User login
For people living with type 2 diabetes mellitus (T2D), exogenous insulin, whether given early or later in T2D diagnosis, can provide many pharmacologically desirable effects. But it has always been clear, and is now more widely recognized, that insulin treatments are not completely risk-free for the patient. There are now newer, non-insulin therapy options that could be used, along with certain patient lifestyle changes in diet and activity levels, that have been shown to achieve desired glucose control—without the associated risks that insulin can bring.
But is it possible to markedly reduce the need for insulin in some 90% of T2D patients and to reduce the doses in the others? Yes—if patients have sufficient beta-cell function and are willing to change their lifestyle. This mode of treatment has been slowly gaining momentum as of late in the medical community because of the benefits it ultimately provides for the patient. In my practice, I personally have done this by using an evidence-based approach that includes thinking inside a larger box. It is a 2-way street, and each should drive the other: the right drugs (in the right doses), and in the right patients.
Why avoid early insulin therapy?
Is the requirement of early insulin therapy in many or most patients a myth?
Yes. It resulted from “old logic,” which was to use insulin early to reduce glucotoxicity and lipotoxicity. The American Diabetes Association guidelines recommend that glycated hemoglobin (HbA1c) should not exceed 8.0% and consider a fasting blood glucose level >250 mg/dL as high, with a need to start insulin treatment right away; other guidelines recommend initiating insulin immediately in patients with HbA1c >9% and postprandial glucose 300 mg/dL. But this was at a time when oral agents were not as effective and took time to titrate or engender good control. We now have agents that are more effective and start working right away.
However, the main problem in early insulin treatment is the significant risk of over-insulinization—a vicious cycle of insulin-caused increased appetite, hypoglycemia-resultant increased weight gain, insulin resistance (poorer control), increased circulating insulin, etc. Moreover, weight gain and individual hypoglycemic events can cause an increase in the risk of cardiovascular (CV) events.
I believe clinicians must start as early as possible in the natural history of T2D to prevent progressive beta-cell failure. Do not believe in “first-, second-, or third-line”; in other words, do not prioritize, so there is no competition between classes. The goal I have for my patients is to provide therapies that aim for the lowest HbA1c possible without hypoglycemia, provide the greatest CV benefit, and assist in weight reduction.
My protocol, “the egregious eleven,” involves using the least number of agents in combinations that treat the greatest number of mechanisms of hyperglycemia—without the use of sulfonylureas (which cause beta-cell apoptosis, hypoglycemia, and weight gain). Fortunately, newer agents, such as glucagon-like peptide 1 receptor agonist (GLP-1 RA) and sodium-glucose cotransporter 1 (SGLT-2) inhibitors, work right away, cause weight reduction, and have side benefits of CV risk reduction—as well as preserve beta-cell function. Metformin remains a valuable agent and has its own potential side benefits, and bromocriptine-QR and pioglitazone have CV side benefits. So, there is really no need for early insulin in true T2D patients (ie, those that are non-ketosis prone and have sufficient beta-cell reserve).
Why reduce insulin in patients who are already on insulin?
Prior protocols resulted in 40%-50% of T2D patients being placed on insulin unnecessarily. As discussed, the side effects of insulin are many; they include weight gain, insulin resistance, hypoglycemia, and CV complications—all of which have been associated with a decline in quality of life.
What is your approach to reduce or eliminate insulin in those already on it (unnecessarily)?
First, I pick the right patient. Physicians should use sound clinical judgment to identify patients with likely residual beta-cell function. It is not just the “insulin-resistant patient," as 30%-50% of type 1 diabetes mellitus patients also have insulin resistance.
It needs to be a definite T2D patient: not ketosis prone, a family history T2D, no islet cell antibodies (if one has any concerns, check for them). They were often started on insulin in the emergency department with no ketosis and never received non-insulin therapy.
Patients need to be willing to commit to my strict, no-concentrated-sweets diet, to perform careful glucose monitoring, and to check their ketones. Patients should be willing to contact me if their sugar level is >250 mg/dL for 2 measurements in a row, while testing 4 times a day or using a continuous glucose-monitoring (CGM) device.
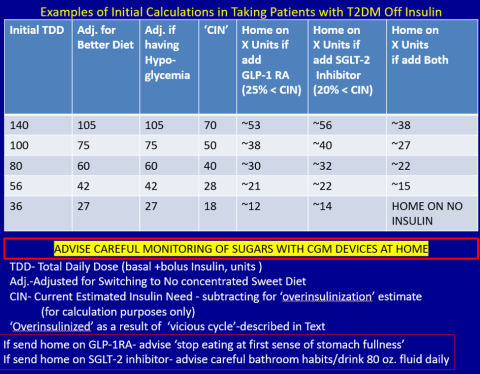
First, estimate a patient’s “current insulin need” (CIN), or the dose they might be on if they had not been subject to over-insulinization (ie, if they had not been subject to the “vicious cycle” discussed above). I do this by taking their total basal and bolus insulin dose, then reducing it by ~25% as the patient changes to a no-concentrated-sweets diet with an additional up-to-25% dose reduction if the patient has been experiencing symptomatic or asymptomatic hypoglycemia.
Next, I reduce this CIN number by ~25% upon starting a rapid-acting subcutaneous GLP-1 RA (liraglutide or oral semaglutide) and reduce the CIN another 20% as they start the SGLT-2 inhibitor. If patients come into my office on <40 U/d, I stop insulin as I start a GLP-1 RA and an SGLT-2 inhibitor and have them monitor home glucose levels to assure reasonable results as they go off the insulin and on their new therapy.
If patients come into my office on >40 U/d, they go home on a GLP-1 RA and an SGLT-2 inhibitor and ~30% of their presenting dose, apportioned between basal/bolus dosing based on when they are currently getting hypoglycemic.
The rapid initial reduction in their insulin doses, with initial adjustments in estimated insulin doses as needed based on home glucose monitoring, and rapid stabilization of glycemic levels by the effectiveness of these 2 agents give patients great motivation to keep up with the diet/program.
Then, as patients lose weight, they are told to report any glucose measurements <80 mg/dL, so that further reduction in insulin doses can be made. When patients achieve a new steady state of glycemia, weight, and GLP-1 RA and SGLT-2 inhibitor doses, you can add bromocriptine-QR, pioglitazone, and/or metformin as needed to allow for a further reduction of insulin. And, as you see the delayed effects of subsequently adding these new agents (eg, glucose <80 mg/dL), you can ultimately stop insulin when they get to <10-12 U/d. The process works very well, even in those starting on up to 300 units of insulin daily. Patients love the outcome and will greatly appreciate your care.
Feel free to contact Dr. Schwartz at [email protected] with any questions about his protocol or diet.
For people living with type 2 diabetes mellitus (T2D), exogenous insulin, whether given early or later in T2D diagnosis, can provide many pharmacologically desirable effects. But it has always been clear, and is now more widely recognized, that insulin treatments are not completely risk-free for the patient. There are now newer, non-insulin therapy options that could be used, along with certain patient lifestyle changes in diet and activity levels, that have been shown to achieve desired glucose control—without the associated risks that insulin can bring.
But is it possible to markedly reduce the need for insulin in some 90% of T2D patients and to reduce the doses in the others? Yes—if patients have sufficient beta-cell function and are willing to change their lifestyle. This mode of treatment has been slowly gaining momentum as of late in the medical community because of the benefits it ultimately provides for the patient. In my practice, I personally have done this by using an evidence-based approach that includes thinking inside a larger box. It is a 2-way street, and each should drive the other: the right drugs (in the right doses), and in the right patients.
Why avoid early insulin therapy?
Is the requirement of early insulin therapy in many or most patients a myth?
Yes. It resulted from “old logic,” which was to use insulin early to reduce glucotoxicity and lipotoxicity. The American Diabetes Association guidelines recommend that glycated hemoglobin (HbA1c) should not exceed 8.0% and consider a fasting blood glucose level >250 mg/dL as high, with a need to start insulin treatment right away; other guidelines recommend initiating insulin immediately in patients with HbA1c >9% and postprandial glucose 300 mg/dL. But this was at a time when oral agents were not as effective and took time to titrate or engender good control. We now have agents that are more effective and start working right away.
However, the main problem in early insulin treatment is the significant risk of over-insulinization—a vicious cycle of insulin-caused increased appetite, hypoglycemia-resultant increased weight gain, insulin resistance (poorer control), increased circulating insulin, etc. Moreover, weight gain and individual hypoglycemic events can cause an increase in the risk of cardiovascular (CV) events.
I believe clinicians must start as early as possible in the natural history of T2D to prevent progressive beta-cell failure. Do not believe in “first-, second-, or third-line”; in other words, do not prioritize, so there is no competition between classes. The goal I have for my patients is to provide therapies that aim for the lowest HbA1c possible without hypoglycemia, provide the greatest CV benefit, and assist in weight reduction.
My protocol, “the egregious eleven,” involves using the least number of agents in combinations that treat the greatest number of mechanisms of hyperglycemia—without the use of sulfonylureas (which cause beta-cell apoptosis, hypoglycemia, and weight gain). Fortunately, newer agents, such as glucagon-like peptide 1 receptor agonist (GLP-1 RA) and sodium-glucose cotransporter 1 (SGLT-2) inhibitors, work right away, cause weight reduction, and have side benefits of CV risk reduction—as well as preserve beta-cell function. Metformin remains a valuable agent and has its own potential side benefits, and bromocriptine-QR and pioglitazone have CV side benefits. So, there is really no need for early insulin in true T2D patients (ie, those that are non-ketosis prone and have sufficient beta-cell reserve).
Why reduce insulin in patients who are already on insulin?
Prior protocols resulted in 40%-50% of T2D patients being placed on insulin unnecessarily. As discussed, the side effects of insulin are many; they include weight gain, insulin resistance, hypoglycemia, and CV complications—all of which have been associated with a decline in quality of life.
What is your approach to reduce or eliminate insulin in those already on it (unnecessarily)?
First, I pick the right patient. Physicians should use sound clinical judgment to identify patients with likely residual beta-cell function. It is not just the “insulin-resistant patient," as 30%-50% of type 1 diabetes mellitus patients also have insulin resistance.
It needs to be a definite T2D patient: not ketosis prone, a family history T2D, no islet cell antibodies (if one has any concerns, check for them). They were often started on insulin in the emergency department with no ketosis and never received non-insulin therapy.
Patients need to be willing to commit to my strict, no-concentrated-sweets diet, to perform careful glucose monitoring, and to check their ketones. Patients should be willing to contact me if their sugar level is >250 mg/dL for 2 measurements in a row, while testing 4 times a day or using a continuous glucose-monitoring (CGM) device.
First, estimate a patient’s “current insulin need” (CIN), or the dose they might be on if they had not been subject to over-insulinization (ie, if they had not been subject to the “vicious cycle” discussed above). I do this by taking their total basal and bolus insulin dose, then reducing it by ~25% as the patient changes to a no-concentrated-sweets diet with an additional up-to-25% dose reduction if the patient has been experiencing symptomatic or asymptomatic hypoglycemia.
Next, I reduce this CIN number by ~25% upon starting a rapid-acting subcutaneous GLP-1 RA (liraglutide or oral semaglutide) and reduce the CIN another 20% as they start the SGLT-2 inhibitor. If patients come into my office on <40 U/d, I stop insulin as I start a GLP-1 RA and an SGLT-2 inhibitor and have them monitor home glucose levels to assure reasonable results as they go off the insulin and on their new therapy.
If patients come into my office on >40 U/d, they go home on a GLP-1 RA and an SGLT-2 inhibitor and ~30% of their presenting dose, apportioned between basal/bolus dosing based on when they are currently getting hypoglycemic.
The rapid initial reduction in their insulin doses, with initial adjustments in estimated insulin doses as needed based on home glucose monitoring, and rapid stabilization of glycemic levels by the effectiveness of these 2 agents give patients great motivation to keep up with the diet/program.
Then, as patients lose weight, they are told to report any glucose measurements <80 mg/dL, so that further reduction in insulin doses can be made. When patients achieve a new steady state of glycemia, weight, and GLP-1 RA and SGLT-2 inhibitor doses, you can add bromocriptine-QR, pioglitazone, and/or metformin as needed to allow for a further reduction of insulin. And, as you see the delayed effects of subsequently adding these new agents (eg, glucose <80 mg/dL), you can ultimately stop insulin when they get to <10-12 U/d. The process works very well, even in those starting on up to 300 units of insulin daily. Patients love the outcome and will greatly appreciate your care.
Feel free to contact Dr. Schwartz at [email protected] with any questions about his protocol or diet.
For people living with type 2 diabetes mellitus (T2D), exogenous insulin, whether given early or later in T2D diagnosis, can provide many pharmacologically desirable effects. But it has always been clear, and is now more widely recognized, that insulin treatments are not completely risk-free for the patient. There are now newer, non-insulin therapy options that could be used, along with certain patient lifestyle changes in diet and activity levels, that have been shown to achieve desired glucose control—without the associated risks that insulin can bring.
But is it possible to markedly reduce the need for insulin in some 90% of T2D patients and to reduce the doses in the others? Yes—if patients have sufficient beta-cell function and are willing to change their lifestyle. This mode of treatment has been slowly gaining momentum as of late in the medical community because of the benefits it ultimately provides for the patient. In my practice, I personally have done this by using an evidence-based approach that includes thinking inside a larger box. It is a 2-way street, and each should drive the other: the right drugs (in the right doses), and in the right patients.
Why avoid early insulin therapy?
Is the requirement of early insulin therapy in many or most patients a myth?
Yes. It resulted from “old logic,” which was to use insulin early to reduce glucotoxicity and lipotoxicity. The American Diabetes Association guidelines recommend that glycated hemoglobin (HbA1c) should not exceed 8.0% and consider a fasting blood glucose level >250 mg/dL as high, with a need to start insulin treatment right away; other guidelines recommend initiating insulin immediately in patients with HbA1c >9% and postprandial glucose 300 mg/dL. But this was at a time when oral agents were not as effective and took time to titrate or engender good control. We now have agents that are more effective and start working right away.
However, the main problem in early insulin treatment is the significant risk of over-insulinization—a vicious cycle of insulin-caused increased appetite, hypoglycemia-resultant increased weight gain, insulin resistance (poorer control), increased circulating insulin, etc. Moreover, weight gain and individual hypoglycemic events can cause an increase in the risk of cardiovascular (CV) events.
I believe clinicians must start as early as possible in the natural history of T2D to prevent progressive beta-cell failure. Do not believe in “first-, second-, or third-line”; in other words, do not prioritize, so there is no competition between classes. The goal I have for my patients is to provide therapies that aim for the lowest HbA1c possible without hypoglycemia, provide the greatest CV benefit, and assist in weight reduction.
My protocol, “the egregious eleven,” involves using the least number of agents in combinations that treat the greatest number of mechanisms of hyperglycemia—without the use of sulfonylureas (which cause beta-cell apoptosis, hypoglycemia, and weight gain). Fortunately, newer agents, such as glucagon-like peptide 1 receptor agonist (GLP-1 RA) and sodium-glucose cotransporter 1 (SGLT-2) inhibitors, work right away, cause weight reduction, and have side benefits of CV risk reduction—as well as preserve beta-cell function. Metformin remains a valuable agent and has its own potential side benefits, and bromocriptine-QR and pioglitazone have CV side benefits. So, there is really no need for early insulin in true T2D patients (ie, those that are non-ketosis prone and have sufficient beta-cell reserve).
Why reduce insulin in patients who are already on insulin?
Prior protocols resulted in 40%-50% of T2D patients being placed on insulin unnecessarily. As discussed, the side effects of insulin are many; they include weight gain, insulin resistance, hypoglycemia, and CV complications—all of which have been associated with a decline in quality of life.
What is your approach to reduce or eliminate insulin in those already on it (unnecessarily)?
First, I pick the right patient. Physicians should use sound clinical judgment to identify patients with likely residual beta-cell function. It is not just the “insulin-resistant patient," as 30%-50% of type 1 diabetes mellitus patients also have insulin resistance.
It needs to be a definite T2D patient: not ketosis prone, a family history T2D, no islet cell antibodies (if one has any concerns, check for them). They were often started on insulin in the emergency department with no ketosis and never received non-insulin therapy.
Patients need to be willing to commit to my strict, no-concentrated-sweets diet, to perform careful glucose monitoring, and to check their ketones. Patients should be willing to contact me if their sugar level is >250 mg/dL for 2 measurements in a row, while testing 4 times a day or using a continuous glucose-monitoring (CGM) device.
First, estimate a patient’s “current insulin need” (CIN), or the dose they might be on if they had not been subject to over-insulinization (ie, if they had not been subject to the “vicious cycle” discussed above). I do this by taking their total basal and bolus insulin dose, then reducing it by ~25% as the patient changes to a no-concentrated-sweets diet with an additional up-to-25% dose reduction if the patient has been experiencing symptomatic or asymptomatic hypoglycemia.
Next, I reduce this CIN number by ~25% upon starting a rapid-acting subcutaneous GLP-1 RA (liraglutide or oral semaglutide) and reduce the CIN another 20% as they start the SGLT-2 inhibitor. If patients come into my office on <40 U/d, I stop insulin as I start a GLP-1 RA and an SGLT-2 inhibitor and have them monitor home glucose levels to assure reasonable results as they go off the insulin and on their new therapy.
If patients come into my office on >40 U/d, they go home on a GLP-1 RA and an SGLT-2 inhibitor and ~30% of their presenting dose, apportioned between basal/bolus dosing based on when they are currently getting hypoglycemic.
The rapid initial reduction in their insulin doses, with initial adjustments in estimated insulin doses as needed based on home glucose monitoring, and rapid stabilization of glycemic levels by the effectiveness of these 2 agents give patients great motivation to keep up with the diet/program.
Then, as patients lose weight, they are told to report any glucose measurements <80 mg/dL, so that further reduction in insulin doses can be made. When patients achieve a new steady state of glycemia, weight, and GLP-1 RA and SGLT-2 inhibitor doses, you can add bromocriptine-QR, pioglitazone, and/or metformin as needed to allow for a further reduction of insulin. And, as you see the delayed effects of subsequently adding these new agents (eg, glucose <80 mg/dL), you can ultimately stop insulin when they get to <10-12 U/d. The process works very well, even in those starting on up to 300 units of insulin daily. Patients love the outcome and will greatly appreciate your care.
Feel free to contact Dr. Schwartz at [email protected] with any questions about his protocol or diet.