User login
Time and study make a difference. So does careful review and reappraisal of existing data. In the past year, the pendulum has swung away from fear of hormone therapy to a better understanding of indications, risks and benefits—an understanding driven largely by the evidence-based position statements of the North American Menopause Society (NAMS).
A meta-analysis of randomized controlled trials of phytoestrogens attested to lack of efficacy or weak effect, which helps clear the picture on soy and red clover, but the researchers stressed that the lack of quality control does not rule out the possibility that some products might carry steroidal effects and potential risk.
And another year has brought even more evidence that diet, exercise, smoking cessation and the like really do improve health and quality of life.
Advisory on hormone therapy and “bio-identicals”
The NAMS Hormone Therapy Panel concluded definitively that bio-identical hormones should be considered in the same category as all the sex steroids, which, in the absence of specific safety and efficacy studies, carry the same risks and benefits as related products.
On the other hand, alternatives do exist for specific indications, such as bisphosphonates for bone conservation.
The new NAMSPosition Statement stresses individualized treatment based on the recommendations below.
The full report is available at www.menopause.org.
- Treatment of moderate to severe menopausal symptoms is the primary indications for systemic therapy. Every systemic product is FDA-approved for this indication.
- Every systemic and local product is approved for moderate vulvar and vaginal atrophy. For this indication alone, local ET is generally advised.
- Duration should be for the lowest effective dose and shortest time consistent with treatment goals.
- If the woman is well aware of potential risks and benefits, and if there is clinical supervision, extended use of the lowest effective ET/EPT dose for treatment goals is acceptable in women who believe the benefits outweigh the risks, for those at high risk of osteoporotic fracture who also have moderate to severe menopause symptoms, for further prevention of established bone loss when alternate therapies are not appropriate or cause side effects, or when outcomes of extended use of those therapies are not known.
- Although specific compounds, doses, and routes of administration may have different outcomes, clinical trial results for one agent should be generalized to all agents within the same family in the absence of data for each specific product. This proviso also applies to the so-called bioidentical products.
Question marks
The Hormone Therapy panel could not agree unanimously on these questions:
- Should women who are doing well on long-term HT discontinue?
- What is the best way to discontinue HT, abrupt cessation or tapering?
- Is the effect of continuous-combined EPT different from that of continuous estrogen with sequential progestogen?
- How definitive is the evidence on early increased CHD risk with HT?
- Conflicting data precluded a consensus on adverse breast cancer and cardiovascular outcomes associated with ET/EPT.
ACKNOWLEDGMENTS
The following commentaries on key papers are from the NAMS First To Knowemail program for members. I thank the members of NAMS who have taken time out to provide these objective reviews of the studies presented here, and Phil Lammers, NAMS Medical Editor.
OSTEOPOROSISCurb your enthusiasm—no need to rush bone drugs if risk is low
McClung MR, Wasnich RD, Hosking DJ, et al, on behalf of the Early Postmenopausal Intervention Cohort (EPIC) study group. Prevention of postmenopausal bone loss: six-year results from the early postmenopausal intervention cohort study. J Clin Endocrinol Metab 2004;89:4879-4885. LEVEL 1 EVIDENCE: Randomized, controlled trial
In this 6-year study of women in their 50s, the placebo group lost an inconsequential amount of bone mass. Not surprisingly, women using alendronate had some increase in BMD and some reduction in bone turnover markers.
Women in their 50s are not melting away. Their bones are not dissolving out from under them, contrary to what many media reports would have ObGyns and patients believe. Still, many clinicians are enthusiastic about prescribing bone drugs like bisphosphonates to women in their 50s who are generally healthy. (And there is no doubt that we do have bone drugs found to be safe and effective in well-designed trials, including the EPIC study.)
Yet there has been a major shift away from starting osteoporosis prevention drugs soon after menopause. EPIC data add support for a “go slow” strategy for drug intervention in healthy women in their 50s.
The EPIC study involved a total of 1,609 women ages 45 to 59, who received alendronate or placebo in a double-blind, randomized design. BMD was measured annually. The 4-year results were reported previously, and the 6-year results were published just last fall. Not surprisingly, women using alendronate had some increase in BMD and some reduction in bone turnover markers. But the results in the women who took placebo are of singular interest.
After 6 years, women on placebo had lost very little bone. The amount lost was statistically significant, but clinically inconsequential. The average BMD in women on placebo decreased 3% in the spine and 2% in the hip. Thus, the average rate of bone loss was about 0.5% per year.
A bone mass decrease of this extent represents a decline of about -0.3 T score, which is negligible. In the EPIC study, the 6-year fracture benefit, based on any type of fracture, boils down to lowering the risk from 1 in 11 on placebo to 1 in 9 on alendronate. These healthy women in their 50s had a very low risk of fracture, and taking a drug for 6 years had very little benefit for fracture reduction.
Women in their 50s typically have about 10% to 15% less bone mass than women of 25 to 30, when bone mass is at its peak. That 10% to 15% lower BMD translates to a T score of –1 to –1.2 , which is currently being labeled as osteopenic. Many patients and physicians have come to feel that osteopenia must always be treated with our newer drugs.
We are discovering that starting healthy women in their 50s on osteoporosis prevention drugs carries an extremely high cost per fracture avoided. During the 10 years since the startup of the EPIC study, support for early drug intervention in healthy women still in their 50s has dwindled. Now, expert groups, including the National Osteoporosis Foundation and the US Preventive Services Task Force, advise waiting until age 65 before starting osteoporosis risk evaluation or considering drug intervention in women who are otherwise healthy.
In my practice, I give healthy women in their 50s permission not to take drugs if their risk of fracture within the next 5 to 10 years is low. The picture is quite different in postmenopausal women in their 50s who do have high fracture risk, such as those who have already had a fracture, or who have very low bone density or high exposure to glucocorticoids.
EPIC data support the concept that the rate of bone loss is quite slow after a year or 2 has elapsed after menopause.
We need to avoid medicalizing these patients simply because we have drugs that reduce bone loss or because women in their 50s have less bone mass than 25-year-olds.
BIBLIOGRAPHY
Hosking D, Chilvers CE, Christiansen C, et al. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med. 1998;338:485-492.
Wasnich RD, Bagger YZ, Hosking DJ, et al. Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause. 2004;11:622-630.
Siris ES, Bilezikian JP, Rubin MR, et al. Pins and plasters aren’t enough: a call for the evaluation and treatment of patients with osteoporotic fractures. J Clin Endocrinol Metab. 2003;88:3482-3486.
Rosen CJ, Black DM, Greenspan SL. Vignettes in osteoporosis: a road map to successful therapeutics. J Bone Miner Res. 2004;19:3-10.
HORMONE THERAPYDoes age affect mortality rate in postmenopausal women using HT?
Salpeter SR, Walsh JME, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women. J Gen Intern Med. 2004;19:791–804. META-ANALYSIS
- This study is sure to incite yet another round of debate about postmenopausal hormone therapy, but it does suggest that we can provide substantial reassurance about safety in younger women considering hormone therapy for menopause-related symptoms.
This study attempted to discover whether the age of the postmenopausal woman using hormone therapy affects mortality. Investigators performed a meta-analysis of clinical trials that reported mortality rates associated with use of postmenopausal hormone therapy, and analyzed the results based on mean ages.
They reported a significant trend between increasing risk of mortality and increasing mean age of the women using hormone therapy—raising the possibility of a health benefit for younger postmenopausal women.
The studies included in the metaanalysis varied in entry criteria, outcomes assessed, number of subjects, and HT type and dosage. Furthermore, because age groups were defined by mean age in each trial rather than actual age of pooled participants, some overlap in ages likely occurred between the analyses of younger and older women.
In postmenopausal women younger than 60, the total mortality rate was reduced by 39% in women taking estrogen-containing hormone therapy, which was significant; in women older than 60, there was no significant effect on total mortality.
The data were from 30 randomized, controlled clinical trials published between 1966 and 2002, and included 26,708 women taking estrogen (ET) or estrogen plus progestogen (EPT). Data were pooled to determine total mortality and mortality due to specific causes such as cardiovascular disease and cancer. The mean trial duration was 4.5 years, and the mean age was 62.2 years.
When the study population was divided into younger and older age groups based on mean ages, it was found that those younger than 60 (mean age, 53.9) had a significantly reduced OR for total mortality of 0.61 (95% CI, 0.39–0.95) and those older than age 60 (mean age, 64.6) had an OR of 1.03 (95% CI, 0.90–1.18).
For specific causes, the OR for cardiovascular disease mortality associated with ET/EPT was 1.10 (95% CI, 0.90–1.34). For overall cancer mortality, the OR was 1.03 (95% CI, 0.82–1.29) and for breast cancer mortality, the OR was 1.03 (95% CI, 0.29–3.67).
For causes other than cardiovascular disease or cancer, mortality was significantly lower in women on HT: OR 0.67 (95% CI, 0.51–0.88). When divided into younger and older age groups, ET/EPT was not associated with a significant change in mortality, with the exception of reduced mortality from causes other than cardiovascular disease and cancer in the older age group (OR, 0.68; 95% CI, 0.56–0.91).
Does HT improve insulin resistance?
Margolis KL, Bonds DE, Rodabough RJ, et al, for the Women’s Health Initiative Investigators. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. LEVEL 1 EVIDENCE: Randomized, controlled trial
Decreased insulin and fasting glucose
- Combined estrogen plus progestogen may reduce the incidence of diabetes, possibly by mediating a decrease in insulin resistance.
Hormone therapy, compared with placebo, was associated with 15 fewer cases of diabetes per 10,000 women per year. Fasting glucose and insulin decreased compared with placebo, and may suggest improved insulin resistance. Although others have reported similar results, it is unlikely that hormone therapy will be prescribed to prevent diabetes, given its greater risk than benefit for other outcomes observed in other WHI analyses.
In the EPT part of WHI, a total of 15,641 postmenopausal women aged 50 to 79 were assigned to placebo or continuous-combined EPT (0.625 mg/day conjugated equine estrogens plus 2.5 mg/day medroxyprogesterone acetate). The incidence of diabetes was based on self-reports of insulin or oral diabetes drug treatment. Fasting glucose, insulin, and lipoproteins were measured at 1 and 3 years. After 5.6 years, the incidence of treated diabetes was 3.5% in the EPT group and 4.2% in the placebo group (hazard ratio, 0.79; 95% CI, 0.67–0.93; P= 0.004). Decreases in fasting glucose and insulin, suggesting decreased insulin resistance, were significant at 1 year in EPT users compared with placebo. The authors concluded that EPT reduces the incidence of diabetes possibly through a decrease in insulin resistance.
To be sure that a drug prevents a disease, everyone with the disease should be excluded at baseline, and at the end of the trial, everyone should be tested for the disease—if it is commonly undiagnosed. To study the incidence of new diabetes, all women (6%) with self-reported diabetes at baseline were excluded, and correctly so. But half of US adults with diabetes are undiagnosed. The WHI 6% prevalence is half of the assumed 12% prevalence in older overweight women. In the WHI, average age was 63 years and average body mass index was 28. Thus, it is not certain that the reduced risk occurred in women who were diabetes-free at baseline.
Fasting glucose was reduced in the EPT part of WHI, as in the Postmenopausal Estrogen Progestin Intervention (PEPI) trial. But 2-hour glucose levels were elevated by hormone treatment in PEPI, and were not measured in the WHI. Many studies have shown that postprandial or post-challenge glucose is a stronger risk factor for cardiovascular disease than fasting hyperglycemia.
Could an elevated post-challenge glucose have played a role in the unexpected excess cardiovascular disease observed with hormone therapy in healthy women in WHI and with hormone therapy in women with documented coronary heart disease in the Heart and Estrogen/progestin Replacement Study (HERS)?
Will transdermal estrogen reduce both fasting and post-challenge glucose? These and other questions remain. (EBC)
Lifestyle changes work best
- This report raises the possibility but does not justify prescribing EPT for diabetes prevention.
Postmenopausal women randomized to EPT had a lower incidence of treated diabetes, by self-report, than women assigned to placebo: a 21% relative risk reduction over 3 years. At 1 year, a comparison of changes from baseline in estimated insulin resistance (HOMA model) in a subgroup indicated a significant reduction with EPT compared with placebo group, but no significant difference at 3 years.
Because of the far-reaching morbidity and mortality due to Type 2 diabetes, particularly from cardiovascular disease, prevention would have major benefits, but the authors acknowledge that this report does not justify prescribing this therapy for this purpose, given hazards previously reported in the WHI.
Still, we can bear in mind other means of reducing risk for diabetes. In the Diabetes Prevention Program,1metformin reduced type 2 diabetes risk by 31%, and a diet plus exercise program reduced it even more: by 58% over approximately 3 years of follow-up in high-risk persons. People at risk for diabetes should be counseled to make lifestyle changes that can reduce this risk far more, and more safely, than might EPT. (CGS)
Consider diabetes implications
- EPT can reduce the incidence of diabetes to the same degree as medications used for cardiovascular disease prevention.2
Growing evidence indicates that reducing insulin resistance in women can prevent onset of diabetes,3and that improving insulin resistance can slow the progression of atherosclerosis.4 Observational studies5—the Heart and Estrogen/progestin Replacement Study (HERS),6 and now the WHI—strongly indicate that EPT reduces the incidence of diabetes in postmenopausal women. Notably, HERS and WHI findings were with continuous-combined estrogen with progestin, the latter often viewed as antagonistic to the beneficial effects of estrogen on carbohydrate metabolism.) Diabetes is much more devastating in women, and more likely to strike. The risk (3,000 of 10,000) in postmenopausal women equals or exceeds that of postmenopausal breast cancer, coronary disease, or hip fracture.7The time has come to consider health and cost implications of long-term HT, especially in women with diabetes risk factors: age, obesity, high systolic BP, high nonfasting glucose, antihypertensive drug use, low HDL, or Hispanic or African-American ethnicity. Clinical trials confirming HT’s benefit add to the totality of evidence that the benefits outweigh the risks.8Since long-term effects (>10 years) reflect only observational data, we urgently need studies designed to understand long-term benefits and risks. (HNH)
1. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
2. Pepine CJ, Cooper-Dehoff RM. Cardiovascular therapies and risk for development of diabetes. J Am Coll Cardiol. 2004;44:509-512.
3. Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance inhigh risk Hispanic women. Diabetes. 2002;51:2796-2803.
4. Xiang AH, Peters RK, Kjos SL, et al. Effect of thiazolidinedione treatment on progression of subclinical atherosclerosis in premenopausal women at high risk for type 2 diabetes. J Clin Endocrinol Metab. 2005;90:1986-1991.
5. Manson JE, Rimm EB, Colditz GA, et al. A prospective study of postmenopausal estrogen therapy and subsequent incidence of non-insulin-dependent diabetes mellitus. Ann Epidemiol. 1992;2:665-673.
6. Kanaya AM, Herrington D, et al. Glycemic effects of postmenopausal hormone therapy: Heart and Estrogen/progestin Replacement Study. Randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:1-9.
7. Narayan KMV, Boyle JP, et al. Lifetime risk for diabetes mellitus in the US. JAMA 2003;290:1884-1890.
8. Philips LS, Langer RD. Postmenopausal hormone therapy: critical reappraisal and a unified hypothesis. Fertil Steril. 2005;83:558-566.
Soy versus placebo: Underwhelming
Red clover, likewise
Trebs EE, Ensrud KE, MacDonald R, Wilt TJ. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstet Gynecol. 2004;104:824–836. META-ANALYSIS
- Phytoestrogens did not significantly improve hot flashes, night sweats, and vaginal dryness, compared to placebo, in this meta-analysis of randomized, controlled clinical trials.
Data on 2,348 women (mean age, 53.1 years) experiencing a mean of 7.1 hot flashes per week were analyzed. Only randomized controlled trials reporting menopausal symptoms of hot flashes, night sweats, and vaginal dryness were included. Mean trial duration was 17 weeks.
- The 11 soy food or beverage supplementtrials (N = 995 women) found no improvement compared with placebo.
- Of the 8 soy food trialsreporting hot flash outcomes, only 1 showed a significant improvement compared with placebo.
- In the 9 soy extract trials, overall results (N = 854) were mixed. In 5 trials using soy extracts and reporting hot flash frequency, 3 found no significant difference in symptoms between the soy and placebo groups; the other 2 (total 114 subjects) found significant improvements.
- The 5 red clover trials(N = 400) showed no improvement over placebo.
Many women in these studies appear to have been perimenopausal rather than postmenopausal. Nine studies included women who had had a menstrual period within the previous 3 to 6 months (late perimenopausal). A subgroup analysis of perimenopausal women would have been useful, since their endocrinologic status is quite different from that of postmenopausal women.
Does soy improve cognition, bone density, or lipids?
Kreijkamp-Kaspers S, Kok L, Grobbee DE, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. 2004;292:65–74. LEVEL 1 EVIDENCE: Randomized, controlled trial
- Soy did not benefit cognition, bone, or lipids in 60-to-75-year-old women.
This careful trial raises the question of how to reconcile these results with animal and observational studies. In all, 202 postmenopausal women aged 60 to 75 years received 25.6 g/day of a soy protein supplement containing 99 mg isoflavones or a milk protein powder for 1 year. Adherence was monitored by serum genistein. There were no notable differences in:
- Memory, verbal skills, or concentration.
- Bone mineral density or bone-specific alkaline phosphatase, calcium, or phosphorus levels.
- Cholesterol, triglycerides, and lipoprotein plasma levels. These findings may not relate to perimenopausal women, in whom soy has been seen to significantly reduce LDL, but only during midfollicular and periovulatory phases.1 Premenopausal2 but not postmenopausal3monkeys given soy have had beneficial effects on bone quality.
REFERENCES
1. Merz-Demlow BE, Duncan AM, Wangen KE, et al. Soy isoflavones improve plasma lipids in normocholes-terolemic, premenopausal women. Am J Clin Nutr. 2000;71:1462-1469.
2. Kaplan JR, et al. Supplementation reduces the trajectory of atherogenesis in premenopausal monkeys at high risk for development of extensive postmenopausal coronary artery plaques. Menopause. 2004;11:653. Abstract S-17.
3. Register TC, Jayo MJ, Anthony MS. Soy phytoestrogens do not prevent bone loss in postmenopausal monkeys. J Clin Endocrinol Metab. 2003;88:4362-4370.
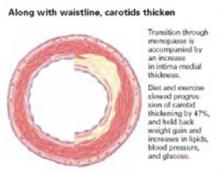
LIFESTYLE THERAPYThe secret to keeping those girlish carotids
Wildman RP, Schott LL, Brockwell S, Kuller LH, Sutton-Tyrrell K. A dietary and exercise intervention slows menopause-associated progression of subclinical atherosclerosis as measured by intima-media thickness of the carotid arteries. J Am Coll Cardiol. 2004;44:579–585. LEVEL 1 EVIDENCE: Randomized, controlled trial
- Carotid artery intima media thickens during the transition through menopause, but diet and exercise can reduce this progression by almost 50%.
- A diet-and-exercise regimen staves off menopause-associated weight gain and increases in lipids, blood pressure, and blood glucose.
These important findings are strong evidence that diet and exercise can slow the subclinical atherosclerosis progression that accompanies the menopause transition.
The Women’s Healthy Lifestyle Project previously found that weight gain and increased lipids, glucose, and blood pressure often accompany the menopause transition.1 This report describes improvements with diet and exercise intervention, compared with controls.
A total of 535 women aged 44 to 50 years were randomized to lifestyle intervention or assessment-only. All were premenopausal, and all had normal to high-normal body mass index, diastolic blood pressure, and fasting glucose and cholesterol levels.
The diet and exercise regimen used in the study was designed to reduce fat and cholesterol, prevent weight gain, and increase physical activity.
End points were progression of intimamedia thickness in the common carotid artery, internal carotid artery, and bulb segments.
The control group had significantly greater increases in intima-media thickness in women who became postmenopausal compared with those who remained premenopausal.
For women who became perimenopausal or postmenopausal during this 4-year study, diet and exercise slowed the progression of intima-media thickness by a 47% average reduction (P< 0.05), but had no effect on carotid segments in the women who remained premenopausal.
No benefit in intima media thickness was seen in women who remained premenopausal during the trial. Nevertheless, there are many well-documented benefits of healthy diet and exercise in premenopausal women.
Also of note, hormone therapy initiated after baseline measurements did not alter the results.
The message for patients, especially perimenopausal patients is that there is no time like the present to start a healthy lifestyle.
No downside
We’ve learned from the Nurse’s Health Study,2an observational study, that women who eat a healthy diet, do not smoke, and who exercise can reduce their risk of coronary heart disease by 57%.
We learned from the randomized controlled trial by the Diabetes Prevention Program Research Group3 that diet and exercise in high-risk women for 3 years can reduce incidence of new diabetes by 58%.
Now, in this trial, we learn that diet and exercise can reduce the progression of atherosclerosis in perimenopausal women by nearly 50%.
Since there is little, if any, downside to healthy living, why wait?
DISCLOSURES
Dr. Utian has served as an advisor/consultant for Eli Lilly, Pfizer, and Novartis. He has received research funding from Amylin, 3m, Barr, Berlex, BMS, Eli Lilly, Forest, Galen, Glaxo Smith Kline, Neurocrine Biosciences, Novartis, Novo Nordisk, Organon, Pharmacia, P&G, Pfizer, Roche, Sepracor, Solvay, Wyeth, and Yamanouchi.
Dr. Ettinger has served as an advisor for Berlex, Duramed-Barr, Glaxo Smith Kline, and P&G.
REFERENCES
1. Kuller LH, Simkin-Silverman LR, Wing RR, Meilahn EN, Ives DG. Women’s Healthy Lifestyle Project: a randomized clinical trial: results at 54 months. Circulation. 2001;103:32-37.
2. Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16-22.
3. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
Time and study make a difference. So does careful review and reappraisal of existing data. In the past year, the pendulum has swung away from fear of hormone therapy to a better understanding of indications, risks and benefits—an understanding driven largely by the evidence-based position statements of the North American Menopause Society (NAMS).
A meta-analysis of randomized controlled trials of phytoestrogens attested to lack of efficacy or weak effect, which helps clear the picture on soy and red clover, but the researchers stressed that the lack of quality control does not rule out the possibility that some products might carry steroidal effects and potential risk.
And another year has brought even more evidence that diet, exercise, smoking cessation and the like really do improve health and quality of life.
Advisory on hormone therapy and “bio-identicals”
The NAMS Hormone Therapy Panel concluded definitively that bio-identical hormones should be considered in the same category as all the sex steroids, which, in the absence of specific safety and efficacy studies, carry the same risks and benefits as related products.
On the other hand, alternatives do exist for specific indications, such as bisphosphonates for bone conservation.
The new NAMSPosition Statement stresses individualized treatment based on the recommendations below.
The full report is available at www.menopause.org.
- Treatment of moderate to severe menopausal symptoms is the primary indications for systemic therapy. Every systemic product is FDA-approved for this indication.
- Every systemic and local product is approved for moderate vulvar and vaginal atrophy. For this indication alone, local ET is generally advised.
- Duration should be for the lowest effective dose and shortest time consistent with treatment goals.
- If the woman is well aware of potential risks and benefits, and if there is clinical supervision, extended use of the lowest effective ET/EPT dose for treatment goals is acceptable in women who believe the benefits outweigh the risks, for those at high risk of osteoporotic fracture who also have moderate to severe menopause symptoms, for further prevention of established bone loss when alternate therapies are not appropriate or cause side effects, or when outcomes of extended use of those therapies are not known.
- Although specific compounds, doses, and routes of administration may have different outcomes, clinical trial results for one agent should be generalized to all agents within the same family in the absence of data for each specific product. This proviso also applies to the so-called bioidentical products.
Question marks
The Hormone Therapy panel could not agree unanimously on these questions:
- Should women who are doing well on long-term HT discontinue?
- What is the best way to discontinue HT, abrupt cessation or tapering?
- Is the effect of continuous-combined EPT different from that of continuous estrogen with sequential progestogen?
- How definitive is the evidence on early increased CHD risk with HT?
- Conflicting data precluded a consensus on adverse breast cancer and cardiovascular outcomes associated with ET/EPT.
ACKNOWLEDGMENTS
The following commentaries on key papers are from the NAMS First To Knowemail program for members. I thank the members of NAMS who have taken time out to provide these objective reviews of the studies presented here, and Phil Lammers, NAMS Medical Editor.
OSTEOPOROSISCurb your enthusiasm—no need to rush bone drugs if risk is low
McClung MR, Wasnich RD, Hosking DJ, et al, on behalf of the Early Postmenopausal Intervention Cohort (EPIC) study group. Prevention of postmenopausal bone loss: six-year results from the early postmenopausal intervention cohort study. J Clin Endocrinol Metab 2004;89:4879-4885. LEVEL 1 EVIDENCE: Randomized, controlled trial
In this 6-year study of women in their 50s, the placebo group lost an inconsequential amount of bone mass. Not surprisingly, women using alendronate had some increase in BMD and some reduction in bone turnover markers.
Women in their 50s are not melting away. Their bones are not dissolving out from under them, contrary to what many media reports would have ObGyns and patients believe. Still, many clinicians are enthusiastic about prescribing bone drugs like bisphosphonates to women in their 50s who are generally healthy. (And there is no doubt that we do have bone drugs found to be safe and effective in well-designed trials, including the EPIC study.)
Yet there has been a major shift away from starting osteoporosis prevention drugs soon after menopause. EPIC data add support for a “go slow” strategy for drug intervention in healthy women in their 50s.
The EPIC study involved a total of 1,609 women ages 45 to 59, who received alendronate or placebo in a double-blind, randomized design. BMD was measured annually. The 4-year results were reported previously, and the 6-year results were published just last fall. Not surprisingly, women using alendronate had some increase in BMD and some reduction in bone turnover markers. But the results in the women who took placebo are of singular interest.
After 6 years, women on placebo had lost very little bone. The amount lost was statistically significant, but clinically inconsequential. The average BMD in women on placebo decreased 3% in the spine and 2% in the hip. Thus, the average rate of bone loss was about 0.5% per year.
A bone mass decrease of this extent represents a decline of about -0.3 T score, which is negligible. In the EPIC study, the 6-year fracture benefit, based on any type of fracture, boils down to lowering the risk from 1 in 11 on placebo to 1 in 9 on alendronate. These healthy women in their 50s had a very low risk of fracture, and taking a drug for 6 years had very little benefit for fracture reduction.
Women in their 50s typically have about 10% to 15% less bone mass than women of 25 to 30, when bone mass is at its peak. That 10% to 15% lower BMD translates to a T score of –1 to –1.2 , which is currently being labeled as osteopenic. Many patients and physicians have come to feel that osteopenia must always be treated with our newer drugs.
We are discovering that starting healthy women in their 50s on osteoporosis prevention drugs carries an extremely high cost per fracture avoided. During the 10 years since the startup of the EPIC study, support for early drug intervention in healthy women still in their 50s has dwindled. Now, expert groups, including the National Osteoporosis Foundation and the US Preventive Services Task Force, advise waiting until age 65 before starting osteoporosis risk evaluation or considering drug intervention in women who are otherwise healthy.
In my practice, I give healthy women in their 50s permission not to take drugs if their risk of fracture within the next 5 to 10 years is low. The picture is quite different in postmenopausal women in their 50s who do have high fracture risk, such as those who have already had a fracture, or who have very low bone density or high exposure to glucocorticoids.
EPIC data support the concept that the rate of bone loss is quite slow after a year or 2 has elapsed after menopause.
We need to avoid medicalizing these patients simply because we have drugs that reduce bone loss or because women in their 50s have less bone mass than 25-year-olds.
BIBLIOGRAPHY
Hosking D, Chilvers CE, Christiansen C, et al. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med. 1998;338:485-492.
Wasnich RD, Bagger YZ, Hosking DJ, et al. Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause. 2004;11:622-630.
Siris ES, Bilezikian JP, Rubin MR, et al. Pins and plasters aren’t enough: a call for the evaluation and treatment of patients with osteoporotic fractures. J Clin Endocrinol Metab. 2003;88:3482-3486.
Rosen CJ, Black DM, Greenspan SL. Vignettes in osteoporosis: a road map to successful therapeutics. J Bone Miner Res. 2004;19:3-10.
HORMONE THERAPYDoes age affect mortality rate in postmenopausal women using HT?
Salpeter SR, Walsh JME, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women. J Gen Intern Med. 2004;19:791–804. META-ANALYSIS
- This study is sure to incite yet another round of debate about postmenopausal hormone therapy, but it does suggest that we can provide substantial reassurance about safety in younger women considering hormone therapy for menopause-related symptoms.
This study attempted to discover whether the age of the postmenopausal woman using hormone therapy affects mortality. Investigators performed a meta-analysis of clinical trials that reported mortality rates associated with use of postmenopausal hormone therapy, and analyzed the results based on mean ages.
They reported a significant trend between increasing risk of mortality and increasing mean age of the women using hormone therapy—raising the possibility of a health benefit for younger postmenopausal women.
The studies included in the metaanalysis varied in entry criteria, outcomes assessed, number of subjects, and HT type and dosage. Furthermore, because age groups were defined by mean age in each trial rather than actual age of pooled participants, some overlap in ages likely occurred between the analyses of younger and older women.
In postmenopausal women younger than 60, the total mortality rate was reduced by 39% in women taking estrogen-containing hormone therapy, which was significant; in women older than 60, there was no significant effect on total mortality.
The data were from 30 randomized, controlled clinical trials published between 1966 and 2002, and included 26,708 women taking estrogen (ET) or estrogen plus progestogen (EPT). Data were pooled to determine total mortality and mortality due to specific causes such as cardiovascular disease and cancer. The mean trial duration was 4.5 years, and the mean age was 62.2 years.
When the study population was divided into younger and older age groups based on mean ages, it was found that those younger than 60 (mean age, 53.9) had a significantly reduced OR for total mortality of 0.61 (95% CI, 0.39–0.95) and those older than age 60 (mean age, 64.6) had an OR of 1.03 (95% CI, 0.90–1.18).
For specific causes, the OR for cardiovascular disease mortality associated with ET/EPT was 1.10 (95% CI, 0.90–1.34). For overall cancer mortality, the OR was 1.03 (95% CI, 0.82–1.29) and for breast cancer mortality, the OR was 1.03 (95% CI, 0.29–3.67).
For causes other than cardiovascular disease or cancer, mortality was significantly lower in women on HT: OR 0.67 (95% CI, 0.51–0.88). When divided into younger and older age groups, ET/EPT was not associated with a significant change in mortality, with the exception of reduced mortality from causes other than cardiovascular disease and cancer in the older age group (OR, 0.68; 95% CI, 0.56–0.91).
Does HT improve insulin resistance?
Margolis KL, Bonds DE, Rodabough RJ, et al, for the Women’s Health Initiative Investigators. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. LEVEL 1 EVIDENCE: Randomized, controlled trial
Decreased insulin and fasting glucose
- Combined estrogen plus progestogen may reduce the incidence of diabetes, possibly by mediating a decrease in insulin resistance.
Hormone therapy, compared with placebo, was associated with 15 fewer cases of diabetes per 10,000 women per year. Fasting glucose and insulin decreased compared with placebo, and may suggest improved insulin resistance. Although others have reported similar results, it is unlikely that hormone therapy will be prescribed to prevent diabetes, given its greater risk than benefit for other outcomes observed in other WHI analyses.
In the EPT part of WHI, a total of 15,641 postmenopausal women aged 50 to 79 were assigned to placebo or continuous-combined EPT (0.625 mg/day conjugated equine estrogens plus 2.5 mg/day medroxyprogesterone acetate). The incidence of diabetes was based on self-reports of insulin or oral diabetes drug treatment. Fasting glucose, insulin, and lipoproteins were measured at 1 and 3 years. After 5.6 years, the incidence of treated diabetes was 3.5% in the EPT group and 4.2% in the placebo group (hazard ratio, 0.79; 95% CI, 0.67–0.93; P= 0.004). Decreases in fasting glucose and insulin, suggesting decreased insulin resistance, were significant at 1 year in EPT users compared with placebo. The authors concluded that EPT reduces the incidence of diabetes possibly through a decrease in insulin resistance.
To be sure that a drug prevents a disease, everyone with the disease should be excluded at baseline, and at the end of the trial, everyone should be tested for the disease—if it is commonly undiagnosed. To study the incidence of new diabetes, all women (6%) with self-reported diabetes at baseline were excluded, and correctly so. But half of US adults with diabetes are undiagnosed. The WHI 6% prevalence is half of the assumed 12% prevalence in older overweight women. In the WHI, average age was 63 years and average body mass index was 28. Thus, it is not certain that the reduced risk occurred in women who were diabetes-free at baseline.
Fasting glucose was reduced in the EPT part of WHI, as in the Postmenopausal Estrogen Progestin Intervention (PEPI) trial. But 2-hour glucose levels were elevated by hormone treatment in PEPI, and were not measured in the WHI. Many studies have shown that postprandial or post-challenge glucose is a stronger risk factor for cardiovascular disease than fasting hyperglycemia.
Could an elevated post-challenge glucose have played a role in the unexpected excess cardiovascular disease observed with hormone therapy in healthy women in WHI and with hormone therapy in women with documented coronary heart disease in the Heart and Estrogen/progestin Replacement Study (HERS)?
Will transdermal estrogen reduce both fasting and post-challenge glucose? These and other questions remain. (EBC)
Lifestyle changes work best
- This report raises the possibility but does not justify prescribing EPT for diabetes prevention.
Postmenopausal women randomized to EPT had a lower incidence of treated diabetes, by self-report, than women assigned to placebo: a 21% relative risk reduction over 3 years. At 1 year, a comparison of changes from baseline in estimated insulin resistance (HOMA model) in a subgroup indicated a significant reduction with EPT compared with placebo group, but no significant difference at 3 years.
Because of the far-reaching morbidity and mortality due to Type 2 diabetes, particularly from cardiovascular disease, prevention would have major benefits, but the authors acknowledge that this report does not justify prescribing this therapy for this purpose, given hazards previously reported in the WHI.
Still, we can bear in mind other means of reducing risk for diabetes. In the Diabetes Prevention Program,1metformin reduced type 2 diabetes risk by 31%, and a diet plus exercise program reduced it even more: by 58% over approximately 3 years of follow-up in high-risk persons. People at risk for diabetes should be counseled to make lifestyle changes that can reduce this risk far more, and more safely, than might EPT. (CGS)
Consider diabetes implications
- EPT can reduce the incidence of diabetes to the same degree as medications used for cardiovascular disease prevention.2
Growing evidence indicates that reducing insulin resistance in women can prevent onset of diabetes,3and that improving insulin resistance can slow the progression of atherosclerosis.4 Observational studies5—the Heart and Estrogen/progestin Replacement Study (HERS),6 and now the WHI—strongly indicate that EPT reduces the incidence of diabetes in postmenopausal women. Notably, HERS and WHI findings were with continuous-combined estrogen with progestin, the latter often viewed as antagonistic to the beneficial effects of estrogen on carbohydrate metabolism.) Diabetes is much more devastating in women, and more likely to strike. The risk (3,000 of 10,000) in postmenopausal women equals or exceeds that of postmenopausal breast cancer, coronary disease, or hip fracture.7The time has come to consider health and cost implications of long-term HT, especially in women with diabetes risk factors: age, obesity, high systolic BP, high nonfasting glucose, antihypertensive drug use, low HDL, or Hispanic or African-American ethnicity. Clinical trials confirming HT’s benefit add to the totality of evidence that the benefits outweigh the risks.8Since long-term effects (>10 years) reflect only observational data, we urgently need studies designed to understand long-term benefits and risks. (HNH)
1. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
2. Pepine CJ, Cooper-Dehoff RM. Cardiovascular therapies and risk for development of diabetes. J Am Coll Cardiol. 2004;44:509-512.
3. Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance inhigh risk Hispanic women. Diabetes. 2002;51:2796-2803.
4. Xiang AH, Peters RK, Kjos SL, et al. Effect of thiazolidinedione treatment on progression of subclinical atherosclerosis in premenopausal women at high risk for type 2 diabetes. J Clin Endocrinol Metab. 2005;90:1986-1991.
5. Manson JE, Rimm EB, Colditz GA, et al. A prospective study of postmenopausal estrogen therapy and subsequent incidence of non-insulin-dependent diabetes mellitus. Ann Epidemiol. 1992;2:665-673.
6. Kanaya AM, Herrington D, et al. Glycemic effects of postmenopausal hormone therapy: Heart and Estrogen/progestin Replacement Study. Randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:1-9.
7. Narayan KMV, Boyle JP, et al. Lifetime risk for diabetes mellitus in the US. JAMA 2003;290:1884-1890.
8. Philips LS, Langer RD. Postmenopausal hormone therapy: critical reappraisal and a unified hypothesis. Fertil Steril. 2005;83:558-566.
Soy versus placebo: Underwhelming
Red clover, likewise
Trebs EE, Ensrud KE, MacDonald R, Wilt TJ. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstet Gynecol. 2004;104:824–836. META-ANALYSIS
- Phytoestrogens did not significantly improve hot flashes, night sweats, and vaginal dryness, compared to placebo, in this meta-analysis of randomized, controlled clinical trials.
Data on 2,348 women (mean age, 53.1 years) experiencing a mean of 7.1 hot flashes per week were analyzed. Only randomized controlled trials reporting menopausal symptoms of hot flashes, night sweats, and vaginal dryness were included. Mean trial duration was 17 weeks.
- The 11 soy food or beverage supplementtrials (N = 995 women) found no improvement compared with placebo.
- Of the 8 soy food trialsreporting hot flash outcomes, only 1 showed a significant improvement compared with placebo.
- In the 9 soy extract trials, overall results (N = 854) were mixed. In 5 trials using soy extracts and reporting hot flash frequency, 3 found no significant difference in symptoms between the soy and placebo groups; the other 2 (total 114 subjects) found significant improvements.
- The 5 red clover trials(N = 400) showed no improvement over placebo.
Many women in these studies appear to have been perimenopausal rather than postmenopausal. Nine studies included women who had had a menstrual period within the previous 3 to 6 months (late perimenopausal). A subgroup analysis of perimenopausal women would have been useful, since their endocrinologic status is quite different from that of postmenopausal women.
Does soy improve cognition, bone density, or lipids?
Kreijkamp-Kaspers S, Kok L, Grobbee DE, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. 2004;292:65–74. LEVEL 1 EVIDENCE: Randomized, controlled trial
- Soy did not benefit cognition, bone, or lipids in 60-to-75-year-old women.
This careful trial raises the question of how to reconcile these results with animal and observational studies. In all, 202 postmenopausal women aged 60 to 75 years received 25.6 g/day of a soy protein supplement containing 99 mg isoflavones or a milk protein powder for 1 year. Adherence was monitored by serum genistein. There were no notable differences in:
- Memory, verbal skills, or concentration.
- Bone mineral density or bone-specific alkaline phosphatase, calcium, or phosphorus levels.
- Cholesterol, triglycerides, and lipoprotein plasma levels. These findings may not relate to perimenopausal women, in whom soy has been seen to significantly reduce LDL, but only during midfollicular and periovulatory phases.1 Premenopausal2 but not postmenopausal3monkeys given soy have had beneficial effects on bone quality.
REFERENCES
1. Merz-Demlow BE, Duncan AM, Wangen KE, et al. Soy isoflavones improve plasma lipids in normocholes-terolemic, premenopausal women. Am J Clin Nutr. 2000;71:1462-1469.
2. Kaplan JR, et al. Supplementation reduces the trajectory of atherogenesis in premenopausal monkeys at high risk for development of extensive postmenopausal coronary artery plaques. Menopause. 2004;11:653. Abstract S-17.
3. Register TC, Jayo MJ, Anthony MS. Soy phytoestrogens do not prevent bone loss in postmenopausal monkeys. J Clin Endocrinol Metab. 2003;88:4362-4370.
LIFESTYLE THERAPYThe secret to keeping those girlish carotids
Wildman RP, Schott LL, Brockwell S, Kuller LH, Sutton-Tyrrell K. A dietary and exercise intervention slows menopause-associated progression of subclinical atherosclerosis as measured by intima-media thickness of the carotid arteries. J Am Coll Cardiol. 2004;44:579–585. LEVEL 1 EVIDENCE: Randomized, controlled trial
- Carotid artery intima media thickens during the transition through menopause, but diet and exercise can reduce this progression by almost 50%.
- A diet-and-exercise regimen staves off menopause-associated weight gain and increases in lipids, blood pressure, and blood glucose.
These important findings are strong evidence that diet and exercise can slow the subclinical atherosclerosis progression that accompanies the menopause transition.
The Women’s Healthy Lifestyle Project previously found that weight gain and increased lipids, glucose, and blood pressure often accompany the menopause transition.1 This report describes improvements with diet and exercise intervention, compared with controls.
A total of 535 women aged 44 to 50 years were randomized to lifestyle intervention or assessment-only. All were premenopausal, and all had normal to high-normal body mass index, diastolic blood pressure, and fasting glucose and cholesterol levels.
The diet and exercise regimen used in the study was designed to reduce fat and cholesterol, prevent weight gain, and increase physical activity.
End points were progression of intimamedia thickness in the common carotid artery, internal carotid artery, and bulb segments.
The control group had significantly greater increases in intima-media thickness in women who became postmenopausal compared with those who remained premenopausal.
For women who became perimenopausal or postmenopausal during this 4-year study, diet and exercise slowed the progression of intima-media thickness by a 47% average reduction (P< 0.05), but had no effect on carotid segments in the women who remained premenopausal.
No benefit in intima media thickness was seen in women who remained premenopausal during the trial. Nevertheless, there are many well-documented benefits of healthy diet and exercise in premenopausal women.
Also of note, hormone therapy initiated after baseline measurements did not alter the results.
The message for patients, especially perimenopausal patients is that there is no time like the present to start a healthy lifestyle.
No downside
We’ve learned from the Nurse’s Health Study,2an observational study, that women who eat a healthy diet, do not smoke, and who exercise can reduce their risk of coronary heart disease by 57%.
We learned from the randomized controlled trial by the Diabetes Prevention Program Research Group3 that diet and exercise in high-risk women for 3 years can reduce incidence of new diabetes by 58%.
Now, in this trial, we learn that diet and exercise can reduce the progression of atherosclerosis in perimenopausal women by nearly 50%.
Since there is little, if any, downside to healthy living, why wait?
DISCLOSURES
Dr. Utian has served as an advisor/consultant for Eli Lilly, Pfizer, and Novartis. He has received research funding from Amylin, 3m, Barr, Berlex, BMS, Eli Lilly, Forest, Galen, Glaxo Smith Kline, Neurocrine Biosciences, Novartis, Novo Nordisk, Organon, Pharmacia, P&G, Pfizer, Roche, Sepracor, Solvay, Wyeth, and Yamanouchi.
Dr. Ettinger has served as an advisor for Berlex, Duramed-Barr, Glaxo Smith Kline, and P&G.
REFERENCES
1. Kuller LH, Simkin-Silverman LR, Wing RR, Meilahn EN, Ives DG. Women’s Healthy Lifestyle Project: a randomized clinical trial: results at 54 months. Circulation. 2001;103:32-37.
2. Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16-22.
3. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
Time and study make a difference. So does careful review and reappraisal of existing data. In the past year, the pendulum has swung away from fear of hormone therapy to a better understanding of indications, risks and benefits—an understanding driven largely by the evidence-based position statements of the North American Menopause Society (NAMS).
A meta-analysis of randomized controlled trials of phytoestrogens attested to lack of efficacy or weak effect, which helps clear the picture on soy and red clover, but the researchers stressed that the lack of quality control does not rule out the possibility that some products might carry steroidal effects and potential risk.
And another year has brought even more evidence that diet, exercise, smoking cessation and the like really do improve health and quality of life.
Advisory on hormone therapy and “bio-identicals”
The NAMS Hormone Therapy Panel concluded definitively that bio-identical hormones should be considered in the same category as all the sex steroids, which, in the absence of specific safety and efficacy studies, carry the same risks and benefits as related products.
On the other hand, alternatives do exist for specific indications, such as bisphosphonates for bone conservation.
The new NAMSPosition Statement stresses individualized treatment based on the recommendations below.
The full report is available at www.menopause.org.
- Treatment of moderate to severe menopausal symptoms is the primary indications for systemic therapy. Every systemic product is FDA-approved for this indication.
- Every systemic and local product is approved for moderate vulvar and vaginal atrophy. For this indication alone, local ET is generally advised.
- Duration should be for the lowest effective dose and shortest time consistent with treatment goals.
- If the woman is well aware of potential risks and benefits, and if there is clinical supervision, extended use of the lowest effective ET/EPT dose for treatment goals is acceptable in women who believe the benefits outweigh the risks, for those at high risk of osteoporotic fracture who also have moderate to severe menopause symptoms, for further prevention of established bone loss when alternate therapies are not appropriate or cause side effects, or when outcomes of extended use of those therapies are not known.
- Although specific compounds, doses, and routes of administration may have different outcomes, clinical trial results for one agent should be generalized to all agents within the same family in the absence of data for each specific product. This proviso also applies to the so-called bioidentical products.
Question marks
The Hormone Therapy panel could not agree unanimously on these questions:
- Should women who are doing well on long-term HT discontinue?
- What is the best way to discontinue HT, abrupt cessation or tapering?
- Is the effect of continuous-combined EPT different from that of continuous estrogen with sequential progestogen?
- How definitive is the evidence on early increased CHD risk with HT?
- Conflicting data precluded a consensus on adverse breast cancer and cardiovascular outcomes associated with ET/EPT.
ACKNOWLEDGMENTS
The following commentaries on key papers are from the NAMS First To Knowemail program for members. I thank the members of NAMS who have taken time out to provide these objective reviews of the studies presented here, and Phil Lammers, NAMS Medical Editor.
OSTEOPOROSISCurb your enthusiasm—no need to rush bone drugs if risk is low
McClung MR, Wasnich RD, Hosking DJ, et al, on behalf of the Early Postmenopausal Intervention Cohort (EPIC) study group. Prevention of postmenopausal bone loss: six-year results from the early postmenopausal intervention cohort study. J Clin Endocrinol Metab 2004;89:4879-4885. LEVEL 1 EVIDENCE: Randomized, controlled trial
In this 6-year study of women in their 50s, the placebo group lost an inconsequential amount of bone mass. Not surprisingly, women using alendronate had some increase in BMD and some reduction in bone turnover markers.
Women in their 50s are not melting away. Their bones are not dissolving out from under them, contrary to what many media reports would have ObGyns and patients believe. Still, many clinicians are enthusiastic about prescribing bone drugs like bisphosphonates to women in their 50s who are generally healthy. (And there is no doubt that we do have bone drugs found to be safe and effective in well-designed trials, including the EPIC study.)
Yet there has been a major shift away from starting osteoporosis prevention drugs soon after menopause. EPIC data add support for a “go slow” strategy for drug intervention in healthy women in their 50s.
The EPIC study involved a total of 1,609 women ages 45 to 59, who received alendronate or placebo in a double-blind, randomized design. BMD was measured annually. The 4-year results were reported previously, and the 6-year results were published just last fall. Not surprisingly, women using alendronate had some increase in BMD and some reduction in bone turnover markers. But the results in the women who took placebo are of singular interest.
After 6 years, women on placebo had lost very little bone. The amount lost was statistically significant, but clinically inconsequential. The average BMD in women on placebo decreased 3% in the spine and 2% in the hip. Thus, the average rate of bone loss was about 0.5% per year.
A bone mass decrease of this extent represents a decline of about -0.3 T score, which is negligible. In the EPIC study, the 6-year fracture benefit, based on any type of fracture, boils down to lowering the risk from 1 in 11 on placebo to 1 in 9 on alendronate. These healthy women in their 50s had a very low risk of fracture, and taking a drug for 6 years had very little benefit for fracture reduction.
Women in their 50s typically have about 10% to 15% less bone mass than women of 25 to 30, when bone mass is at its peak. That 10% to 15% lower BMD translates to a T score of –1 to –1.2 , which is currently being labeled as osteopenic. Many patients and physicians have come to feel that osteopenia must always be treated with our newer drugs.
We are discovering that starting healthy women in their 50s on osteoporosis prevention drugs carries an extremely high cost per fracture avoided. During the 10 years since the startup of the EPIC study, support for early drug intervention in healthy women still in their 50s has dwindled. Now, expert groups, including the National Osteoporosis Foundation and the US Preventive Services Task Force, advise waiting until age 65 before starting osteoporosis risk evaluation or considering drug intervention in women who are otherwise healthy.
In my practice, I give healthy women in their 50s permission not to take drugs if their risk of fracture within the next 5 to 10 years is low. The picture is quite different in postmenopausal women in their 50s who do have high fracture risk, such as those who have already had a fracture, or who have very low bone density or high exposure to glucocorticoids.
EPIC data support the concept that the rate of bone loss is quite slow after a year or 2 has elapsed after menopause.
We need to avoid medicalizing these patients simply because we have drugs that reduce bone loss or because women in their 50s have less bone mass than 25-year-olds.
BIBLIOGRAPHY
Hosking D, Chilvers CE, Christiansen C, et al. Prevention of bone loss with alendronate in postmenopausal women under 60 years of age. Early Postmenopausal Intervention Cohort Study Group. N Engl J Med. 1998;338:485-492.
Wasnich RD, Bagger YZ, Hosking DJ, et al. Changes in bone density and turnover after alendronate or estrogen withdrawal. Menopause. 2004;11:622-630.
Siris ES, Bilezikian JP, Rubin MR, et al. Pins and plasters aren’t enough: a call for the evaluation and treatment of patients with osteoporotic fractures. J Clin Endocrinol Metab. 2003;88:3482-3486.
Rosen CJ, Black DM, Greenspan SL. Vignettes in osteoporosis: a road map to successful therapeutics. J Bone Miner Res. 2004;19:3-10.
HORMONE THERAPYDoes age affect mortality rate in postmenopausal women using HT?
Salpeter SR, Walsh JME, Greyber E, Ormiston TM, Salpeter EE. Mortality associated with hormone replacement therapy in younger and older women. J Gen Intern Med. 2004;19:791–804. META-ANALYSIS
- This study is sure to incite yet another round of debate about postmenopausal hormone therapy, but it does suggest that we can provide substantial reassurance about safety in younger women considering hormone therapy for menopause-related symptoms.
This study attempted to discover whether the age of the postmenopausal woman using hormone therapy affects mortality. Investigators performed a meta-analysis of clinical trials that reported mortality rates associated with use of postmenopausal hormone therapy, and analyzed the results based on mean ages.
They reported a significant trend between increasing risk of mortality and increasing mean age of the women using hormone therapy—raising the possibility of a health benefit for younger postmenopausal women.
The studies included in the metaanalysis varied in entry criteria, outcomes assessed, number of subjects, and HT type and dosage. Furthermore, because age groups were defined by mean age in each trial rather than actual age of pooled participants, some overlap in ages likely occurred between the analyses of younger and older women.
In postmenopausal women younger than 60, the total mortality rate was reduced by 39% in women taking estrogen-containing hormone therapy, which was significant; in women older than 60, there was no significant effect on total mortality.
The data were from 30 randomized, controlled clinical trials published between 1966 and 2002, and included 26,708 women taking estrogen (ET) or estrogen plus progestogen (EPT). Data were pooled to determine total mortality and mortality due to specific causes such as cardiovascular disease and cancer. The mean trial duration was 4.5 years, and the mean age was 62.2 years.
When the study population was divided into younger and older age groups based on mean ages, it was found that those younger than 60 (mean age, 53.9) had a significantly reduced OR for total mortality of 0.61 (95% CI, 0.39–0.95) and those older than age 60 (mean age, 64.6) had an OR of 1.03 (95% CI, 0.90–1.18).
For specific causes, the OR for cardiovascular disease mortality associated with ET/EPT was 1.10 (95% CI, 0.90–1.34). For overall cancer mortality, the OR was 1.03 (95% CI, 0.82–1.29) and for breast cancer mortality, the OR was 1.03 (95% CI, 0.29–3.67).
For causes other than cardiovascular disease or cancer, mortality was significantly lower in women on HT: OR 0.67 (95% CI, 0.51–0.88). When divided into younger and older age groups, ET/EPT was not associated with a significant change in mortality, with the exception of reduced mortality from causes other than cardiovascular disease and cancer in the older age group (OR, 0.68; 95% CI, 0.56–0.91).
Does HT improve insulin resistance?
Margolis KL, Bonds DE, Rodabough RJ, et al, for the Women’s Health Initiative Investigators. Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the Women’s Health Initiative Hormone Trial. Diabetologia. 2004;47:1175–1187. LEVEL 1 EVIDENCE: Randomized, controlled trial
Decreased insulin and fasting glucose
- Combined estrogen plus progestogen may reduce the incidence of diabetes, possibly by mediating a decrease in insulin resistance.
Hormone therapy, compared with placebo, was associated with 15 fewer cases of diabetes per 10,000 women per year. Fasting glucose and insulin decreased compared with placebo, and may suggest improved insulin resistance. Although others have reported similar results, it is unlikely that hormone therapy will be prescribed to prevent diabetes, given its greater risk than benefit for other outcomes observed in other WHI analyses.
In the EPT part of WHI, a total of 15,641 postmenopausal women aged 50 to 79 were assigned to placebo or continuous-combined EPT (0.625 mg/day conjugated equine estrogens plus 2.5 mg/day medroxyprogesterone acetate). The incidence of diabetes was based on self-reports of insulin or oral diabetes drug treatment. Fasting glucose, insulin, and lipoproteins were measured at 1 and 3 years. After 5.6 years, the incidence of treated diabetes was 3.5% in the EPT group and 4.2% in the placebo group (hazard ratio, 0.79; 95% CI, 0.67–0.93; P= 0.004). Decreases in fasting glucose and insulin, suggesting decreased insulin resistance, were significant at 1 year in EPT users compared with placebo. The authors concluded that EPT reduces the incidence of diabetes possibly through a decrease in insulin resistance.
To be sure that a drug prevents a disease, everyone with the disease should be excluded at baseline, and at the end of the trial, everyone should be tested for the disease—if it is commonly undiagnosed. To study the incidence of new diabetes, all women (6%) with self-reported diabetes at baseline were excluded, and correctly so. But half of US adults with diabetes are undiagnosed. The WHI 6% prevalence is half of the assumed 12% prevalence in older overweight women. In the WHI, average age was 63 years and average body mass index was 28. Thus, it is not certain that the reduced risk occurred in women who were diabetes-free at baseline.
Fasting glucose was reduced in the EPT part of WHI, as in the Postmenopausal Estrogen Progestin Intervention (PEPI) trial. But 2-hour glucose levels were elevated by hormone treatment in PEPI, and were not measured in the WHI. Many studies have shown that postprandial or post-challenge glucose is a stronger risk factor for cardiovascular disease than fasting hyperglycemia.
Could an elevated post-challenge glucose have played a role in the unexpected excess cardiovascular disease observed with hormone therapy in healthy women in WHI and with hormone therapy in women with documented coronary heart disease in the Heart and Estrogen/progestin Replacement Study (HERS)?
Will transdermal estrogen reduce both fasting and post-challenge glucose? These and other questions remain. (EBC)
Lifestyle changes work best
- This report raises the possibility but does not justify prescribing EPT for diabetes prevention.
Postmenopausal women randomized to EPT had a lower incidence of treated diabetes, by self-report, than women assigned to placebo: a 21% relative risk reduction over 3 years. At 1 year, a comparison of changes from baseline in estimated insulin resistance (HOMA model) in a subgroup indicated a significant reduction with EPT compared with placebo group, but no significant difference at 3 years.
Because of the far-reaching morbidity and mortality due to Type 2 diabetes, particularly from cardiovascular disease, prevention would have major benefits, but the authors acknowledge that this report does not justify prescribing this therapy for this purpose, given hazards previously reported in the WHI.
Still, we can bear in mind other means of reducing risk for diabetes. In the Diabetes Prevention Program,1metformin reduced type 2 diabetes risk by 31%, and a diet plus exercise program reduced it even more: by 58% over approximately 3 years of follow-up in high-risk persons. People at risk for diabetes should be counseled to make lifestyle changes that can reduce this risk far more, and more safely, than might EPT. (CGS)
Consider diabetes implications
- EPT can reduce the incidence of diabetes to the same degree as medications used for cardiovascular disease prevention.2
Growing evidence indicates that reducing insulin resistance in women can prevent onset of diabetes,3and that improving insulin resistance can slow the progression of atherosclerosis.4 Observational studies5—the Heart and Estrogen/progestin Replacement Study (HERS),6 and now the WHI—strongly indicate that EPT reduces the incidence of diabetes in postmenopausal women. Notably, HERS and WHI findings were with continuous-combined estrogen with progestin, the latter often viewed as antagonistic to the beneficial effects of estrogen on carbohydrate metabolism.) Diabetes is much more devastating in women, and more likely to strike. The risk (3,000 of 10,000) in postmenopausal women equals or exceeds that of postmenopausal breast cancer, coronary disease, or hip fracture.7The time has come to consider health and cost implications of long-term HT, especially in women with diabetes risk factors: age, obesity, high systolic BP, high nonfasting glucose, antihypertensive drug use, low HDL, or Hispanic or African-American ethnicity. Clinical trials confirming HT’s benefit add to the totality of evidence that the benefits outweigh the risks.8Since long-term effects (>10 years) reflect only observational data, we urgently need studies designed to understand long-term benefits and risks. (HNH)
1. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.
2. Pepine CJ, Cooper-Dehoff RM. Cardiovascular therapies and risk for development of diabetes. J Am Coll Cardiol. 2004;44:509-512.
3. Buchanan TA, Xiang AH, Peters RK, et al. Preservation of pancreatic beta-cell function and prevention of type 2 diabetes by pharmacological treatment of insulin resistance inhigh risk Hispanic women. Diabetes. 2002;51:2796-2803.
4. Xiang AH, Peters RK, Kjos SL, et al. Effect of thiazolidinedione treatment on progression of subclinical atherosclerosis in premenopausal women at high risk for type 2 diabetes. J Clin Endocrinol Metab. 2005;90:1986-1991.
5. Manson JE, Rimm EB, Colditz GA, et al. A prospective study of postmenopausal estrogen therapy and subsequent incidence of non-insulin-dependent diabetes mellitus. Ann Epidemiol. 1992;2:665-673.
6. Kanaya AM, Herrington D, et al. Glycemic effects of postmenopausal hormone therapy: Heart and Estrogen/progestin Replacement Study. Randomized, double-blind, placebo-controlled trial. Ann Intern Med. 2003;138:1-9.
7. Narayan KMV, Boyle JP, et al. Lifetime risk for diabetes mellitus in the US. JAMA 2003;290:1884-1890.
8. Philips LS, Langer RD. Postmenopausal hormone therapy: critical reappraisal and a unified hypothesis. Fertil Steril. 2005;83:558-566.
Soy versus placebo: Underwhelming
Red clover, likewise
Trebs EE, Ensrud KE, MacDonald R, Wilt TJ. Phytoestrogens for treatment of menopausal symptoms: a systematic review. Obstet Gynecol. 2004;104:824–836. META-ANALYSIS
- Phytoestrogens did not significantly improve hot flashes, night sweats, and vaginal dryness, compared to placebo, in this meta-analysis of randomized, controlled clinical trials.
Data on 2,348 women (mean age, 53.1 years) experiencing a mean of 7.1 hot flashes per week were analyzed. Only randomized controlled trials reporting menopausal symptoms of hot flashes, night sweats, and vaginal dryness were included. Mean trial duration was 17 weeks.
- The 11 soy food or beverage supplementtrials (N = 995 women) found no improvement compared with placebo.
- Of the 8 soy food trialsreporting hot flash outcomes, only 1 showed a significant improvement compared with placebo.
- In the 9 soy extract trials, overall results (N = 854) were mixed. In 5 trials using soy extracts and reporting hot flash frequency, 3 found no significant difference in symptoms between the soy and placebo groups; the other 2 (total 114 subjects) found significant improvements.
- The 5 red clover trials(N = 400) showed no improvement over placebo.
Many women in these studies appear to have been perimenopausal rather than postmenopausal. Nine studies included women who had had a menstrual period within the previous 3 to 6 months (late perimenopausal). A subgroup analysis of perimenopausal women would have been useful, since their endocrinologic status is quite different from that of postmenopausal women.
Does soy improve cognition, bone density, or lipids?
Kreijkamp-Kaspers S, Kok L, Grobbee DE, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA. 2004;292:65–74. LEVEL 1 EVIDENCE: Randomized, controlled trial
- Soy did not benefit cognition, bone, or lipids in 60-to-75-year-old women.
This careful trial raises the question of how to reconcile these results with animal and observational studies. In all, 202 postmenopausal women aged 60 to 75 years received 25.6 g/day of a soy protein supplement containing 99 mg isoflavones or a milk protein powder for 1 year. Adherence was monitored by serum genistein. There were no notable differences in:
- Memory, verbal skills, or concentration.
- Bone mineral density or bone-specific alkaline phosphatase, calcium, or phosphorus levels.
- Cholesterol, triglycerides, and lipoprotein plasma levels. These findings may not relate to perimenopausal women, in whom soy has been seen to significantly reduce LDL, but only during midfollicular and periovulatory phases.1 Premenopausal2 but not postmenopausal3monkeys given soy have had beneficial effects on bone quality.
REFERENCES
1. Merz-Demlow BE, Duncan AM, Wangen KE, et al. Soy isoflavones improve plasma lipids in normocholes-terolemic, premenopausal women. Am J Clin Nutr. 2000;71:1462-1469.
2. Kaplan JR, et al. Supplementation reduces the trajectory of atherogenesis in premenopausal monkeys at high risk for development of extensive postmenopausal coronary artery plaques. Menopause. 2004;11:653. Abstract S-17.
3. Register TC, Jayo MJ, Anthony MS. Soy phytoestrogens do not prevent bone loss in postmenopausal monkeys. J Clin Endocrinol Metab. 2003;88:4362-4370.
LIFESTYLE THERAPYThe secret to keeping those girlish carotids
Wildman RP, Schott LL, Brockwell S, Kuller LH, Sutton-Tyrrell K. A dietary and exercise intervention slows menopause-associated progression of subclinical atherosclerosis as measured by intima-media thickness of the carotid arteries. J Am Coll Cardiol. 2004;44:579–585. LEVEL 1 EVIDENCE: Randomized, controlled trial
- Carotid artery intima media thickens during the transition through menopause, but diet and exercise can reduce this progression by almost 50%.
- A diet-and-exercise regimen staves off menopause-associated weight gain and increases in lipids, blood pressure, and blood glucose.
These important findings are strong evidence that diet and exercise can slow the subclinical atherosclerosis progression that accompanies the menopause transition.
The Women’s Healthy Lifestyle Project previously found that weight gain and increased lipids, glucose, and blood pressure often accompany the menopause transition.1 This report describes improvements with diet and exercise intervention, compared with controls.
A total of 535 women aged 44 to 50 years were randomized to lifestyle intervention or assessment-only. All were premenopausal, and all had normal to high-normal body mass index, diastolic blood pressure, and fasting glucose and cholesterol levels.
The diet and exercise regimen used in the study was designed to reduce fat and cholesterol, prevent weight gain, and increase physical activity.
End points were progression of intimamedia thickness in the common carotid artery, internal carotid artery, and bulb segments.
The control group had significantly greater increases in intima-media thickness in women who became postmenopausal compared with those who remained premenopausal.
For women who became perimenopausal or postmenopausal during this 4-year study, diet and exercise slowed the progression of intima-media thickness by a 47% average reduction (P< 0.05), but had no effect on carotid segments in the women who remained premenopausal.
No benefit in intima media thickness was seen in women who remained premenopausal during the trial. Nevertheless, there are many well-documented benefits of healthy diet and exercise in premenopausal women.
Also of note, hormone therapy initiated after baseline measurements did not alter the results.
The message for patients, especially perimenopausal patients is that there is no time like the present to start a healthy lifestyle.
No downside
We’ve learned from the Nurse’s Health Study,2an observational study, that women who eat a healthy diet, do not smoke, and who exercise can reduce their risk of coronary heart disease by 57%.
We learned from the randomized controlled trial by the Diabetes Prevention Program Research Group3 that diet and exercise in high-risk women for 3 years can reduce incidence of new diabetes by 58%.
Now, in this trial, we learn that diet and exercise can reduce the progression of atherosclerosis in perimenopausal women by nearly 50%.
Since there is little, if any, downside to healthy living, why wait?
DISCLOSURES
Dr. Utian has served as an advisor/consultant for Eli Lilly, Pfizer, and Novartis. He has received research funding from Amylin, 3m, Barr, Berlex, BMS, Eli Lilly, Forest, Galen, Glaxo Smith Kline, Neurocrine Biosciences, Novartis, Novo Nordisk, Organon, Pharmacia, P&G, Pfizer, Roche, Sepracor, Solvay, Wyeth, and Yamanouchi.
Dr. Ettinger has served as an advisor for Berlex, Duramed-Barr, Glaxo Smith Kline, and P&G.
REFERENCES
1. Kuller LH, Simkin-Silverman LR, Wing RR, Meilahn EN, Ives DG. Women’s Healthy Lifestyle Project: a randomized clinical trial: results at 54 months. Circulation. 2001;103:32-37.
2. Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16-22.
3. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393-403.