User login
The European Medicines Agency’s (EMA) Pharmacovigilance and Risk Assessment Committee (PRAC) has recommended suspending marketing authorizations for 4 linear gadolinium contrast agents because of evidence that small amounts of the gadolinium they contain are deposited in the brain.
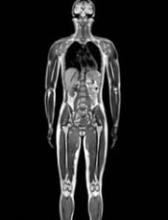
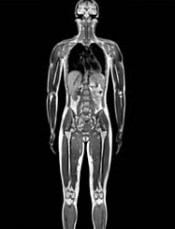
The agents concerned are intravenous injections of gadobenic acid, gadodiamide, gadopentetic acid, and gadoversetamide, which are given to patients to enhance images from magnetic resonance imaging (MRI) body scans.
The PRAC’s review of gadolinium agents found “convincing evidence” of accumulation of gadolinium in the brain from studies directly measuring gadolinium in brain tissues and areas of increased signal intensity seen on MRI scan images many months after the last injection of a gadolinium contrast agent.
Although no symptoms or diseases linked to gadolinium in the brain have been reported, the PRAC took a precautionary approach, noting that data on the long-term effects in the brain are limited.
Companies concerned by this review have the right to request that the PRAC re-examine its recommendations.
The PRAC’s recommendations will be sent to the Committee for Medicinal Products for Human Use (CHMP) for its opinion. Further details will be published at the time of the CHMP opinion.
The final stage of the review procedure is the adoption by the European Commission of a legally binding decision applicable in all European Union member states.
Details of the review, recommendations
The PRAC’s review was initiated on March 17, 2016, at the request of the European Commission, under Article 31 of Directive 2001/83/EC.
The review covers agents containing the following active substances: gadobenic acid, gadobutrol, gadodiamide, gadopentetic acid, gadoteric acid, gadoteridol, gadoversetamide, and gadoxetic acid.
The 4 agents recommended for suspension (gadobenic acid, gadodiamide, gadopentetic acid, and gadoversetamide) are linear agents. They have a structure more likely to release gadolinium, which can build up in body tissues.
Other agents, known as macrocyclic agents, are more stable and have a much lower propensity to release gadolinium.
The PRAC recommends that macrocyclic agents (gadobutrol, gadoteric acid, and gadoteridol) be used at the lowest dose that enhances images sufficiently to make diagnoses and only when unenhanced body scans are not suitable.
The PRAC has recommended that some linear agents remain available. The committee said that gadoxetic acid, a linear agent used at a low dose for liver scans, can remain on the market as it meets an important diagnostic need in patients with few alternatives.
In addition, a formulation of gadopentetic acid injected directly into joints should remain available because its gadolinium concentration is very low—around 200 times lower than those of intravenous products.
Both agents should be used at the lowest dose that enhances images sufficiently to make diagnoses and only if unenhanced scans are not suitable.
For those marketing authorizations recommended for suspension, the suspensions can be lifted if the respective companies provide evidence of new benefits in an identified patient group that outweigh its risks or show that their product (modified or not) does not release gadolinium significantly or lead to its retention in tissues. ![]()
The European Medicines Agency’s (EMA) Pharmacovigilance and Risk Assessment Committee (PRAC) has recommended suspending marketing authorizations for 4 linear gadolinium contrast agents because of evidence that small amounts of the gadolinium they contain are deposited in the brain.
The agents concerned are intravenous injections of gadobenic acid, gadodiamide, gadopentetic acid, and gadoversetamide, which are given to patients to enhance images from magnetic resonance imaging (MRI) body scans.
The PRAC’s review of gadolinium agents found “convincing evidence” of accumulation of gadolinium in the brain from studies directly measuring gadolinium in brain tissues and areas of increased signal intensity seen on MRI scan images many months after the last injection of a gadolinium contrast agent.
Although no symptoms or diseases linked to gadolinium in the brain have been reported, the PRAC took a precautionary approach, noting that data on the long-term effects in the brain are limited.
Companies concerned by this review have the right to request that the PRAC re-examine its recommendations.
The PRAC’s recommendations will be sent to the Committee for Medicinal Products for Human Use (CHMP) for its opinion. Further details will be published at the time of the CHMP opinion.
The final stage of the review procedure is the adoption by the European Commission of a legally binding decision applicable in all European Union member states.
Details of the review, recommendations
The PRAC’s review was initiated on March 17, 2016, at the request of the European Commission, under Article 31 of Directive 2001/83/EC.
The review covers agents containing the following active substances: gadobenic acid, gadobutrol, gadodiamide, gadopentetic acid, gadoteric acid, gadoteridol, gadoversetamide, and gadoxetic acid.
The 4 agents recommended for suspension (gadobenic acid, gadodiamide, gadopentetic acid, and gadoversetamide) are linear agents. They have a structure more likely to release gadolinium, which can build up in body tissues.
Other agents, known as macrocyclic agents, are more stable and have a much lower propensity to release gadolinium.
The PRAC recommends that macrocyclic agents (gadobutrol, gadoteric acid, and gadoteridol) be used at the lowest dose that enhances images sufficiently to make diagnoses and only when unenhanced body scans are not suitable.
The PRAC has recommended that some linear agents remain available. The committee said that gadoxetic acid, a linear agent used at a low dose for liver scans, can remain on the market as it meets an important diagnostic need in patients with few alternatives.
In addition, a formulation of gadopentetic acid injected directly into joints should remain available because its gadolinium concentration is very low—around 200 times lower than those of intravenous products.
Both agents should be used at the lowest dose that enhances images sufficiently to make diagnoses and only if unenhanced scans are not suitable.
For those marketing authorizations recommended for suspension, the suspensions can be lifted if the respective companies provide evidence of new benefits in an identified patient group that outweigh its risks or show that their product (modified or not) does not release gadolinium significantly or lead to its retention in tissues. ![]()
The European Medicines Agency’s (EMA) Pharmacovigilance and Risk Assessment Committee (PRAC) has recommended suspending marketing authorizations for 4 linear gadolinium contrast agents because of evidence that small amounts of the gadolinium they contain are deposited in the brain.
The agents concerned are intravenous injections of gadobenic acid, gadodiamide, gadopentetic acid, and gadoversetamide, which are given to patients to enhance images from magnetic resonance imaging (MRI) body scans.
The PRAC’s review of gadolinium agents found “convincing evidence” of accumulation of gadolinium in the brain from studies directly measuring gadolinium in brain tissues and areas of increased signal intensity seen on MRI scan images many months after the last injection of a gadolinium contrast agent.
Although no symptoms or diseases linked to gadolinium in the brain have been reported, the PRAC took a precautionary approach, noting that data on the long-term effects in the brain are limited.
Companies concerned by this review have the right to request that the PRAC re-examine its recommendations.
The PRAC’s recommendations will be sent to the Committee for Medicinal Products for Human Use (CHMP) for its opinion. Further details will be published at the time of the CHMP opinion.
The final stage of the review procedure is the adoption by the European Commission of a legally binding decision applicable in all European Union member states.
Details of the review, recommendations
The PRAC’s review was initiated on March 17, 2016, at the request of the European Commission, under Article 31 of Directive 2001/83/EC.
The review covers agents containing the following active substances: gadobenic acid, gadobutrol, gadodiamide, gadopentetic acid, gadoteric acid, gadoteridol, gadoversetamide, and gadoxetic acid.
The 4 agents recommended for suspension (gadobenic acid, gadodiamide, gadopentetic acid, and gadoversetamide) are linear agents. They have a structure more likely to release gadolinium, which can build up in body tissues.
Other agents, known as macrocyclic agents, are more stable and have a much lower propensity to release gadolinium.
The PRAC recommends that macrocyclic agents (gadobutrol, gadoteric acid, and gadoteridol) be used at the lowest dose that enhances images sufficiently to make diagnoses and only when unenhanced body scans are not suitable.
The PRAC has recommended that some linear agents remain available. The committee said that gadoxetic acid, a linear agent used at a low dose for liver scans, can remain on the market as it meets an important diagnostic need in patients with few alternatives.
In addition, a formulation of gadopentetic acid injected directly into joints should remain available because its gadolinium concentration is very low—around 200 times lower than those of intravenous products.
Both agents should be used at the lowest dose that enhances images sufficiently to make diagnoses and only if unenhanced scans are not suitable.
For those marketing authorizations recommended for suspension, the suspensions can be lifted if the respective companies provide evidence of new benefits in an identified patient group that outweigh its risks or show that their product (modified or not) does not release gadolinium significantly or lead to its retention in tissues. ![]()