User login
Understanding of respiratory syncytial virus (RSV) infection has increased substantially since its discovery, but a curative treatment remains elusive. Insights into the impact of gestational age, epidemiologic patterns, virus incubation and proliferation, pathogenesis and pathophysiology, and host immune response have set the stage for preventive measures and effective therapy. In today’s clinical practice, a specific humanized antibody is administered to high-risk infants, which is safe and effective in preventing RSV-related acute and postacute symptoms.
VIRUS STRUCTURE AND CLASSIFICATION
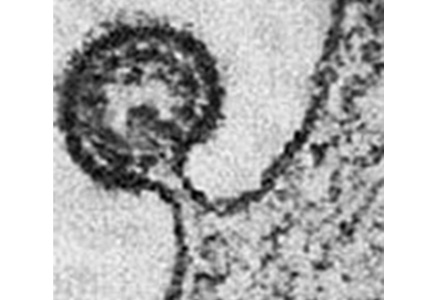
Knowledge of the molecular structure of RSV is important because it gives clues as to how it infects the human airways (Figure 1). The virus is made of a single strand of RNA contained in a nucleocapsid made of only 11 proteins and covered by a lipid envelope. Essential for the virulence of RSV are the surface glycoproteins G and F (fusion) that attach to the host airway epithelial cells and merge the viral envelope to the membranes of multiple adjacent cells, creating the “syncytia” that give RSV its name. G and F proteins are also the principal antigens exposed to the host immune system, and therefore the patient’s neutralizing antibodies are primarily directed against these targets.1
Human RSV is a Pneumovirus belonging to the Paramyxoviridae family. There are two strains of RSV (A and B) found in infants. Approximately 60% of all lower respiratory infections in preschool-aged children worldwide are caused by RSV (Figure 2).2 All the other viruses—including influenza, parainfluenza, metapneumovirus, and adenoviruses—as well as bacterial infections are much less common during the first years of life.
VIROLOGY
The burden of RSV continues to grow worldwide, particularly among the youngest segment of the population.2 During the first year of life, infants are not adequately protected from RSV by maternal immunoglobulins. Thus, approximately 80% of lower respiratory infections in children younger than 1 year are due to RSV with peak incidence occurring at 2 to 3 months of age. In the United States, bronchiolitis-associated hospitalizations in infants younger than 12 months more than doubled between 1980 and 1996, and in 1996, accounted for approximately 3% of all pediatric hospitalizations. Up to 120,000 RSV-related pediatric hospitalizations per year occur during a typical season.3
Mortality rates have improved significantly—at least in industrialized countries—and now are probably less than 1%.4 In the United States, RSV is estimated to cause fewer than 100 deaths annually, primarily because of better supportive care and the use of mechanical ventilation.
The seasonality of RSV varies by region. In the northern United States from October through March, RSV causes up to 90% of lower respiratory infection cases in infants. In the southern United States and farther into the southern hemisphere, the virus becomes endemic and can be present throughout the year with peak incidence occurring from August to September.2
RSV RISK FACTORS
The severity and frequency of RSV infection is influenced by well-known risk factors including environmental overcrowding, absence of breastfeeding, and immunosuppression. Children with chronic lung disease are predisposed to clinically significant RSV infection by their limited respiratory reserve, and 60% of those affected will be hospitalized. Hemodynamically significant congenital heart defects associated with higher pulmonary blood flow also increase risk for severe RSV disease, with more than 50% of children infected requiring hospitalization.4
Prematurity and increased RSV risk
Premature-born infants have a 10-fold increased risk of RSV infection and account for 25% to 30% of RSV hospitalizations annually.4 Prematurity as a risk factor for RSV is primarily tied to the physiology of placental immunoglobulin G (IgG) transfer. The human placenta is not permeable to IgG during the first half of pregnancy because of low expression of the Fc receptor needed to bind immunoglobulins and transfer them into the fetal circulation. Further, maternal IgG is recognized as a foreign protein by the newborn and is progressively removed from the circulation by the liver.5
Thus, the IgG decline continues postnatally, reaching the lowest concentration at 2 to 3 months of age because newborns are unable to synthesize their own antibodies. During this time, full-term born babies are at the highest risk for developing RSV infection. The risk is logically higher for premature infants who lack the full benefit of IgG transfer occurring during the last trimester, rendering antibody levels even lower at nadir. The greater the prematurity, the less the antibody protection and the more the predisposition to RSV infection.5
CLINICAL MANIFESTATIONS
The hallmark signs of RSV bronchiolitis are wheezing, cough, and increased work of breathing caused by infection of the bronchiolar airways. However, the nasal phase of the infection may cause irritation and trigeminal nerve activation associated with sneezing, congestion, and apnea. Indeed, approximately 20% of infants manifest an apnea episode as the first symptom of infection.6
The specific etiology of RSV can be confirmed by antigen detection tests, currently being replaced by more sensitive polymerase chain reaction (PCR)-based assays. Chest radiography reveals obvious signs of RSV lower respiratory infection, typically patch atelectasis, increased peribronchial markings, and bilateral hyperinflation with flattening of the diaphragm. Current guidelines indicate that the diagnosis of acute bronchiolitis should be based exclusively on the history and physical exam; it does not require radiographic or laboratory studies.
Correct etiologic diagnosis, however, is crucial to avoid unnecessary therapies and rule out rare conditions that could be worsened by therapies commonly used for bronchiolitis. For example, infants with dilated cardiomyopathy and congestive heart failure may present with symptoms of wheezing that mimic an acute respiratory infection, but these patients are at risk of developing supraventricular tachycardia and even cardiopulmonary collapse associated with administration of beta-agonist agents. In such cases, a chest radiograph will show cardiomegaly suggesting a different diagnosis and therapy, and thus avoid significant complications.
Recurrent wheezing
Children infected with RSV during their first year of life develop a higher risk of subsequent episodes of bronchial obstruction. Several retrospective studies conducted in the 1980s explored the incidence of lower respiratory symptoms continuing for years after initial RSV infection. Particularly unexpected was the finding that at least one-third of children hospitalized with RSV bronchiolitis will continue to wheeze beyond 6 to 8 years of age (Figure 3).7–9
More recent prospective studies of recurrent wheezing following RSV infection have been conducted. A Swedish study of 47 infants hospitalized with culture-proven RSV bronchiolitis showed significantly increased physician-diagnosed asthma (38%) compared with the 93 controls (2%) at age 7.5 years (P < .0001).10 Follow-up studies of the same cohort found increased risk for recurrent wheezing or asthma in the RSV group still present at age 13 years11 and 18 years12 compared with controls.
Asthma
History of RSV infection is important both as a trigger of asthma attacks and in the inception of asthma. Family history of asthma is the most important marker of a genetic predisposition to develop asthma. A multivariate analysis of the Swedish cohort discussed above showed that children with neither previous RSV infection nor family history of asthma had approximately a 5% risk of developing asthma by age 7.5 years. None of the children with a family history of asthma but no history of RSV infection developed asthma during follow-up. Children who had an RSV infection without a familial predisposition to asthma had approximately a 20% risk of asthma development. Children with both previous RSV infection and a family history of asthma had the highest (~40%) risk of developing asthma.10
These results provide important information about asthma pathogenesis in early life. The main implication is that even if a child has a genetic predisposition to asthma development, clinical manifestations will not develop without exposure to environmental agents that allow the actual expression of the predisposing genes, such as pollution, unbalanced diet, or early-life RSV infection. On the other hand, children without genetic predisposition can present with clinical manifestations undistinguishable from atopic asthma if their respiratory tract is exposed to specific viral pathogens during crucial developmental windows in early life. Finally, the combination of genetic predisposition and adverse environmental exposures in infancy carries the highest risk of asthma.
RSV MANAGEMENT
Ribavirin
The life cycle of RSV after the initial infection affects treatment strategies. RSV produces no symptoms for at least 3 to 5 days following infection (“eclipse phase”), during which time it reproduces exponentially and reaches the lungs, causing the first symptoms to be observable after 5 to 7 days. By the time the first symptoms emerge, the virus is already rapidly disappearing from the system.13 If ribavirin is administered at this point, the pediatrician has provided a virostatic agent against a virus that is no longer present, and therapy will not only be ineffective, but it may trigger bronchospasms.
The American Academy of Pediatrics (AAP) Committee on Infectious Diseases supported the use of ribavirin in 1993 guidelines,14 but changed their recommendation to “may be considered” in 1996.15 The only exception for ribavirin use is in the immunocompromised patient with RSV. In this scenario, the virus continues to replicate for months after the initial infection unopposed by the host defense mechanisms; therefore, aerosolized ribavirin therapy should be considered, either alone or in combination with humanized anti-RSV antibody.
Other treatments
Corticosteroids have not been shown to have a significant effect on RSV bronchiolitis.16 Alpha- and beta-adrenergic bronchodilators, such as epinephrine and albuterol, have shown very little or no effect on RSV symptoms in multiple controlled trials. The only effective treatment with proven efficacy is supportive therapy with adequate fluid intake and oxygen. Oral feeding should be withheld in patients with high respiratory rates to prevent aspiration.
PROPHYLAXIS
Vaccines
Vaccines have been ineffective against RSV and can be dangerous in children. RSV is not a strongly cytopathic virus; illness results primarily from the host immune response against the infection rather than from the virus itself.16 Therefore, any vaccine carries the risk of creating a stronger and potentially dangerous immune response to the next infection.
In the 1960s, a formalin-inactivated RSV vaccine was introduced with deleterious outcomes—it was minimally protective and was responsible for infant deaths. Because mortality was not immediate, the vaccine continued to be administered throughout the season. Unfortunately, with their immunity modified, vaccinated children became severely ill when they came in contact with the wild-type virus during the year following vaccination. The withdrawal of the formalin-inactivated vaccine still represents an important precedent that makes investigators and regulatory bodies very cautious about active immunization against this virus.
Palivizumab
Palivizumab is a 96% human monoclonal antibody targeting the RSV F protein, and it offers passive immunity for infants at risk for severe infection. The Impact-RSV clinical trial of palivizumab showed that five monthly intramuscular injections effectively reduced RSV hospitalizations by 78% in premature infants from 32 to 35 weeks gestation without bronchopulmonary dysplasia (BPD). Treatment offered only a 39% reduction in premature infants with BPD.17
The most recent AAP guidelines18 recommend palivizumab prophylaxis with a maximum of five monthly doses only in the first year of life for otherwise healthy infants born before 29 weeks gestation and for infants born before 32 weeks gestation with chronic lung disease of prematurity (CLD) defined as a requirement for supplemental oxygen for at least 28 days after birth. Prophylaxis is no longer recommended in the second year of life, except for infants with CLD still requiring oxygen, corticosteroids, or diuretics. Palivizumab prophylaxis should be discontinued if a breakthrough RSV hospitalization occurs because the likelihood of a second RSV hospitalization in the same season is low.
Palivizumab also should be considered for children with hemodynamically significant congenital heart defects, profound immunodeficiency, and pulmonary or neuromuscular pathologies impairing airway clearance; however, no formal recommendation was made for patients with Down syndrome or cystic fibrosis due to insufficient data.18 The protective effect of palivizumab appears to be cumulative, with almost half of the breakthrough infections observed in the clinical trials occurring after the first injection or immediately following the second.17
A recent randomized, double-blind, placebo-controlled trial has shown that palivizumab given to premature infants during the first year of life provides a 40% to 60% reduction of wheezing episodes.19 This trial confirms the results of previous retrospective studies; however, further large multicenter trials of palivizumab prophylaxis in both premature and full-term infants are needed to assess protection against recurrent wheezing and asthma, and to formulate evidence-based recommendations.
FUTURE DIRECTIONS
Nebulization of 3% saline improves mucociliary clearance and is increasingly being used in airway diseases involving mucus plugging (eg, cystic fibrosis). It also has been reported to reduce length of hospital stay and provide symptomatic relief in patients with bronchiolitis, but its use remains controversial. In particular, it has not been shown to be effective at reducing hospitalization when used in emergency settings. Therefore, based on current evidence, the administration of hypertonic saline for bronchiolitis should be limited to hospitalized infants.
Anti-leukotrienes used during the acute phase of RSV bronchiolitis have been shown to improve post-bronchiolitis respiratory symptoms, especially in younger patients with high urinary leukotriene E4 (LTE4). However, a large, multicenter, randomized, double-blind, placebo-controlled trial with montelukast failed to show statistically significant clinical improvement. A post-hoc data analysis revealed that children with persistent respiratory symptoms after the acute phase of the infection may benefit from montelukast, but the manufacturer (Merck) is no longer pursuing this indication.
There is not enough scientific evidence at present to support the use of DNAse or exogenous surfactant in the setting of acute bronchiolitis. New generations of specific antivirals and vaccines based on immunogens offer hope as new options for prophylaxis and/or management of RSV infection in the not too distant future.
Recent basic science investigations have provided new findings that could influence future therapies. Some of these studies have suggested that the tropism of RSV is not solely for the airway epithelium; rather, this virus can spread hematogenously to infect extrapulmonary targets, particularly the bone marrow stromal cells where RSV finds a sanctuary niche shielding it from immune protection and allowing subclinical latency. In one study, RSV was found in the bone marrow of every child and in about 80% of adults tested.20 Similar findings were replicated with other pathogens like Mycobacterium tuberculosis.21 This discovery could direct future treatment strategies for a number of viral, bacterial, and parasitic infections.
In another study, transplacental transmission of RSV from the mother’s airways to fetal lung tissues was shown for the first time in an animal model of infection. When the virus enters fetal respiratory cells, it persists and induces the expression of neurotrophic factors and receptors resulting in postnatal airways with dramatically increased parasympathetic innervation and methacholine reactivity. These structural and functional changes provide a suitable model to explain the development of long-term bronchial hyperreactivity following re-exposure to RSV in early life.22
In conclusion, the search for a cure for the most common respiratory infection in children has humbled several generations of investigators through the more than 50 years since RSV was discovered. Nevertheless, the rapid evolution in the fields of virology and immunology and recent breakthrough discoveries promise to deliver new strategies that may make a difference in the management of this common infection and its long-term sequelae.
- Peters TR, Crowe JE Jr. Respiratory syncytial virus. In: Long SS, Pickering LK, Prober CG, eds. Principles and Practice of Pediatric Infectious Diseases. 3rd ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2008.
- Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev 2014; 35:519–530.
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999; 282:1440–1446.
- Simoes EA, Rieger CHL. RSV infection in developed and developing countries. Infect Med 1999; 16:S11–S17.
- Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1,500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res 1986; 20:899 –904.
- Sabogal C, Auais A, Napchan G, et al. Effect of respiratory syncytial virus on apnea in weanling rats. Pediatr Res 2005; 57:819–825.
- Hall CB, Hall WJ, Gala CL, MaGill FB, Leddy JP. Long-term prospective study in children after respiratory syncytial virus infection. J Pediatr 1984; 105:358–364.
- Henry RL, Hodges IGC, Milner AD, Stokes GM. Respiratory problems 2 years after acute bronchiolitis in infancy. Arch Dis Child 1983; 58:713–716.
- Webb MSC, Henry RL, Milner AD, Stokes GM, Swarbrick AS. Continuing respiratory problems three and a half years after acute viral bronchiolitis. Arch Dis Child 1985; 60:1064–1067.
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161:1501–1507.
- Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005; 171:137–141.
- Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65:1045–1052.
- McIntosh K, Chanock RM. Respiratory syncytial virus. In: Fields BN, Knipe DM, eds. Virology. New York: Raven Press; 1990:1045.
- Committee on Infectious Diseases. American Academy of Pediatrics: use of ribavirin in the treatment of respiratory syncytial virus infection. Pediatrics 1993; 92:501–504.
- Committee on Infectious Diseases. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 1996; 97:137–140.
- Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol 2011; 46:324–347.
- The Impact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory synctial virus infection in high-risk infants. Pediatrics 1998; 102(3 Pt 1):531–537.
- Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134:e1474–e1502.
- Blanken MO, Rovers MM, Molenaar JM, et al; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–1799.
- Rezaee F, Gibson LF, Piktel D, Othumpangat S, Piedimonte G. Respiratory syncytial virus infection in human bone marrow stromal cells. Am J Respir Cell Mol Biol 2011; 45:277–286.
- Das B, Kashino SS, Pulu I, et al. CD271+ bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med 2013; 5:170ra13.
- Piedimonte G, Perez MK. Alternative mechanisms for respiratory syncytial virus (RSV) infection and persistence: could RSV be transmitted through the placenta and persist into developing fetal lungs? Curr Opin Pharmacol 2014; 16:82–88.
Understanding of respiratory syncytial virus (RSV) infection has increased substantially since its discovery, but a curative treatment remains elusive. Insights into the impact of gestational age, epidemiologic patterns, virus incubation and proliferation, pathogenesis and pathophysiology, and host immune response have set the stage for preventive measures and effective therapy. In today’s clinical practice, a specific humanized antibody is administered to high-risk infants, which is safe and effective in preventing RSV-related acute and postacute symptoms.
VIRUS STRUCTURE AND CLASSIFICATION
Knowledge of the molecular structure of RSV is important because it gives clues as to how it infects the human airways (Figure 1). The virus is made of a single strand of RNA contained in a nucleocapsid made of only 11 proteins and covered by a lipid envelope. Essential for the virulence of RSV are the surface glycoproteins G and F (fusion) that attach to the host airway epithelial cells and merge the viral envelope to the membranes of multiple adjacent cells, creating the “syncytia” that give RSV its name. G and F proteins are also the principal antigens exposed to the host immune system, and therefore the patient’s neutralizing antibodies are primarily directed against these targets.1
Human RSV is a Pneumovirus belonging to the Paramyxoviridae family. There are two strains of RSV (A and B) found in infants. Approximately 60% of all lower respiratory infections in preschool-aged children worldwide are caused by RSV (Figure 2).2 All the other viruses—including influenza, parainfluenza, metapneumovirus, and adenoviruses—as well as bacterial infections are much less common during the first years of life.
VIROLOGY
The burden of RSV continues to grow worldwide, particularly among the youngest segment of the population.2 During the first year of life, infants are not adequately protected from RSV by maternal immunoglobulins. Thus, approximately 80% of lower respiratory infections in children younger than 1 year are due to RSV with peak incidence occurring at 2 to 3 months of age. In the United States, bronchiolitis-associated hospitalizations in infants younger than 12 months more than doubled between 1980 and 1996, and in 1996, accounted for approximately 3% of all pediatric hospitalizations. Up to 120,000 RSV-related pediatric hospitalizations per year occur during a typical season.3
Mortality rates have improved significantly—at least in industrialized countries—and now are probably less than 1%.4 In the United States, RSV is estimated to cause fewer than 100 deaths annually, primarily because of better supportive care and the use of mechanical ventilation.
The seasonality of RSV varies by region. In the northern United States from October through March, RSV causes up to 90% of lower respiratory infection cases in infants. In the southern United States and farther into the southern hemisphere, the virus becomes endemic and can be present throughout the year with peak incidence occurring from August to September.2
RSV RISK FACTORS
The severity and frequency of RSV infection is influenced by well-known risk factors including environmental overcrowding, absence of breastfeeding, and immunosuppression. Children with chronic lung disease are predisposed to clinically significant RSV infection by their limited respiratory reserve, and 60% of those affected will be hospitalized. Hemodynamically significant congenital heart defects associated with higher pulmonary blood flow also increase risk for severe RSV disease, with more than 50% of children infected requiring hospitalization.4
Prematurity and increased RSV risk
Premature-born infants have a 10-fold increased risk of RSV infection and account for 25% to 30% of RSV hospitalizations annually.4 Prematurity as a risk factor for RSV is primarily tied to the physiology of placental immunoglobulin G (IgG) transfer. The human placenta is not permeable to IgG during the first half of pregnancy because of low expression of the Fc receptor needed to bind immunoglobulins and transfer them into the fetal circulation. Further, maternal IgG is recognized as a foreign protein by the newborn and is progressively removed from the circulation by the liver.5
Thus, the IgG decline continues postnatally, reaching the lowest concentration at 2 to 3 months of age because newborns are unable to synthesize their own antibodies. During this time, full-term born babies are at the highest risk for developing RSV infection. The risk is logically higher for premature infants who lack the full benefit of IgG transfer occurring during the last trimester, rendering antibody levels even lower at nadir. The greater the prematurity, the less the antibody protection and the more the predisposition to RSV infection.5
CLINICAL MANIFESTATIONS
The hallmark signs of RSV bronchiolitis are wheezing, cough, and increased work of breathing caused by infection of the bronchiolar airways. However, the nasal phase of the infection may cause irritation and trigeminal nerve activation associated with sneezing, congestion, and apnea. Indeed, approximately 20% of infants manifest an apnea episode as the first symptom of infection.6
The specific etiology of RSV can be confirmed by antigen detection tests, currently being replaced by more sensitive polymerase chain reaction (PCR)-based assays. Chest radiography reveals obvious signs of RSV lower respiratory infection, typically patch atelectasis, increased peribronchial markings, and bilateral hyperinflation with flattening of the diaphragm. Current guidelines indicate that the diagnosis of acute bronchiolitis should be based exclusively on the history and physical exam; it does not require radiographic or laboratory studies.
Correct etiologic diagnosis, however, is crucial to avoid unnecessary therapies and rule out rare conditions that could be worsened by therapies commonly used for bronchiolitis. For example, infants with dilated cardiomyopathy and congestive heart failure may present with symptoms of wheezing that mimic an acute respiratory infection, but these patients are at risk of developing supraventricular tachycardia and even cardiopulmonary collapse associated with administration of beta-agonist agents. In such cases, a chest radiograph will show cardiomegaly suggesting a different diagnosis and therapy, and thus avoid significant complications.
Recurrent wheezing
Children infected with RSV during their first year of life develop a higher risk of subsequent episodes of bronchial obstruction. Several retrospective studies conducted in the 1980s explored the incidence of lower respiratory symptoms continuing for years after initial RSV infection. Particularly unexpected was the finding that at least one-third of children hospitalized with RSV bronchiolitis will continue to wheeze beyond 6 to 8 years of age (Figure 3).7–9
More recent prospective studies of recurrent wheezing following RSV infection have been conducted. A Swedish study of 47 infants hospitalized with culture-proven RSV bronchiolitis showed significantly increased physician-diagnosed asthma (38%) compared with the 93 controls (2%) at age 7.5 years (P < .0001).10 Follow-up studies of the same cohort found increased risk for recurrent wheezing or asthma in the RSV group still present at age 13 years11 and 18 years12 compared with controls.
Asthma
History of RSV infection is important both as a trigger of asthma attacks and in the inception of asthma. Family history of asthma is the most important marker of a genetic predisposition to develop asthma. A multivariate analysis of the Swedish cohort discussed above showed that children with neither previous RSV infection nor family history of asthma had approximately a 5% risk of developing asthma by age 7.5 years. None of the children with a family history of asthma but no history of RSV infection developed asthma during follow-up. Children who had an RSV infection without a familial predisposition to asthma had approximately a 20% risk of asthma development. Children with both previous RSV infection and a family history of asthma had the highest (~40%) risk of developing asthma.10
These results provide important information about asthma pathogenesis in early life. The main implication is that even if a child has a genetic predisposition to asthma development, clinical manifestations will not develop without exposure to environmental agents that allow the actual expression of the predisposing genes, such as pollution, unbalanced diet, or early-life RSV infection. On the other hand, children without genetic predisposition can present with clinical manifestations undistinguishable from atopic asthma if their respiratory tract is exposed to specific viral pathogens during crucial developmental windows in early life. Finally, the combination of genetic predisposition and adverse environmental exposures in infancy carries the highest risk of asthma.
RSV MANAGEMENT
Ribavirin
The life cycle of RSV after the initial infection affects treatment strategies. RSV produces no symptoms for at least 3 to 5 days following infection (“eclipse phase”), during which time it reproduces exponentially and reaches the lungs, causing the first symptoms to be observable after 5 to 7 days. By the time the first symptoms emerge, the virus is already rapidly disappearing from the system.13 If ribavirin is administered at this point, the pediatrician has provided a virostatic agent against a virus that is no longer present, and therapy will not only be ineffective, but it may trigger bronchospasms.
The American Academy of Pediatrics (AAP) Committee on Infectious Diseases supported the use of ribavirin in 1993 guidelines,14 but changed their recommendation to “may be considered” in 1996.15 The only exception for ribavirin use is in the immunocompromised patient with RSV. In this scenario, the virus continues to replicate for months after the initial infection unopposed by the host defense mechanisms; therefore, aerosolized ribavirin therapy should be considered, either alone or in combination with humanized anti-RSV antibody.
Other treatments
Corticosteroids have not been shown to have a significant effect on RSV bronchiolitis.16 Alpha- and beta-adrenergic bronchodilators, such as epinephrine and albuterol, have shown very little or no effect on RSV symptoms in multiple controlled trials. The only effective treatment with proven efficacy is supportive therapy with adequate fluid intake and oxygen. Oral feeding should be withheld in patients with high respiratory rates to prevent aspiration.
PROPHYLAXIS
Vaccines
Vaccines have been ineffective against RSV and can be dangerous in children. RSV is not a strongly cytopathic virus; illness results primarily from the host immune response against the infection rather than from the virus itself.16 Therefore, any vaccine carries the risk of creating a stronger and potentially dangerous immune response to the next infection.
In the 1960s, a formalin-inactivated RSV vaccine was introduced with deleterious outcomes—it was minimally protective and was responsible for infant deaths. Because mortality was not immediate, the vaccine continued to be administered throughout the season. Unfortunately, with their immunity modified, vaccinated children became severely ill when they came in contact with the wild-type virus during the year following vaccination. The withdrawal of the formalin-inactivated vaccine still represents an important precedent that makes investigators and regulatory bodies very cautious about active immunization against this virus.
Palivizumab
Palivizumab is a 96% human monoclonal antibody targeting the RSV F protein, and it offers passive immunity for infants at risk for severe infection. The Impact-RSV clinical trial of palivizumab showed that five monthly intramuscular injections effectively reduced RSV hospitalizations by 78% in premature infants from 32 to 35 weeks gestation without bronchopulmonary dysplasia (BPD). Treatment offered only a 39% reduction in premature infants with BPD.17
The most recent AAP guidelines18 recommend palivizumab prophylaxis with a maximum of five monthly doses only in the first year of life for otherwise healthy infants born before 29 weeks gestation and for infants born before 32 weeks gestation with chronic lung disease of prematurity (CLD) defined as a requirement for supplemental oxygen for at least 28 days after birth. Prophylaxis is no longer recommended in the second year of life, except for infants with CLD still requiring oxygen, corticosteroids, or diuretics. Palivizumab prophylaxis should be discontinued if a breakthrough RSV hospitalization occurs because the likelihood of a second RSV hospitalization in the same season is low.
Palivizumab also should be considered for children with hemodynamically significant congenital heart defects, profound immunodeficiency, and pulmonary or neuromuscular pathologies impairing airway clearance; however, no formal recommendation was made for patients with Down syndrome or cystic fibrosis due to insufficient data.18 The protective effect of palivizumab appears to be cumulative, with almost half of the breakthrough infections observed in the clinical trials occurring after the first injection or immediately following the second.17
A recent randomized, double-blind, placebo-controlled trial has shown that palivizumab given to premature infants during the first year of life provides a 40% to 60% reduction of wheezing episodes.19 This trial confirms the results of previous retrospective studies; however, further large multicenter trials of palivizumab prophylaxis in both premature and full-term infants are needed to assess protection against recurrent wheezing and asthma, and to formulate evidence-based recommendations.
FUTURE DIRECTIONS
Nebulization of 3% saline improves mucociliary clearance and is increasingly being used in airway diseases involving mucus plugging (eg, cystic fibrosis). It also has been reported to reduce length of hospital stay and provide symptomatic relief in patients with bronchiolitis, but its use remains controversial. In particular, it has not been shown to be effective at reducing hospitalization when used in emergency settings. Therefore, based on current evidence, the administration of hypertonic saline for bronchiolitis should be limited to hospitalized infants.
Anti-leukotrienes used during the acute phase of RSV bronchiolitis have been shown to improve post-bronchiolitis respiratory symptoms, especially in younger patients with high urinary leukotriene E4 (LTE4). However, a large, multicenter, randomized, double-blind, placebo-controlled trial with montelukast failed to show statistically significant clinical improvement. A post-hoc data analysis revealed that children with persistent respiratory symptoms after the acute phase of the infection may benefit from montelukast, but the manufacturer (Merck) is no longer pursuing this indication.
There is not enough scientific evidence at present to support the use of DNAse or exogenous surfactant in the setting of acute bronchiolitis. New generations of specific antivirals and vaccines based on immunogens offer hope as new options for prophylaxis and/or management of RSV infection in the not too distant future.
Recent basic science investigations have provided new findings that could influence future therapies. Some of these studies have suggested that the tropism of RSV is not solely for the airway epithelium; rather, this virus can spread hematogenously to infect extrapulmonary targets, particularly the bone marrow stromal cells where RSV finds a sanctuary niche shielding it from immune protection and allowing subclinical latency. In one study, RSV was found in the bone marrow of every child and in about 80% of adults tested.20 Similar findings were replicated with other pathogens like Mycobacterium tuberculosis.21 This discovery could direct future treatment strategies for a number of viral, bacterial, and parasitic infections.
In another study, transplacental transmission of RSV from the mother’s airways to fetal lung tissues was shown for the first time in an animal model of infection. When the virus enters fetal respiratory cells, it persists and induces the expression of neurotrophic factors and receptors resulting in postnatal airways with dramatically increased parasympathetic innervation and methacholine reactivity. These structural and functional changes provide a suitable model to explain the development of long-term bronchial hyperreactivity following re-exposure to RSV in early life.22
In conclusion, the search for a cure for the most common respiratory infection in children has humbled several generations of investigators through the more than 50 years since RSV was discovered. Nevertheless, the rapid evolution in the fields of virology and immunology and recent breakthrough discoveries promise to deliver new strategies that may make a difference in the management of this common infection and its long-term sequelae.
Understanding of respiratory syncytial virus (RSV) infection has increased substantially since its discovery, but a curative treatment remains elusive. Insights into the impact of gestational age, epidemiologic patterns, virus incubation and proliferation, pathogenesis and pathophysiology, and host immune response have set the stage for preventive measures and effective therapy. In today’s clinical practice, a specific humanized antibody is administered to high-risk infants, which is safe and effective in preventing RSV-related acute and postacute symptoms.
VIRUS STRUCTURE AND CLASSIFICATION
Knowledge of the molecular structure of RSV is important because it gives clues as to how it infects the human airways (Figure 1). The virus is made of a single strand of RNA contained in a nucleocapsid made of only 11 proteins and covered by a lipid envelope. Essential for the virulence of RSV are the surface glycoproteins G and F (fusion) that attach to the host airway epithelial cells and merge the viral envelope to the membranes of multiple adjacent cells, creating the “syncytia” that give RSV its name. G and F proteins are also the principal antigens exposed to the host immune system, and therefore the patient’s neutralizing antibodies are primarily directed against these targets.1
Human RSV is a Pneumovirus belonging to the Paramyxoviridae family. There are two strains of RSV (A and B) found in infants. Approximately 60% of all lower respiratory infections in preschool-aged children worldwide are caused by RSV (Figure 2).2 All the other viruses—including influenza, parainfluenza, metapneumovirus, and adenoviruses—as well as bacterial infections are much less common during the first years of life.
VIROLOGY
The burden of RSV continues to grow worldwide, particularly among the youngest segment of the population.2 During the first year of life, infants are not adequately protected from RSV by maternal immunoglobulins. Thus, approximately 80% of lower respiratory infections in children younger than 1 year are due to RSV with peak incidence occurring at 2 to 3 months of age. In the United States, bronchiolitis-associated hospitalizations in infants younger than 12 months more than doubled between 1980 and 1996, and in 1996, accounted for approximately 3% of all pediatric hospitalizations. Up to 120,000 RSV-related pediatric hospitalizations per year occur during a typical season.3
Mortality rates have improved significantly—at least in industrialized countries—and now are probably less than 1%.4 In the United States, RSV is estimated to cause fewer than 100 deaths annually, primarily because of better supportive care and the use of mechanical ventilation.
The seasonality of RSV varies by region. In the northern United States from October through March, RSV causes up to 90% of lower respiratory infection cases in infants. In the southern United States and farther into the southern hemisphere, the virus becomes endemic and can be present throughout the year with peak incidence occurring from August to September.2
RSV RISK FACTORS
The severity and frequency of RSV infection is influenced by well-known risk factors including environmental overcrowding, absence of breastfeeding, and immunosuppression. Children with chronic lung disease are predisposed to clinically significant RSV infection by their limited respiratory reserve, and 60% of those affected will be hospitalized. Hemodynamically significant congenital heart defects associated with higher pulmonary blood flow also increase risk for severe RSV disease, with more than 50% of children infected requiring hospitalization.4
Prematurity and increased RSV risk
Premature-born infants have a 10-fold increased risk of RSV infection and account for 25% to 30% of RSV hospitalizations annually.4 Prematurity as a risk factor for RSV is primarily tied to the physiology of placental immunoglobulin G (IgG) transfer. The human placenta is not permeable to IgG during the first half of pregnancy because of low expression of the Fc receptor needed to bind immunoglobulins and transfer them into the fetal circulation. Further, maternal IgG is recognized as a foreign protein by the newborn and is progressively removed from the circulation by the liver.5
Thus, the IgG decline continues postnatally, reaching the lowest concentration at 2 to 3 months of age because newborns are unable to synthesize their own antibodies. During this time, full-term born babies are at the highest risk for developing RSV infection. The risk is logically higher for premature infants who lack the full benefit of IgG transfer occurring during the last trimester, rendering antibody levels even lower at nadir. The greater the prematurity, the less the antibody protection and the more the predisposition to RSV infection.5
CLINICAL MANIFESTATIONS
The hallmark signs of RSV bronchiolitis are wheezing, cough, and increased work of breathing caused by infection of the bronchiolar airways. However, the nasal phase of the infection may cause irritation and trigeminal nerve activation associated with sneezing, congestion, and apnea. Indeed, approximately 20% of infants manifest an apnea episode as the first symptom of infection.6
The specific etiology of RSV can be confirmed by antigen detection tests, currently being replaced by more sensitive polymerase chain reaction (PCR)-based assays. Chest radiography reveals obvious signs of RSV lower respiratory infection, typically patch atelectasis, increased peribronchial markings, and bilateral hyperinflation with flattening of the diaphragm. Current guidelines indicate that the diagnosis of acute bronchiolitis should be based exclusively on the history and physical exam; it does not require radiographic or laboratory studies.
Correct etiologic diagnosis, however, is crucial to avoid unnecessary therapies and rule out rare conditions that could be worsened by therapies commonly used for bronchiolitis. For example, infants with dilated cardiomyopathy and congestive heart failure may present with symptoms of wheezing that mimic an acute respiratory infection, but these patients are at risk of developing supraventricular tachycardia and even cardiopulmonary collapse associated with administration of beta-agonist agents. In such cases, a chest radiograph will show cardiomegaly suggesting a different diagnosis and therapy, and thus avoid significant complications.
Recurrent wheezing
Children infected with RSV during their first year of life develop a higher risk of subsequent episodes of bronchial obstruction. Several retrospective studies conducted in the 1980s explored the incidence of lower respiratory symptoms continuing for years after initial RSV infection. Particularly unexpected was the finding that at least one-third of children hospitalized with RSV bronchiolitis will continue to wheeze beyond 6 to 8 years of age (Figure 3).7–9
More recent prospective studies of recurrent wheezing following RSV infection have been conducted. A Swedish study of 47 infants hospitalized with culture-proven RSV bronchiolitis showed significantly increased physician-diagnosed asthma (38%) compared with the 93 controls (2%) at age 7.5 years (P < .0001).10 Follow-up studies of the same cohort found increased risk for recurrent wheezing or asthma in the RSV group still present at age 13 years11 and 18 years12 compared with controls.
Asthma
History of RSV infection is important both as a trigger of asthma attacks and in the inception of asthma. Family history of asthma is the most important marker of a genetic predisposition to develop asthma. A multivariate analysis of the Swedish cohort discussed above showed that children with neither previous RSV infection nor family history of asthma had approximately a 5% risk of developing asthma by age 7.5 years. None of the children with a family history of asthma but no history of RSV infection developed asthma during follow-up. Children who had an RSV infection without a familial predisposition to asthma had approximately a 20% risk of asthma development. Children with both previous RSV infection and a family history of asthma had the highest (~40%) risk of developing asthma.10
These results provide important information about asthma pathogenesis in early life. The main implication is that even if a child has a genetic predisposition to asthma development, clinical manifestations will not develop without exposure to environmental agents that allow the actual expression of the predisposing genes, such as pollution, unbalanced diet, or early-life RSV infection. On the other hand, children without genetic predisposition can present with clinical manifestations undistinguishable from atopic asthma if their respiratory tract is exposed to specific viral pathogens during crucial developmental windows in early life. Finally, the combination of genetic predisposition and adverse environmental exposures in infancy carries the highest risk of asthma.
RSV MANAGEMENT
Ribavirin
The life cycle of RSV after the initial infection affects treatment strategies. RSV produces no symptoms for at least 3 to 5 days following infection (“eclipse phase”), during which time it reproduces exponentially and reaches the lungs, causing the first symptoms to be observable after 5 to 7 days. By the time the first symptoms emerge, the virus is already rapidly disappearing from the system.13 If ribavirin is administered at this point, the pediatrician has provided a virostatic agent against a virus that is no longer present, and therapy will not only be ineffective, but it may trigger bronchospasms.
The American Academy of Pediatrics (AAP) Committee on Infectious Diseases supported the use of ribavirin in 1993 guidelines,14 but changed their recommendation to “may be considered” in 1996.15 The only exception for ribavirin use is in the immunocompromised patient with RSV. In this scenario, the virus continues to replicate for months after the initial infection unopposed by the host defense mechanisms; therefore, aerosolized ribavirin therapy should be considered, either alone or in combination with humanized anti-RSV antibody.
Other treatments
Corticosteroids have not been shown to have a significant effect on RSV bronchiolitis.16 Alpha- and beta-adrenergic bronchodilators, such as epinephrine and albuterol, have shown very little or no effect on RSV symptoms in multiple controlled trials. The only effective treatment with proven efficacy is supportive therapy with adequate fluid intake and oxygen. Oral feeding should be withheld in patients with high respiratory rates to prevent aspiration.
PROPHYLAXIS
Vaccines
Vaccines have been ineffective against RSV and can be dangerous in children. RSV is not a strongly cytopathic virus; illness results primarily from the host immune response against the infection rather than from the virus itself.16 Therefore, any vaccine carries the risk of creating a stronger and potentially dangerous immune response to the next infection.
In the 1960s, a formalin-inactivated RSV vaccine was introduced with deleterious outcomes—it was minimally protective and was responsible for infant deaths. Because mortality was not immediate, the vaccine continued to be administered throughout the season. Unfortunately, with their immunity modified, vaccinated children became severely ill when they came in contact with the wild-type virus during the year following vaccination. The withdrawal of the formalin-inactivated vaccine still represents an important precedent that makes investigators and regulatory bodies very cautious about active immunization against this virus.
Palivizumab
Palivizumab is a 96% human monoclonal antibody targeting the RSV F protein, and it offers passive immunity for infants at risk for severe infection. The Impact-RSV clinical trial of palivizumab showed that five monthly intramuscular injections effectively reduced RSV hospitalizations by 78% in premature infants from 32 to 35 weeks gestation without bronchopulmonary dysplasia (BPD). Treatment offered only a 39% reduction in premature infants with BPD.17
The most recent AAP guidelines18 recommend palivizumab prophylaxis with a maximum of five monthly doses only in the first year of life for otherwise healthy infants born before 29 weeks gestation and for infants born before 32 weeks gestation with chronic lung disease of prematurity (CLD) defined as a requirement for supplemental oxygen for at least 28 days after birth. Prophylaxis is no longer recommended in the second year of life, except for infants with CLD still requiring oxygen, corticosteroids, or diuretics. Palivizumab prophylaxis should be discontinued if a breakthrough RSV hospitalization occurs because the likelihood of a second RSV hospitalization in the same season is low.
Palivizumab also should be considered for children with hemodynamically significant congenital heart defects, profound immunodeficiency, and pulmonary or neuromuscular pathologies impairing airway clearance; however, no formal recommendation was made for patients with Down syndrome or cystic fibrosis due to insufficient data.18 The protective effect of palivizumab appears to be cumulative, with almost half of the breakthrough infections observed in the clinical trials occurring after the first injection or immediately following the second.17
A recent randomized, double-blind, placebo-controlled trial has shown that palivizumab given to premature infants during the first year of life provides a 40% to 60% reduction of wheezing episodes.19 This trial confirms the results of previous retrospective studies; however, further large multicenter trials of palivizumab prophylaxis in both premature and full-term infants are needed to assess protection against recurrent wheezing and asthma, and to formulate evidence-based recommendations.
FUTURE DIRECTIONS
Nebulization of 3% saline improves mucociliary clearance and is increasingly being used in airway diseases involving mucus plugging (eg, cystic fibrosis). It also has been reported to reduce length of hospital stay and provide symptomatic relief in patients with bronchiolitis, but its use remains controversial. In particular, it has not been shown to be effective at reducing hospitalization when used in emergency settings. Therefore, based on current evidence, the administration of hypertonic saline for bronchiolitis should be limited to hospitalized infants.
Anti-leukotrienes used during the acute phase of RSV bronchiolitis have been shown to improve post-bronchiolitis respiratory symptoms, especially in younger patients with high urinary leukotriene E4 (LTE4). However, a large, multicenter, randomized, double-blind, placebo-controlled trial with montelukast failed to show statistically significant clinical improvement. A post-hoc data analysis revealed that children with persistent respiratory symptoms after the acute phase of the infection may benefit from montelukast, but the manufacturer (Merck) is no longer pursuing this indication.
There is not enough scientific evidence at present to support the use of DNAse or exogenous surfactant in the setting of acute bronchiolitis. New generations of specific antivirals and vaccines based on immunogens offer hope as new options for prophylaxis and/or management of RSV infection in the not too distant future.
Recent basic science investigations have provided new findings that could influence future therapies. Some of these studies have suggested that the tropism of RSV is not solely for the airway epithelium; rather, this virus can spread hematogenously to infect extrapulmonary targets, particularly the bone marrow stromal cells where RSV finds a sanctuary niche shielding it from immune protection and allowing subclinical latency. In one study, RSV was found in the bone marrow of every child and in about 80% of adults tested.20 Similar findings were replicated with other pathogens like Mycobacterium tuberculosis.21 This discovery could direct future treatment strategies for a number of viral, bacterial, and parasitic infections.
In another study, transplacental transmission of RSV from the mother’s airways to fetal lung tissues was shown for the first time in an animal model of infection. When the virus enters fetal respiratory cells, it persists and induces the expression of neurotrophic factors and receptors resulting in postnatal airways with dramatically increased parasympathetic innervation and methacholine reactivity. These structural and functional changes provide a suitable model to explain the development of long-term bronchial hyperreactivity following re-exposure to RSV in early life.22
In conclusion, the search for a cure for the most common respiratory infection in children has humbled several generations of investigators through the more than 50 years since RSV was discovered. Nevertheless, the rapid evolution in the fields of virology and immunology and recent breakthrough discoveries promise to deliver new strategies that may make a difference in the management of this common infection and its long-term sequelae.
- Peters TR, Crowe JE Jr. Respiratory syncytial virus. In: Long SS, Pickering LK, Prober CG, eds. Principles and Practice of Pediatric Infectious Diseases. 3rd ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2008.
- Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev 2014; 35:519–530.
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999; 282:1440–1446.
- Simoes EA, Rieger CHL. RSV infection in developed and developing countries. Infect Med 1999; 16:S11–S17.
- Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1,500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res 1986; 20:899 –904.
- Sabogal C, Auais A, Napchan G, et al. Effect of respiratory syncytial virus on apnea in weanling rats. Pediatr Res 2005; 57:819–825.
- Hall CB, Hall WJ, Gala CL, MaGill FB, Leddy JP. Long-term prospective study in children after respiratory syncytial virus infection. J Pediatr 1984; 105:358–364.
- Henry RL, Hodges IGC, Milner AD, Stokes GM. Respiratory problems 2 years after acute bronchiolitis in infancy. Arch Dis Child 1983; 58:713–716.
- Webb MSC, Henry RL, Milner AD, Stokes GM, Swarbrick AS. Continuing respiratory problems three and a half years after acute viral bronchiolitis. Arch Dis Child 1985; 60:1064–1067.
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161:1501–1507.
- Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005; 171:137–141.
- Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65:1045–1052.
- McIntosh K, Chanock RM. Respiratory syncytial virus. In: Fields BN, Knipe DM, eds. Virology. New York: Raven Press; 1990:1045.
- Committee on Infectious Diseases. American Academy of Pediatrics: use of ribavirin in the treatment of respiratory syncytial virus infection. Pediatrics 1993; 92:501–504.
- Committee on Infectious Diseases. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 1996; 97:137–140.
- Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol 2011; 46:324–347.
- The Impact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory synctial virus infection in high-risk infants. Pediatrics 1998; 102(3 Pt 1):531–537.
- Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134:e1474–e1502.
- Blanken MO, Rovers MM, Molenaar JM, et al; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–1799.
- Rezaee F, Gibson LF, Piktel D, Othumpangat S, Piedimonte G. Respiratory syncytial virus infection in human bone marrow stromal cells. Am J Respir Cell Mol Biol 2011; 45:277–286.
- Das B, Kashino SS, Pulu I, et al. CD271+ bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med 2013; 5:170ra13.
- Piedimonte G, Perez MK. Alternative mechanisms for respiratory syncytial virus (RSV) infection and persistence: could RSV be transmitted through the placenta and persist into developing fetal lungs? Curr Opin Pharmacol 2014; 16:82–88.
- Peters TR, Crowe JE Jr. Respiratory syncytial virus. In: Long SS, Pickering LK, Prober CG, eds. Principles and Practice of Pediatric Infectious Diseases. 3rd ed. Philadelphia, PA: Churchill Livingstone/Elsevier; 2008.
- Piedimonte G, Perez MK. Respiratory syncytial virus infection and bronchiolitis. Pediatr Rev 2014; 35:519–530.
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. JAMA 1999; 282:1440–1446.
- Simoes EA, Rieger CHL. RSV infection in developed and developing countries. Infect Med 1999; 16:S11–S17.
- Ballow M, Cates KL, Rowe JC, Goetz C, Desbonnet C. Development of the immune system in very low birth weight (less than 1,500 g) premature infants: concentrations of plasma immunoglobulins and patterns of infections. Pediatr Res 1986; 20:899 –904.
- Sabogal C, Auais A, Napchan G, et al. Effect of respiratory syncytial virus on apnea in weanling rats. Pediatr Res 2005; 57:819–825.
- Hall CB, Hall WJ, Gala CL, MaGill FB, Leddy JP. Long-term prospective study in children after respiratory syncytial virus infection. J Pediatr 1984; 105:358–364.
- Henry RL, Hodges IGC, Milner AD, Stokes GM. Respiratory problems 2 years after acute bronchiolitis in infancy. Arch Dis Child 1983; 58:713–716.
- Webb MSC, Henry RL, Milner AD, Stokes GM, Swarbrick AS. Continuing respiratory problems three and a half years after acute viral bronchiolitis. Arch Dis Child 1985; 60:1064–1067.
- Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am J Respir Crit Care Med 2000; 161:1501–1507.
- Sigurs N, Gustafsson PM, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005; 171:137–141.
- Sigurs N, Aljassim F, Kjellman B, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax 2010; 65:1045–1052.
- McIntosh K, Chanock RM. Respiratory syncytial virus. In: Fields BN, Knipe DM, eds. Virology. New York: Raven Press; 1990:1045.
- Committee on Infectious Diseases. American Academy of Pediatrics: use of ribavirin in the treatment of respiratory syncytial virus infection. Pediatrics 1993; 92:501–504.
- Committee on Infectious Diseases. Reassessment of the indications for ribavirin therapy in respiratory syncytial virus infections. Pediatrics 1996; 97:137–140.
- Wright M, Piedimonte G. Respiratory syncytial virus prevention and therapy: past, present, and future. Pediatr Pulmonol 2011; 46:324–347.
- The Impact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory synctial virus infection in high-risk infants. Pediatrics 1998; 102(3 Pt 1):531–537.
- Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics 2014; 134:e1474–e1502.
- Blanken MO, Rovers MM, Molenaar JM, et al; Dutch RSV Neonatal Network. Respiratory syncytial virus and recurrent wheeze in healthy preterm infants. N Engl J Med 2013; 368:1791–1799.
- Rezaee F, Gibson LF, Piktel D, Othumpangat S, Piedimonte G. Respiratory syncytial virus infection in human bone marrow stromal cells. Am J Respir Cell Mol Biol 2011; 45:277–286.
- Das B, Kashino SS, Pulu I, et al. CD271+ bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis. Sci Transl Med 2013; 5:170ra13.
- Piedimonte G, Perez MK. Alternative mechanisms for respiratory syncytial virus (RSV) infection and persistence: could RSV be transmitted through the placenta and persist into developing fetal lungs? Curr Opin Pharmacol 2014; 16:82–88.