User login
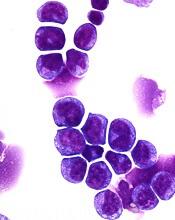
Preclinical research has revealed a potential therapeutic target for MLL-rearranged acute myeloid leukemia (AML).
The target—F-box protein S-phase kinase-associated protein 2 (Skp2)—degrades another protein called p27Kip1 that is important to the formation of healthy blood cells.
This finding was published in the Journal of Experimental Medicine.
“Our work provides a complete mechanistic look into the function of genetic and molecular programs driving this leukemia, and it exploits these processes to identify actionable therapeutic targets,’’ said study author H. Leighton Grimes, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
For this work, Dr Grimes and his colleagues performed biochemical analyses of cells from AML patients. This gave the researchers comprehensive information about the targets and functions of the miR-196 molecular signaling pathway.
The team inserted mimics of miR-196 into MLL-AF9 leukemia cells to incorporate them into the cellular machinery. The group then lysed the cells for analyses, which revealed molecular targets of miR-196 in the leukemia cells.
Next, the researchers screened AML cells in mice for miR-196 targets. These experiments showed that certain microRNA targets are more important than others in the maintenance and spread of leukemia stem cells (LSCs).
Computer-assisted analysis of the Molecular Signature Database (a shared multi-institutional resource) allowed the researchers to identify sets of genes that show up in high numbers in MLL-AF9 leukemia.
Additional biochemical testing revealed that miR-196 directly targets and inhibits Cdkn1b/p27Kip1, which controls molecular programming in LSCs that allows them to maintain aggressive MLL-AF9 leukemia.
When miR-196 targets Cdkn1b/p27Kip1, it accelerates MLL-AF9 progression by abnormally linking stem cell activity with the growth of leukemia cells.
With the data suggesting that elevation of p27Kip1 protein levels may be therapeutic to AML patients, the researchers investigated a related molecular pathway that also regulates p27Kip1.
This investigation yielded the treatment target Skp2, which degrades the p27 protein and lowers its expression.
The researchers tested an experimental Skp2 inhibitor, SLZ P1041, on human AML cell lines and found the drug killed AML cells in a dose-dependent manner.
The team also tested SLZ P1041 in combination with other inhibitors—IBET-151, palbociclib, and MI-1. The most consistent synergies were with the combination of SLZ P1041 and MI-1, an inhibitor of the interaction between Menin and MLL.
“We still have extensive additional testing to conduct in laboratory animal models of AML before knowing if [targeting Skp2] will translate to patient care,” Dr Grimes said.
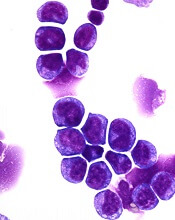
Preclinical research has revealed a potential therapeutic target for MLL-rearranged acute myeloid leukemia (AML).
The target—F-box protein S-phase kinase-associated protein 2 (Skp2)—degrades another protein called p27Kip1 that is important to the formation of healthy blood cells.
This finding was published in the Journal of Experimental Medicine.
“Our work provides a complete mechanistic look into the function of genetic and molecular programs driving this leukemia, and it exploits these processes to identify actionable therapeutic targets,’’ said study author H. Leighton Grimes, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
For this work, Dr Grimes and his colleagues performed biochemical analyses of cells from AML patients. This gave the researchers comprehensive information about the targets and functions of the miR-196 molecular signaling pathway.
The team inserted mimics of miR-196 into MLL-AF9 leukemia cells to incorporate them into the cellular machinery. The group then lysed the cells for analyses, which revealed molecular targets of miR-196 in the leukemia cells.
Next, the researchers screened AML cells in mice for miR-196 targets. These experiments showed that certain microRNA targets are more important than others in the maintenance and spread of leukemia stem cells (LSCs).
Computer-assisted analysis of the Molecular Signature Database (a shared multi-institutional resource) allowed the researchers to identify sets of genes that show up in high numbers in MLL-AF9 leukemia.
Additional biochemical testing revealed that miR-196 directly targets and inhibits Cdkn1b/p27Kip1, which controls molecular programming in LSCs that allows them to maintain aggressive MLL-AF9 leukemia.
When miR-196 targets Cdkn1b/p27Kip1, it accelerates MLL-AF9 progression by abnormally linking stem cell activity with the growth of leukemia cells.
With the data suggesting that elevation of p27Kip1 protein levels may be therapeutic to AML patients, the researchers investigated a related molecular pathway that also regulates p27Kip1.
This investigation yielded the treatment target Skp2, which degrades the p27 protein and lowers its expression.
The researchers tested an experimental Skp2 inhibitor, SLZ P1041, on human AML cell lines and found the drug killed AML cells in a dose-dependent manner.
The team also tested SLZ P1041 in combination with other inhibitors—IBET-151, palbociclib, and MI-1. The most consistent synergies were with the combination of SLZ P1041 and MI-1, an inhibitor of the interaction between Menin and MLL.
“We still have extensive additional testing to conduct in laboratory animal models of AML before knowing if [targeting Skp2] will translate to patient care,” Dr Grimes said.
Preclinical research has revealed a potential therapeutic target for MLL-rearranged acute myeloid leukemia (AML).
The target—F-box protein S-phase kinase-associated protein 2 (Skp2)—degrades another protein called p27Kip1 that is important to the formation of healthy blood cells.
This finding was published in the Journal of Experimental Medicine.
“Our work provides a complete mechanistic look into the function of genetic and molecular programs driving this leukemia, and it exploits these processes to identify actionable therapeutic targets,’’ said study author H. Leighton Grimes, PhD, of Cincinnati Children’s Hospital Medical Center in Ohio.
For this work, Dr Grimes and his colleagues performed biochemical analyses of cells from AML patients. This gave the researchers comprehensive information about the targets and functions of the miR-196 molecular signaling pathway.
The team inserted mimics of miR-196 into MLL-AF9 leukemia cells to incorporate them into the cellular machinery. The group then lysed the cells for analyses, which revealed molecular targets of miR-196 in the leukemia cells.
Next, the researchers screened AML cells in mice for miR-196 targets. These experiments showed that certain microRNA targets are more important than others in the maintenance and spread of leukemia stem cells (LSCs).
Computer-assisted analysis of the Molecular Signature Database (a shared multi-institutional resource) allowed the researchers to identify sets of genes that show up in high numbers in MLL-AF9 leukemia.
Additional biochemical testing revealed that miR-196 directly targets and inhibits Cdkn1b/p27Kip1, which controls molecular programming in LSCs that allows them to maintain aggressive MLL-AF9 leukemia.
When miR-196 targets Cdkn1b/p27Kip1, it accelerates MLL-AF9 progression by abnormally linking stem cell activity with the growth of leukemia cells.
With the data suggesting that elevation of p27Kip1 protein levels may be therapeutic to AML patients, the researchers investigated a related molecular pathway that also regulates p27Kip1.
This investigation yielded the treatment target Skp2, which degrades the p27 protein and lowers its expression.
The researchers tested an experimental Skp2 inhibitor, SLZ P1041, on human AML cell lines and found the drug killed AML cells in a dose-dependent manner.
The team also tested SLZ P1041 in combination with other inhibitors—IBET-151, palbociclib, and MI-1. The most consistent synergies were with the combination of SLZ P1041 and MI-1, an inhibitor of the interaction between Menin and MLL.
“We still have extensive additional testing to conduct in laboratory animal models of AML before knowing if [targeting Skp2] will translate to patient care,” Dr Grimes said.