User login
From the Division of Gastroenterology University of Washington, Seattle, WA (Dr. Tiderington), and the Division of Gastroenterology, Hepatology and Nutrition, The Ohio State University Wexner Medical Center and The Ohio State University Inflammatory Bowel Disease Center, Columbus, OH (Dr. Afzali).
Abstract
- Objective: To provide a practical approach to the management of patients with inflammatory bowel disease (IBD) following tumor necrosis factor (TNF) alpha antagonist failure.
- Methods: Review of the literature.
- Results: TNF alpha antagonists play a central role in the treatment of IBD. Unfortunately, some patients will not respond to therapy with TNF antagonists, and others will lose response during treatment. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. In this paper we review the mechanisms of drug failure, the use of reactive therapeutic drug monitoring to guide clinical decision making, and propose an evidence-based method for managing this common clinical scenario.
- Conclusion: Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Key words: TNF antagonists; therapeutic drug monitoring; biologic failure; Crohn’s disease treatment; ulcerative colitis treatment.
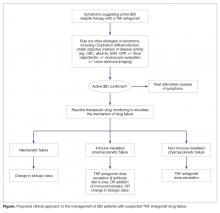
Ulcerative colitis and Crohn’s disease are the two types of inflammatory bowel disease (IBD), and they are characterized by chronic, immunologically mediated inflammation involving the gastrointestinal tract [1]. Guided by an understanding of the role of tumor necrosis factor (TNF) alpha in the pathogenesis of IBD, TNF antagonists have played a central role in modern treatment algorithms [2]. Unfortunately, roughly one third of patients will not have a clinical response when given induction dosing of the currently available anti-TNF agents, and of those who do respond to treatment, up to one half will lose response to treatment within the first year [3]. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. Once the clinician has confirmed that active IBD is present based on endoscopic, cross-sectional imaging and/or biochemical markers of inflammation, the next step is to identify the cause of the treatment failure, as this guides management. Here we review the body of literature that guides our understanding of treatment failure as well as therapeutic drug monitoring and propose an evidence-based algorithm for managing this common clinical scenario.
Defining Treatment Failure
Patients who receive anti-TNF therapy but demonstrate active IBD should be classified as having either primary nonresponse or secondary loss of response. Primary nonresponse is defined as having either no response, or only partial response, to induction with anti-TNF therapy [4]. Data from pivotal trials and meta-analyses suggest that about one third of patients will not show any clinical response to induction with anti-TNF therapies, with response typically being defined using composite endpoints favoring clinical symptoms and only sometimes incorporating endoscopic findings [5]. An additional one third of patients will have only a partial response, without remission. Patients with ulcerative colitis are at a slightly increased risk of primary nonresponse compared to patients with Crohn’s disease. Though the time frame for defining primary nonresponse is different for each agent because each agent has a slightly different induction schedule, in general the maximal response to therapy is typically seen by week 12, and it is reasonable to use this as a time cutoff [6].
Secondary loss of response is likewise defined as recrudescence of clinically active disease after an initial response. In general, the presence of secondary loss of response should not be invoked until week 12 of therapy. In most pivotal trials, secondary loss of response was seen in roughly half of patients at 1 year. In clinical practice, however, particularly as therapeutic drug monitoring has become more common, the observed rates of secondary loss of response have been lower [6].
Applying these definitions appropriately is important because it dictates the next steps in management. When a patient presents with symptoms suggesting active IBD while on anti-TNF therapy, either during induction when primary nonresponse is possible, or in maintenance when secondary loss of response would be invoked, the first step is to determine if active IBD is the etiology for the presenting symptoms. The initial evaluation should rule out common infectious causes of symptoms mimicking IBD. In particular, Clostridium difficile infection should be ruled out with stool testing. In certain circumstances, ruling out cytomegalovirus (CMV) colitis is important, so an endoscopic evaluation with colonic biopsies should be considered. In the absence of infectious colitis, the presence of active inflammation can often be identified endoscopically, or can be inferred from noninvasive markers with a fair degree of certainty. Fecal calprotectin is an ideal choice for this purpose. In ulcerative colitis it is estimated to have a sensitivity of 0.88 and a specificity of 0.79 for the prediction of endoscopically active disease. The estimated sensitivity for detecting endoscopically active Crohn’s disease is essentially the same (0.87), and the specificity is only slightly lower (0.67). C-reactive protein demonstrates a better specificity (0.92), but has a marginal sensitivity (0.49) [7]. Other etiologies for the patient’s symptoms should also be considered, including medication side effects including use of nonsteroidal anti-inflammatory medications, bile acid malabsorption, small intestinal bacterial overgrowth (SIBO), irritable bowel syndrome (IBS), diverticular disease, ischemic colitis, fibrostenotic strictures, and cancer, depending on comorbidities and the history of present illness.
Once it has been determined that active IBD is the etiology for the patient’s symptoms, the patient should be classified as having either primary nonresponse or secondary loss of response as described above. For the clinician, the next question is how to alter or optimize therapy.
The decision of how to optimize therapy will largely depend on which anti-TNF therapy the patient is currently receiving, and whether they are receiving it as monotherapy or as combination therapy with an immunomodulator. Optimizing therapy will involve either increasing the dose or frequency of administration of the anti-TNF therapy, increasing the dose of azathioprine if indicated, adding an immunomodulator if the patient is on anti-TNF monotherapy, changing to a different anti-TNF agent, or changing to a different class of medication with a different mechanism of action. The recently released American Gastroenterological Association (AGA) guidelines on therapeutic drug monitoring in IBD provide a framework for making these decisions [8]. In general, the clinical choice will be dictated by the etiology of the drug failure.
Types of TNF Antagonist Drug Failure
Our understanding of the causes of biologic treatment failure are evolving but are typically classified as due to mechanistic failure, non-immune-mediated pharmacokinetic failure, or immune-mediated pharmacokinetic failure [9]. Differentiating between these classes of treatment failure requires therapeutic drug monitoring (TDM), which will be discussed in more detail below.
Mechanistic failure is encountered when the underlying biology does not favor a response to a particular therapy. Studies indicate a strong association between particular genetic phenotypes and the probability of a response to induction with anti-TNF agents [10]. This suggests that some individuals have IBD driven by a biochemical inflammatory cascade in which TNF features prominently, while others have alternative mechanistic drivers of inflammation without significantly elevated TNF levels. Mechanistic failure will typically present as primary nonresponse, but can also be seen in patients with secondary loss of response. Mechanistic failure can be elucidated clinically by the use of TDM. In the case of mechanistic failure, active disease is seen in the presence of adequate drug level, without the presence of anti-drug antibodies. The AGA recommends considering switching to a biologic with a different mechanism of action when mechanistic failure is identified [8].
Non-immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to a drug at adequate drug levels experiences suboptimal drug levels because of pharmacokinetic factors. In the case of anti-TNF therapy, this can be conceptualized as either an increased clearance of anti-TNF from the body (eg, in patients with significant hypoalbuminemia or severe colitis), a reduction in the average serum anti-TNF level because of the redistribution of drug in patients with a large body mass index, or inadequate saturation of the total body burden of TNF-alpha in subjects with a high baseline level of inflammation [11]. Non-immune-mediated pharmacokinetic failure can also be identified clinically through TDM. In this case, active disease is seen in the presence of a suboptimal drug level, without the presence of anti-drug antibodies. The AGA recommends considering dose-escalation of the current TNF antagonist when non-immune-mediated pharmacokinetic failure is identified [8], as this can improve clinical response in an estimated 82% of patients [9].
Finally, immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to the current biologic therapy when at adequate drug concentration levels experiences suboptimal drug levels because of increased drug clearance mediated by anti-drug antibodies [9]. Because anti-TNF agents are monoclonal antibodies, they are inherently immunogenic, and it is well established that episodic dosing and lower serum drug concentrations are strong risk factors for the development of anti-drug antibodies [12]. When anti-drug antibodies are present, and are associated with both a decreased serum drug concentration and active inflammatory bowel disease, immune-mediate pharmacokinetic failure can be invoked. When anti-drug antibodies are present, but at a low level, the AGA recommends dose escalation of current TNF antagonist. When anti-drug antibodies are present at a high level, the AGA recommends considering either the addition of an immunomodulator (if not already being used), or changing to a different class of biologic therapy [8]. This recommendation is based in part on data showing that the proportion of patients with sustained anti-drug antibodies during the first year of therapy with an TNF antagonist is likely between 14% and 20% for those on monotherapy, but between 1% and 5% for those on concomitant immunomodulatory therapy [13,14].
Therapeutic Drug Monitoring of Anti-TNF Agents
As described above, TDM, which is the process of testing the patient’s serum for both the concentration of the TNF antagonist and for the presence and concentration of anti-drug antibodies, can help differentiate between mechanistic failure, non-immune-mediated pharmacokinetic failure, and immune-mediated pharmacokinetic failure (Table 1).
Therapeutic drug monitoring can be classified as either proactive or reactive. Proactive TDM is performed during induction or maintenance therapy when the patient does not have signs or symptoms of active disease to suggest a loss of response. Theoretically, this would allow dose modification and optimization, including dose de-escalation in certain circumstances, and could thus provide cost savings with minimal impact on clinical outcomes. The TAXIT trial provides the most robust evaluation of proactive TDM in TNF antagonist therapy. In this study, patients with Crohn’s disease or ulcerative colitis who had a stable clinical response while on maintenance infliximab were first dose optimized proactively to a target trough concentration of 3–7 μg/mL, then randomized to having dose modifications made based on clinical factors alone, defined as reactive monitoring, or dose modifications based on proactive monitoring, performed by checking the drug concentration and antibody levels before each infusion. At 1 year there was no statistically significant difference in the proportion of patients in remission. In addition, some patients in the proactive TDM group were able to have a dose reduction without a subsequent flare of disease, thus providing cost savings [15]. This study suggests that proactive TDM may have a role in drug optimization, particularly with respect to cost-effectiveness, but provides only indirect evidence of a clinical benefit, since all subjects enrolled in the study were proactively dose optimized prior to randomization. This study had a limited follow-up time of 1 year so was not able to assess for longer-term benefits and risks associated with proactive TDM.
More recently, a large, multicenter, retrospective cohort study provided additional evidence that proactive TDM may provide a clinical benefit in addition to cost savings. This study retrospectively evaluated consecutive patients receiving maintenance infliximab for Crohn’s disease between 2006 and 2015, with a median follow-up time of 2.4 years. They were classified as having had either proactive TDM or reactive TDM. Proactive TDM was associated with statistically significant reductions in the risk of treatment failure (hazard ratio [HR] 0.16, 95% confidence interval [CI] 0.09–0.27), the need for surgery (HR 0.30, 95% CI 0.11–0.80), hospitalization (HR 0.16, 95% CI 0.07–0.33), and anti-drug antibody formation (HR 0.25, 95% CI 0.07–0.84) [16].
To date, however, no randomized controlled trials have been published comparing proactive TDM to reactive TDM in treatment-naive patients. Because of the paucity of prospective studies, the AGA currently makes no recommendation regarding the use of proactive TDM in clinical practice. However, the current AGA guidelines do recommend reactive TDM in the setting of secondary loss of response based on the results of one randomized controlled trial (RCT) and several observational studies. The RCT was small (n = 69), and enrolled patients with Crohn’s disease on maintenance therapy with infliximab. Similar to the TAXIT trial, the study did not show a statistically significant difference in rates of clinical remission when subjects were randomized to either empiric dose escalation (to 5 mg/kg every 4 weeks) based on symptoms, or to dose escalations based on the results of reactive TDM. Also similar to the TAXIT trial, it showed an estimated cost savings of about 34% based on local prices in Denmark for reactive TDM over empiric dose escalation [17].
Meanwhile, the observational studies for reactive TDM provided additional support to the clinical benefit of reactive TDM, but also to the underlying hypotheses that drive reactive TDM, namely that subjects with mechanistic failure benefit from a change in drug class, those with non-immune-mediated pharmacokinetic failure benefit from dose escalation, and that those with immune-mediated pharmacokinetic failure may benefit from either dose escalation or a change in mechanism of action, depending on antibody titers. Specifically, on pooled analysis of 2 of these studies, 82% of subjects who were found to have non-immune-mediated pharmacokinetic failure responded to empiric dose escalation, whereas only 8% of subjects who were found to have immune-mediated pharmacokinetic failure with high anti-drug antibody titers responded to dose escalation [9]. Likewise, in a retrospective study involving subjects who were being treated with infliximab and then had reactive TDM performed, when non-immune-mediated pharmacokinetic failure was identified, a clinical response was seen in 86% of subjects who underwent dose escalation, and only 33% among those who were switched to a different anti-TNF (P < 0.016). Conversely, dose escalation resulted in a clinical response only 17% of the time when anti-drug antibodies were detectable, compared to a 92% response rate when the subject was switched to a different anti-TNF (P < 0.004) [18].
Interpreting the Results of Reactive Therapeutic Drug Monitoring
The implementation of reactive TDM involves obtaining a serum drug and antibody level and then interpreting what those results suggest about the mechanism of drug failure, in order to decide on a course of action. The serum drug level should be a trough concentration, so it should be obtained just prior to a timed dose, while on a stable treatment regimen. Exactly what serum drug concentration we should be targeting in reactive therapeutic drug monitoring remains an area of investigation. No RCTs have been published. There is ample observational, cross-sectional data from cohorts of patients on maintenance therapy, though heterogeneity in study design and study populations, as well as use of different assays, limit interpretation of the data. In a secondary analysis of data from 6 observational studies of patients on infliximab maintenance therapy, there was a highly statistically significant concentration-dependent trend in rates of clinical remission depending on the measured infliximab trough concentration, with 96% of those with infliximab > 7 μg/mL in remission, 92% of those with infliximab > 5 μg/mL in remission, and 75% of those with infliximab > 1 μg/mL in remission. Likewise, data from 4 studies of patients receiving adalimumab showed a statistically significant concentration-dependent trend in clinical remission, with 90% of those with adalimumab trough concentrations > 7.5 μg/mL being in clinical remission, compared with only 83% of those with concentrations > 5 μg/mL. Similarly, data from 9 studies suggested that a certolizumab trough concentration > 20 μg/mL was associated with a 75% probability of being in clinical remission, compared to a 60% probability when the trough concentration was > 10 μg/mL [9]. Based on these analyses, the AGA suggests target trough concentrations for reactive therapeutic drug monitoring of anti-TNF agents of ≥ 5 μg/mL for infliximab, ≥ 7.5 μg/mL for adalimumamb, and ≥ 20 μg/mL for certolizumab. They did not suggest a target trough concentration for golimumab because of insufficient evidence [8].
When interpreting TDM test results, it is important to know if the test you have used is drug-sensitive or drug-tolerant (Table 2). Drug-sensitive tests will be less likely to reveal the presence of anti-drug antibodies when the drug level is above a certain threshold. A post-hoc analysis of the TAXIT trial recently suggested that subjects who have antibodies detected on a drug-tolerant test which were not detected on a drug-sensitive test are more likely to respond to higher doses of infliximab [19]. It follows that there should be a threshold anti-drug antibody titer below which someone who has immune-mediated pharmacokinetic failure will still respond to TNF antagonist dose escalation, but above which they will fail to respond to dose escalation. To be sure, our understanding of the clinical implications of a drug-tolerant test demonstrating an adequate drug level while also detectable anti-drug antibodies is evolving. Complicating the issue further is the fact that anti-drug antibody concentrations cannot be compared between assays because of assay-specific characteristics. As such, though the presence of low antibody titers and high antibody titers seems to be clinically important, recommendations cannot yet be made on how to interpret specific thresholds. Furthermore, development of transient versus sustained antibodies requires further clinical investigation to determine impact and treatment algorithms.
Optimizing Therapy
Once you have determined the most likely cause of drug failure, the next step is to make a change in medical therapy.
When switching within class (to another anti-TNF agent), the choice of which agent to use next will largely depend on patient preference (route of administration, infusion versus injection), insurance, and costs of treatment. When making the decision to switch within class, it should be kept in mind that the probability of achieving remission is modestly reduced compared to the probability seen in anti-TNF-naive patients [20], and even more so when the patient is switching to their third anti-TNF agent [21]. Thus, for the patient who has already previously switched from one TNF antagonist to a second TNF antagonist, it may be better to switch to a different class of biologic rather than attempting to capture a clinical remission with a third TNF antagonist.
When adding an immunomodulator (azathioprine or methotrexate), the expectation is that the therapy will increase the serum concentration of the anti-TNF agent [14] and/or reduce the ongoing risk of anti-drug antibody formation [22]. There could also be a direct treatment effect on the bowel disease by the immunomodulator.
When switching to an alternate mechanism of action, the currently FDA-approved options include the biologic agents vedolizumab (for both moderate-to-severe ulcerative colitis and moderate-to-severe Crohn’s disease) and ustekinumab (for moderate-to-severe Crohn’s disease), as well as the recently FDA-approved oral, small-molecule JAK1 and JAK3 inhibitor tofacitinib (for moderate-to-severe ulcerative colitis). Prospective comparative effectiveness studies for these agents are lacking and are unlikely to be performed in part due to the cost and time required to accomplish these studies. A recent post-hoc analysis of clinical trials data suggests that there are no significant differences in the rates of clinical response, clinical remission, or in adverse outcomes to vedolizumab or ustekinumab when they are used in patients who have failed anti-TNF therapy [23]. Thus, one cannot be recommended over the other, and the decision of which to use is usually guided by patient preference and insurance coverage.
Meanwhile, the role of tofacitinib in the treatment algorithm of patients who have failed anti-TNF therapy remains unclear. The phase III clinical trials OCTAVE 1, OCTAVE 2, and OCTAVE Sustain showed efficacy for both the induction and maintenance of remission in patients with moderate-to-severe ulcerative colitis who had previously failed anti-TNF agents. However, there remain concerns about the safety profile of tofacitinib compared to vedolizumab and ustekinumab, particularly regarding herpes zoster infection, dyslipidemia, and adverse cardiovascular events. Notable findings from the tofacitinib induction trials include robust rates of clinical remission (18.5% vs 8.2% for placebo in Octave 1, and 16.6% vs 3.6% in Octave 2, P < 0.001 for both comparisons) and mucosal healing (31.3% vs 15.6% for placebo in Octave 1, and 28.4% and 11.6% in Octave 2, P < 0.001 for both comparisons) after 8 weeks of induction therapy [24]. These results suggest that tofacitinib, or other JAK inhibitors that become approved in the future, may be excellent oral agents for the induction of remission in moderate-to-severe ulcerative colitis, and may demonstrate a better side effect profile than steroids. Whether cost factors (compared to steroid therapy) will limit the role of JAK-inhibitor therapy in induction, and whether safety concerns will limit their use in maintenance therapy, remains to be seen.
Off-Label Rescue Therapy and Surgery
Though the armamentarium of IBD therapies has expanded greatly over the past 2 decades, and will continue to do so for the foreseeable future, there are still a limited selection of therapies available to patients. Patients who have failed anti-TNF therapy, and subsequently fail vedolizumab and/or ustekinumab, have limited options. These options include clinical trials, off-label small molecule rescue therapy, and surgery. Patients who are felt to require any of these options should be referred to a tertiary center for evaluation by a gastroenterologist specializing in the treatment of IBD and/or a colorectal surgeon specializing in the surgical management of IBD.
Tacrolimus
Tacrolimus, a macrolide calcineurin inhibitor, has been studied as a small molecule therapy for IBD, though not in randomized controlled trials. There is biological plausibility for its use as a disease modifying agent. Mucosal T cells in subjects with active Crohn’s disease have been found to express increased levels of mRNA encoding IL-2, and tacrolimus acts primarily by reducing IL-2 production [25]. The largest observational cohort evaluating the use of tacrolimus, published by Thin et al, included patients with both ulcerative colitis (n = 24) and Crohn’s disease (n = 11) who had moderate to severely active IBD. All patients had failed dose-optimized thiopurine therapy, 89% had primary nonresponse or secondary loss of response to at least one anti-TNF agent, and 74% were either steroid-refractory or steroid-dependent at the time tacrolimus was started. With close monitoring, they targeted a tacrolimus trough of 8–12 ng/mL. At 30 days, 66% had a clinical response, and 40% were in clinical remission. At 90 days, 60% had a clinical response, and 37% were in clinical remission. At 1 year, 31% had a clinical response, and 23% were in clinical remission. Of those in clinical remission at 1 year, 88% were either off of steroids or on less than 5 mg of prednisone per day. Renal impairment was seen in 25% of patients, including severe renal impairment in 11%, requiring drug cessation. Infectious complications were seen in 9% of patients. Headaches, tremor, and pancreatitis were also observed, though less commonly. The majority of patients ultimately had a surgical intervention, particularly if they were steroid-refractory at baseline, but the time to surgery was delayed in those who achieved a response or remission in the first 90 days of tacrolimus therapy. The authors suggested that while tacrolimus may lack clear long-term benefit in patients with medically refractory IBD, a therapeutic trial should be considered in select patients with the goal of medical and nutritional optimization before surgical intervention [26].
Cyclosporine
Cyclosporine, which also exerts its effect by inhibiting IL-2 production, has an established role in the management of severe ulcerative colitis. Data from randomized, placebo-controlled trials, along with numerous open label observational studies, have shown that intravenous cyclosporine can induce remission and potentially obviate the need for urgent/emergent colectomy in steroid-refractory patients who are hospitalized with severe ulcerative colitis [27,28]. Its use in maintenance therapy remains controversial, however. Older observational data suggest that even among those who have an initial clinical response to cyclosporine induction, 33% will undergo colectomy by 1 year, and 88% will undergo colectomy by 7 years [27). Though the concomitant administration of a thiopurine may delay the need for colectomy [29,30], cyclosporine seems to be, at best, a temporizing therapy for patients with severe ulcerative colitis. Studies on the use of cyclosporine for the induction of remission in Crohn’s disease have been less robust, as have studies on the use of cyclosporine for the maintenance of remission in Crohn’s disease [31]. Dose-dependent toxicity also remains a concern, particularly when being considered as maintenance therapy. Though some observational data suggest that the absolute risks of serious side effects from maintenance cyclosporine are small, cyclosporine is still generally avoided as a maintenance therapy [30].
Mycophenolate Mofetil
Mycophenolate mofetil (MMF), which inhibits both B and T cell proliferation by inhibiting de novo purine synthesis, has been studied in both Crohn’s disease and ulcerative colitis. Studies have been small, observational, and heterogeneous. On the whole, they suggest that MMF does have some efficacy, but it is not necessarily more effective than azathioprine and may have a slightly increased risk of side effects [32]. Given that the side effects of MMF include diarrhea, and an IBD-like enterocolitis (MMF-induced colitis) when given to subjects without an established diagnosis of IBD, it is likely best to avoid using the drug in patients with IBD [33].
Surgery
Finally, when medical management has failed, or when fibrostenotic and/or penetrating complications of inflammatory bowel disease are present, surgery should be considered. Surgery can provide a cure in patients with ulcerative colitis, and can induce remission in patients with Crohn’s disease. Managing IBD medications around the time of surgery is always challenging. Multiple large, retrospective cohort studies have suggested that the risk for postoperative infectious complications, anastomotic leaks, and thrombotic complications do not differ between those who receive anti-TNF therapy within several months of surgery and those who do not. Nevertheless, some surgeons may prefer to time elective surgery halfway between doses of anti-TNF therapy. Additionally, there is some data to suggest that patients who are on both thiopurines and anti-TNF agents have an increased risk of postoperative complications compared to those who are on anti-TNF agents alone [34].
After a surgical evaluation, a plan of action should be formulated in a multidisciplinary fashion to determine how medical management will proceed. For those with an established diagnosis of ulcerative colitis, medical therapy can often be stopped postoperatively and the patient can be monitored prospectively for pouch complications including possible new-onset Crohn’s disease. For those who undergo surgery for the management of Crohn’s disease, though a resection completed with negative margins does induce remission, nearly 90% can be expected to have histologic, endoscopic, or clinical recurrence by 1 year. A randomized controlled trial showed that postoperative anti-TNF therapy can reduce this risk to 9% [35]. Unfortunately, a subsequently conducted large, multicenter, randomized controlled trial comparing postoperative infliximab to placebo was terminated early because of a lack of a statistically significant difference in clinical recurrence between the 2 groups at week 74. However, this lack of demonstrated efficacy may have been obscured by the relatively mild phenotype of the enrolled participants, who had a median CDAI score of 105.5 at baseline [36]. Based on available data, the AGA does conditionally recommend postoperative anti-TNF and/or thiopurine therapy for those patients with Crohn’s disease who are in a surgically induced remission [37]. The patients who are most likely to benefit from postoperative medical therapy are those who have the highest risk of recurrence, namely those who were young at the time of diagnosis, had a short disease duration prior to surgery, have multiple sites of disease, and who use tobacco products [34].
Emerging and Future Options
Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Based on our improved, and evolving understanding of the pathogenesis of IBD, several new biochemical targets have emerged, offering novel ways to modulate the cytokine cascade which drives IBD [38]. Well over a dozen phase II and phase III trials for IBD therapeutic agents are ongoing, including biologic agents targeting interleukin-23, β7-Integrin, and MAdCAM-1, as well as small molecule agents targeting the JAK/STAT pathway and the sphingosine-1-phosphate receptor modulators [39]. As new agents are approved, it may be possible to develop a more patient-centered approach to care by targeting therapies to the particular pathogenesis of each patient’s disease. Nevertheless, integrating these therapies into practice algorithms will remain a challenge in the absence of meaningful comparative effectiveness trials [40].
Conclusion
When evaluating a patient who seems to have failed anti-TNF therapy for IBD, the first step is to confirm that active inflammatory disease is present. This process includes ruling out other potential causes of the patient’s symptoms, including infectious colitis, and ideally includes obtaining objective evidence of inflammation, whether through non-invasive biomarkers, an endoscopic evaluation and/or cross-sectional imaging. Once active IBD is confirmed, reactive therapeutic drug monitoring can help elucidate the likely mechanism of drug failure, which in turn can guide medical decision making.
Corresponding author: Anita Afzali MD, MPH, The Ohio State University Wexner Medical Center, 395 West 12th Ave, Room 280, Columbus, OH 43210, [email protected].
Financial disclosures: Dr. Afzali has served as a speaker/consultant for Abbvie, UCB, Takeda, Pfizer, Janssen; on the advisory board of Abbvie, UCB; received grant support from UCB; and is a board member of IBD Horizons.
1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78.
2. Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 2012;61:918–32.
3. Mitrev N, Leong RW. Therapeutic drug monitoring of anti-tumour necrosis factor-α agents in inflammatory bowel disease. Expert Opin Drug Saf 2017;16:303–17.
4. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015;21:182–97.
5. Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015;148:37–51.
6. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis 2010;4:355–66.
7. Mosli MH, Zou G, Garg SK, et al. C-Reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:802–19.
8. Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–34.
9. Vande Casteele N, Herfarth H, Katz J, et al. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology 2017;153:835–57.
10. López-Hernández R, Valdés M, Campillo JA, et al. Genetic polymorphisms of tumour necrosis factor alpha (TNF-α) promoter gene and response to TNF-α inhibitors in Spanish patients with inflammatory bowel disease. Int J Immunogenet 2014;41:63–8.
11. Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012;91:635–46.
12. Hindryckx P, Novak G, Vande Casteele N, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: a practical overview. Drugs 2017;77:363–77.
13. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95.
14. Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther 2009;30:210–26.
15. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9.
16. Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–8.
17. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27.
18. Afif W, Loftus EV Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9.
19. Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut 2017.
20. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther 2015;41:613–23.
21. Gisbert JP, Chaparro M. Use of a third anti-TNF after failure of two previous anti-TNFs in patients with inflammatory bowel disease: is it worth it? Scand J Gastroenterol 2015;50:379–86.
22. Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012;10:1079–87.
23. Kawalec P, Moćko P. An indirect comparison of ustekinumab and vedolizumab in the therapy of TNF-failure Crohn’s disease patients. J Comp Eff Res 2017;7:101–11.
24. Sandborn WJ, Su C, Panes J, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:1723–36.
25. Yarkoni S, Sagiv Y, Kaminitz A, Askenasy N. Interleukin 2 targeted therapy in inflammatory bowel disease. Gut 2009;58:1705–6.
26. Thin LW, Murray K, Lawrance IC. Oral tacrolimus for the treatment of refractory inflammatory bowel disease in the biologic era. Inflamm Bowel Dis 2013;19:1490–8.
27. Moskovitz DN, Van Assche G, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:760–5.
28. Arts J, D’Haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis 2004;10:73–8.
29. Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol 1999;94:1587–92.
30. Cheifetz AS, Stern J, Garud S, et al. Cyclosporine is safe and effective in patients with severe ulcerative colitis. J Clin Gastroenterol 2011;45:107–12.
31. Lazarev M, Present DH, Lichtiger S, et al. The effect of intravenous cyclosporine on rates of colonic surgery in hospitalized patients with severe Crohn’s colitis. J Clin Gastroenterol 2012;46:764–7.
32. Renna S, Cottone M, Orlando A. Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease. World J Gastroenterol 2014;20:9675–90.
33. Izower MA, Rahman M, Molmenti EP, et al. Correlation of abnormal histology with endoscopic findings among mycophenolate mofetil treated patients. World J Gastrointest Endosc 2017;9:405–10.
34. Ferrari L, Krane MK, Fichera A. Inflammatory bowel disease surgery in the biologic era. World J Gastrointest Surg 2016;8:363–70.
35. Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441–50.
36. Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology 2016;150:1568–78.
37. Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute technical review on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:277–95.
38. Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology 2017;152:374–88.
39. Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci 2017;38:127–42.
40. Khanna R, Feagan BG. Emerging therapies for inflammatory bowel diseases. Dig Dis 2016;34 Suppl 1:67–
From the Division of Gastroenterology University of Washington, Seattle, WA (Dr. Tiderington), and the Division of Gastroenterology, Hepatology and Nutrition, The Ohio State University Wexner Medical Center and The Ohio State University Inflammatory Bowel Disease Center, Columbus, OH (Dr. Afzali).
Abstract
- Objective: To provide a practical approach to the management of patients with inflammatory bowel disease (IBD) following tumor necrosis factor (TNF) alpha antagonist failure.
- Methods: Review of the literature.
- Results: TNF alpha antagonists play a central role in the treatment of IBD. Unfortunately, some patients will not respond to therapy with TNF antagonists, and others will lose response during treatment. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. In this paper we review the mechanisms of drug failure, the use of reactive therapeutic drug monitoring to guide clinical decision making, and propose an evidence-based method for managing this common clinical scenario.
- Conclusion: Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Key words: TNF antagonists; therapeutic drug monitoring; biologic failure; Crohn’s disease treatment; ulcerative colitis treatment.
Ulcerative colitis and Crohn’s disease are the two types of inflammatory bowel disease (IBD), and they are characterized by chronic, immunologically mediated inflammation involving the gastrointestinal tract [1]. Guided by an understanding of the role of tumor necrosis factor (TNF) alpha in the pathogenesis of IBD, TNF antagonists have played a central role in modern treatment algorithms [2]. Unfortunately, roughly one third of patients will not have a clinical response when given induction dosing of the currently available anti-TNF agents, and of those who do respond to treatment, up to one half will lose response to treatment within the first year [3]. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. Once the clinician has confirmed that active IBD is present based on endoscopic, cross-sectional imaging and/or biochemical markers of inflammation, the next step is to identify the cause of the treatment failure, as this guides management. Here we review the body of literature that guides our understanding of treatment failure as well as therapeutic drug monitoring and propose an evidence-based algorithm for managing this common clinical scenario.
Defining Treatment Failure
Patients who receive anti-TNF therapy but demonstrate active IBD should be classified as having either primary nonresponse or secondary loss of response. Primary nonresponse is defined as having either no response, or only partial response, to induction with anti-TNF therapy [4]. Data from pivotal trials and meta-analyses suggest that about one third of patients will not show any clinical response to induction with anti-TNF therapies, with response typically being defined using composite endpoints favoring clinical symptoms and only sometimes incorporating endoscopic findings [5]. An additional one third of patients will have only a partial response, without remission. Patients with ulcerative colitis are at a slightly increased risk of primary nonresponse compared to patients with Crohn’s disease. Though the time frame for defining primary nonresponse is different for each agent because each agent has a slightly different induction schedule, in general the maximal response to therapy is typically seen by week 12, and it is reasonable to use this as a time cutoff [6].
Secondary loss of response is likewise defined as recrudescence of clinically active disease after an initial response. In general, the presence of secondary loss of response should not be invoked until week 12 of therapy. In most pivotal trials, secondary loss of response was seen in roughly half of patients at 1 year. In clinical practice, however, particularly as therapeutic drug monitoring has become more common, the observed rates of secondary loss of response have been lower [6].
Applying these definitions appropriately is important because it dictates the next steps in management. When a patient presents with symptoms suggesting active IBD while on anti-TNF therapy, either during induction when primary nonresponse is possible, or in maintenance when secondary loss of response would be invoked, the first step is to determine if active IBD is the etiology for the presenting symptoms. The initial evaluation should rule out common infectious causes of symptoms mimicking IBD. In particular, Clostridium difficile infection should be ruled out with stool testing. In certain circumstances, ruling out cytomegalovirus (CMV) colitis is important, so an endoscopic evaluation with colonic biopsies should be considered. In the absence of infectious colitis, the presence of active inflammation can often be identified endoscopically, or can be inferred from noninvasive markers with a fair degree of certainty. Fecal calprotectin is an ideal choice for this purpose. In ulcerative colitis it is estimated to have a sensitivity of 0.88 and a specificity of 0.79 for the prediction of endoscopically active disease. The estimated sensitivity for detecting endoscopically active Crohn’s disease is essentially the same (0.87), and the specificity is only slightly lower (0.67). C-reactive protein demonstrates a better specificity (0.92), but has a marginal sensitivity (0.49) [7]. Other etiologies for the patient’s symptoms should also be considered, including medication side effects including use of nonsteroidal anti-inflammatory medications, bile acid malabsorption, small intestinal bacterial overgrowth (SIBO), irritable bowel syndrome (IBS), diverticular disease, ischemic colitis, fibrostenotic strictures, and cancer, depending on comorbidities and the history of present illness.
Once it has been determined that active IBD is the etiology for the patient’s symptoms, the patient should be classified as having either primary nonresponse or secondary loss of response as described above. For the clinician, the next question is how to alter or optimize therapy.
The decision of how to optimize therapy will largely depend on which anti-TNF therapy the patient is currently receiving, and whether they are receiving it as monotherapy or as combination therapy with an immunomodulator. Optimizing therapy will involve either increasing the dose or frequency of administration of the anti-TNF therapy, increasing the dose of azathioprine if indicated, adding an immunomodulator if the patient is on anti-TNF monotherapy, changing to a different anti-TNF agent, or changing to a different class of medication with a different mechanism of action. The recently released American Gastroenterological Association (AGA) guidelines on therapeutic drug monitoring in IBD provide a framework for making these decisions [8]. In general, the clinical choice will be dictated by the etiology of the drug failure.
Types of TNF Antagonist Drug Failure
Our understanding of the causes of biologic treatment failure are evolving but are typically classified as due to mechanistic failure, non-immune-mediated pharmacokinetic failure, or immune-mediated pharmacokinetic failure [9]. Differentiating between these classes of treatment failure requires therapeutic drug monitoring (TDM), which will be discussed in more detail below.
Mechanistic failure is encountered when the underlying biology does not favor a response to a particular therapy. Studies indicate a strong association between particular genetic phenotypes and the probability of a response to induction with anti-TNF agents [10]. This suggests that some individuals have IBD driven by a biochemical inflammatory cascade in which TNF features prominently, while others have alternative mechanistic drivers of inflammation without significantly elevated TNF levels. Mechanistic failure will typically present as primary nonresponse, but can also be seen in patients with secondary loss of response. Mechanistic failure can be elucidated clinically by the use of TDM. In the case of mechanistic failure, active disease is seen in the presence of adequate drug level, without the presence of anti-drug antibodies. The AGA recommends considering switching to a biologic with a different mechanism of action when mechanistic failure is identified [8].
Non-immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to a drug at adequate drug levels experiences suboptimal drug levels because of pharmacokinetic factors. In the case of anti-TNF therapy, this can be conceptualized as either an increased clearance of anti-TNF from the body (eg, in patients with significant hypoalbuminemia or severe colitis), a reduction in the average serum anti-TNF level because of the redistribution of drug in patients with a large body mass index, or inadequate saturation of the total body burden of TNF-alpha in subjects with a high baseline level of inflammation [11]. Non-immune-mediated pharmacokinetic failure can also be identified clinically through TDM. In this case, active disease is seen in the presence of a suboptimal drug level, without the presence of anti-drug antibodies. The AGA recommends considering dose-escalation of the current TNF antagonist when non-immune-mediated pharmacokinetic failure is identified [8], as this can improve clinical response in an estimated 82% of patients [9].
Finally, immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to the current biologic therapy when at adequate drug concentration levels experiences suboptimal drug levels because of increased drug clearance mediated by anti-drug antibodies [9]. Because anti-TNF agents are monoclonal antibodies, they are inherently immunogenic, and it is well established that episodic dosing and lower serum drug concentrations are strong risk factors for the development of anti-drug antibodies [12]. When anti-drug antibodies are present, and are associated with both a decreased serum drug concentration and active inflammatory bowel disease, immune-mediate pharmacokinetic failure can be invoked. When anti-drug antibodies are present, but at a low level, the AGA recommends dose escalation of current TNF antagonist. When anti-drug antibodies are present at a high level, the AGA recommends considering either the addition of an immunomodulator (if not already being used), or changing to a different class of biologic therapy [8]. This recommendation is based in part on data showing that the proportion of patients with sustained anti-drug antibodies during the first year of therapy with an TNF antagonist is likely between 14% and 20% for those on monotherapy, but between 1% and 5% for those on concomitant immunomodulatory therapy [13,14].
Therapeutic Drug Monitoring of Anti-TNF Agents
As described above, TDM, which is the process of testing the patient’s serum for both the concentration of the TNF antagonist and for the presence and concentration of anti-drug antibodies, can help differentiate between mechanistic failure, non-immune-mediated pharmacokinetic failure, and immune-mediated pharmacokinetic failure (Table 1).
Therapeutic drug monitoring can be classified as either proactive or reactive. Proactive TDM is performed during induction or maintenance therapy when the patient does not have signs or symptoms of active disease to suggest a loss of response. Theoretically, this would allow dose modification and optimization, including dose de-escalation in certain circumstances, and could thus provide cost savings with minimal impact on clinical outcomes. The TAXIT trial provides the most robust evaluation of proactive TDM in TNF antagonist therapy. In this study, patients with Crohn’s disease or ulcerative colitis who had a stable clinical response while on maintenance infliximab were first dose optimized proactively to a target trough concentration of 3–7 μg/mL, then randomized to having dose modifications made based on clinical factors alone, defined as reactive monitoring, or dose modifications based on proactive monitoring, performed by checking the drug concentration and antibody levels before each infusion. At 1 year there was no statistically significant difference in the proportion of patients in remission. In addition, some patients in the proactive TDM group were able to have a dose reduction without a subsequent flare of disease, thus providing cost savings [15]. This study suggests that proactive TDM may have a role in drug optimization, particularly with respect to cost-effectiveness, but provides only indirect evidence of a clinical benefit, since all subjects enrolled in the study were proactively dose optimized prior to randomization. This study had a limited follow-up time of 1 year so was not able to assess for longer-term benefits and risks associated with proactive TDM.
More recently, a large, multicenter, retrospective cohort study provided additional evidence that proactive TDM may provide a clinical benefit in addition to cost savings. This study retrospectively evaluated consecutive patients receiving maintenance infliximab for Crohn’s disease between 2006 and 2015, with a median follow-up time of 2.4 years. They were classified as having had either proactive TDM or reactive TDM. Proactive TDM was associated with statistically significant reductions in the risk of treatment failure (hazard ratio [HR] 0.16, 95% confidence interval [CI] 0.09–0.27), the need for surgery (HR 0.30, 95% CI 0.11–0.80), hospitalization (HR 0.16, 95% CI 0.07–0.33), and anti-drug antibody formation (HR 0.25, 95% CI 0.07–0.84) [16].
To date, however, no randomized controlled trials have been published comparing proactive TDM to reactive TDM in treatment-naive patients. Because of the paucity of prospective studies, the AGA currently makes no recommendation regarding the use of proactive TDM in clinical practice. However, the current AGA guidelines do recommend reactive TDM in the setting of secondary loss of response based on the results of one randomized controlled trial (RCT) and several observational studies. The RCT was small (n = 69), and enrolled patients with Crohn’s disease on maintenance therapy with infliximab. Similar to the TAXIT trial, the study did not show a statistically significant difference in rates of clinical remission when subjects were randomized to either empiric dose escalation (to 5 mg/kg every 4 weeks) based on symptoms, or to dose escalations based on the results of reactive TDM. Also similar to the TAXIT trial, it showed an estimated cost savings of about 34% based on local prices in Denmark for reactive TDM over empiric dose escalation [17].
Meanwhile, the observational studies for reactive TDM provided additional support to the clinical benefit of reactive TDM, but also to the underlying hypotheses that drive reactive TDM, namely that subjects with mechanistic failure benefit from a change in drug class, those with non-immune-mediated pharmacokinetic failure benefit from dose escalation, and that those with immune-mediated pharmacokinetic failure may benefit from either dose escalation or a change in mechanism of action, depending on antibody titers. Specifically, on pooled analysis of 2 of these studies, 82% of subjects who were found to have non-immune-mediated pharmacokinetic failure responded to empiric dose escalation, whereas only 8% of subjects who were found to have immune-mediated pharmacokinetic failure with high anti-drug antibody titers responded to dose escalation [9]. Likewise, in a retrospective study involving subjects who were being treated with infliximab and then had reactive TDM performed, when non-immune-mediated pharmacokinetic failure was identified, a clinical response was seen in 86% of subjects who underwent dose escalation, and only 33% among those who were switched to a different anti-TNF (P < 0.016). Conversely, dose escalation resulted in a clinical response only 17% of the time when anti-drug antibodies were detectable, compared to a 92% response rate when the subject was switched to a different anti-TNF (P < 0.004) [18].
Interpreting the Results of Reactive Therapeutic Drug Monitoring
The implementation of reactive TDM involves obtaining a serum drug and antibody level and then interpreting what those results suggest about the mechanism of drug failure, in order to decide on a course of action. The serum drug level should be a trough concentration, so it should be obtained just prior to a timed dose, while on a stable treatment regimen. Exactly what serum drug concentration we should be targeting in reactive therapeutic drug monitoring remains an area of investigation. No RCTs have been published. There is ample observational, cross-sectional data from cohorts of patients on maintenance therapy, though heterogeneity in study design and study populations, as well as use of different assays, limit interpretation of the data. In a secondary analysis of data from 6 observational studies of patients on infliximab maintenance therapy, there was a highly statistically significant concentration-dependent trend in rates of clinical remission depending on the measured infliximab trough concentration, with 96% of those with infliximab > 7 μg/mL in remission, 92% of those with infliximab > 5 μg/mL in remission, and 75% of those with infliximab > 1 μg/mL in remission. Likewise, data from 4 studies of patients receiving adalimumab showed a statistically significant concentration-dependent trend in clinical remission, with 90% of those with adalimumab trough concentrations > 7.5 μg/mL being in clinical remission, compared with only 83% of those with concentrations > 5 μg/mL. Similarly, data from 9 studies suggested that a certolizumab trough concentration > 20 μg/mL was associated with a 75% probability of being in clinical remission, compared to a 60% probability when the trough concentration was > 10 μg/mL [9]. Based on these analyses, the AGA suggests target trough concentrations for reactive therapeutic drug monitoring of anti-TNF agents of ≥ 5 μg/mL for infliximab, ≥ 7.5 μg/mL for adalimumamb, and ≥ 20 μg/mL for certolizumab. They did not suggest a target trough concentration for golimumab because of insufficient evidence [8].
When interpreting TDM test results, it is important to know if the test you have used is drug-sensitive or drug-tolerant (Table 2). Drug-sensitive tests will be less likely to reveal the presence of anti-drug antibodies when the drug level is above a certain threshold. A post-hoc analysis of the TAXIT trial recently suggested that subjects who have antibodies detected on a drug-tolerant test which were not detected on a drug-sensitive test are more likely to respond to higher doses of infliximab [19]. It follows that there should be a threshold anti-drug antibody titer below which someone who has immune-mediated pharmacokinetic failure will still respond to TNF antagonist dose escalation, but above which they will fail to respond to dose escalation. To be sure, our understanding of the clinical implications of a drug-tolerant test demonstrating an adequate drug level while also detectable anti-drug antibodies is evolving. Complicating the issue further is the fact that anti-drug antibody concentrations cannot be compared between assays because of assay-specific characteristics. As such, though the presence of low antibody titers and high antibody titers seems to be clinically important, recommendations cannot yet be made on how to interpret specific thresholds. Furthermore, development of transient versus sustained antibodies requires further clinical investigation to determine impact and treatment algorithms.
Optimizing Therapy
Once you have determined the most likely cause of drug failure, the next step is to make a change in medical therapy.
When switching within class (to another anti-TNF agent), the choice of which agent to use next will largely depend on patient preference (route of administration, infusion versus injection), insurance, and costs of treatment. When making the decision to switch within class, it should be kept in mind that the probability of achieving remission is modestly reduced compared to the probability seen in anti-TNF-naive patients [20], and even more so when the patient is switching to their third anti-TNF agent [21]. Thus, for the patient who has already previously switched from one TNF antagonist to a second TNF antagonist, it may be better to switch to a different class of biologic rather than attempting to capture a clinical remission with a third TNF antagonist.
When adding an immunomodulator (azathioprine or methotrexate), the expectation is that the therapy will increase the serum concentration of the anti-TNF agent [14] and/or reduce the ongoing risk of anti-drug antibody formation [22]. There could also be a direct treatment effect on the bowel disease by the immunomodulator.
When switching to an alternate mechanism of action, the currently FDA-approved options include the biologic agents vedolizumab (for both moderate-to-severe ulcerative colitis and moderate-to-severe Crohn’s disease) and ustekinumab (for moderate-to-severe Crohn’s disease), as well as the recently FDA-approved oral, small-molecule JAK1 and JAK3 inhibitor tofacitinib (for moderate-to-severe ulcerative colitis). Prospective comparative effectiveness studies for these agents are lacking and are unlikely to be performed in part due to the cost and time required to accomplish these studies. A recent post-hoc analysis of clinical trials data suggests that there are no significant differences in the rates of clinical response, clinical remission, or in adverse outcomes to vedolizumab or ustekinumab when they are used in patients who have failed anti-TNF therapy [23]. Thus, one cannot be recommended over the other, and the decision of which to use is usually guided by patient preference and insurance coverage.
Meanwhile, the role of tofacitinib in the treatment algorithm of patients who have failed anti-TNF therapy remains unclear. The phase III clinical trials OCTAVE 1, OCTAVE 2, and OCTAVE Sustain showed efficacy for both the induction and maintenance of remission in patients with moderate-to-severe ulcerative colitis who had previously failed anti-TNF agents. However, there remain concerns about the safety profile of tofacitinib compared to vedolizumab and ustekinumab, particularly regarding herpes zoster infection, dyslipidemia, and adverse cardiovascular events. Notable findings from the tofacitinib induction trials include robust rates of clinical remission (18.5% vs 8.2% for placebo in Octave 1, and 16.6% vs 3.6% in Octave 2, P < 0.001 for both comparisons) and mucosal healing (31.3% vs 15.6% for placebo in Octave 1, and 28.4% and 11.6% in Octave 2, P < 0.001 for both comparisons) after 8 weeks of induction therapy [24]. These results suggest that tofacitinib, or other JAK inhibitors that become approved in the future, may be excellent oral agents for the induction of remission in moderate-to-severe ulcerative colitis, and may demonstrate a better side effect profile than steroids. Whether cost factors (compared to steroid therapy) will limit the role of JAK-inhibitor therapy in induction, and whether safety concerns will limit their use in maintenance therapy, remains to be seen.
Off-Label Rescue Therapy and Surgery
Though the armamentarium of IBD therapies has expanded greatly over the past 2 decades, and will continue to do so for the foreseeable future, there are still a limited selection of therapies available to patients. Patients who have failed anti-TNF therapy, and subsequently fail vedolizumab and/or ustekinumab, have limited options. These options include clinical trials, off-label small molecule rescue therapy, and surgery. Patients who are felt to require any of these options should be referred to a tertiary center for evaluation by a gastroenterologist specializing in the treatment of IBD and/or a colorectal surgeon specializing in the surgical management of IBD.
Tacrolimus
Tacrolimus, a macrolide calcineurin inhibitor, has been studied as a small molecule therapy for IBD, though not in randomized controlled trials. There is biological plausibility for its use as a disease modifying agent. Mucosal T cells in subjects with active Crohn’s disease have been found to express increased levels of mRNA encoding IL-2, and tacrolimus acts primarily by reducing IL-2 production [25]. The largest observational cohort evaluating the use of tacrolimus, published by Thin et al, included patients with both ulcerative colitis (n = 24) and Crohn’s disease (n = 11) who had moderate to severely active IBD. All patients had failed dose-optimized thiopurine therapy, 89% had primary nonresponse or secondary loss of response to at least one anti-TNF agent, and 74% were either steroid-refractory or steroid-dependent at the time tacrolimus was started. With close monitoring, they targeted a tacrolimus trough of 8–12 ng/mL. At 30 days, 66% had a clinical response, and 40% were in clinical remission. At 90 days, 60% had a clinical response, and 37% were in clinical remission. At 1 year, 31% had a clinical response, and 23% were in clinical remission. Of those in clinical remission at 1 year, 88% were either off of steroids or on less than 5 mg of prednisone per day. Renal impairment was seen in 25% of patients, including severe renal impairment in 11%, requiring drug cessation. Infectious complications were seen in 9% of patients. Headaches, tremor, and pancreatitis were also observed, though less commonly. The majority of patients ultimately had a surgical intervention, particularly if they were steroid-refractory at baseline, but the time to surgery was delayed in those who achieved a response or remission in the first 90 days of tacrolimus therapy. The authors suggested that while tacrolimus may lack clear long-term benefit in patients with medically refractory IBD, a therapeutic trial should be considered in select patients with the goal of medical and nutritional optimization before surgical intervention [26].
Cyclosporine
Cyclosporine, which also exerts its effect by inhibiting IL-2 production, has an established role in the management of severe ulcerative colitis. Data from randomized, placebo-controlled trials, along with numerous open label observational studies, have shown that intravenous cyclosporine can induce remission and potentially obviate the need for urgent/emergent colectomy in steroid-refractory patients who are hospitalized with severe ulcerative colitis [27,28]. Its use in maintenance therapy remains controversial, however. Older observational data suggest that even among those who have an initial clinical response to cyclosporine induction, 33% will undergo colectomy by 1 year, and 88% will undergo colectomy by 7 years [27). Though the concomitant administration of a thiopurine may delay the need for colectomy [29,30], cyclosporine seems to be, at best, a temporizing therapy for patients with severe ulcerative colitis. Studies on the use of cyclosporine for the induction of remission in Crohn’s disease have been less robust, as have studies on the use of cyclosporine for the maintenance of remission in Crohn’s disease [31]. Dose-dependent toxicity also remains a concern, particularly when being considered as maintenance therapy. Though some observational data suggest that the absolute risks of serious side effects from maintenance cyclosporine are small, cyclosporine is still generally avoided as a maintenance therapy [30].
Mycophenolate Mofetil
Mycophenolate mofetil (MMF), which inhibits both B and T cell proliferation by inhibiting de novo purine synthesis, has been studied in both Crohn’s disease and ulcerative colitis. Studies have been small, observational, and heterogeneous. On the whole, they suggest that MMF does have some efficacy, but it is not necessarily more effective than azathioprine and may have a slightly increased risk of side effects [32]. Given that the side effects of MMF include diarrhea, and an IBD-like enterocolitis (MMF-induced colitis) when given to subjects without an established diagnosis of IBD, it is likely best to avoid using the drug in patients with IBD [33].
Surgery
Finally, when medical management has failed, or when fibrostenotic and/or penetrating complications of inflammatory bowel disease are present, surgery should be considered. Surgery can provide a cure in patients with ulcerative colitis, and can induce remission in patients with Crohn’s disease. Managing IBD medications around the time of surgery is always challenging. Multiple large, retrospective cohort studies have suggested that the risk for postoperative infectious complications, anastomotic leaks, and thrombotic complications do not differ between those who receive anti-TNF therapy within several months of surgery and those who do not. Nevertheless, some surgeons may prefer to time elective surgery halfway between doses of anti-TNF therapy. Additionally, there is some data to suggest that patients who are on both thiopurines and anti-TNF agents have an increased risk of postoperative complications compared to those who are on anti-TNF agents alone [34].
After a surgical evaluation, a plan of action should be formulated in a multidisciplinary fashion to determine how medical management will proceed. For those with an established diagnosis of ulcerative colitis, medical therapy can often be stopped postoperatively and the patient can be monitored prospectively for pouch complications including possible new-onset Crohn’s disease. For those who undergo surgery for the management of Crohn’s disease, though a resection completed with negative margins does induce remission, nearly 90% can be expected to have histologic, endoscopic, or clinical recurrence by 1 year. A randomized controlled trial showed that postoperative anti-TNF therapy can reduce this risk to 9% [35]. Unfortunately, a subsequently conducted large, multicenter, randomized controlled trial comparing postoperative infliximab to placebo was terminated early because of a lack of a statistically significant difference in clinical recurrence between the 2 groups at week 74. However, this lack of demonstrated efficacy may have been obscured by the relatively mild phenotype of the enrolled participants, who had a median CDAI score of 105.5 at baseline [36]. Based on available data, the AGA does conditionally recommend postoperative anti-TNF and/or thiopurine therapy for those patients with Crohn’s disease who are in a surgically induced remission [37]. The patients who are most likely to benefit from postoperative medical therapy are those who have the highest risk of recurrence, namely those who were young at the time of diagnosis, had a short disease duration prior to surgery, have multiple sites of disease, and who use tobacco products [34].
Emerging and Future Options
Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Based on our improved, and evolving understanding of the pathogenesis of IBD, several new biochemical targets have emerged, offering novel ways to modulate the cytokine cascade which drives IBD [38]. Well over a dozen phase II and phase III trials for IBD therapeutic agents are ongoing, including biologic agents targeting interleukin-23, β7-Integrin, and MAdCAM-1, as well as small molecule agents targeting the JAK/STAT pathway and the sphingosine-1-phosphate receptor modulators [39]. As new agents are approved, it may be possible to develop a more patient-centered approach to care by targeting therapies to the particular pathogenesis of each patient’s disease. Nevertheless, integrating these therapies into practice algorithms will remain a challenge in the absence of meaningful comparative effectiveness trials [40].
Conclusion
When evaluating a patient who seems to have failed anti-TNF therapy for IBD, the first step is to confirm that active inflammatory disease is present. This process includes ruling out other potential causes of the patient’s symptoms, including infectious colitis, and ideally includes obtaining objective evidence of inflammation, whether through non-invasive biomarkers, an endoscopic evaluation and/or cross-sectional imaging. Once active IBD is confirmed, reactive therapeutic drug monitoring can help elucidate the likely mechanism of drug failure, which in turn can guide medical decision making.
Corresponding author: Anita Afzali MD, MPH, The Ohio State University Wexner Medical Center, 395 West 12th Ave, Room 280, Columbus, OH 43210, [email protected].
Financial disclosures: Dr. Afzali has served as a speaker/consultant for Abbvie, UCB, Takeda, Pfizer, Janssen; on the advisory board of Abbvie, UCB; received grant support from UCB; and is a board member of IBD Horizons.
From the Division of Gastroenterology University of Washington, Seattle, WA (Dr. Tiderington), and the Division of Gastroenterology, Hepatology and Nutrition, The Ohio State University Wexner Medical Center and The Ohio State University Inflammatory Bowel Disease Center, Columbus, OH (Dr. Afzali).
Abstract
- Objective: To provide a practical approach to the management of patients with inflammatory bowel disease (IBD) following tumor necrosis factor (TNF) alpha antagonist failure.
- Methods: Review of the literature.
- Results: TNF alpha antagonists play a central role in the treatment of IBD. Unfortunately, some patients will not respond to therapy with TNF antagonists, and others will lose response during treatment. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. In this paper we review the mechanisms of drug failure, the use of reactive therapeutic drug monitoring to guide clinical decision making, and propose an evidence-based method for managing this common clinical scenario.
- Conclusion: Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Key words: TNF antagonists; therapeutic drug monitoring; biologic failure; Crohn’s disease treatment; ulcerative colitis treatment.
Ulcerative colitis and Crohn’s disease are the two types of inflammatory bowel disease (IBD), and they are characterized by chronic, immunologically mediated inflammation involving the gastrointestinal tract [1]. Guided by an understanding of the role of tumor necrosis factor (TNF) alpha in the pathogenesis of IBD, TNF antagonists have played a central role in modern treatment algorithms [2]. Unfortunately, roughly one third of patients will not have a clinical response when given induction dosing of the currently available anti-TNF agents, and of those who do respond to treatment, up to one half will lose response to treatment within the first year [3]. When patients present with persistent or recurrent symptoms suggesting active IBD while on anti-TNF therapy it can present a dilemma for the clinician. Once the clinician has confirmed that active IBD is present based on endoscopic, cross-sectional imaging and/or biochemical markers of inflammation, the next step is to identify the cause of the treatment failure, as this guides management. Here we review the body of literature that guides our understanding of treatment failure as well as therapeutic drug monitoring and propose an evidence-based algorithm for managing this common clinical scenario.
Defining Treatment Failure
Patients who receive anti-TNF therapy but demonstrate active IBD should be classified as having either primary nonresponse or secondary loss of response. Primary nonresponse is defined as having either no response, or only partial response, to induction with anti-TNF therapy [4]. Data from pivotal trials and meta-analyses suggest that about one third of patients will not show any clinical response to induction with anti-TNF therapies, with response typically being defined using composite endpoints favoring clinical symptoms and only sometimes incorporating endoscopic findings [5]. An additional one third of patients will have only a partial response, without remission. Patients with ulcerative colitis are at a slightly increased risk of primary nonresponse compared to patients with Crohn’s disease. Though the time frame for defining primary nonresponse is different for each agent because each agent has a slightly different induction schedule, in general the maximal response to therapy is typically seen by week 12, and it is reasonable to use this as a time cutoff [6].
Secondary loss of response is likewise defined as recrudescence of clinically active disease after an initial response. In general, the presence of secondary loss of response should not be invoked until week 12 of therapy. In most pivotal trials, secondary loss of response was seen in roughly half of patients at 1 year. In clinical practice, however, particularly as therapeutic drug monitoring has become more common, the observed rates of secondary loss of response have been lower [6].
Applying these definitions appropriately is important because it dictates the next steps in management. When a patient presents with symptoms suggesting active IBD while on anti-TNF therapy, either during induction when primary nonresponse is possible, or in maintenance when secondary loss of response would be invoked, the first step is to determine if active IBD is the etiology for the presenting symptoms. The initial evaluation should rule out common infectious causes of symptoms mimicking IBD. In particular, Clostridium difficile infection should be ruled out with stool testing. In certain circumstances, ruling out cytomegalovirus (CMV) colitis is important, so an endoscopic evaluation with colonic biopsies should be considered. In the absence of infectious colitis, the presence of active inflammation can often be identified endoscopically, or can be inferred from noninvasive markers with a fair degree of certainty. Fecal calprotectin is an ideal choice for this purpose. In ulcerative colitis it is estimated to have a sensitivity of 0.88 and a specificity of 0.79 for the prediction of endoscopically active disease. The estimated sensitivity for detecting endoscopically active Crohn’s disease is essentially the same (0.87), and the specificity is only slightly lower (0.67). C-reactive protein demonstrates a better specificity (0.92), but has a marginal sensitivity (0.49) [7]. Other etiologies for the patient’s symptoms should also be considered, including medication side effects including use of nonsteroidal anti-inflammatory medications, bile acid malabsorption, small intestinal bacterial overgrowth (SIBO), irritable bowel syndrome (IBS), diverticular disease, ischemic colitis, fibrostenotic strictures, and cancer, depending on comorbidities and the history of present illness.
Once it has been determined that active IBD is the etiology for the patient’s symptoms, the patient should be classified as having either primary nonresponse or secondary loss of response as described above. For the clinician, the next question is how to alter or optimize therapy.
The decision of how to optimize therapy will largely depend on which anti-TNF therapy the patient is currently receiving, and whether they are receiving it as monotherapy or as combination therapy with an immunomodulator. Optimizing therapy will involve either increasing the dose or frequency of administration of the anti-TNF therapy, increasing the dose of azathioprine if indicated, adding an immunomodulator if the patient is on anti-TNF monotherapy, changing to a different anti-TNF agent, or changing to a different class of medication with a different mechanism of action. The recently released American Gastroenterological Association (AGA) guidelines on therapeutic drug monitoring in IBD provide a framework for making these decisions [8]. In general, the clinical choice will be dictated by the etiology of the drug failure.
Types of TNF Antagonist Drug Failure
Our understanding of the causes of biologic treatment failure are evolving but are typically classified as due to mechanistic failure, non-immune-mediated pharmacokinetic failure, or immune-mediated pharmacokinetic failure [9]. Differentiating between these classes of treatment failure requires therapeutic drug monitoring (TDM), which will be discussed in more detail below.
Mechanistic failure is encountered when the underlying biology does not favor a response to a particular therapy. Studies indicate a strong association between particular genetic phenotypes and the probability of a response to induction with anti-TNF agents [10]. This suggests that some individuals have IBD driven by a biochemical inflammatory cascade in which TNF features prominently, while others have alternative mechanistic drivers of inflammation without significantly elevated TNF levels. Mechanistic failure will typically present as primary nonresponse, but can also be seen in patients with secondary loss of response. Mechanistic failure can be elucidated clinically by the use of TDM. In the case of mechanistic failure, active disease is seen in the presence of adequate drug level, without the presence of anti-drug antibodies. The AGA recommends considering switching to a biologic with a different mechanism of action when mechanistic failure is identified [8].
Non-immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to a drug at adequate drug levels experiences suboptimal drug levels because of pharmacokinetic factors. In the case of anti-TNF therapy, this can be conceptualized as either an increased clearance of anti-TNF from the body (eg, in patients with significant hypoalbuminemia or severe colitis), a reduction in the average serum anti-TNF level because of the redistribution of drug in patients with a large body mass index, or inadequate saturation of the total body burden of TNF-alpha in subjects with a high baseline level of inflammation [11]. Non-immune-mediated pharmacokinetic failure can also be identified clinically through TDM. In this case, active disease is seen in the presence of a suboptimal drug level, without the presence of anti-drug antibodies. The AGA recommends considering dose-escalation of the current TNF antagonist when non-immune-mediated pharmacokinetic failure is identified [8], as this can improve clinical response in an estimated 82% of patients [9].
Finally, immune-mediated pharmacokinetic failure is encountered when a patient who would otherwise respond to the current biologic therapy when at adequate drug concentration levels experiences suboptimal drug levels because of increased drug clearance mediated by anti-drug antibodies [9]. Because anti-TNF agents are monoclonal antibodies, they are inherently immunogenic, and it is well established that episodic dosing and lower serum drug concentrations are strong risk factors for the development of anti-drug antibodies [12]. When anti-drug antibodies are present, and are associated with both a decreased serum drug concentration and active inflammatory bowel disease, immune-mediate pharmacokinetic failure can be invoked. When anti-drug antibodies are present, but at a low level, the AGA recommends dose escalation of current TNF antagonist. When anti-drug antibodies are present at a high level, the AGA recommends considering either the addition of an immunomodulator (if not already being used), or changing to a different class of biologic therapy [8]. This recommendation is based in part on data showing that the proportion of patients with sustained anti-drug antibodies during the first year of therapy with an TNF antagonist is likely between 14% and 20% for those on monotherapy, but between 1% and 5% for those on concomitant immunomodulatory therapy [13,14].
Therapeutic Drug Monitoring of Anti-TNF Agents
As described above, TDM, which is the process of testing the patient’s serum for both the concentration of the TNF antagonist and for the presence and concentration of anti-drug antibodies, can help differentiate between mechanistic failure, non-immune-mediated pharmacokinetic failure, and immune-mediated pharmacokinetic failure (Table 1).
Therapeutic drug monitoring can be classified as either proactive or reactive. Proactive TDM is performed during induction or maintenance therapy when the patient does not have signs or symptoms of active disease to suggest a loss of response. Theoretically, this would allow dose modification and optimization, including dose de-escalation in certain circumstances, and could thus provide cost savings with minimal impact on clinical outcomes. The TAXIT trial provides the most robust evaluation of proactive TDM in TNF antagonist therapy. In this study, patients with Crohn’s disease or ulcerative colitis who had a stable clinical response while on maintenance infliximab were first dose optimized proactively to a target trough concentration of 3–7 μg/mL, then randomized to having dose modifications made based on clinical factors alone, defined as reactive monitoring, or dose modifications based on proactive monitoring, performed by checking the drug concentration and antibody levels before each infusion. At 1 year there was no statistically significant difference in the proportion of patients in remission. In addition, some patients in the proactive TDM group were able to have a dose reduction without a subsequent flare of disease, thus providing cost savings [15]. This study suggests that proactive TDM may have a role in drug optimization, particularly with respect to cost-effectiveness, but provides only indirect evidence of a clinical benefit, since all subjects enrolled in the study were proactively dose optimized prior to randomization. This study had a limited follow-up time of 1 year so was not able to assess for longer-term benefits and risks associated with proactive TDM.
More recently, a large, multicenter, retrospective cohort study provided additional evidence that proactive TDM may provide a clinical benefit in addition to cost savings. This study retrospectively evaluated consecutive patients receiving maintenance infliximab for Crohn’s disease between 2006 and 2015, with a median follow-up time of 2.4 years. They were classified as having had either proactive TDM or reactive TDM. Proactive TDM was associated with statistically significant reductions in the risk of treatment failure (hazard ratio [HR] 0.16, 95% confidence interval [CI] 0.09–0.27), the need for surgery (HR 0.30, 95% CI 0.11–0.80), hospitalization (HR 0.16, 95% CI 0.07–0.33), and anti-drug antibody formation (HR 0.25, 95% CI 0.07–0.84) [16].
To date, however, no randomized controlled trials have been published comparing proactive TDM to reactive TDM in treatment-naive patients. Because of the paucity of prospective studies, the AGA currently makes no recommendation regarding the use of proactive TDM in clinical practice. However, the current AGA guidelines do recommend reactive TDM in the setting of secondary loss of response based on the results of one randomized controlled trial (RCT) and several observational studies. The RCT was small (n = 69), and enrolled patients with Crohn’s disease on maintenance therapy with infliximab. Similar to the TAXIT trial, the study did not show a statistically significant difference in rates of clinical remission when subjects were randomized to either empiric dose escalation (to 5 mg/kg every 4 weeks) based on symptoms, or to dose escalations based on the results of reactive TDM. Also similar to the TAXIT trial, it showed an estimated cost savings of about 34% based on local prices in Denmark for reactive TDM over empiric dose escalation [17].
Meanwhile, the observational studies for reactive TDM provided additional support to the clinical benefit of reactive TDM, but also to the underlying hypotheses that drive reactive TDM, namely that subjects with mechanistic failure benefit from a change in drug class, those with non-immune-mediated pharmacokinetic failure benefit from dose escalation, and that those with immune-mediated pharmacokinetic failure may benefit from either dose escalation or a change in mechanism of action, depending on antibody titers. Specifically, on pooled analysis of 2 of these studies, 82% of subjects who were found to have non-immune-mediated pharmacokinetic failure responded to empiric dose escalation, whereas only 8% of subjects who were found to have immune-mediated pharmacokinetic failure with high anti-drug antibody titers responded to dose escalation [9]. Likewise, in a retrospective study involving subjects who were being treated with infliximab and then had reactive TDM performed, when non-immune-mediated pharmacokinetic failure was identified, a clinical response was seen in 86% of subjects who underwent dose escalation, and only 33% among those who were switched to a different anti-TNF (P < 0.016). Conversely, dose escalation resulted in a clinical response only 17% of the time when anti-drug antibodies were detectable, compared to a 92% response rate when the subject was switched to a different anti-TNF (P < 0.004) [18].
Interpreting the Results of Reactive Therapeutic Drug Monitoring
The implementation of reactive TDM involves obtaining a serum drug and antibody level and then interpreting what those results suggest about the mechanism of drug failure, in order to decide on a course of action. The serum drug level should be a trough concentration, so it should be obtained just prior to a timed dose, while on a stable treatment regimen. Exactly what serum drug concentration we should be targeting in reactive therapeutic drug monitoring remains an area of investigation. No RCTs have been published. There is ample observational, cross-sectional data from cohorts of patients on maintenance therapy, though heterogeneity in study design and study populations, as well as use of different assays, limit interpretation of the data. In a secondary analysis of data from 6 observational studies of patients on infliximab maintenance therapy, there was a highly statistically significant concentration-dependent trend in rates of clinical remission depending on the measured infliximab trough concentration, with 96% of those with infliximab > 7 μg/mL in remission, 92% of those with infliximab > 5 μg/mL in remission, and 75% of those with infliximab > 1 μg/mL in remission. Likewise, data from 4 studies of patients receiving adalimumab showed a statistically significant concentration-dependent trend in clinical remission, with 90% of those with adalimumab trough concentrations > 7.5 μg/mL being in clinical remission, compared with only 83% of those with concentrations > 5 μg/mL. Similarly, data from 9 studies suggested that a certolizumab trough concentration > 20 μg/mL was associated with a 75% probability of being in clinical remission, compared to a 60% probability when the trough concentration was > 10 μg/mL [9]. Based on these analyses, the AGA suggests target trough concentrations for reactive therapeutic drug monitoring of anti-TNF agents of ≥ 5 μg/mL for infliximab, ≥ 7.5 μg/mL for adalimumamb, and ≥ 20 μg/mL for certolizumab. They did not suggest a target trough concentration for golimumab because of insufficient evidence [8].
When interpreting TDM test results, it is important to know if the test you have used is drug-sensitive or drug-tolerant (Table 2). Drug-sensitive tests will be less likely to reveal the presence of anti-drug antibodies when the drug level is above a certain threshold. A post-hoc analysis of the TAXIT trial recently suggested that subjects who have antibodies detected on a drug-tolerant test which were not detected on a drug-sensitive test are more likely to respond to higher doses of infliximab [19]. It follows that there should be a threshold anti-drug antibody titer below which someone who has immune-mediated pharmacokinetic failure will still respond to TNF antagonist dose escalation, but above which they will fail to respond to dose escalation. To be sure, our understanding of the clinical implications of a drug-tolerant test demonstrating an adequate drug level while also detectable anti-drug antibodies is evolving. Complicating the issue further is the fact that anti-drug antibody concentrations cannot be compared between assays because of assay-specific characteristics. As such, though the presence of low antibody titers and high antibody titers seems to be clinically important, recommendations cannot yet be made on how to interpret specific thresholds. Furthermore, development of transient versus sustained antibodies requires further clinical investigation to determine impact and treatment algorithms.
Optimizing Therapy
Once you have determined the most likely cause of drug failure, the next step is to make a change in medical therapy.
When switching within class (to another anti-TNF agent), the choice of which agent to use next will largely depend on patient preference (route of administration, infusion versus injection), insurance, and costs of treatment. When making the decision to switch within class, it should be kept in mind that the probability of achieving remission is modestly reduced compared to the probability seen in anti-TNF-naive patients [20], and even more so when the patient is switching to their third anti-TNF agent [21]. Thus, for the patient who has already previously switched from one TNF antagonist to a second TNF antagonist, it may be better to switch to a different class of biologic rather than attempting to capture a clinical remission with a third TNF antagonist.
When adding an immunomodulator (azathioprine or methotrexate), the expectation is that the therapy will increase the serum concentration of the anti-TNF agent [14] and/or reduce the ongoing risk of anti-drug antibody formation [22]. There could also be a direct treatment effect on the bowel disease by the immunomodulator.
When switching to an alternate mechanism of action, the currently FDA-approved options include the biologic agents vedolizumab (for both moderate-to-severe ulcerative colitis and moderate-to-severe Crohn’s disease) and ustekinumab (for moderate-to-severe Crohn’s disease), as well as the recently FDA-approved oral, small-molecule JAK1 and JAK3 inhibitor tofacitinib (for moderate-to-severe ulcerative colitis). Prospective comparative effectiveness studies for these agents are lacking and are unlikely to be performed in part due to the cost and time required to accomplish these studies. A recent post-hoc analysis of clinical trials data suggests that there are no significant differences in the rates of clinical response, clinical remission, or in adverse outcomes to vedolizumab or ustekinumab when they are used in patients who have failed anti-TNF therapy [23]. Thus, one cannot be recommended over the other, and the decision of which to use is usually guided by patient preference and insurance coverage.
Meanwhile, the role of tofacitinib in the treatment algorithm of patients who have failed anti-TNF therapy remains unclear. The phase III clinical trials OCTAVE 1, OCTAVE 2, and OCTAVE Sustain showed efficacy for both the induction and maintenance of remission in patients with moderate-to-severe ulcerative colitis who had previously failed anti-TNF agents. However, there remain concerns about the safety profile of tofacitinib compared to vedolizumab and ustekinumab, particularly regarding herpes zoster infection, dyslipidemia, and adverse cardiovascular events. Notable findings from the tofacitinib induction trials include robust rates of clinical remission (18.5% vs 8.2% for placebo in Octave 1, and 16.6% vs 3.6% in Octave 2, P < 0.001 for both comparisons) and mucosal healing (31.3% vs 15.6% for placebo in Octave 1, and 28.4% and 11.6% in Octave 2, P < 0.001 for both comparisons) after 8 weeks of induction therapy [24]. These results suggest that tofacitinib, or other JAK inhibitors that become approved in the future, may be excellent oral agents for the induction of remission in moderate-to-severe ulcerative colitis, and may demonstrate a better side effect profile than steroids. Whether cost factors (compared to steroid therapy) will limit the role of JAK-inhibitor therapy in induction, and whether safety concerns will limit their use in maintenance therapy, remains to be seen.
Off-Label Rescue Therapy and Surgery
Though the armamentarium of IBD therapies has expanded greatly over the past 2 decades, and will continue to do so for the foreseeable future, there are still a limited selection of therapies available to patients. Patients who have failed anti-TNF therapy, and subsequently fail vedolizumab and/or ustekinumab, have limited options. These options include clinical trials, off-label small molecule rescue therapy, and surgery. Patients who are felt to require any of these options should be referred to a tertiary center for evaluation by a gastroenterologist specializing in the treatment of IBD and/or a colorectal surgeon specializing in the surgical management of IBD.
Tacrolimus
Tacrolimus, a macrolide calcineurin inhibitor, has been studied as a small molecule therapy for IBD, though not in randomized controlled trials. There is biological plausibility for its use as a disease modifying agent. Mucosal T cells in subjects with active Crohn’s disease have been found to express increased levels of mRNA encoding IL-2, and tacrolimus acts primarily by reducing IL-2 production [25]. The largest observational cohort evaluating the use of tacrolimus, published by Thin et al, included patients with both ulcerative colitis (n = 24) and Crohn’s disease (n = 11) who had moderate to severely active IBD. All patients had failed dose-optimized thiopurine therapy, 89% had primary nonresponse or secondary loss of response to at least one anti-TNF agent, and 74% were either steroid-refractory or steroid-dependent at the time tacrolimus was started. With close monitoring, they targeted a tacrolimus trough of 8–12 ng/mL. At 30 days, 66% had a clinical response, and 40% were in clinical remission. At 90 days, 60% had a clinical response, and 37% were in clinical remission. At 1 year, 31% had a clinical response, and 23% were in clinical remission. Of those in clinical remission at 1 year, 88% were either off of steroids or on less than 5 mg of prednisone per day. Renal impairment was seen in 25% of patients, including severe renal impairment in 11%, requiring drug cessation. Infectious complications were seen in 9% of patients. Headaches, tremor, and pancreatitis were also observed, though less commonly. The majority of patients ultimately had a surgical intervention, particularly if they were steroid-refractory at baseline, but the time to surgery was delayed in those who achieved a response or remission in the first 90 days of tacrolimus therapy. The authors suggested that while tacrolimus may lack clear long-term benefit in patients with medically refractory IBD, a therapeutic trial should be considered in select patients with the goal of medical and nutritional optimization before surgical intervention [26].
Cyclosporine
Cyclosporine, which also exerts its effect by inhibiting IL-2 production, has an established role in the management of severe ulcerative colitis. Data from randomized, placebo-controlled trials, along with numerous open label observational studies, have shown that intravenous cyclosporine can induce remission and potentially obviate the need for urgent/emergent colectomy in steroid-refractory patients who are hospitalized with severe ulcerative colitis [27,28]. Its use in maintenance therapy remains controversial, however. Older observational data suggest that even among those who have an initial clinical response to cyclosporine induction, 33% will undergo colectomy by 1 year, and 88% will undergo colectomy by 7 years [27). Though the concomitant administration of a thiopurine may delay the need for colectomy [29,30], cyclosporine seems to be, at best, a temporizing therapy for patients with severe ulcerative colitis. Studies on the use of cyclosporine for the induction of remission in Crohn’s disease have been less robust, as have studies on the use of cyclosporine for the maintenance of remission in Crohn’s disease [31]. Dose-dependent toxicity also remains a concern, particularly when being considered as maintenance therapy. Though some observational data suggest that the absolute risks of serious side effects from maintenance cyclosporine are small, cyclosporine is still generally avoided as a maintenance therapy [30].
Mycophenolate Mofetil
Mycophenolate mofetil (MMF), which inhibits both B and T cell proliferation by inhibiting de novo purine synthesis, has been studied in both Crohn’s disease and ulcerative colitis. Studies have been small, observational, and heterogeneous. On the whole, they suggest that MMF does have some efficacy, but it is not necessarily more effective than azathioprine and may have a slightly increased risk of side effects [32]. Given that the side effects of MMF include diarrhea, and an IBD-like enterocolitis (MMF-induced colitis) when given to subjects without an established diagnosis of IBD, it is likely best to avoid using the drug in patients with IBD [33].
Surgery
Finally, when medical management has failed, or when fibrostenotic and/or penetrating complications of inflammatory bowel disease are present, surgery should be considered. Surgery can provide a cure in patients with ulcerative colitis, and can induce remission in patients with Crohn’s disease. Managing IBD medications around the time of surgery is always challenging. Multiple large, retrospective cohort studies have suggested that the risk for postoperative infectious complications, anastomotic leaks, and thrombotic complications do not differ between those who receive anti-TNF therapy within several months of surgery and those who do not. Nevertheless, some surgeons may prefer to time elective surgery halfway between doses of anti-TNF therapy. Additionally, there is some data to suggest that patients who are on both thiopurines and anti-TNF agents have an increased risk of postoperative complications compared to those who are on anti-TNF agents alone [34].
After a surgical evaluation, a plan of action should be formulated in a multidisciplinary fashion to determine how medical management will proceed. For those with an established diagnosis of ulcerative colitis, medical therapy can often be stopped postoperatively and the patient can be monitored prospectively for pouch complications including possible new-onset Crohn’s disease. For those who undergo surgery for the management of Crohn’s disease, though a resection completed with negative margins does induce remission, nearly 90% can be expected to have histologic, endoscopic, or clinical recurrence by 1 year. A randomized controlled trial showed that postoperative anti-TNF therapy can reduce this risk to 9% [35]. Unfortunately, a subsequently conducted large, multicenter, randomized controlled trial comparing postoperative infliximab to placebo was terminated early because of a lack of a statistically significant difference in clinical recurrence between the 2 groups at week 74. However, this lack of demonstrated efficacy may have been obscured by the relatively mild phenotype of the enrolled participants, who had a median CDAI score of 105.5 at baseline [36]. Based on available data, the AGA does conditionally recommend postoperative anti-TNF and/or thiopurine therapy for those patients with Crohn’s disease who are in a surgically induced remission [37]. The patients who are most likely to benefit from postoperative medical therapy are those who have the highest risk of recurrence, namely those who were young at the time of diagnosis, had a short disease duration prior to surgery, have multiple sites of disease, and who use tobacco products [34].
Emerging and Future Options
Despite the improved clinical outcomes seen since the introduction of TNF antagonists for the management of IBD, there remains a significant need for additional medical therapies. Fortunately, the armamentarium is expected to expand dramatically over the next decade.
Based on our improved, and evolving understanding of the pathogenesis of IBD, several new biochemical targets have emerged, offering novel ways to modulate the cytokine cascade which drives IBD [38]. Well over a dozen phase II and phase III trials for IBD therapeutic agents are ongoing, including biologic agents targeting interleukin-23, β7-Integrin, and MAdCAM-1, as well as small molecule agents targeting the JAK/STAT pathway and the sphingosine-1-phosphate receptor modulators [39]. As new agents are approved, it may be possible to develop a more patient-centered approach to care by targeting therapies to the particular pathogenesis of each patient’s disease. Nevertheless, integrating these therapies into practice algorithms will remain a challenge in the absence of meaningful comparative effectiveness trials [40].
Conclusion
When evaluating a patient who seems to have failed anti-TNF therapy for IBD, the first step is to confirm that active inflammatory disease is present. This process includes ruling out other potential causes of the patient’s symptoms, including infectious colitis, and ideally includes obtaining objective evidence of inflammation, whether through non-invasive biomarkers, an endoscopic evaluation and/or cross-sectional imaging. Once active IBD is confirmed, reactive therapeutic drug monitoring can help elucidate the likely mechanism of drug failure, which in turn can guide medical decision making.
Corresponding author: Anita Afzali MD, MPH, The Ohio State University Wexner Medical Center, 395 West 12th Ave, Room 280, Columbus, OH 43210, [email protected].
Financial disclosures: Dr. Afzali has served as a speaker/consultant for Abbvie, UCB, Takeda, Pfizer, Janssen; on the advisory board of Abbvie, UCB; received grant support from UCB; and is a board member of IBD Horizons.
1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78.
2. Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 2012;61:918–32.
3. Mitrev N, Leong RW. Therapeutic drug monitoring of anti-tumour necrosis factor-α agents in inflammatory bowel disease. Expert Opin Drug Saf 2017;16:303–17.
4. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015;21:182–97.
5. Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015;148:37–51.
6. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis 2010;4:355–66.
7. Mosli MH, Zou G, Garg SK, et al. C-Reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:802–19.
8. Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–34.
9. Vande Casteele N, Herfarth H, Katz J, et al. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology 2017;153:835–57.
10. López-Hernández R, Valdés M, Campillo JA, et al. Genetic polymorphisms of tumour necrosis factor alpha (TNF-α) promoter gene and response to TNF-α inhibitors in Spanish patients with inflammatory bowel disease. Int J Immunogenet 2014;41:63–8.
11. Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012;91:635–46.
12. Hindryckx P, Novak G, Vande Casteele N, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: a practical overview. Drugs 2017;77:363–77.
13. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95.
14. Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther 2009;30:210–26.
15. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9.
16. Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–8.
17. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27.
18. Afif W, Loftus EV Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9.
19. Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut 2017.
20. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther 2015;41:613–23.
21. Gisbert JP, Chaparro M. Use of a third anti-TNF after failure of two previous anti-TNFs in patients with inflammatory bowel disease: is it worth it? Scand J Gastroenterol 2015;50:379–86.
22. Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012;10:1079–87.
23. Kawalec P, Moćko P. An indirect comparison of ustekinumab and vedolizumab in the therapy of TNF-failure Crohn’s disease patients. J Comp Eff Res 2017;7:101–11.
24. Sandborn WJ, Su C, Panes J, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:1723–36.
25. Yarkoni S, Sagiv Y, Kaminitz A, Askenasy N. Interleukin 2 targeted therapy in inflammatory bowel disease. Gut 2009;58:1705–6.
26. Thin LW, Murray K, Lawrance IC. Oral tacrolimus for the treatment of refractory inflammatory bowel disease in the biologic era. Inflamm Bowel Dis 2013;19:1490–8.
27. Moskovitz DN, Van Assche G, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:760–5.
28. Arts J, D’Haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis 2004;10:73–8.
29. Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol 1999;94:1587–92.
30. Cheifetz AS, Stern J, Garud S, et al. Cyclosporine is safe and effective in patients with severe ulcerative colitis. J Clin Gastroenterol 2011;45:107–12.
31. Lazarev M, Present DH, Lichtiger S, et al. The effect of intravenous cyclosporine on rates of colonic surgery in hospitalized patients with severe Crohn’s colitis. J Clin Gastroenterol 2012;46:764–7.
32. Renna S, Cottone M, Orlando A. Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease. World J Gastroenterol 2014;20:9675–90.
33. Izower MA, Rahman M, Molmenti EP, et al. Correlation of abnormal histology with endoscopic findings among mycophenolate mofetil treated patients. World J Gastrointest Endosc 2017;9:405–10.
34. Ferrari L, Krane MK, Fichera A. Inflammatory bowel disease surgery in the biologic era. World J Gastrointest Surg 2016;8:363–70.
35. Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441–50.
36. Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology 2016;150:1568–78.
37. Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute technical review on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:277–95.
38. Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology 2017;152:374–88.
39. Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci 2017;38:127–42.
40. Khanna R, Feagan BG. Emerging therapies for inflammatory bowel diseases. Dig Dis 2016;34 Suppl 1:67–
1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med 2009;361:2066–78.
2. Danese S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 2012;61:918–32.
3. Mitrev N, Leong RW. Therapeutic drug monitoring of anti-tumour necrosis factor-α agents in inflammatory bowel disease. Expert Opin Drug Saf 2017;16:303–17.
4. Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: evolution in the definition and management of primary nonresponse. Inflamm Bowel Dis 2015;21:182–97.
5. Levesque BG, Sandborn WJ, Ruel J, et al. Converging goals of treatment of inflammatory bowel disease from clinical trials and practice. Gastroenterology 2015;148:37–51.
6. Allez M, Karmiris K, Louis E, et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: definitions, frequency and pharmacological aspects. J Crohns Colitis 2010;4:355–66.
7. Mosli MH, Zou G, Garg SK, et al. C-Reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: a systematic review and meta-analysis. Am J Gastroenterol 2015;110:802–19.
8. Feuerstein JD, Nguyen GC, Kupfer SS, et al. American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology 2017;153:827–34.
9. Vande Casteele N, Herfarth H, Katz J, et al. American Gastroenterological Association Institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology 2017;153:835–57.
10. López-Hernández R, Valdés M, Campillo JA, et al. Genetic polymorphisms of tumour necrosis factor alpha (TNF-α) promoter gene and response to TNF-α inhibitors in Spanish patients with inflammatory bowel disease. Int J Immunogenet 2014;41:63–8.
11. Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther 2012;91:635–46.
12. Hindryckx P, Novak G, Vande Casteele N, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: a practical overview. Drugs 2017;77:363–77.
13. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95.
14. Lichtenstein GR, Diamond RH, Wagner CL, et al. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther 2009;30:210–26.
15. Vande Casteele N, Ferrante M, Van Assche G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148:1320–9.
16. Papamichael K, Chachu KA, Vajravelu RK, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol 2017;15:1580–8.
17. Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn’s disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27.
18. Afif W, Loftus EV Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010;105:1133–9.
19. Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut 2017.
20. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther 2015;41:613–23.
21. Gisbert JP, Chaparro M. Use of a third anti-TNF after failure of two previous anti-TNFs in patients with inflammatory bowel disease: is it worth it? Scand J Gastroenterol 2015;50:379–86.
22. Ordás I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012;10:1079–87.
23. Kawalec P, Moćko P. An indirect comparison of ustekinumab and vedolizumab in the therapy of TNF-failure Crohn’s disease patients. J Comp Eff Res 2017;7:101–11.
24. Sandborn WJ, Su C, Panes J, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;377:1723–36.
25. Yarkoni S, Sagiv Y, Kaminitz A, Askenasy N. Interleukin 2 targeted therapy in inflammatory bowel disease. Gut 2009;58:1705–6.
26. Thin LW, Murray K, Lawrance IC. Oral tacrolimus for the treatment of refractory inflammatory bowel disease in the biologic era. Inflamm Bowel Dis 2013;19:1490–8.
27. Moskovitz DN, Van Assche G, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 2006;4:760–5.
28. Arts J, D’Haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis 2004;10:73–8.
29. Cohen RD, Stein R, Hanauer SB. Intravenous cyclosporin in ulcerative colitis: a five-year experience. Am J Gastroenterol 1999;94:1587–92.
30. Cheifetz AS, Stern J, Garud S, et al. Cyclosporine is safe and effective in patients with severe ulcerative colitis. J Clin Gastroenterol 2011;45:107–12.
31. Lazarev M, Present DH, Lichtiger S, et al. The effect of intravenous cyclosporine on rates of colonic surgery in hospitalized patients with severe Crohn’s colitis. J Clin Gastroenterol 2012;46:764–7.
32. Renna S, Cottone M, Orlando A. Optimization of the treatment with immunosuppressants and biologics in inflammatory bowel disease. World J Gastroenterol 2014;20:9675–90.
33. Izower MA, Rahman M, Molmenti EP, et al. Correlation of abnormal histology with endoscopic findings among mycophenolate mofetil treated patients. World J Gastrointest Endosc 2017;9:405–10.
34. Ferrari L, Krane MK, Fichera A. Inflammatory bowel disease surgery in the biologic era. World J Gastrointest Surg 2016;8:363–70.
35. Regueiro M, Schraut W, Baidoo L, et al. Infliximab prevents Crohn’s disease recurrence after ileal resection. Gastroenterology 2009;136:441–50.
36. Regueiro M, Feagan BG, Zou B, et al. Infliximab reduces endoscopic, but not clinical, recurrence of Crohn’s disease after ileocolonic resection. Gastroenterology 2016;150:1568–78.
37. Regueiro M, Velayos F, Greer JB, et al. American Gastroenterological Association Institute technical review on the management of Crohn’s disease after surgical resection. Gastroenterology 2017;152:277–95.
38. Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology 2017;152:374–88.
39. Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci 2017;38:127–42.
40. Khanna R, Feagan BG. Emerging therapies for inflammatory bowel diseases. Dig Dis 2016;34 Suppl 1:67–