User login
Hyperuricemia is a metabolic problem that has become quite common over the past several decades. The main clinical issues associated with hyperuricemia are gouty arthritis, gouty tophi, and uric acid kidney stones. For decades, these have been the main indications for lowering serum urate levels. Well-established nonarticular associations of hyperuricemia in gout include chronic kidney disease, coronary artery disease, and hypertension.1 Recent animal studies and epidemiologic studies have shed new light on the relationship between urate and comorbid disease processes. This article describes our evolving understanding of the association of hyperuricemia and gout with kidney disease, hypertension, and cardiovascular disease, and also reviews the kidney’s role in regulation of urate levels in the body.
SOURCES, DISTRIBUTION, AND ELIMINATION OF URATE
Uric acid is the end product of purine metabolism in humans. Sources of purine are either endogenous, from de novo synthesis and nucleic acid breakdown (approximately 600 mg/day), or exogenous, from dietary purine intake (approximately 100 mg/day).2 In the steady state, this daily production and ingestion of approximately 700 mg of uric acid is balanced by daily elimination of an equal amount of uric acid from the body. Approximately 30% of this daily loss is through the gut, with subsequent bacterial intestinal uricolysis. The other 70% (roughly 500 mg daily) needs to be excreted by the kidneys.
In humans, plasma urate is freely filtered at the glomerulus, but the fractional excretion of the filtered uric acid is less than 10%. This demonstrates a dominance of reabsorptive processes in humans, and these processes are handled primarily by the selective urate transport protein known as URAT1 in the proximal convoluted tubules. In normouricemic individuals, there is a balance between the daily production and ingestion of uric acid and the daily excretion of it. In most patients with primary hyperuricemia and gout, the fractional excretion of uric acid by the kidneys is relatively diminished, resulting in an imbalance of uric acid homeostasis, and serum urate exceeds saturability (6.8 mg/dL at physiologic temperature and pH). As this imbalance persists over years and decades, the miscible serum uric acid pool expands and urate may be deposited as part of an insoluble urate pool in the joints and soft tissues, referred to as tophi.
In the past, most investigators have focused on the destructive and proinflammatory role of insoluble deposited urate crystals, but new evidence is accumulating that rising levels of soluble urate in body fluids may also be harmful and lead to kidney disease, hypertension, and cardiovascular disease.
RENAL MANIFESTATIONS OF HYPERURICEMIA
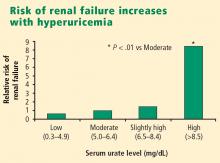
Urate is strongly associated with renal disease but traditionally has not been considered to have a causal role in kidney dysfunction. The exceptions have been uric acid kidney stones and the acute uric acid nephropathy associated with chemotherapy and tumor lysis syndrome. New epidemiologic studies in humans, as well as an animal model of mild hyperuricemia leading to microvascular changes in the glomerular afferent arterioles, shed new light on a possible direct role of urate in the genesis of idiopathic chronic kidney disease.
Longstanding reluctance to implicate urate in kidney disease
Significant impairment of renal function was reported in up to 40% of patients with gout in studies conducted before the advent of effective urate-lowering therapies.3,4 In these older studies, renal failure was the eventual cause of death in 18% to 25% of patients with gout.3,4 However, any primary causal relationship for urate in this very high incidence of kidney disease was questioned for decades in light of the many other conditions and factors associated with hyperuricemia and gout that may contribute to kidney disease, such as hypertension, diabetes mellitus, alcohol abuse, non-steroidal anti-inflammatory drug use, and lead toxicity.
Recent epidemiologic studies establish the connection
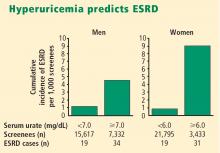
More recently, two large prospective population studies from Japan examined the relationship between serum urate level and development of kidney disease using multiple covariate analysis to adjust for age, blood pressure, body mass index, proteinuria, hematocrit, hyperlipidemia, fasting glucose, and serum creatinine.5,6
ASSOCIATION OF HYPERTENSION AND HYPERURICEMIA
The strong association between hypertension and hyperuricemia has been recognized for more than a century. In his original description of essential hypertension in 1879, Frederick A. Mohamed noted that many of his subjects came from gouty families.7 Studies from the 1950s and 1960s showed the prevalence of hyperuricemia in hypertensive subjects to be between 20% and 40%.8,9 The prevalence of hypertension among gouty patients is Hyperuricemia predicts ESRD between 25% and 50%.10 In 1972, Kahn et al found that a rising level of serum urate is an independent risk factor for hypertension.11 A year later, Klein et al demonstrated a linear relationship between serum urate level and systolic blood pressure in both black and white subjects.12
Six large epidemiologic studies published over the past 7 years have found that serum urate level predicts the later development of hypertension.13–18 The most recent of these is the Normative Aging Study,18 which showed that the serum urate level independently predicts the development of hypertension when using age-adjusted and multivariate models that include body mass, abdominal girth, alcohol use, serum lipid levels, plasma glucose level, and smoking status.
A potential mechanism emerges
No clear causal or mechanistic link between elevated serum urate and the development of hypertension was evident until a rat model of mild hyperuricemia was found to be associated with the development of an initial salt-insensitive hypertension that was reversible with restoration of normal urate levels.19 However, if the urate-induced hypertension was allowed to persist, the rat would develop a salt-sensitive hypertension that was irreversible even if normouricemia was reestablished.19
This mechanism was tested in a small pilot study of 5 children with previously untreated essential hypertension who received monotherapy with allopurinol for 1 month.20 All 5 children had substantial drops in their continuously monitored ambulatory blood pressure, and 4 of the 5 developed normal blood pressure (as assessed by continuous monitoring). After the allopurinol was stopped, the blood pressure of all 5 children rebounded to baseline levels.20 A larger blinded, randomized, placebo-controlled crossover trial using the same intervention with similar results has been presented recently at national meetings.21
ASSOCIATION BETWEEN CARDIOVASCULAR DISEASE AND HYPERURICEMIA
There is considerable documentation that urate levels correlate with many recognized cardiovascular risk factors, including age, male gender, hypertension, diabetes mellitus, hypertriglyceridemia, obesity, and insulin resistance. Because of these relationships, the observed association between serum urate elevations and cardiovascular disease was considered to be “epiphenomenal” and not causal. Many older epidemiologic studies that demonstrated the increased cardiovascular risk associated with higher urate levels used traditional statistical techniques that could not prove the “independence” of urate as a risk factor.22
An independent effect is finally documented
Three studies published in the past several years demonstrate an independent risk relationship between hyperuricemia and cardiovascular disease, including acute myocardial infarction.23–25
Multivariate regression analysis of the Multiple Risk Factor Intervention Trial (MRFIT) database demonstrated hyperuricemia to be an independent risk factor for acute myocardial infarction.23
Similarly, the Italian Progetto Ipertensione Umbria Monitoraggio Ambulatoriale (PIUMA) study showed that after adjustment for age, sex, diabetes, serum lipid levels, serum creatinine, left ventricular hypertrophy, blood pressure, and diuretic use, serum urate levels in the highest quartile were associated with increased risk of all cardiovascular events (relative risk [RR] = 1.73) and fatal cardiovascular events (RR = 1.96) compared with urate levels in the second quartile.24
Finally, follow-up of 4,385 participants in the Rotterdam Study over an average of 8.4 years revealed that high urate levels were a strong predictor of both myocardial infarction and stroke, even after adjustment for other vascular risk factors.25
CONCLUSIONS
Based on the preponderance of recent epidemiologic studies, it appears that an elevated serum urate level is an independent risk factor for kidney disease, hypertension, and cardiovascular disease. The precise mechanisms for urate-induced tissue injury remain unclear, and no large study to date has convincingly demonstrated that lowering serum urate levels can prevent or lessen the risk of these potentially severe complications of hyperuricemia.
It should also be emphasized that the treatment of hyperuricemia with medications such as allopurinol or probenecid is currently not indicated in patients with hypertension, kidney disease, or heart disease. The use of these agents is supported only in the treatment of gout, the treatment of uric acid kidney stones, and the prevention of tumor lysis syndrome.
- Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR Jr, Saag KG. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis 2005; 64:267–272.
- Richards J, Weinman EJ. Uric acid and renal disease. J Nephrol 1966; 9:160–166.
- Berger L, Yü TF. Renal function in gout. IV. An analysis of 524 gouty subjects including long-term follow-up studies. Am J Med 1975; 59:605–613.
- Talbot JH, Terplan KL. The kidney in gout. Medicine 1960; 39:405–468.
- Tomita M, Mizuno S, Yamanaka H, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol 2000; 10:403–409.
- Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis 2004; 44:642–650.
- Mohamed FA. On chronic Bright’s disease, and its essential symptoms. Lancet 1879; 1:399–401.
- Weiss TE, Segaloff A, Moore C. Gout and diabetes. Metabolism 1957; 6:103.
- Kinsey D, Walther R, Sise HS, Whitelaw G, Smithwick R. Incidence of hyperuricemia in 400 hypertensive patients. Circulation 1961; 24:972.
- Becker MA, Jolly M. Hyperuricemia and associated diseases. Rheum Dis Clin North Am 2006; 32:275–293.
- Kahn HA, Medalie JH, Neufeld HN, Riss E, Goldbourt U. The incidence of hypertension and associated factors: the Israel ischemic heart disease study. Am Heart J 1972; 84:171–182.
- Klein R, Klein BE, Cornoni JC, Maready J, Cassel JC, Tyroler HA. Serum uric acid. Its relationships to coronary heart disease risk factors and cardiovascular disease, Evans County, Georgia. Arch Intern Med 1973; 132:401–410.
- Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or type II diabetes in Japanese male office workers. Eur J Epidemiol 2003; 18:523–530.
- Alper AB Jr, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension 2005; 45:34–38.
- Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 2003; 42:474–480.
- Nagahama K, Inoue T, Iseki K, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res 2004; 27:835–841.
- Sundström J, Sullivan L, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 2005; 45:28–33.
- Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the Normative Aging Study. Hypertension 2006; 48:1031–1036.
- Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001; 38:1101–1106.
- Feig DI, Nakagawa T, Karumanchi SA, et al. Hypothesis: uric acid, nephron number, and the pathogenesis of essential hypertension. Kidney Int 2004; 66:281–287.
- Feig DI, Mazzali M, Kang DH, et al. Serum uric acid: a risk factor and a target for treatment? J Am Soc Nephrol 2006; 17(4 Suppl):S69–S73.
- Rich MW. Uric acid: is it a risk factor for cardiovascular disease? Am J Cardiol 2000; 85:1018–1021.
- Krishnan E, Baker JF, Furst DE, Schumacher HR Jr. Gout and the risk of acute myocardial infarction. Arthritis Rheum 2006; 54:2688–2696.
- Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 2000; 36:1072–1078.
- Bos MJ , Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam Study. Stroke 2006; 37:1503–1507.
Hyperuricemia is a metabolic problem that has become quite common over the past several decades. The main clinical issues associated with hyperuricemia are gouty arthritis, gouty tophi, and uric acid kidney stones. For decades, these have been the main indications for lowering serum urate levels. Well-established nonarticular associations of hyperuricemia in gout include chronic kidney disease, coronary artery disease, and hypertension.1 Recent animal studies and epidemiologic studies have shed new light on the relationship between urate and comorbid disease processes. This article describes our evolving understanding of the association of hyperuricemia and gout with kidney disease, hypertension, and cardiovascular disease, and also reviews the kidney’s role in regulation of urate levels in the body.
SOURCES, DISTRIBUTION, AND ELIMINATION OF URATE
Uric acid is the end product of purine metabolism in humans. Sources of purine are either endogenous, from de novo synthesis and nucleic acid breakdown (approximately 600 mg/day), or exogenous, from dietary purine intake (approximately 100 mg/day).2 In the steady state, this daily production and ingestion of approximately 700 mg of uric acid is balanced by daily elimination of an equal amount of uric acid from the body. Approximately 30% of this daily loss is through the gut, with subsequent bacterial intestinal uricolysis. The other 70% (roughly 500 mg daily) needs to be excreted by the kidneys.
In humans, plasma urate is freely filtered at the glomerulus, but the fractional excretion of the filtered uric acid is less than 10%. This demonstrates a dominance of reabsorptive processes in humans, and these processes are handled primarily by the selective urate transport protein known as URAT1 in the proximal convoluted tubules. In normouricemic individuals, there is a balance between the daily production and ingestion of uric acid and the daily excretion of it. In most patients with primary hyperuricemia and gout, the fractional excretion of uric acid by the kidneys is relatively diminished, resulting in an imbalance of uric acid homeostasis, and serum urate exceeds saturability (6.8 mg/dL at physiologic temperature and pH). As this imbalance persists over years and decades, the miscible serum uric acid pool expands and urate may be deposited as part of an insoluble urate pool in the joints and soft tissues, referred to as tophi.
In the past, most investigators have focused on the destructive and proinflammatory role of insoluble deposited urate crystals, but new evidence is accumulating that rising levels of soluble urate in body fluids may also be harmful and lead to kidney disease, hypertension, and cardiovascular disease.
RENAL MANIFESTATIONS OF HYPERURICEMIA
Urate is strongly associated with renal disease but traditionally has not been considered to have a causal role in kidney dysfunction. The exceptions have been uric acid kidney stones and the acute uric acid nephropathy associated with chemotherapy and tumor lysis syndrome. New epidemiologic studies in humans, as well as an animal model of mild hyperuricemia leading to microvascular changes in the glomerular afferent arterioles, shed new light on a possible direct role of urate in the genesis of idiopathic chronic kidney disease.
Longstanding reluctance to implicate urate in kidney disease
Significant impairment of renal function was reported in up to 40% of patients with gout in studies conducted before the advent of effective urate-lowering therapies.3,4 In these older studies, renal failure was the eventual cause of death in 18% to 25% of patients with gout.3,4 However, any primary causal relationship for urate in this very high incidence of kidney disease was questioned for decades in light of the many other conditions and factors associated with hyperuricemia and gout that may contribute to kidney disease, such as hypertension, diabetes mellitus, alcohol abuse, non-steroidal anti-inflammatory drug use, and lead toxicity.
Recent epidemiologic studies establish the connection
More recently, two large prospective population studies from Japan examined the relationship between serum urate level and development of kidney disease using multiple covariate analysis to adjust for age, blood pressure, body mass index, proteinuria, hematocrit, hyperlipidemia, fasting glucose, and serum creatinine.5,6
ASSOCIATION OF HYPERTENSION AND HYPERURICEMIA
The strong association between hypertension and hyperuricemia has been recognized for more than a century. In his original description of essential hypertension in 1879, Frederick A. Mohamed noted that many of his subjects came from gouty families.7 Studies from the 1950s and 1960s showed the prevalence of hyperuricemia in hypertensive subjects to be between 20% and 40%.8,9 The prevalence of hypertension among gouty patients is Hyperuricemia predicts ESRD between 25% and 50%.10 In 1972, Kahn et al found that a rising level of serum urate is an independent risk factor for hypertension.11 A year later, Klein et al demonstrated a linear relationship between serum urate level and systolic blood pressure in both black and white subjects.12
Six large epidemiologic studies published over the past 7 years have found that serum urate level predicts the later development of hypertension.13–18 The most recent of these is the Normative Aging Study,18 which showed that the serum urate level independently predicts the development of hypertension when using age-adjusted and multivariate models that include body mass, abdominal girth, alcohol use, serum lipid levels, plasma glucose level, and smoking status.
A potential mechanism emerges
No clear causal or mechanistic link between elevated serum urate and the development of hypertension was evident until a rat model of mild hyperuricemia was found to be associated with the development of an initial salt-insensitive hypertension that was reversible with restoration of normal urate levels.19 However, if the urate-induced hypertension was allowed to persist, the rat would develop a salt-sensitive hypertension that was irreversible even if normouricemia was reestablished.19
This mechanism was tested in a small pilot study of 5 children with previously untreated essential hypertension who received monotherapy with allopurinol for 1 month.20 All 5 children had substantial drops in their continuously monitored ambulatory blood pressure, and 4 of the 5 developed normal blood pressure (as assessed by continuous monitoring). After the allopurinol was stopped, the blood pressure of all 5 children rebounded to baseline levels.20 A larger blinded, randomized, placebo-controlled crossover trial using the same intervention with similar results has been presented recently at national meetings.21
ASSOCIATION BETWEEN CARDIOVASCULAR DISEASE AND HYPERURICEMIA
There is considerable documentation that urate levels correlate with many recognized cardiovascular risk factors, including age, male gender, hypertension, diabetes mellitus, hypertriglyceridemia, obesity, and insulin resistance. Because of these relationships, the observed association between serum urate elevations and cardiovascular disease was considered to be “epiphenomenal” and not causal. Many older epidemiologic studies that demonstrated the increased cardiovascular risk associated with higher urate levels used traditional statistical techniques that could not prove the “independence” of urate as a risk factor.22
An independent effect is finally documented
Three studies published in the past several years demonstrate an independent risk relationship between hyperuricemia and cardiovascular disease, including acute myocardial infarction.23–25
Multivariate regression analysis of the Multiple Risk Factor Intervention Trial (MRFIT) database demonstrated hyperuricemia to be an independent risk factor for acute myocardial infarction.23
Similarly, the Italian Progetto Ipertensione Umbria Monitoraggio Ambulatoriale (PIUMA) study showed that after adjustment for age, sex, diabetes, serum lipid levels, serum creatinine, left ventricular hypertrophy, blood pressure, and diuretic use, serum urate levels in the highest quartile were associated with increased risk of all cardiovascular events (relative risk [RR] = 1.73) and fatal cardiovascular events (RR = 1.96) compared with urate levels in the second quartile.24
Finally, follow-up of 4,385 participants in the Rotterdam Study over an average of 8.4 years revealed that high urate levels were a strong predictor of both myocardial infarction and stroke, even after adjustment for other vascular risk factors.25
CONCLUSIONS
Based on the preponderance of recent epidemiologic studies, it appears that an elevated serum urate level is an independent risk factor for kidney disease, hypertension, and cardiovascular disease. The precise mechanisms for urate-induced tissue injury remain unclear, and no large study to date has convincingly demonstrated that lowering serum urate levels can prevent or lessen the risk of these potentially severe complications of hyperuricemia.
It should also be emphasized that the treatment of hyperuricemia with medications such as allopurinol or probenecid is currently not indicated in patients with hypertension, kidney disease, or heart disease. The use of these agents is supported only in the treatment of gout, the treatment of uric acid kidney stones, and the prevention of tumor lysis syndrome.
Hyperuricemia is a metabolic problem that has become quite common over the past several decades. The main clinical issues associated with hyperuricemia are gouty arthritis, gouty tophi, and uric acid kidney stones. For decades, these have been the main indications for lowering serum urate levels. Well-established nonarticular associations of hyperuricemia in gout include chronic kidney disease, coronary artery disease, and hypertension.1 Recent animal studies and epidemiologic studies have shed new light on the relationship between urate and comorbid disease processes. This article describes our evolving understanding of the association of hyperuricemia and gout with kidney disease, hypertension, and cardiovascular disease, and also reviews the kidney’s role in regulation of urate levels in the body.
SOURCES, DISTRIBUTION, AND ELIMINATION OF URATE
Uric acid is the end product of purine metabolism in humans. Sources of purine are either endogenous, from de novo synthesis and nucleic acid breakdown (approximately 600 mg/day), or exogenous, from dietary purine intake (approximately 100 mg/day).2 In the steady state, this daily production and ingestion of approximately 700 mg of uric acid is balanced by daily elimination of an equal amount of uric acid from the body. Approximately 30% of this daily loss is through the gut, with subsequent bacterial intestinal uricolysis. The other 70% (roughly 500 mg daily) needs to be excreted by the kidneys.
In humans, plasma urate is freely filtered at the glomerulus, but the fractional excretion of the filtered uric acid is less than 10%. This demonstrates a dominance of reabsorptive processes in humans, and these processes are handled primarily by the selective urate transport protein known as URAT1 in the proximal convoluted tubules. In normouricemic individuals, there is a balance between the daily production and ingestion of uric acid and the daily excretion of it. In most patients with primary hyperuricemia and gout, the fractional excretion of uric acid by the kidneys is relatively diminished, resulting in an imbalance of uric acid homeostasis, and serum urate exceeds saturability (6.8 mg/dL at physiologic temperature and pH). As this imbalance persists over years and decades, the miscible serum uric acid pool expands and urate may be deposited as part of an insoluble urate pool in the joints and soft tissues, referred to as tophi.
In the past, most investigators have focused on the destructive and proinflammatory role of insoluble deposited urate crystals, but new evidence is accumulating that rising levels of soluble urate in body fluids may also be harmful and lead to kidney disease, hypertension, and cardiovascular disease.
RENAL MANIFESTATIONS OF HYPERURICEMIA
Urate is strongly associated with renal disease but traditionally has not been considered to have a causal role in kidney dysfunction. The exceptions have been uric acid kidney stones and the acute uric acid nephropathy associated with chemotherapy and tumor lysis syndrome. New epidemiologic studies in humans, as well as an animal model of mild hyperuricemia leading to microvascular changes in the glomerular afferent arterioles, shed new light on a possible direct role of urate in the genesis of idiopathic chronic kidney disease.
Longstanding reluctance to implicate urate in kidney disease
Significant impairment of renal function was reported in up to 40% of patients with gout in studies conducted before the advent of effective urate-lowering therapies.3,4 In these older studies, renal failure was the eventual cause of death in 18% to 25% of patients with gout.3,4 However, any primary causal relationship for urate in this very high incidence of kidney disease was questioned for decades in light of the many other conditions and factors associated with hyperuricemia and gout that may contribute to kidney disease, such as hypertension, diabetes mellitus, alcohol abuse, non-steroidal anti-inflammatory drug use, and lead toxicity.
Recent epidemiologic studies establish the connection
More recently, two large prospective population studies from Japan examined the relationship between serum urate level and development of kidney disease using multiple covariate analysis to adjust for age, blood pressure, body mass index, proteinuria, hematocrit, hyperlipidemia, fasting glucose, and serum creatinine.5,6
ASSOCIATION OF HYPERTENSION AND HYPERURICEMIA
The strong association between hypertension and hyperuricemia has been recognized for more than a century. In his original description of essential hypertension in 1879, Frederick A. Mohamed noted that many of his subjects came from gouty families.7 Studies from the 1950s and 1960s showed the prevalence of hyperuricemia in hypertensive subjects to be between 20% and 40%.8,9 The prevalence of hypertension among gouty patients is Hyperuricemia predicts ESRD between 25% and 50%.10 In 1972, Kahn et al found that a rising level of serum urate is an independent risk factor for hypertension.11 A year later, Klein et al demonstrated a linear relationship between serum urate level and systolic blood pressure in both black and white subjects.12
Six large epidemiologic studies published over the past 7 years have found that serum urate level predicts the later development of hypertension.13–18 The most recent of these is the Normative Aging Study,18 which showed that the serum urate level independently predicts the development of hypertension when using age-adjusted and multivariate models that include body mass, abdominal girth, alcohol use, serum lipid levels, plasma glucose level, and smoking status.
A potential mechanism emerges
No clear causal or mechanistic link between elevated serum urate and the development of hypertension was evident until a rat model of mild hyperuricemia was found to be associated with the development of an initial salt-insensitive hypertension that was reversible with restoration of normal urate levels.19 However, if the urate-induced hypertension was allowed to persist, the rat would develop a salt-sensitive hypertension that was irreversible even if normouricemia was reestablished.19
This mechanism was tested in a small pilot study of 5 children with previously untreated essential hypertension who received monotherapy with allopurinol for 1 month.20 All 5 children had substantial drops in their continuously monitored ambulatory blood pressure, and 4 of the 5 developed normal blood pressure (as assessed by continuous monitoring). After the allopurinol was stopped, the blood pressure of all 5 children rebounded to baseline levels.20 A larger blinded, randomized, placebo-controlled crossover trial using the same intervention with similar results has been presented recently at national meetings.21
ASSOCIATION BETWEEN CARDIOVASCULAR DISEASE AND HYPERURICEMIA
There is considerable documentation that urate levels correlate with many recognized cardiovascular risk factors, including age, male gender, hypertension, diabetes mellitus, hypertriglyceridemia, obesity, and insulin resistance. Because of these relationships, the observed association between serum urate elevations and cardiovascular disease was considered to be “epiphenomenal” and not causal. Many older epidemiologic studies that demonstrated the increased cardiovascular risk associated with higher urate levels used traditional statistical techniques that could not prove the “independence” of urate as a risk factor.22
An independent effect is finally documented
Three studies published in the past several years demonstrate an independent risk relationship between hyperuricemia and cardiovascular disease, including acute myocardial infarction.23–25
Multivariate regression analysis of the Multiple Risk Factor Intervention Trial (MRFIT) database demonstrated hyperuricemia to be an independent risk factor for acute myocardial infarction.23
Similarly, the Italian Progetto Ipertensione Umbria Monitoraggio Ambulatoriale (PIUMA) study showed that after adjustment for age, sex, diabetes, serum lipid levels, serum creatinine, left ventricular hypertrophy, blood pressure, and diuretic use, serum urate levels in the highest quartile were associated with increased risk of all cardiovascular events (relative risk [RR] = 1.73) and fatal cardiovascular events (RR = 1.96) compared with urate levels in the second quartile.24
Finally, follow-up of 4,385 participants in the Rotterdam Study over an average of 8.4 years revealed that high urate levels were a strong predictor of both myocardial infarction and stroke, even after adjustment for other vascular risk factors.25
CONCLUSIONS
Based on the preponderance of recent epidemiologic studies, it appears that an elevated serum urate level is an independent risk factor for kidney disease, hypertension, and cardiovascular disease. The precise mechanisms for urate-induced tissue injury remain unclear, and no large study to date has convincingly demonstrated that lowering serum urate levels can prevent or lessen the risk of these potentially severe complications of hyperuricemia.
It should also be emphasized that the treatment of hyperuricemia with medications such as allopurinol or probenecid is currently not indicated in patients with hypertension, kidney disease, or heart disease. The use of these agents is supported only in the treatment of gout, the treatment of uric acid kidney stones, and the prevention of tumor lysis syndrome.
- Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR Jr, Saag KG. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis 2005; 64:267–272.
- Richards J, Weinman EJ. Uric acid and renal disease. J Nephrol 1966; 9:160–166.
- Berger L, Yü TF. Renal function in gout. IV. An analysis of 524 gouty subjects including long-term follow-up studies. Am J Med 1975; 59:605–613.
- Talbot JH, Terplan KL. The kidney in gout. Medicine 1960; 39:405–468.
- Tomita M, Mizuno S, Yamanaka H, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol 2000; 10:403–409.
- Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis 2004; 44:642–650.
- Mohamed FA. On chronic Bright’s disease, and its essential symptoms. Lancet 1879; 1:399–401.
- Weiss TE, Segaloff A, Moore C. Gout and diabetes. Metabolism 1957; 6:103.
- Kinsey D, Walther R, Sise HS, Whitelaw G, Smithwick R. Incidence of hyperuricemia in 400 hypertensive patients. Circulation 1961; 24:972.
- Becker MA, Jolly M. Hyperuricemia and associated diseases. Rheum Dis Clin North Am 2006; 32:275–293.
- Kahn HA, Medalie JH, Neufeld HN, Riss E, Goldbourt U. The incidence of hypertension and associated factors: the Israel ischemic heart disease study. Am Heart J 1972; 84:171–182.
- Klein R, Klein BE, Cornoni JC, Maready J, Cassel JC, Tyroler HA. Serum uric acid. Its relationships to coronary heart disease risk factors and cardiovascular disease, Evans County, Georgia. Arch Intern Med 1973; 132:401–410.
- Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or type II diabetes in Japanese male office workers. Eur J Epidemiol 2003; 18:523–530.
- Alper AB Jr, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension 2005; 45:34–38.
- Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 2003; 42:474–480.
- Nagahama K, Inoue T, Iseki K, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res 2004; 27:835–841.
- Sundström J, Sullivan L, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 2005; 45:28–33.
- Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the Normative Aging Study. Hypertension 2006; 48:1031–1036.
- Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001; 38:1101–1106.
- Feig DI, Nakagawa T, Karumanchi SA, et al. Hypothesis: uric acid, nephron number, and the pathogenesis of essential hypertension. Kidney Int 2004; 66:281–287.
- Feig DI, Mazzali M, Kang DH, et al. Serum uric acid: a risk factor and a target for treatment? J Am Soc Nephrol 2006; 17(4 Suppl):S69–S73.
- Rich MW. Uric acid: is it a risk factor for cardiovascular disease? Am J Cardiol 2000; 85:1018–1021.
- Krishnan E, Baker JF, Furst DE, Schumacher HR Jr. Gout and the risk of acute myocardial infarction. Arthritis Rheum 2006; 54:2688–2696.
- Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 2000; 36:1072–1078.
- Bos MJ , Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam Study. Stroke 2006; 37:1503–1507.
- Mikuls TR, Farrar JT, Bilker WB, Fernandes S, Schumacher HR Jr, Saag KG. Gout epidemiology: results from the UK General Practice Research Database, 1990–1999. Ann Rheum Dis 2005; 64:267–272.
- Richards J, Weinman EJ. Uric acid and renal disease. J Nephrol 1966; 9:160–166.
- Berger L, Yü TF. Renal function in gout. IV. An analysis of 524 gouty subjects including long-term follow-up studies. Am J Med 1975; 59:605–613.
- Talbot JH, Terplan KL. The kidney in gout. Medicine 1960; 39:405–468.
- Tomita M, Mizuno S, Yamanaka H, et al. Does hyperuricemia affect mortality? A prospective cohort study of Japanese male workers. J Epidemiol 2000; 10:403–409.
- Iseki K, Ikemiya Y, Inoue T, Iseki C, Kinjo K, Takishita S. Significance of hyperuricemia as a risk factor for developing ESRD in a screened cohort. Am J Kidney Dis 2004; 44:642–650.
- Mohamed FA. On chronic Bright’s disease, and its essential symptoms. Lancet 1879; 1:399–401.
- Weiss TE, Segaloff A, Moore C. Gout and diabetes. Metabolism 1957; 6:103.
- Kinsey D, Walther R, Sise HS, Whitelaw G, Smithwick R. Incidence of hyperuricemia in 400 hypertensive patients. Circulation 1961; 24:972.
- Becker MA, Jolly M. Hyperuricemia and associated diseases. Rheum Dis Clin North Am 2006; 32:275–293.
- Kahn HA, Medalie JH, Neufeld HN, Riss E, Goldbourt U. The incidence of hypertension and associated factors: the Israel ischemic heart disease study. Am Heart J 1972; 84:171–182.
- Klein R, Klein BE, Cornoni JC, Maready J, Cassel JC, Tyroler HA. Serum uric acid. Its relationships to coronary heart disease risk factors and cardiovascular disease, Evans County, Georgia. Arch Intern Med 1973; 132:401–410.
- Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or type II diabetes in Japanese male office workers. Eur J Epidemiol 2003; 18:523–530.
- Alper AB Jr, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL. Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension 2005; 45:34–38.
- Masuo K, Kawaguchi H, Mikami H, Ogihara T, Tuck ML. Serum uric acid and plasma norepinephrine concentrations predict subsequent weight gain and blood pressure elevation. Hypertension 2003; 42:474–480.
- Nagahama K, Inoue T, Iseki K, et al. Hyperuricemia as a predictor of hypertension in a screened cohort in Okinawa, Japan. Hypertens Res 2004; 27:835–841.
- Sundström J, Sullivan L, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Relations of serum uric acid to longitudinal blood pressure tracking and hypertension incidence. Hypertension 2005; 45:28–33.
- Perlstein TS, Gumieniak O, Williams GH, et al. Uric acid and the development of hypertension: the Normative Aging Study. Hypertension 2006; 48:1031–1036.
- Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001; 38:1101–1106.
- Feig DI, Nakagawa T, Karumanchi SA, et al. Hypothesis: uric acid, nephron number, and the pathogenesis of essential hypertension. Kidney Int 2004; 66:281–287.
- Feig DI, Mazzali M, Kang DH, et al. Serum uric acid: a risk factor and a target for treatment? J Am Soc Nephrol 2006; 17(4 Suppl):S69–S73.
- Rich MW. Uric acid: is it a risk factor for cardiovascular disease? Am J Cardiol 2000; 85:1018–1021.
- Krishnan E, Baker JF, Furst DE, Schumacher HR Jr. Gout and the risk of acute myocardial infarction. Arthritis Rheum 2006; 54:2688–2696.
- Verdecchia P, Schillaci G, Reboldi G, Santeusanio F, Porcellati C, Brunetti P. Relation between serum uric acid and risk of cardiovascular disease in essential hypertension. The PIUMA study. Hypertension 2000; 36:1072–1078.
- Bos MJ , Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam Study. Stroke 2006; 37:1503–1507.
KEY POINTS
- Hyperuricemia significantly increases the risk of renal failure and end-stage renal disease.
- Larger, more rigorous trials are under way to assess preliminary findings suggesting that urate-lowering therapy might normalize blood pressure in hypertensive adolescent patients.
- Use of urate-lowering therapies to treat hyperuricemia is not currently supported in patients with kidney disease, heart disease, or hypertension in the absence of gout or uric acid kidney stones.