User login
Shock has been defined as failure to deliver and/or utilize adequate amounts of oxygen1 and is a common cause of critical illness. Few studies have examined the predictive utility of bedside clinical examination in predicting the category of shock. Scholars have suggested a bedside approach that uses simple examination techniques and applied physiology to rapidly identify a patients' circulation as high vs. low cardiac output. Those with a high‐output examination are designated as high‐output, most often septic shock. Low‐output patients are further categorized as heart full or heart empty to distinguish cardiogenic from hypovolemic categories of shock, respectively.2 The predictive characteristics of this simple algorithm have not been studied. In this study, we examine the operating characteristics of selected elements of this algorithm when administered at the bedside by trainees in Internal Medicine.
Methods
This study was performed after approval of the Institutional Review Board; informed consent was waived. Patients with nonsurgical problems who present to the hospital or who develop sustained hypotension are managed by medical house officers on the intensive care and/or rapid response team with the supervision of patients' attending physicians. All house officers were asked to document explicitly in their assessment notes the following examination findings: finger capillary refill (same/quicker vs. slower than examiner's), hand skin temperature (same/warmer vs. cooler than examiner's) and pulse pressure (ie, same/wider vs. thinner than examiner's), presence or absence of crackles >1/3 from base on bilateral lung examination and jugular venous pressure (JVP) vs. 8 cmH2O. The documented examinations of either the rapid response team (PGY2; n = 14) or intensive care unit (ICU) resident (PGY3; n = 14) for patients evaluated between September 2008 and February 2009 were used for this study. Resuscitation was administered entirely by house officers, occasionally guided in person, but always supervised by attending physicians.
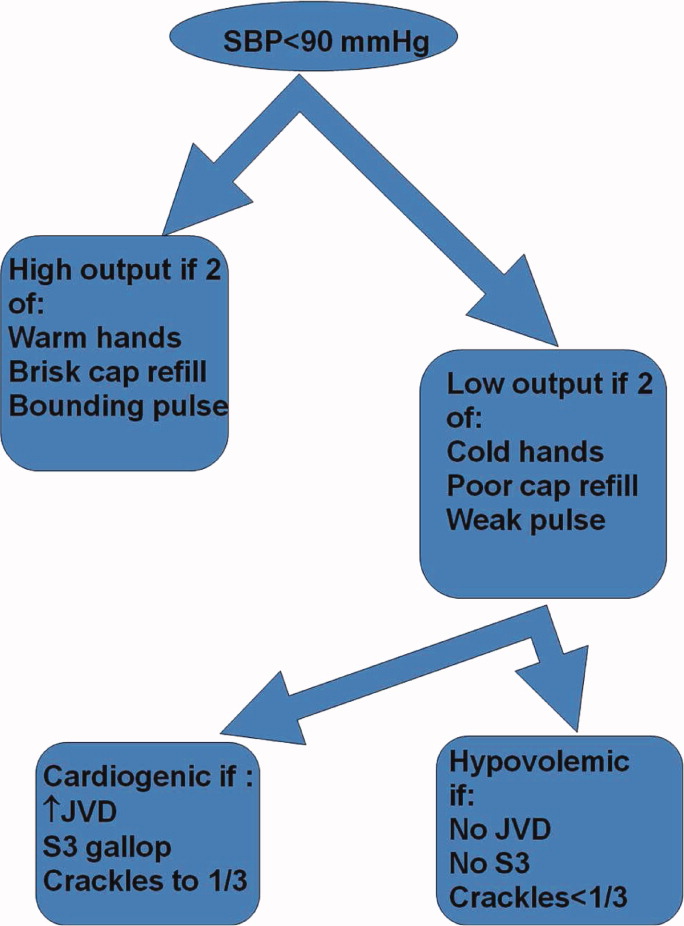
In May 2009, clinical data, including electrocardiograms/echocardiograms and laboratory (eg, cardiac enzymes, culture) results were abstracted from medical records of subjects. These were presented to a blinded senior clinician (DK) to review and apply evidence‐based or consensus criteria,36 whenever possible, to categorize the type of shock (septic vs. cardiogenic vs. hypovolemic) based on data acquired after the onset of shock. For example, patients with microbiologic and/or radiologic evidence of infection were classified as septic shock,1, 3, 4 those with acute left or right ventricular dysfunction on echocardiogram were classified as cardiogenic shock,1, 6 and those with clinical evidence of acute hemorrhage with hypovolemic shock.1, 5 While some of the patients were examined by DK as part of clinical care, he was blinded to the identity of patients and their algorithm‐related physical examination findings when he reviewed the abstracted data (>2 months after study closure) to adjudicate the final diagnosis of shock. These diagnoses were considered the reference standard for this study. The operating characteristics (sensitivity = true positive/true positive + false negative; specificity = true negative/true negative + false positive; negative predictive value (NPV) = true negative/all negatives; positive predictive value (PPV) = true positive/all positives; accuracy = true results/all results) were calculated for combinations of physical examination findings and correct final diagnosis (Figure 1).
Results
A total of 68 patients, averaging 71 16 years, were studied; 57% were male, and 66% were White, and 20% were Black. Table 1 lists characteristics of patients. A total of 37 patients were diagnosed as having septic shock, 11 had cardiogenic shock and 10 hypovolemic shock. Operating characteristics of the bedside examination techniques for predicting mechanism of shock are listed in Table 2. Capillary refill and skin temperature were 100% concordant yielding sensitivity of 89% (95% confidence interval [CI], 75‐97%), specificity of 68% (95% CI, 46‐83%), PPV of 77% (95% CI, 61‐88%), NPV of 84% (95% CI, 64‐96%) and overall accuracy of 79% (95% CI, 68‐88%) for diagnosis of high output (ie, septic shock). JVP 8 cmH2O was more accurate than crackles for predicting cardiogenic shock in low‐output patients with sensitivity of 82% (95% CI, 48‐98%), specificity of 79% (95% CI, 41‐95%), PPV of 75% (95% CI, 43‐95%), NPV of 85% (95% CI, 55‐98%), and overall accuracy of 80% (95%CI, 59‐93%). Using just skin temperature and JVP, the bedside approach misdiagnosed 19 of 75 cases (overall accuracy, 75%; 95% CI, 16‐37%).
| n Total | |
|---|---|
| |
| Gender, n (%) | n = 68 |
| Male | 39 (57) |
| Age, years | 71 16 |
| Race, n (%) | |
| White | 45 (66) |
| Black | 15 (22) |
| Hispanic | 7 (10) |
| Other | 1 (2) |
| High output, n (%) | n = 37 |
| Sepsis | |
| Pneumonia | 10 (27) |
| Urinary tract | 17 (46) |
| Skin | 3 (8) |
| Gastrointestinal | 5 (14) |
| Non‐infectious SIRS | 2 (5) |
| Low output heart full, n (%) | n = 18 |
| Pulmonary embolism | 3 (16) |
| AMI | 7 (40) |
| Cardiomyopathy | 5 (28) |
| Rhythm disturbance | 3 (16) |
| Low output heart empty, n (%) | n = 13 |
| Hemorrhagic | 9 (70) |
| NPO | 1 (7) |
| Diarrhea | 2 (14) |
| Adrenal crisis | 1 (7) |
| Prediction of SIRS | Capillary Refill Same/Faster (%) | Skin Same/ Warm (%) | Bounding Pulses (%) |
|---|---|---|---|
| |||
| Sensitivity | 89 | 89 | 65 |
| Specificity | 68 | 68 | 74 |
| Accuracy | 79 | 79 | 69 |
| Prediction of SIRS | Capillary Refill Same/Faster + Warm Skin + Bounding Pulse (%) | Capillary Refill Same/Faster + Warm Skin (%) | Any Other Combination of 2 (%) |
| Sensitivity | 62 | 89 | 62 |
| Specificity | 74 | 68 | 74 |
| Accuracy | 67 | 79 | 67 |
| Prediction of Cardiogenic | JVP (%) | Crackles (%) | JVP + Crackles (%) |
| Sensitivity | 82 | 55 | 55 |
| Specificity | 79 | 71 | 100 |
| Accuracy | 80 | 64 | 80 |
Discussion
This is the first study to examine the predictive characteristics of simple bedside physical examination techniques in correctly predicting the category/mechanism of shock. Overall, the algorithm performed well, and accurately predicted the category of shock in three‐quarters of patients. It also has the benefit of being very rapid, taking only seconds to complete, using bedside techniques that even inexperienced clinicians can apply.
Very few studies have examined the accuracy of examination techniques specifically for diagnosis of shock. In humans injected with endotoxin, body temperature and cardiac output increased, but skin temperature and capillary refill times are not well described.79 Schriger and Baraff10 reported that capillary refill >2 seconds was only 59% sensitive for diagnosing hypovolemia in patients with hypovolemic shock or orthostatic changes in blood pressure. Sensitivity was 77% in 13 patients with hypovolemic shock.10 However, some studies have demonstrated that age, sex, external temperature11 and fever12 can affect capillary refill times. Otieno et al.13 demonstrated a kappa statistic value of 0.49 for capillary refill 4 seconds, suggesting that reproducibility of this technique could be a major drawback. McGee et al.14 reviewed examination techniques for diagnosing hypovolemic states and concluded that postural changes in heart rate and blood pressure were the most accurate; capillary refill was not recommended. Stevenson and Perloff15 demonstrated that crackles and elevated JVP were absent in 18 of 43 patients with pulmonary capillary wedge pressures >22 mmHg. Butman et al.16 showed that elevated JVP was 82% accurate for predicting a wedge pressure >18 mmHg. Connors et al.17 demonstrated that clinicians' predictions of heart filling pressures and cardiac output were accurate (relative to pulmonary artery catheter measurements) in less than 50% of cases, though the examination techniques used were not qualified or quantified. No previous study has combined simple, semiobjective physical examination techniques for the purpose of distinguishing categories of shock.
Since identification of the pathogenesis of shock has important treatment/prognostic implications (eg, fluid and vasopressor therapies, early search for drainable focus of infection in sepsis, reestablishing vessel patency in myocardial infarction and pulmonary embolus), we believe that this simple, rapidly administered algorithm will prove useful in clinical medicine. In some clinical situations, the approach can lead to timely identification of the causative mechanism, allowing prompt definitive treatment. For example, a patient presenting with high‐output hypotension is so often sepsis/septic shock that treatment with antibiotics is justified (since success is time‐sensitive) even when the exact site/microbe has not yet been identified. Acute right heart overfilled low‐output hypotension should be considered pulmonary embolism (which also requires time‐sensitive therapies) until proven otherwise. Yet, a sizeable number of cases do not fit neatly into a single category. For example, 11% of patients with septic shock presented with cool extremities in the early phases of illness. In clinical decision‐making, 2 diagnostic‐therapeutic paradigms are common. In the first, the diagnosis is relatively certain and narrowly‐directed, mechanism‐specific treatment is appropriate. The second paradigm is 1 of significant uncertainty, when clinicians must treat empirically the most likely causes until more data become available to permit safe narrowing of therapies. For example, a patient presenting with hypotension, cool extremities, leukocytosis and apparent pneumonia should be treated empirically for septic shock while exploring explanations for the incongruous low‐output state (eg, profound hypovolemia, adrenal insufficiency, concurrent or precedent myocardial dysfunction). Patients often have several mechanisms contributing to hypotension. Since patients are not ideal forms, there can be no perfect decision‐tool; clinicians would be fool‐hardy to prematurely close decision‐making prior to definitive diagnosis. In the case of shock, such diagnostic arrogance would delay time‐sensitive therapies and thus contribute to morbidity and mortality. Nonetheless, this physical examination algorithmunderstanding its operating characteristics and limitationsmay add to the bedside clinician's diagnostic armamentarium.
Our study has several notable limitations. First, bedside examinations were performed by multiple observers who had limited (1 electronic mail) instruction on how to perform and document the data gathered for this study. So these results should be generalized cautiously until reproduced at other centers with greater numbers of observers (than the 28 of this study). The central supposition, that skin cooler, capillary refill longer, and pulse pressure more narrow than theirs, presupposes reasonable homogeneity of the normal state which is not necessarily true.11 Interobserver variability of physical examination further compromises the fidelity of findings recorded for this study.13 Since we conducted a retrospective review, and because of the emergency nature of the clinical problem, it would be difficult to conduct a study in which multiple examiners performed the same physical examinations to quantify interobserver variability. Irrespective, we would expect interobserver variability to systematically reduce accuracy; it is all‐the‐more impressive that trainees' examination results correctly diagnosed mechanism of shock in three‐quarters of cases. Also, examiners were not blinded to clinical history, so results of their examination could have been biased by their pre‐examination hypotheses of pathogenesis. Of course, they were not aware of the expert's final categorization of mechanism performed much later in time. Since there is no absolute reference standard for classification of the pathogenesis of shock, we depended upon careful review of selected data (same parameters for each patient) by a single senior investigatoralbeit armed with evidence‐based or consensus‐based standards of diagnosing shock. Finally, it can be argued that all forms of shock are mixed (with hypovolemia) early in the course; sepsis requires refilling of a leaky and dilated vasculature and the noncompliant ischemic ventricle often requires a higher filling pressure than normal to empty. To complicate even more, patients may have preexistent conditions (eg, chronic congestive heart failure, cirrhosis) that limit cardiovascular responses to acute shock. Our diagnostic approach was to identify the principal cause of the acute decompensation, assuming that many patients will have more than 1 single mechanism accounting for hypotension.
In conclusion, this is the first study to examine the utility of this simple physical examination algorithm to diagnose the mechanism of shock. Some have discounted or underemphasized examination techniques in favor of more time‐intensive and labor‐intensive diagnostic modalities, such as bedside echocardiography, which may waste precious time and resources. The simple physical examination algorithm assessed in this study has favorable operating characteristics and can be performed readily by even novice clinicians. If replicated at other centers and by greater numbers of observers, this approach could assist clinicians and teachers who train clinicians to rapidly diagnose and manage patients with shock.
- ,,, et al.Hemodynamic monitoring in shock and implications for management. International consensus conference. Paris, France, 27–28 April 2006.Intensive Care Med.2007;33:575–590.
- .The pathophysiology of the circulation in critical illness. In:Principles of Critical Care.New York:McGraw Hill;2005.
- ,,, et al.2001 SCCM/ESICM/ACCP/ATS/SIS. International Sepsis Definitions Conference.Crit Care Med.2003;31:1250–1256.
- ,,, et al.Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.Chest.1992;101:1644–1655;
- ,,, et al.Resuscitation from severe hemorrhage.Crit Care Med.1996;24(2 Suppl):S12–S23.
- ,.Cardiogenic shock: current concepts and improving outcomes circulation.Circulation.2008;117:686–697.
- ,,, et al.The cardiovascular response of normal humans to the administration of endotoxin.N Engl J Med.1989;321:280–287.
- ,,,,,.Experimental endotoxemia in humans: análysis of cytokine release and coagulation, fibrinolytic, an complement pathways.Blood.1990;76:2520–2526.
- ,,,,.Peripheral resistance changes during shock in man.Angiology.1968;19:268–276.
- ,.Capillary refill—is it a useful predictor of hypovolemic states?Ann Emerg Med.1991;20:601–605.
- ,.Defining normal capillary refill: variation with age, sex, and temperature.Ann Emerg Med.1988;17:113–116.
- ,,,.Effect of fever on capillary refill time.Pediatr Emerg Care.1997;13:305–307.
- ,,,,,.Are bedside features of shock reproducible between different observers?Arch Dis Child.2004;89:977–979.
- ,,.Is this patient hypovolemic?JAMA.1999;281:1022–1029.
- ,.The limited reliability of physical signs for estimating hemodynamics in chronic heart failure.JAMA.1989;261:884–888.
- ,,,,.Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension.J Am Coll Cardiol.1993;22:968–974.
- ,,.Evaluation of right‐heart catheterization in the critically ill patients without acute myocardial infarction.N Engl J Med.1983;308(5):263–267.
Shock has been defined as failure to deliver and/or utilize adequate amounts of oxygen1 and is a common cause of critical illness. Few studies have examined the predictive utility of bedside clinical examination in predicting the category of shock. Scholars have suggested a bedside approach that uses simple examination techniques and applied physiology to rapidly identify a patients' circulation as high vs. low cardiac output. Those with a high‐output examination are designated as high‐output, most often septic shock. Low‐output patients are further categorized as heart full or heart empty to distinguish cardiogenic from hypovolemic categories of shock, respectively.2 The predictive characteristics of this simple algorithm have not been studied. In this study, we examine the operating characteristics of selected elements of this algorithm when administered at the bedside by trainees in Internal Medicine.
Methods
This study was performed after approval of the Institutional Review Board; informed consent was waived. Patients with nonsurgical problems who present to the hospital or who develop sustained hypotension are managed by medical house officers on the intensive care and/or rapid response team with the supervision of patients' attending physicians. All house officers were asked to document explicitly in their assessment notes the following examination findings: finger capillary refill (same/quicker vs. slower than examiner's), hand skin temperature (same/warmer vs. cooler than examiner's) and pulse pressure (ie, same/wider vs. thinner than examiner's), presence or absence of crackles >1/3 from base on bilateral lung examination and jugular venous pressure (JVP) vs. 8 cmH2O. The documented examinations of either the rapid response team (PGY2; n = 14) or intensive care unit (ICU) resident (PGY3; n = 14) for patients evaluated between September 2008 and February 2009 were used for this study. Resuscitation was administered entirely by house officers, occasionally guided in person, but always supervised by attending physicians.
In May 2009, clinical data, including electrocardiograms/echocardiograms and laboratory (eg, cardiac enzymes, culture) results were abstracted from medical records of subjects. These were presented to a blinded senior clinician (DK) to review and apply evidence‐based or consensus criteria,36 whenever possible, to categorize the type of shock (septic vs. cardiogenic vs. hypovolemic) based on data acquired after the onset of shock. For example, patients with microbiologic and/or radiologic evidence of infection were classified as septic shock,1, 3, 4 those with acute left or right ventricular dysfunction on echocardiogram were classified as cardiogenic shock,1, 6 and those with clinical evidence of acute hemorrhage with hypovolemic shock.1, 5 While some of the patients were examined by DK as part of clinical care, he was blinded to the identity of patients and their algorithm‐related physical examination findings when he reviewed the abstracted data (>2 months after study closure) to adjudicate the final diagnosis of shock. These diagnoses were considered the reference standard for this study. The operating characteristics (sensitivity = true positive/true positive + false negative; specificity = true negative/true negative + false positive; negative predictive value (NPV) = true negative/all negatives; positive predictive value (PPV) = true positive/all positives; accuracy = true results/all results) were calculated for combinations of physical examination findings and correct final diagnosis (Figure 1).

Results
A total of 68 patients, averaging 71 16 years, were studied; 57% were male, and 66% were White, and 20% were Black. Table 1 lists characteristics of patients. A total of 37 patients were diagnosed as having septic shock, 11 had cardiogenic shock and 10 hypovolemic shock. Operating characteristics of the bedside examination techniques for predicting mechanism of shock are listed in Table 2. Capillary refill and skin temperature were 100% concordant yielding sensitivity of 89% (95% confidence interval [CI], 75‐97%), specificity of 68% (95% CI, 46‐83%), PPV of 77% (95% CI, 61‐88%), NPV of 84% (95% CI, 64‐96%) and overall accuracy of 79% (95% CI, 68‐88%) for diagnosis of high output (ie, septic shock). JVP 8 cmH2O was more accurate than crackles for predicting cardiogenic shock in low‐output patients with sensitivity of 82% (95% CI, 48‐98%), specificity of 79% (95% CI, 41‐95%), PPV of 75% (95% CI, 43‐95%), NPV of 85% (95% CI, 55‐98%), and overall accuracy of 80% (95%CI, 59‐93%). Using just skin temperature and JVP, the bedside approach misdiagnosed 19 of 75 cases (overall accuracy, 75%; 95% CI, 16‐37%).
| n Total | |
|---|---|
| |
| Gender, n (%) | n = 68 |
| Male | 39 (57) |
| Age, years | 71 16 |
| Race, n (%) | |
| White | 45 (66) |
| Black | 15 (22) |
| Hispanic | 7 (10) |
| Other | 1 (2) |
| High output, n (%) | n = 37 |
| Sepsis | |
| Pneumonia | 10 (27) |
| Urinary tract | 17 (46) |
| Skin | 3 (8) |
| Gastrointestinal | 5 (14) |
| Non‐infectious SIRS | 2 (5) |
| Low output heart full, n (%) | n = 18 |
| Pulmonary embolism | 3 (16) |
| AMI | 7 (40) |
| Cardiomyopathy | 5 (28) |
| Rhythm disturbance | 3 (16) |
| Low output heart empty, n (%) | n = 13 |
| Hemorrhagic | 9 (70) |
| NPO | 1 (7) |
| Diarrhea | 2 (14) |
| Adrenal crisis | 1 (7) |
| Prediction of SIRS | Capillary Refill Same/Faster (%) | Skin Same/ Warm (%) | Bounding Pulses (%) |
|---|---|---|---|
| |||
| Sensitivity | 89 | 89 | 65 |
| Specificity | 68 | 68 | 74 |
| Accuracy | 79 | 79 | 69 |
| Prediction of SIRS | Capillary Refill Same/Faster + Warm Skin + Bounding Pulse (%) | Capillary Refill Same/Faster + Warm Skin (%) | Any Other Combination of 2 (%) |
| Sensitivity | 62 | 89 | 62 |
| Specificity | 74 | 68 | 74 |
| Accuracy | 67 | 79 | 67 |
| Prediction of Cardiogenic | JVP (%) | Crackles (%) | JVP + Crackles (%) |
| Sensitivity | 82 | 55 | 55 |
| Specificity | 79 | 71 | 100 |
| Accuracy | 80 | 64 | 80 |
Discussion
This is the first study to examine the predictive characteristics of simple bedside physical examination techniques in correctly predicting the category/mechanism of shock. Overall, the algorithm performed well, and accurately predicted the category of shock in three‐quarters of patients. It also has the benefit of being very rapid, taking only seconds to complete, using bedside techniques that even inexperienced clinicians can apply.
Very few studies have examined the accuracy of examination techniques specifically for diagnosis of shock. In humans injected with endotoxin, body temperature and cardiac output increased, but skin temperature and capillary refill times are not well described.79 Schriger and Baraff10 reported that capillary refill >2 seconds was only 59% sensitive for diagnosing hypovolemia in patients with hypovolemic shock or orthostatic changes in blood pressure. Sensitivity was 77% in 13 patients with hypovolemic shock.10 However, some studies have demonstrated that age, sex, external temperature11 and fever12 can affect capillary refill times. Otieno et al.13 demonstrated a kappa statistic value of 0.49 for capillary refill 4 seconds, suggesting that reproducibility of this technique could be a major drawback. McGee et al.14 reviewed examination techniques for diagnosing hypovolemic states and concluded that postural changes in heart rate and blood pressure were the most accurate; capillary refill was not recommended. Stevenson and Perloff15 demonstrated that crackles and elevated JVP were absent in 18 of 43 patients with pulmonary capillary wedge pressures >22 mmHg. Butman et al.16 showed that elevated JVP was 82% accurate for predicting a wedge pressure >18 mmHg. Connors et al.17 demonstrated that clinicians' predictions of heart filling pressures and cardiac output were accurate (relative to pulmonary artery catheter measurements) in less than 50% of cases, though the examination techniques used were not qualified or quantified. No previous study has combined simple, semiobjective physical examination techniques for the purpose of distinguishing categories of shock.
Since identification of the pathogenesis of shock has important treatment/prognostic implications (eg, fluid and vasopressor therapies, early search for drainable focus of infection in sepsis, reestablishing vessel patency in myocardial infarction and pulmonary embolus), we believe that this simple, rapidly administered algorithm will prove useful in clinical medicine. In some clinical situations, the approach can lead to timely identification of the causative mechanism, allowing prompt definitive treatment. For example, a patient presenting with high‐output hypotension is so often sepsis/septic shock that treatment with antibiotics is justified (since success is time‐sensitive) even when the exact site/microbe has not yet been identified. Acute right heart overfilled low‐output hypotension should be considered pulmonary embolism (which also requires time‐sensitive therapies) until proven otherwise. Yet, a sizeable number of cases do not fit neatly into a single category. For example, 11% of patients with septic shock presented with cool extremities in the early phases of illness. In clinical decision‐making, 2 diagnostic‐therapeutic paradigms are common. In the first, the diagnosis is relatively certain and narrowly‐directed, mechanism‐specific treatment is appropriate. The second paradigm is 1 of significant uncertainty, when clinicians must treat empirically the most likely causes until more data become available to permit safe narrowing of therapies. For example, a patient presenting with hypotension, cool extremities, leukocytosis and apparent pneumonia should be treated empirically for septic shock while exploring explanations for the incongruous low‐output state (eg, profound hypovolemia, adrenal insufficiency, concurrent or precedent myocardial dysfunction). Patients often have several mechanisms contributing to hypotension. Since patients are not ideal forms, there can be no perfect decision‐tool; clinicians would be fool‐hardy to prematurely close decision‐making prior to definitive diagnosis. In the case of shock, such diagnostic arrogance would delay time‐sensitive therapies and thus contribute to morbidity and mortality. Nonetheless, this physical examination algorithmunderstanding its operating characteristics and limitationsmay add to the bedside clinician's diagnostic armamentarium.
Our study has several notable limitations. First, bedside examinations were performed by multiple observers who had limited (1 electronic mail) instruction on how to perform and document the data gathered for this study. So these results should be generalized cautiously until reproduced at other centers with greater numbers of observers (than the 28 of this study). The central supposition, that skin cooler, capillary refill longer, and pulse pressure more narrow than theirs, presupposes reasonable homogeneity of the normal state which is not necessarily true.11 Interobserver variability of physical examination further compromises the fidelity of findings recorded for this study.13 Since we conducted a retrospective review, and because of the emergency nature of the clinical problem, it would be difficult to conduct a study in which multiple examiners performed the same physical examinations to quantify interobserver variability. Irrespective, we would expect interobserver variability to systematically reduce accuracy; it is all‐the‐more impressive that trainees' examination results correctly diagnosed mechanism of shock in three‐quarters of cases. Also, examiners were not blinded to clinical history, so results of their examination could have been biased by their pre‐examination hypotheses of pathogenesis. Of course, they were not aware of the expert's final categorization of mechanism performed much later in time. Since there is no absolute reference standard for classification of the pathogenesis of shock, we depended upon careful review of selected data (same parameters for each patient) by a single senior investigatoralbeit armed with evidence‐based or consensus‐based standards of diagnosing shock. Finally, it can be argued that all forms of shock are mixed (with hypovolemia) early in the course; sepsis requires refilling of a leaky and dilated vasculature and the noncompliant ischemic ventricle often requires a higher filling pressure than normal to empty. To complicate even more, patients may have preexistent conditions (eg, chronic congestive heart failure, cirrhosis) that limit cardiovascular responses to acute shock. Our diagnostic approach was to identify the principal cause of the acute decompensation, assuming that many patients will have more than 1 single mechanism accounting for hypotension.
In conclusion, this is the first study to examine the utility of this simple physical examination algorithm to diagnose the mechanism of shock. Some have discounted or underemphasized examination techniques in favor of more time‐intensive and labor‐intensive diagnostic modalities, such as bedside echocardiography, which may waste precious time and resources. The simple physical examination algorithm assessed in this study has favorable operating characteristics and can be performed readily by even novice clinicians. If replicated at other centers and by greater numbers of observers, this approach could assist clinicians and teachers who train clinicians to rapidly diagnose and manage patients with shock.
Shock has been defined as failure to deliver and/or utilize adequate amounts of oxygen1 and is a common cause of critical illness. Few studies have examined the predictive utility of bedside clinical examination in predicting the category of shock. Scholars have suggested a bedside approach that uses simple examination techniques and applied physiology to rapidly identify a patients' circulation as high vs. low cardiac output. Those with a high‐output examination are designated as high‐output, most often septic shock. Low‐output patients are further categorized as heart full or heart empty to distinguish cardiogenic from hypovolemic categories of shock, respectively.2 The predictive characteristics of this simple algorithm have not been studied. In this study, we examine the operating characteristics of selected elements of this algorithm when administered at the bedside by trainees in Internal Medicine.
Methods
This study was performed after approval of the Institutional Review Board; informed consent was waived. Patients with nonsurgical problems who present to the hospital or who develop sustained hypotension are managed by medical house officers on the intensive care and/or rapid response team with the supervision of patients' attending physicians. All house officers were asked to document explicitly in their assessment notes the following examination findings: finger capillary refill (same/quicker vs. slower than examiner's), hand skin temperature (same/warmer vs. cooler than examiner's) and pulse pressure (ie, same/wider vs. thinner than examiner's), presence or absence of crackles >1/3 from base on bilateral lung examination and jugular venous pressure (JVP) vs. 8 cmH2O. The documented examinations of either the rapid response team (PGY2; n = 14) or intensive care unit (ICU) resident (PGY3; n = 14) for patients evaluated between September 2008 and February 2009 were used for this study. Resuscitation was administered entirely by house officers, occasionally guided in person, but always supervised by attending physicians.
In May 2009, clinical data, including electrocardiograms/echocardiograms and laboratory (eg, cardiac enzymes, culture) results were abstracted from medical records of subjects. These were presented to a blinded senior clinician (DK) to review and apply evidence‐based or consensus criteria,36 whenever possible, to categorize the type of shock (septic vs. cardiogenic vs. hypovolemic) based on data acquired after the onset of shock. For example, patients with microbiologic and/or radiologic evidence of infection were classified as septic shock,1, 3, 4 those with acute left or right ventricular dysfunction on echocardiogram were classified as cardiogenic shock,1, 6 and those with clinical evidence of acute hemorrhage with hypovolemic shock.1, 5 While some of the patients were examined by DK as part of clinical care, he was blinded to the identity of patients and their algorithm‐related physical examination findings when he reviewed the abstracted data (>2 months after study closure) to adjudicate the final diagnosis of shock. These diagnoses were considered the reference standard for this study. The operating characteristics (sensitivity = true positive/true positive + false negative; specificity = true negative/true negative + false positive; negative predictive value (NPV) = true negative/all negatives; positive predictive value (PPV) = true positive/all positives; accuracy = true results/all results) were calculated for combinations of physical examination findings and correct final diagnosis (Figure 1).

Results
A total of 68 patients, averaging 71 16 years, were studied; 57% were male, and 66% were White, and 20% were Black. Table 1 lists characteristics of patients. A total of 37 patients were diagnosed as having septic shock, 11 had cardiogenic shock and 10 hypovolemic shock. Operating characteristics of the bedside examination techniques for predicting mechanism of shock are listed in Table 2. Capillary refill and skin temperature were 100% concordant yielding sensitivity of 89% (95% confidence interval [CI], 75‐97%), specificity of 68% (95% CI, 46‐83%), PPV of 77% (95% CI, 61‐88%), NPV of 84% (95% CI, 64‐96%) and overall accuracy of 79% (95% CI, 68‐88%) for diagnosis of high output (ie, septic shock). JVP 8 cmH2O was more accurate than crackles for predicting cardiogenic shock in low‐output patients with sensitivity of 82% (95% CI, 48‐98%), specificity of 79% (95% CI, 41‐95%), PPV of 75% (95% CI, 43‐95%), NPV of 85% (95% CI, 55‐98%), and overall accuracy of 80% (95%CI, 59‐93%). Using just skin temperature and JVP, the bedside approach misdiagnosed 19 of 75 cases (overall accuracy, 75%; 95% CI, 16‐37%).
| n Total | |
|---|---|
| |
| Gender, n (%) | n = 68 |
| Male | 39 (57) |
| Age, years | 71 16 |
| Race, n (%) | |
| White | 45 (66) |
| Black | 15 (22) |
| Hispanic | 7 (10) |
| Other | 1 (2) |
| High output, n (%) | n = 37 |
| Sepsis | |
| Pneumonia | 10 (27) |
| Urinary tract | 17 (46) |
| Skin | 3 (8) |
| Gastrointestinal | 5 (14) |
| Non‐infectious SIRS | 2 (5) |
| Low output heart full, n (%) | n = 18 |
| Pulmonary embolism | 3 (16) |
| AMI | 7 (40) |
| Cardiomyopathy | 5 (28) |
| Rhythm disturbance | 3 (16) |
| Low output heart empty, n (%) | n = 13 |
| Hemorrhagic | 9 (70) |
| NPO | 1 (7) |
| Diarrhea | 2 (14) |
| Adrenal crisis | 1 (7) |
| Prediction of SIRS | Capillary Refill Same/Faster (%) | Skin Same/ Warm (%) | Bounding Pulses (%) |
|---|---|---|---|
| |||
| Sensitivity | 89 | 89 | 65 |
| Specificity | 68 | 68 | 74 |
| Accuracy | 79 | 79 | 69 |
| Prediction of SIRS | Capillary Refill Same/Faster + Warm Skin + Bounding Pulse (%) | Capillary Refill Same/Faster + Warm Skin (%) | Any Other Combination of 2 (%) |
| Sensitivity | 62 | 89 | 62 |
| Specificity | 74 | 68 | 74 |
| Accuracy | 67 | 79 | 67 |
| Prediction of Cardiogenic | JVP (%) | Crackles (%) | JVP + Crackles (%) |
| Sensitivity | 82 | 55 | 55 |
| Specificity | 79 | 71 | 100 |
| Accuracy | 80 | 64 | 80 |
Discussion
This is the first study to examine the predictive characteristics of simple bedside physical examination techniques in correctly predicting the category/mechanism of shock. Overall, the algorithm performed well, and accurately predicted the category of shock in three‐quarters of patients. It also has the benefit of being very rapid, taking only seconds to complete, using bedside techniques that even inexperienced clinicians can apply.
Very few studies have examined the accuracy of examination techniques specifically for diagnosis of shock. In humans injected with endotoxin, body temperature and cardiac output increased, but skin temperature and capillary refill times are not well described.79 Schriger and Baraff10 reported that capillary refill >2 seconds was only 59% sensitive for diagnosing hypovolemia in patients with hypovolemic shock or orthostatic changes in blood pressure. Sensitivity was 77% in 13 patients with hypovolemic shock.10 However, some studies have demonstrated that age, sex, external temperature11 and fever12 can affect capillary refill times. Otieno et al.13 demonstrated a kappa statistic value of 0.49 for capillary refill 4 seconds, suggesting that reproducibility of this technique could be a major drawback. McGee et al.14 reviewed examination techniques for diagnosing hypovolemic states and concluded that postural changes in heart rate and blood pressure were the most accurate; capillary refill was not recommended. Stevenson and Perloff15 demonstrated that crackles and elevated JVP were absent in 18 of 43 patients with pulmonary capillary wedge pressures >22 mmHg. Butman et al.16 showed that elevated JVP was 82% accurate for predicting a wedge pressure >18 mmHg. Connors et al.17 demonstrated that clinicians' predictions of heart filling pressures and cardiac output were accurate (relative to pulmonary artery catheter measurements) in less than 50% of cases, though the examination techniques used were not qualified or quantified. No previous study has combined simple, semiobjective physical examination techniques for the purpose of distinguishing categories of shock.
Since identification of the pathogenesis of shock has important treatment/prognostic implications (eg, fluid and vasopressor therapies, early search for drainable focus of infection in sepsis, reestablishing vessel patency in myocardial infarction and pulmonary embolus), we believe that this simple, rapidly administered algorithm will prove useful in clinical medicine. In some clinical situations, the approach can lead to timely identification of the causative mechanism, allowing prompt definitive treatment. For example, a patient presenting with high‐output hypotension is so often sepsis/septic shock that treatment with antibiotics is justified (since success is time‐sensitive) even when the exact site/microbe has not yet been identified. Acute right heart overfilled low‐output hypotension should be considered pulmonary embolism (which also requires time‐sensitive therapies) until proven otherwise. Yet, a sizeable number of cases do not fit neatly into a single category. For example, 11% of patients with septic shock presented with cool extremities in the early phases of illness. In clinical decision‐making, 2 diagnostic‐therapeutic paradigms are common. In the first, the diagnosis is relatively certain and narrowly‐directed, mechanism‐specific treatment is appropriate. The second paradigm is 1 of significant uncertainty, when clinicians must treat empirically the most likely causes until more data become available to permit safe narrowing of therapies. For example, a patient presenting with hypotension, cool extremities, leukocytosis and apparent pneumonia should be treated empirically for septic shock while exploring explanations for the incongruous low‐output state (eg, profound hypovolemia, adrenal insufficiency, concurrent or precedent myocardial dysfunction). Patients often have several mechanisms contributing to hypotension. Since patients are not ideal forms, there can be no perfect decision‐tool; clinicians would be fool‐hardy to prematurely close decision‐making prior to definitive diagnosis. In the case of shock, such diagnostic arrogance would delay time‐sensitive therapies and thus contribute to morbidity and mortality. Nonetheless, this physical examination algorithmunderstanding its operating characteristics and limitationsmay add to the bedside clinician's diagnostic armamentarium.
Our study has several notable limitations. First, bedside examinations were performed by multiple observers who had limited (1 electronic mail) instruction on how to perform and document the data gathered for this study. So these results should be generalized cautiously until reproduced at other centers with greater numbers of observers (than the 28 of this study). The central supposition, that skin cooler, capillary refill longer, and pulse pressure more narrow than theirs, presupposes reasonable homogeneity of the normal state which is not necessarily true.11 Interobserver variability of physical examination further compromises the fidelity of findings recorded for this study.13 Since we conducted a retrospective review, and because of the emergency nature of the clinical problem, it would be difficult to conduct a study in which multiple examiners performed the same physical examinations to quantify interobserver variability. Irrespective, we would expect interobserver variability to systematically reduce accuracy; it is all‐the‐more impressive that trainees' examination results correctly diagnosed mechanism of shock in three‐quarters of cases. Also, examiners were not blinded to clinical history, so results of their examination could have been biased by their pre‐examination hypotheses of pathogenesis. Of course, they were not aware of the expert's final categorization of mechanism performed much later in time. Since there is no absolute reference standard for classification of the pathogenesis of shock, we depended upon careful review of selected data (same parameters for each patient) by a single senior investigatoralbeit armed with evidence‐based or consensus‐based standards of diagnosing shock. Finally, it can be argued that all forms of shock are mixed (with hypovolemia) early in the course; sepsis requires refilling of a leaky and dilated vasculature and the noncompliant ischemic ventricle often requires a higher filling pressure than normal to empty. To complicate even more, patients may have preexistent conditions (eg, chronic congestive heart failure, cirrhosis) that limit cardiovascular responses to acute shock. Our diagnostic approach was to identify the principal cause of the acute decompensation, assuming that many patients will have more than 1 single mechanism accounting for hypotension.
In conclusion, this is the first study to examine the utility of this simple physical examination algorithm to diagnose the mechanism of shock. Some have discounted or underemphasized examination techniques in favor of more time‐intensive and labor‐intensive diagnostic modalities, such as bedside echocardiography, which may waste precious time and resources. The simple physical examination algorithm assessed in this study has favorable operating characteristics and can be performed readily by even novice clinicians. If replicated at other centers and by greater numbers of observers, this approach could assist clinicians and teachers who train clinicians to rapidly diagnose and manage patients with shock.
- ,,, et al.Hemodynamic monitoring in shock and implications for management. International consensus conference. Paris, France, 27–28 April 2006.Intensive Care Med.2007;33:575–590.
- .The pathophysiology of the circulation in critical illness. In:Principles of Critical Care.New York:McGraw Hill;2005.
- ,,, et al.2001 SCCM/ESICM/ACCP/ATS/SIS. International Sepsis Definitions Conference.Crit Care Med.2003;31:1250–1256.
- ,,, et al.Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.Chest.1992;101:1644–1655;
- ,,, et al.Resuscitation from severe hemorrhage.Crit Care Med.1996;24(2 Suppl):S12–S23.
- ,.Cardiogenic shock: current concepts and improving outcomes circulation.Circulation.2008;117:686–697.
- ,,, et al.The cardiovascular response of normal humans to the administration of endotoxin.N Engl J Med.1989;321:280–287.
- ,,,,,.Experimental endotoxemia in humans: análysis of cytokine release and coagulation, fibrinolytic, an complement pathways.Blood.1990;76:2520–2526.
- ,,,,.Peripheral resistance changes during shock in man.Angiology.1968;19:268–276.
- ,.Capillary refill—is it a useful predictor of hypovolemic states?Ann Emerg Med.1991;20:601–605.
- ,.Defining normal capillary refill: variation with age, sex, and temperature.Ann Emerg Med.1988;17:113–116.
- ,,,.Effect of fever on capillary refill time.Pediatr Emerg Care.1997;13:305–307.
- ,,,,,.Are bedside features of shock reproducible between different observers?Arch Dis Child.2004;89:977–979.
- ,,.Is this patient hypovolemic?JAMA.1999;281:1022–1029.
- ,.The limited reliability of physical signs for estimating hemodynamics in chronic heart failure.JAMA.1989;261:884–888.
- ,,,,.Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension.J Am Coll Cardiol.1993;22:968–974.
- ,,.Evaluation of right‐heart catheterization in the critically ill patients without acute myocardial infarction.N Engl J Med.1983;308(5):263–267.
- ,,, et al.Hemodynamic monitoring in shock and implications for management. International consensus conference. Paris, France, 27–28 April 2006.Intensive Care Med.2007;33:575–590.
- .The pathophysiology of the circulation in critical illness. In:Principles of Critical Care.New York:McGraw Hill;2005.
- ,,, et al.2001 SCCM/ESICM/ACCP/ATS/SIS. International Sepsis Definitions Conference.Crit Care Med.2003;31:1250–1256.
- ,,, et al.Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis.Chest.1992;101:1644–1655;
- ,,, et al.Resuscitation from severe hemorrhage.Crit Care Med.1996;24(2 Suppl):S12–S23.
- ,.Cardiogenic shock: current concepts and improving outcomes circulation.Circulation.2008;117:686–697.
- ,,, et al.The cardiovascular response of normal humans to the administration of endotoxin.N Engl J Med.1989;321:280–287.
- ,,,,,.Experimental endotoxemia in humans: análysis of cytokine release and coagulation, fibrinolytic, an complement pathways.Blood.1990;76:2520–2526.
- ,,,,.Peripheral resistance changes during shock in man.Angiology.1968;19:268–276.
- ,.Capillary refill—is it a useful predictor of hypovolemic states?Ann Emerg Med.1991;20:601–605.
- ,.Defining normal capillary refill: variation with age, sex, and temperature.Ann Emerg Med.1988;17:113–116.
- ,,,.Effect of fever on capillary refill time.Pediatr Emerg Care.1997;13:305–307.
- ,,,,,.Are bedside features of shock reproducible between different observers?Arch Dis Child.2004;89:977–979.
- ,,.Is this patient hypovolemic?JAMA.1999;281:1022–1029.
- ,.The limited reliability of physical signs for estimating hemodynamics in chronic heart failure.JAMA.1989;261:884–888.
- ,,,,.Bedside cardiovascular examination in patients with severe chronic heart failure: importance of rest or inducible jugular venous distension.J Am Coll Cardiol.1993;22:968–974.
- ,,.Evaluation of right‐heart catheterization in the critically ill patients without acute myocardial infarction.N Engl J Med.1983;308(5):263–267.