User login
Pyogenic granuloma
BY ALLISON HAN AND LAWRENCE F. EICHENFIELD, MD
Frontline Medical News
Pyogenic granuloma (PG), also known as lobular capillary hemangioma, is a benign, acquired vascular neoplasm of the skin and mucous membranes, and is fairly commonly found in children.1 Though most variants are superficial, PGs rarely may be discovered in subcutaneous, dermal, or satellite locations as well.2 Despite its name, no infectious or granulomatous disorder association with the vascular tumor has been discovered.
PGs are characterized by small, erythematous to purple papules that rapidly grow to become pedunculated or sessile within weeks, and then stabilize. They may bleed profusely because of their friable vascular nature, ulcerate, and crust.3 They rarely resolve on their own, thus, removal frequently is sought.4 PGs most commonly develop on the head and neck, and slightly less commonly on the fingers, periungual areas, upper extremities, lips, gingiva, and conjunctiva. Most PGs grow to about 1 to 10 mm.5
Histologically, PGs are organized in a characteristic lobular pattern of hyperplastic capillary clusters after which they are named (lobular capillary hemangioma). Fibrous bands separate the lobules. Mitotic activity is often seen, likely reflecting the rapid growth of the tumor.2 Immunohistochemistry staining is notably negative for glucose transporter 1 (GLUT-1), unlike hemangiomas, which aids in differentiating the two lesions.6
The pathogenesis of PGs is poorly understood; some hypothesize that a traumatic event, such as an insect bite, may trigger aberrant overgrowth of granulation tissue, but this theory is contested as many PGs appear to arise de novo.5 PGs have a tendency to develop within vascular lesions, suggesting an angiogenic stimuli of development, and increased expression of angiogenic growth factors (vascular endothelial growth factor and mitogen-activated protein kinase pathways) have been found in PGs.7,8
PGs also arise frequently during pregnancy and resolve after childbirth, leading many to support a causal role of estrogen in PG development. A link between both steroid hormone and angiogenesis may exist, as a beta isoform of estrogen receptor has been discovered to have consistent expression in a variety of vascular proliferations, possibly underlying PG pathogenesis.2
Drug-induced cases of PGs have been reported as well, with use of systemic medications such as acitretin, human immunodeficiency virus protease inhibitors, and epidermal growth factor receptor inhibitors.9
Diagnosis and differential
The diagnosis of PGs is based on the characteristic history of a rapidly growing, red to purple nodule that frequently bleeds and often ulcerates, accompanied by the physical findings of a typical exophytic, friable, vascular lesion. Patients often present with the “band aid sign,” an irritated portion of skin from the adhesive of bandages worn to protect the bleeding lesion.
The classic history of profuse and recurrent bleeding with little agitation often enables differentiation of PG from other disorders. In young infants, PGs may look similar to infantile hemangiomas, however hemangiomas regress and involute spontaneously unlike PGs.10 Other disorders that may be differentiated from PGs by history include Spitz nevi, glomus tumors, and warts. Melanoma in children may be amelanotic and present as red papules or nodules as well.11 An association between Bartonella infection and PGs had been questioned in the past because of reported increased rates of Bartonella seropositivity in patients, but this has since been debunked.12
Treatment
Almost all PGs undergo treatment; 85%-90% of PGs are treated by localized numbing, followed by superficial excision and electrodessication of the base.13 This technique allows the PG to be removed in less than 5 minutes and avoids suture use. Alternative treatments include surgical excision, pulsed dye laser, cryotherapy with liquid nitrogen, timolol solution, imiquimod, or a combination of methods.3,4,5 Methods that produce tissue for histopathologic analysis are ideal to rule out malignancy or other lesions that may mimic PGs such as Spitz nevi. Pulsed dye laser offers low recurrence, but often requires multiple treatments, and young children may not tolerate the treatment well. Cryotherapy has few side effects, but has higher recurrence rates and requires multiple treatment sessions.14
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego, as well as vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Allison Han is a medical student at the university. Neither Dr. Eichenfield nor Ms. Han have any relevant financial disclosures. Email them at [email protected].
References
1. Br J Dermatol. 2014;171(3):466-73.
2. Am J Dermatopathol. 2007;29(4):408-11.
3. J Plast Reconstr Aesthet Surg. 2011;64(9):1216-20.
4. Br J Dermatol. 2014;171(6):1537-8.
5. Pediatr Dermatol. 2004;21(1):10-3.
6. Pediatr Dermatol. 2009;26(3):323-7.
7. J Am Acad Dermatol. 2000;42(2 Pt 1):275-9.
8. J Am Acad Dermatol. 2001;44(2):193-7.
9. Br J Dermatol. 2010;163(5):941-53.
10. Pediatrics. 1992;90(6):989-91.
11. J Am Acad Dermatol. 2013;68(6):913-25.
12. J Am Acad Dermatol. 2005;53(6):1065-6.
13. “Hurwitz Clinical Pediatric Dermatology,” 4th Edition: A Textbook of Skin Disorders of Childhood and Adolescence (New York: Elsevier Health Sciences, 2011).
14. Dermatol Surg. 2013;39(8):1137-46.
Pyogenic granuloma
BY ALLISON HAN AND LAWRENCE F. EICHENFIELD, MD
Frontline Medical News
Pyogenic granuloma (PG), also known as lobular capillary hemangioma, is a benign, acquired vascular neoplasm of the skin and mucous membranes, and is fairly commonly found in children.1 Though most variants are superficial, PGs rarely may be discovered in subcutaneous, dermal, or satellite locations as well.2 Despite its name, no infectious or granulomatous disorder association with the vascular tumor has been discovered.
PGs are characterized by small, erythematous to purple papules that rapidly grow to become pedunculated or sessile within weeks, and then stabilize. They may bleed profusely because of their friable vascular nature, ulcerate, and crust.3 They rarely resolve on their own, thus, removal frequently is sought.4 PGs most commonly develop on the head and neck, and slightly less commonly on the fingers, periungual areas, upper extremities, lips, gingiva, and conjunctiva. Most PGs grow to about 1 to 10 mm.5
Histologically, PGs are organized in a characteristic lobular pattern of hyperplastic capillary clusters after which they are named (lobular capillary hemangioma). Fibrous bands separate the lobules. Mitotic activity is often seen, likely reflecting the rapid growth of the tumor.2 Immunohistochemistry staining is notably negative for glucose transporter 1 (GLUT-1), unlike hemangiomas, which aids in differentiating the two lesions.6
The pathogenesis of PGs is poorly understood; some hypothesize that a traumatic event, such as an insect bite, may trigger aberrant overgrowth of granulation tissue, but this theory is contested as many PGs appear to arise de novo.5 PGs have a tendency to develop within vascular lesions, suggesting an angiogenic stimuli of development, and increased expression of angiogenic growth factors (vascular endothelial growth factor and mitogen-activated protein kinase pathways) have been found in PGs.7,8
PGs also arise frequently during pregnancy and resolve after childbirth, leading many to support a causal role of estrogen in PG development. A link between both steroid hormone and angiogenesis may exist, as a beta isoform of estrogen receptor has been discovered to have consistent expression in a variety of vascular proliferations, possibly underlying PG pathogenesis.2
Drug-induced cases of PGs have been reported as well, with use of systemic medications such as acitretin, human immunodeficiency virus protease inhibitors, and epidermal growth factor receptor inhibitors.9
Diagnosis and differential
The diagnosis of PGs is based on the characteristic history of a rapidly growing, red to purple nodule that frequently bleeds and often ulcerates, accompanied by the physical findings of a typical exophytic, friable, vascular lesion. Patients often present with the “band aid sign,” an irritated portion of skin from the adhesive of bandages worn to protect the bleeding lesion.
The classic history of profuse and recurrent bleeding with little agitation often enables differentiation of PG from other disorders. In young infants, PGs may look similar to infantile hemangiomas, however hemangiomas regress and involute spontaneously unlike PGs.10 Other disorders that may be differentiated from PGs by history include Spitz nevi, glomus tumors, and warts. Melanoma in children may be amelanotic and present as red papules or nodules as well.11 An association between Bartonella infection and PGs had been questioned in the past because of reported increased rates of Bartonella seropositivity in patients, but this has since been debunked.12
Treatment
Almost all PGs undergo treatment; 85%-90% of PGs are treated by localized numbing, followed by superficial excision and electrodessication of the base.13 This technique allows the PG to be removed in less than 5 minutes and avoids suture use. Alternative treatments include surgical excision, pulsed dye laser, cryotherapy with liquid nitrogen, timolol solution, imiquimod, or a combination of methods.3,4,5 Methods that produce tissue for histopathologic analysis are ideal to rule out malignancy or other lesions that may mimic PGs such as Spitz nevi. Pulsed dye laser offers low recurrence, but often requires multiple treatments, and young children may not tolerate the treatment well. Cryotherapy has few side effects, but has higher recurrence rates and requires multiple treatment sessions.14
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego, as well as vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Allison Han is a medical student at the university. Neither Dr. Eichenfield nor Ms. Han have any relevant financial disclosures. Email them at [email protected].
References
1. Br J Dermatol. 2014;171(3):466-73.
2. Am J Dermatopathol. 2007;29(4):408-11.
3. J Plast Reconstr Aesthet Surg. 2011;64(9):1216-20.
4. Br J Dermatol. 2014;171(6):1537-8.
5. Pediatr Dermatol. 2004;21(1):10-3.
6. Pediatr Dermatol. 2009;26(3):323-7.
7. J Am Acad Dermatol. 2000;42(2 Pt 1):275-9.
8. J Am Acad Dermatol. 2001;44(2):193-7.
9. Br J Dermatol. 2010;163(5):941-53.
10. Pediatrics. 1992;90(6):989-91.
11. J Am Acad Dermatol. 2013;68(6):913-25.
12. J Am Acad Dermatol. 2005;53(6):1065-6.
13. “Hurwitz Clinical Pediatric Dermatology,” 4th Edition: A Textbook of Skin Disorders of Childhood and Adolescence (New York: Elsevier Health Sciences, 2011).
14. Dermatol Surg. 2013;39(8):1137-46.
Pyogenic granuloma
BY ALLISON HAN AND LAWRENCE F. EICHENFIELD, MD
Frontline Medical News
Pyogenic granuloma (PG), also known as lobular capillary hemangioma, is a benign, acquired vascular neoplasm of the skin and mucous membranes, and is fairly commonly found in children.1 Though most variants are superficial, PGs rarely may be discovered in subcutaneous, dermal, or satellite locations as well.2 Despite its name, no infectious or granulomatous disorder association with the vascular tumor has been discovered.
PGs are characterized by small, erythematous to purple papules that rapidly grow to become pedunculated or sessile within weeks, and then stabilize. They may bleed profusely because of their friable vascular nature, ulcerate, and crust.3 They rarely resolve on their own, thus, removal frequently is sought.4 PGs most commonly develop on the head and neck, and slightly less commonly on the fingers, periungual areas, upper extremities, lips, gingiva, and conjunctiva. Most PGs grow to about 1 to 10 mm.5
Histologically, PGs are organized in a characteristic lobular pattern of hyperplastic capillary clusters after which they are named (lobular capillary hemangioma). Fibrous bands separate the lobules. Mitotic activity is often seen, likely reflecting the rapid growth of the tumor.2 Immunohistochemistry staining is notably negative for glucose transporter 1 (GLUT-1), unlike hemangiomas, which aids in differentiating the two lesions.6
The pathogenesis of PGs is poorly understood; some hypothesize that a traumatic event, such as an insect bite, may trigger aberrant overgrowth of granulation tissue, but this theory is contested as many PGs appear to arise de novo.5 PGs have a tendency to develop within vascular lesions, suggesting an angiogenic stimuli of development, and increased expression of angiogenic growth factors (vascular endothelial growth factor and mitogen-activated protein kinase pathways) have been found in PGs.7,8
PGs also arise frequently during pregnancy and resolve after childbirth, leading many to support a causal role of estrogen in PG development. A link between both steroid hormone and angiogenesis may exist, as a beta isoform of estrogen receptor has been discovered to have consistent expression in a variety of vascular proliferations, possibly underlying PG pathogenesis.2
Drug-induced cases of PGs have been reported as well, with use of systemic medications such as acitretin, human immunodeficiency virus protease inhibitors, and epidermal growth factor receptor inhibitors.9
Diagnosis and differential
The diagnosis of PGs is based on the characteristic history of a rapidly growing, red to purple nodule that frequently bleeds and often ulcerates, accompanied by the physical findings of a typical exophytic, friable, vascular lesion. Patients often present with the “band aid sign,” an irritated portion of skin from the adhesive of bandages worn to protect the bleeding lesion.
The classic history of profuse and recurrent bleeding with little agitation often enables differentiation of PG from other disorders. In young infants, PGs may look similar to infantile hemangiomas, however hemangiomas regress and involute spontaneously unlike PGs.10 Other disorders that may be differentiated from PGs by history include Spitz nevi, glomus tumors, and warts. Melanoma in children may be amelanotic and present as red papules or nodules as well.11 An association between Bartonella infection and PGs had been questioned in the past because of reported increased rates of Bartonella seropositivity in patients, but this has since been debunked.12
Treatment
Almost all PGs undergo treatment; 85%-90% of PGs are treated by localized numbing, followed by superficial excision and electrodessication of the base.13 This technique allows the PG to be removed in less than 5 minutes and avoids suture use. Alternative treatments include surgical excision, pulsed dye laser, cryotherapy with liquid nitrogen, timolol solution, imiquimod, or a combination of methods.3,4,5 Methods that produce tissue for histopathologic analysis are ideal to rule out malignancy or other lesions that may mimic PGs such as Spitz nevi. Pulsed dye laser offers low recurrence, but often requires multiple treatments, and young children may not tolerate the treatment well. Cryotherapy has few side effects, but has higher recurrence rates and requires multiple treatment sessions.14
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego, as well as vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Allison Han is a medical student at the university. Neither Dr. Eichenfield nor Ms. Han have any relevant financial disclosures. Email them at [email protected].
References
1. Br J Dermatol. 2014;171(3):466-73.
2. Am J Dermatopathol. 2007;29(4):408-11.
3. J Plast Reconstr Aesthet Surg. 2011;64(9):1216-20.
4. Br J Dermatol. 2014;171(6):1537-8.
5. Pediatr Dermatol. 2004;21(1):10-3.
6. Pediatr Dermatol. 2009;26(3):323-7.
7. J Am Acad Dermatol. 2000;42(2 Pt 1):275-9.
8. J Am Acad Dermatol. 2001;44(2):193-7.
9. Br J Dermatol. 2010;163(5):941-53.
10. Pediatrics. 1992;90(6):989-91.
11. J Am Acad Dermatol. 2013;68(6):913-25.
12. J Am Acad Dermatol. 2005;53(6):1065-6.
13. “Hurwitz Clinical Pediatric Dermatology,” 4th Edition: A Textbook of Skin Disorders of Childhood and Adolescence (New York: Elsevier Health Sciences, 2011).
14. Dermatol Surg. 2013;39(8):1137-46.
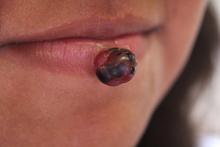
A 7-year-old healthy female presents to the dermatology clinic for evaluation of a bump on her lower lip that has been present for 3 months. It started as a small red papule and within a couple months rapidly increased in size, but now has stabilized. She denies pain and itching, but she notes that it frequently bleeds, and that the bleeding can be difficult to stop. She denies any trauma to the area. Review of systems was otherwise negative.
On examination, the patient is a well-developed, healthy appearing female. In the middle of her lower lip, there is a 1-cm pedunculated, raspberry-like exophytic nodule. It is reddish-purple with slight ulceration at the surface. There is no active bleeding. The remainder of the physical examination is normal.