User login
Summary of guidelines published in the Journal of Hospital Medicine
When on call for admissions, a hospitalist receives a request from a colleague to admit an octogenarian man with an acute uncomplicated deep vein thrombosis to start heparin, bridging to warfarin. The patient has no evidence of postphlebitic syndrome, pulmonary embolism, or right-sided heart strain. The hospitalist asks her colleague if he had considered treating the patient in the ambulatory setting using a direct-acting oral anticoagulant (DOAC). After all, this would save the patient an unnecessary hospitalization, weekly international normalized ratio checks, and other important lifestyle changes. In response, the colleague voices concern that the “new drugs don’t have antidotes.”
DOACs have several benefits over vitamin K antagonists (VKAs) and heparins. DOACs have quicker onset of action, can be taken by mouth, in general do not require dosage adjustment, and have fewer dietary and lifestyle modifications, compared with VKAs and heparins. In atrial fibrillation, DOACs have been shown to have lower all-cause and bleeding-related mortality than warfarin (see Table 1).1 Observational studies also suggest less risk of major bleeding with DOACs over warfarin but no difference in overall mortality when used to treat venous thromboembolism (see Table 2).2 Because of these combined advantages, DOACs are increasingly prescribed, accounting for approximately half of all oral anticoagulant prescriptions in 2014.3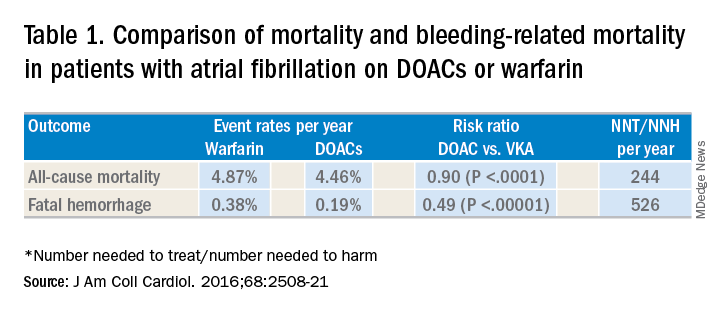
Although DOACs have been shown to be as good if not superior to VKAs and heparins in these circumstances, there are situations where a DOAC should not be used. There is limited data on the safety of DOACs in patients with mechanical heart valves, liver failure, and chronic kidney disease with a creatinine clearance less than 30 mL/min.4 Therefore, warfarin is still the preferred agent in these settings. There is some data that apixaban may be safe in patients with a creatinine clearance of greater than 10 mL/min, but long-term safety studies have not been performed in patients with end-stage renal disease on hemodialysis.5 Finally, in patients requiring concomitant inducers or inhibitors of the P-glycoprotein or cytochrome P450 enzymes like antiepileptics and protease inhibitors, VKAs and heparins are favored.4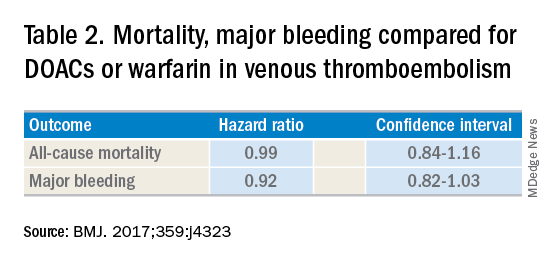
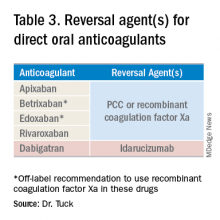
Notwithstanding their advantages, when DOACs first hit the market there were concerns that reversal agents were not available. In the August issue of the Journal of Hospital Medicine’s Clinical Guideline Highlights for the Hospitalist, Emily Gottenborg, MD, and Gregory Misky, MD, summarized guideline recommendations for reversal of the newer agents.6 This includes use of idarucizumab for patients on dabigatran and use of prothrombin complex concentrate (PCC) or recombinant coagulation factor Xa (andexanet alfa) for patients on apixaban or rivaroxaban for the treatment of life-threatening bleeding.
Idarucizumab is a monoclonal antibody developed to reverse the effects of dabigatran, the only DOAC that directly inhibits thrombin. In 2017, researchers reported on a cohort of subjects receiving idarucizumab for uncontrolled bleeding or who were on dabigatran and about to undergo an urgent procedure.7 Of those with uncontrolled bleeding, two-thirds had confirmed bleeding cessation within 24 hours. Periprocedural hemostasis was achieved in 93.4% of patients undergoing urgent procedures. However, it should be noted that use of idarucizumab conferred an increase risk (6.3%) of thrombosis within 90 days. Based on these findings, guidelines recommend use of idarucizumab in patients experiencing life-threatening bleeding, balanced against the risk of thrombosis.8
In 2018, the Food and Drug Administration approved recombinant coagulation factor Xa for treatment of life-threatening or uncontrolled bleeding in patients on apixaban or rivaroxaban.9 The approval came after a study by the ANNEXA-4 investigators showed that recombinant coagulation factor Xa quickly and effectively achieved hemostasis.10 Full study results were published in April 2019, demonstrating 82% of patients receiving the drug attained clinical hemostasis.11 However, as with idarucizumab, up to 10% of patients had a thrombotic event in the follow-up period. Use of recombinant coagulation factor Xa for treatment of life-threatening bleeding related to betrixaban and edoxaban is considered off label but is recommended by guidelines.8 Studies on investigational reversal agents for betrixaban and edoxaban are ongoing.
Both unactivated and activated PCC contain clotting factor X. Their use to control bleeding related to DOAC use is based on observational studies. In a systematic review of the nonrandomized studies, the efficacy of PCC to stem major bleeding was 69% and the risk for thromboembolism was 4%.12 There are no head-to-head studies comparing use of recombinant coagulation factor Xa and PCC. Therefore, guidelines are to use either recombinant factor Xa or PCC for the treatment of life-threatening bleeding related to DOAC use.7
As thrombosis risk heightens after use of any reversal agent, the recommendations are to resume anticoagulation within 90 days if the patient is at moderate or high risk for recurrent thromboembolism.8
After discussion with the hospitalist about the new agents available to reverse anticoagulation, the colleague decided to place the patient on a DOAC and keep the patient in his nursing home. Thankfully, the patient did not thereafter experience sustained bleeding necessitating use of these reversal agents. More importantly for the patient, he was able to stay in the comfort of his home.
Dr. Tuck is associate section chief for hospital medicine at the Veterans Affairs Medical Center in Washington, D.C.
References
1. Gómez-Outes A et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508-21.
2. Jun M et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population-based, observational study. BMJ. 2017;359:j4323.
3. Barnes GD et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:(1300-5).e2.
4. Reddy P et al. Practical approach to VTE management in hospitalized patients. Am J Ther. 2017;24(4):e442-67.
5. Kimachi M et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017 Nov 6;11:CD011373.
6. Gottenborg E et al. Clinical guideline highlights for the hospitalist: The management of anticoagulation in the hospitalized adult. J Hosp Med. 2019; 14(8):499-500.
7. Pollack CV Jr et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med. 2017;377(5):431-41.
8. Witt DM et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-91.
9. Malarky M et al. FDA accelerated approval letter. Retrieved July 15, 2019. https://www.fda.gov/media/113285/download
10. Connolly SJ et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-41.
11. Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med. 2019;380(14):1326-35.
12. Piran S et al. Management of direct factor Xa inhibitor–related major bleeding with prothrombin complex concentrate: A meta-analysis. Blood Adv. 2019;3(2):158-67.
Summary of guidelines published in the Journal of Hospital Medicine
Summary of guidelines published in the Journal of Hospital Medicine
When on call for admissions, a hospitalist receives a request from a colleague to admit an octogenarian man with an acute uncomplicated deep vein thrombosis to start heparin, bridging to warfarin. The patient has no evidence of postphlebitic syndrome, pulmonary embolism, or right-sided heart strain. The hospitalist asks her colleague if he had considered treating the patient in the ambulatory setting using a direct-acting oral anticoagulant (DOAC). After all, this would save the patient an unnecessary hospitalization, weekly international normalized ratio checks, and other important lifestyle changes. In response, the colleague voices concern that the “new drugs don’t have antidotes.”
DOACs have several benefits over vitamin K antagonists (VKAs) and heparins. DOACs have quicker onset of action, can be taken by mouth, in general do not require dosage adjustment, and have fewer dietary and lifestyle modifications, compared with VKAs and heparins. In atrial fibrillation, DOACs have been shown to have lower all-cause and bleeding-related mortality than warfarin (see Table 1).1 Observational studies also suggest less risk of major bleeding with DOACs over warfarin but no difference in overall mortality when used to treat venous thromboembolism (see Table 2).2 Because of these combined advantages, DOACs are increasingly prescribed, accounting for approximately half of all oral anticoagulant prescriptions in 2014.3
Although DOACs have been shown to be as good if not superior to VKAs and heparins in these circumstances, there are situations where a DOAC should not be used. There is limited data on the safety of DOACs in patients with mechanical heart valves, liver failure, and chronic kidney disease with a creatinine clearance less than 30 mL/min.4 Therefore, warfarin is still the preferred agent in these settings. There is some data that apixaban may be safe in patients with a creatinine clearance of greater than 10 mL/min, but long-term safety studies have not been performed in patients with end-stage renal disease on hemodialysis.5 Finally, in patients requiring concomitant inducers or inhibitors of the P-glycoprotein or cytochrome P450 enzymes like antiepileptics and protease inhibitors, VKAs and heparins are favored.4
Notwithstanding their advantages, when DOACs first hit the market there were concerns that reversal agents were not available. In the August issue of the Journal of Hospital Medicine’s Clinical Guideline Highlights for the Hospitalist, Emily Gottenborg, MD, and Gregory Misky, MD, summarized guideline recommendations for reversal of the newer agents.6 This includes use of idarucizumab for patients on dabigatran and use of prothrombin complex concentrate (PCC) or recombinant coagulation factor Xa (andexanet alfa) for patients on apixaban or rivaroxaban for the treatment of life-threatening bleeding.
Idarucizumab is a monoclonal antibody developed to reverse the effects of dabigatran, the only DOAC that directly inhibits thrombin. In 2017, researchers reported on a cohort of subjects receiving idarucizumab for uncontrolled bleeding or who were on dabigatran and about to undergo an urgent procedure.7 Of those with uncontrolled bleeding, two-thirds had confirmed bleeding cessation within 24 hours. Periprocedural hemostasis was achieved in 93.4% of patients undergoing urgent procedures. However, it should be noted that use of idarucizumab conferred an increase risk (6.3%) of thrombosis within 90 days. Based on these findings, guidelines recommend use of idarucizumab in patients experiencing life-threatening bleeding, balanced against the risk of thrombosis.8
In 2018, the Food and Drug Administration approved recombinant coagulation factor Xa for treatment of life-threatening or uncontrolled bleeding in patients on apixaban or rivaroxaban.9 The approval came after a study by the ANNEXA-4 investigators showed that recombinant coagulation factor Xa quickly and effectively achieved hemostasis.10 Full study results were published in April 2019, demonstrating 82% of patients receiving the drug attained clinical hemostasis.11 However, as with idarucizumab, up to 10% of patients had a thrombotic event in the follow-up period. Use of recombinant coagulation factor Xa for treatment of life-threatening bleeding related to betrixaban and edoxaban is considered off label but is recommended by guidelines.8 Studies on investigational reversal agents for betrixaban and edoxaban are ongoing.
Both unactivated and activated PCC contain clotting factor X. Their use to control bleeding related to DOAC use is based on observational studies. In a systematic review of the nonrandomized studies, the efficacy of PCC to stem major bleeding was 69% and the risk for thromboembolism was 4%.12 There are no head-to-head studies comparing use of recombinant coagulation factor Xa and PCC. Therefore, guidelines are to use either recombinant factor Xa or PCC for the treatment of life-threatening bleeding related to DOAC use.7
As thrombosis risk heightens after use of any reversal agent, the recommendations are to resume anticoagulation within 90 days if the patient is at moderate or high risk for recurrent thromboembolism.8
After discussion with the hospitalist about the new agents available to reverse anticoagulation, the colleague decided to place the patient on a DOAC and keep the patient in his nursing home. Thankfully, the patient did not thereafter experience sustained bleeding necessitating use of these reversal agents. More importantly for the patient, he was able to stay in the comfort of his home.
Dr. Tuck is associate section chief for hospital medicine at the Veterans Affairs Medical Center in Washington, D.C.
References
1. Gómez-Outes A et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508-21.
2. Jun M et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population-based, observational study. BMJ. 2017;359:j4323.
3. Barnes GD et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:(1300-5).e2.
4. Reddy P et al. Practical approach to VTE management in hospitalized patients. Am J Ther. 2017;24(4):e442-67.
5. Kimachi M et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017 Nov 6;11:CD011373.
6. Gottenborg E et al. Clinical guideline highlights for the hospitalist: The management of anticoagulation in the hospitalized adult. J Hosp Med. 2019; 14(8):499-500.
7. Pollack CV Jr et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med. 2017;377(5):431-41.
8. Witt DM et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-91.
9. Malarky M et al. FDA accelerated approval letter. Retrieved July 15, 2019. https://www.fda.gov/media/113285/download
10. Connolly SJ et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-41.
11. Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med. 2019;380(14):1326-35.
12. Piran S et al. Management of direct factor Xa inhibitor–related major bleeding with prothrombin complex concentrate: A meta-analysis. Blood Adv. 2019;3(2):158-67.
When on call for admissions, a hospitalist receives a request from a colleague to admit an octogenarian man with an acute uncomplicated deep vein thrombosis to start heparin, bridging to warfarin. The patient has no evidence of postphlebitic syndrome, pulmonary embolism, or right-sided heart strain. The hospitalist asks her colleague if he had considered treating the patient in the ambulatory setting using a direct-acting oral anticoagulant (DOAC). After all, this would save the patient an unnecessary hospitalization, weekly international normalized ratio checks, and other important lifestyle changes. In response, the colleague voices concern that the “new drugs don’t have antidotes.”
DOACs have several benefits over vitamin K antagonists (VKAs) and heparins. DOACs have quicker onset of action, can be taken by mouth, in general do not require dosage adjustment, and have fewer dietary and lifestyle modifications, compared with VKAs and heparins. In atrial fibrillation, DOACs have been shown to have lower all-cause and bleeding-related mortality than warfarin (see Table 1).1 Observational studies also suggest less risk of major bleeding with DOACs over warfarin but no difference in overall mortality when used to treat venous thromboembolism (see Table 2).2 Because of these combined advantages, DOACs are increasingly prescribed, accounting for approximately half of all oral anticoagulant prescriptions in 2014.3
Although DOACs have been shown to be as good if not superior to VKAs and heparins in these circumstances, there are situations where a DOAC should not be used. There is limited data on the safety of DOACs in patients with mechanical heart valves, liver failure, and chronic kidney disease with a creatinine clearance less than 30 mL/min.4 Therefore, warfarin is still the preferred agent in these settings. There is some data that apixaban may be safe in patients with a creatinine clearance of greater than 10 mL/min, but long-term safety studies have not been performed in patients with end-stage renal disease on hemodialysis.5 Finally, in patients requiring concomitant inducers or inhibitors of the P-glycoprotein or cytochrome P450 enzymes like antiepileptics and protease inhibitors, VKAs and heparins are favored.4
Notwithstanding their advantages, when DOACs first hit the market there were concerns that reversal agents were not available. In the August issue of the Journal of Hospital Medicine’s Clinical Guideline Highlights for the Hospitalist, Emily Gottenborg, MD, and Gregory Misky, MD, summarized guideline recommendations for reversal of the newer agents.6 This includes use of idarucizumab for patients on dabigatran and use of prothrombin complex concentrate (PCC) or recombinant coagulation factor Xa (andexanet alfa) for patients on apixaban or rivaroxaban for the treatment of life-threatening bleeding.
Idarucizumab is a monoclonal antibody developed to reverse the effects of dabigatran, the only DOAC that directly inhibits thrombin. In 2017, researchers reported on a cohort of subjects receiving idarucizumab for uncontrolled bleeding or who were on dabigatran and about to undergo an urgent procedure.7 Of those with uncontrolled bleeding, two-thirds had confirmed bleeding cessation within 24 hours. Periprocedural hemostasis was achieved in 93.4% of patients undergoing urgent procedures. However, it should be noted that use of idarucizumab conferred an increase risk (6.3%) of thrombosis within 90 days. Based on these findings, guidelines recommend use of idarucizumab in patients experiencing life-threatening bleeding, balanced against the risk of thrombosis.8
In 2018, the Food and Drug Administration approved recombinant coagulation factor Xa for treatment of life-threatening or uncontrolled bleeding in patients on apixaban or rivaroxaban.9 The approval came after a study by the ANNEXA-4 investigators showed that recombinant coagulation factor Xa quickly and effectively achieved hemostasis.10 Full study results were published in April 2019, demonstrating 82% of patients receiving the drug attained clinical hemostasis.11 However, as with idarucizumab, up to 10% of patients had a thrombotic event in the follow-up period. Use of recombinant coagulation factor Xa for treatment of life-threatening bleeding related to betrixaban and edoxaban is considered off label but is recommended by guidelines.8 Studies on investigational reversal agents for betrixaban and edoxaban are ongoing.
Both unactivated and activated PCC contain clotting factor X. Their use to control bleeding related to DOAC use is based on observational studies. In a systematic review of the nonrandomized studies, the efficacy of PCC to stem major bleeding was 69% and the risk for thromboembolism was 4%.12 There are no head-to-head studies comparing use of recombinant coagulation factor Xa and PCC. Therefore, guidelines are to use either recombinant factor Xa or PCC for the treatment of life-threatening bleeding related to DOAC use.7
As thrombosis risk heightens after use of any reversal agent, the recommendations are to resume anticoagulation within 90 days if the patient is at moderate or high risk for recurrent thromboembolism.8
After discussion with the hospitalist about the new agents available to reverse anticoagulation, the colleague decided to place the patient on a DOAC and keep the patient in his nursing home. Thankfully, the patient did not thereafter experience sustained bleeding necessitating use of these reversal agents. More importantly for the patient, he was able to stay in the comfort of his home.
Dr. Tuck is associate section chief for hospital medicine at the Veterans Affairs Medical Center in Washington, D.C.
References
1. Gómez-Outes A et al. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2016;68:2508-21.
2. Jun M et al. Comparative safety of direct oral anticoagulants and warfarin in venous thromboembolism: multicentre, population-based, observational study. BMJ. 2017;359:j4323.
3. Barnes GD et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:(1300-5).e2.
4. Reddy P et al. Practical approach to VTE management in hospitalized patients. Am J Ther. 2017;24(4):e442-67.
5. Kimachi M et al. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. 2017 Nov 6;11:CD011373.
6. Gottenborg E et al. Clinical guideline highlights for the hospitalist: The management of anticoagulation in the hospitalized adult. J Hosp Med. 2019; 14(8):499-500.
7. Pollack CV Jr et al. Idarucizumab for dabigatran reversal – full cohort analysis. N Engl J Med. 2017;377(5):431-41.
8. Witt DM et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: Optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257-91.
9. Malarky M et al. FDA accelerated approval letter. Retrieved July 15, 2019. https://www.fda.gov/media/113285/download
10. Connolly SJ et al. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131-41.
11. Connolly SJ et al. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med. 2019;380(14):1326-35.
12. Piran S et al. Management of direct factor Xa inhibitor–related major bleeding with prothrombin complex concentrate: A meta-analysis. Blood Adv. 2019;3(2):158-67.