User login
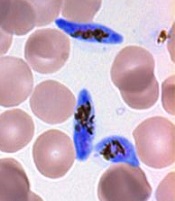
gametocyte stage (blue) and
uninfected red blood cells
Credit: The Llinás lab
Results of 2 new studies suggest that a single regulatory protein acts as a master switch to trigger development of the sexual forms of malaria parasites.
It appears that the protein, AP2-G, is necessary for activating a set of genes that initiate the development of Plasmodium gametocytes, the only forms of the parasite that are infectious to mosquitoes.
This suggests that if researchers can target AP2-G, they can stop sexual parasites from forming.
And if the sexual forms of the parasite never develop in an infected person’s blood, none will enter the mosquito’s gut, and the mosquito will be unable to infect anyone else with malaria.
“Exciting opportunities now lie ahead for finding an effective way to break the chain of malaria transmission by preventing the malaria parasite from completing its full lifecycle,” said Manuel Llinás, PhD, a professor at Pennsylvania State University who was involved in both studies.
The 2 studies, which were published as letters to Nature, had remarkably similar results, despite the fact that the groups worked with 2 different malaria parasites—Plasmodium falciparum and Plasmodium berghei.
In one study, researchers analyzed the whole-genome sequences of 2 P falciparum strains that were unable to produce gametocytes. The only mutated, non-functional gene common to both strains was the AP2-G gene.
In the other study, researchers sequenced P berghei parasites that had lost their ability to make gametocytes. Again, the only common mutated gene in these parasites was AP2-G.
To confirm these observations, both groups of researchers disabled the AP2-G gene in parasites that could generate gametocytes.
As expected, disabling the gene prevented the parasites from producing gametocytes. But the parasites regained their ability to make gametocytes when the mutated gene was repaired.
These results, as well as results of additional experiments, suggest that sexual-stage malaria parasites are produced only when the AP2-G protein is in working order.
“Our research has demonstrated unequivocally that the AP2-G transcription factor protein is essential for flipping the switch that initiates the transformation of malaria parasites in the blood from the asexual stage to the critical sexual stage of their life cycle,” Dr Llinás said.
He and his colleagues believe their discovery is exciting for the future of malaria research. It could spur the development of a sexual-stage vaccine, which would help a person infected with malaria mount an immune response to prevent their parasites from being transmitted to a mosquito, effectively ending the life cycle for that person’s batch of malaria parasites. ![]()

gametocyte stage (blue) and
uninfected red blood cells
Credit: The Llinás lab
Results of 2 new studies suggest that a single regulatory protein acts as a master switch to trigger development of the sexual forms of malaria parasites.
It appears that the protein, AP2-G, is necessary for activating a set of genes that initiate the development of Plasmodium gametocytes, the only forms of the parasite that are infectious to mosquitoes.
This suggests that if researchers can target AP2-G, they can stop sexual parasites from forming.
And if the sexual forms of the parasite never develop in an infected person’s blood, none will enter the mosquito’s gut, and the mosquito will be unable to infect anyone else with malaria.
“Exciting opportunities now lie ahead for finding an effective way to break the chain of malaria transmission by preventing the malaria parasite from completing its full lifecycle,” said Manuel Llinás, PhD, a professor at Pennsylvania State University who was involved in both studies.
The 2 studies, which were published as letters to Nature, had remarkably similar results, despite the fact that the groups worked with 2 different malaria parasites—Plasmodium falciparum and Plasmodium berghei.
In one study, researchers analyzed the whole-genome sequences of 2 P falciparum strains that were unable to produce gametocytes. The only mutated, non-functional gene common to both strains was the AP2-G gene.
In the other study, researchers sequenced P berghei parasites that had lost their ability to make gametocytes. Again, the only common mutated gene in these parasites was AP2-G.
To confirm these observations, both groups of researchers disabled the AP2-G gene in parasites that could generate gametocytes.
As expected, disabling the gene prevented the parasites from producing gametocytes. But the parasites regained their ability to make gametocytes when the mutated gene was repaired.
These results, as well as results of additional experiments, suggest that sexual-stage malaria parasites are produced only when the AP2-G protein is in working order.
“Our research has demonstrated unequivocally that the AP2-G transcription factor protein is essential for flipping the switch that initiates the transformation of malaria parasites in the blood from the asexual stage to the critical sexual stage of their life cycle,” Dr Llinás said.
He and his colleagues believe their discovery is exciting for the future of malaria research. It could spur the development of a sexual-stage vaccine, which would help a person infected with malaria mount an immune response to prevent their parasites from being transmitted to a mosquito, effectively ending the life cycle for that person’s batch of malaria parasites. ![]()

gametocyte stage (blue) and
uninfected red blood cells
Credit: The Llinás lab
Results of 2 new studies suggest that a single regulatory protein acts as a master switch to trigger development of the sexual forms of malaria parasites.
It appears that the protein, AP2-G, is necessary for activating a set of genes that initiate the development of Plasmodium gametocytes, the only forms of the parasite that are infectious to mosquitoes.
This suggests that if researchers can target AP2-G, they can stop sexual parasites from forming.
And if the sexual forms of the parasite never develop in an infected person’s blood, none will enter the mosquito’s gut, and the mosquito will be unable to infect anyone else with malaria.
“Exciting opportunities now lie ahead for finding an effective way to break the chain of malaria transmission by preventing the malaria parasite from completing its full lifecycle,” said Manuel Llinás, PhD, a professor at Pennsylvania State University who was involved in both studies.
The 2 studies, which were published as letters to Nature, had remarkably similar results, despite the fact that the groups worked with 2 different malaria parasites—Plasmodium falciparum and Plasmodium berghei.
In one study, researchers analyzed the whole-genome sequences of 2 P falciparum strains that were unable to produce gametocytes. The only mutated, non-functional gene common to both strains was the AP2-G gene.
In the other study, researchers sequenced P berghei parasites that had lost their ability to make gametocytes. Again, the only common mutated gene in these parasites was AP2-G.
To confirm these observations, both groups of researchers disabled the AP2-G gene in parasites that could generate gametocytes.
As expected, disabling the gene prevented the parasites from producing gametocytes. But the parasites regained their ability to make gametocytes when the mutated gene was repaired.
These results, as well as results of additional experiments, suggest that sexual-stage malaria parasites are produced only when the AP2-G protein is in working order.
“Our research has demonstrated unequivocally that the AP2-G transcription factor protein is essential for flipping the switch that initiates the transformation of malaria parasites in the blood from the asexual stage to the critical sexual stage of their life cycle,” Dr Llinás said.
He and his colleagues believe their discovery is exciting for the future of malaria research. It could spur the development of a sexual-stage vaccine, which would help a person infected with malaria mount an immune response to prevent their parasites from being transmitted to a mosquito, effectively ending the life cycle for that person’s batch of malaria parasites. ![]()