User login
The US Department of Health and Human Services (HHS) Ending the HIV Epidemic framework aims to decrease HIV infections in the United States by 90% before 2030.1 Achieving this goal requires identifying persons at high risk for HIV and ensuring timely and efficient access to HIV preexposure prophylaxis (PrEP).2-5 However, despite its commercial availability since 2012, community uptake of PrEP is low.6 In 2019, < 25% of Americans who could benefit from PrEP were using this preventive therapy.7 Poor uptake of PrEP has also been documented among veterans and US military service members. National data on men in the military and men who have sex with men (MSM) in the military suggest that about 12,000 service members are eligible for PrEP; however, only 2000 service members and their beneficiaries accessed PrEP in February 2017.8
A review of health records of US military service members conducted from 2014 to 2016 indicated that most patients who received PrEP did not receive recommended monitoring in accordance with the Centers for Disease Control and Prevention (CDC) guidelines. Furthermore, 16% of these individuals did not have HIV testing within 14 days of initiating PrEP, and 13% were never evaluated for hepatitis B infection.8
Pharmacists are highly accessible health care professionals (HCPs): More than 90% of Americans live within 5 miles of a community pharmacy.9 Pharmacists play an integral role within the outpatient health care team and have been responsible for improvements in health care outcomes for a variety of chronic conditions and immunization practices.10-13 Additionally, community pharmacists have provided vital access to care during the COVID-19 pandemic.14 The clinical pharmacist practitioner (CPP) is an innovative and advanced role within the Veterans Health Administration (VHA), functioning with a scope of practice and prescribing privileges to provide direct patient care.15
CPPs are well suited to address the need for increased access, capacity, and timely provision of PrEP, especially in areas where HIV acquisition rates are high or in areas with reduced access to care. We describe a model for a pharmacist-led HIV PrEP program (Pharm-PrEP) to increase access to PrEP. A similar program could be adapted to further expand the use of PrEP in other health care systems and community settings.
Pharm-PrEP Program Description
The Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHCS) provides health care services at 11 locations in southern California and serves > 86,000 veterans. The VAGLAHCS pharmacy staff includes 33 CPPs who practice in more than 9 clinical service lines. HIV PrEP services are available through the infectious diseases (ID) service for veterans wishing to begin or continue PrEP or for those identified as high risk. HIV PrEP consultations are placed by the referring HCP to the ID service for scheduling and evaluation. Prior to implementation of the pharmacist-managed PrEP clinic, 2 ID physician assistants (PAs) were responsible for PrEP evaluation, initiation, and follow-up. Each PA had 1 half-day face-to-face clinic and 1 PA had an additional half-day telehealth clinic. About 100 PrEP patients were followed by the ID group.
In July 2019, through collaboration with the ID service, a pharmacy PrEP clinic was created to increase access for veterans to initiate or continue PrEP. This clinic included 1 ID-trained CPP and 1 postgraduate-year-2 pharmacy resident. The CPP initiates and monitors veterans for HIV PrEP with prescribing privileges under a defined scope of practice.
Awareness of this novel service was raised through in-service training sessions for primary care and women’s health clinics. Referrals are generated directly from primary care practitioners (PCPs) or emergency department (ED) visits and are accepted on a continuing basis. Visits with the CPP are conducted in person or through telehealth services based on patient preference. Direct CPP patient care appointments involve a standardized assessment and discussion of patient HIV transmission risk, a review of social and sexual history, sexual practices and HIV risk, clinical evidence of acute HIV or other sexually transmitted infection (STI) symptoms, follow-up PrEP monitoring requirements, and counseling on appropriate PrEP use. CPPs can order laboratory tests, bone densitometry (DEXA scan), immunizations, PrEP, and STI treatment as required. ID service physicians are available during CPP visits for further assessment or consultation. While initially most visits are conducted in person, follow-up visits by telehealth or video have become predominant; most patients prefer these modalities, citing convenience, flexibility, and the ability to obtain laboratory tests in advance. Use of telephone and video is intended to reduce patient loss to follow-up.
All required baseline laboratory panels for PrEP monitoring are ordered and interpreted by the CPP in accordance with CDC guidelines.16 These include screening for syphilis, gonorrhea, and chlamydia; fourth-generation antibody-antigen HIV tests; renal function; viral hepatitis; and pregnancy. After reviewing screening results, the CPP will prescribe tenofovir disproxil fumarate/emtricitabine (TDF/FTC) or tenofovir alafenamide/emtricitabine (TAF/FTC) based on individual patient clinical characteristics, US Food and Drug Administration–approved labeling, and VA Pharmacy Benefits Management Criteria for Use. Initial prescriptions are for a 30-day supply with subsequent prescriptions for 90 days (no refills), providing follow-up HIV testing is completed.
Follow-up PrEP visits are scheduled about every 3 months with some overlap to avoid gaps in medication due to late laboratory testing or delayed receipt of mailed medications. The only laboratory testing strictly required each quarter before PrEP renewal is HIV and pregnancy testing. Other screenings, including STIs and renal function are completed at least every 6 months or more frequently, if indicated, based on individual risk factors. Hepatitis C antibody testing is conducted annually if the patient has ongoing risk factors. Treatment of gonorrhea/chlamydia and syphilis for patients with positive test results is also initiated by the CPP, including recommending antimicrobial regimens. Additional interventions conducted as part of the clinic include indicated vaccinations (meningococcal, human papillomavirus, hepatitis A and B), and DEXA scans. Collaboration with ID service attendings and PAs is conducted on an as-needed basis via direct consultation in the colocated clinic or through email or messaging.
Periodic surveillance of a local dashboard of veterans eligible for HIV PrEP is conducted to re-engage veterans in care who may have been lost to follow-up, along with periodic review of a local STI dashboard. These dashboards capture population-based data to identify patients who may benefit from additional STI screenings as well as potential candidates for HIV PrEP. Clinicians can review their patient panel to target individuals who may be due for specific actions. Patients are identified as needing cotesting if they screen positive for ≥ 1 STIs but have not had a concurrent or subsequent full screening panel (gonorrhea, chlamydia, syphilis, and HIV). Cotesting for bacterial STIs and HIV at the time of an encounter has been promoted to expedite STI identification and treatment and limit community transmission. These reports also identify patients who may be potential candidates for HIV PrEP, based on a history of positive screenings, frequent STI testing, recent prescriptions for postexposure prophylaxis (PEP) or encounters with specific International Classification of Diseases codes associated with high-risk practices.
Clinic Quality of Care
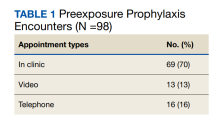
From July 2019 to March 2020, 53 veterans were managed by the pharm-PrEP clinic in 98 encounters. Seventy percent of encounters were in-person (Table 1).
Baseline information collected included demographics, documented patient-reported risk factors, fourth-generation HIV screening test results, STI status, viral hepatitis serologies, and renal function test results. Information collected every 3 to 6 months included STI status, fourth-generation HIV screening test results, renal function test results, adherence to therapy, changes in risk factors, and prescription refill data. Additional interventions conducted as part of clinic workflow included DEXA scans, vaccinations, and active prescriptions for condoms.
Baseline Characteristics
Pharm-PrEP clinic patients were predominantly male (94%), and a majority indicated White race with a median age of 38 years (range, 24-80 years).
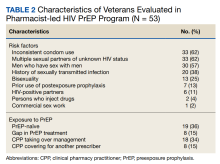
Veterans referred to the clinic had up to 5 risk factors for PrEP initiation. The most common risk factors were inconsistent condom use (62%), multiple sexual partners of unknown HIV status (62%), MSM (57%), STI history (38%), bisexual partners (25%), and HIV-positive sexual partners (11%). One of the 53 individuals referred for PrEP had no risk factors and did not initiate PrEP. Two individuals declined initiation of PrEP after consultation. Twenty six of 53 veterans at baseline continued their use of PrEP following transfer to clinic CPP management; 24 of 27 veterans not currently using PrEP (89%) started or restarted lapsed PrEP use following CPP consultation.
HIV and STI Screening
No individuals tested positive for HIV at baseline (n = 52) or while on PrEP. PrEP was not renewed for 3 patients that did follow through with HIV testing. The median number of days an HIV test was completed prior to initial PrEP and PrEP renewal was 4 days and < 7 days, respectively, both of which are below the recommended maximal interval of 7 days, according to CDC PrEP guidelines. Some postinitiation HIV testing occurred using a longer interval of 14 days, in accordance with VA National Criteria for Use of PrEP. This modification allowed more flexibility as a majority of PrEP prescriptions are sent to veterans via mail. The CPP reviewing HIV test results was able to expedite the processing and mailing of PrEP prescriptions if deemed appropriate, ie, the HIV test was negative. This approach was not used if a patient had high-risk exposures without PrEP during the time between collection of the HIV test and mailing of the prescription.
STI screening is a vital component of the Pharm-PrEP clinic and helped identify 4 patients with gonorrhea/chlamydia at baseline and 1 with syphilis after initiation of PrEP. All patients were prescribed antibiotics at the screening. Los Angeles County has high rates of STI transmission; thus implementation of clinic processes allowing the CPP to screen for STIs, interpret test results, and treat patients with STIs is vital to limit spread in the community.17
Selection of PrEP Regimen
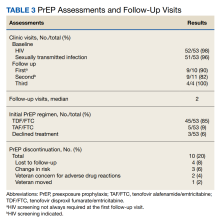
The majority of individuals in the cohort received TDF/FTC for PrEP; TAF/FTC was restricted to individuals who had documented renal dysfunction or bone loss (Table 3).
Six DEXA scans were completed by the end of the evaluation period and 2 had abnormal results. One patient discontinued TDF/FTC and reinitiated with TAF/FTC. The other was switched to TAF/FTC 1 month after initiation.
Follow-Up Visits
The median number of visits per patient was 2. The median time between visits was in accordance with recommended follow-up intervals with 35 days between visits 1 and 2, 60 days between visits 2 and 3, and 88 days thereafter. In all, 10 veterans (20%) stopped PrEP: 4 (8%) were lost to follow-up; 3 (6%) had sustained behavior changes decreasing their HIV exposure risk; 2 (4%) were concerned about ADRs; and 1 (2%) moved out of state. Even after including those patients with a decrease in HIV exposure risk who no longer required PrEP, our 20% discontinuation rate was lower than those reported in other studies that showed a wide variation in PrEP discontinuation rates ranging from 33% to 62%.18-20
Challenges
Some challenges with the implementation of the clinic included logistic and operational barriers, such as developing clinical pathways and managing workflow to facilitate vaccinations or STI treatment for individuals using video or telehealth services, as well as encouraging referrals from PCPs. These challenges were addressed by providing periodic targeted in-service training sessions to primary care teams to increase awareness of the Pharm-PrEP clinic. Collaboration with the ID service and ED allowed implementation of a direct pathway for patients initiated on nonoccupational HIV PEP after a high-risk exposure to be evaluated for transition to HIV PrEP. This PEP-2-PrEP pathway was designed to decrease barriers to follow-up for high-risk individuals who had recently received PEP in the ED. The CPP plays an active, integral role in managing patient care in the PEP-2-PrEP pathway.
Pharmacist-Led PrEP Care
The implementation of the VAGLAHCS Pharm-PrEP clinic demonstrates how CPPs can expand access and manage HIV PrEP with high reliability. Key factors for successfully integrating CPPs as PrEP prescribers include identifying physician champions; using in-services or other training platforms to raise awareness among potential referring HCPs and stimulate referrals; and developing processes to identify high-risk veterans. Also, nontraditional modes of care, such as video or telehealth appointments, can increase access and expand the volume of patient care visits. Such modalities are useful for PrEP management when combined with a well-defined operational process for laboratory specimen collection before appointments. This system is particularly well suited to increasing access to PrEP for patients who live in rural or highly rural areas that are medically underserved or who have difficulty traveling to a clinical facility for an in-person visit.
Although community health care organizations and HCPs face pay barriers not present in the VHA system, several studies have demonstrated feasability of pharmacist-led clinics in private health care systems.21-24 Havens and colleagues described a PrEP program affilitated with an university that assessed patient satisfaction and pharmacist acceptability with this new service.22 The results of surveys reported high patient satisfaction and pharmacist acceptability.23 Tung and colleagues described a PrEP clinic located in a community pharmacy with the ability to bill for pharmacist and laboratory services in addition to medication costs.24 These studies, along with our findings, demonstrate that CPPs are well positioned to manage HIV PrEP in the community. Leveraging the skills and experience of CPPs to address poor uptake and access to PrEP should be a central component in achieving the goals of the Ending the HIV Epidemic initiative, given that pharmacists are one of the most accessible groups of HCPs nationally.
Pharmacist prescriptive authority varies across different states and may depend on collaborative practice agreements, statewide protocols, or class-specific prescribing.25 For example, California was among the first states to authorize initiation and prescription of HIV PrEP and PEP by pharmacists in specified amounts after appropriate training.26 Nationwide support for similar policies in the community and within health care systems will be critical to the successful implementation and functioning of pharmacy-led PrEP clinics.
Conclusions
The success of this Pharm-PrEP clinic was largely due to a collaborative, interdisciplinary effort to implement this new clinic process and incorporate the CPP into the general ID outpatient clinic, while allowing flexibility in scheduling and use of different encounter modalities for patients. Deploying pharmacists as PrEP prescribers can help health care systems increase PrEP access and capacity and improve efforts to achieve the goals of the Ending the HIV Epidemic. This type of program can be a model for other health care organizations and systems to implement pharmacy-led PrEP clinics and expand telehealth modalities to deliver PrEP.
Acknowledgments
The infectious diseases service at the Veterans Affairs Greater Los Angeles Healthcare System and the veterans we serve.
1. Centers for Disease Control and Prevention. About Ending the HIV Epidemic in the U.S. Initiative. Updated September 7, 2021. Accessed April 3, 2023. https://www.cdc.gov/endhiv/about.html
2. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53-60. doi:10.1016/S0140-6736(15)00056-2 3. Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399-410. doi:10.1056/NEJMoa1108524
4. Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083-2090. doi:10.1016/S0140-6736(13)61127-7
5. Effectivenesss of prevention strategies to reduce the risk of acquiring or transmitting HIV. Centers for Disease Control and Prevention. Updated June 17, 2022. Accessed April 3, 2023. https://www.cdc.gov/hiv/risk/estimates/preventionstrategies.html
6. Centers for Disease Control and Prevention. HIV prevention pill not reaching most American who could benefit- especially people of color. Press release. Updated March 6, 2018. Accessed April 3, 2023. https://www.cdc.gov/nchhstp/newsroom/2018/croi-2018-PrEP-press-release.html
7. America’s HIV Epidemic Analysis Dashboard (AHEAD).The Six EHE Indicators: PrEP coverage. Accessed April 3, 2023. https://ahead.hiv.gov
8. Blaylock JM, Hakre S, Okulicz JF, et al. HIV preexposure prophylaxis in the U.S. Military Services - 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67(20):569-574. Published 2018 May 25. doi:10.15585/mmwr.mm6720a1
9. National Association of Chain Drug Stores (NACDS) Foundation. Face-to-Face with Community Pharmacies. Accessed April 14, 2023. https://www.nacds.org/pdfs/about/rximpact-leavebehind.pdf
10. Newman TV, San-Juan-Rodriguez A, Parekh N, et al. Impact of community pharmacist-led interventions in chronic disease management on clinical, utilization, and economic outcomes: an umbrella review. Res Social Adm Pharm. 2020;16(9):1155-1165. doi:10.1016/j.sapharm. 2019.12.016
11. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38(3):309-318. doi:10.1002/phar.2083
12. Matzke GR, Moczygemba LR, Williams KJ, Czar MJ, Lee WT. Impact of a pharmacist-physician collaborative care model on patient outcomes and health services utilization. Am J Health Syst Pharm. 2018;75(14):1039-1047. doi:10.2146/ajhp170789
13. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310(1):46-56. doi:10.1001/jama.2013.6549.
14. Strand MA, Bratberg J, Eukel H, Hardy M, Williams C. Community pharmacists’ contributions to disease management during the COVID-19 pandemic. Prev Chronic Dis. 2020;17:E69. doi:10.5888/pcd17.200317
15. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415. doi:10.2146/ajhp150778
16. Centers for Disease Control and Prevention. US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. March 2018. Accessed April 3, 2023. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
17. County of Los Angeles Public Health. Sexually transmitted diseases in Los Angeles County, 2019. May 2021. Accessed April 3, 2023. http://publichealth.lacounty.gov/dhsp/Reports/STD/2019_LAC_STD_Snapshot_051921Update.pdf
18. Krakower D, Maloney KM, Powell VE, et al. Patterns and clinical consequences of discontinuing HIV preexposure prophylaxis during primary care. J Int AIDS Soc. 2019;22(2):e25250. doi:10.1002/jia2.25250
19. Morgan E, Ryan DT, Newcomb ME, Mustanski B. High rate of discontinuation may diminish PrEP coverage among young men who have sex with men. AIDS Behav. 2018;22(11):3645-3648. doi:10.1007/s10461-018-2125-2
20. Spinelli MA, Scott HM, Vittinghoff E, et al. Missed visits associated with future preexposure prophylaxis (PrEP) discontinuation among PrEP users in a municipal primary care health network. Open Forum Infect Dis. 2019;6(4):ofz101. Published 2019 Feb 26. doi:10.1093/ofid/ofz101
21. Ryan K, Lewis J, Sanchez D, Anderson B, Mercier RC. 1293. The next step in PrEP: evaluating outcomes of a pharmacist-run HIV pre-exposure prophylaxis (PrEP) clinic. Open Forum Infect Dis. 2018;5(suppl 1):S395. doi:10.1093/ofid/ofy210.1126
22. Havens JP, Scarsi KK, Sayles H, Klepser DG, Swindells S, Bares SH. Acceptability and feasibility of a pharmacist-led HIV pre-exposure prophylaxis (PrEP) program in the Midwestern United States. Open Forum Infect Dis. 2019;6(10):ofz365. doi:10.1093/ofid/ofz365
23. Zhao A, Dangerfield DT 2nd, Nunn A, et al. Pharmacy-based interventions to increase use of HIV pre-exposure prophylaxis in the United States: a scoping review. AIDS Behav. 2022;26(5):1377-1392. doi:10.1007/s10461-021-03494-4
24. Tung EL, Thomas A, Eichner A, Shalit P. Implementation of a community pharmacy-based pre-exposure prophylaxis service: a novel model for pre-exposure prophylaxis care. Sex Health. 2018;15(6):556-561. doi:10.1071/SH18084
25. Sachdev G, Kliethermes MA, Vernon V, Leal S, Crabtree G. Current status of prescriptive authority by pharmacists in the United States. J Am Coll Clin Pharm. 2020;3(4):807-817. doi:10.1002/jac5.1245
26. California legislation information: SB-159 HIV: preexposure and postexposure prophylaxis. Accessed April 14, 2023. https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200SB159
The US Department of Health and Human Services (HHS) Ending the HIV Epidemic framework aims to decrease HIV infections in the United States by 90% before 2030.1 Achieving this goal requires identifying persons at high risk for HIV and ensuring timely and efficient access to HIV preexposure prophylaxis (PrEP).2-5 However, despite its commercial availability since 2012, community uptake of PrEP is low.6 In 2019, < 25% of Americans who could benefit from PrEP were using this preventive therapy.7 Poor uptake of PrEP has also been documented among veterans and US military service members. National data on men in the military and men who have sex with men (MSM) in the military suggest that about 12,000 service members are eligible for PrEP; however, only 2000 service members and their beneficiaries accessed PrEP in February 2017.8
A review of health records of US military service members conducted from 2014 to 2016 indicated that most patients who received PrEP did not receive recommended monitoring in accordance with the Centers for Disease Control and Prevention (CDC) guidelines. Furthermore, 16% of these individuals did not have HIV testing within 14 days of initiating PrEP, and 13% were never evaluated for hepatitis B infection.8
Pharmacists are highly accessible health care professionals (HCPs): More than 90% of Americans live within 5 miles of a community pharmacy.9 Pharmacists play an integral role within the outpatient health care team and have been responsible for improvements in health care outcomes for a variety of chronic conditions and immunization practices.10-13 Additionally, community pharmacists have provided vital access to care during the COVID-19 pandemic.14 The clinical pharmacist practitioner (CPP) is an innovative and advanced role within the Veterans Health Administration (VHA), functioning with a scope of practice and prescribing privileges to provide direct patient care.15
CPPs are well suited to address the need for increased access, capacity, and timely provision of PrEP, especially in areas where HIV acquisition rates are high or in areas with reduced access to care. We describe a model for a pharmacist-led HIV PrEP program (Pharm-PrEP) to increase access to PrEP. A similar program could be adapted to further expand the use of PrEP in other health care systems and community settings.
Pharm-PrEP Program Description
The Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHCS) provides health care services at 11 locations in southern California and serves > 86,000 veterans. The VAGLAHCS pharmacy staff includes 33 CPPs who practice in more than 9 clinical service lines. HIV PrEP services are available through the infectious diseases (ID) service for veterans wishing to begin or continue PrEP or for those identified as high risk. HIV PrEP consultations are placed by the referring HCP to the ID service for scheduling and evaluation. Prior to implementation of the pharmacist-managed PrEP clinic, 2 ID physician assistants (PAs) were responsible for PrEP evaluation, initiation, and follow-up. Each PA had 1 half-day face-to-face clinic and 1 PA had an additional half-day telehealth clinic. About 100 PrEP patients were followed by the ID group.
In July 2019, through collaboration with the ID service, a pharmacy PrEP clinic was created to increase access for veterans to initiate or continue PrEP. This clinic included 1 ID-trained CPP and 1 postgraduate-year-2 pharmacy resident. The CPP initiates and monitors veterans for HIV PrEP with prescribing privileges under a defined scope of practice.
Awareness of this novel service was raised through in-service training sessions for primary care and women’s health clinics. Referrals are generated directly from primary care practitioners (PCPs) or emergency department (ED) visits and are accepted on a continuing basis. Visits with the CPP are conducted in person or through telehealth services based on patient preference. Direct CPP patient care appointments involve a standardized assessment and discussion of patient HIV transmission risk, a review of social and sexual history, sexual practices and HIV risk, clinical evidence of acute HIV or other sexually transmitted infection (STI) symptoms, follow-up PrEP monitoring requirements, and counseling on appropriate PrEP use. CPPs can order laboratory tests, bone densitometry (DEXA scan), immunizations, PrEP, and STI treatment as required. ID service physicians are available during CPP visits for further assessment or consultation. While initially most visits are conducted in person, follow-up visits by telehealth or video have become predominant; most patients prefer these modalities, citing convenience, flexibility, and the ability to obtain laboratory tests in advance. Use of telephone and video is intended to reduce patient loss to follow-up.
All required baseline laboratory panels for PrEP monitoring are ordered and interpreted by the CPP in accordance with CDC guidelines.16 These include screening for syphilis, gonorrhea, and chlamydia; fourth-generation antibody-antigen HIV tests; renal function; viral hepatitis; and pregnancy. After reviewing screening results, the CPP will prescribe tenofovir disproxil fumarate/emtricitabine (TDF/FTC) or tenofovir alafenamide/emtricitabine (TAF/FTC) based on individual patient clinical characteristics, US Food and Drug Administration–approved labeling, and VA Pharmacy Benefits Management Criteria for Use. Initial prescriptions are for a 30-day supply with subsequent prescriptions for 90 days (no refills), providing follow-up HIV testing is completed.
Follow-up PrEP visits are scheduled about every 3 months with some overlap to avoid gaps in medication due to late laboratory testing or delayed receipt of mailed medications. The only laboratory testing strictly required each quarter before PrEP renewal is HIV and pregnancy testing. Other screenings, including STIs and renal function are completed at least every 6 months or more frequently, if indicated, based on individual risk factors. Hepatitis C antibody testing is conducted annually if the patient has ongoing risk factors. Treatment of gonorrhea/chlamydia and syphilis for patients with positive test results is also initiated by the CPP, including recommending antimicrobial regimens. Additional interventions conducted as part of the clinic include indicated vaccinations (meningococcal, human papillomavirus, hepatitis A and B), and DEXA scans. Collaboration with ID service attendings and PAs is conducted on an as-needed basis via direct consultation in the colocated clinic or through email or messaging.
Periodic surveillance of a local dashboard of veterans eligible for HIV PrEP is conducted to re-engage veterans in care who may have been lost to follow-up, along with periodic review of a local STI dashboard. These dashboards capture population-based data to identify patients who may benefit from additional STI screenings as well as potential candidates for HIV PrEP. Clinicians can review their patient panel to target individuals who may be due for specific actions. Patients are identified as needing cotesting if they screen positive for ≥ 1 STIs but have not had a concurrent or subsequent full screening panel (gonorrhea, chlamydia, syphilis, and HIV). Cotesting for bacterial STIs and HIV at the time of an encounter has been promoted to expedite STI identification and treatment and limit community transmission. These reports also identify patients who may be potential candidates for HIV PrEP, based on a history of positive screenings, frequent STI testing, recent prescriptions for postexposure prophylaxis (PEP) or encounters with specific International Classification of Diseases codes associated with high-risk practices.
Clinic Quality of Care
From July 2019 to March 2020, 53 veterans were managed by the pharm-PrEP clinic in 98 encounters. Seventy percent of encounters were in-person (Table 1).
Baseline information collected included demographics, documented patient-reported risk factors, fourth-generation HIV screening test results, STI status, viral hepatitis serologies, and renal function test results. Information collected every 3 to 6 months included STI status, fourth-generation HIV screening test results, renal function test results, adherence to therapy, changes in risk factors, and prescription refill data. Additional interventions conducted as part of clinic workflow included DEXA scans, vaccinations, and active prescriptions for condoms.
Baseline Characteristics
Pharm-PrEP clinic patients were predominantly male (94%), and a majority indicated White race with a median age of 38 years (range, 24-80 years).
Veterans referred to the clinic had up to 5 risk factors for PrEP initiation. The most common risk factors were inconsistent condom use (62%), multiple sexual partners of unknown HIV status (62%), MSM (57%), STI history (38%), bisexual partners (25%), and HIV-positive sexual partners (11%). One of the 53 individuals referred for PrEP had no risk factors and did not initiate PrEP. Two individuals declined initiation of PrEP after consultation. Twenty six of 53 veterans at baseline continued their use of PrEP following transfer to clinic CPP management; 24 of 27 veterans not currently using PrEP (89%) started or restarted lapsed PrEP use following CPP consultation.
HIV and STI Screening
No individuals tested positive for HIV at baseline (n = 52) or while on PrEP. PrEP was not renewed for 3 patients that did follow through with HIV testing. The median number of days an HIV test was completed prior to initial PrEP and PrEP renewal was 4 days and < 7 days, respectively, both of which are below the recommended maximal interval of 7 days, according to CDC PrEP guidelines. Some postinitiation HIV testing occurred using a longer interval of 14 days, in accordance with VA National Criteria for Use of PrEP. This modification allowed more flexibility as a majority of PrEP prescriptions are sent to veterans via mail. The CPP reviewing HIV test results was able to expedite the processing and mailing of PrEP prescriptions if deemed appropriate, ie, the HIV test was negative. This approach was not used if a patient had high-risk exposures without PrEP during the time between collection of the HIV test and mailing of the prescription.
STI screening is a vital component of the Pharm-PrEP clinic and helped identify 4 patients with gonorrhea/chlamydia at baseline and 1 with syphilis after initiation of PrEP. All patients were prescribed antibiotics at the screening. Los Angeles County has high rates of STI transmission; thus implementation of clinic processes allowing the CPP to screen for STIs, interpret test results, and treat patients with STIs is vital to limit spread in the community.17
Selection of PrEP Regimen
The majority of individuals in the cohort received TDF/FTC for PrEP; TAF/FTC was restricted to individuals who had documented renal dysfunction or bone loss (Table 3).
Six DEXA scans were completed by the end of the evaluation period and 2 had abnormal results. One patient discontinued TDF/FTC and reinitiated with TAF/FTC. The other was switched to TAF/FTC 1 month after initiation.
Follow-Up Visits
The median number of visits per patient was 2. The median time between visits was in accordance with recommended follow-up intervals with 35 days between visits 1 and 2, 60 days between visits 2 and 3, and 88 days thereafter. In all, 10 veterans (20%) stopped PrEP: 4 (8%) were lost to follow-up; 3 (6%) had sustained behavior changes decreasing their HIV exposure risk; 2 (4%) were concerned about ADRs; and 1 (2%) moved out of state. Even after including those patients with a decrease in HIV exposure risk who no longer required PrEP, our 20% discontinuation rate was lower than those reported in other studies that showed a wide variation in PrEP discontinuation rates ranging from 33% to 62%.18-20
Challenges
Some challenges with the implementation of the clinic included logistic and operational barriers, such as developing clinical pathways and managing workflow to facilitate vaccinations or STI treatment for individuals using video or telehealth services, as well as encouraging referrals from PCPs. These challenges were addressed by providing periodic targeted in-service training sessions to primary care teams to increase awareness of the Pharm-PrEP clinic. Collaboration with the ID service and ED allowed implementation of a direct pathway for patients initiated on nonoccupational HIV PEP after a high-risk exposure to be evaluated for transition to HIV PrEP. This PEP-2-PrEP pathway was designed to decrease barriers to follow-up for high-risk individuals who had recently received PEP in the ED. The CPP plays an active, integral role in managing patient care in the PEP-2-PrEP pathway.
Pharmacist-Led PrEP Care
The implementation of the VAGLAHCS Pharm-PrEP clinic demonstrates how CPPs can expand access and manage HIV PrEP with high reliability. Key factors for successfully integrating CPPs as PrEP prescribers include identifying physician champions; using in-services or other training platforms to raise awareness among potential referring HCPs and stimulate referrals; and developing processes to identify high-risk veterans. Also, nontraditional modes of care, such as video or telehealth appointments, can increase access and expand the volume of patient care visits. Such modalities are useful for PrEP management when combined with a well-defined operational process for laboratory specimen collection before appointments. This system is particularly well suited to increasing access to PrEP for patients who live in rural or highly rural areas that are medically underserved or who have difficulty traveling to a clinical facility for an in-person visit.
Although community health care organizations and HCPs face pay barriers not present in the VHA system, several studies have demonstrated feasability of pharmacist-led clinics in private health care systems.21-24 Havens and colleagues described a PrEP program affilitated with an university that assessed patient satisfaction and pharmacist acceptability with this new service.22 The results of surveys reported high patient satisfaction and pharmacist acceptability.23 Tung and colleagues described a PrEP clinic located in a community pharmacy with the ability to bill for pharmacist and laboratory services in addition to medication costs.24 These studies, along with our findings, demonstrate that CPPs are well positioned to manage HIV PrEP in the community. Leveraging the skills and experience of CPPs to address poor uptake and access to PrEP should be a central component in achieving the goals of the Ending the HIV Epidemic initiative, given that pharmacists are one of the most accessible groups of HCPs nationally.
Pharmacist prescriptive authority varies across different states and may depend on collaborative practice agreements, statewide protocols, or class-specific prescribing.25 For example, California was among the first states to authorize initiation and prescription of HIV PrEP and PEP by pharmacists in specified amounts after appropriate training.26 Nationwide support for similar policies in the community and within health care systems will be critical to the successful implementation and functioning of pharmacy-led PrEP clinics.
Conclusions
The success of this Pharm-PrEP clinic was largely due to a collaborative, interdisciplinary effort to implement this new clinic process and incorporate the CPP into the general ID outpatient clinic, while allowing flexibility in scheduling and use of different encounter modalities for patients. Deploying pharmacists as PrEP prescribers can help health care systems increase PrEP access and capacity and improve efforts to achieve the goals of the Ending the HIV Epidemic. This type of program can be a model for other health care organizations and systems to implement pharmacy-led PrEP clinics and expand telehealth modalities to deliver PrEP.
Acknowledgments
The infectious diseases service at the Veterans Affairs Greater Los Angeles Healthcare System and the veterans we serve.
The US Department of Health and Human Services (HHS) Ending the HIV Epidemic framework aims to decrease HIV infections in the United States by 90% before 2030.1 Achieving this goal requires identifying persons at high risk for HIV and ensuring timely and efficient access to HIV preexposure prophylaxis (PrEP).2-5 However, despite its commercial availability since 2012, community uptake of PrEP is low.6 In 2019, < 25% of Americans who could benefit from PrEP were using this preventive therapy.7 Poor uptake of PrEP has also been documented among veterans and US military service members. National data on men in the military and men who have sex with men (MSM) in the military suggest that about 12,000 service members are eligible for PrEP; however, only 2000 service members and their beneficiaries accessed PrEP in February 2017.8
A review of health records of US military service members conducted from 2014 to 2016 indicated that most patients who received PrEP did not receive recommended monitoring in accordance with the Centers for Disease Control and Prevention (CDC) guidelines. Furthermore, 16% of these individuals did not have HIV testing within 14 days of initiating PrEP, and 13% were never evaluated for hepatitis B infection.8
Pharmacists are highly accessible health care professionals (HCPs): More than 90% of Americans live within 5 miles of a community pharmacy.9 Pharmacists play an integral role within the outpatient health care team and have been responsible for improvements in health care outcomes for a variety of chronic conditions and immunization practices.10-13 Additionally, community pharmacists have provided vital access to care during the COVID-19 pandemic.14 The clinical pharmacist practitioner (CPP) is an innovative and advanced role within the Veterans Health Administration (VHA), functioning with a scope of practice and prescribing privileges to provide direct patient care.15
CPPs are well suited to address the need for increased access, capacity, and timely provision of PrEP, especially in areas where HIV acquisition rates are high or in areas with reduced access to care. We describe a model for a pharmacist-led HIV PrEP program (Pharm-PrEP) to increase access to PrEP. A similar program could be adapted to further expand the use of PrEP in other health care systems and community settings.
Pharm-PrEP Program Description
The Veterans Affairs Greater Los Angeles Healthcare System (VAGLAHCS) provides health care services at 11 locations in southern California and serves > 86,000 veterans. The VAGLAHCS pharmacy staff includes 33 CPPs who practice in more than 9 clinical service lines. HIV PrEP services are available through the infectious diseases (ID) service for veterans wishing to begin or continue PrEP or for those identified as high risk. HIV PrEP consultations are placed by the referring HCP to the ID service for scheduling and evaluation. Prior to implementation of the pharmacist-managed PrEP clinic, 2 ID physician assistants (PAs) were responsible for PrEP evaluation, initiation, and follow-up. Each PA had 1 half-day face-to-face clinic and 1 PA had an additional half-day telehealth clinic. About 100 PrEP patients were followed by the ID group.
In July 2019, through collaboration with the ID service, a pharmacy PrEP clinic was created to increase access for veterans to initiate or continue PrEP. This clinic included 1 ID-trained CPP and 1 postgraduate-year-2 pharmacy resident. The CPP initiates and monitors veterans for HIV PrEP with prescribing privileges under a defined scope of practice.
Awareness of this novel service was raised through in-service training sessions for primary care and women’s health clinics. Referrals are generated directly from primary care practitioners (PCPs) or emergency department (ED) visits and are accepted on a continuing basis. Visits with the CPP are conducted in person or through telehealth services based on patient preference. Direct CPP patient care appointments involve a standardized assessment and discussion of patient HIV transmission risk, a review of social and sexual history, sexual practices and HIV risk, clinical evidence of acute HIV or other sexually transmitted infection (STI) symptoms, follow-up PrEP monitoring requirements, and counseling on appropriate PrEP use. CPPs can order laboratory tests, bone densitometry (DEXA scan), immunizations, PrEP, and STI treatment as required. ID service physicians are available during CPP visits for further assessment or consultation. While initially most visits are conducted in person, follow-up visits by telehealth or video have become predominant; most patients prefer these modalities, citing convenience, flexibility, and the ability to obtain laboratory tests in advance. Use of telephone and video is intended to reduce patient loss to follow-up.
All required baseline laboratory panels for PrEP monitoring are ordered and interpreted by the CPP in accordance with CDC guidelines.16 These include screening for syphilis, gonorrhea, and chlamydia; fourth-generation antibody-antigen HIV tests; renal function; viral hepatitis; and pregnancy. After reviewing screening results, the CPP will prescribe tenofovir disproxil fumarate/emtricitabine (TDF/FTC) or tenofovir alafenamide/emtricitabine (TAF/FTC) based on individual patient clinical characteristics, US Food and Drug Administration–approved labeling, and VA Pharmacy Benefits Management Criteria for Use. Initial prescriptions are for a 30-day supply with subsequent prescriptions for 90 days (no refills), providing follow-up HIV testing is completed.
Follow-up PrEP visits are scheduled about every 3 months with some overlap to avoid gaps in medication due to late laboratory testing or delayed receipt of mailed medications. The only laboratory testing strictly required each quarter before PrEP renewal is HIV and pregnancy testing. Other screenings, including STIs and renal function are completed at least every 6 months or more frequently, if indicated, based on individual risk factors. Hepatitis C antibody testing is conducted annually if the patient has ongoing risk factors. Treatment of gonorrhea/chlamydia and syphilis for patients with positive test results is also initiated by the CPP, including recommending antimicrobial regimens. Additional interventions conducted as part of the clinic include indicated vaccinations (meningococcal, human papillomavirus, hepatitis A and B), and DEXA scans. Collaboration with ID service attendings and PAs is conducted on an as-needed basis via direct consultation in the colocated clinic or through email or messaging.
Periodic surveillance of a local dashboard of veterans eligible for HIV PrEP is conducted to re-engage veterans in care who may have been lost to follow-up, along with periodic review of a local STI dashboard. These dashboards capture population-based data to identify patients who may benefit from additional STI screenings as well as potential candidates for HIV PrEP. Clinicians can review their patient panel to target individuals who may be due for specific actions. Patients are identified as needing cotesting if they screen positive for ≥ 1 STIs but have not had a concurrent or subsequent full screening panel (gonorrhea, chlamydia, syphilis, and HIV). Cotesting for bacterial STIs and HIV at the time of an encounter has been promoted to expedite STI identification and treatment and limit community transmission. These reports also identify patients who may be potential candidates for HIV PrEP, based on a history of positive screenings, frequent STI testing, recent prescriptions for postexposure prophylaxis (PEP) or encounters with specific International Classification of Diseases codes associated with high-risk practices.
Clinic Quality of Care
From July 2019 to March 2020, 53 veterans were managed by the pharm-PrEP clinic in 98 encounters. Seventy percent of encounters were in-person (Table 1).
Baseline information collected included demographics, documented patient-reported risk factors, fourth-generation HIV screening test results, STI status, viral hepatitis serologies, and renal function test results. Information collected every 3 to 6 months included STI status, fourth-generation HIV screening test results, renal function test results, adherence to therapy, changes in risk factors, and prescription refill data. Additional interventions conducted as part of clinic workflow included DEXA scans, vaccinations, and active prescriptions for condoms.
Baseline Characteristics
Pharm-PrEP clinic patients were predominantly male (94%), and a majority indicated White race with a median age of 38 years (range, 24-80 years).
Veterans referred to the clinic had up to 5 risk factors for PrEP initiation. The most common risk factors were inconsistent condom use (62%), multiple sexual partners of unknown HIV status (62%), MSM (57%), STI history (38%), bisexual partners (25%), and HIV-positive sexual partners (11%). One of the 53 individuals referred for PrEP had no risk factors and did not initiate PrEP. Two individuals declined initiation of PrEP after consultation. Twenty six of 53 veterans at baseline continued their use of PrEP following transfer to clinic CPP management; 24 of 27 veterans not currently using PrEP (89%) started or restarted lapsed PrEP use following CPP consultation.
HIV and STI Screening
No individuals tested positive for HIV at baseline (n = 52) or while on PrEP. PrEP was not renewed for 3 patients that did follow through with HIV testing. The median number of days an HIV test was completed prior to initial PrEP and PrEP renewal was 4 days and < 7 days, respectively, both of which are below the recommended maximal interval of 7 days, according to CDC PrEP guidelines. Some postinitiation HIV testing occurred using a longer interval of 14 days, in accordance with VA National Criteria for Use of PrEP. This modification allowed more flexibility as a majority of PrEP prescriptions are sent to veterans via mail. The CPP reviewing HIV test results was able to expedite the processing and mailing of PrEP prescriptions if deemed appropriate, ie, the HIV test was negative. This approach was not used if a patient had high-risk exposures without PrEP during the time between collection of the HIV test and mailing of the prescription.
STI screening is a vital component of the Pharm-PrEP clinic and helped identify 4 patients with gonorrhea/chlamydia at baseline and 1 with syphilis after initiation of PrEP. All patients were prescribed antibiotics at the screening. Los Angeles County has high rates of STI transmission; thus implementation of clinic processes allowing the CPP to screen for STIs, interpret test results, and treat patients with STIs is vital to limit spread in the community.17
Selection of PrEP Regimen
The majority of individuals in the cohort received TDF/FTC for PrEP; TAF/FTC was restricted to individuals who had documented renal dysfunction or bone loss (Table 3).
Six DEXA scans were completed by the end of the evaluation period and 2 had abnormal results. One patient discontinued TDF/FTC and reinitiated with TAF/FTC. The other was switched to TAF/FTC 1 month after initiation.
Follow-Up Visits
The median number of visits per patient was 2. The median time between visits was in accordance with recommended follow-up intervals with 35 days between visits 1 and 2, 60 days between visits 2 and 3, and 88 days thereafter. In all, 10 veterans (20%) stopped PrEP: 4 (8%) were lost to follow-up; 3 (6%) had sustained behavior changes decreasing their HIV exposure risk; 2 (4%) were concerned about ADRs; and 1 (2%) moved out of state. Even after including those patients with a decrease in HIV exposure risk who no longer required PrEP, our 20% discontinuation rate was lower than those reported in other studies that showed a wide variation in PrEP discontinuation rates ranging from 33% to 62%.18-20
Challenges
Some challenges with the implementation of the clinic included logistic and operational barriers, such as developing clinical pathways and managing workflow to facilitate vaccinations or STI treatment for individuals using video or telehealth services, as well as encouraging referrals from PCPs. These challenges were addressed by providing periodic targeted in-service training sessions to primary care teams to increase awareness of the Pharm-PrEP clinic. Collaboration with the ID service and ED allowed implementation of a direct pathway for patients initiated on nonoccupational HIV PEP after a high-risk exposure to be evaluated for transition to HIV PrEP. This PEP-2-PrEP pathway was designed to decrease barriers to follow-up for high-risk individuals who had recently received PEP in the ED. The CPP plays an active, integral role in managing patient care in the PEP-2-PrEP pathway.
Pharmacist-Led PrEP Care
The implementation of the VAGLAHCS Pharm-PrEP clinic demonstrates how CPPs can expand access and manage HIV PrEP with high reliability. Key factors for successfully integrating CPPs as PrEP prescribers include identifying physician champions; using in-services or other training platforms to raise awareness among potential referring HCPs and stimulate referrals; and developing processes to identify high-risk veterans. Also, nontraditional modes of care, such as video or telehealth appointments, can increase access and expand the volume of patient care visits. Such modalities are useful for PrEP management when combined with a well-defined operational process for laboratory specimen collection before appointments. This system is particularly well suited to increasing access to PrEP for patients who live in rural or highly rural areas that are medically underserved or who have difficulty traveling to a clinical facility for an in-person visit.
Although community health care organizations and HCPs face pay barriers not present in the VHA system, several studies have demonstrated feasability of pharmacist-led clinics in private health care systems.21-24 Havens and colleagues described a PrEP program affilitated with an university that assessed patient satisfaction and pharmacist acceptability with this new service.22 The results of surveys reported high patient satisfaction and pharmacist acceptability.23 Tung and colleagues described a PrEP clinic located in a community pharmacy with the ability to bill for pharmacist and laboratory services in addition to medication costs.24 These studies, along with our findings, demonstrate that CPPs are well positioned to manage HIV PrEP in the community. Leveraging the skills and experience of CPPs to address poor uptake and access to PrEP should be a central component in achieving the goals of the Ending the HIV Epidemic initiative, given that pharmacists are one of the most accessible groups of HCPs nationally.
Pharmacist prescriptive authority varies across different states and may depend on collaborative practice agreements, statewide protocols, or class-specific prescribing.25 For example, California was among the first states to authorize initiation and prescription of HIV PrEP and PEP by pharmacists in specified amounts after appropriate training.26 Nationwide support for similar policies in the community and within health care systems will be critical to the successful implementation and functioning of pharmacy-led PrEP clinics.
Conclusions
The success of this Pharm-PrEP clinic was largely due to a collaborative, interdisciplinary effort to implement this new clinic process and incorporate the CPP into the general ID outpatient clinic, while allowing flexibility in scheduling and use of different encounter modalities for patients. Deploying pharmacists as PrEP prescribers can help health care systems increase PrEP access and capacity and improve efforts to achieve the goals of the Ending the HIV Epidemic. This type of program can be a model for other health care organizations and systems to implement pharmacy-led PrEP clinics and expand telehealth modalities to deliver PrEP.
Acknowledgments
The infectious diseases service at the Veterans Affairs Greater Los Angeles Healthcare System and the veterans we serve.
1. Centers for Disease Control and Prevention. About Ending the HIV Epidemic in the U.S. Initiative. Updated September 7, 2021. Accessed April 3, 2023. https://www.cdc.gov/endhiv/about.html
2. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53-60. doi:10.1016/S0140-6736(15)00056-2 3. Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399-410. doi:10.1056/NEJMoa1108524
4. Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083-2090. doi:10.1016/S0140-6736(13)61127-7
5. Effectivenesss of prevention strategies to reduce the risk of acquiring or transmitting HIV. Centers for Disease Control and Prevention. Updated June 17, 2022. Accessed April 3, 2023. https://www.cdc.gov/hiv/risk/estimates/preventionstrategies.html
6. Centers for Disease Control and Prevention. HIV prevention pill not reaching most American who could benefit- especially people of color. Press release. Updated March 6, 2018. Accessed April 3, 2023. https://www.cdc.gov/nchhstp/newsroom/2018/croi-2018-PrEP-press-release.html
7. America’s HIV Epidemic Analysis Dashboard (AHEAD).The Six EHE Indicators: PrEP coverage. Accessed April 3, 2023. https://ahead.hiv.gov
8. Blaylock JM, Hakre S, Okulicz JF, et al. HIV preexposure prophylaxis in the U.S. Military Services - 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67(20):569-574. Published 2018 May 25. doi:10.15585/mmwr.mm6720a1
9. National Association of Chain Drug Stores (NACDS) Foundation. Face-to-Face with Community Pharmacies. Accessed April 14, 2023. https://www.nacds.org/pdfs/about/rximpact-leavebehind.pdf
10. Newman TV, San-Juan-Rodriguez A, Parekh N, et al. Impact of community pharmacist-led interventions in chronic disease management on clinical, utilization, and economic outcomes: an umbrella review. Res Social Adm Pharm. 2020;16(9):1155-1165. doi:10.1016/j.sapharm. 2019.12.016
11. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38(3):309-318. doi:10.1002/phar.2083
12. Matzke GR, Moczygemba LR, Williams KJ, Czar MJ, Lee WT. Impact of a pharmacist-physician collaborative care model on patient outcomes and health services utilization. Am J Health Syst Pharm. 2018;75(14):1039-1047. doi:10.2146/ajhp170789
13. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310(1):46-56. doi:10.1001/jama.2013.6549.
14. Strand MA, Bratberg J, Eukel H, Hardy M, Williams C. Community pharmacists’ contributions to disease management during the COVID-19 pandemic. Prev Chronic Dis. 2020;17:E69. doi:10.5888/pcd17.200317
15. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415. doi:10.2146/ajhp150778
16. Centers for Disease Control and Prevention. US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. March 2018. Accessed April 3, 2023. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
17. County of Los Angeles Public Health. Sexually transmitted diseases in Los Angeles County, 2019. May 2021. Accessed April 3, 2023. http://publichealth.lacounty.gov/dhsp/Reports/STD/2019_LAC_STD_Snapshot_051921Update.pdf
18. Krakower D, Maloney KM, Powell VE, et al. Patterns and clinical consequences of discontinuing HIV preexposure prophylaxis during primary care. J Int AIDS Soc. 2019;22(2):e25250. doi:10.1002/jia2.25250
19. Morgan E, Ryan DT, Newcomb ME, Mustanski B. High rate of discontinuation may diminish PrEP coverage among young men who have sex with men. AIDS Behav. 2018;22(11):3645-3648. doi:10.1007/s10461-018-2125-2
20. Spinelli MA, Scott HM, Vittinghoff E, et al. Missed visits associated with future preexposure prophylaxis (PrEP) discontinuation among PrEP users in a municipal primary care health network. Open Forum Infect Dis. 2019;6(4):ofz101. Published 2019 Feb 26. doi:10.1093/ofid/ofz101
21. Ryan K, Lewis J, Sanchez D, Anderson B, Mercier RC. 1293. The next step in PrEP: evaluating outcomes of a pharmacist-run HIV pre-exposure prophylaxis (PrEP) clinic. Open Forum Infect Dis. 2018;5(suppl 1):S395. doi:10.1093/ofid/ofy210.1126
22. Havens JP, Scarsi KK, Sayles H, Klepser DG, Swindells S, Bares SH. Acceptability and feasibility of a pharmacist-led HIV pre-exposure prophylaxis (PrEP) program in the Midwestern United States. Open Forum Infect Dis. 2019;6(10):ofz365. doi:10.1093/ofid/ofz365
23. Zhao A, Dangerfield DT 2nd, Nunn A, et al. Pharmacy-based interventions to increase use of HIV pre-exposure prophylaxis in the United States: a scoping review. AIDS Behav. 2022;26(5):1377-1392. doi:10.1007/s10461-021-03494-4
24. Tung EL, Thomas A, Eichner A, Shalit P. Implementation of a community pharmacy-based pre-exposure prophylaxis service: a novel model for pre-exposure prophylaxis care. Sex Health. 2018;15(6):556-561. doi:10.1071/SH18084
25. Sachdev G, Kliethermes MA, Vernon V, Leal S, Crabtree G. Current status of prescriptive authority by pharmacists in the United States. J Am Coll Clin Pharm. 2020;3(4):807-817. doi:10.1002/jac5.1245
26. California legislation information: SB-159 HIV: preexposure and postexposure prophylaxis. Accessed April 14, 2023. https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200SB159
1. Centers for Disease Control and Prevention. About Ending the HIV Epidemic in the U.S. Initiative. Updated September 7, 2021. Accessed April 3, 2023. https://www.cdc.gov/endhiv/about.html
2. McCormack S, Dunn DT, Desai M, et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53-60. doi:10.1016/S0140-6736(15)00056-2 3. Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399-410. doi:10.1056/NEJMoa1108524
4. Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083-2090. doi:10.1016/S0140-6736(13)61127-7
5. Effectivenesss of prevention strategies to reduce the risk of acquiring or transmitting HIV. Centers for Disease Control and Prevention. Updated June 17, 2022. Accessed April 3, 2023. https://www.cdc.gov/hiv/risk/estimates/preventionstrategies.html
6. Centers for Disease Control and Prevention. HIV prevention pill not reaching most American who could benefit- especially people of color. Press release. Updated March 6, 2018. Accessed April 3, 2023. https://www.cdc.gov/nchhstp/newsroom/2018/croi-2018-PrEP-press-release.html
7. America’s HIV Epidemic Analysis Dashboard (AHEAD).The Six EHE Indicators: PrEP coverage. Accessed April 3, 2023. https://ahead.hiv.gov
8. Blaylock JM, Hakre S, Okulicz JF, et al. HIV preexposure prophylaxis in the U.S. Military Services - 2014-2016. MMWR Morb Mortal Wkly Rep. 2018;67(20):569-574. Published 2018 May 25. doi:10.15585/mmwr.mm6720a1
9. National Association of Chain Drug Stores (NACDS) Foundation. Face-to-Face with Community Pharmacies. Accessed April 14, 2023. https://www.nacds.org/pdfs/about/rximpact-leavebehind.pdf
10. Newman TV, San-Juan-Rodriguez A, Parekh N, et al. Impact of community pharmacist-led interventions in chronic disease management on clinical, utilization, and economic outcomes: an umbrella review. Res Social Adm Pharm. 2020;16(9):1155-1165. doi:10.1016/j.sapharm. 2019.12.016
11. Anderegg MD, Gums TH, Uribe L, et al. Pharmacist intervention for blood pressure control in patients with diabetes and/or chronic kidney disease. Pharmacotherapy. 2018;38(3):309-318. doi:10.1002/phar.2083
12. Matzke GR, Moczygemba LR, Williams KJ, Czar MJ, Lee WT. Impact of a pharmacist-physician collaborative care model on patient outcomes and health services utilization. Am J Health Syst Pharm. 2018;75(14):1039-1047. doi:10.2146/ajhp170789
13. Margolis KL, Asche SE, Bergdall AR, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310(1):46-56. doi:10.1001/jama.2013.6549.
14. Strand MA, Bratberg J, Eukel H, Hardy M, Williams C. Community pharmacists’ contributions to disease management during the COVID-19 pandemic. Prev Chronic Dis. 2020;17:E69. doi:10.5888/pcd17.200317
15. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415. doi:10.2146/ajhp150778
16. Centers for Disease Control and Prevention. US Public Health Service: Preexposure prophylaxis for the prevention of HIV infection in the United States—2017 update: a clinical practice guideline. March 2018. Accessed April 3, 2023. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
17. County of Los Angeles Public Health. Sexually transmitted diseases in Los Angeles County, 2019. May 2021. Accessed April 3, 2023. http://publichealth.lacounty.gov/dhsp/Reports/STD/2019_LAC_STD_Snapshot_051921Update.pdf
18. Krakower D, Maloney KM, Powell VE, et al. Patterns and clinical consequences of discontinuing HIV preexposure prophylaxis during primary care. J Int AIDS Soc. 2019;22(2):e25250. doi:10.1002/jia2.25250
19. Morgan E, Ryan DT, Newcomb ME, Mustanski B. High rate of discontinuation may diminish PrEP coverage among young men who have sex with men. AIDS Behav. 2018;22(11):3645-3648. doi:10.1007/s10461-018-2125-2
20. Spinelli MA, Scott HM, Vittinghoff E, et al. Missed visits associated with future preexposure prophylaxis (PrEP) discontinuation among PrEP users in a municipal primary care health network. Open Forum Infect Dis. 2019;6(4):ofz101. Published 2019 Feb 26. doi:10.1093/ofid/ofz101
21. Ryan K, Lewis J, Sanchez D, Anderson B, Mercier RC. 1293. The next step in PrEP: evaluating outcomes of a pharmacist-run HIV pre-exposure prophylaxis (PrEP) clinic. Open Forum Infect Dis. 2018;5(suppl 1):S395. doi:10.1093/ofid/ofy210.1126
22. Havens JP, Scarsi KK, Sayles H, Klepser DG, Swindells S, Bares SH. Acceptability and feasibility of a pharmacist-led HIV pre-exposure prophylaxis (PrEP) program in the Midwestern United States. Open Forum Infect Dis. 2019;6(10):ofz365. doi:10.1093/ofid/ofz365
23. Zhao A, Dangerfield DT 2nd, Nunn A, et al. Pharmacy-based interventions to increase use of HIV pre-exposure prophylaxis in the United States: a scoping review. AIDS Behav. 2022;26(5):1377-1392. doi:10.1007/s10461-021-03494-4
24. Tung EL, Thomas A, Eichner A, Shalit P. Implementation of a community pharmacy-based pre-exposure prophylaxis service: a novel model for pre-exposure prophylaxis care. Sex Health. 2018;15(6):556-561. doi:10.1071/SH18084
25. Sachdev G, Kliethermes MA, Vernon V, Leal S, Crabtree G. Current status of prescriptive authority by pharmacists in the United States. J Am Coll Clin Pharm. 2020;3(4):807-817. doi:10.1002/jac5.1245
26. California legislation information: SB-159 HIV: preexposure and postexposure prophylaxis. Accessed April 14, 2023. https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201920200SB159