User login
Pulmonary arterial hypertension (PAH) is a rare disease that is associated with high mortality and is characterized by pulmonary vascular remodeling. Portopulmonary hypertension (POPH) is a form of PAH that occurs in patients with portal hypertension where no alternative cause of PAH can be identified. POPH is documented in approximately 4.5% to 8.5% of liver transplant candidates,1,2 but there is no relationship between the existence or severity of POPH and the severity of liver dysfunction.3 Mantz and Craig described the first case of POPH in a 53-year-old woman with enlarged pulmonary arteries that exhibited forceful pulsations more characteristic of the aorta than a low-pressure pulmonary trunk.4 Autopsy revealed findings of chronic liver disease including a stenotic portal vein, portocaval shunt, and esophageal varices. In both PAH and POPH, pre-capillary pulmonary arteries have characteristic lesions, such as intimal thickening, endothelial proliferation, and thrombotic changes. This 2-part article reviews the diagnosis and treatment of patients with POPH. Here, we review the epidemiology, prognosis, pathogenesis, and diagnosis of POPH; current treatment options for POPH are reviewed in a separate article.
Definition
The term POPH was first used by Yoshida et al in 1993 to describe the first successful liver transplant in a patient with POPH, a 39-year-old man with chronic hepatitis.5 The World Health Organization (WHO) classifies POPH as a form of Group 1 PAH.6 The criteria that must be met to make a diagnosis of POPH are shown in the Table 1.7
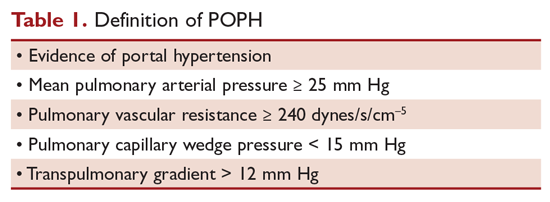
Moderate POPH is defined as a mean pulmonary artery pressure (MPAP) between 35 mm Hg and < 45 mm Hg, whereas severe POPH is MPAP ≥ 45 mm Hg. Moderate and severe POPH are considered contraindications to liver transplant because of high perioperative and postoperative mortality rates.8 In 2000, the Mayo Clinic retrospectively reviewed 43 patients with POPH who underwent attempted liver transplantation.9 The cardiopulmonary-related mortality rate in patients with a MPAP of 35 to < 50 mm Hg was 50% and 100% for those with MPAP > 50 mm Hg. No mortality was noted in patients with a pre-liver transplant MPAP of < 35 mm Hg and transpulmonary gradient (TPG) < 15 mm Hg.
Epidemiology
In 1983, a series of 17,901 autopsied patients showed a primary pulmonary hypertension prevalence of 0.13% and a prevalence of 0.73% in patients with cirrhosis.10 In 1987, Rich et al published data from the National Institutes of Health’s national registry of primary pulmonary hypertension.11 The registry included data from 187 patients from 32 centers. Further analyses by Groves et al concluded that 8.3% of the patients likely had POPH.12 Humbert et al published data on the French pulmonary hypertension registry experience in 2006.13 The French registry included 674 patients from 17 university hospitals; 10.4% of these patients had POPH. The largest prospective study was published by Hadengue et al in 1991.14 In this study, 507 patients hospitalized with portal hypertension but without known pulmonary hypertension underwent cardiac catheterization; 10 patients (2%) had pulmonary hypertension and more than half were clinically asymptomatic. Finally, the Registry to Evaluate Early And Long-term pulmonary arterial hypertension disease management (REVEAL registry) documented a 5.3% frequency of POPH (174 of 3525) in the United States.15
Prognosis
Individuals with POPH have worse outcomes compared to other forms of PAH. Median survival prior to the introduction of vasodilator therapy was a dismal 6 months and mean survival was 15 months.16 The cause of death in patients with POPH is equally distributed between right heart failure from POPH and direct complications of chronic liver disease.1 Le Pavec et al retrospectively analyzed all patients referred to the French Referral Center with POPH between 1984 and 2004 (154 patients).1 Approximately 50% of the patients were Child-Turcotte-Pugh class B or C, and 60% were classified as New York Health Association (NYHA) class III or IV. In these patients, 1-, 3-, and 5-year survival rates were 88%, 75%, and 68%, respectively. Major independent prognostic risk factors were presence and severity of cirrhosis and preservation of right ventricular function. Interestingly, NYHA functional class was not related to survival in this study, although it has clearly been identified as a strong prognostic factor in idiopathic PAH.
Krowka et al evaluated 174 patients with POPH enrolled in the REVEAL Registry,15 a multicenter, observational, US-based study comprised of more than 3500 patients with PAH. Despite having better hemodynamic parameters at diagnosis, patients with POPH had significantly poorer survival and all-cause hospitalization compared with patients with idiopathic PAH (IPAH) or hereditary PAH (HPAH). Two-year survival from enrollment was 67% in POPH versus 85% in those with IPAH/HPAH (P < 0.001). Five-year survival from time of diagnosis was 40% versus 64% (P < 0.001). Additionally, patients with POPH were less likely to be on PAH-specific therapy at enrollment, with only 25% on treatment at the time of entry. These findings were replicated in 2005 when Kawut et al retrospectively compared 13 patients with POPH with 33 patients with IPAH.17 Despite having a higher cardiac index and lower pulmonary vascular resistance than patients with IPAH, patients with POPH had a higher risk of death (hazard ratio, 2.8, P = 0.04), likely reflecting the combination of 2 serious diseases.
In 2008 the Mayo Clinic published their retrospective analysis of patients with POPH to determine the natural history of POPH.18 Patients were categorized into 3 groups: (1) no medical therapy for POPH and no liver transplant; (2) medical therapy for POPH alone; (3) medical therapy for POPH followed by liver transplant. The study included 74 patients between 1994 through 2007; 19 patients who did not receive treatment for POPH or liver transplant truly represented the natural history of POPH. Their 5-year survival was only 14%, and over half were deceased 1 year after diagnosis. The largest group consisted of patients who received therapy for POPH but no liver transplant. This group did remarkably better than those who received no therapy at all, with a 5-year survival of 45%. However, the patients with the overall best survival were those who received a combination of treatment for POPH followed by liver transplant. Their 5-year survival was 67%. Survival at 5 years was only 25% for the small group of patients who received transplant without PAH therapy. Once again, mortality did not correlate with the severity of hepatic dysfunction or baseline hemodynamic data.
Pathogenesis
The pathogenesis of POPH is unclear. Multiple studies have shown that there is minimal, if any, association with pulmonary hypertension and the severity of liver disease or portal hypertension.19,20 Portal hypertension is the result of an increase in intrahepatic resistance and an increase in blood flow into the portal circulation. Collateral vessels develop and blood from the splanchnic circulation is allowed directly into the systemic venous circulation, bypassing the liver. One of the most widely accepted theories is that a humoral substance, that would otherwise be metabolized by the liver, is able to reach the pulmonary circulation through collaterals, resulting in POPH.21 Pelicelli et al evaluated the possible role of endothelin-1, interleukin-6, interleukin 1β, and tumor necrosis factor in the pathogenesis of POPH.22 Plasma concentrations of these cytokines were compared between patients with POPH and patients with cirrhosis but no POPH. Patients with POPH had higher concentrations of endothelin-1 and interleukin-6, suggesting antagonists for these cytokines may have a role in the treatment of POPH. The role of endothelin-1 was further supported by Kamath et al in 200023 when they determined the pulmonary vascular bed is exposed to increased levels of circulating endothelin-1a in the setting of cirrhosis. Endothelin-1 is a potent vasoconstrictor and facilitator of smooth muscle proliferation.
In addition to collateral circulation allowing mediators to reach the pulmonary arterial bed in portal hypertension, high flow may trigger a vasoproliferative process in the pulmonary vascular bed. Patients with advanced liver disease have a low systemic vascular resistance, with a compensatory increase in cardiac output. An increase in cardiac output can lead to shear stress of the pulmonary vascular endothelial layer. Although the resistance of the pulmonary vasculature may decrease rapidly to help normalize pulmonary pressures, persistent circulatory overload could result in irreversible vascular changes. Autopsy and lung explant studies show that POPH is characterized by obstructive and remodeling changes in the pulmonary arterial bed.24 Initially, medial hypertrophy with smooth muscle proliferation is present. As the disease advances, platelet aggregates, in situ thrombosis, and intimal fibrosis develop. Finally, web-like lesions involving the entire pulmonary wall develop with recanalization for the passage of pulmonary arterial flow. These changes are identical to the changes observed in patients with other forms of PAH.
Not all patients with portal hypertension develop POPH, suggesting that genetic predisposition may play a role in POPH development. The Pulmonary Vascular Complications of Liver Study Group published a multicenter case-control study that attempted to identify genetic risk factors for POPH in patients with advanced liver disease.25 More than 1000 common single nucleotide polymorphisms (SNPs) in 93 candidate genes were genotyped in each patient. When compared to controls, multiple SNPs in the genes coding for estrogen receptor 1, aromatase, phosphodiesterase 5, angiopoietin 1, and calcium binding protein A4 were associated with an increased risk of POPH. One year earlier, the same study group concluded that female sex (adjusted odds ratio [OR], 2.90) and autoimmune hepatitis (adjusted OR, 4.02) were associated with a higher risk for POPH, whereas hepatitis C was associated with a decreased risk.20
Clinical Presentation
Clinical presentation is variable in POPH. Patients referred to a pulmonologist will usually present with symptoms similar to patients with other forms of PAH. In a retrospective analysis of patients referred to the French Referral Center for Pulmonary Hypertension, 60% of the patients belonged to NYHA functional class III or IV.1 In a series of 78 patients with POPH, the most common presenting pulmonary symptom was dyspnea on exertion (81%), followed by syncope, chest pain, and fatigue (< 33%).16 Symptoms such as syncope and chest pain are usually markers of severe POPH.3 Stigmata of portal hypertension, such as ascites, spider angiomata, and palmar erythema, may be present on exam. An accentuated pulmonary component of the second heart sound can be seen in 82% of patients and a systolic murmur caused by tricuspid regurgitation in 69% of patients.16 Patients with severe POPH may have jugular vein distention, peripheral edema, and a third heart sound.
Diagnostic Evaluation
Chest x-rays may show prominent pulmonary arteries and cardiomegaly in patients with POPH, whereas electrocardiogram can suggest right ventricular hypertrophy and right axis deviation. The best screening test for POPH in patients with portal hypertension is echocardiography. Routine screening for POPH is recommended during liver transplant evaluation in the practice guidelines from the American Association for the Study of Liver Disease.26 Right-sided cardiac chamber enlargement and right ventricular pressure or volume overload can be assessed on echocardiography. Colle et al followed 165 patients evaluated for liver transplantation who underwent transthoracic Doppler echocardiography and right heart catheterization.27 Seventeen patients met the criteria for POPH on echocardiography (presence of tricuspid regurgitation and calculated systolic pulmonary artery pressure over 30 mm Hg) and right heart catheterization confirmed the diagnosis in 10 patients. Right ventricular systolic pressure (RVSP) estimate of ≤ 30 mm Hg on 2-dimensional echo had a 100% sensitivity and negative predictive value. Positive predictive value was poor at 59%, reiterating the need for right heart catheterization in the diagnosis of POPH. When Kim et al used a RVSP threshold of 50 mm Hg, 72% had at least moderate pulmonary hypertension, including 30% with severe pulmonary hypertension.28 Raevens et al analyzed data from 152 patients who underwent pretransplant echocardiography and catheterization.2 Their data show a RVSP threshold of greater than 38 mm Hg by echocardiography had a specificity of 82% and sensitivity and negative predictive value of 100%. The European Respiratory Society recommendations state that PAH should be considered unlikely if echocardiography estimates a RVSP ≤36 mm Hg and likely if the RVSP is estimated at > 50 mm Hg.29 We recommend repeating echocardiography every 6 to 12 months in patients listed for liver transplantation, as pulmonary hemodynamics may change over time.
Computed tomography (CT) may have a complementary role in the future for the noninvasive detection of POPH. In a study published in 2014, 49 patients referred for liver transplantation were retrospectively reviewed.30 Measured CT signs included the main pulmonary artery/ascending aorta diameter ratio, the mean left and right main pulmonary artery diameter, and the enlargement of the pulmonary artery compared to the ascending aorta. Compared to the transthoracic echocardiography alone, an algorithm incorporating CT and echocardiography improved the detection of POPH (area under curve = 0.8, P < 0.0001).
A diagnosis of POPH can only be confirmed when PAH exists in a patient with portal hypertension, as determined by right heart catheterization, and no other cause of PAH can be identified. MPAP should be 25 mm Hg or greater, PVR of 240 dynes/s/cm–5, wedge pressure of 15 mm Hg or less, and TPG greater than 12 mm Hg. Krowka et al showed the value of right heart catheterization in their 10-year prospective, echocardiography-catheterization algorithm study.19 Of 1235 liver transplant candidates who underwent echocardiography, 104 patients had a RVSP exceeding 50 mm Hg. Almost all of these patients had a right heart catheterization. All cause pulmonary hypertension (MPAP > 25 mm Hg) was confirmed in 90% of the patients, and 35% had a PVR < 240 dynes/s/cm–5 and pulmonary capillary wedge pressure (PCWP) > 15 mm Hg, suggesting fluid overload. Forty-one patients had significant POPH, with a PVR > 400 dynes/s/cm–5, and 24% also had an elevated PCWP. TPG was > 12 mm Hg in all of these patients, confirming POPH. As demonstrated by this study, right heart catheterization is required to confirm the diagnosis of POPH because high flow and fluid overload can lead to elevated pulmonary artery pressures.
Patients with POPH have a unique clinical profile with characteristics common to patients with primary pulmonary hypertension and chronic liver disease. In a retrospective review that compared 30 patients with PAH, 30 patients with chronic liver disease only, and 30 patients with catheterization-proved POPH,31 patients with POPH had elevated MPAP similar to those with primary PAH, but they also had reduced SVR and elevated cardiac index similar to those with chronic liver disease alone.
Besides POPH, 2 other common causes can lead to increased pulmonary arterial blood flow in patients with portal hypertension. First is a high-flow condition caused by increased cardiac output but with a normal PVR and PCWP. Fluid overload can also lead to pulmonary venous hypertension with increased PCWP, normal cardiac output, and normal PVR. Up to 25% of patients with POPH may present with marked excess volume caused by fluid retention.3 There can be an increase in both PCWP and PVR depending on the presence and the degree of fluid retention. TPG (MPAP – PCWP) > 12 mm Hg was introduced to make such patients less confusing and to help correct for increased PCWP secondary to fluid overload. Obstruction to pulmonary arterial flow is manifest by an increased TPG (Table 2).
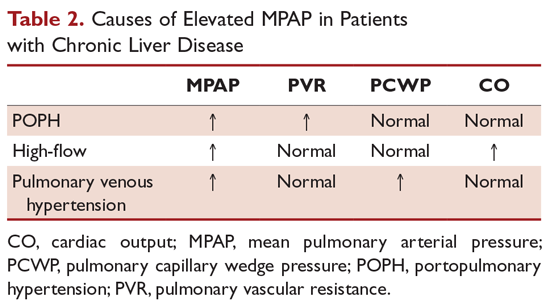
POPH should be distinguished from hepatopulmonary syndrome (HPS), which is another pulmonary vascular consequence of liver disease. Unlike POPH, HPS is characterized by a defect in arterial oxygenation induced by pulmonary vascular dilation.32 Similar to other patients with liver disease, patients with HPS have a normal PVR and increased cardiac output secondary to a high-flow state. HPS is diagnosed by confirmation of an intrapulmonary shunt by echocardiogram. Injection of agitated saline results in saline bubbles being visualized in the left atrium 3 or more cardiac cycles after they appear in the right atrium. Currently, there is no effective medical treatment for HPS and liver transplantation is the only successful treatment.
Conclusion
POPH is an uncommon complication of chronic liver disease. It is defined as PAH in a patient with portal hypertension excluding other causes of PAH. The following criteria must be met to make a diagnosis of POPH: (1) evidence of portal hypertension; (2) MPAP ≥ 35 mm Hg; (3) PVR ≥ 240 dynes/s/cm5; (4) pulmonary capillary wedge pressure ≤ 15 mm Hg; and (5) TPG > 12 mm Hg. Individuals with POPH have worse outcomes compared to other forms of PAH, with a median survival of 6 months without medical therapy. The pathogenesis of POPH is unclear but may be related to a genetic predisposition since not all patients with portal hypertension develop POPH. Echocardiography is an excellent screening test for POPH, but a right heart catheterization must be performed to confirm the diagnosis.
1. Le Pavec J, Souza R, Herve P, et al. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med. 2008;178:637-643.
2. Raevens S, Colle I, Reyntjens K, et al. Echocardiography for the detection of portopulmonary hypertension in liver transplant candidates: An analysis of cutoff values. Liver Transplant. 2013;19:602-610.
3. Krowka MJ. Portopulmonary hypertension. Semin Respir Crit Care Med. 2012;33:17-25.
4. Mantz F. Portal axis thrombosis with spontaneous portocaval shunt and resultant cor pulmonale. AMA Arch Pathol. 1951;52:91-97.
5. Yoshida EM, Erb SR, Pflugfelder PW, et al. Single-lung versus liver transplantation for the treatment of portopulmonary hypertension--a comparison of two patients. Transplantation. 1993;55:688-690.
6. Badesch DB, Champion HC, Gomez Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54 54(1 Suppl):S55-66.
7. Cartin-Ceba R, Krowka MJ. Portopulmonary hypertension. Clin Liver Dis. 2014;18:421-438.
8. Ramsay M, Simpson BR, Nguyen T, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3:494-500.
9. Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6:443-450.
10. McDonnell P, Toye P, Hutchins G. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis. 1983;127:437-441.
11. Rich S, Dantzker D, Ayres S, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216-223.
12. Groves B. Pulmonary Hypertension Associated with Cirrhosis. Philadelphia: University of Pennsylvania Press; 1990.
13. Habib G, Gressin V, Yaici A, et al. Pulmonary arterial hypertension in France results from a national registry. Am J Respir Crit Care Med. 2006;173:1023-1030.
14. Hadengue A, Benhayoun M, Lebrec D, Benhamou J. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology. 1991;100:520-528.
15. Krowka MJ, Miller DP, Barst RJ, et al. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest. 2012;141:906-915.
16. Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol. 1991;17:492-498.
17. Kawut SM, Taichman DB, Ahya VN, et al. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transpl. 2005;11:1107-1111.
18. Swanson KL, Wiesner RH, Nyberg SL, et al. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445-2453.
19. Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: Results from a 10-year screening algorithm. Hepatology. 2006;44:1502-1510.
20. Kawut SM, Krowka MJ, Trotter JF, et al. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48:196-203.
21. Lebrec D, Capron JP, Dhumeaux D, Benhamou JP. pulmonary hypertension complicating portal hypertension. Am J Rev Resp Dis. 1979;120:849-856.
22. Pellicelli AM, Barbaro G, Puoti C, et al. Plasma cytokines and portopulmonary hypertension in patients with cirrhosis waiting for orthotopic liver transplantation. Angiology. 2010;61:802-806.
23. Kamath PS, Carpenter HA, Lloyd RV, et al. Hepatic localization of endothelin-1 in patients with idiopathic portal hypertension and cirrhosis of the liver. Liver Transpl. 2000;6:596-602.
24. Krowka MJ, Edwards WD. A spectrum of pulmonary vascular pathology in portopulmonary hypertension. Liver Transpl. 2000;6:241-242.
25. Roberts KE, Fallon MB, Krowka MJ, et al. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care Med. 2009;179:835-842.
26. Murray KF, Carithers RL. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407-1432.
27. Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology. 2003;37:401-409.
28. Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transpl. 2000;6:453-458.
29. Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263.
30. Devaraj A, Loveridge R, Bosanac D, et al. Portopulmonary hypertension: Improved detection using CT and echocardiography in combination. Eur Radiol. 2014;24:2385-2393.
31. Kuo P, Plotkin J, Johnson L, et al. Distinctive clinical features of portopulmonary hypertension. Chest. 1997;112:980-986.
32. Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378-2387.
Pulmonary arterial hypertension (PAH) is a rare disease that is associated with high mortality and is characterized by pulmonary vascular remodeling. Portopulmonary hypertension (POPH) is a form of PAH that occurs in patients with portal hypertension where no alternative cause of PAH can be identified. POPH is documented in approximately 4.5% to 8.5% of liver transplant candidates,1,2 but there is no relationship between the existence or severity of POPH and the severity of liver dysfunction.3 Mantz and Craig described the first case of POPH in a 53-year-old woman with enlarged pulmonary arteries that exhibited forceful pulsations more characteristic of the aorta than a low-pressure pulmonary trunk.4 Autopsy revealed findings of chronic liver disease including a stenotic portal vein, portocaval shunt, and esophageal varices. In both PAH and POPH, pre-capillary pulmonary arteries have characteristic lesions, such as intimal thickening, endothelial proliferation, and thrombotic changes. This 2-part article reviews the diagnosis and treatment of patients with POPH. Here, we review the epidemiology, prognosis, pathogenesis, and diagnosis of POPH; current treatment options for POPH are reviewed in a separate article.
Definition
The term POPH was first used by Yoshida et al in 1993 to describe the first successful liver transplant in a patient with POPH, a 39-year-old man with chronic hepatitis.5 The World Health Organization (WHO) classifies POPH as a form of Group 1 PAH.6 The criteria that must be met to make a diagnosis of POPH are shown in the Table 1.7

Moderate POPH is defined as a mean pulmonary artery pressure (MPAP) between 35 mm Hg and < 45 mm Hg, whereas severe POPH is MPAP ≥ 45 mm Hg. Moderate and severe POPH are considered contraindications to liver transplant because of high perioperative and postoperative mortality rates.8 In 2000, the Mayo Clinic retrospectively reviewed 43 patients with POPH who underwent attempted liver transplantation.9 The cardiopulmonary-related mortality rate in patients with a MPAP of 35 to < 50 mm Hg was 50% and 100% for those with MPAP > 50 mm Hg. No mortality was noted in patients with a pre-liver transplant MPAP of < 35 mm Hg and transpulmonary gradient (TPG) < 15 mm Hg.
Epidemiology
In 1983, a series of 17,901 autopsied patients showed a primary pulmonary hypertension prevalence of 0.13% and a prevalence of 0.73% in patients with cirrhosis.10 In 1987, Rich et al published data from the National Institutes of Health’s national registry of primary pulmonary hypertension.11 The registry included data from 187 patients from 32 centers. Further analyses by Groves et al concluded that 8.3% of the patients likely had POPH.12 Humbert et al published data on the French pulmonary hypertension registry experience in 2006.13 The French registry included 674 patients from 17 university hospitals; 10.4% of these patients had POPH. The largest prospective study was published by Hadengue et al in 1991.14 In this study, 507 patients hospitalized with portal hypertension but without known pulmonary hypertension underwent cardiac catheterization; 10 patients (2%) had pulmonary hypertension and more than half were clinically asymptomatic. Finally, the Registry to Evaluate Early And Long-term pulmonary arterial hypertension disease management (REVEAL registry) documented a 5.3% frequency of POPH (174 of 3525) in the United States.15
Prognosis
Individuals with POPH have worse outcomes compared to other forms of PAH. Median survival prior to the introduction of vasodilator therapy was a dismal 6 months and mean survival was 15 months.16 The cause of death in patients with POPH is equally distributed between right heart failure from POPH and direct complications of chronic liver disease.1 Le Pavec et al retrospectively analyzed all patients referred to the French Referral Center with POPH between 1984 and 2004 (154 patients).1 Approximately 50% of the patients were Child-Turcotte-Pugh class B or C, and 60% were classified as New York Health Association (NYHA) class III or IV. In these patients, 1-, 3-, and 5-year survival rates were 88%, 75%, and 68%, respectively. Major independent prognostic risk factors were presence and severity of cirrhosis and preservation of right ventricular function. Interestingly, NYHA functional class was not related to survival in this study, although it has clearly been identified as a strong prognostic factor in idiopathic PAH.
Krowka et al evaluated 174 patients with POPH enrolled in the REVEAL Registry,15 a multicenter, observational, US-based study comprised of more than 3500 patients with PAH. Despite having better hemodynamic parameters at diagnosis, patients with POPH had significantly poorer survival and all-cause hospitalization compared with patients with idiopathic PAH (IPAH) or hereditary PAH (HPAH). Two-year survival from enrollment was 67% in POPH versus 85% in those with IPAH/HPAH (P < 0.001). Five-year survival from time of diagnosis was 40% versus 64% (P < 0.001). Additionally, patients with POPH were less likely to be on PAH-specific therapy at enrollment, with only 25% on treatment at the time of entry. These findings were replicated in 2005 when Kawut et al retrospectively compared 13 patients with POPH with 33 patients with IPAH.17 Despite having a higher cardiac index and lower pulmonary vascular resistance than patients with IPAH, patients with POPH had a higher risk of death (hazard ratio, 2.8, P = 0.04), likely reflecting the combination of 2 serious diseases.
In 2008 the Mayo Clinic published their retrospective analysis of patients with POPH to determine the natural history of POPH.18 Patients were categorized into 3 groups: (1) no medical therapy for POPH and no liver transplant; (2) medical therapy for POPH alone; (3) medical therapy for POPH followed by liver transplant. The study included 74 patients between 1994 through 2007; 19 patients who did not receive treatment for POPH or liver transplant truly represented the natural history of POPH. Their 5-year survival was only 14%, and over half were deceased 1 year after diagnosis. The largest group consisted of patients who received therapy for POPH but no liver transplant. This group did remarkably better than those who received no therapy at all, with a 5-year survival of 45%. However, the patients with the overall best survival were those who received a combination of treatment for POPH followed by liver transplant. Their 5-year survival was 67%. Survival at 5 years was only 25% for the small group of patients who received transplant without PAH therapy. Once again, mortality did not correlate with the severity of hepatic dysfunction or baseline hemodynamic data.
Pathogenesis
The pathogenesis of POPH is unclear. Multiple studies have shown that there is minimal, if any, association with pulmonary hypertension and the severity of liver disease or portal hypertension.19,20 Portal hypertension is the result of an increase in intrahepatic resistance and an increase in blood flow into the portal circulation. Collateral vessels develop and blood from the splanchnic circulation is allowed directly into the systemic venous circulation, bypassing the liver. One of the most widely accepted theories is that a humoral substance, that would otherwise be metabolized by the liver, is able to reach the pulmonary circulation through collaterals, resulting in POPH.21 Pelicelli et al evaluated the possible role of endothelin-1, interleukin-6, interleukin 1β, and tumor necrosis factor in the pathogenesis of POPH.22 Plasma concentrations of these cytokines were compared between patients with POPH and patients with cirrhosis but no POPH. Patients with POPH had higher concentrations of endothelin-1 and interleukin-6, suggesting antagonists for these cytokines may have a role in the treatment of POPH. The role of endothelin-1 was further supported by Kamath et al in 200023 when they determined the pulmonary vascular bed is exposed to increased levels of circulating endothelin-1a in the setting of cirrhosis. Endothelin-1 is a potent vasoconstrictor and facilitator of smooth muscle proliferation.
In addition to collateral circulation allowing mediators to reach the pulmonary arterial bed in portal hypertension, high flow may trigger a vasoproliferative process in the pulmonary vascular bed. Patients with advanced liver disease have a low systemic vascular resistance, with a compensatory increase in cardiac output. An increase in cardiac output can lead to shear stress of the pulmonary vascular endothelial layer. Although the resistance of the pulmonary vasculature may decrease rapidly to help normalize pulmonary pressures, persistent circulatory overload could result in irreversible vascular changes. Autopsy and lung explant studies show that POPH is characterized by obstructive and remodeling changes in the pulmonary arterial bed.24 Initially, medial hypertrophy with smooth muscle proliferation is present. As the disease advances, platelet aggregates, in situ thrombosis, and intimal fibrosis develop. Finally, web-like lesions involving the entire pulmonary wall develop with recanalization for the passage of pulmonary arterial flow. These changes are identical to the changes observed in patients with other forms of PAH.
Not all patients with portal hypertension develop POPH, suggesting that genetic predisposition may play a role in POPH development. The Pulmonary Vascular Complications of Liver Study Group published a multicenter case-control study that attempted to identify genetic risk factors for POPH in patients with advanced liver disease.25 More than 1000 common single nucleotide polymorphisms (SNPs) in 93 candidate genes were genotyped in each patient. When compared to controls, multiple SNPs in the genes coding for estrogen receptor 1, aromatase, phosphodiesterase 5, angiopoietin 1, and calcium binding protein A4 were associated with an increased risk of POPH. One year earlier, the same study group concluded that female sex (adjusted odds ratio [OR], 2.90) and autoimmune hepatitis (adjusted OR, 4.02) were associated with a higher risk for POPH, whereas hepatitis C was associated with a decreased risk.20
Clinical Presentation
Clinical presentation is variable in POPH. Patients referred to a pulmonologist will usually present with symptoms similar to patients with other forms of PAH. In a retrospective analysis of patients referred to the French Referral Center for Pulmonary Hypertension, 60% of the patients belonged to NYHA functional class III or IV.1 In a series of 78 patients with POPH, the most common presenting pulmonary symptom was dyspnea on exertion (81%), followed by syncope, chest pain, and fatigue (< 33%).16 Symptoms such as syncope and chest pain are usually markers of severe POPH.3 Stigmata of portal hypertension, such as ascites, spider angiomata, and palmar erythema, may be present on exam. An accentuated pulmonary component of the second heart sound can be seen in 82% of patients and a systolic murmur caused by tricuspid regurgitation in 69% of patients.16 Patients with severe POPH may have jugular vein distention, peripheral edema, and a third heart sound.
Diagnostic Evaluation
Chest x-rays may show prominent pulmonary arteries and cardiomegaly in patients with POPH, whereas electrocardiogram can suggest right ventricular hypertrophy and right axis deviation. The best screening test for POPH in patients with portal hypertension is echocardiography. Routine screening for POPH is recommended during liver transplant evaluation in the practice guidelines from the American Association for the Study of Liver Disease.26 Right-sided cardiac chamber enlargement and right ventricular pressure or volume overload can be assessed on echocardiography. Colle et al followed 165 patients evaluated for liver transplantation who underwent transthoracic Doppler echocardiography and right heart catheterization.27 Seventeen patients met the criteria for POPH on echocardiography (presence of tricuspid regurgitation and calculated systolic pulmonary artery pressure over 30 mm Hg) and right heart catheterization confirmed the diagnosis in 10 patients. Right ventricular systolic pressure (RVSP) estimate of ≤ 30 mm Hg on 2-dimensional echo had a 100% sensitivity and negative predictive value. Positive predictive value was poor at 59%, reiterating the need for right heart catheterization in the diagnosis of POPH. When Kim et al used a RVSP threshold of 50 mm Hg, 72% had at least moderate pulmonary hypertension, including 30% with severe pulmonary hypertension.28 Raevens et al analyzed data from 152 patients who underwent pretransplant echocardiography and catheterization.2 Their data show a RVSP threshold of greater than 38 mm Hg by echocardiography had a specificity of 82% and sensitivity and negative predictive value of 100%. The European Respiratory Society recommendations state that PAH should be considered unlikely if echocardiography estimates a RVSP ≤36 mm Hg and likely if the RVSP is estimated at > 50 mm Hg.29 We recommend repeating echocardiography every 6 to 12 months in patients listed for liver transplantation, as pulmonary hemodynamics may change over time.
Computed tomography (CT) may have a complementary role in the future for the noninvasive detection of POPH. In a study published in 2014, 49 patients referred for liver transplantation were retrospectively reviewed.30 Measured CT signs included the main pulmonary artery/ascending aorta diameter ratio, the mean left and right main pulmonary artery diameter, and the enlargement of the pulmonary artery compared to the ascending aorta. Compared to the transthoracic echocardiography alone, an algorithm incorporating CT and echocardiography improved the detection of POPH (area under curve = 0.8, P < 0.0001).
A diagnosis of POPH can only be confirmed when PAH exists in a patient with portal hypertension, as determined by right heart catheterization, and no other cause of PAH can be identified. MPAP should be 25 mm Hg or greater, PVR of 240 dynes/s/cm–5, wedge pressure of 15 mm Hg or less, and TPG greater than 12 mm Hg. Krowka et al showed the value of right heart catheterization in their 10-year prospective, echocardiography-catheterization algorithm study.19 Of 1235 liver transplant candidates who underwent echocardiography, 104 patients had a RVSP exceeding 50 mm Hg. Almost all of these patients had a right heart catheterization. All cause pulmonary hypertension (MPAP > 25 mm Hg) was confirmed in 90% of the patients, and 35% had a PVR < 240 dynes/s/cm–5 and pulmonary capillary wedge pressure (PCWP) > 15 mm Hg, suggesting fluid overload. Forty-one patients had significant POPH, with a PVR > 400 dynes/s/cm–5, and 24% also had an elevated PCWP. TPG was > 12 mm Hg in all of these patients, confirming POPH. As demonstrated by this study, right heart catheterization is required to confirm the diagnosis of POPH because high flow and fluid overload can lead to elevated pulmonary artery pressures.
Patients with POPH have a unique clinical profile with characteristics common to patients with primary pulmonary hypertension and chronic liver disease. In a retrospective review that compared 30 patients with PAH, 30 patients with chronic liver disease only, and 30 patients with catheterization-proved POPH,31 patients with POPH had elevated MPAP similar to those with primary PAH, but they also had reduced SVR and elevated cardiac index similar to those with chronic liver disease alone.
Besides POPH, 2 other common causes can lead to increased pulmonary arterial blood flow in patients with portal hypertension. First is a high-flow condition caused by increased cardiac output but with a normal PVR and PCWP. Fluid overload can also lead to pulmonary venous hypertension with increased PCWP, normal cardiac output, and normal PVR. Up to 25% of patients with POPH may present with marked excess volume caused by fluid retention.3 There can be an increase in both PCWP and PVR depending on the presence and the degree of fluid retention. TPG (MPAP – PCWP) > 12 mm Hg was introduced to make such patients less confusing and to help correct for increased PCWP secondary to fluid overload. Obstruction to pulmonary arterial flow is manifest by an increased TPG (Table 2).

POPH should be distinguished from hepatopulmonary syndrome (HPS), which is another pulmonary vascular consequence of liver disease. Unlike POPH, HPS is characterized by a defect in arterial oxygenation induced by pulmonary vascular dilation.32 Similar to other patients with liver disease, patients with HPS have a normal PVR and increased cardiac output secondary to a high-flow state. HPS is diagnosed by confirmation of an intrapulmonary shunt by echocardiogram. Injection of agitated saline results in saline bubbles being visualized in the left atrium 3 or more cardiac cycles after they appear in the right atrium. Currently, there is no effective medical treatment for HPS and liver transplantation is the only successful treatment.
Conclusion
POPH is an uncommon complication of chronic liver disease. It is defined as PAH in a patient with portal hypertension excluding other causes of PAH. The following criteria must be met to make a diagnosis of POPH: (1) evidence of portal hypertension; (2) MPAP ≥ 35 mm Hg; (3) PVR ≥ 240 dynes/s/cm5; (4) pulmonary capillary wedge pressure ≤ 15 mm Hg; and (5) TPG > 12 mm Hg. Individuals with POPH have worse outcomes compared to other forms of PAH, with a median survival of 6 months without medical therapy. The pathogenesis of POPH is unclear but may be related to a genetic predisposition since not all patients with portal hypertension develop POPH. Echocardiography is an excellent screening test for POPH, but a right heart catheterization must be performed to confirm the diagnosis.
Pulmonary arterial hypertension (PAH) is a rare disease that is associated with high mortality and is characterized by pulmonary vascular remodeling. Portopulmonary hypertension (POPH) is a form of PAH that occurs in patients with portal hypertension where no alternative cause of PAH can be identified. POPH is documented in approximately 4.5% to 8.5% of liver transplant candidates,1,2 but there is no relationship between the existence or severity of POPH and the severity of liver dysfunction.3 Mantz and Craig described the first case of POPH in a 53-year-old woman with enlarged pulmonary arteries that exhibited forceful pulsations more characteristic of the aorta than a low-pressure pulmonary trunk.4 Autopsy revealed findings of chronic liver disease including a stenotic portal vein, portocaval shunt, and esophageal varices. In both PAH and POPH, pre-capillary pulmonary arteries have characteristic lesions, such as intimal thickening, endothelial proliferation, and thrombotic changes. This 2-part article reviews the diagnosis and treatment of patients with POPH. Here, we review the epidemiology, prognosis, pathogenesis, and diagnosis of POPH; current treatment options for POPH are reviewed in a separate article.
Definition
The term POPH was first used by Yoshida et al in 1993 to describe the first successful liver transplant in a patient with POPH, a 39-year-old man with chronic hepatitis.5 The World Health Organization (WHO) classifies POPH as a form of Group 1 PAH.6 The criteria that must be met to make a diagnosis of POPH are shown in the Table 1.7

Moderate POPH is defined as a mean pulmonary artery pressure (MPAP) between 35 mm Hg and < 45 mm Hg, whereas severe POPH is MPAP ≥ 45 mm Hg. Moderate and severe POPH are considered contraindications to liver transplant because of high perioperative and postoperative mortality rates.8 In 2000, the Mayo Clinic retrospectively reviewed 43 patients with POPH who underwent attempted liver transplantation.9 The cardiopulmonary-related mortality rate in patients with a MPAP of 35 to < 50 mm Hg was 50% and 100% for those with MPAP > 50 mm Hg. No mortality was noted in patients with a pre-liver transplant MPAP of < 35 mm Hg and transpulmonary gradient (TPG) < 15 mm Hg.
Epidemiology
In 1983, a series of 17,901 autopsied patients showed a primary pulmonary hypertension prevalence of 0.13% and a prevalence of 0.73% in patients with cirrhosis.10 In 1987, Rich et al published data from the National Institutes of Health’s national registry of primary pulmonary hypertension.11 The registry included data from 187 patients from 32 centers. Further analyses by Groves et al concluded that 8.3% of the patients likely had POPH.12 Humbert et al published data on the French pulmonary hypertension registry experience in 2006.13 The French registry included 674 patients from 17 university hospitals; 10.4% of these patients had POPH. The largest prospective study was published by Hadengue et al in 1991.14 In this study, 507 patients hospitalized with portal hypertension but without known pulmonary hypertension underwent cardiac catheterization; 10 patients (2%) had pulmonary hypertension and more than half were clinically asymptomatic. Finally, the Registry to Evaluate Early And Long-term pulmonary arterial hypertension disease management (REVEAL registry) documented a 5.3% frequency of POPH (174 of 3525) in the United States.15
Prognosis
Individuals with POPH have worse outcomes compared to other forms of PAH. Median survival prior to the introduction of vasodilator therapy was a dismal 6 months and mean survival was 15 months.16 The cause of death in patients with POPH is equally distributed between right heart failure from POPH and direct complications of chronic liver disease.1 Le Pavec et al retrospectively analyzed all patients referred to the French Referral Center with POPH between 1984 and 2004 (154 patients).1 Approximately 50% of the patients were Child-Turcotte-Pugh class B or C, and 60% were classified as New York Health Association (NYHA) class III or IV. In these patients, 1-, 3-, and 5-year survival rates were 88%, 75%, and 68%, respectively. Major independent prognostic risk factors were presence and severity of cirrhosis and preservation of right ventricular function. Interestingly, NYHA functional class was not related to survival in this study, although it has clearly been identified as a strong prognostic factor in idiopathic PAH.
Krowka et al evaluated 174 patients with POPH enrolled in the REVEAL Registry,15 a multicenter, observational, US-based study comprised of more than 3500 patients with PAH. Despite having better hemodynamic parameters at diagnosis, patients with POPH had significantly poorer survival and all-cause hospitalization compared with patients with idiopathic PAH (IPAH) or hereditary PAH (HPAH). Two-year survival from enrollment was 67% in POPH versus 85% in those with IPAH/HPAH (P < 0.001). Five-year survival from time of diagnosis was 40% versus 64% (P < 0.001). Additionally, patients with POPH were less likely to be on PAH-specific therapy at enrollment, with only 25% on treatment at the time of entry. These findings were replicated in 2005 when Kawut et al retrospectively compared 13 patients with POPH with 33 patients with IPAH.17 Despite having a higher cardiac index and lower pulmonary vascular resistance than patients with IPAH, patients with POPH had a higher risk of death (hazard ratio, 2.8, P = 0.04), likely reflecting the combination of 2 serious diseases.
In 2008 the Mayo Clinic published their retrospective analysis of patients with POPH to determine the natural history of POPH.18 Patients were categorized into 3 groups: (1) no medical therapy for POPH and no liver transplant; (2) medical therapy for POPH alone; (3) medical therapy for POPH followed by liver transplant. The study included 74 patients between 1994 through 2007; 19 patients who did not receive treatment for POPH or liver transplant truly represented the natural history of POPH. Their 5-year survival was only 14%, and over half were deceased 1 year after diagnosis. The largest group consisted of patients who received therapy for POPH but no liver transplant. This group did remarkably better than those who received no therapy at all, with a 5-year survival of 45%. However, the patients with the overall best survival were those who received a combination of treatment for POPH followed by liver transplant. Their 5-year survival was 67%. Survival at 5 years was only 25% for the small group of patients who received transplant without PAH therapy. Once again, mortality did not correlate with the severity of hepatic dysfunction or baseline hemodynamic data.
Pathogenesis
The pathogenesis of POPH is unclear. Multiple studies have shown that there is minimal, if any, association with pulmonary hypertension and the severity of liver disease or portal hypertension.19,20 Portal hypertension is the result of an increase in intrahepatic resistance and an increase in blood flow into the portal circulation. Collateral vessels develop and blood from the splanchnic circulation is allowed directly into the systemic venous circulation, bypassing the liver. One of the most widely accepted theories is that a humoral substance, that would otherwise be metabolized by the liver, is able to reach the pulmonary circulation through collaterals, resulting in POPH.21 Pelicelli et al evaluated the possible role of endothelin-1, interleukin-6, interleukin 1β, and tumor necrosis factor in the pathogenesis of POPH.22 Plasma concentrations of these cytokines were compared between patients with POPH and patients with cirrhosis but no POPH. Patients with POPH had higher concentrations of endothelin-1 and interleukin-6, suggesting antagonists for these cytokines may have a role in the treatment of POPH. The role of endothelin-1 was further supported by Kamath et al in 200023 when they determined the pulmonary vascular bed is exposed to increased levels of circulating endothelin-1a in the setting of cirrhosis. Endothelin-1 is a potent vasoconstrictor and facilitator of smooth muscle proliferation.
In addition to collateral circulation allowing mediators to reach the pulmonary arterial bed in portal hypertension, high flow may trigger a vasoproliferative process in the pulmonary vascular bed. Patients with advanced liver disease have a low systemic vascular resistance, with a compensatory increase in cardiac output. An increase in cardiac output can lead to shear stress of the pulmonary vascular endothelial layer. Although the resistance of the pulmonary vasculature may decrease rapidly to help normalize pulmonary pressures, persistent circulatory overload could result in irreversible vascular changes. Autopsy and lung explant studies show that POPH is characterized by obstructive and remodeling changes in the pulmonary arterial bed.24 Initially, medial hypertrophy with smooth muscle proliferation is present. As the disease advances, platelet aggregates, in situ thrombosis, and intimal fibrosis develop. Finally, web-like lesions involving the entire pulmonary wall develop with recanalization for the passage of pulmonary arterial flow. These changes are identical to the changes observed in patients with other forms of PAH.
Not all patients with portal hypertension develop POPH, suggesting that genetic predisposition may play a role in POPH development. The Pulmonary Vascular Complications of Liver Study Group published a multicenter case-control study that attempted to identify genetic risk factors for POPH in patients with advanced liver disease.25 More than 1000 common single nucleotide polymorphisms (SNPs) in 93 candidate genes were genotyped in each patient. When compared to controls, multiple SNPs in the genes coding for estrogen receptor 1, aromatase, phosphodiesterase 5, angiopoietin 1, and calcium binding protein A4 were associated with an increased risk of POPH. One year earlier, the same study group concluded that female sex (adjusted odds ratio [OR], 2.90) and autoimmune hepatitis (adjusted OR, 4.02) were associated with a higher risk for POPH, whereas hepatitis C was associated with a decreased risk.20
Clinical Presentation
Clinical presentation is variable in POPH. Patients referred to a pulmonologist will usually present with symptoms similar to patients with other forms of PAH. In a retrospective analysis of patients referred to the French Referral Center for Pulmonary Hypertension, 60% of the patients belonged to NYHA functional class III or IV.1 In a series of 78 patients with POPH, the most common presenting pulmonary symptom was dyspnea on exertion (81%), followed by syncope, chest pain, and fatigue (< 33%).16 Symptoms such as syncope and chest pain are usually markers of severe POPH.3 Stigmata of portal hypertension, such as ascites, spider angiomata, and palmar erythema, may be present on exam. An accentuated pulmonary component of the second heart sound can be seen in 82% of patients and a systolic murmur caused by tricuspid regurgitation in 69% of patients.16 Patients with severe POPH may have jugular vein distention, peripheral edema, and a third heart sound.
Diagnostic Evaluation
Chest x-rays may show prominent pulmonary arteries and cardiomegaly in patients with POPH, whereas electrocardiogram can suggest right ventricular hypertrophy and right axis deviation. The best screening test for POPH in patients with portal hypertension is echocardiography. Routine screening for POPH is recommended during liver transplant evaluation in the practice guidelines from the American Association for the Study of Liver Disease.26 Right-sided cardiac chamber enlargement and right ventricular pressure or volume overload can be assessed on echocardiography. Colle et al followed 165 patients evaluated for liver transplantation who underwent transthoracic Doppler echocardiography and right heart catheterization.27 Seventeen patients met the criteria for POPH on echocardiography (presence of tricuspid regurgitation and calculated systolic pulmonary artery pressure over 30 mm Hg) and right heart catheterization confirmed the diagnosis in 10 patients. Right ventricular systolic pressure (RVSP) estimate of ≤ 30 mm Hg on 2-dimensional echo had a 100% sensitivity and negative predictive value. Positive predictive value was poor at 59%, reiterating the need for right heart catheterization in the diagnosis of POPH. When Kim et al used a RVSP threshold of 50 mm Hg, 72% had at least moderate pulmonary hypertension, including 30% with severe pulmonary hypertension.28 Raevens et al analyzed data from 152 patients who underwent pretransplant echocardiography and catheterization.2 Their data show a RVSP threshold of greater than 38 mm Hg by echocardiography had a specificity of 82% and sensitivity and negative predictive value of 100%. The European Respiratory Society recommendations state that PAH should be considered unlikely if echocardiography estimates a RVSP ≤36 mm Hg and likely if the RVSP is estimated at > 50 mm Hg.29 We recommend repeating echocardiography every 6 to 12 months in patients listed for liver transplantation, as pulmonary hemodynamics may change over time.
Computed tomography (CT) may have a complementary role in the future for the noninvasive detection of POPH. In a study published in 2014, 49 patients referred for liver transplantation were retrospectively reviewed.30 Measured CT signs included the main pulmonary artery/ascending aorta diameter ratio, the mean left and right main pulmonary artery diameter, and the enlargement of the pulmonary artery compared to the ascending aorta. Compared to the transthoracic echocardiography alone, an algorithm incorporating CT and echocardiography improved the detection of POPH (area under curve = 0.8, P < 0.0001).
A diagnosis of POPH can only be confirmed when PAH exists in a patient with portal hypertension, as determined by right heart catheterization, and no other cause of PAH can be identified. MPAP should be 25 mm Hg or greater, PVR of 240 dynes/s/cm–5, wedge pressure of 15 mm Hg or less, and TPG greater than 12 mm Hg. Krowka et al showed the value of right heart catheterization in their 10-year prospective, echocardiography-catheterization algorithm study.19 Of 1235 liver transplant candidates who underwent echocardiography, 104 patients had a RVSP exceeding 50 mm Hg. Almost all of these patients had a right heart catheterization. All cause pulmonary hypertension (MPAP > 25 mm Hg) was confirmed in 90% of the patients, and 35% had a PVR < 240 dynes/s/cm–5 and pulmonary capillary wedge pressure (PCWP) > 15 mm Hg, suggesting fluid overload. Forty-one patients had significant POPH, with a PVR > 400 dynes/s/cm–5, and 24% also had an elevated PCWP. TPG was > 12 mm Hg in all of these patients, confirming POPH. As demonstrated by this study, right heart catheterization is required to confirm the diagnosis of POPH because high flow and fluid overload can lead to elevated pulmonary artery pressures.
Patients with POPH have a unique clinical profile with characteristics common to patients with primary pulmonary hypertension and chronic liver disease. In a retrospective review that compared 30 patients with PAH, 30 patients with chronic liver disease only, and 30 patients with catheterization-proved POPH,31 patients with POPH had elevated MPAP similar to those with primary PAH, but they also had reduced SVR and elevated cardiac index similar to those with chronic liver disease alone.
Besides POPH, 2 other common causes can lead to increased pulmonary arterial blood flow in patients with portal hypertension. First is a high-flow condition caused by increased cardiac output but with a normal PVR and PCWP. Fluid overload can also lead to pulmonary venous hypertension with increased PCWP, normal cardiac output, and normal PVR. Up to 25% of patients with POPH may present with marked excess volume caused by fluid retention.3 There can be an increase in both PCWP and PVR depending on the presence and the degree of fluid retention. TPG (MPAP – PCWP) > 12 mm Hg was introduced to make such patients less confusing and to help correct for increased PCWP secondary to fluid overload. Obstruction to pulmonary arterial flow is manifest by an increased TPG (Table 2).

POPH should be distinguished from hepatopulmonary syndrome (HPS), which is another pulmonary vascular consequence of liver disease. Unlike POPH, HPS is characterized by a defect in arterial oxygenation induced by pulmonary vascular dilation.32 Similar to other patients with liver disease, patients with HPS have a normal PVR and increased cardiac output secondary to a high-flow state. HPS is diagnosed by confirmation of an intrapulmonary shunt by echocardiogram. Injection of agitated saline results in saline bubbles being visualized in the left atrium 3 or more cardiac cycles after they appear in the right atrium. Currently, there is no effective medical treatment for HPS and liver transplantation is the only successful treatment.
Conclusion
POPH is an uncommon complication of chronic liver disease. It is defined as PAH in a patient with portal hypertension excluding other causes of PAH. The following criteria must be met to make a diagnosis of POPH: (1) evidence of portal hypertension; (2) MPAP ≥ 35 mm Hg; (3) PVR ≥ 240 dynes/s/cm5; (4) pulmonary capillary wedge pressure ≤ 15 mm Hg; and (5) TPG > 12 mm Hg. Individuals with POPH have worse outcomes compared to other forms of PAH, with a median survival of 6 months without medical therapy. The pathogenesis of POPH is unclear but may be related to a genetic predisposition since not all patients with portal hypertension develop POPH. Echocardiography is an excellent screening test for POPH, but a right heart catheterization must be performed to confirm the diagnosis.
1. Le Pavec J, Souza R, Herve P, et al. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med. 2008;178:637-643.
2. Raevens S, Colle I, Reyntjens K, et al. Echocardiography for the detection of portopulmonary hypertension in liver transplant candidates: An analysis of cutoff values. Liver Transplant. 2013;19:602-610.
3. Krowka MJ. Portopulmonary hypertension. Semin Respir Crit Care Med. 2012;33:17-25.
4. Mantz F. Portal axis thrombosis with spontaneous portocaval shunt and resultant cor pulmonale. AMA Arch Pathol. 1951;52:91-97.
5. Yoshida EM, Erb SR, Pflugfelder PW, et al. Single-lung versus liver transplantation for the treatment of portopulmonary hypertension--a comparison of two patients. Transplantation. 1993;55:688-690.
6. Badesch DB, Champion HC, Gomez Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54 54(1 Suppl):S55-66.
7. Cartin-Ceba R, Krowka MJ. Portopulmonary hypertension. Clin Liver Dis. 2014;18:421-438.
8. Ramsay M, Simpson BR, Nguyen T, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3:494-500.
9. Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6:443-450.
10. McDonnell P, Toye P, Hutchins G. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis. 1983;127:437-441.
11. Rich S, Dantzker D, Ayres S, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216-223.
12. Groves B. Pulmonary Hypertension Associated with Cirrhosis. Philadelphia: University of Pennsylvania Press; 1990.
13. Habib G, Gressin V, Yaici A, et al. Pulmonary arterial hypertension in France results from a national registry. Am J Respir Crit Care Med. 2006;173:1023-1030.
14. Hadengue A, Benhayoun M, Lebrec D, Benhamou J. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology. 1991;100:520-528.
15. Krowka MJ, Miller DP, Barst RJ, et al. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest. 2012;141:906-915.
16. Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol. 1991;17:492-498.
17. Kawut SM, Taichman DB, Ahya VN, et al. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transpl. 2005;11:1107-1111.
18. Swanson KL, Wiesner RH, Nyberg SL, et al. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445-2453.
19. Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: Results from a 10-year screening algorithm. Hepatology. 2006;44:1502-1510.
20. Kawut SM, Krowka MJ, Trotter JF, et al. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48:196-203.
21. Lebrec D, Capron JP, Dhumeaux D, Benhamou JP. pulmonary hypertension complicating portal hypertension. Am J Rev Resp Dis. 1979;120:849-856.
22. Pellicelli AM, Barbaro G, Puoti C, et al. Plasma cytokines and portopulmonary hypertension in patients with cirrhosis waiting for orthotopic liver transplantation. Angiology. 2010;61:802-806.
23. Kamath PS, Carpenter HA, Lloyd RV, et al. Hepatic localization of endothelin-1 in patients with idiopathic portal hypertension and cirrhosis of the liver. Liver Transpl. 2000;6:596-602.
24. Krowka MJ, Edwards WD. A spectrum of pulmonary vascular pathology in portopulmonary hypertension. Liver Transpl. 2000;6:241-242.
25. Roberts KE, Fallon MB, Krowka MJ, et al. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care Med. 2009;179:835-842.
26. Murray KF, Carithers RL. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407-1432.
27. Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology. 2003;37:401-409.
28. Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transpl. 2000;6:453-458.
29. Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263.
30. Devaraj A, Loveridge R, Bosanac D, et al. Portopulmonary hypertension: Improved detection using CT and echocardiography in combination. Eur Radiol. 2014;24:2385-2393.
31. Kuo P, Plotkin J, Johnson L, et al. Distinctive clinical features of portopulmonary hypertension. Chest. 1997;112:980-986.
32. Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378-2387.
1. Le Pavec J, Souza R, Herve P, et al. Portopulmonary hypertension: survival and prognostic factors. Am J Respir Crit Care Med. 2008;178:637-643.
2. Raevens S, Colle I, Reyntjens K, et al. Echocardiography for the detection of portopulmonary hypertension in liver transplant candidates: An analysis of cutoff values. Liver Transplant. 2013;19:602-610.
3. Krowka MJ. Portopulmonary hypertension. Semin Respir Crit Care Med. 2012;33:17-25.
4. Mantz F. Portal axis thrombosis with spontaneous portocaval shunt and resultant cor pulmonale. AMA Arch Pathol. 1951;52:91-97.
5. Yoshida EM, Erb SR, Pflugfelder PW, et al. Single-lung versus liver transplantation for the treatment of portopulmonary hypertension--a comparison of two patients. Transplantation. 1993;55:688-690.
6. Badesch DB, Champion HC, Gomez Sanchez MA, et al. Diagnosis and assessment of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54 54(1 Suppl):S55-66.
7. Cartin-Ceba R, Krowka MJ. Portopulmonary hypertension. Clin Liver Dis. 2014;18:421-438.
8. Ramsay M, Simpson BR, Nguyen T, et al. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3:494-500.
9. Krowka MJ, Plevak DJ, Findlay JY, et al. Pulmonary hemodynamics and perioperative cardiopulmonary-related mortality in patients with portopulmonary hypertension undergoing liver transplantation. Liver Transpl. 2000;6:443-450.
10. McDonnell P, Toye P, Hutchins G. Primary pulmonary hypertension and cirrhosis: are they related? Am Rev Respir Dis. 1983;127:437-441.
11. Rich S, Dantzker D, Ayres S, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med. 1987;107:216-223.
12. Groves B. Pulmonary Hypertension Associated with Cirrhosis. Philadelphia: University of Pennsylvania Press; 1990.
13. Habib G, Gressin V, Yaici A, et al. Pulmonary arterial hypertension in France results from a national registry. Am J Respir Crit Care Med. 2006;173:1023-1030.
14. Hadengue A, Benhayoun M, Lebrec D, Benhamou J. Pulmonary hypertension complicating portal hypertension: prevalence and relation to splanchnic hemodynamics. Gastroenterology. 1991;100:520-528.
15. Krowka MJ, Miller DP, Barst RJ, et al. Portopulmonary hypertension: a report from the US-based REVEAL Registry. Chest. 2012;141:906-915.
16. Robalino BD, Moodie DS. Association between primary pulmonary hypertension and portal hypertension: analysis of its pathophysiology and clinical, laboratory and hemodynamic manifestations. J Am Coll Cardiol. 1991;17:492-498.
17. Kawut SM, Taichman DB, Ahya VN, et al. Hemodynamics and survival of patients with portopulmonary hypertension. Liver Transpl. 2005;11:1107-1111.
18. Swanson KL, Wiesner RH, Nyberg SL, et al. Survival in portopulmonary hypertension: Mayo Clinic experience categorized by treatment subgroups. Am J Transplant. 2008;8:2445-2453.
19. Krowka MJ, Swanson KL, Frantz RP, et al. Portopulmonary hypertension: Results from a 10-year screening algorithm. Hepatology. 2006;44:1502-1510.
20. Kawut SM, Krowka MJ, Trotter JF, et al. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48:196-203.
21. Lebrec D, Capron JP, Dhumeaux D, Benhamou JP. pulmonary hypertension complicating portal hypertension. Am J Rev Resp Dis. 1979;120:849-856.
22. Pellicelli AM, Barbaro G, Puoti C, et al. Plasma cytokines and portopulmonary hypertension in patients with cirrhosis waiting for orthotopic liver transplantation. Angiology. 2010;61:802-806.
23. Kamath PS, Carpenter HA, Lloyd RV, et al. Hepatic localization of endothelin-1 in patients with idiopathic portal hypertension and cirrhosis of the liver. Liver Transpl. 2000;6:596-602.
24. Krowka MJ, Edwards WD. A spectrum of pulmonary vascular pathology in portopulmonary hypertension. Liver Transpl. 2000;6:241-242.
25. Roberts KE, Fallon MB, Krowka MJ, et al. Genetic risk factors for portopulmonary hypertension in patients with advanced liver disease. Am J Respir Crit Care Med. 2009;179:835-842.
26. Murray KF, Carithers RL. AASLD practice guidelines: Evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407-1432.
27. Colle IO, Moreau R, Godinho E, et al. Diagnosis of portopulmonary hypertension in candidates for liver transplantation: a prospective study. Hepatology. 2003;37:401-409.
28. Kim WR, Krowka MJ, Plevak DJ, et al. Accuracy of Doppler echocardiography in the assessment of pulmonary hypertension in liver transplant candidates. Liver Transpl. 2000;6:453-458.
29. Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219-1263.
30. Devaraj A, Loveridge R, Bosanac D, et al. Portopulmonary hypertension: Improved detection using CT and echocardiography in combination. Eur Radiol. 2014;24:2385-2393.
31. Kuo P, Plotkin J, Johnson L, et al. Distinctive clinical features of portopulmonary hypertension. Chest. 1997;112:980-986.
32. Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378-2387.