User login
Delirium presents as an acute change in mentation characterized by reduced attention, clouding of awareness, and typically an altered level of arousal. It can be caused by a host of medical conditions, medications, or other psychoactive substances and is therefore encountered primarily in acute and postacute medical settings.1 More than a quarter of all hospitalized patients develop delirium,2 with rates up to 80% in the critically ill.3 Similarly, delirium occurs in more than one-third of patients who transition to postacute care.4 These high prevalence rates are alarming, especially because delirium is a risk factor for mortality, prolonged hospitalization, institutionalization, and overall higher cost of care.5 However, more than a quarter of delirium is preventable.6 Evidence-based guidelines for delirium uniformly call for multicomponent prevention strategies,7 and these are best delivered through collaborative models of care. In short, delirium impacts healthcare systems; therefore, interventions aimed at preventing delirium and its consequences ought to be systems-based.
Since the Institute of Medicine issued its 1999 report highlighting the critical role of medical errors in healthcare, healthcare systems have increasingly become team-based.8 “Medical care is inherently interdependent,”9 and this implies that delirium prevention rests not only on individuals but also on broader systems of care. Although nonpharmacological interventions are efficacious at preventing delirium,10 previous reviews have focused on specific interventions or multiple interventions rather than the systems of care needed to deliver them. Indeed, teams and the quality of their teamwork impact outcomes.11
Herein, we provide a systematic review and meta-analysis of integrated models of care designed to prevent delirium. What distinguishes this review from previous reviews of nonpharmacological interventions to prevent delirium is our focus on discrete models of care that involve collaboration among clinicians. Our goal is to identify the most promising models that deserve further development, investigation, and dissemination. Viewing delirium prevention through a collaborative care lens is consistent with efforts to achieve value-based care and may encourage drawing from the expanding literature outlining the benefits of mental healthcare integration.12,13 Specifically, a systems perspective highlights the potential for system-wide benefits such as reducing readmissions14,15 and cost savings.16
METHODS
This systematic review and meta-analysis follows PRISMA guidelines. A search of OVID, MEDLINE, CINAHL, Cochrane Database of Systematic Reviews, EMBASE, and PsycINFO was completed by a medical librarian for clinical studies in which models of care were implemented to prevent delirium using PICO (P patient, problem or population; I, intervention; C, comparison, control or comparator; O, outcome) inquiries. Search terms included delirium, acute confusional state, altered mental status, prevention, and control (“delirium”/exp OR “acute confusion”/exp OR “altered mental status”/exp) AND “prevention and control”/exp AND [English]/lim AND [embase]/lim).
One researcher (AK) screened articles by title for relevance. Relevant articles were then divided among four authors (AK, MO, NF, and OB), and the abstracts were screened for eligibility. The authors reviewed the full texts of any potentially eligible studies. Each full text was assigned to two authors for full review. Discrepancies were adjudicated by conference among all authors. In addition, references within all full-text publications were scanned for potential additional articles.
The inclusion criteria for review of full-text articles required English-language description of a model of care with multiple interventions, delirium reported as an outcome, and presence of a comparator group.
“Model of care” was defined by the Cochrane Effective Practice and Organization of Care Review Group as follows: (1) revision of professional roles, including shifting of professional roles or expansion of roles to new tasks; (2) creation of clinical multidisciplinary teams or addition of new members to the team who collaborate inpatient care; (3) delivery of multiple interventions across multiple domains (ie, studies involving a single intervention such as physical therapy or targeting a single domain such as sleep were excluded); and (4) formal integration of services whereby teams work together in collaboration with existing services to enhance care.17 For this review, we required that studies include a comparator group so that effectiveness of the intervention could be assessed. Quality improvement studies that lacked a comparator group were excluded.
Delirium incidence was the primary outcome and was evaluated by meta-analysis. Heterogeneity was assessed using I2 and visual inspection of forest plots. I2 values of 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively. The studies were pooled according to study type as follows: randomized controlled trials, pre–post design, and other nonrandomized prospective studies. Random effects models were used to calculate estimates using the Comprehensive Meta-Analysis software (Version 3, Biostat, Englewood, New Jersey), which also generated forest plots.
Risk of bias was assessed using criteria established by the Cochrane Collaborative Review Criteria, which lists six categories of potential bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting.17 Each study was assessed by two authors (either MO and AK or MO-P. and OB) for bias and a numerical value was assigned to each of the six categories as follows: 1 = low risk, 2 = unknown/moderate risk, and 3 = high risk. Where scorers disagreed, all authors jointly conferred, and a consensus score was given. The values for each of these six categories were added to create a composite risk-of-bias score for each study, with 6 being the lowest possible score and 18 the highest. Overall risk was classified as follows: <9 = low risk, 9-12 = moderate risk, and >12 = high risk.
RESULTS
Study Selection Process
An initial literature search identified 352 articles. After reviewing the titles, 308 articles were excluded for irrelevance, and 44 abstracts were screened for eligibility. We excluded 27 articles upon abstract review, and the full texts of 17 were obtained for detailed review. In addition, we identified another 10 potentially eligible articles through review of references and obtained full texts of these as well. Of the 27 full-text articles reviewed, 15 were included in this systematic review, 10 of which were suitable for meta-analysis. The Figure shows the PRISMA flow chart.
Study Characteristics
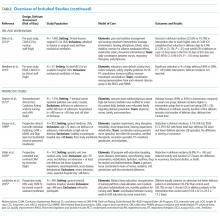
The 15 studies that met the inclusion criteria are summarized in the Table.18-32 Delirium prevention was among the primary outcomes of 13 studies; delirium outcomes were reported in the other two studies as well, which were primarily designed to assess feasibility.26,27 Six studies were conducted in the United States, three in Sweden, two in Spain, two in the United Kingdom, and one each conducted in Korea and Canada. Healthcare settings among the included studies involved the intensive care unit (six studies), medical floors (four studies), surgical floors (three studies), a long-term care unit (one study), and
Outcomes Reported
All but one of the studies reported delirium incidence. The most commonly used delirium screening instrument was the Confusion Assessment Method (CAM) or its modified version, the CAM-ICU (11 studies).33,34 Other methods used to assess mentation included the Richmond Agitation Sedation Scale,35 the Organic Brain Syndrome scale,36 the revised Delirium Rating Scale,37 the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition,38 and the Confusion Rating Scale.39 (Details regarding delirium screening tools can be found in the systematic review by De and Wand.40) Researchers performed delirium assessment in nine studies, whereas assessments were performed by clinical staff in the remaining studies. Other outcomes reported included length of stay (LOS), mortality, number of days ventilated, and functional decline. None of the included studies reported cost effectiveness.
Risk of Bias Assessment
Risk of bias assessment identified only two studies—both randomized controlled trials—as low risk (Table). The remaining studies had moderate (four studies) or high risk (nine studies).
Results from Individual Studies
Of the 15 studies, nine reported a statistically significant reduction in delirium incidence, and another two reported a statistically insignificant reduction. In addition, seven of the eight studies that assessed delirium duration found reduced duration in the intervention cohort, and two of the three studies that reported delirium severity found a reduction in the intervention group.
Results of Meta-Analysis
Random effects models were created to meta-analyze groups of studies based on design as follows: randomized controlled trials (three studies18,19,25), pre–post intervention studies (four of six studies included28-31), and other nonrandomized studies (three of four studies included21-23). Meta-analysis was not completed for the two feasibility studies26,27 because delirium outcome data were limited due to the feasibility study design. The study of Dale et al.32 was excluded from the meta-analysis because the rates of CAM-ICU completion differed substantially between control and intervention groups (0.35 vs 1.49 per 24 hours, respectively), leading to imbalanced between-group sensitivity in delirium detection and Needham et al.20 was also excluded because it reported only days of delirium, not delirium incidence. The study by Lundström et al.24 was also excluded from the meta-analysis because delirium incidence was measured on days 1, 3, and 5, whereas the other studies reported delirium daily.
Meta-analysis of the three randomized controlled trials revealed a pooled odds ratio of 0.56 (95% CI: 0.37-0.85; P = .006) for delirium incidence among intervention group subjects relative to those in comparator groups. The heterogeneity across studies was low (I2 = 29%). Pooling data from four pre–post studies found that the odds ratio for delirium incidence was 0.63 (95% CI: 0.37-1.07; P = .09). The heterogeneity across these studies was moderate (I2 = 65%). Results from the three eligible, nonrandomized prospective studies were also pooled. The odds ratio for developing delirium among study subjects was 0.79 (95% CI: 0.46-1.37; P = .40), and the heterogeneity among these studies was high (I2 = 85%).
DISCUSSION
We provide a systematic review and meta-analysis of delirium preventive models of care. Meta-analysis of the three randomized controlled trials found that these models of care led to a statistically significant reduction in delirium incidence; study subjects had an 11.5% reduction in absolute delirium incidence. The pooled odds ratios for both of the other sets of nonrandomized studies favored the intervention group but were not significant, each because of one included study. The pre–post meta-analysis failed to reach significance as one of the included studies found a trend toward higher delirium incidence; however, interestingly, in that same study, the overall delirium-free days were significantly reduced overall (24 vs 27, P = .002). Similarly, meta-analysis of the three additional nonrandomized prospective studies failed to reach significance because the largest included study found higher rates of delirium among intervention group subjects. Despite considerable risk of bias in several of these studies, their findings were broadly consistent; all but one study (Gagnon 201221) reported a trend or a significant reduction in delirium incidence, duration, severity, or number of delirium episodes. Moreover, the value of such models of care extended beyond preventing delirium; for instance, other positive outcomes included reduced LOS and fewer medical complications.
Models of care ranged widely with respect to specific interventions, though several common elements highlighted their relevance for delirium care and as potential delirium prevention strategies in future studies. For example, two of the randomized controlled trials18,19 employed early mobilization, enhanced nutrition, sleep hygiene, early reduction of invasive procedures (eg, urinary catheterization), and pain control in their multicomponent models. Five additional studies also incorporated early mobilization,20,22,23,31,32 and three sought to improve sleep quality.22,28,30 Among other important strategies were delirium screening,18,20,22,30,31 monitoring medication,18,20,22,26,28,30,32 orientation,18,21,23,28 addressing vision and hearing impairment,18,22,23,32 hydration,18,22,23 avoiding hypoxia,18,20,30 and staff, patient, and caretaker education.19,21,23,27-30 Unique strategies were implemented in certain studies. For instance, one study used massage therapy,28 preventing delays in transfer logistics in another,30 and a third addressed psychosocial problems.25 Overall, the selection of strategies depended on the patient setting; thus, no one care bundle should be expected to emerge as a universal model for delirium prevention. Rather, these results should be interpreted within their specific care contexts and judged on the quality of evidence (eg, effect size and statistically significant findings, low risk of bias, sound experimental design). The one study that failed to find any positive effect on delirium, that of Gagnon et al.,21 was conducted on an inpatient palliative care service in Canada, and its negative finding may reflect the unique delirium risk factors in patients who are nearing end of life.
This current review differs from previous delirium prevention reviews in operationally defining a “model of care.” We identified a great deal of variation in specific models and team composition. For example, some interventions were carried out by nurses18-20,31 and physicians,20,21,25,32 whereas others involved physical therapists,20,22,28 medical residents,23 geriatricians,22,23,25 pharmacists,26 researchers,18 and trained volunteers.22 In all cases, the staff roles were expanded to include new tasks, and the clinical team worked collaboratively to administer interventions across multiple domains. Team-related considerations are critical because modern medical care is inherently interdependent.9 These broad differences in team composition across studies demonstrate the number of potential options for team structure and function. They also highlight the number of “moving parts” to be considered when designing and implementing delirium care bundles.
Most of the delirium prevention studies implementing models of care are characterized by a substantial risk of bias. We evaluated risk of bias along six categories of potential sources, including random assignment to groups, ability to foresee future group allocation, blinding of participants and personnel to group assignment, blinding of outcome assessment, completeness of outcome data, selective reporting, and other potential sources of bias.17 Two of the three studies that used randomization had a low risk of bias, and four additional studies had a moderate risk of bias. Allocation concealment was accomplished only in randomized controlled trials, whereas blinding of both subjects and study personnel was not implemented in any of the studies. Although some studies relied on data analysis by research personnel blinded to group membership or the nature of the intervention, others failed to do so or failed to describe data analysis in sufficient detail. Studies also failed to report the percentage of unscorable or otherwise omitted delirium assessments necessary to calculate attrition rates or to understand the comprehensiveness of outcome assessment in a systematic manner. Other potential sources of bias included systematic differences between the intervention and control groups (such as differences in gender composition, age, or delirium risk) at study outset.
A primary limitation of this review is the heterogeneity of settings, interventions, and models of care across included studies. We excluded several studies from this review for being delivered by a single individual or service line (eg, introduction of a geriatric consult service, physical therapy, or volunteers), for providing a single intervention (eg, early ambulation alone), or for multiple interventions targeting a single domain (eg, sleep). We did so because the future of value-based care lies in collaboration of providers and services, and in a way the complexity across and within these studies ultimately reflects the complexity of medical settings as well as the multifactorial nature of delirium. The broader message is a call for increasing the integration of delirium-related care services. As discussed earlier, the high risk of bias across these studies is a limitation of our findings; high-quality evidence on the value of delirium prevention models of care remains limited. Thus, although our review suggests that there are multicomponent models of care that hold promise in mitigating delirium and its outcomes, additional randomized studies are required to confirm the efficacy of such models of care and to test which services, interventions, and clinical domains deserve priority.
CONCLUSION
To our knowledge, this is the first systematic review and meta-analysis of delirium preventive models of care. Models of care, as defined here, necessarily included a multidisciplinary team in which traditional staff roles had been revised to implement a multicomponent, multidomain intervention. Other recent reviews are available for multicomponent pharmacological and nonpharmacological interventions to prevent and manage delirium,41-49 but just as important as which interventions are being delivered is the team that delivers them. Care delivery in a complex medical system is more than handing a patient a medication or facilitating ambulation; it requires a choreographed dance of teamwork and integration across services. This review identifies promising models of care that deserve further recognition, refinement, and ultimately widespread implementation.
Acknowledgments
The authors comprise a writing group created through the Delirium Boot Camp, an annual meeting originally sponsored by the Center of Excellence for Delirium in Aging: Research, Training, and Educational Enhancement (CEDARTREE, Boston, Massachusetts); it is currently supported by the Network for Investigation of Delirium: Unifying Scientists (NIDUS, Boston, Massachusetts). The authors would like to thank medical librarian Rita Mitchell (Aurora Health Care, Milwaukee, Wisconsin) for the literature search, senior scientific writer and editor Joe Grundle (Aurora Research Institute, Milwaukee, Wisconsin) for editorial assistance, and graphics specialist Brian Miller (Aurora Research Institute, Milwaukee, Wisconsin) for help with the figures.
Disclosures
The authors report no relevant conflicts of interest.
Funding
No funding was dedicated to the conduct of this review.
1. American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing, Inc.
2. Schubert M, Schürch R, Boettger S, et al. A hospital-wide evaluation of delirium prevalence and outcomes in acute care patients - A cohort study. BMC Health Serv Res. 2018;18(1):550. https://doi.org/10.1186/s12913-018-3345-x.
3. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the Intensive Care Unit. JAMA. 2004;291(14):1753-1762. https://doi.org/10.1001/jama.291.14.1753.
4. Gual N, Morandi A, Pérez LM, et al. Risk factors and outcomes of delirium in older patients admitted to postacute care with and without dementia. Dement Geriatr Cogn Disord. 2018;45(1-2):121-129. https://doi.org/10.1159/000485794.
5. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. https://doi.org/10.1056/NEJMcp1605501.
6. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. https://doi.org/10.1016/S0140-6736(13)60688-1.
7. Bush SH, Marchington KL, Agar M, et al. Quality of clinical practice guidelines in delirium: A systematic appraisal. BMJ Open. 2017;7(3):e013809. https://doi.org/10.1136/bmjopen-2016-013809.
8. Institute of Medicine. 2000. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press. https://doi.org/10.17226/9728.
9. Rosen MA, DiazGranados D, Dietz AS, et al. Teamwork in healthcare: key discoveries enabling safer, high-quality care. Am Psychol. 2018;73(4):433-450. https://doi.org/10.1037/amp0000298.
10. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: A systematic overview. The SENATOR project ONTOP series. PLOS ONE. 2015;10(6):e0123090. https://doi.org/10.1371/journal.pone.0123090.
11. Thomas EJ. Improving teamwork in healthcare: current approaches and the path forward. BMJ Qual Saf. 2011;20(8):647-650. https://doi.org/10.1136/bmjqs-2011-000117.
12. Sledge W, Bozzo J, White-McCullum B, Lee H. The cost-benefit from the perspective of the hospital of a proactive psychiatric consultation service on inpatient general medicine services. Health Econ Outcome -Res. 2016;2:2-6.
13. Unützer J, Katon WJ, Fan MY, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14(2):95-100. PubMed
14. Lee E, Kim J. Cost-benefit analysis of a delirium prevention strategy in the intensive care unit. Nurs Crit Care. 2014;21:367-373. https://doi.org/10.1111/nicc.12124.
15. Rubin FH, Bellon J, Bilderback A, Urda K, Inouye SK. Effect of the hospital elder life program on risk of 30-day readmission. J Am Geriatr Soc. 2018;66(1):145-149. https://doi.org/10.1111/jgs.15132.
16. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the hospital elder life program in a community hospital. Psychosomatics. 2013;54(3):219-226. https://doi.org/10.1016/j.psym.2013.01.010.
17. Cochrane effective practice and organisation of Care Group (EPOC). Data collection Checklistist. Chochrane Effective Practice and Organisation of Care Group (EPOC) Methods Papers. . https://methods.cochrane.org/sites/methods.cochrane.org.bias/files/public/uploads/EPOC Data Collection Checklist.pdf. Accessed May 27, 2014.
18. Moon KJ, Lee SM. The effects of a tailored intensive care unit delirium prevention protocol: A randomized controlled trial. Int J Nurs Stud. 2015;52(9):1423-1432. https://doi.org/10.1016/j.ijnurstu.2015.04.021.
19. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res-. 2007;19(3):178-186. https://doi.org/10.1007/BF03324687.
20. Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients With acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536-542. https://doi.org/10.1016/j.apmr.2010.01.002.
21. Gagnon P, Allard P, Gagnon B, Mérette C, Tardif F. Delirium prevention in terminal cancer: assessment of a multicomponent intervention. Psychooncology. 2012;21(2):187-194. https://doi.org/10.1002/pon.1881.
22. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901.
23. Vidán MT, Sánchez E, Alonso M, et al. An intervention integrated into daily clinical practice reduces the incidence of delirium during hospitalization in elderly patients. J Am Geriatr Soc. 2009;57(11):2029-2036. https://doi.org/10.1111/j.1532-5415.2009.02485.x.
24. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. https://doi.org/10.1111/j.1532-5415.2005.53210.x.
25. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: A randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x.
26. Rice KL, Bennett MJ, Berger L, et al. A pilot randomized controlled trial of the feasibility of a multicomponent delirium prevention intervention versus usual care in acute stroke. J Cardiovasc Nurs. 2017;32(1):E1-E10. https://doi.org/10.1097/JCN.0000000000000356.
27. Siddiqi N, Cheater F, Collinson M, et al. The PiTSTOP study: a feasibility cluster randomized trial of delirium prevention in care homes for older people. Age Ageing. 2016;45(5):652-661. https://doi.org/10.1093/ageing/afw091.
28. Bryczkowski SB, Lopreiato MC, Yonclas PP, Sacca JJ, Mosenthal AC. Delirium prevention program in the surgical intensive care unit (SICU) improved the outcomes of older adults. J Surg Res. 2014;186:519. https://doi.org/10.1016/j.jss.2013.11.352
29. Holt R, Young J, Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing. 2013;42(6):721-727. https://doi.org/10.1093/ageing/aft120.
30. Björkelund KB, Hommel A, Thorngren KG, et al. Reducing delirium in elderly patients with hip fracture: A multi-factorial intervention study. Acta Anaesthesiol-Scand. 2010;54(6):678-688. https://doi.org/10.1111/j.1399-6576.2010.02232.x.
31. Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility (ABCDE) bundle. Crit Care Med. 2014;42(5):1024-1036. https://doi.org/10.1097/CCM.0000000000000129.
32. Dale CR, Kannas DA, Fan VS, et al. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am Thorac Soc. 2014;11(3):367-374. https://doi.org/10.1513/AnnalsATS.201306-210OC.
33. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. https://doi.org/10.7326/0003-4819-113-12-941.
34. Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370-1379. https://doi.org/10.1097/00003246-200107000-00012.
35. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. https://doi.org/10.1164/rccm.2107138.
36. Jensen E, Dehlin O, Gustafson L. A comparison between three psychogeriatric rating scales. Int J Geriatr Psychiatry. 1993;8(3):215-229. https://doi.org/10.1002/gps.930080305.
37. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatr Clin Neurosci. 2001;13(2):229-242. https://doi.org/10.1176/jnp.13.2.229.
38. American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Arlington, VA, US: American Psychiatric Publishing, Inc.
39. Williams MA. Delirium/acute confusional states: evaluation devices in nursing. Int Psychogeriatr. 1991;3(2):301-308. PubMed
40. De J, Wand APF. Delirium screening: A systematic review of delirium screening tools in hospitalized patients. Gerontologist-. 2015;55(6):1079-1099. https://doi.org/10.1093/geront/gnv100.
41. Martinez F, Tobar C, Hill N. Preventing delirium: should non-pharmacological,
multicomponent interventions be used? A systematic review and meta-analysis of the literature. Age Ageing. 2015;44(2):196-204. https://doi.org/10.1093/ageing/afu173.
42. Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5):375-380. https://doi.org/10.7326/0003-4819-158-5-201303051-00003.
43. Rivosecchi RM, Smithburger PL, Svec S, Campbell S, Kane-Gill SL. Nonpharmacological interventions to prevent delirium: an evidence-based systematic review. Crit Care Nurse. 2015;35(1):39-50; quiz 51. https://doi.org/10.4037/ccn2015423.
44. Trogrlić Z, van der Jagt M, Bakker J, et al. A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Crit Care. 2015;19:157. https://doi.org/10.1186/s13054-015-0886-9.
45. Wang Y, Tang J, Zhou F, Yang L, Wu J. Comprehensive geriatric care reduces acute perioperative delirium in elderly patients with hip fractures: A meta-analysis. Medicine (Baltimore). 2017;96(26):e7361. https://doi.org/10.1097/MD.0000000000007361.
46. Shields L, Henderson V, Caslake R. Comprehensive geriatric assessment for prevention of delirium After hip fracture: A systematic review of randomized controlled trials. J Am Geriatr Soc. 2017;65(7):1559-1565. https://doi.org/10.1111/jgs.14846.
47. Oberai T, Lizarondo L, Ruurd J. Effectiveness of multi-component interventions on incidence of delirium in hospitalized older patients with hip fracture: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2017;15(2):259-268. https://doi.org/10.11124/JBISRIR-2016-002943.
48. Collinsworth AW, Priest EL, Campbell CR, Vasilevskis EE, Masica AL. A review of multifaceted care approaches for the prevention and mitigation of delirium in intensive care units. J Intensive Care Med. 2016;31(2):127-141. https://doi.org/10.1177/0885066614553925.
49. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological
delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. https://doi.org/10.1001/jamainternmed.2014.7779.
Delirium presents as an acute change in mentation characterized by reduced attention, clouding of awareness, and typically an altered level of arousal. It can be caused by a host of medical conditions, medications, or other psychoactive substances and is therefore encountered primarily in acute and postacute medical settings.1 More than a quarter of all hospitalized patients develop delirium,2 with rates up to 80% in the critically ill.3 Similarly, delirium occurs in more than one-third of patients who transition to postacute care.4 These high prevalence rates are alarming, especially because delirium is a risk factor for mortality, prolonged hospitalization, institutionalization, and overall higher cost of care.5 However, more than a quarter of delirium is preventable.6 Evidence-based guidelines for delirium uniformly call for multicomponent prevention strategies,7 and these are best delivered through collaborative models of care. In short, delirium impacts healthcare systems; therefore, interventions aimed at preventing delirium and its consequences ought to be systems-based.
Since the Institute of Medicine issued its 1999 report highlighting the critical role of medical errors in healthcare, healthcare systems have increasingly become team-based.8 “Medical care is inherently interdependent,”9 and this implies that delirium prevention rests not only on individuals but also on broader systems of care. Although nonpharmacological interventions are efficacious at preventing delirium,10 previous reviews have focused on specific interventions or multiple interventions rather than the systems of care needed to deliver them. Indeed, teams and the quality of their teamwork impact outcomes.11
Herein, we provide a systematic review and meta-analysis of integrated models of care designed to prevent delirium. What distinguishes this review from previous reviews of nonpharmacological interventions to prevent delirium is our focus on discrete models of care that involve collaboration among clinicians. Our goal is to identify the most promising models that deserve further development, investigation, and dissemination. Viewing delirium prevention through a collaborative care lens is consistent with efforts to achieve value-based care and may encourage drawing from the expanding literature outlining the benefits of mental healthcare integration.12,13 Specifically, a systems perspective highlights the potential for system-wide benefits such as reducing readmissions14,15 and cost savings.16
METHODS
This systematic review and meta-analysis follows PRISMA guidelines. A search of OVID, MEDLINE, CINAHL, Cochrane Database of Systematic Reviews, EMBASE, and PsycINFO was completed by a medical librarian for clinical studies in which models of care were implemented to prevent delirium using PICO (P patient, problem or population; I, intervention; C, comparison, control or comparator; O, outcome) inquiries. Search terms included delirium, acute confusional state, altered mental status, prevention, and control (“delirium”/exp OR “acute confusion”/exp OR “altered mental status”/exp) AND “prevention and control”/exp AND [English]/lim AND [embase]/lim).
One researcher (AK) screened articles by title for relevance. Relevant articles were then divided among four authors (AK, MO, NF, and OB), and the abstracts were screened for eligibility. The authors reviewed the full texts of any potentially eligible studies. Each full text was assigned to two authors for full review. Discrepancies were adjudicated by conference among all authors. In addition, references within all full-text publications were scanned for potential additional articles.
The inclusion criteria for review of full-text articles required English-language description of a model of care with multiple interventions, delirium reported as an outcome, and presence of a comparator group.
“Model of care” was defined by the Cochrane Effective Practice and Organization of Care Review Group as follows: (1) revision of professional roles, including shifting of professional roles or expansion of roles to new tasks; (2) creation of clinical multidisciplinary teams or addition of new members to the team who collaborate inpatient care; (3) delivery of multiple interventions across multiple domains (ie, studies involving a single intervention such as physical therapy or targeting a single domain such as sleep were excluded); and (4) formal integration of services whereby teams work together in collaboration with existing services to enhance care.17 For this review, we required that studies include a comparator group so that effectiveness of the intervention could be assessed. Quality improvement studies that lacked a comparator group were excluded.
Delirium incidence was the primary outcome and was evaluated by meta-analysis. Heterogeneity was assessed using I2 and visual inspection of forest plots. I2 values of 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively. The studies were pooled according to study type as follows: randomized controlled trials, pre–post design, and other nonrandomized prospective studies. Random effects models were used to calculate estimates using the Comprehensive Meta-Analysis software (Version 3, Biostat, Englewood, New Jersey), which also generated forest plots.
Risk of bias was assessed using criteria established by the Cochrane Collaborative Review Criteria, which lists six categories of potential bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting.17 Each study was assessed by two authors (either MO and AK or MO-P. and OB) for bias and a numerical value was assigned to each of the six categories as follows: 1 = low risk, 2 = unknown/moderate risk, and 3 = high risk. Where scorers disagreed, all authors jointly conferred, and a consensus score was given. The values for each of these six categories were added to create a composite risk-of-bias score for each study, with 6 being the lowest possible score and 18 the highest. Overall risk was classified as follows: <9 = low risk, 9-12 = moderate risk, and >12 = high risk.
RESULTS
Study Selection Process
An initial literature search identified 352 articles. After reviewing the titles, 308 articles were excluded for irrelevance, and 44 abstracts were screened for eligibility. We excluded 27 articles upon abstract review, and the full texts of 17 were obtained for detailed review. In addition, we identified another 10 potentially eligible articles through review of references and obtained full texts of these as well. Of the 27 full-text articles reviewed, 15 were included in this systematic review, 10 of which were suitable for meta-analysis. The Figure shows the PRISMA flow chart.
Study Characteristics
The 15 studies that met the inclusion criteria are summarized in the Table.18-32 Delirium prevention was among the primary outcomes of 13 studies; delirium outcomes were reported in the other two studies as well, which were primarily designed to assess feasibility.26,27 Six studies were conducted in the United States, three in Sweden, two in Spain, two in the United Kingdom, and one each conducted in Korea and Canada. Healthcare settings among the included studies involved the intensive care unit (six studies), medical floors (four studies), surgical floors (three studies), a long-term care unit (one study), and
Outcomes Reported
All but one of the studies reported delirium incidence. The most commonly used delirium screening instrument was the Confusion Assessment Method (CAM) or its modified version, the CAM-ICU (11 studies).33,34 Other methods used to assess mentation included the Richmond Agitation Sedation Scale,35 the Organic Brain Syndrome scale,36 the revised Delirium Rating Scale,37 the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition,38 and the Confusion Rating Scale.39 (Details regarding delirium screening tools can be found in the systematic review by De and Wand.40) Researchers performed delirium assessment in nine studies, whereas assessments were performed by clinical staff in the remaining studies. Other outcomes reported included length of stay (LOS), mortality, number of days ventilated, and functional decline. None of the included studies reported cost effectiveness.
Risk of Bias Assessment
Risk of bias assessment identified only two studies—both randomized controlled trials—as low risk (Table). The remaining studies had moderate (four studies) or high risk (nine studies).
Results from Individual Studies
Of the 15 studies, nine reported a statistically significant reduction in delirium incidence, and another two reported a statistically insignificant reduction. In addition, seven of the eight studies that assessed delirium duration found reduced duration in the intervention cohort, and two of the three studies that reported delirium severity found a reduction in the intervention group.
Results of Meta-Analysis
Random effects models were created to meta-analyze groups of studies based on design as follows: randomized controlled trials (three studies18,19,25), pre–post intervention studies (four of six studies included28-31), and other nonrandomized studies (three of four studies included21-23). Meta-analysis was not completed for the two feasibility studies26,27 because delirium outcome data were limited due to the feasibility study design. The study of Dale et al.32 was excluded from the meta-analysis because the rates of CAM-ICU completion differed substantially between control and intervention groups (0.35 vs 1.49 per 24 hours, respectively), leading to imbalanced between-group sensitivity in delirium detection and Needham et al.20 was also excluded because it reported only days of delirium, not delirium incidence. The study by Lundström et al.24 was also excluded from the meta-analysis because delirium incidence was measured on days 1, 3, and 5, whereas the other studies reported delirium daily.
Meta-analysis of the three randomized controlled trials revealed a pooled odds ratio of 0.56 (95% CI: 0.37-0.85; P = .006) for delirium incidence among intervention group subjects relative to those in comparator groups. The heterogeneity across studies was low (I2 = 29%). Pooling data from four pre–post studies found that the odds ratio for delirium incidence was 0.63 (95% CI: 0.37-1.07; P = .09). The heterogeneity across these studies was moderate (I2 = 65%). Results from the three eligible, nonrandomized prospective studies were also pooled. The odds ratio for developing delirium among study subjects was 0.79 (95% CI: 0.46-1.37; P = .40), and the heterogeneity among these studies was high (I2 = 85%).
DISCUSSION
We provide a systematic review and meta-analysis of delirium preventive models of care. Meta-analysis of the three randomized controlled trials found that these models of care led to a statistically significant reduction in delirium incidence; study subjects had an 11.5% reduction in absolute delirium incidence. The pooled odds ratios for both of the other sets of nonrandomized studies favored the intervention group but were not significant, each because of one included study. The pre–post meta-analysis failed to reach significance as one of the included studies found a trend toward higher delirium incidence; however, interestingly, in that same study, the overall delirium-free days were significantly reduced overall (24 vs 27, P = .002). Similarly, meta-analysis of the three additional nonrandomized prospective studies failed to reach significance because the largest included study found higher rates of delirium among intervention group subjects. Despite considerable risk of bias in several of these studies, their findings were broadly consistent; all but one study (Gagnon 201221) reported a trend or a significant reduction in delirium incidence, duration, severity, or number of delirium episodes. Moreover, the value of such models of care extended beyond preventing delirium; for instance, other positive outcomes included reduced LOS and fewer medical complications.
Models of care ranged widely with respect to specific interventions, though several common elements highlighted their relevance for delirium care and as potential delirium prevention strategies in future studies. For example, two of the randomized controlled trials18,19 employed early mobilization, enhanced nutrition, sleep hygiene, early reduction of invasive procedures (eg, urinary catheterization), and pain control in their multicomponent models. Five additional studies also incorporated early mobilization,20,22,23,31,32 and three sought to improve sleep quality.22,28,30 Among other important strategies were delirium screening,18,20,22,30,31 monitoring medication,18,20,22,26,28,30,32 orientation,18,21,23,28 addressing vision and hearing impairment,18,22,23,32 hydration,18,22,23 avoiding hypoxia,18,20,30 and staff, patient, and caretaker education.19,21,23,27-30 Unique strategies were implemented in certain studies. For instance, one study used massage therapy,28 preventing delays in transfer logistics in another,30 and a third addressed psychosocial problems.25 Overall, the selection of strategies depended on the patient setting; thus, no one care bundle should be expected to emerge as a universal model for delirium prevention. Rather, these results should be interpreted within their specific care contexts and judged on the quality of evidence (eg, effect size and statistically significant findings, low risk of bias, sound experimental design). The one study that failed to find any positive effect on delirium, that of Gagnon et al.,21 was conducted on an inpatient palliative care service in Canada, and its negative finding may reflect the unique delirium risk factors in patients who are nearing end of life.
This current review differs from previous delirium prevention reviews in operationally defining a “model of care.” We identified a great deal of variation in specific models and team composition. For example, some interventions were carried out by nurses18-20,31 and physicians,20,21,25,32 whereas others involved physical therapists,20,22,28 medical residents,23 geriatricians,22,23,25 pharmacists,26 researchers,18 and trained volunteers.22 In all cases, the staff roles were expanded to include new tasks, and the clinical team worked collaboratively to administer interventions across multiple domains. Team-related considerations are critical because modern medical care is inherently interdependent.9 These broad differences in team composition across studies demonstrate the number of potential options for team structure and function. They also highlight the number of “moving parts” to be considered when designing and implementing delirium care bundles.
Most of the delirium prevention studies implementing models of care are characterized by a substantial risk of bias. We evaluated risk of bias along six categories of potential sources, including random assignment to groups, ability to foresee future group allocation, blinding of participants and personnel to group assignment, blinding of outcome assessment, completeness of outcome data, selective reporting, and other potential sources of bias.17 Two of the three studies that used randomization had a low risk of bias, and four additional studies had a moderate risk of bias. Allocation concealment was accomplished only in randomized controlled trials, whereas blinding of both subjects and study personnel was not implemented in any of the studies. Although some studies relied on data analysis by research personnel blinded to group membership or the nature of the intervention, others failed to do so or failed to describe data analysis in sufficient detail. Studies also failed to report the percentage of unscorable or otherwise omitted delirium assessments necessary to calculate attrition rates or to understand the comprehensiveness of outcome assessment in a systematic manner. Other potential sources of bias included systematic differences between the intervention and control groups (such as differences in gender composition, age, or delirium risk) at study outset.
A primary limitation of this review is the heterogeneity of settings, interventions, and models of care across included studies. We excluded several studies from this review for being delivered by a single individual or service line (eg, introduction of a geriatric consult service, physical therapy, or volunteers), for providing a single intervention (eg, early ambulation alone), or for multiple interventions targeting a single domain (eg, sleep). We did so because the future of value-based care lies in collaboration of providers and services, and in a way the complexity across and within these studies ultimately reflects the complexity of medical settings as well as the multifactorial nature of delirium. The broader message is a call for increasing the integration of delirium-related care services. As discussed earlier, the high risk of bias across these studies is a limitation of our findings; high-quality evidence on the value of delirium prevention models of care remains limited. Thus, although our review suggests that there are multicomponent models of care that hold promise in mitigating delirium and its outcomes, additional randomized studies are required to confirm the efficacy of such models of care and to test which services, interventions, and clinical domains deserve priority.
CONCLUSION
To our knowledge, this is the first systematic review and meta-analysis of delirium preventive models of care. Models of care, as defined here, necessarily included a multidisciplinary team in which traditional staff roles had been revised to implement a multicomponent, multidomain intervention. Other recent reviews are available for multicomponent pharmacological and nonpharmacological interventions to prevent and manage delirium,41-49 but just as important as which interventions are being delivered is the team that delivers them. Care delivery in a complex medical system is more than handing a patient a medication or facilitating ambulation; it requires a choreographed dance of teamwork and integration across services. This review identifies promising models of care that deserve further recognition, refinement, and ultimately widespread implementation.
Acknowledgments
The authors comprise a writing group created through the Delirium Boot Camp, an annual meeting originally sponsored by the Center of Excellence for Delirium in Aging: Research, Training, and Educational Enhancement (CEDARTREE, Boston, Massachusetts); it is currently supported by the Network for Investigation of Delirium: Unifying Scientists (NIDUS, Boston, Massachusetts). The authors would like to thank medical librarian Rita Mitchell (Aurora Health Care, Milwaukee, Wisconsin) for the literature search, senior scientific writer and editor Joe Grundle (Aurora Research Institute, Milwaukee, Wisconsin) for editorial assistance, and graphics specialist Brian Miller (Aurora Research Institute, Milwaukee, Wisconsin) for help with the figures.
Disclosures
The authors report no relevant conflicts of interest.
Funding
No funding was dedicated to the conduct of this review.
Delirium presents as an acute change in mentation characterized by reduced attention, clouding of awareness, and typically an altered level of arousal. It can be caused by a host of medical conditions, medications, or other psychoactive substances and is therefore encountered primarily in acute and postacute medical settings.1 More than a quarter of all hospitalized patients develop delirium,2 with rates up to 80% in the critically ill.3 Similarly, delirium occurs in more than one-third of patients who transition to postacute care.4 These high prevalence rates are alarming, especially because delirium is a risk factor for mortality, prolonged hospitalization, institutionalization, and overall higher cost of care.5 However, more than a quarter of delirium is preventable.6 Evidence-based guidelines for delirium uniformly call for multicomponent prevention strategies,7 and these are best delivered through collaborative models of care. In short, delirium impacts healthcare systems; therefore, interventions aimed at preventing delirium and its consequences ought to be systems-based.
Since the Institute of Medicine issued its 1999 report highlighting the critical role of medical errors in healthcare, healthcare systems have increasingly become team-based.8 “Medical care is inherently interdependent,”9 and this implies that delirium prevention rests not only on individuals but also on broader systems of care. Although nonpharmacological interventions are efficacious at preventing delirium,10 previous reviews have focused on specific interventions or multiple interventions rather than the systems of care needed to deliver them. Indeed, teams and the quality of their teamwork impact outcomes.11
Herein, we provide a systematic review and meta-analysis of integrated models of care designed to prevent delirium. What distinguishes this review from previous reviews of nonpharmacological interventions to prevent delirium is our focus on discrete models of care that involve collaboration among clinicians. Our goal is to identify the most promising models that deserve further development, investigation, and dissemination. Viewing delirium prevention through a collaborative care lens is consistent with efforts to achieve value-based care and may encourage drawing from the expanding literature outlining the benefits of mental healthcare integration.12,13 Specifically, a systems perspective highlights the potential for system-wide benefits such as reducing readmissions14,15 and cost savings.16
METHODS
This systematic review and meta-analysis follows PRISMA guidelines. A search of OVID, MEDLINE, CINAHL, Cochrane Database of Systematic Reviews, EMBASE, and PsycINFO was completed by a medical librarian for clinical studies in which models of care were implemented to prevent delirium using PICO (P patient, problem or population; I, intervention; C, comparison, control or comparator; O, outcome) inquiries. Search terms included delirium, acute confusional state, altered mental status, prevention, and control (“delirium”/exp OR “acute confusion”/exp OR “altered mental status”/exp) AND “prevention and control”/exp AND [English]/lim AND [embase]/lim).
One researcher (AK) screened articles by title for relevance. Relevant articles were then divided among four authors (AK, MO, NF, and OB), and the abstracts were screened for eligibility. The authors reviewed the full texts of any potentially eligible studies. Each full text was assigned to two authors for full review. Discrepancies were adjudicated by conference among all authors. In addition, references within all full-text publications were scanned for potential additional articles.
The inclusion criteria for review of full-text articles required English-language description of a model of care with multiple interventions, delirium reported as an outcome, and presence of a comparator group.
“Model of care” was defined by the Cochrane Effective Practice and Organization of Care Review Group as follows: (1) revision of professional roles, including shifting of professional roles or expansion of roles to new tasks; (2) creation of clinical multidisciplinary teams or addition of new members to the team who collaborate inpatient care; (3) delivery of multiple interventions across multiple domains (ie, studies involving a single intervention such as physical therapy or targeting a single domain such as sleep were excluded); and (4) formal integration of services whereby teams work together in collaboration with existing services to enhance care.17 For this review, we required that studies include a comparator group so that effectiveness of the intervention could be assessed. Quality improvement studies that lacked a comparator group were excluded.
Delirium incidence was the primary outcome and was evaluated by meta-analysis. Heterogeneity was assessed using I2 and visual inspection of forest plots. I2 values of 25%, 50%, and 75% represent low, moderate, and high heterogeneity, respectively. The studies were pooled according to study type as follows: randomized controlled trials, pre–post design, and other nonrandomized prospective studies. Random effects models were used to calculate estimates using the Comprehensive Meta-Analysis software (Version 3, Biostat, Englewood, New Jersey), which also generated forest plots.
Risk of bias was assessed using criteria established by the Cochrane Collaborative Review Criteria, which lists six categories of potential bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting.17 Each study was assessed by two authors (either MO and AK or MO-P. and OB) for bias and a numerical value was assigned to each of the six categories as follows: 1 = low risk, 2 = unknown/moderate risk, and 3 = high risk. Where scorers disagreed, all authors jointly conferred, and a consensus score was given. The values for each of these six categories were added to create a composite risk-of-bias score for each study, with 6 being the lowest possible score and 18 the highest. Overall risk was classified as follows: <9 = low risk, 9-12 = moderate risk, and >12 = high risk.
RESULTS
Study Selection Process
An initial literature search identified 352 articles. After reviewing the titles, 308 articles were excluded for irrelevance, and 44 abstracts were screened for eligibility. We excluded 27 articles upon abstract review, and the full texts of 17 were obtained for detailed review. In addition, we identified another 10 potentially eligible articles through review of references and obtained full texts of these as well. Of the 27 full-text articles reviewed, 15 were included in this systematic review, 10 of which were suitable for meta-analysis. The Figure shows the PRISMA flow chart.
Study Characteristics
The 15 studies that met the inclusion criteria are summarized in the Table.18-32 Delirium prevention was among the primary outcomes of 13 studies; delirium outcomes were reported in the other two studies as well, which were primarily designed to assess feasibility.26,27 Six studies were conducted in the United States, three in Sweden, two in Spain, two in the United Kingdom, and one each conducted in Korea and Canada. Healthcare settings among the included studies involved the intensive care unit (six studies), medical floors (four studies), surgical floors (three studies), a long-term care unit (one study), and
Outcomes Reported
All but one of the studies reported delirium incidence. The most commonly used delirium screening instrument was the Confusion Assessment Method (CAM) or its modified version, the CAM-ICU (11 studies).33,34 Other methods used to assess mentation included the Richmond Agitation Sedation Scale,35 the Organic Brain Syndrome scale,36 the revised Delirium Rating Scale,37 the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition,38 and the Confusion Rating Scale.39 (Details regarding delirium screening tools can be found in the systematic review by De and Wand.40) Researchers performed delirium assessment in nine studies, whereas assessments were performed by clinical staff in the remaining studies. Other outcomes reported included length of stay (LOS), mortality, number of days ventilated, and functional decline. None of the included studies reported cost effectiveness.
Risk of Bias Assessment
Risk of bias assessment identified only two studies—both randomized controlled trials—as low risk (Table). The remaining studies had moderate (four studies) or high risk (nine studies).
Results from Individual Studies
Of the 15 studies, nine reported a statistically significant reduction in delirium incidence, and another two reported a statistically insignificant reduction. In addition, seven of the eight studies that assessed delirium duration found reduced duration in the intervention cohort, and two of the three studies that reported delirium severity found a reduction in the intervention group.
Results of Meta-Analysis
Random effects models were created to meta-analyze groups of studies based on design as follows: randomized controlled trials (three studies18,19,25), pre–post intervention studies (four of six studies included28-31), and other nonrandomized studies (three of four studies included21-23). Meta-analysis was not completed for the two feasibility studies26,27 because delirium outcome data were limited due to the feasibility study design. The study of Dale et al.32 was excluded from the meta-analysis because the rates of CAM-ICU completion differed substantially between control and intervention groups (0.35 vs 1.49 per 24 hours, respectively), leading to imbalanced between-group sensitivity in delirium detection and Needham et al.20 was also excluded because it reported only days of delirium, not delirium incidence. The study by Lundström et al.24 was also excluded from the meta-analysis because delirium incidence was measured on days 1, 3, and 5, whereas the other studies reported delirium daily.
Meta-analysis of the three randomized controlled trials revealed a pooled odds ratio of 0.56 (95% CI: 0.37-0.85; P = .006) for delirium incidence among intervention group subjects relative to those in comparator groups. The heterogeneity across studies was low (I2 = 29%). Pooling data from four pre–post studies found that the odds ratio for delirium incidence was 0.63 (95% CI: 0.37-1.07; P = .09). The heterogeneity across these studies was moderate (I2 = 65%). Results from the three eligible, nonrandomized prospective studies were also pooled. The odds ratio for developing delirium among study subjects was 0.79 (95% CI: 0.46-1.37; P = .40), and the heterogeneity among these studies was high (I2 = 85%).
DISCUSSION
We provide a systematic review and meta-analysis of delirium preventive models of care. Meta-analysis of the three randomized controlled trials found that these models of care led to a statistically significant reduction in delirium incidence; study subjects had an 11.5% reduction in absolute delirium incidence. The pooled odds ratios for both of the other sets of nonrandomized studies favored the intervention group but were not significant, each because of one included study. The pre–post meta-analysis failed to reach significance as one of the included studies found a trend toward higher delirium incidence; however, interestingly, in that same study, the overall delirium-free days were significantly reduced overall (24 vs 27, P = .002). Similarly, meta-analysis of the three additional nonrandomized prospective studies failed to reach significance because the largest included study found higher rates of delirium among intervention group subjects. Despite considerable risk of bias in several of these studies, their findings were broadly consistent; all but one study (Gagnon 201221) reported a trend or a significant reduction in delirium incidence, duration, severity, or number of delirium episodes. Moreover, the value of such models of care extended beyond preventing delirium; for instance, other positive outcomes included reduced LOS and fewer medical complications.
Models of care ranged widely with respect to specific interventions, though several common elements highlighted their relevance for delirium care and as potential delirium prevention strategies in future studies. For example, two of the randomized controlled trials18,19 employed early mobilization, enhanced nutrition, sleep hygiene, early reduction of invasive procedures (eg, urinary catheterization), and pain control in their multicomponent models. Five additional studies also incorporated early mobilization,20,22,23,31,32 and three sought to improve sleep quality.22,28,30 Among other important strategies were delirium screening,18,20,22,30,31 monitoring medication,18,20,22,26,28,30,32 orientation,18,21,23,28 addressing vision and hearing impairment,18,22,23,32 hydration,18,22,23 avoiding hypoxia,18,20,30 and staff, patient, and caretaker education.19,21,23,27-30 Unique strategies were implemented in certain studies. For instance, one study used massage therapy,28 preventing delays in transfer logistics in another,30 and a third addressed psychosocial problems.25 Overall, the selection of strategies depended on the patient setting; thus, no one care bundle should be expected to emerge as a universal model for delirium prevention. Rather, these results should be interpreted within their specific care contexts and judged on the quality of evidence (eg, effect size and statistically significant findings, low risk of bias, sound experimental design). The one study that failed to find any positive effect on delirium, that of Gagnon et al.,21 was conducted on an inpatient palliative care service in Canada, and its negative finding may reflect the unique delirium risk factors in patients who are nearing end of life.
This current review differs from previous delirium prevention reviews in operationally defining a “model of care.” We identified a great deal of variation in specific models and team composition. For example, some interventions were carried out by nurses18-20,31 and physicians,20,21,25,32 whereas others involved physical therapists,20,22,28 medical residents,23 geriatricians,22,23,25 pharmacists,26 researchers,18 and trained volunteers.22 In all cases, the staff roles were expanded to include new tasks, and the clinical team worked collaboratively to administer interventions across multiple domains. Team-related considerations are critical because modern medical care is inherently interdependent.9 These broad differences in team composition across studies demonstrate the number of potential options for team structure and function. They also highlight the number of “moving parts” to be considered when designing and implementing delirium care bundles.
Most of the delirium prevention studies implementing models of care are characterized by a substantial risk of bias. We evaluated risk of bias along six categories of potential sources, including random assignment to groups, ability to foresee future group allocation, blinding of participants and personnel to group assignment, blinding of outcome assessment, completeness of outcome data, selective reporting, and other potential sources of bias.17 Two of the three studies that used randomization had a low risk of bias, and four additional studies had a moderate risk of bias. Allocation concealment was accomplished only in randomized controlled trials, whereas blinding of both subjects and study personnel was not implemented in any of the studies. Although some studies relied on data analysis by research personnel blinded to group membership or the nature of the intervention, others failed to do so or failed to describe data analysis in sufficient detail. Studies also failed to report the percentage of unscorable or otherwise omitted delirium assessments necessary to calculate attrition rates or to understand the comprehensiveness of outcome assessment in a systematic manner. Other potential sources of bias included systematic differences between the intervention and control groups (such as differences in gender composition, age, or delirium risk) at study outset.
A primary limitation of this review is the heterogeneity of settings, interventions, and models of care across included studies. We excluded several studies from this review for being delivered by a single individual or service line (eg, introduction of a geriatric consult service, physical therapy, or volunteers), for providing a single intervention (eg, early ambulation alone), or for multiple interventions targeting a single domain (eg, sleep). We did so because the future of value-based care lies in collaboration of providers and services, and in a way the complexity across and within these studies ultimately reflects the complexity of medical settings as well as the multifactorial nature of delirium. The broader message is a call for increasing the integration of delirium-related care services. As discussed earlier, the high risk of bias across these studies is a limitation of our findings; high-quality evidence on the value of delirium prevention models of care remains limited. Thus, although our review suggests that there are multicomponent models of care that hold promise in mitigating delirium and its outcomes, additional randomized studies are required to confirm the efficacy of such models of care and to test which services, interventions, and clinical domains deserve priority.
CONCLUSION
To our knowledge, this is the first systematic review and meta-analysis of delirium preventive models of care. Models of care, as defined here, necessarily included a multidisciplinary team in which traditional staff roles had been revised to implement a multicomponent, multidomain intervention. Other recent reviews are available for multicomponent pharmacological and nonpharmacological interventions to prevent and manage delirium,41-49 but just as important as which interventions are being delivered is the team that delivers them. Care delivery in a complex medical system is more than handing a patient a medication or facilitating ambulation; it requires a choreographed dance of teamwork and integration across services. This review identifies promising models of care that deserve further recognition, refinement, and ultimately widespread implementation.
Acknowledgments
The authors comprise a writing group created through the Delirium Boot Camp, an annual meeting originally sponsored by the Center of Excellence for Delirium in Aging: Research, Training, and Educational Enhancement (CEDARTREE, Boston, Massachusetts); it is currently supported by the Network for Investigation of Delirium: Unifying Scientists (NIDUS, Boston, Massachusetts). The authors would like to thank medical librarian Rita Mitchell (Aurora Health Care, Milwaukee, Wisconsin) for the literature search, senior scientific writer and editor Joe Grundle (Aurora Research Institute, Milwaukee, Wisconsin) for editorial assistance, and graphics specialist Brian Miller (Aurora Research Institute, Milwaukee, Wisconsin) for help with the figures.
Disclosures
The authors report no relevant conflicts of interest.
Funding
No funding was dedicated to the conduct of this review.
1. American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing, Inc.
2. Schubert M, Schürch R, Boettger S, et al. A hospital-wide evaluation of delirium prevalence and outcomes in acute care patients - A cohort study. BMC Health Serv Res. 2018;18(1):550. https://doi.org/10.1186/s12913-018-3345-x.
3. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the Intensive Care Unit. JAMA. 2004;291(14):1753-1762. https://doi.org/10.1001/jama.291.14.1753.
4. Gual N, Morandi A, Pérez LM, et al. Risk factors and outcomes of delirium in older patients admitted to postacute care with and without dementia. Dement Geriatr Cogn Disord. 2018;45(1-2):121-129. https://doi.org/10.1159/000485794.
5. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. https://doi.org/10.1056/NEJMcp1605501.
6. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. https://doi.org/10.1016/S0140-6736(13)60688-1.
7. Bush SH, Marchington KL, Agar M, et al. Quality of clinical practice guidelines in delirium: A systematic appraisal. BMJ Open. 2017;7(3):e013809. https://doi.org/10.1136/bmjopen-2016-013809.
8. Institute of Medicine. 2000. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press. https://doi.org/10.17226/9728.
9. Rosen MA, DiazGranados D, Dietz AS, et al. Teamwork in healthcare: key discoveries enabling safer, high-quality care. Am Psychol. 2018;73(4):433-450. https://doi.org/10.1037/amp0000298.
10. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: A systematic overview. The SENATOR project ONTOP series. PLOS ONE. 2015;10(6):e0123090. https://doi.org/10.1371/journal.pone.0123090.
11. Thomas EJ. Improving teamwork in healthcare: current approaches and the path forward. BMJ Qual Saf. 2011;20(8):647-650. https://doi.org/10.1136/bmjqs-2011-000117.
12. Sledge W, Bozzo J, White-McCullum B, Lee H. The cost-benefit from the perspective of the hospital of a proactive psychiatric consultation service on inpatient general medicine services. Health Econ Outcome -Res. 2016;2:2-6.
13. Unützer J, Katon WJ, Fan MY, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14(2):95-100. PubMed
14. Lee E, Kim J. Cost-benefit analysis of a delirium prevention strategy in the intensive care unit. Nurs Crit Care. 2014;21:367-373. https://doi.org/10.1111/nicc.12124.
15. Rubin FH, Bellon J, Bilderback A, Urda K, Inouye SK. Effect of the hospital elder life program on risk of 30-day readmission. J Am Geriatr Soc. 2018;66(1):145-149. https://doi.org/10.1111/jgs.15132.
16. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the hospital elder life program in a community hospital. Psychosomatics. 2013;54(3):219-226. https://doi.org/10.1016/j.psym.2013.01.010.
17. Cochrane effective practice and organisation of Care Group (EPOC). Data collection Checklistist. Chochrane Effective Practice and Organisation of Care Group (EPOC) Methods Papers. . https://methods.cochrane.org/sites/methods.cochrane.org.bias/files/public/uploads/EPOC Data Collection Checklist.pdf. Accessed May 27, 2014.
18. Moon KJ, Lee SM. The effects of a tailored intensive care unit delirium prevention protocol: A randomized controlled trial. Int J Nurs Stud. 2015;52(9):1423-1432. https://doi.org/10.1016/j.ijnurstu.2015.04.021.
19. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res-. 2007;19(3):178-186. https://doi.org/10.1007/BF03324687.
20. Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients With acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536-542. https://doi.org/10.1016/j.apmr.2010.01.002.
21. Gagnon P, Allard P, Gagnon B, Mérette C, Tardif F. Delirium prevention in terminal cancer: assessment of a multicomponent intervention. Psychooncology. 2012;21(2):187-194. https://doi.org/10.1002/pon.1881.
22. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901.
23. Vidán MT, Sánchez E, Alonso M, et al. An intervention integrated into daily clinical practice reduces the incidence of delirium during hospitalization in elderly patients. J Am Geriatr Soc. 2009;57(11):2029-2036. https://doi.org/10.1111/j.1532-5415.2009.02485.x.
24. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. https://doi.org/10.1111/j.1532-5415.2005.53210.x.
25. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: A randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x.
26. Rice KL, Bennett MJ, Berger L, et al. A pilot randomized controlled trial of the feasibility of a multicomponent delirium prevention intervention versus usual care in acute stroke. J Cardiovasc Nurs. 2017;32(1):E1-E10. https://doi.org/10.1097/JCN.0000000000000356.
27. Siddiqi N, Cheater F, Collinson M, et al. The PiTSTOP study: a feasibility cluster randomized trial of delirium prevention in care homes for older people. Age Ageing. 2016;45(5):652-661. https://doi.org/10.1093/ageing/afw091.
28. Bryczkowski SB, Lopreiato MC, Yonclas PP, Sacca JJ, Mosenthal AC. Delirium prevention program in the surgical intensive care unit (SICU) improved the outcomes of older adults. J Surg Res. 2014;186:519. https://doi.org/10.1016/j.jss.2013.11.352
29. Holt R, Young J, Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing. 2013;42(6):721-727. https://doi.org/10.1093/ageing/aft120.
30. Björkelund KB, Hommel A, Thorngren KG, et al. Reducing delirium in elderly patients with hip fracture: A multi-factorial intervention study. Acta Anaesthesiol-Scand. 2010;54(6):678-688. https://doi.org/10.1111/j.1399-6576.2010.02232.x.
31. Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility (ABCDE) bundle. Crit Care Med. 2014;42(5):1024-1036. https://doi.org/10.1097/CCM.0000000000000129.
32. Dale CR, Kannas DA, Fan VS, et al. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am Thorac Soc. 2014;11(3):367-374. https://doi.org/10.1513/AnnalsATS.201306-210OC.
33. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. https://doi.org/10.7326/0003-4819-113-12-941.
34. Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370-1379. https://doi.org/10.1097/00003246-200107000-00012.
35. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. https://doi.org/10.1164/rccm.2107138.
36. Jensen E, Dehlin O, Gustafson L. A comparison between three psychogeriatric rating scales. Int J Geriatr Psychiatry. 1993;8(3):215-229. https://doi.org/10.1002/gps.930080305.
37. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatr Clin Neurosci. 2001;13(2):229-242. https://doi.org/10.1176/jnp.13.2.229.
38. American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Arlington, VA, US: American Psychiatric Publishing, Inc.
39. Williams MA. Delirium/acute confusional states: evaluation devices in nursing. Int Psychogeriatr. 1991;3(2):301-308. PubMed
40. De J, Wand APF. Delirium screening: A systematic review of delirium screening tools in hospitalized patients. Gerontologist-. 2015;55(6):1079-1099. https://doi.org/10.1093/geront/gnv100.
41. Martinez F, Tobar C, Hill N. Preventing delirium: should non-pharmacological,
multicomponent interventions be used? A systematic review and meta-analysis of the literature. Age Ageing. 2015;44(2):196-204. https://doi.org/10.1093/ageing/afu173.
42. Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5):375-380. https://doi.org/10.7326/0003-4819-158-5-201303051-00003.
43. Rivosecchi RM, Smithburger PL, Svec S, Campbell S, Kane-Gill SL. Nonpharmacological interventions to prevent delirium: an evidence-based systematic review. Crit Care Nurse. 2015;35(1):39-50; quiz 51. https://doi.org/10.4037/ccn2015423.
44. Trogrlić Z, van der Jagt M, Bakker J, et al. A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Crit Care. 2015;19:157. https://doi.org/10.1186/s13054-015-0886-9.
45. Wang Y, Tang J, Zhou F, Yang L, Wu J. Comprehensive geriatric care reduces acute perioperative delirium in elderly patients with hip fractures: A meta-analysis. Medicine (Baltimore). 2017;96(26):e7361. https://doi.org/10.1097/MD.0000000000007361.
46. Shields L, Henderson V, Caslake R. Comprehensive geriatric assessment for prevention of delirium After hip fracture: A systematic review of randomized controlled trials. J Am Geriatr Soc. 2017;65(7):1559-1565. https://doi.org/10.1111/jgs.14846.
47. Oberai T, Lizarondo L, Ruurd J. Effectiveness of multi-component interventions on incidence of delirium in hospitalized older patients with hip fracture: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2017;15(2):259-268. https://doi.org/10.11124/JBISRIR-2016-002943.
48. Collinsworth AW, Priest EL, Campbell CR, Vasilevskis EE, Masica AL. A review of multifaceted care approaches for the prevention and mitigation of delirium in intensive care units. J Intensive Care Med. 2016;31(2):127-141. https://doi.org/10.1177/0885066614553925.
49. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological
delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. https://doi.org/10.1001/jamainternmed.2014.7779.
1. American Psychiatric Association; 2013. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Publishing, Inc.
2. Schubert M, Schürch R, Boettger S, et al. A hospital-wide evaluation of delirium prevalence and outcomes in acute care patients - A cohort study. BMC Health Serv Res. 2018;18(1):550. https://doi.org/10.1186/s12913-018-3345-x.
3. Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the Intensive Care Unit. JAMA. 2004;291(14):1753-1762. https://doi.org/10.1001/jama.291.14.1753.
4. Gual N, Morandi A, Pérez LM, et al. Risk factors and outcomes of delirium in older patients admitted to postacute care with and without dementia. Dement Geriatr Cogn Disord. 2018;45(1-2):121-129. https://doi.org/10.1159/000485794.
5. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. https://doi.org/10.1056/NEJMcp1605501.
6. Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. https://doi.org/10.1016/S0140-6736(13)60688-1.
7. Bush SH, Marchington KL, Agar M, et al. Quality of clinical practice guidelines in delirium: A systematic appraisal. BMJ Open. 2017;7(3):e013809. https://doi.org/10.1136/bmjopen-2016-013809.
8. Institute of Medicine. 2000. To Err Is Human: Building a Safer Health System. Washington, DC: The National Academies Press. https://doi.org/10.17226/9728.
9. Rosen MA, DiazGranados D, Dietz AS, et al. Teamwork in healthcare: key discoveries enabling safer, high-quality care. Am Psychol. 2018;73(4):433-450. https://doi.org/10.1037/amp0000298.
10. Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: A systematic overview. The SENATOR project ONTOP series. PLOS ONE. 2015;10(6):e0123090. https://doi.org/10.1371/journal.pone.0123090.
11. Thomas EJ. Improving teamwork in healthcare: current approaches and the path forward. BMJ Qual Saf. 2011;20(8):647-650. https://doi.org/10.1136/bmjqs-2011-000117.
12. Sledge W, Bozzo J, White-McCullum B, Lee H. The cost-benefit from the perspective of the hospital of a proactive psychiatric consultation service on inpatient general medicine services. Health Econ Outcome -Res. 2016;2:2-6.
13. Unützer J, Katon WJ, Fan MY, et al. Long-term cost effects of collaborative care for late-life depression. Am J Manag Care. 2008;14(2):95-100. PubMed
14. Lee E, Kim J. Cost-benefit analysis of a delirium prevention strategy in the intensive care unit. Nurs Crit Care. 2014;21:367-373. https://doi.org/10.1111/nicc.12124.
15. Rubin FH, Bellon J, Bilderback A, Urda K, Inouye SK. Effect of the hospital elder life program on risk of 30-day readmission. J Am Geriatr Soc. 2018;66(1):145-149. https://doi.org/10.1111/jgs.15132.
16. Zaubler TS, Murphy K, Rizzuto L, et al. Quality improvement and cost savings with multicomponent delirium interventions: replication of the hospital elder life program in a community hospital. Psychosomatics. 2013;54(3):219-226. https://doi.org/10.1016/j.psym.2013.01.010.
17. Cochrane effective practice and organisation of Care Group (EPOC). Data collection Checklistist. Chochrane Effective Practice and Organisation of Care Group (EPOC) Methods Papers. . https://methods.cochrane.org/sites/methods.cochrane.org.bias/files/public/uploads/EPOC Data Collection Checklist.pdf. Accessed May 27, 2014.
18. Moon KJ, Lee SM. The effects of a tailored intensive care unit delirium prevention protocol: A randomized controlled trial. Int J Nurs Stud. 2015;52(9):1423-1432. https://doi.org/10.1016/j.ijnurstu.2015.04.021.
19. Lundström M, Olofsson B, Stenvall M, et al. Postoperative delirium in old patients with femoral neck fracture: a randomized intervention study. Aging Clin Exp Res-. 2007;19(3):178-186. https://doi.org/10.1007/BF03324687.
20. Needham DM, Korupolu R, Zanni JM, et al. Early physical medicine and rehabilitation for patients With acute respiratory failure: a quality improvement project. Arch Phys Med Rehabil. 2010;91(4):536-542. https://doi.org/10.1016/j.apmr.2010.01.002.
21. Gagnon P, Allard P, Gagnon B, Mérette C, Tardif F. Delirium prevention in terminal cancer: assessment of a multicomponent intervention. Psychooncology. 2012;21(2):187-194. https://doi.org/10.1002/pon.1881.
22. Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901.
23. Vidán MT, Sánchez E, Alonso M, et al. An intervention integrated into daily clinical practice reduces the incidence of delirium during hospitalization in elderly patients. J Am Geriatr Soc. 2009;57(11):2029-2036. https://doi.org/10.1111/j.1532-5415.2009.02485.x.
24. Lundström M, Edlund A, Karlsson S, et al. A multifactorial intervention program reduces the duration of delirium, length of hospitalization, and mortality in delirious patients. J Am Geriatr Soc. 2005;53(4):622-628. https://doi.org/10.1111/j.1532-5415.2005.53210.x.
25. Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: A randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482. https://doi.org/10.1111/j.1532-5415.2005.53466.x.
26. Rice KL, Bennett MJ, Berger L, et al. A pilot randomized controlled trial of the feasibility of a multicomponent delirium prevention intervention versus usual care in acute stroke. J Cardiovasc Nurs. 2017;32(1):E1-E10. https://doi.org/10.1097/JCN.0000000000000356.
27. Siddiqi N, Cheater F, Collinson M, et al. The PiTSTOP study: a feasibility cluster randomized trial of delirium prevention in care homes for older people. Age Ageing. 2016;45(5):652-661. https://doi.org/10.1093/ageing/afw091.
28. Bryczkowski SB, Lopreiato MC, Yonclas PP, Sacca JJ, Mosenthal AC. Delirium prevention program in the surgical intensive care unit (SICU) improved the outcomes of older adults. J Surg Res. 2014;186:519. https://doi.org/10.1016/j.jss.2013.11.352
29. Holt R, Young J, Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing. 2013;42(6):721-727. https://doi.org/10.1093/ageing/aft120.
30. Björkelund KB, Hommel A, Thorngren KG, et al. Reducing delirium in elderly patients with hip fracture: A multi-factorial intervention study. Acta Anaesthesiol-Scand. 2010;54(6):678-688. https://doi.org/10.1111/j.1399-6576.2010.02232.x.
31. Balas MC, Vasilevskis EE, Olsen KM, et al. Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility (ABCDE) bundle. Crit Care Med. 2014;42(5):1024-1036. https://doi.org/10.1097/CCM.0000000000000129.
32. Dale CR, Kannas DA, Fan VS, et al. Improved analgesia, sedation, and delirium protocol associated with decreased duration of delirium and mechanical ventilation. Ann Am Thorac Soc. 2014;11(3):367-374. https://doi.org/10.1513/AnnalsATS.201306-210OC.
33. Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. https://doi.org/10.7326/0003-4819-113-12-941.
34. Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370-1379. https://doi.org/10.1097/00003246-200107000-00012.
35. Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338-1344. https://doi.org/10.1164/rccm.2107138.
36. Jensen E, Dehlin O, Gustafson L. A comparison between three psychogeriatric rating scales. Int J Geriatr Psychiatry. 1993;8(3):215-229. https://doi.org/10.1002/gps.930080305.
37. Trzepacz PT, Mittal D, Torres R, et al. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatr Clin Neurosci. 2001;13(2):229-242. https://doi.org/10.1176/jnp.13.2.229.
38. American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Arlington, VA, US: American Psychiatric Publishing, Inc.
39. Williams MA. Delirium/acute confusional states: evaluation devices in nursing. Int Psychogeriatr. 1991;3(2):301-308. PubMed
40. De J, Wand APF. Delirium screening: A systematic review of delirium screening tools in hospitalized patients. Gerontologist-. 2015;55(6):1079-1099. https://doi.org/10.1093/geront/gnv100.
41. Martinez F, Tobar C, Hill N. Preventing delirium: should non-pharmacological,
multicomponent interventions be used? A systematic review and meta-analysis of the literature. Age Ageing. 2015;44(2):196-204. https://doi.org/10.1093/ageing/afu173.
42. Reston JT, Schoelles KM. In-facility delirium prevention programs as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5):375-380. https://doi.org/10.7326/0003-4819-158-5-201303051-00003.
43. Rivosecchi RM, Smithburger PL, Svec S, Campbell S, Kane-Gill SL. Nonpharmacological interventions to prevent delirium: an evidence-based systematic review. Crit Care Nurse. 2015;35(1):39-50; quiz 51. https://doi.org/10.4037/ccn2015423.
44. Trogrlić Z, van der Jagt M, Bakker J, et al. A systematic review of implementation strategies for assessment, prevention, and management of ICU delirium and their effect on clinical outcomes. Crit Care. 2015;19:157. https://doi.org/10.1186/s13054-015-0886-9.
45. Wang Y, Tang J, Zhou F, Yang L, Wu J. Comprehensive geriatric care reduces acute perioperative delirium in elderly patients with hip fractures: A meta-analysis. Medicine (Baltimore). 2017;96(26):e7361. https://doi.org/10.1097/MD.0000000000007361.
46. Shields L, Henderson V, Caslake R. Comprehensive geriatric assessment for prevention of delirium After hip fracture: A systematic review of randomized controlled trials. J Am Geriatr Soc. 2017;65(7):1559-1565. https://doi.org/10.1111/jgs.14846.
47. Oberai T, Lizarondo L, Ruurd J. Effectiveness of multi-component interventions on incidence of delirium in hospitalized older patients with hip fracture: a systematic review protocol. JBI Database Syst Rev Implement Rep. 2017;15(2):259-268. https://doi.org/10.11124/JBISRIR-2016-002943.
48. Collinsworth AW, Priest EL, Campbell CR, Vasilevskis EE, Masica AL. A review of multifaceted care approaches for the prevention and mitigation of delirium in intensive care units. J Intensive Care Med. 2016;31(2):127-141. https://doi.org/10.1177/0885066614553925.
49. Hshieh TT, Yue J, Oh E, et al. Effectiveness of multicomponent nonpharmacological
delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175(4):512-520. https://doi.org/10.1001/jamainternmed.2014.7779.
© 2019 Society of Hospital Medicine