User login
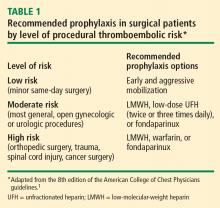
Most surgical patients who require hospitalization should be considered at high risk for venous thromboembolism (VTE) and be given appropriate prophylaxis. For lower-risk procedures such as knee arthroscopy, prophylaxis is needed for those with individual risk factors such as morbid obesity, limited mobility after surgery, or a history of deep vein thrombosis (DVT) or malignancy. Too often, however, prophylaxis is not provided appropriately or not given at all.
This review surveys the essentials of perioperative VTE prophylaxis and important new developments in the field, which include the 2008 release of new evidence-based clinical practice guidelines on antithrombotic and thrombolytic therapy from the American College of Chest Physicians (ACCP). This 8th edition of the guidelines updates the previous edition, published in 2004, and includes a section by Geerts et al devoted to VTE prevention.1 Other major guidelines are also discussed, as are developments in VTE-related quality measurement, management of special patient populations (those with renal impairment or morbid obesity), and emerging therapies for VTE prophylaxis.
IMPETUS FOR QUALITY IMPROVEMENT IN VTE
A new seriousness about VTE quality measures
The 8th edition of the ACCP guidelines recommends that every hospital develop a formal, active strategy to consistently identify medical and surgical patients at risk for VTE and to prevent VTE occurrence.1 Although prior editions of the ACCP guidelines have made this recommendation for more than 2 decades, fewer than 1 in 10 acute care hospitals had any such strategy in place as recently as 5 years ago. Now, however, most US hospitals have implemented such a strategy, thanks to the growing national emphasis on health care quality measurement in recent years.
The Surgical Care Improvement Project (SCIP) has been at the forefront of this recent quality measures movement. SCIP, a joint project of the American Medical Association and federal government agencies, set a goal to reduce surgical complications in the United States by 25% from 2005 to 2010.2 Two SCIP process measures relate to improving VTE prophylaxis2,3:
- The proportion of surgical patients for whom recommended VTE prophylaxis is ordered
- The proportion of surgical patients who actually receive appropriate VTE prophylaxis within 24 hours before or after surgery.
The Joint Commission and the National Quality Forum recently endorsed these two SCIP performance measures for perioperative VTE prophylaxis along with several others relating to VTE treatment.
CMS raises the stakes with reimbursement restrictions
More significantly, the federal government’s Centers for Medicare and Medicaid Services (CMS) will soon refuse to reimburse for hospital treatment of a primary diagnosis of DVT or pulmonary embolism (PE) following recent (within 30 days) total hip or knee arthroplasty. Effective October 1, 2009, a primary VTE diagnosis following these joint replacement procedures will be added to CMS’ current list of “never events,” or hospital-acquired conditions for which CMS will not provide reimbursement because they are considered the result of preventable medical errors. (Notably, treatment of DVT or PE as a secondary diagnosis will still be reimbursed—for example, if a joint replacement patient develops nosocomial pneumonia, is transferred to the intensive care unit, and then develops VTE.) This addition of DVT and PE to the list is highly controversial since these events sometimes develop even if prophylactic therapy is appropriate and aggressive.
Strategies to promote best practices
In the update for the new 8th edition of its guidelines, the ACCP added recommendations on specific ways for hospitals to identify patients at high risk for VTE and ensure that they receive appropriate prophylaxis. These include the use of computer decision-support systems, preprinted orders, and periodic audit and feedback.1
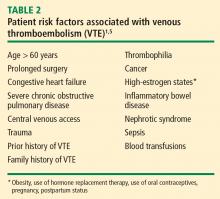
Researchers at Brigham and Women’s Hospital evaluated the effectiveness of a computer alert system for notifying physicians of newly hospitalized patients at risk for DVT who were not receiving prevention therapy within the first 24 hours of hospital admission.4 These patients presumably “fell through the cracks” and warranted prophylaxis but were otherwise not recognized by the health care team. Risk was determined by a scoring system based on multiple variables, including malignancy, previous DVT or PE, hypercoagulability, major surgery, advanced age, obesity, ordered bed rest, and treatment with hormone replacement therapy or oral contraceptives. Study physicians had to acknowledge having received the alert but could choose whether or not to order VTE prophylaxis. Prophylaxis was used in considerably more patients from the intervention group than from a control group of high-risk patients whose physicians did not receive alerts (34% vs 14%, respectively); accordingly, the risk of a symptomatic DVT or PE event at 90 days was reduced by 41% in the intervention group.
Despite this evidence of improved practice under the alert system, the study begs the question of why the percentage of patients at risk for VTE who were given prophylaxis was still so low (34%), demonstrating how much progress in improving practice remains to be achieved.
PROPHYLAXIS STRATEGIES: MATCHING THERAPY TO RISK
NONPHARMACOLOGIC PROPHYLAXIS STRATEGIES
Does ambulation prevent DVT?
Although it is commonly accepted that walking prevents DVT, this has never been directly tested. Walking may simply be a marker of health, and healthy people are less prone to develop thromboses. We have almost no evidence to show that forcing an unhealthy person to walk helps prevent DVT. Early ambulation offers many benefits and should be encouraged, but it should not be considered DVT prophylaxis; it is simply good hospital care.
Mechanical devices: Adherence is key
Amaragiri and Lees conducted a systematic literature review of randomized controlled trials evaluating the effectiveness of graduated compression stockings (elastic stockings) for preventing DVT in various groups of hospitalized patients.6 The analysis demonstrated a statistically significant reduction in DVT incidence with graduated compression stockings compared with control both among the nine trials in which stockings were used alone (odds ratio = 0.34) and among the seven trials in which stockings were used in addition to another method of thromboprophylaxis (odds ratio = 0.24). Although benefit was demonstrated, many of the trials in this review involved patients undergoing gynecologic surgery and date from the 1970s and 1980s (when obesity was less prevalent), so the applicability of their results today may be limited.
The 8th edition of the ACCP guidelines recommends that mechanical methods of VTE prophylaxis be used primarily in patients who are at high risk of bleeding and that careful attention be directed to ensuring their proper use and optimal adherence.1 The latter point about adherence cannot be emphasized enough, as graduated compression stockings and other mechanical devices have been shown not to be effective unless they are worn at least 18 to 20 hours a day. This degree of adherence is difficult to achieve, as it can severely limit patient mobility and leave patients susceptible to development of pressure ulcers.
Mechanical compression should be initiated prior to induction of anesthesia and continue intraoperatively and then into the postanesthesia care unit. Orders for use of mechanical devices should include instructions in the patient’s medical chart specifying how—and for how many hours per day—they are to be worn. Not doing so leaves the physician vulnerable to litigation, especially as the ACCP guidelines include language on optimal adherence to these devices (“they should be removed for only a short time each day when the patient is actually walking or for bathing”1).
Continuous external compression therapy
Newer mechanical device options include a continuous external compression therapy system that allows patients to be mobile while wearing it and provides rhythmic compression that results in good peak venous flows. Ideally such a device could be put on the patient preoperatively and worn during surgery, throughout the hospital stay, and even at home during recovery. Anecdotally, however, I see patients turn these new devices off at the side of the bed just as often as they do with traditional devices.
Vena caval interruption
Vena caval interruption involves placement of a retrievable vena cava filter before surgery and removal some time later; it offers the potential for VTE prophylaxis in patients who could not tolerate even minor amounts of bleeding, such as certain trauma patients. The Eastern Association for the Surgery of Trauma has put forth a consensus recommendation to consider vena caval interruption in high-risk trauma patients who cannot receive pharmacologic prophylaxis.7 A randomized trial evaluating the usefulness of vena caval interruption for patients undergoing surgery is needed. For now, this intervention should be regarded as experimental and considered only on a highly individualized basis.
PHARMACOLOGIC PROPHYLAXIS
Timing of initiation
Pharmacologic VTE prophylaxis generally should begin 8 to 24 hours postoperatively. Of course, adequate hemostasis is required before initiation, and the net risk/benefit tradeoff with regard to timing of anticoagulant initiation has still not been well studied in many surgical patient populations.
Extended prophylaxis
In the update for the 8th edition of its guidelines, the ACCP added an explicit recommendation for extended outpatient prophylaxis with low-molecular-weight heparin (LMWH) for up to 28 days postoperatively in selected high-risk patients undergoing general or gynecologic surgery, including those with cancer or a history of VTE.1 This recommendation was based largely on studies of extended prophylaxis in patients with cancer undergoing colorectal surgery.8
Increased appreciation of the value of extended VTE prophylaxis after discharge is linked to a growing recognition that DVT and PE episodes in the community setting are often related to a recent hospital stay for either medical illness or surgery. A population-based study found that 59% of all community cases of a first lifetime VTE event in residents of Olmsted County, Minn., over a 15-year period could be linked to current or recent (< 30 days) hospitalization or nursing home residence.9 A similar population-based study in the Worcester, Mass., area found that three-fourths of all VTE events in a 3-year period occurred in the outpatient setting.10 Among patients with these outpatient VTE events, a large proportion had undergone surgery (23%) or hospitalization (37%) in the prior 3 months; among those, 67% experienced their VTE within 1 month of their time in the hospital.
These findings are no surprise, since surgery induces a hypercoagulable state that, when combined with individual risk factors such as obesity, old age, or poor heart function, cannot be assumed to return to baseline on postoperative day 4 or 5 just because the patient is being discharged.
Orthopedic surgery
For patients undergoing major orthopedic procedures, the ACCP guidelines recommend against routine screening for VTE with Doppler ultrasonography before discharge if the patient is asymptomatic.1 Such screening is not considered cost-effective because asymptomatic clots often are found, for which treatment is uncertain, and proximal clots may be missed, giving a false sense of security.
New to the ACCP guidelines in the 8th edition is the recommendation that patients undergoing knee arthroscopy who have risk factors for VTE (or whose procedure is complicated) should receive 1 week of prophylaxis with LMWH.1 Also new are recommendations for patients with risk factors undergoing single- or multilevel laminectomy (Table 4).
Recommendations unchanged in neurosurgery, spinal injury, trauma, burns
Recommendations for neurosurgery remain unchanged from the prior (2004) edition of the ACCP guidelines and are still based on the 2000 meta-analysis by Iorio and Agnelli of LMWH prophylaxis in neurosurgery cases.11 In the United States, the standard is overwhelmingly to use mechanical devices for thromboprophylaxis in neurosurgery, even for patients with cancer.
For prophylaxis in surgical patients with spinal cord injury, multisystem trauma, or burns, LMWH is predominantly used, and the ACCP recommendations are unchanged from 2004.
Drug-specific considerations
LMWH vs vitamin K antagonist. Although vitamin K antagonists (warfarin) still appear in the latest ACCP recommendations,1 LMWH is preferable. A 2004 meta-analysis of studies comparing vitamin K antagonists with LMWH for prophylaxis in patients undergoing orthopedic surgery found that vitamin K antagonists were associated with more episodes of total DVT (relative risk [RR] = 1.51; 95% CI, 1.27–1.79) and proximal DVT (RR = 1.51; 95% CI, 1.04–2.17) compared with LMWH.12 No difference was found in rates of wound hematoma or major bleeding. This finding of inferiority for vitamin K antagonists came despite the likelihood that warfarin was more often administered correctly (ie, with dose adjustment to achieve an international normalized ratio [INR] of 2.0 to 3.0 within 72 hours after surgery) in the studies in this analysis than it is in real-world practice.
Fondaparinux. The indirect factor Xa–specific inhibitor fondaparinux has had a surprisingly limited clinical adoption despite having been widely studied and found to be safe and effective. This is likely attributable in part to its 17-hour half-life, which raises concerns that it may take 3 days for its effects to stop if a patient begins to bleed. Large phase 3 studies have found fondaparinux to be equivalent to LMWH in VTE prevention after hip replacement, marginally superior to LMWH after knee replacement, and superior to LMWH following hip fracture repair.13 Fondaparinux was associated with an increase in bleeding events and instances of transfusion requirement, but only in one of the studies, which was in the setting of knee replacement surgery.14
Aspirin not recommended by ACCP. Although aspirin reduces the risk of VTE, practice guidelines from both the ACCP1 and the International Union of Angiology15 contain no recommendation for its use as prophylaxis because it is considered less effective and more risky than other therapies. In contrast, clinical practice guidelines from the American Academy of Orthopaedic Surgeons suggest that aspirin is reasonable for VTE prophylaxis.16 The varying recommendations reflect differences in perspective among these different specialties.
Aspirin has the advantages of ease of use and low cost, but it is clearly not the best evidence-based approach for VTE prophylaxis. The only recent randomized trial evidence in support of aspirin comes from the Pulmonary Embolism Prevention trial, a study with a flawed design involving more than 13,000 patients undergoing surgery for hip fracture or elective arthroplasty in five countries.17 Patients were randomized to receive aspirin 160 mg daily or placebo for 35 days along with any other prophylaxis deemed necessary (an important potential confounder). Aspirin was associated with an absolute reduction in symptomatic events of less than 1% relative to placebo, and no benefit was observed within the first week. The best results with aspirin were among patients with hip fracture. No benefit was shown among patients undergoing hip arthroplasty or knee arthroplasty; in those groups, both the aspirin and placebo recipients were also treated with LMWH. An absolute increase in rates of wound bleeding (0.6% increase) and gastrointestinal bleeding (1.0% increase) was observed in the aspirin group. The absolute increase in complications was greater than the absolute reduction in episodes of symptomatic DVT: for every episode of symptomatic DVT averted, one wound bleed and 10 gastrointestinal bleeds occurred.
SPECIAL PATIENT POPULATIONS
Renal impairment
The 8th edition of the ACCP guidelines recommends that renal function be kept in mind when considering LMWH, fondaparinux, and other antithrombotic drugs that are cleared by the kidneys. Fondaparinux and LMWH can bioaccumulate in patients with renal insufficiency, who have a higher risk of bleeding to begin with, thereby compounding the risk. Options for patients with renal compromise include avoiding drugs that bioaccumulate, using a lower dosage, and monitoring the drug level or anticoagulant effect.1
Fondaparinux is explicitly contraindicated in patients with low body weight (< 50 kg) or renal impairment (creatinine clearance < 30 mL/min). Renal function should be assessed periodically in any patients receiving the drug.18
I also would not use fondaparinux or LMWH in patients with rapidly changing renal function. For patients with chronic, stable renal impairment, one can reduce the dose of LMWH empirically; one LMWH, enoxaparin, has specific dosing guidelines in its package insert (one-third reduction in dose), but this option does not hold for patients with rapidly changing renal function.19
Obesity
The 8th edition of the ACCP guidelines recommends weight-based dosing of thromboprophylactic agents in obese patients. The guidelines particularly recommend that patients undergoing inpatient bariatric surgery be given higher doses of LMWH or unfractionated heparin.1,20
Frederiksen et al measured the anticoagulant effect of a single fixed dose of a LMWH (using anti-factor Xa heparin activity levels) and found that it was dependent on body weight.21 This suggests that fixed doses that are effective in normal-weight patients may have no detectable anti-coagulant effect in patients with very high body weight.
Weight-based dosing: mounting nonprospective evidence. Weight-based dosage adjustment for the morbidly obese has not been directly studied in a prospective, randomized fashion. A nonrandomized study by Scholten et al compared two regimens of enoxaparin (30 mg twice daily vs 40 mg twice daily) among 481 obese patients undergoing bariatric surgery; each regimen was used along with mechanical thromboprophylaxis.22 They found that the higher-dose regimen was associated with significantly fewer postoperative DVT complications (0.6% vs 5.4%; P < .01) without an increase in bleeding complications.
Separately, Shepherd et al used weight-adjusted doses of unfractionated heparin (started on the evening of surgery) to achieve subtherapeutic peak anti–factor Xa heparin activity levels of 0.11 to 0.25 IU/mL in a series of 700 patients undergoing laparoscopic gastric bypass surgery.23 The resulting doses were greater than those in traditional fixed-rate protocols, but rates of bleeding and VTE events were low and comparable to those reported in patients receiving standard doses.
Don’t rule out multimodal approaches. Multimodal prophylaxis can also be used in obese patients and need not be abandoned as a result of size considerations. For instance, two intermittent compression therapy devices can be pieced together with a Velcro binder if a single device is too small to be worn.
EMERGING ANTICOAGULANT OPTIONS
For many years, unfractionated heparin was the only available parenteral anticoagulant. While heparin has broad anticoagulant properties, it also has well-established limitations, including the need for parenteral delivery, recent problems related to contamination (it is derived from pig intestines), and of course heparin-induced thrombocytopenia (HIT). HIT is an immune-mediated form of platelet activation that can lead to widespread thrombosis throughout the body. It is more commonly associated with venous thrombosis, but arterial events with limb-threatening ischemia may also occur. LMWH is associated with a reduced risk of HIT, but LMWH does not avoid the risk entirely.
Beyond the issue of avoiding HIT, newer anticoagulant therapies are being developed with the aim of oral administration and more targeted inhibition of coagulation factors IIa (thrombin) and Xa.24
Oral direct thrombin inhibitors
One of the two most promising classes of emerging anticoagulants is the direct thrombin inhibitors, most of which are being developed for oral administration. There were high hopes for the initial compound in this class, ximelagatran, but it was abandoned about 5 years ago because of hepatotoxicity.
Dabigatran is the direct thrombin inhibitor furthest along in development today. Currently approved in Europe for prevention of VTE in patients undergoing total hip or knee replacement surgery, dabigatran is likely to be available soon in the United States. It is administered orally, has a rapid onset of action (< 1 hour), and has a predictable anticoagulant response that requires no monitoring.24 Because dabigatran is excreted essentially unchanged by the kidneys and may bioaccumulate, it should not be used in patients with renal impairment or rapidly changing renal function.
In phase 3 clinical trials for VTE prevention in knee replacement surgery, dabigatran was at least as effective as enoxaparin 40 mg once daily and had a comparable safety profile,25 but it was slightly less effective than enoxaparin 30 mg twice daily.26 In a phase 3 trial in patients undergoing hip replacement surgery, dabigatran was equivalent in efficacy and safety to enoxaparin 40 mg once daily.27
Oral direct factor Xa inhibitors
A key rationale for direct inhibition of factor Xa is that it results in inhibition of thrombin production on the activated platelet. Whereas fondaparinux is an indirect inhibitor of factor Xa, direct factor Xa inhibitors offer an advantage in that they inhibit factor Xa within the prothrombinase complex, which occurs on the surface of a platelet and is the main site for thrombin development (very little thrombin is actually produced on endothelial cells). Recall the adage that “thrombin begets more thrombin”: it activates not only platelets but the intrinsic and extrinsic pathways.28
Factor Xa may be a better target than thrombin for a number of other reasons:
- Factor Xa is believed to have few functions (compared with thrombin) outside of coagulation
- In vitro studies show that factor Xa has a wider therapeutic window than thrombin, which translates to greater separation between drug levels that will confer efficacy and bleeding
- Thrombin inhibitors are associated with rebound thrombin generation (there is no evidence of this with factor Xa inhibitors)
- The efficacy of heparin-based anticoagulants improves as selectivity for factor Xa increases (unfractionated heparin is less effective than LMWH, which is less effective than fondaparinux).
Two direct factor Xa inhibitors—both administered orally—are far along in development, as detailed below.
Apixaban has shown promise, but the phase 3 ADVANCE-1 study of apixaban for VTE prevention in patients undergoing knee surgery did not meet statistical criteria for noninferiority compared with enoxaparin 30 mg twice daily.29 This prompted a delay in regulatory filings for apixaban in the United States, and the drug’s prospects for approval for VTE prevention may be unclear until release of results from two other comparative phase 3 trials with enoxaparin in 2009 and 2010.
Rivaroxaban is more likely to become clinically available soon, in light of recent results from the phase 3 RECORD4 trial demonstrating that it was significantly superior to enoxaparin 30 mg twice daily in preventing VTE following knee replacement surgery with comparable rates of major bleeding.30
DISCUSSION
Question from the audience: Some surgeons in my hospital prescribe warfarin immediately after surgery without a bridge of LMWH. Is that appropriate?
Dr. Michota: Warfarin is an option for prophylaxis in orthopedic surgery, beginning on the day of surgery. It could even be started the day before surgery, but the dose should be monitored to achieve an INR between 2.0 and 3.0 within 72 hours of the procedure. If the INR is not in this optimum range, prophylactic doses of LMWH can be given until it is therapeutic.
Follow-up question: In practice, do you actually encourage INR monitoring? Usually we just put patients on a certain dose without monitoring. When we do check the INR, it’s usually 1.4 or 1.5.
Dr. Michota: Warfarin was shown to be effective in reducing VTE risk in orthopedic surgery with dose adjustment based on INR monitoring. On that basis, warfarin remains in the guideline recommendations. Unmonitored, warfarin has not been shown to reduce risk, so to give it that way would not be evidence-based.
Question from the audience: I work with several plastic surgeons who use compression stockings intraoperatively because they’ve heard of several patients who developed a PE during surgery. Is there any benefit to using compression stockings for 2 to 3 hours and then sending the patient home?
Dr. Michota: I don’t know. Theoretically, a device that is on and working before induction may reduce stasis.
The plastic surgery societies do have guidelines. Risk depends on the type of plastic surgery procedure; for example, risk probably increases due to inflammation in procedures that involve scraping the fat pads.
This is an area where we don’t have much data. These patients may be at risk, but we don’t know the best way to mitigate it. It is important that risks be discussed with patients in the informed-consent process and be documented. If the surgeon thinks it is reasonable to give pharmacologic prophylaxis after surgery, I wouldn’t hesitate to do that, but any form of bleeding in the setting of plastic surgery is catastrophic because it defeats the reason for which the surgery was done in the first place.
Question from the audience: How do the guidelines address being aggressive with pharmacologic thromboprophylaxis when a patient is already taking dual antiplatelet therapy?
Dr. Michota: For patients with an indication for VTE prophylaxis in a setting for which there is a specific strategy, the ACCP guidelines recommend that they be put on that regimen whether they are on antiplatelet agents or not. For example, consider a high-risk patient having colorectal surgery who should get unfractionated heparin or LMWH postoperatively and who is currently taking clopidogrel and aspirin. There is no evidence that the dual aspirin–clopidogrel therapy alone is effective in decreasing the risk of DVT. However, we do know that if we add on additional agents, the risk of bleeding is increased. The guidelines consider risk and benefit, and they recommend adding the agents that we know work to prevent DVT.
Question from the audience: You briefly mentioned prophylaxis for knee arthroscopy, which is the most frequently performed orthopedic procedure. Do these recommendations apply to all patients undergoing knee arthroscopy?
Dr. Michota: No. Prophylaxis is indicated only for patients with what the ACCP considers to be additional risk factors for thrombosis. They didn’t specify which risk factors, but good indications for prophylaxis would include morbid obesity, limited mobility after the procedure, a personal history of DVT, features of stasis noted on physical examination, stasis dermatitis (or other features that could indicate prior thrombosis), advanced age, and malignancy. If a patient undergoing knee arthroscopy has other nonmodifiable risk factors, you should also think about prophylaxis. But the vast majority of patients do not need it.
Question from the audience: I’m an academic hospitalist who works closely with orthopedic surgeons. Certain surgeons will only use aspirin for prophylaxis, and it is nonnegotiable. Where does that leave me from a medicolegal standpoint? Our model is to follow ACCP recommendations, but these orthopedic surgeons still use only aspirin.
Dr. Michota: You must do everything you can to come to a consensus with your surgeon colleagues. If you are uncomfortable, as a group you must say to the surgeons, “We are uncomfortable. This is how we view the data. How do you view the data?” If they answer, “We’re doing it because it’s easy, and the American Academy of Orthopaedic Surgeons says we can do it,” I don’t have a good response. But it is more likely that their use of aspirin is based on their own observations; they may not see many clots. Of course, the problem with observational data is that the numbers are not large and they are not generated in a randomized and prospective fashion. Perhaps you can come to some middle ground, but you could always make the difficult choice and say, “I’m just not going to follow your patients.”
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133:381S–453S.
- Medicare Quality Improvement Community (MedQIC) Web site. http://www.medqic.org. Accessed June 1, 2009.
- Surgical Care Improvement Project (SCIP). Colorado Foundation for Medical Care Web site. http://www.cfmc.org/hospital/hospital_scip.htm. Accessed June 1, 2009.
- Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med 2005; 352:969–977.
- Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107:I-9–I-16.
- Amaragiri SV, Lees TA. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev 2000; (3):CD001484.
- Rogers FB, Cipolle MD, Velmahos G, et al. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST Practice Management Work Group. J Trauma 2002; 53:142–164.
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002; 346:975–980.
- Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med 2002; 162:1245–1248.
- Spencer FA, Lessard D, Emery C, et al. Venous thromboembolism in the outpatient setting. Arch Intern Med 2007; 167:1471–1475.
- Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med 2000; 160:2327–2332.
- Mismetti P, Laporte S, Zufferey P, et al. Prevention of venous thromboembolism in orthopedic surgery with vitamin K antagonists: a meta-analysis. J Thromb Haemost 2004; 2:1058–1070.
- Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studies. Arch Intern Med 2002; 162:1833–1840.
- Bauer KA, Eriksson BI, Lassen MR, Turpie AG; Steering Committee of the Pentasaccharide in Major Knee Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med 2001; 345:1305–1310.
- Cardiovascular Disease Educational and Research Trust; Cyprus Cardiovascular Disease Educational and Research Trust; European Venous Forum; International Surgical Thrombosis Forum; International Union of Angiology; Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006; 25:101–161.
- American Academy of Orthopaedic Surgeons clinical guideline on prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. http://www.aaos.org/research/guidelines/PE_summary.pdf. Accessed June 5, 2009.
- Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355:1295-1302.
- Arixtra injection [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2008.
- Sanderink GJ, Guimart C, Jariwala N, et al. Enoxaparin pharmacokinetics and pharmacodynamics in renal impairment. J Am Coll Cardiol 2001; 37(suppl A):229A. Abstract.
- Hirsh J, Bauer KA, Donati MB, et al; American College of Chest Physicians. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) [published correction appears in Chest 2008; 134:473]. Chest 2008; 133:141S–159S.
- Frederiksen SG, Hedenbro JL, Norgren L. Enoxaparin effect depends on body-weight and current doses may be inadequate in obese patients. Br J Surg 2003; 90:547–548.
- Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg 2002; 12:19–24.
- Shepherd MF, Rosborough TK, Schwartz ML. Heparin thromboprophylaxis in gastric bypass surgery. Obes Surg 2003; 13:249–253.
- Weitz JI, Bates SM. New anticoagulants. J Thromb Haemost 2005; 3:1843–1853.
- Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009; 24:1–9.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial [published correction appears in Lancet 2007; 370:2004]. Lancet 2007: 370:949–956.
- Hoffman M, Monroe DM 3rd, Roberts HR. Activated factor VII activates factors IX and X on the surface of activated platelets: thoughts on the mechanism of action of high-dose activated factor VII. Blood Coagul Fibrinolysis 1998; 9(suppl 1):S61–S65.
- Bristol-Myers Squibb and Pfizer provide update on apixaban clinical development program [press release]. New York, NY: August 27, 2008.
- Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009; 373:1673–1680.
Most surgical patients who require hospitalization should be considered at high risk for venous thromboembolism (VTE) and be given appropriate prophylaxis. For lower-risk procedures such as knee arthroscopy, prophylaxis is needed for those with individual risk factors such as morbid obesity, limited mobility after surgery, or a history of deep vein thrombosis (DVT) or malignancy. Too often, however, prophylaxis is not provided appropriately or not given at all.
This review surveys the essentials of perioperative VTE prophylaxis and important new developments in the field, which include the 2008 release of new evidence-based clinical practice guidelines on antithrombotic and thrombolytic therapy from the American College of Chest Physicians (ACCP). This 8th edition of the guidelines updates the previous edition, published in 2004, and includes a section by Geerts et al devoted to VTE prevention.1 Other major guidelines are also discussed, as are developments in VTE-related quality measurement, management of special patient populations (those with renal impairment or morbid obesity), and emerging therapies for VTE prophylaxis.
IMPETUS FOR QUALITY IMPROVEMENT IN VTE
A new seriousness about VTE quality measures
The 8th edition of the ACCP guidelines recommends that every hospital develop a formal, active strategy to consistently identify medical and surgical patients at risk for VTE and to prevent VTE occurrence.1 Although prior editions of the ACCP guidelines have made this recommendation for more than 2 decades, fewer than 1 in 10 acute care hospitals had any such strategy in place as recently as 5 years ago. Now, however, most US hospitals have implemented such a strategy, thanks to the growing national emphasis on health care quality measurement in recent years.
The Surgical Care Improvement Project (SCIP) has been at the forefront of this recent quality measures movement. SCIP, a joint project of the American Medical Association and federal government agencies, set a goal to reduce surgical complications in the United States by 25% from 2005 to 2010.2 Two SCIP process measures relate to improving VTE prophylaxis2,3:
- The proportion of surgical patients for whom recommended VTE prophylaxis is ordered
- The proportion of surgical patients who actually receive appropriate VTE prophylaxis within 24 hours before or after surgery.
The Joint Commission and the National Quality Forum recently endorsed these two SCIP performance measures for perioperative VTE prophylaxis along with several others relating to VTE treatment.
CMS raises the stakes with reimbursement restrictions
More significantly, the federal government’s Centers for Medicare and Medicaid Services (CMS) will soon refuse to reimburse for hospital treatment of a primary diagnosis of DVT or pulmonary embolism (PE) following recent (within 30 days) total hip or knee arthroplasty. Effective October 1, 2009, a primary VTE diagnosis following these joint replacement procedures will be added to CMS’ current list of “never events,” or hospital-acquired conditions for which CMS will not provide reimbursement because they are considered the result of preventable medical errors. (Notably, treatment of DVT or PE as a secondary diagnosis will still be reimbursed—for example, if a joint replacement patient develops nosocomial pneumonia, is transferred to the intensive care unit, and then develops VTE.) This addition of DVT and PE to the list is highly controversial since these events sometimes develop even if prophylactic therapy is appropriate and aggressive.
Strategies to promote best practices
In the update for the new 8th edition of its guidelines, the ACCP added recommendations on specific ways for hospitals to identify patients at high risk for VTE and ensure that they receive appropriate prophylaxis. These include the use of computer decision-support systems, preprinted orders, and periodic audit and feedback.1
Researchers at Brigham and Women’s Hospital evaluated the effectiveness of a computer alert system for notifying physicians of newly hospitalized patients at risk for DVT who were not receiving prevention therapy within the first 24 hours of hospital admission.4 These patients presumably “fell through the cracks” and warranted prophylaxis but were otherwise not recognized by the health care team. Risk was determined by a scoring system based on multiple variables, including malignancy, previous DVT or PE, hypercoagulability, major surgery, advanced age, obesity, ordered bed rest, and treatment with hormone replacement therapy or oral contraceptives. Study physicians had to acknowledge having received the alert but could choose whether or not to order VTE prophylaxis. Prophylaxis was used in considerably more patients from the intervention group than from a control group of high-risk patients whose physicians did not receive alerts (34% vs 14%, respectively); accordingly, the risk of a symptomatic DVT or PE event at 90 days was reduced by 41% in the intervention group.
Despite this evidence of improved practice under the alert system, the study begs the question of why the percentage of patients at risk for VTE who were given prophylaxis was still so low (34%), demonstrating how much progress in improving practice remains to be achieved.
PROPHYLAXIS STRATEGIES: MATCHING THERAPY TO RISK
NONPHARMACOLOGIC PROPHYLAXIS STRATEGIES
Does ambulation prevent DVT?
Although it is commonly accepted that walking prevents DVT, this has never been directly tested. Walking may simply be a marker of health, and healthy people are less prone to develop thromboses. We have almost no evidence to show that forcing an unhealthy person to walk helps prevent DVT. Early ambulation offers many benefits and should be encouraged, but it should not be considered DVT prophylaxis; it is simply good hospital care.
Mechanical devices: Adherence is key
Amaragiri and Lees conducted a systematic literature review of randomized controlled trials evaluating the effectiveness of graduated compression stockings (elastic stockings) for preventing DVT in various groups of hospitalized patients.6 The analysis demonstrated a statistically significant reduction in DVT incidence with graduated compression stockings compared with control both among the nine trials in which stockings were used alone (odds ratio = 0.34) and among the seven trials in which stockings were used in addition to another method of thromboprophylaxis (odds ratio = 0.24). Although benefit was demonstrated, many of the trials in this review involved patients undergoing gynecologic surgery and date from the 1970s and 1980s (when obesity was less prevalent), so the applicability of their results today may be limited.
The 8th edition of the ACCP guidelines recommends that mechanical methods of VTE prophylaxis be used primarily in patients who are at high risk of bleeding and that careful attention be directed to ensuring their proper use and optimal adherence.1 The latter point about adherence cannot be emphasized enough, as graduated compression stockings and other mechanical devices have been shown not to be effective unless they are worn at least 18 to 20 hours a day. This degree of adherence is difficult to achieve, as it can severely limit patient mobility and leave patients susceptible to development of pressure ulcers.
Mechanical compression should be initiated prior to induction of anesthesia and continue intraoperatively and then into the postanesthesia care unit. Orders for use of mechanical devices should include instructions in the patient’s medical chart specifying how—and for how many hours per day—they are to be worn. Not doing so leaves the physician vulnerable to litigation, especially as the ACCP guidelines include language on optimal adherence to these devices (“they should be removed for only a short time each day when the patient is actually walking or for bathing”1).
Continuous external compression therapy
Newer mechanical device options include a continuous external compression therapy system that allows patients to be mobile while wearing it and provides rhythmic compression that results in good peak venous flows. Ideally such a device could be put on the patient preoperatively and worn during surgery, throughout the hospital stay, and even at home during recovery. Anecdotally, however, I see patients turn these new devices off at the side of the bed just as often as they do with traditional devices.
Vena caval interruption
Vena caval interruption involves placement of a retrievable vena cava filter before surgery and removal some time later; it offers the potential for VTE prophylaxis in patients who could not tolerate even minor amounts of bleeding, such as certain trauma patients. The Eastern Association for the Surgery of Trauma has put forth a consensus recommendation to consider vena caval interruption in high-risk trauma patients who cannot receive pharmacologic prophylaxis.7 A randomized trial evaluating the usefulness of vena caval interruption for patients undergoing surgery is needed. For now, this intervention should be regarded as experimental and considered only on a highly individualized basis.
PHARMACOLOGIC PROPHYLAXIS
Timing of initiation
Pharmacologic VTE prophylaxis generally should begin 8 to 24 hours postoperatively. Of course, adequate hemostasis is required before initiation, and the net risk/benefit tradeoff with regard to timing of anticoagulant initiation has still not been well studied in many surgical patient populations.
Extended prophylaxis
In the update for the 8th edition of its guidelines, the ACCP added an explicit recommendation for extended outpatient prophylaxis with low-molecular-weight heparin (LMWH) for up to 28 days postoperatively in selected high-risk patients undergoing general or gynecologic surgery, including those with cancer or a history of VTE.1 This recommendation was based largely on studies of extended prophylaxis in patients with cancer undergoing colorectal surgery.8
Increased appreciation of the value of extended VTE prophylaxis after discharge is linked to a growing recognition that DVT and PE episodes in the community setting are often related to a recent hospital stay for either medical illness or surgery. A population-based study found that 59% of all community cases of a first lifetime VTE event in residents of Olmsted County, Minn., over a 15-year period could be linked to current or recent (< 30 days) hospitalization or nursing home residence.9 A similar population-based study in the Worcester, Mass., area found that three-fourths of all VTE events in a 3-year period occurred in the outpatient setting.10 Among patients with these outpatient VTE events, a large proportion had undergone surgery (23%) or hospitalization (37%) in the prior 3 months; among those, 67% experienced their VTE within 1 month of their time in the hospital.
These findings are no surprise, since surgery induces a hypercoagulable state that, when combined with individual risk factors such as obesity, old age, or poor heart function, cannot be assumed to return to baseline on postoperative day 4 or 5 just because the patient is being discharged.
Orthopedic surgery
For patients undergoing major orthopedic procedures, the ACCP guidelines recommend against routine screening for VTE with Doppler ultrasonography before discharge if the patient is asymptomatic.1 Such screening is not considered cost-effective because asymptomatic clots often are found, for which treatment is uncertain, and proximal clots may be missed, giving a false sense of security.
New to the ACCP guidelines in the 8th edition is the recommendation that patients undergoing knee arthroscopy who have risk factors for VTE (or whose procedure is complicated) should receive 1 week of prophylaxis with LMWH.1 Also new are recommendations for patients with risk factors undergoing single- or multilevel laminectomy (Table 4).
Recommendations unchanged in neurosurgery, spinal injury, trauma, burns
Recommendations for neurosurgery remain unchanged from the prior (2004) edition of the ACCP guidelines and are still based on the 2000 meta-analysis by Iorio and Agnelli of LMWH prophylaxis in neurosurgery cases.11 In the United States, the standard is overwhelmingly to use mechanical devices for thromboprophylaxis in neurosurgery, even for patients with cancer.
For prophylaxis in surgical patients with spinal cord injury, multisystem trauma, or burns, LMWH is predominantly used, and the ACCP recommendations are unchanged from 2004.
Drug-specific considerations
LMWH vs vitamin K antagonist. Although vitamin K antagonists (warfarin) still appear in the latest ACCP recommendations,1 LMWH is preferable. A 2004 meta-analysis of studies comparing vitamin K antagonists with LMWH for prophylaxis in patients undergoing orthopedic surgery found that vitamin K antagonists were associated with more episodes of total DVT (relative risk [RR] = 1.51; 95% CI, 1.27–1.79) and proximal DVT (RR = 1.51; 95% CI, 1.04–2.17) compared with LMWH.12 No difference was found in rates of wound hematoma or major bleeding. This finding of inferiority for vitamin K antagonists came despite the likelihood that warfarin was more often administered correctly (ie, with dose adjustment to achieve an international normalized ratio [INR] of 2.0 to 3.0 within 72 hours after surgery) in the studies in this analysis than it is in real-world practice.
Fondaparinux. The indirect factor Xa–specific inhibitor fondaparinux has had a surprisingly limited clinical adoption despite having been widely studied and found to be safe and effective. This is likely attributable in part to its 17-hour half-life, which raises concerns that it may take 3 days for its effects to stop if a patient begins to bleed. Large phase 3 studies have found fondaparinux to be equivalent to LMWH in VTE prevention after hip replacement, marginally superior to LMWH after knee replacement, and superior to LMWH following hip fracture repair.13 Fondaparinux was associated with an increase in bleeding events and instances of transfusion requirement, but only in one of the studies, which was in the setting of knee replacement surgery.14
Aspirin not recommended by ACCP. Although aspirin reduces the risk of VTE, practice guidelines from both the ACCP1 and the International Union of Angiology15 contain no recommendation for its use as prophylaxis because it is considered less effective and more risky than other therapies. In contrast, clinical practice guidelines from the American Academy of Orthopaedic Surgeons suggest that aspirin is reasonable for VTE prophylaxis.16 The varying recommendations reflect differences in perspective among these different specialties.
Aspirin has the advantages of ease of use and low cost, but it is clearly not the best evidence-based approach for VTE prophylaxis. The only recent randomized trial evidence in support of aspirin comes from the Pulmonary Embolism Prevention trial, a study with a flawed design involving more than 13,000 patients undergoing surgery for hip fracture or elective arthroplasty in five countries.17 Patients were randomized to receive aspirin 160 mg daily or placebo for 35 days along with any other prophylaxis deemed necessary (an important potential confounder). Aspirin was associated with an absolute reduction in symptomatic events of less than 1% relative to placebo, and no benefit was observed within the first week. The best results with aspirin were among patients with hip fracture. No benefit was shown among patients undergoing hip arthroplasty or knee arthroplasty; in those groups, both the aspirin and placebo recipients were also treated with LMWH. An absolute increase in rates of wound bleeding (0.6% increase) and gastrointestinal bleeding (1.0% increase) was observed in the aspirin group. The absolute increase in complications was greater than the absolute reduction in episodes of symptomatic DVT: for every episode of symptomatic DVT averted, one wound bleed and 10 gastrointestinal bleeds occurred.
SPECIAL PATIENT POPULATIONS
Renal impairment
The 8th edition of the ACCP guidelines recommends that renal function be kept in mind when considering LMWH, fondaparinux, and other antithrombotic drugs that are cleared by the kidneys. Fondaparinux and LMWH can bioaccumulate in patients with renal insufficiency, who have a higher risk of bleeding to begin with, thereby compounding the risk. Options for patients with renal compromise include avoiding drugs that bioaccumulate, using a lower dosage, and monitoring the drug level or anticoagulant effect.1
Fondaparinux is explicitly contraindicated in patients with low body weight (< 50 kg) or renal impairment (creatinine clearance < 30 mL/min). Renal function should be assessed periodically in any patients receiving the drug.18
I also would not use fondaparinux or LMWH in patients with rapidly changing renal function. For patients with chronic, stable renal impairment, one can reduce the dose of LMWH empirically; one LMWH, enoxaparin, has specific dosing guidelines in its package insert (one-third reduction in dose), but this option does not hold for patients with rapidly changing renal function.19
Obesity
The 8th edition of the ACCP guidelines recommends weight-based dosing of thromboprophylactic agents in obese patients. The guidelines particularly recommend that patients undergoing inpatient bariatric surgery be given higher doses of LMWH or unfractionated heparin.1,20
Frederiksen et al measured the anticoagulant effect of a single fixed dose of a LMWH (using anti-factor Xa heparin activity levels) and found that it was dependent on body weight.21 This suggests that fixed doses that are effective in normal-weight patients may have no detectable anti-coagulant effect in patients with very high body weight.
Weight-based dosing: mounting nonprospective evidence. Weight-based dosage adjustment for the morbidly obese has not been directly studied in a prospective, randomized fashion. A nonrandomized study by Scholten et al compared two regimens of enoxaparin (30 mg twice daily vs 40 mg twice daily) among 481 obese patients undergoing bariatric surgery; each regimen was used along with mechanical thromboprophylaxis.22 They found that the higher-dose regimen was associated with significantly fewer postoperative DVT complications (0.6% vs 5.4%; P < .01) without an increase in bleeding complications.
Separately, Shepherd et al used weight-adjusted doses of unfractionated heparin (started on the evening of surgery) to achieve subtherapeutic peak anti–factor Xa heparin activity levels of 0.11 to 0.25 IU/mL in a series of 700 patients undergoing laparoscopic gastric bypass surgery.23 The resulting doses were greater than those in traditional fixed-rate protocols, but rates of bleeding and VTE events were low and comparable to those reported in patients receiving standard doses.
Don’t rule out multimodal approaches. Multimodal prophylaxis can also be used in obese patients and need not be abandoned as a result of size considerations. For instance, two intermittent compression therapy devices can be pieced together with a Velcro binder if a single device is too small to be worn.
EMERGING ANTICOAGULANT OPTIONS
For many years, unfractionated heparin was the only available parenteral anticoagulant. While heparin has broad anticoagulant properties, it also has well-established limitations, including the need for parenteral delivery, recent problems related to contamination (it is derived from pig intestines), and of course heparin-induced thrombocytopenia (HIT). HIT is an immune-mediated form of platelet activation that can lead to widespread thrombosis throughout the body. It is more commonly associated with venous thrombosis, but arterial events with limb-threatening ischemia may also occur. LMWH is associated with a reduced risk of HIT, but LMWH does not avoid the risk entirely.
Beyond the issue of avoiding HIT, newer anticoagulant therapies are being developed with the aim of oral administration and more targeted inhibition of coagulation factors IIa (thrombin) and Xa.24
Oral direct thrombin inhibitors
One of the two most promising classes of emerging anticoagulants is the direct thrombin inhibitors, most of which are being developed for oral administration. There were high hopes for the initial compound in this class, ximelagatran, but it was abandoned about 5 years ago because of hepatotoxicity.
Dabigatran is the direct thrombin inhibitor furthest along in development today. Currently approved in Europe for prevention of VTE in patients undergoing total hip or knee replacement surgery, dabigatran is likely to be available soon in the United States. It is administered orally, has a rapid onset of action (< 1 hour), and has a predictable anticoagulant response that requires no monitoring.24 Because dabigatran is excreted essentially unchanged by the kidneys and may bioaccumulate, it should not be used in patients with renal impairment or rapidly changing renal function.
In phase 3 clinical trials for VTE prevention in knee replacement surgery, dabigatran was at least as effective as enoxaparin 40 mg once daily and had a comparable safety profile,25 but it was slightly less effective than enoxaparin 30 mg twice daily.26 In a phase 3 trial in patients undergoing hip replacement surgery, dabigatran was equivalent in efficacy and safety to enoxaparin 40 mg once daily.27
Oral direct factor Xa inhibitors
A key rationale for direct inhibition of factor Xa is that it results in inhibition of thrombin production on the activated platelet. Whereas fondaparinux is an indirect inhibitor of factor Xa, direct factor Xa inhibitors offer an advantage in that they inhibit factor Xa within the prothrombinase complex, which occurs on the surface of a platelet and is the main site for thrombin development (very little thrombin is actually produced on endothelial cells). Recall the adage that “thrombin begets more thrombin”: it activates not only platelets but the intrinsic and extrinsic pathways.28
Factor Xa may be a better target than thrombin for a number of other reasons:
- Factor Xa is believed to have few functions (compared with thrombin) outside of coagulation
- In vitro studies show that factor Xa has a wider therapeutic window than thrombin, which translates to greater separation between drug levels that will confer efficacy and bleeding
- Thrombin inhibitors are associated with rebound thrombin generation (there is no evidence of this with factor Xa inhibitors)
- The efficacy of heparin-based anticoagulants improves as selectivity for factor Xa increases (unfractionated heparin is less effective than LMWH, which is less effective than fondaparinux).
Two direct factor Xa inhibitors—both administered orally—are far along in development, as detailed below.
Apixaban has shown promise, but the phase 3 ADVANCE-1 study of apixaban for VTE prevention in patients undergoing knee surgery did not meet statistical criteria for noninferiority compared with enoxaparin 30 mg twice daily.29 This prompted a delay in regulatory filings for apixaban in the United States, and the drug’s prospects for approval for VTE prevention may be unclear until release of results from two other comparative phase 3 trials with enoxaparin in 2009 and 2010.
Rivaroxaban is more likely to become clinically available soon, in light of recent results from the phase 3 RECORD4 trial demonstrating that it was significantly superior to enoxaparin 30 mg twice daily in preventing VTE following knee replacement surgery with comparable rates of major bleeding.30
DISCUSSION
Question from the audience: Some surgeons in my hospital prescribe warfarin immediately after surgery without a bridge of LMWH. Is that appropriate?
Dr. Michota: Warfarin is an option for prophylaxis in orthopedic surgery, beginning on the day of surgery. It could even be started the day before surgery, but the dose should be monitored to achieve an INR between 2.0 and 3.0 within 72 hours of the procedure. If the INR is not in this optimum range, prophylactic doses of LMWH can be given until it is therapeutic.
Follow-up question: In practice, do you actually encourage INR monitoring? Usually we just put patients on a certain dose without monitoring. When we do check the INR, it’s usually 1.4 or 1.5.
Dr. Michota: Warfarin was shown to be effective in reducing VTE risk in orthopedic surgery with dose adjustment based on INR monitoring. On that basis, warfarin remains in the guideline recommendations. Unmonitored, warfarin has not been shown to reduce risk, so to give it that way would not be evidence-based.
Question from the audience: I work with several plastic surgeons who use compression stockings intraoperatively because they’ve heard of several patients who developed a PE during surgery. Is there any benefit to using compression stockings for 2 to 3 hours and then sending the patient home?
Dr. Michota: I don’t know. Theoretically, a device that is on and working before induction may reduce stasis.
The plastic surgery societies do have guidelines. Risk depends on the type of plastic surgery procedure; for example, risk probably increases due to inflammation in procedures that involve scraping the fat pads.
This is an area where we don’t have much data. These patients may be at risk, but we don’t know the best way to mitigate it. It is important that risks be discussed with patients in the informed-consent process and be documented. If the surgeon thinks it is reasonable to give pharmacologic prophylaxis after surgery, I wouldn’t hesitate to do that, but any form of bleeding in the setting of plastic surgery is catastrophic because it defeats the reason for which the surgery was done in the first place.
Question from the audience: How do the guidelines address being aggressive with pharmacologic thromboprophylaxis when a patient is already taking dual antiplatelet therapy?
Dr. Michota: For patients with an indication for VTE prophylaxis in a setting for which there is a specific strategy, the ACCP guidelines recommend that they be put on that regimen whether they are on antiplatelet agents or not. For example, consider a high-risk patient having colorectal surgery who should get unfractionated heparin or LMWH postoperatively and who is currently taking clopidogrel and aspirin. There is no evidence that the dual aspirin–clopidogrel therapy alone is effective in decreasing the risk of DVT. However, we do know that if we add on additional agents, the risk of bleeding is increased. The guidelines consider risk and benefit, and they recommend adding the agents that we know work to prevent DVT.
Question from the audience: You briefly mentioned prophylaxis for knee arthroscopy, which is the most frequently performed orthopedic procedure. Do these recommendations apply to all patients undergoing knee arthroscopy?
Dr. Michota: No. Prophylaxis is indicated only for patients with what the ACCP considers to be additional risk factors for thrombosis. They didn’t specify which risk factors, but good indications for prophylaxis would include morbid obesity, limited mobility after the procedure, a personal history of DVT, features of stasis noted on physical examination, stasis dermatitis (or other features that could indicate prior thrombosis), advanced age, and malignancy. If a patient undergoing knee arthroscopy has other nonmodifiable risk factors, you should also think about prophylaxis. But the vast majority of patients do not need it.
Question from the audience: I’m an academic hospitalist who works closely with orthopedic surgeons. Certain surgeons will only use aspirin for prophylaxis, and it is nonnegotiable. Where does that leave me from a medicolegal standpoint? Our model is to follow ACCP recommendations, but these orthopedic surgeons still use only aspirin.
Dr. Michota: You must do everything you can to come to a consensus with your surgeon colleagues. If you are uncomfortable, as a group you must say to the surgeons, “We are uncomfortable. This is how we view the data. How do you view the data?” If they answer, “We’re doing it because it’s easy, and the American Academy of Orthopaedic Surgeons says we can do it,” I don’t have a good response. But it is more likely that their use of aspirin is based on their own observations; they may not see many clots. Of course, the problem with observational data is that the numbers are not large and they are not generated in a randomized and prospective fashion. Perhaps you can come to some middle ground, but you could always make the difficult choice and say, “I’m just not going to follow your patients.”
Most surgical patients who require hospitalization should be considered at high risk for venous thromboembolism (VTE) and be given appropriate prophylaxis. For lower-risk procedures such as knee arthroscopy, prophylaxis is needed for those with individual risk factors such as morbid obesity, limited mobility after surgery, or a history of deep vein thrombosis (DVT) or malignancy. Too often, however, prophylaxis is not provided appropriately or not given at all.
This review surveys the essentials of perioperative VTE prophylaxis and important new developments in the field, which include the 2008 release of new evidence-based clinical practice guidelines on antithrombotic and thrombolytic therapy from the American College of Chest Physicians (ACCP). This 8th edition of the guidelines updates the previous edition, published in 2004, and includes a section by Geerts et al devoted to VTE prevention.1 Other major guidelines are also discussed, as are developments in VTE-related quality measurement, management of special patient populations (those with renal impairment or morbid obesity), and emerging therapies for VTE prophylaxis.
IMPETUS FOR QUALITY IMPROVEMENT IN VTE
A new seriousness about VTE quality measures
The 8th edition of the ACCP guidelines recommends that every hospital develop a formal, active strategy to consistently identify medical and surgical patients at risk for VTE and to prevent VTE occurrence.1 Although prior editions of the ACCP guidelines have made this recommendation for more than 2 decades, fewer than 1 in 10 acute care hospitals had any such strategy in place as recently as 5 years ago. Now, however, most US hospitals have implemented such a strategy, thanks to the growing national emphasis on health care quality measurement in recent years.
The Surgical Care Improvement Project (SCIP) has been at the forefront of this recent quality measures movement. SCIP, a joint project of the American Medical Association and federal government agencies, set a goal to reduce surgical complications in the United States by 25% from 2005 to 2010.2 Two SCIP process measures relate to improving VTE prophylaxis2,3:
- The proportion of surgical patients for whom recommended VTE prophylaxis is ordered
- The proportion of surgical patients who actually receive appropriate VTE prophylaxis within 24 hours before or after surgery.
The Joint Commission and the National Quality Forum recently endorsed these two SCIP performance measures for perioperative VTE prophylaxis along with several others relating to VTE treatment.
CMS raises the stakes with reimbursement restrictions
More significantly, the federal government’s Centers for Medicare and Medicaid Services (CMS) will soon refuse to reimburse for hospital treatment of a primary diagnosis of DVT or pulmonary embolism (PE) following recent (within 30 days) total hip or knee arthroplasty. Effective October 1, 2009, a primary VTE diagnosis following these joint replacement procedures will be added to CMS’ current list of “never events,” or hospital-acquired conditions for which CMS will not provide reimbursement because they are considered the result of preventable medical errors. (Notably, treatment of DVT or PE as a secondary diagnosis will still be reimbursed—for example, if a joint replacement patient develops nosocomial pneumonia, is transferred to the intensive care unit, and then develops VTE.) This addition of DVT and PE to the list is highly controversial since these events sometimes develop even if prophylactic therapy is appropriate and aggressive.
Strategies to promote best practices
In the update for the new 8th edition of its guidelines, the ACCP added recommendations on specific ways for hospitals to identify patients at high risk for VTE and ensure that they receive appropriate prophylaxis. These include the use of computer decision-support systems, preprinted orders, and periodic audit and feedback.1
Researchers at Brigham and Women’s Hospital evaluated the effectiveness of a computer alert system for notifying physicians of newly hospitalized patients at risk for DVT who were not receiving prevention therapy within the first 24 hours of hospital admission.4 These patients presumably “fell through the cracks” and warranted prophylaxis but were otherwise not recognized by the health care team. Risk was determined by a scoring system based on multiple variables, including malignancy, previous DVT or PE, hypercoagulability, major surgery, advanced age, obesity, ordered bed rest, and treatment with hormone replacement therapy or oral contraceptives. Study physicians had to acknowledge having received the alert but could choose whether or not to order VTE prophylaxis. Prophylaxis was used in considerably more patients from the intervention group than from a control group of high-risk patients whose physicians did not receive alerts (34% vs 14%, respectively); accordingly, the risk of a symptomatic DVT or PE event at 90 days was reduced by 41% in the intervention group.
Despite this evidence of improved practice under the alert system, the study begs the question of why the percentage of patients at risk for VTE who were given prophylaxis was still so low (34%), demonstrating how much progress in improving practice remains to be achieved.
PROPHYLAXIS STRATEGIES: MATCHING THERAPY TO RISK
NONPHARMACOLOGIC PROPHYLAXIS STRATEGIES
Does ambulation prevent DVT?
Although it is commonly accepted that walking prevents DVT, this has never been directly tested. Walking may simply be a marker of health, and healthy people are less prone to develop thromboses. We have almost no evidence to show that forcing an unhealthy person to walk helps prevent DVT. Early ambulation offers many benefits and should be encouraged, but it should not be considered DVT prophylaxis; it is simply good hospital care.
Mechanical devices: Adherence is key
Amaragiri and Lees conducted a systematic literature review of randomized controlled trials evaluating the effectiveness of graduated compression stockings (elastic stockings) for preventing DVT in various groups of hospitalized patients.6 The analysis demonstrated a statistically significant reduction in DVT incidence with graduated compression stockings compared with control both among the nine trials in which stockings were used alone (odds ratio = 0.34) and among the seven trials in which stockings were used in addition to another method of thromboprophylaxis (odds ratio = 0.24). Although benefit was demonstrated, many of the trials in this review involved patients undergoing gynecologic surgery and date from the 1970s and 1980s (when obesity was less prevalent), so the applicability of their results today may be limited.
The 8th edition of the ACCP guidelines recommends that mechanical methods of VTE prophylaxis be used primarily in patients who are at high risk of bleeding and that careful attention be directed to ensuring their proper use and optimal adherence.1 The latter point about adherence cannot be emphasized enough, as graduated compression stockings and other mechanical devices have been shown not to be effective unless they are worn at least 18 to 20 hours a day. This degree of adherence is difficult to achieve, as it can severely limit patient mobility and leave patients susceptible to development of pressure ulcers.
Mechanical compression should be initiated prior to induction of anesthesia and continue intraoperatively and then into the postanesthesia care unit. Orders for use of mechanical devices should include instructions in the patient’s medical chart specifying how—and for how many hours per day—they are to be worn. Not doing so leaves the physician vulnerable to litigation, especially as the ACCP guidelines include language on optimal adherence to these devices (“they should be removed for only a short time each day when the patient is actually walking or for bathing”1).
Continuous external compression therapy
Newer mechanical device options include a continuous external compression therapy system that allows patients to be mobile while wearing it and provides rhythmic compression that results in good peak venous flows. Ideally such a device could be put on the patient preoperatively and worn during surgery, throughout the hospital stay, and even at home during recovery. Anecdotally, however, I see patients turn these new devices off at the side of the bed just as often as they do with traditional devices.
Vena caval interruption
Vena caval interruption involves placement of a retrievable vena cava filter before surgery and removal some time later; it offers the potential for VTE prophylaxis in patients who could not tolerate even minor amounts of bleeding, such as certain trauma patients. The Eastern Association for the Surgery of Trauma has put forth a consensus recommendation to consider vena caval interruption in high-risk trauma patients who cannot receive pharmacologic prophylaxis.7 A randomized trial evaluating the usefulness of vena caval interruption for patients undergoing surgery is needed. For now, this intervention should be regarded as experimental and considered only on a highly individualized basis.
PHARMACOLOGIC PROPHYLAXIS
Timing of initiation
Pharmacologic VTE prophylaxis generally should begin 8 to 24 hours postoperatively. Of course, adequate hemostasis is required before initiation, and the net risk/benefit tradeoff with regard to timing of anticoagulant initiation has still not been well studied in many surgical patient populations.
Extended prophylaxis
In the update for the 8th edition of its guidelines, the ACCP added an explicit recommendation for extended outpatient prophylaxis with low-molecular-weight heparin (LMWH) for up to 28 days postoperatively in selected high-risk patients undergoing general or gynecologic surgery, including those with cancer or a history of VTE.1 This recommendation was based largely on studies of extended prophylaxis in patients with cancer undergoing colorectal surgery.8
Increased appreciation of the value of extended VTE prophylaxis after discharge is linked to a growing recognition that DVT and PE episodes in the community setting are often related to a recent hospital stay for either medical illness or surgery. A population-based study found that 59% of all community cases of a first lifetime VTE event in residents of Olmsted County, Minn., over a 15-year period could be linked to current or recent (< 30 days) hospitalization or nursing home residence.9 A similar population-based study in the Worcester, Mass., area found that three-fourths of all VTE events in a 3-year period occurred in the outpatient setting.10 Among patients with these outpatient VTE events, a large proportion had undergone surgery (23%) or hospitalization (37%) in the prior 3 months; among those, 67% experienced their VTE within 1 month of their time in the hospital.
These findings are no surprise, since surgery induces a hypercoagulable state that, when combined with individual risk factors such as obesity, old age, or poor heart function, cannot be assumed to return to baseline on postoperative day 4 or 5 just because the patient is being discharged.
Orthopedic surgery
For patients undergoing major orthopedic procedures, the ACCP guidelines recommend against routine screening for VTE with Doppler ultrasonography before discharge if the patient is asymptomatic.1 Such screening is not considered cost-effective because asymptomatic clots often are found, for which treatment is uncertain, and proximal clots may be missed, giving a false sense of security.
New to the ACCP guidelines in the 8th edition is the recommendation that patients undergoing knee arthroscopy who have risk factors for VTE (or whose procedure is complicated) should receive 1 week of prophylaxis with LMWH.1 Also new are recommendations for patients with risk factors undergoing single- or multilevel laminectomy (Table 4).
Recommendations unchanged in neurosurgery, spinal injury, trauma, burns
Recommendations for neurosurgery remain unchanged from the prior (2004) edition of the ACCP guidelines and are still based on the 2000 meta-analysis by Iorio and Agnelli of LMWH prophylaxis in neurosurgery cases.11 In the United States, the standard is overwhelmingly to use mechanical devices for thromboprophylaxis in neurosurgery, even for patients with cancer.
For prophylaxis in surgical patients with spinal cord injury, multisystem trauma, or burns, LMWH is predominantly used, and the ACCP recommendations are unchanged from 2004.
Drug-specific considerations
LMWH vs vitamin K antagonist. Although vitamin K antagonists (warfarin) still appear in the latest ACCP recommendations,1 LMWH is preferable. A 2004 meta-analysis of studies comparing vitamin K antagonists with LMWH for prophylaxis in patients undergoing orthopedic surgery found that vitamin K antagonists were associated with more episodes of total DVT (relative risk [RR] = 1.51; 95% CI, 1.27–1.79) and proximal DVT (RR = 1.51; 95% CI, 1.04–2.17) compared with LMWH.12 No difference was found in rates of wound hematoma or major bleeding. This finding of inferiority for vitamin K antagonists came despite the likelihood that warfarin was more often administered correctly (ie, with dose adjustment to achieve an international normalized ratio [INR] of 2.0 to 3.0 within 72 hours after surgery) in the studies in this analysis than it is in real-world practice.
Fondaparinux. The indirect factor Xa–specific inhibitor fondaparinux has had a surprisingly limited clinical adoption despite having been widely studied and found to be safe and effective. This is likely attributable in part to its 17-hour half-life, which raises concerns that it may take 3 days for its effects to stop if a patient begins to bleed. Large phase 3 studies have found fondaparinux to be equivalent to LMWH in VTE prevention after hip replacement, marginally superior to LMWH after knee replacement, and superior to LMWH following hip fracture repair.13 Fondaparinux was associated with an increase in bleeding events and instances of transfusion requirement, but only in one of the studies, which was in the setting of knee replacement surgery.14
Aspirin not recommended by ACCP. Although aspirin reduces the risk of VTE, practice guidelines from both the ACCP1 and the International Union of Angiology15 contain no recommendation for its use as prophylaxis because it is considered less effective and more risky than other therapies. In contrast, clinical practice guidelines from the American Academy of Orthopaedic Surgeons suggest that aspirin is reasonable for VTE prophylaxis.16 The varying recommendations reflect differences in perspective among these different specialties.
Aspirin has the advantages of ease of use and low cost, but it is clearly not the best evidence-based approach for VTE prophylaxis. The only recent randomized trial evidence in support of aspirin comes from the Pulmonary Embolism Prevention trial, a study with a flawed design involving more than 13,000 patients undergoing surgery for hip fracture or elective arthroplasty in five countries.17 Patients were randomized to receive aspirin 160 mg daily or placebo for 35 days along with any other prophylaxis deemed necessary (an important potential confounder). Aspirin was associated with an absolute reduction in symptomatic events of less than 1% relative to placebo, and no benefit was observed within the first week. The best results with aspirin were among patients with hip fracture. No benefit was shown among patients undergoing hip arthroplasty or knee arthroplasty; in those groups, both the aspirin and placebo recipients were also treated with LMWH. An absolute increase in rates of wound bleeding (0.6% increase) and gastrointestinal bleeding (1.0% increase) was observed in the aspirin group. The absolute increase in complications was greater than the absolute reduction in episodes of symptomatic DVT: for every episode of symptomatic DVT averted, one wound bleed and 10 gastrointestinal bleeds occurred.
SPECIAL PATIENT POPULATIONS
Renal impairment
The 8th edition of the ACCP guidelines recommends that renal function be kept in mind when considering LMWH, fondaparinux, and other antithrombotic drugs that are cleared by the kidneys. Fondaparinux and LMWH can bioaccumulate in patients with renal insufficiency, who have a higher risk of bleeding to begin with, thereby compounding the risk. Options for patients with renal compromise include avoiding drugs that bioaccumulate, using a lower dosage, and monitoring the drug level or anticoagulant effect.1
Fondaparinux is explicitly contraindicated in patients with low body weight (< 50 kg) or renal impairment (creatinine clearance < 30 mL/min). Renal function should be assessed periodically in any patients receiving the drug.18
I also would not use fondaparinux or LMWH in patients with rapidly changing renal function. For patients with chronic, stable renal impairment, one can reduce the dose of LMWH empirically; one LMWH, enoxaparin, has specific dosing guidelines in its package insert (one-third reduction in dose), but this option does not hold for patients with rapidly changing renal function.19
Obesity
The 8th edition of the ACCP guidelines recommends weight-based dosing of thromboprophylactic agents in obese patients. The guidelines particularly recommend that patients undergoing inpatient bariatric surgery be given higher doses of LMWH or unfractionated heparin.1,20
Frederiksen et al measured the anticoagulant effect of a single fixed dose of a LMWH (using anti-factor Xa heparin activity levels) and found that it was dependent on body weight.21 This suggests that fixed doses that are effective in normal-weight patients may have no detectable anti-coagulant effect in patients with very high body weight.
Weight-based dosing: mounting nonprospective evidence. Weight-based dosage adjustment for the morbidly obese has not been directly studied in a prospective, randomized fashion. A nonrandomized study by Scholten et al compared two regimens of enoxaparin (30 mg twice daily vs 40 mg twice daily) among 481 obese patients undergoing bariatric surgery; each regimen was used along with mechanical thromboprophylaxis.22 They found that the higher-dose regimen was associated with significantly fewer postoperative DVT complications (0.6% vs 5.4%; P < .01) without an increase in bleeding complications.
Separately, Shepherd et al used weight-adjusted doses of unfractionated heparin (started on the evening of surgery) to achieve subtherapeutic peak anti–factor Xa heparin activity levels of 0.11 to 0.25 IU/mL in a series of 700 patients undergoing laparoscopic gastric bypass surgery.23 The resulting doses were greater than those in traditional fixed-rate protocols, but rates of bleeding and VTE events were low and comparable to those reported in patients receiving standard doses.
Don’t rule out multimodal approaches. Multimodal prophylaxis can also be used in obese patients and need not be abandoned as a result of size considerations. For instance, two intermittent compression therapy devices can be pieced together with a Velcro binder if a single device is too small to be worn.
EMERGING ANTICOAGULANT OPTIONS
For many years, unfractionated heparin was the only available parenteral anticoagulant. While heparin has broad anticoagulant properties, it also has well-established limitations, including the need for parenteral delivery, recent problems related to contamination (it is derived from pig intestines), and of course heparin-induced thrombocytopenia (HIT). HIT is an immune-mediated form of platelet activation that can lead to widespread thrombosis throughout the body. It is more commonly associated with venous thrombosis, but arterial events with limb-threatening ischemia may also occur. LMWH is associated with a reduced risk of HIT, but LMWH does not avoid the risk entirely.
Beyond the issue of avoiding HIT, newer anticoagulant therapies are being developed with the aim of oral administration and more targeted inhibition of coagulation factors IIa (thrombin) and Xa.24
Oral direct thrombin inhibitors
One of the two most promising classes of emerging anticoagulants is the direct thrombin inhibitors, most of which are being developed for oral administration. There were high hopes for the initial compound in this class, ximelagatran, but it was abandoned about 5 years ago because of hepatotoxicity.
Dabigatran is the direct thrombin inhibitor furthest along in development today. Currently approved in Europe for prevention of VTE in patients undergoing total hip or knee replacement surgery, dabigatran is likely to be available soon in the United States. It is administered orally, has a rapid onset of action (< 1 hour), and has a predictable anticoagulant response that requires no monitoring.24 Because dabigatran is excreted essentially unchanged by the kidneys and may bioaccumulate, it should not be used in patients with renal impairment or rapidly changing renal function.
In phase 3 clinical trials for VTE prevention in knee replacement surgery, dabigatran was at least as effective as enoxaparin 40 mg once daily and had a comparable safety profile,25 but it was slightly less effective than enoxaparin 30 mg twice daily.26 In a phase 3 trial in patients undergoing hip replacement surgery, dabigatran was equivalent in efficacy and safety to enoxaparin 40 mg once daily.27
Oral direct factor Xa inhibitors
A key rationale for direct inhibition of factor Xa is that it results in inhibition of thrombin production on the activated platelet. Whereas fondaparinux is an indirect inhibitor of factor Xa, direct factor Xa inhibitors offer an advantage in that they inhibit factor Xa within the prothrombinase complex, which occurs on the surface of a platelet and is the main site for thrombin development (very little thrombin is actually produced on endothelial cells). Recall the adage that “thrombin begets more thrombin”: it activates not only platelets but the intrinsic and extrinsic pathways.28
Factor Xa may be a better target than thrombin for a number of other reasons:
- Factor Xa is believed to have few functions (compared with thrombin) outside of coagulation
- In vitro studies show that factor Xa has a wider therapeutic window than thrombin, which translates to greater separation between drug levels that will confer efficacy and bleeding
- Thrombin inhibitors are associated with rebound thrombin generation (there is no evidence of this with factor Xa inhibitors)
- The efficacy of heparin-based anticoagulants improves as selectivity for factor Xa increases (unfractionated heparin is less effective than LMWH, which is less effective than fondaparinux).
Two direct factor Xa inhibitors—both administered orally—are far along in development, as detailed below.
Apixaban has shown promise, but the phase 3 ADVANCE-1 study of apixaban for VTE prevention in patients undergoing knee surgery did not meet statistical criteria for noninferiority compared with enoxaparin 30 mg twice daily.29 This prompted a delay in regulatory filings for apixaban in the United States, and the drug’s prospects for approval for VTE prevention may be unclear until release of results from two other comparative phase 3 trials with enoxaparin in 2009 and 2010.
Rivaroxaban is more likely to become clinically available soon, in light of recent results from the phase 3 RECORD4 trial demonstrating that it was significantly superior to enoxaparin 30 mg twice daily in preventing VTE following knee replacement surgery with comparable rates of major bleeding.30
DISCUSSION
Question from the audience: Some surgeons in my hospital prescribe warfarin immediately after surgery without a bridge of LMWH. Is that appropriate?
Dr. Michota: Warfarin is an option for prophylaxis in orthopedic surgery, beginning on the day of surgery. It could even be started the day before surgery, but the dose should be monitored to achieve an INR between 2.0 and 3.0 within 72 hours of the procedure. If the INR is not in this optimum range, prophylactic doses of LMWH can be given until it is therapeutic.
Follow-up question: In practice, do you actually encourage INR monitoring? Usually we just put patients on a certain dose without monitoring. When we do check the INR, it’s usually 1.4 or 1.5.
Dr. Michota: Warfarin was shown to be effective in reducing VTE risk in orthopedic surgery with dose adjustment based on INR monitoring. On that basis, warfarin remains in the guideline recommendations. Unmonitored, warfarin has not been shown to reduce risk, so to give it that way would not be evidence-based.
Question from the audience: I work with several plastic surgeons who use compression stockings intraoperatively because they’ve heard of several patients who developed a PE during surgery. Is there any benefit to using compression stockings for 2 to 3 hours and then sending the patient home?
Dr. Michota: I don’t know. Theoretically, a device that is on and working before induction may reduce stasis.
The plastic surgery societies do have guidelines. Risk depends on the type of plastic surgery procedure; for example, risk probably increases due to inflammation in procedures that involve scraping the fat pads.
This is an area where we don’t have much data. These patients may be at risk, but we don’t know the best way to mitigate it. It is important that risks be discussed with patients in the informed-consent process and be documented. If the surgeon thinks it is reasonable to give pharmacologic prophylaxis after surgery, I wouldn’t hesitate to do that, but any form of bleeding in the setting of plastic surgery is catastrophic because it defeats the reason for which the surgery was done in the first place.
Question from the audience: How do the guidelines address being aggressive with pharmacologic thromboprophylaxis when a patient is already taking dual antiplatelet therapy?
Dr. Michota: For patients with an indication for VTE prophylaxis in a setting for which there is a specific strategy, the ACCP guidelines recommend that they be put on that regimen whether they are on antiplatelet agents or not. For example, consider a high-risk patient having colorectal surgery who should get unfractionated heparin or LMWH postoperatively and who is currently taking clopidogrel and aspirin. There is no evidence that the dual aspirin–clopidogrel therapy alone is effective in decreasing the risk of DVT. However, we do know that if we add on additional agents, the risk of bleeding is increased. The guidelines consider risk and benefit, and they recommend adding the agents that we know work to prevent DVT.
Question from the audience: You briefly mentioned prophylaxis for knee arthroscopy, which is the most frequently performed orthopedic procedure. Do these recommendations apply to all patients undergoing knee arthroscopy?
Dr. Michota: No. Prophylaxis is indicated only for patients with what the ACCP considers to be additional risk factors for thrombosis. They didn’t specify which risk factors, but good indications for prophylaxis would include morbid obesity, limited mobility after the procedure, a personal history of DVT, features of stasis noted on physical examination, stasis dermatitis (or other features that could indicate prior thrombosis), advanced age, and malignancy. If a patient undergoing knee arthroscopy has other nonmodifiable risk factors, you should also think about prophylaxis. But the vast majority of patients do not need it.
Question from the audience: I’m an academic hospitalist who works closely with orthopedic surgeons. Certain surgeons will only use aspirin for prophylaxis, and it is nonnegotiable. Where does that leave me from a medicolegal standpoint? Our model is to follow ACCP recommendations, but these orthopedic surgeons still use only aspirin.
Dr. Michota: You must do everything you can to come to a consensus with your surgeon colleagues. If you are uncomfortable, as a group you must say to the surgeons, “We are uncomfortable. This is how we view the data. How do you view the data?” If they answer, “We’re doing it because it’s easy, and the American Academy of Orthopaedic Surgeons says we can do it,” I don’t have a good response. But it is more likely that their use of aspirin is based on their own observations; they may not see many clots. Of course, the problem with observational data is that the numbers are not large and they are not generated in a randomized and prospective fashion. Perhaps you can come to some middle ground, but you could always make the difficult choice and say, “I’m just not going to follow your patients.”
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133:381S–453S.
- Medicare Quality Improvement Community (MedQIC) Web site. http://www.medqic.org. Accessed June 1, 2009.
- Surgical Care Improvement Project (SCIP). Colorado Foundation for Medical Care Web site. http://www.cfmc.org/hospital/hospital_scip.htm. Accessed June 1, 2009.
- Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med 2005; 352:969–977.
- Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107:I-9–I-16.
- Amaragiri SV, Lees TA. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev 2000; (3):CD001484.
- Rogers FB, Cipolle MD, Velmahos G, et al. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST Practice Management Work Group. J Trauma 2002; 53:142–164.
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002; 346:975–980.
- Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med 2002; 162:1245–1248.
- Spencer FA, Lessard D, Emery C, et al. Venous thromboembolism in the outpatient setting. Arch Intern Med 2007; 167:1471–1475.
- Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med 2000; 160:2327–2332.
- Mismetti P, Laporte S, Zufferey P, et al. Prevention of venous thromboembolism in orthopedic surgery with vitamin K antagonists: a meta-analysis. J Thromb Haemost 2004; 2:1058–1070.
- Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studies. Arch Intern Med 2002; 162:1833–1840.
- Bauer KA, Eriksson BI, Lassen MR, Turpie AG; Steering Committee of the Pentasaccharide in Major Knee Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med 2001; 345:1305–1310.
- Cardiovascular Disease Educational and Research Trust; Cyprus Cardiovascular Disease Educational and Research Trust; European Venous Forum; International Surgical Thrombosis Forum; International Union of Angiology; Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006; 25:101–161.
- American Academy of Orthopaedic Surgeons clinical guideline on prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. http://www.aaos.org/research/guidelines/PE_summary.pdf. Accessed June 5, 2009.
- Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355:1295-1302.
- Arixtra injection [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2008.
- Sanderink GJ, Guimart C, Jariwala N, et al. Enoxaparin pharmacokinetics and pharmacodynamics in renal impairment. J Am Coll Cardiol 2001; 37(suppl A):229A. Abstract.
- Hirsh J, Bauer KA, Donati MB, et al; American College of Chest Physicians. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) [published correction appears in Chest 2008; 134:473]. Chest 2008; 133:141S–159S.
- Frederiksen SG, Hedenbro JL, Norgren L. Enoxaparin effect depends on body-weight and current doses may be inadequate in obese patients. Br J Surg 2003; 90:547–548.
- Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg 2002; 12:19–24.
- Shepherd MF, Rosborough TK, Schwartz ML. Heparin thromboprophylaxis in gastric bypass surgery. Obes Surg 2003; 13:249–253.
- Weitz JI, Bates SM. New anticoagulants. J Thromb Haemost 2005; 3:1843–1853.
- Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009; 24:1–9.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial [published correction appears in Lancet 2007; 370:2004]. Lancet 2007: 370:949–956.
- Hoffman M, Monroe DM 3rd, Roberts HR. Activated factor VII activates factors IX and X on the surface of activated platelets: thoughts on the mechanism of action of high-dose activated factor VII. Blood Coagul Fibrinolysis 1998; 9(suppl 1):S61–S65.
- Bristol-Myers Squibb and Pfizer provide update on apixaban clinical development program [press release]. New York, NY: August 27, 2008.
- Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009; 373:1673–1680.
- Geerts WH, Bergqvist D, Pineo GF, et al; American College of Chest Physicians. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008; 133:381S–453S.
- Medicare Quality Improvement Community (MedQIC) Web site. http://www.medqic.org. Accessed June 1, 2009.
- Surgical Care Improvement Project (SCIP). Colorado Foundation for Medical Care Web site. http://www.cfmc.org/hospital/hospital_scip.htm. Accessed June 1, 2009.
- Kucher N, Koo S, Quiroz R, et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med 2005; 352:969–977.
- Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107:I-9–I-16.
- Amaragiri SV, Lees TA. Elastic compression stockings for prevention of deep vein thrombosis. Cochrane Database Syst Rev 2000; (3):CD001484.
- Rogers FB, Cipolle MD, Velmahos G, et al. Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST Practice Management Work Group. J Trauma 2002; 53:142–164.
- Bergqvist D, Agnelli G, Cohen AT, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 2002; 346:975–980.
- Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med 2002; 162:1245–1248.
- Spencer FA, Lessard D, Emery C, et al. Venous thromboembolism in the outpatient setting. Arch Intern Med 2007; 167:1471–1475.
- Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: a meta-analysis. Arch Intern Med 2000; 160:2327–2332.
- Mismetti P, Laporte S, Zufferey P, et al. Prevention of venous thromboembolism in orthopedic surgery with vitamin K antagonists: a meta-analysis. J Thromb Haemost 2004; 2:1058–1070.
- Turpie AG, Bauer KA, Eriksson BI, Lassen MR. Fondaparinux vs enoxaparin for the prevention of venous thromboembolism in major orthopedic surgery: a meta-analysis of 4 randomized double-blind studies. Arch Intern Med 2002; 162:1833–1840.
- Bauer KA, Eriksson BI, Lassen MR, Turpie AG; Steering Committee of the Pentasaccharide in Major Knee Surgery Study. Fondaparinux compared with enoxaparin for the prevention of venous thromboembolism after elective major knee surgery. N Engl J Med 2001; 345:1305–1310.
- Cardiovascular Disease Educational and Research Trust; Cyprus Cardiovascular Disease Educational and Research Trust; European Venous Forum; International Surgical Thrombosis Forum; International Union of Angiology; Union Internationale de Phlébologie. Prevention and treatment of venous thromboembolism. International Consensus Statement (guidelines according to scientific evidence). Int Angiol 2006; 25:101–161.
- American Academy of Orthopaedic Surgeons clinical guideline on prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. http://www.aaos.org/research/guidelines/PE_summary.pdf. Accessed June 5, 2009.
- Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary Embolism Prevention (PEP) trial. Lancet 2000; 355:1295-1302.
- Arixtra injection [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2008.
- Sanderink GJ, Guimart C, Jariwala N, et al. Enoxaparin pharmacokinetics and pharmacodynamics in renal impairment. J Am Coll Cardiol 2001; 37(suppl A):229A. Abstract.
- Hirsh J, Bauer KA, Donati MB, et al; American College of Chest Physicians. Parenteral anticoagulants: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) [published correction appears in Chest 2008; 134:473]. Chest 2008; 133:141S–159S.
- Frederiksen SG, Hedenbro JL, Norgren L. Enoxaparin effect depends on body-weight and current doses may be inadequate in obese patients. Br J Surg 2003; 90:547–548.
- Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg 2002; 12:19–24.
- Shepherd MF, Rosborough TK, Schwartz ML. Heparin thromboprophylaxis in gastric bypass surgery. Obes Surg 2003; 13:249–253.
- Weitz JI, Bates SM. New anticoagulants. J Thromb Haemost 2005; 3:1843–1853.
- Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost 2007; 5:2178–2185.
- RE-MOBILIZE Writing Committee, Ginsberg JS, Davidson BL, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty 2009; 24:1–9.
- Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial [published correction appears in Lancet 2007; 370:2004]. Lancet 2007: 370:949–956.
- Hoffman M, Monroe DM 3rd, Roberts HR. Activated factor VII activates factors IX and X on the surface of activated platelets: thoughts on the mechanism of action of high-dose activated factor VII. Blood Coagul Fibrinolysis 1998; 9(suppl 1):S61–S65.
- Bristol-Myers Squibb and Pfizer provide update on apixaban clinical development program [press release]. New York, NY: August 27, 2008.
- Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009; 373:1673–1680.
KEY POINTS
- Effective October 1, 2009, the Centers for Medicare and Medicaid Services is refusing to reimburse for hospital treatment of a primary diagnosis of deep vein thrombosis or pulmonary embolism following recent (within 30 days) hip or knee replacement surgery.
- Mechanical methods of thromboprophylaxis are not effective unless used for at least 18 to 20 hours a day.
- The latest ACCP guidelines recommend extended pharmacologic VTE prophylaxis for up to 28 days in select high-risk patients undergoing general or gynecologic surgery. Extended prophylaxis of varying duration is recommended for patients undergoing major orthopedic procedures.
- Aspirin alone is not recommended for perioperative VTE prophylaxis in any patient group by the ACCP or the International Union of Angiology.
- Patients with renal impairment have fewer anticoagulant options and may require dose adjustment. Weight-based dosing appears to be safe and effective for obese surgical patients.
- New selective and orally administered direct thrombin inhibitors and oral direct factor Xa inhibitors may soon be available for perioperative VTE prophylaxis.