User login
A hospital quality improvement initiative reduced antibiotic use by more than half when well-appearing newborns exposed to chorioamnionitis were initially monitored for symptoms instead of routinely given antibiotics, found a study in Pediatrics.
“The reduction in both antibiotic use and laboratory testing occurred without clinically relevant delays in care or poor outcomes,” wrote Neha S. Joshi, MD, of Stanford (Calif.) University and her associates.
At Lucile Packard Children’s Hospital Stanford, about half of all antibiotic use for late-preterm or term infants went to newborns exposed to chorioamnionitis. The hospital developed a quality improvement initiative to safely reduce unnecessary antibiotic use in these patients and to decrease unnecessary lab testing given the weak clinical relevance of CBC counts and C-reactive protein labs for determining whether to give a well-appearing child antibiotics, the study authors explained.
Before the initiative began, standard practice included admitting all infants to the neonatal ICU who were at least 34 weeks’ gestation and exposed to chorioamnionitis. They were treated with ampicillin and gentamicin until early-onset sepsis was excluded. Lab evaluations included a CBC count, blood culture, and multiple C-reactive protein labs.
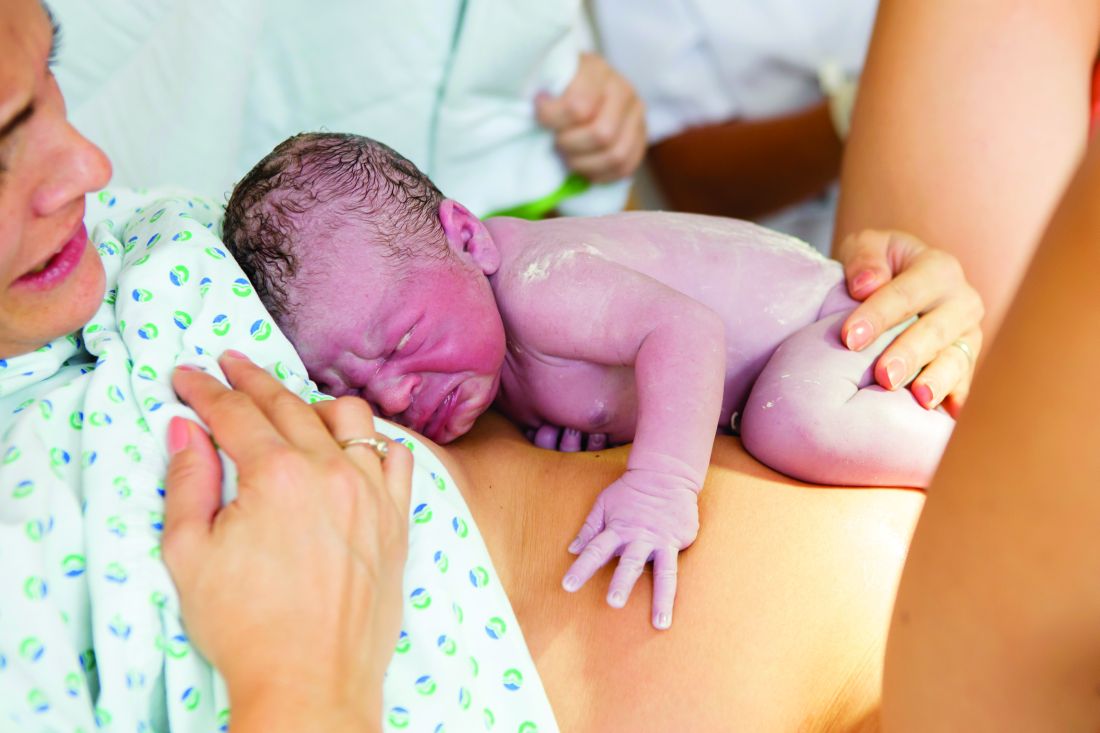
Under the new protocol, symptomatic newborns still had the same labs and received empirical antibiotics. Well-appearing, late-preterm or term infants exposed to chorioamnionitis first spent 2 hours of skin-to-skin contact with their mothers and then were monitored clinically in a level II nursery for at least 24 hours. Unless clinical symptoms developed in that time, the infants then were returned to their mothers until discharge without labs or antibiotics. Those who did develop potentially septic signs/symptoms, as determined by the treating physician, were evaluated and then received antibiotics if deemed appropriate.
During the first 15 months of the quality improvement initiative, 310 infants (5.7% of the 5,425 total births with at least 34 weeks’ gestation) were exposed to chorioamnionitis. Of these, 23 (7.4%) were symptomatic and began antibiotics; another 10 (3.2%) were admitted to the neonatal ICU for a congenital anomaly.
The researchers collected data on antibiotic use, lab tests, cultures, and clinical outcomes from the remaining 277 well-appearing newborns; 88% did not receive antibiotics during their hospital stay, and 83% underwent no laboratory testing. Only 17% of infants had lab testing for sepsis; none had culture result–positive, early-onset sepsis.
Only 12% of infants who initially appeared well developed signs/symptoms of sepsis, underwent laboratory testing, and received antibiotics. Nearly half of these (5% of all infants) received antibiotic treatment for at least 5 days despite negative cultures, while the other 7% received antibiotics for less than 48 hours, Dr. Joshi and her colleagues reported.
Infants with at least 34 weeks’ gestation receiving antibiotics at the hospital dropped from 12.3% before the initiative to 5.5% afterward, a 55% decrease (95% confidence interval, 40%-60%), the researchers said. Study limitations included a lack of postdischarge follow-up, the variability in physician decisions about which infants were symptomatic and which ones needed antibiotics, and an inability to generalize findings to institutions without 24/7 availability of neonatal hospitalists.
Past studies have found that all newborns with positive cultures showed symptoms at birth and needed resuscitation, continuous positive airway pressure, or intubation.
“An infant who is well-appearing at birth likely has an even lower risk of early-onset sepsis even in the setting of chorioamnionitis, and an empirical antibiotic treatment strategy for chorioamnionitis-exposed infants will result in a large number of uninfected infants being treated,” Dr. Joshi and her associates said. “Updated treatment approaches are needed to reduce unnecessary antibiotic exposure and provide higher-value care in this population.”
The study did not use external funding. The authors had no disclosures.
SOURCE: Joshi NS et al. Pediatrics. 2018;141(4):e20172056.
A hospital quality improvement initiative reduced antibiotic use by more than half when well-appearing newborns exposed to chorioamnionitis were initially monitored for symptoms instead of routinely given antibiotics, found a study in Pediatrics.
“The reduction in both antibiotic use and laboratory testing occurred without clinically relevant delays in care or poor outcomes,” wrote Neha S. Joshi, MD, of Stanford (Calif.) University and her associates.
At Lucile Packard Children’s Hospital Stanford, about half of all antibiotic use for late-preterm or term infants went to newborns exposed to chorioamnionitis. The hospital developed a quality improvement initiative to safely reduce unnecessary antibiotic use in these patients and to decrease unnecessary lab testing given the weak clinical relevance of CBC counts and C-reactive protein labs for determining whether to give a well-appearing child antibiotics, the study authors explained.
Before the initiative began, standard practice included admitting all infants to the neonatal ICU who were at least 34 weeks’ gestation and exposed to chorioamnionitis. They were treated with ampicillin and gentamicin until early-onset sepsis was excluded. Lab evaluations included a CBC count, blood culture, and multiple C-reactive protein labs.
Under the new protocol, symptomatic newborns still had the same labs and received empirical antibiotics. Well-appearing, late-preterm or term infants exposed to chorioamnionitis first spent 2 hours of skin-to-skin contact with their mothers and then were monitored clinically in a level II nursery for at least 24 hours. Unless clinical symptoms developed in that time, the infants then were returned to their mothers until discharge without labs or antibiotics. Those who did develop potentially septic signs/symptoms, as determined by the treating physician, were evaluated and then received antibiotics if deemed appropriate.
During the first 15 months of the quality improvement initiative, 310 infants (5.7% of the 5,425 total births with at least 34 weeks’ gestation) were exposed to chorioamnionitis. Of these, 23 (7.4%) were symptomatic and began antibiotics; another 10 (3.2%) were admitted to the neonatal ICU for a congenital anomaly.
The researchers collected data on antibiotic use, lab tests, cultures, and clinical outcomes from the remaining 277 well-appearing newborns; 88% did not receive antibiotics during their hospital stay, and 83% underwent no laboratory testing. Only 17% of infants had lab testing for sepsis; none had culture result–positive, early-onset sepsis.
Only 12% of infants who initially appeared well developed signs/symptoms of sepsis, underwent laboratory testing, and received antibiotics. Nearly half of these (5% of all infants) received antibiotic treatment for at least 5 days despite negative cultures, while the other 7% received antibiotics for less than 48 hours, Dr. Joshi and her colleagues reported.
Infants with at least 34 weeks’ gestation receiving antibiotics at the hospital dropped from 12.3% before the initiative to 5.5% afterward, a 55% decrease (95% confidence interval, 40%-60%), the researchers said. Study limitations included a lack of postdischarge follow-up, the variability in physician decisions about which infants were symptomatic and which ones needed antibiotics, and an inability to generalize findings to institutions without 24/7 availability of neonatal hospitalists.
Past studies have found that all newborns with positive cultures showed symptoms at birth and needed resuscitation, continuous positive airway pressure, or intubation.
“An infant who is well-appearing at birth likely has an even lower risk of early-onset sepsis even in the setting of chorioamnionitis, and an empirical antibiotic treatment strategy for chorioamnionitis-exposed infants will result in a large number of uninfected infants being treated,” Dr. Joshi and her associates said. “Updated treatment approaches are needed to reduce unnecessary antibiotic exposure and provide higher-value care in this population.”
The study did not use external funding. The authors had no disclosures.
SOURCE: Joshi NS et al. Pediatrics. 2018;141(4):e20172056.
A hospital quality improvement initiative reduced antibiotic use by more than half when well-appearing newborns exposed to chorioamnionitis were initially monitored for symptoms instead of routinely given antibiotics, found a study in Pediatrics.
“The reduction in both antibiotic use and laboratory testing occurred without clinically relevant delays in care or poor outcomes,” wrote Neha S. Joshi, MD, of Stanford (Calif.) University and her associates.
At Lucile Packard Children’s Hospital Stanford, about half of all antibiotic use for late-preterm or term infants went to newborns exposed to chorioamnionitis. The hospital developed a quality improvement initiative to safely reduce unnecessary antibiotic use in these patients and to decrease unnecessary lab testing given the weak clinical relevance of CBC counts and C-reactive protein labs for determining whether to give a well-appearing child antibiotics, the study authors explained.
Before the initiative began, standard practice included admitting all infants to the neonatal ICU who were at least 34 weeks’ gestation and exposed to chorioamnionitis. They were treated with ampicillin and gentamicin until early-onset sepsis was excluded. Lab evaluations included a CBC count, blood culture, and multiple C-reactive protein labs.
Under the new protocol, symptomatic newborns still had the same labs and received empirical antibiotics. Well-appearing, late-preterm or term infants exposed to chorioamnionitis first spent 2 hours of skin-to-skin contact with their mothers and then were monitored clinically in a level II nursery for at least 24 hours. Unless clinical symptoms developed in that time, the infants then were returned to their mothers until discharge without labs or antibiotics. Those who did develop potentially septic signs/symptoms, as determined by the treating physician, were evaluated and then received antibiotics if deemed appropriate.
During the first 15 months of the quality improvement initiative, 310 infants (5.7% of the 5,425 total births with at least 34 weeks’ gestation) were exposed to chorioamnionitis. Of these, 23 (7.4%) were symptomatic and began antibiotics; another 10 (3.2%) were admitted to the neonatal ICU for a congenital anomaly.
The researchers collected data on antibiotic use, lab tests, cultures, and clinical outcomes from the remaining 277 well-appearing newborns; 88% did not receive antibiotics during their hospital stay, and 83% underwent no laboratory testing. Only 17% of infants had lab testing for sepsis; none had culture result–positive, early-onset sepsis.
Only 12% of infants who initially appeared well developed signs/symptoms of sepsis, underwent laboratory testing, and received antibiotics. Nearly half of these (5% of all infants) received antibiotic treatment for at least 5 days despite negative cultures, while the other 7% received antibiotics for less than 48 hours, Dr. Joshi and her colleagues reported.
Infants with at least 34 weeks’ gestation receiving antibiotics at the hospital dropped from 12.3% before the initiative to 5.5% afterward, a 55% decrease (95% confidence interval, 40%-60%), the researchers said. Study limitations included a lack of postdischarge follow-up, the variability in physician decisions about which infants were symptomatic and which ones needed antibiotics, and an inability to generalize findings to institutions without 24/7 availability of neonatal hospitalists.
Past studies have found that all newborns with positive cultures showed symptoms at birth and needed resuscitation, continuous positive airway pressure, or intubation.
“An infant who is well-appearing at birth likely has an even lower risk of early-onset sepsis even in the setting of chorioamnionitis, and an empirical antibiotic treatment strategy for chorioamnionitis-exposed infants will result in a large number of uninfected infants being treated,” Dr. Joshi and her associates said. “Updated treatment approaches are needed to reduce unnecessary antibiotic exposure and provide higher-value care in this population.”
The study did not use external funding. The authors had no disclosures.
SOURCE: Joshi NS et al. Pediatrics. 2018;141(4):e20172056.
FROM PEDIATRICS
Key clinical point:
Major finding: After a quality improvement initiative was implemented, 55% fewer late-preterm and term, chorioamnionitis-exposed infants received antibiotics without an increase in negative outcomes.
Data source: A study of 310 chorioamnionitis-exposed newborns who were late preterm or term at a California hospital.
Disclosures: The study did not use external funding. The authors had no relevant financial disclosures.
Source: Joshi NS et al. Pediatrics. 2018;141(4):e20172056.