User login
- The CDC no longer recommends the use of fluoroquinolones for the treatment of gonococcal infections and associated conditions such as pelvic inflammatory disease (PID).
- Consequently, only one class of drugs, the cephalosporins, is still recommended and available for the treatment of gonorrhea.
- The CDC now recommends ceftriaxone, 125 mg IM, in a single dose, as the preferred treatment.
- For patients with cephalosporin allergies, azithromycin, 2 g orally, as a single dose, remains an option. The CDC discourages widespread use, however, because of concerns about resistance.
The Centers for Disease Control and Prevention (CDC) recently released an update to its treatment guidelines for sexually transmitted diseases, stating that fluoroquinolones are no longer recommended for treatment of gonococcal infections.1 This change resulted from a progressive increase in the rate of resistance to quinolones among gonorrhea isolated from publicly funded treatment centers across the country.
The new advisory applies to all quinolones previously recommended: ciprofloxacin, ofloxacin, and levofloxacin.
Epidemiology. Gonorrhea remains common in the United States, with nearly 340,000 cases reported in 2005. Since it is under-reported, estimates are that more than 600,000 cases occur each year.2
Neisseria gonorrhoeae causes infection of the cervix, urethra, rectum, pharynx, and adnexa. It can also cause disseminated disease that can affect joints, heart, and the meninges.
Tracking the spread of resistant cases
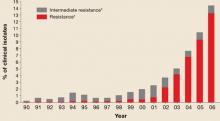
Since the early 1990s, fluoroquinolones have been one of the recommended treatments for gonorrhea because of their availability as effective, single-dose oral regimens. Fluoroquinolone-resistant N gonorrhea began to emerge at the end of the century and has progressed rapidly since. FIGURE 1 illustrates the proportion of fluoroquinolone-resistant N gonorrhea from the CDC’s Gonococcal Isolate Surveillance Project (GISP) by year, from 1990 to 2006.
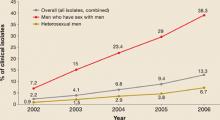
Resistance began to emerge first among gonorrhea isolates from men who have sex with men (MSM), and resistance rates among MSM continue to be higher than in heterosexual men (FIGURE 2).
Geographic trends. In 2000, the CDC recommended that quinolones should no longer be used to treat gonorrhea in persons who contracted the infection in Asia or the Pacific. In 2002, California was added to this list. In 2004, the recommendation against quinolone use was extended to all MSM in the US.
The new recommendation against general use is based on resistance surpassing 5% of total isolates.
FIGURE 1
Percentage of N gonorrhoeae isolates with intermediate resistance or resistance to ciprofloxacin
Data for 2006 are preliminary (January-June only).
* Demonstrating ciprofloxacin minimum inhibitory concentration of 0.125–0.500 mcg/mL.
† Demonstrating ciprofloxacin minimum inhibitory concentration of ≥1.0 mcg/ml.
Source: Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinalones no longer recommended for treatment of gonococcal infections.
MMWR Recomm Rep 2007; 56:332-336.
FIGURE 2
Progressive increase of fluoroquinolone resistance
Percent of isolates from the CDC Gonococcal Isolate Surveillance Project found to be resistant to fluoroquinalones, 2002 through June 2006
Source: GISP report. Centers for Disease Control and Prevention.
Sexually Transmitted Disease Surveillance 2005 Supplement, Gonoccal Isolate Surveillance Project (GISP) Annual Report 2005. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, January 2007.
Ceftriaxone, the default treatment of choice
The loss of quinolones as a recommended gonorrhea treatment leaves only ceftriaxone, 125 mg intramuscularly (IM), as the only readily available treatment for urogenital, anorectal, and pharyngeal gonorrhea. Cefixime 400 mg as a single dose is also recommended, but is not currently available in tablet form in the US. It is available as a suspension with 100 mg per 5 cc.
Other options
Possible oral options include cefpodoxime 400 mg or cefuroxime axetil 1 g. However, neither has the official endorsement of the CDC, and neither appears effective against pharyngeal infection.
Spectinomycin 2 g intramuscularly is recommended for those with cephalosporin allergy—but, like cefixime, it is not currently available in the US, and it also is not considered effective against pharyngeal infection.
Azithromycin 2 g orally as a single dose is currently effective against gonorrhea and is an option for those with cephalosporin allergies. The CDC discourages its widespread use because of concerns about resistance.
New information regarding the availability of spectinomycin and cefixime can be obtained from local health departments or the CDC’s sexually transmitted diseases web site (www.cdc.gov/std).3
Recommended regimens for treatment of gonorrhea
| Uncomplicated gonococcal infections of the cervix, urethra, and rectum* |
| Recommended regimens† |
| Ceftriaxone 125 mg in a single IM dose |
| or |
| Cefixime‡ 400 mg in a single oral dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| Uncomplicated gonococcal infections of the pharynx* |
| Recommended regimens |
| Ceftriaxone 125 mg in a single IM dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| * For all adult and adolescent patients, regardless of travel history or sexual behavior. For those allergic to penicillins or cephalosporins, or for treatment of disseminated gonococcal infections, PID, and epididymitis, see www.cdc.gov/std/treatment. |
| † Alternative regimens: Spectinomycin 2 g in a single IM dose (not currently available in US) or cephalosporin single-dose regimens. |
| Other single-dose cephalosporin regimens that are considered alternative treatment regimens against uncomplicated urogenital and anorectal gonococcal infections include ceftizoxime 500 mg IM; or cefoxitin 2 g IM, administered with probenecid 1 g orally; or cefotaxime 500 mg IM. Some evidence indicates that cefpodoxime 400 mg and cefuroxime axetil 1 g might be oral alternatives. |
| ‡ 400 mg by suspension; tablets are no longer available in the US. |
| Source: www.cdc.gov/mmwr/PDF/rr/rr5511.pdf.2 |
Associated conditions
Treat for chlamydia if chlamydial infection is not ruled out
The CDC continues to recommend concurrent treatment for chlamydia for all persons who have gonorrhea, unless coinfection has been ruled out.
Therapies for chlamydia include azithromycin 1 g as a single dose or doxycycline 100 mg twice a day for 7 days.
Pelvic inflammatory disease and epididymitis
The treatment of both pelvic inflammatory disease (PID) and epididymitis include an option of ceftriaxone 250 mg IM plus doxycycline for either 7 days (for epididymitis) or 10 days (for PID). There are several parenteral options for PID and disseminated gonorrhea; these can be found on the CDC’s STD web site.3
Should you always retest to ensure a cure?
It is still not necessary to retest patients who have had the recommended treatments. However, patients with persistent symptoms or rapidly recurring symptoms should be retested by cultures so that drug-resistance patterns can be checked if gonorrhea is documented.
Retest for recurrence
Consider retesting all treated patients after 3 to 6 months, since anyone with a sexually transmitted infection is at risk of being reinfected.
Summary
The ongoing challenges with the evolving resistance patterns of gonorrhea illustrate the importance of physicians accurately diagnosing gonorrhea, treating with recommended regimens, reporting positive cases to the local public health department, and assisting with partner evaluation and treatment.
1. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007;56:332-336.Available at: www.cdc.gov/mmwr/pdf/wk/mm5614.pdf. Accessed on June 15, 2007.
2. CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11).-Available at www.cdc.gov/mmwr/PDF/rr/rr5511.pdf. Accessed on June 15, 2007.
3. Updated recommended treatment regimens for gonococcal infections and associated conditions—United States, April 2007. Available at: www.cdc.gov/std/treatment/2006/updated-regimens.htm. Accessed on June 15, 2007.
- The CDC no longer recommends the use of fluoroquinolones for the treatment of gonococcal infections and associated conditions such as pelvic inflammatory disease (PID).
- Consequently, only one class of drugs, the cephalosporins, is still recommended and available for the treatment of gonorrhea.
- The CDC now recommends ceftriaxone, 125 mg IM, in a single dose, as the preferred treatment.
- For patients with cephalosporin allergies, azithromycin, 2 g orally, as a single dose, remains an option. The CDC discourages widespread use, however, because of concerns about resistance.
The Centers for Disease Control and Prevention (CDC) recently released an update to its treatment guidelines for sexually transmitted diseases, stating that fluoroquinolones are no longer recommended for treatment of gonococcal infections.1 This change resulted from a progressive increase in the rate of resistance to quinolones among gonorrhea isolated from publicly funded treatment centers across the country.
The new advisory applies to all quinolones previously recommended: ciprofloxacin, ofloxacin, and levofloxacin.
Epidemiology. Gonorrhea remains common in the United States, with nearly 340,000 cases reported in 2005. Since it is under-reported, estimates are that more than 600,000 cases occur each year.2
Neisseria gonorrhoeae causes infection of the cervix, urethra, rectum, pharynx, and adnexa. It can also cause disseminated disease that can affect joints, heart, and the meninges.
Tracking the spread of resistant cases
Since the early 1990s, fluoroquinolones have been one of the recommended treatments for gonorrhea because of their availability as effective, single-dose oral regimens. Fluoroquinolone-resistant N gonorrhea began to emerge at the end of the century and has progressed rapidly since. FIGURE 1 illustrates the proportion of fluoroquinolone-resistant N gonorrhea from the CDC’s Gonococcal Isolate Surveillance Project (GISP) by year, from 1990 to 2006.
Resistance began to emerge first among gonorrhea isolates from men who have sex with men (MSM), and resistance rates among MSM continue to be higher than in heterosexual men (FIGURE 2).
Geographic trends. In 2000, the CDC recommended that quinolones should no longer be used to treat gonorrhea in persons who contracted the infection in Asia or the Pacific. In 2002, California was added to this list. In 2004, the recommendation against quinolone use was extended to all MSM in the US.
The new recommendation against general use is based on resistance surpassing 5% of total isolates.
FIGURE 1
Percentage of N gonorrhoeae isolates with intermediate resistance or resistance to ciprofloxacin
Data for 2006 are preliminary (January-June only).
* Demonstrating ciprofloxacin minimum inhibitory concentration of 0.125–0.500 mcg/mL.
† Demonstrating ciprofloxacin minimum inhibitory concentration of ≥1.0 mcg/ml.
Source: Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinalones no longer recommended for treatment of gonococcal infections.
MMWR Recomm Rep 2007; 56:332-336.
FIGURE 2
Progressive increase of fluoroquinolone resistance
Percent of isolates from the CDC Gonococcal Isolate Surveillance Project found to be resistant to fluoroquinalones, 2002 through June 2006
Source: GISP report. Centers for Disease Control and Prevention.
Sexually Transmitted Disease Surveillance 2005 Supplement, Gonoccal Isolate Surveillance Project (GISP) Annual Report 2005. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, January 2007.
Ceftriaxone, the default treatment of choice
The loss of quinolones as a recommended gonorrhea treatment leaves only ceftriaxone, 125 mg intramuscularly (IM), as the only readily available treatment for urogenital, anorectal, and pharyngeal gonorrhea. Cefixime 400 mg as a single dose is also recommended, but is not currently available in tablet form in the US. It is available as a suspension with 100 mg per 5 cc.
Other options
Possible oral options include cefpodoxime 400 mg or cefuroxime axetil 1 g. However, neither has the official endorsement of the CDC, and neither appears effective against pharyngeal infection.
Spectinomycin 2 g intramuscularly is recommended for those with cephalosporin allergy—but, like cefixime, it is not currently available in the US, and it also is not considered effective against pharyngeal infection.
Azithromycin 2 g orally as a single dose is currently effective against gonorrhea and is an option for those with cephalosporin allergies. The CDC discourages its widespread use because of concerns about resistance.
New information regarding the availability of spectinomycin and cefixime can be obtained from local health departments or the CDC’s sexually transmitted diseases web site (www.cdc.gov/std).3
Recommended regimens for treatment of gonorrhea
| Uncomplicated gonococcal infections of the cervix, urethra, and rectum* |
| Recommended regimens† |
| Ceftriaxone 125 mg in a single IM dose |
| or |
| Cefixime‡ 400 mg in a single oral dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| Uncomplicated gonococcal infections of the pharynx* |
| Recommended regimens |
| Ceftriaxone 125 mg in a single IM dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| * For all adult and adolescent patients, regardless of travel history or sexual behavior. For those allergic to penicillins or cephalosporins, or for treatment of disseminated gonococcal infections, PID, and epididymitis, see www.cdc.gov/std/treatment. |
| † Alternative regimens: Spectinomycin 2 g in a single IM dose (not currently available in US) or cephalosporin single-dose regimens. |
| Other single-dose cephalosporin regimens that are considered alternative treatment regimens against uncomplicated urogenital and anorectal gonococcal infections include ceftizoxime 500 mg IM; or cefoxitin 2 g IM, administered with probenecid 1 g orally; or cefotaxime 500 mg IM. Some evidence indicates that cefpodoxime 400 mg and cefuroxime axetil 1 g might be oral alternatives. |
| ‡ 400 mg by suspension; tablets are no longer available in the US. |
| Source: www.cdc.gov/mmwr/PDF/rr/rr5511.pdf.2 |
Associated conditions
Treat for chlamydia if chlamydial infection is not ruled out
The CDC continues to recommend concurrent treatment for chlamydia for all persons who have gonorrhea, unless coinfection has been ruled out.
Therapies for chlamydia include azithromycin 1 g as a single dose or doxycycline 100 mg twice a day for 7 days.
Pelvic inflammatory disease and epididymitis
The treatment of both pelvic inflammatory disease (PID) and epididymitis include an option of ceftriaxone 250 mg IM plus doxycycline for either 7 days (for epididymitis) or 10 days (for PID). There are several parenteral options for PID and disseminated gonorrhea; these can be found on the CDC’s STD web site.3
Should you always retest to ensure a cure?
It is still not necessary to retest patients who have had the recommended treatments. However, patients with persistent symptoms or rapidly recurring symptoms should be retested by cultures so that drug-resistance patterns can be checked if gonorrhea is documented.
Retest for recurrence
Consider retesting all treated patients after 3 to 6 months, since anyone with a sexually transmitted infection is at risk of being reinfected.
Summary
The ongoing challenges with the evolving resistance patterns of gonorrhea illustrate the importance of physicians accurately diagnosing gonorrhea, treating with recommended regimens, reporting positive cases to the local public health department, and assisting with partner evaluation and treatment.
- The CDC no longer recommends the use of fluoroquinolones for the treatment of gonococcal infections and associated conditions such as pelvic inflammatory disease (PID).
- Consequently, only one class of drugs, the cephalosporins, is still recommended and available for the treatment of gonorrhea.
- The CDC now recommends ceftriaxone, 125 mg IM, in a single dose, as the preferred treatment.
- For patients with cephalosporin allergies, azithromycin, 2 g orally, as a single dose, remains an option. The CDC discourages widespread use, however, because of concerns about resistance.
The Centers for Disease Control and Prevention (CDC) recently released an update to its treatment guidelines for sexually transmitted diseases, stating that fluoroquinolones are no longer recommended for treatment of gonococcal infections.1 This change resulted from a progressive increase in the rate of resistance to quinolones among gonorrhea isolated from publicly funded treatment centers across the country.
The new advisory applies to all quinolones previously recommended: ciprofloxacin, ofloxacin, and levofloxacin.
Epidemiology. Gonorrhea remains common in the United States, with nearly 340,000 cases reported in 2005. Since it is under-reported, estimates are that more than 600,000 cases occur each year.2
Neisseria gonorrhoeae causes infection of the cervix, urethra, rectum, pharynx, and adnexa. It can also cause disseminated disease that can affect joints, heart, and the meninges.
Tracking the spread of resistant cases
Since the early 1990s, fluoroquinolones have been one of the recommended treatments for gonorrhea because of their availability as effective, single-dose oral regimens. Fluoroquinolone-resistant N gonorrhea began to emerge at the end of the century and has progressed rapidly since. FIGURE 1 illustrates the proportion of fluoroquinolone-resistant N gonorrhea from the CDC’s Gonococcal Isolate Surveillance Project (GISP) by year, from 1990 to 2006.
Resistance began to emerge first among gonorrhea isolates from men who have sex with men (MSM), and resistance rates among MSM continue to be higher than in heterosexual men (FIGURE 2).
Geographic trends. In 2000, the CDC recommended that quinolones should no longer be used to treat gonorrhea in persons who contracted the infection in Asia or the Pacific. In 2002, California was added to this list. In 2004, the recommendation against quinolone use was extended to all MSM in the US.
The new recommendation against general use is based on resistance surpassing 5% of total isolates.
FIGURE 1
Percentage of N gonorrhoeae isolates with intermediate resistance or resistance to ciprofloxacin
Data for 2006 are preliminary (January-June only).
* Demonstrating ciprofloxacin minimum inhibitory concentration of 0.125–0.500 mcg/mL.
† Demonstrating ciprofloxacin minimum inhibitory concentration of ≥1.0 mcg/ml.
Source: Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinalones no longer recommended for treatment of gonococcal infections.
MMWR Recomm Rep 2007; 56:332-336.
FIGURE 2
Progressive increase of fluoroquinolone resistance
Percent of isolates from the CDC Gonococcal Isolate Surveillance Project found to be resistant to fluoroquinalones, 2002 through June 2006
Source: GISP report. Centers for Disease Control and Prevention.
Sexually Transmitted Disease Surveillance 2005 Supplement, Gonoccal Isolate Surveillance Project (GISP) Annual Report 2005. Atlanta, Ga: US Department of Health and Human Services, Centers for Disease Control and Prevention, January 2007.
Ceftriaxone, the default treatment of choice
The loss of quinolones as a recommended gonorrhea treatment leaves only ceftriaxone, 125 mg intramuscularly (IM), as the only readily available treatment for urogenital, anorectal, and pharyngeal gonorrhea. Cefixime 400 mg as a single dose is also recommended, but is not currently available in tablet form in the US. It is available as a suspension with 100 mg per 5 cc.
Other options
Possible oral options include cefpodoxime 400 mg or cefuroxime axetil 1 g. However, neither has the official endorsement of the CDC, and neither appears effective against pharyngeal infection.
Spectinomycin 2 g intramuscularly is recommended for those with cephalosporin allergy—but, like cefixime, it is not currently available in the US, and it also is not considered effective against pharyngeal infection.
Azithromycin 2 g orally as a single dose is currently effective against gonorrhea and is an option for those with cephalosporin allergies. The CDC discourages its widespread use because of concerns about resistance.
New information regarding the availability of spectinomycin and cefixime can be obtained from local health departments or the CDC’s sexually transmitted diseases web site (www.cdc.gov/std).3
Recommended regimens for treatment of gonorrhea
| Uncomplicated gonococcal infections of the cervix, urethra, and rectum* |
| Recommended regimens† |
| Ceftriaxone 125 mg in a single IM dose |
| or |
| Cefixime‡ 400 mg in a single oral dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| Uncomplicated gonococcal infections of the pharynx* |
| Recommended regimens |
| Ceftriaxone 125 mg in a single IM dose |
| plus |
| Treatment for chlamydia if chlamydial infection has not been ruled out |
| * For all adult and adolescent patients, regardless of travel history or sexual behavior. For those allergic to penicillins or cephalosporins, or for treatment of disseminated gonococcal infections, PID, and epididymitis, see www.cdc.gov/std/treatment. |
| † Alternative regimens: Spectinomycin 2 g in a single IM dose (not currently available in US) or cephalosporin single-dose regimens. |
| Other single-dose cephalosporin regimens that are considered alternative treatment regimens against uncomplicated urogenital and anorectal gonococcal infections include ceftizoxime 500 mg IM; or cefoxitin 2 g IM, administered with probenecid 1 g orally; or cefotaxime 500 mg IM. Some evidence indicates that cefpodoxime 400 mg and cefuroxime axetil 1 g might be oral alternatives. |
| ‡ 400 mg by suspension; tablets are no longer available in the US. |
| Source: www.cdc.gov/mmwr/PDF/rr/rr5511.pdf.2 |
Associated conditions
Treat for chlamydia if chlamydial infection is not ruled out
The CDC continues to recommend concurrent treatment for chlamydia for all persons who have gonorrhea, unless coinfection has been ruled out.
Therapies for chlamydia include azithromycin 1 g as a single dose or doxycycline 100 mg twice a day for 7 days.
Pelvic inflammatory disease and epididymitis
The treatment of both pelvic inflammatory disease (PID) and epididymitis include an option of ceftriaxone 250 mg IM plus doxycycline for either 7 days (for epididymitis) or 10 days (for PID). There are several parenteral options for PID and disseminated gonorrhea; these can be found on the CDC’s STD web site.3
Should you always retest to ensure a cure?
It is still not necessary to retest patients who have had the recommended treatments. However, patients with persistent symptoms or rapidly recurring symptoms should be retested by cultures so that drug-resistance patterns can be checked if gonorrhea is documented.
Retest for recurrence
Consider retesting all treated patients after 3 to 6 months, since anyone with a sexually transmitted infection is at risk of being reinfected.
Summary
The ongoing challenges with the evolving resistance patterns of gonorrhea illustrate the importance of physicians accurately diagnosing gonorrhea, treating with recommended regimens, reporting positive cases to the local public health department, and assisting with partner evaluation and treatment.
1. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007;56:332-336.Available at: www.cdc.gov/mmwr/pdf/wk/mm5614.pdf. Accessed on June 15, 2007.
2. CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11).-Available at www.cdc.gov/mmwr/PDF/rr/rr5511.pdf. Accessed on June 15, 2007.
3. Updated recommended treatment regimens for gonococcal infections and associated conditions—United States, April 2007. Available at: www.cdc.gov/std/treatment/2006/updated-regimens.htm. Accessed on June 15, 2007.
1. CDC. Update to CDC’s sexually transmitted diseases treatment guidelines, 2006: Fluoroquinolones no longer recommended for treatment of gonococcal infections. MMWR Morb Mortal Wkly Rep 2007;56:332-336.Available at: www.cdc.gov/mmwr/pdf/wk/mm5614.pdf. Accessed on June 15, 2007.
2. CDC. Sexually transmitted diseases treatment guidelines, 2006. MMWR Recomm Rep 2006;55(RR-11).-Available at www.cdc.gov/mmwr/PDF/rr/rr5511.pdf. Accessed on June 15, 2007.
3. Updated recommended treatment regimens for gonococcal infections and associated conditions—United States, April 2007. Available at: www.cdc.gov/std/treatment/2006/updated-regimens.htm. Accessed on June 15, 2007.