updated September 27, 2016. Accessed October 23, 2016.
- Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: The ODYSSEY COMBO I study. Am Heart J 2015; 169:906–915.
- Efficacy and Safety of Alirocumab (SAR236553/REGN727) Versus Ezetimibe on Top of Statin in High Cardiovascular Risk Patients With Hypercholesterolemia (ODYSSEY COMBO II). U.S. National Institutes of Health website. Updated June 23, 2016. https://clinicaltrials.gov/ct2/show/NCT01644188. Accessed October 23, 2016.
- Roth EM, Moriarty P, Bergeron J, et al; ODYSSEY CHOICE I investigators. A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add-on to statin: ODYSSEY CHOICE I. Atherosclerosis 2016, doi: 10.1016/j.atherosclerosis.2016.08.043.
- Phase III Study To Evaluate Alirocumab in Patients With Hypercholesterolemia Not Treated With a Statin (ODYSSEY CHOICE II). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT02023879. Updated November 2, 2015. Accessed October 23, 2016.
- Monthly and twice monthly subcutaneous dosing of PF-04950615 (RN316) in hypercholesterolemic subjects on a statin. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/results?term=NCT01592240. Updated October 14, 2014. Accessed October 23, 2016.
- Zhang XL, Zhu QQ, Zhu L, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med 2015; 13:123.
- Sabatine MS, Giugliano RP, Wiviott SD, et al; OSLER Investigators. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1500–1509.
- Robinson JG, Farnier M, Krempf M, et al; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1489–1499.
- Navarese EP, Kolodziejczak M, Schulze V, et al. Effects of proprotein convertase subtilisin/kexin type 9 antibodies in adults with hypercholesterolemia: a systematic review and meta-analysis. Ann Intern Med 2015; 163:40–51.
- Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk (FOURIER). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01764633. Updated July 26, 2016. Accessed October 23, 2016.
- ODYSSEY Outcomes: Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab. U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01663402. Updated October 23, 2016. Accessed September 13, 2016.
- The Evaluation of Bococizumab (PF-04950615;RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-1). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01975376. Updated September 22, 2016. Accessed October 23, 2016.
- The Evaluation of Bococizumab (PF-04950615; RN316) in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects (SPIRE-2). U.S. National Institutes of Health website. https://clinicaltrials.gov/ct2/show/NCT01975389. Updated July 26, 2016. Accessed October 23, 2016.
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al; Writing Committee. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 2016; 68:92–125.
KEY POINTS
- Potential candidates for PCSK9 inhibitor therapy are patients with familial hypercholesterolemia with a lifetime burden of elevated low-density-lipoprotein cholesterol (LDL-C) and thus a low likelihood of optimal control on current therapies; patients with complete or partial statin intolerance, with high-intensity statin dosing limited by adverse effects; and patients at high CVD risk with LDL-C goals not achieved with current therapies.
- Subcutaneously administered monoclonal antibodies targeting PCSK9 are currently the only PCSK9 inhibitors with FDA approval.
- PCSK9 inhibitors under study include agents with more durable effect and that require less frequent injections, RNA-interference therapies, vaccinations, antisense therapies, and oral formulations.