User login
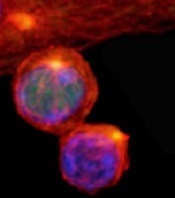
(with c-Myc in green)
Image courtesy of
Katherine Verbist and St. Jude
New research published in Nature helps explain how 2 types of cells arise from activated T cells.
Investigators found that distribution of the regulatory protein c-Myc during asymmetric cell division impacts an activated T cell’s fate, determining whether it will become an effector T cell or a memory T cell.
The team therefore believes that manipulating c-Myc levels could make vaccines more effective or advance immunotherapies for cancer treatment.
“Our work suggests that it may be possible to manipulate the immune response by nudging production of c-Myc in one direction or the other,” said study author Douglas Green, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Potentially, that could mean more effective vaccines or help to advance T-cell immune therapy for cancer treatment.”
Through a series of experiments, Dr Green and his colleagues found that, during asymmetric cell division of activated T cells, high levels of c-Myc accumulated in one daughter cell.
There, c-Myc launched and sustained the rapid proliferation of effector T cells, including those in mice infected with the influenza virus.
In contrast, daughter cells with low levels of c-Myc functioned like memory T cells, proliferating to mount an immune response a month later when mice were again exposed to the virus.
The investigators also identified the metabolic and signaling pathways that serve as a positive feedback loop to sustain the high levels of c-Myc that effector T cells require to maintain their identities and function.
The team showed that disrupting certain components of the system disturbed c-Myc production, which altered the fate of T cells and caused effector T cells to operate like memory T cells.
“While daughter cells of activated T cells seem to have very different fates, we showed their behavior could be altered by manipulating these metabolic and regulatory pathways to increase or decrease c-Myc levels,” Dr Green said. ![]()

(with c-Myc in green)
Image courtesy of
Katherine Verbist and St. Jude
New research published in Nature helps explain how 2 types of cells arise from activated T cells.
Investigators found that distribution of the regulatory protein c-Myc during asymmetric cell division impacts an activated T cell’s fate, determining whether it will become an effector T cell or a memory T cell.
The team therefore believes that manipulating c-Myc levels could make vaccines more effective or advance immunotherapies for cancer treatment.
“Our work suggests that it may be possible to manipulate the immune response by nudging production of c-Myc in one direction or the other,” said study author Douglas Green, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Potentially, that could mean more effective vaccines or help to advance T-cell immune therapy for cancer treatment.”
Through a series of experiments, Dr Green and his colleagues found that, during asymmetric cell division of activated T cells, high levels of c-Myc accumulated in one daughter cell.
There, c-Myc launched and sustained the rapid proliferation of effector T cells, including those in mice infected with the influenza virus.
In contrast, daughter cells with low levels of c-Myc functioned like memory T cells, proliferating to mount an immune response a month later when mice were again exposed to the virus.
The investigators also identified the metabolic and signaling pathways that serve as a positive feedback loop to sustain the high levels of c-Myc that effector T cells require to maintain their identities and function.
The team showed that disrupting certain components of the system disturbed c-Myc production, which altered the fate of T cells and caused effector T cells to operate like memory T cells.
“While daughter cells of activated T cells seem to have very different fates, we showed their behavior could be altered by manipulating these metabolic and regulatory pathways to increase or decrease c-Myc levels,” Dr Green said. ![]()

(with c-Myc in green)
Image courtesy of
Katherine Verbist and St. Jude
New research published in Nature helps explain how 2 types of cells arise from activated T cells.
Investigators found that distribution of the regulatory protein c-Myc during asymmetric cell division impacts an activated T cell’s fate, determining whether it will become an effector T cell or a memory T cell.
The team therefore believes that manipulating c-Myc levels could make vaccines more effective or advance immunotherapies for cancer treatment.
“Our work suggests that it may be possible to manipulate the immune response by nudging production of c-Myc in one direction or the other,” said study author Douglas Green, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“Potentially, that could mean more effective vaccines or help to advance T-cell immune therapy for cancer treatment.”
Through a series of experiments, Dr Green and his colleagues found that, during asymmetric cell division of activated T cells, high levels of c-Myc accumulated in one daughter cell.
There, c-Myc launched and sustained the rapid proliferation of effector T cells, including those in mice infected with the influenza virus.
In contrast, daughter cells with low levels of c-Myc functioned like memory T cells, proliferating to mount an immune response a month later when mice were again exposed to the virus.
The investigators also identified the metabolic and signaling pathways that serve as a positive feedback loop to sustain the high levels of c-Myc that effector T cells require to maintain their identities and function.
The team showed that disrupting certain components of the system disturbed c-Myc production, which altered the fate of T cells and caused effector T cells to operate like memory T cells.
“While daughter cells of activated T cells seem to have very different fates, we showed their behavior could be altered by manipulating these metabolic and regulatory pathways to increase or decrease c-Myc levels,” Dr Green said. ![]()