User login
Pathophysiology of type 2 diabetes mellitus: potential role of incretin-based therapies
Glucose-lowering effects of incretin-based therapies
Safety, tolerability, and nonglycemic effects of incretin-based therapies
- The glucagon-like peptide (GLP)-1 agonists promote a 1 kg to 4 kg weight loss and satiety, while the dipeptidyl peptidase (DPP)-4 inhibitors are usually weight neutral
- The GLP-1 agonists and DPP-4 inhibitors have a favorable safety profile, including rare occurrence of severe hypoglycemia and a low incidence of mild to moderate hypoglycemia
- Mild to moderate nausea associated with GLP-1 agonists generally resolves over 4 to 8 weeks and can be minimized by dose escalation strategies
- Hypersensitivity reactions occur infrequently with DPP-4 inhibitors
- Active surveillance and investigation are ongoing regarding the possible association of GLP-1 agonists and/or DPP-4 inhibitors with acute pancreatitis; thyroid medullary cancer; and with cardiovascular disease
The authors received editorial assistance from the Primary Care Education Consortium and WriteHealth, LLC in the development of this activity and honoraria from the Primary Care Education Consortium. They have disclosed that Dr Campbell is on the advisory board for Daiichi-Sankyo and the speakers bureau for Eli Lilly and Co; Dr Cobble is on the advisory board for Abbott Laboratories, AstraZeneca, and Eli Lilly and Co and speakers bureau for Abbott Laboratories, AstraZeneca/Bristol Myers Squibb, Eli Lilly and Co, GlaxoSmithKline, and Novo Nordisk Inc; Dr Reid is on the advisory board and speakers bureau for Amylin Pharmaceuticals, Medtronic, Novo Nordisk Inc, and sanofi-aventis; and Dr Shomali is on the advisory board for Novo Nordisk Inc and speakers bureau for Amylin Pharmaceuticals, Eli Lilly and Co, sanofi-aventis, and Takeda Pharmaceuticals.
Introduction
The GLP-1 agonists and DPP-4 inhibitors have important benefits beyond lowering glycosylated hemoglobin, fasting plasma glucose, and postprandial glucose. Although an important treatment goal for type 2 diabetes mellitus (T2DM) is to reduce the risk of other diabetes-related diseases, eg, cardiovascular disease, many glucose-lowering agents cause weight gain, thereby adding to the burgeoning problem of obesity and related long-term consequences, as shown in our 3 case studies. In addition, cardiovascular risks associated with thiazolidinediones have become a major concern.
Side effects and their impact on patient tolerability are also important considerations when selecting and titrating therapy. Concerns about hypoglycemia can affect patient adherence to a medication regimen and a patient’s willingness to continue and intensify therapy, especially with insulin and sulfonylureas.1-3 As seen in Cases 1 (metformin-related diarrhea) and 2 (pioglitazone-related edema), patient adherence and willingness to continue therapy can be jeopardized by medication-related side effects.
Given these issues, the use of glucose-lowering medications that reduce related risk factors and have a favorable safety profile is advantageous. The GLP-1 agonists and DPP-4 inhibitors are desirable options, based on these considerations.
Reducing risk
The importance of weight
It is well recognized that weight gain is a major risk factor for T2DM and other disorders. It is also clear that concerns about weight gain adversely affect a patient’s willingness to begin treatment with glucose-lowering medications such as thiazolidinediones (TZDs), insulin, and sulfonylureas.1-3 The other side of the story is probably less appreciated—that is, weight loss can be a significant motivating factor for patients with T2DM that, in our experience, can improve adherence to lifestyle intervention and a medication regimen. In fact, improved quality of life, as assessed by physical and emotional domains, has been reported as a result of liraglutide-associated weight loss.4 In addition, multidimensional assessment of patient satisfaction generally has shown similar improvement with liraglutide 1.2 mg once daily and sitagliptin 100 mg once daily, and significantly greater improvement with liraglutide 1.8 mg once daily.5 Consequently, the ability of GLP-1 agonists to promote weight loss in most patients and of DPP-4 inhibitors to be weight neutral offers important benefits. Weight is an issue in all 3 of our patient cases, especially in Cases 1 and 2, where the patients’ body mass indices (BMIs) are ≥30 kg/m2.
Either as monotherapy or when added to glucose-lowering therapy, twice-daily exenatide or once-daily liraglutide generally promotes a 1 kg to 4 kg weight loss.4,6-11 The addition of exenatide or liraglutide to metformin, a sulfonylurea, or both resulted in a mean 2.9 kg weight loss with exenatide and a 3.2 kg loss with liraglutide after 26 weeks.12 Patients who continued liraglutide for an additional 14 weeks lost an additional 0.4 kg, while patients switched from exenatide to liraglutide lost an additional 0.9 kg.13 The amount of weight lost is greater with a higher baseline BMI.14,15 It is important to note, however, that 16% of patients did not experience any weight loss,14 possibly because no specific caloric restriction was required.14,16 Slight increases to slight decreases in weight have been observed in clinical trials with sitagliptin and saxagliptin.17-23 Comparison of liraglutide with sitagliptin showed a mean weight loss of 2.9 kg and 3.4 kg for liraglutide 1.2 mg and 1.8 mg once daily, respectively, over 26 weeks and a 1.0 kg weight loss with sitagliptin 100 mg once daily.5 Analysis of a large cohort database that followed patients for up to 1 year showed that patients treated with exenatide lost a mean of 3.0 kg, while patients treated with sitagliptin lost 1.1 kg and those treated with insulin gained 0.6 kg.24 Accordingly, the DPP-4 inhibitors are considered weight neutral.
The reason for the difference between GLP-1 agonists and DPP-4 inhibitors with respect to weight remains unclear, but may result from the direct action of GLP-1 agonists on the GLP-1 receptor compared to the indirect action of DPP-4 inhibitors, which slow the clearance of endogenous GLP-1.25 This may explain the ability of GLP-1 agonists—but not DPP-4 inhibitors—to promote satiety and reduce caloric intake.26,27 In a crossover comparison of exenatide with sitagliptin, caloric intake during a standardized meal decreased with exenatide (–134 kcal) and increased with sitagliptin (+130 kcal) (P=.0227).16
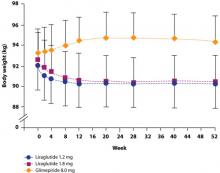
The possibility that the nausea associated with GLP-1 agonists but not DPP-4 inhibitors is the reason for the difference in weight effects has been investigated. Analyses have shown, however, that the weight loss observed with GLP-1 agonists is independent of nausea.4,10,28 For example, in a study by Garber et al,4 there was no difference in the amount of weight lost among patients who experienced liraglutide-associated nausea for >7 days, for 1 to 7 days, or not at all. The finding that nausea generally resolved within the first few weeks of liraglutide treatment, while weight loss was maintained over the 52 weeks of the trial, provides further evidence that nausea is not the cause of weight loss (FIGURE 1).4 Similar long-term weight loss has been observed with exenatide: weight loss was achieved through 30 weeks (–3.0 kg; P<.05 vs baseline) of double-blind treatment and maintained during an additional 52 weeks of open-label treatment (–5.3 kg; P<.05 vs baseline).29
FIGURE 1 Change in body weight (kg) over 52 weeks with liraglutide 1.2 mg and 1.8 mg vs glimepiride 8.0 mg4
cardiovascular benefits
The effects of incretin therapy on markers of cardiovascular disease have been assessed in several clinical trials (TABLE 1).4,8-12,17,22,30,31 In general, these trials demonstrate small but significant reductions in systolic blood pressure (1 to 7 mm Hg) with the GLP-1 agonists; diastolic blood pressure, however, is not significantly affected.4,8-12,30,31 There are insufficient data regarding the DPP-4 inhibitors.17,22
Effects on the lipid profile also have been investigated,4,5,8-12,17,22,30,31 but differences among the agents are difficult to assess because of different baseline lipid levels and the limited number of direct comparative studies. In general, clinical studies show that low-density lipoprotein (LDL) cholesterol is reduced by 1 to 17 mg/dL with the GLP-1 agonists and increased by 3 to 9 mg/dL with the DPP-4 inhibitors. Changes in high-density lipoprotein (HDL) cholesterol are generally small for both GLP-1 agonists and DPP-4 inhibitors, ranging from an increase of 5 mg/dL to a decrease of 2 mg/dL. The greatest change in the lipid profile is in the triglyceride level, with a reduction of 12 to 40 mg/dL with the GLP-1 agonists, while the triglyceride changes observed with the DPP-4 inhibitors range from a reduction of 35 mg/dL to an increase of 16 mg/dL.
Two studies have directly compared the effects on the lipid profile of 2 incretin agents over 26 weeks. In the first study, exenatide and liraglutide showed similar changes in LDL cholesterol (–7 vs –8 mg/dL, respectively) and HDL cholesterol (–1 vs –1 mg/dL, respectively) levels, but liraglutide showed a significantly greater reduction in triglyceride levels than exenatide (–36 vs –20 mg/dL, respectively; P=.0485).12 The second study compared liraglutide 1.2 mg and 1.8 mg once daily and sitagliptin 100 mg once daily.5 LDL cholesterol increased by 1 mg/dL in both liraglutide groups and by 2 mg/dL in the sitagliptin group, while there was no change in HDL cholesterol with either dose of liraglutide or sitagliptin. The triglyceride level decreased by 17 mg/dL in the liraglutide 1.2 mg group, 38 mg/dL in the liraglutide 1.8 mg group, and 35 mg/dL in the sitagliptin group. None of the differences between either liraglutide group and sitagliptin were statistically significant.
Effects on the lipid profile appear to be durable. Klonoff et al showed that the effects of exenatide on the lipid profile were sustained over 3.5 years of follow-up (in a 30-week randomized, double-blind trial followed by a 3-year open-label extension).14 In patients younger than 65 years, changes from baseline were as follows: total cholesterol, –10 mg/dL (P=.0056); LDL cholesterol, –11 mg/dL (P=.0012); HDL cholesterol, +8 mg/dL (P<.0001); and triglycerides, –44 mg/dL (P=.0042). Similar changes were observed in those 65 years and older.
The cardiovascular benefits of incretin therapy may extend beyond blood pressure and the lipid profile. An exploratory subanalysis of a randomized controlled trial with liraglutide showed a significant decrease in plasminogen activator inhibitor-1, an inflammatory biomarker, and B-type natriuretic peptide, a marker of left ventricular dysfunction.32 No significant effect on high-sensitivity C-reactive protein, interleukin-6, or tumor necrosis factor-α was observed.
While not appropriate as primary therapy, the effect of the GLP-1 agonists on blood pressure and of the GLP-1 agonists and DPP-4 inhibitors on the lipid profile could be an added benefit for all patients with T2DM because of the strong association between T2DM and cardiovascular disease. This can be seen in all 3 of our cases: Case 1 (hypertriglyceridemia), Case 2 (essential hypertension), and Case 3 (peripheral arterial disease).
TABLE 1
Selected studies with GLP-1 agonists and DPP-4 inhibitors assessing cardiovascular end points4,8-12,17,22,30,31
| Agent/clinical trial | Concomitant treatment; duration (wk) | Change from baseline | |||
|---|---|---|---|---|---|
| Systolic blood pressure (mm Hg) | LDL cholesterol (mg/dL) | HDL cholesterol (mg/dL) | Triglycerides (mg/dL) | ||
| Exenatide (E) | |||||
| Moretto, 200830 | Diet/exercise; | ||||
| E, 5 μg BID | 24 | -4 | NR | NR | NR |
| E, 10 μg BID | -4 | NR | NR | NR | |
| Placebo | 0 | NR | NR | NR | |
| Blonde, 20068 | MET + SU; | ||||
| E, 5 μg BID | 82 wk | -1 | -2 | +5 | -39 |
| E, 10 μg BID | -1 | -2 | +5 | -39 | |
| Nauck, 200731 | MET + SU; | ||||
| E, 10 μg BID | 52 wk | -5 | NC | NR | NC |
| Premix aspart 70/30 BID | +1 | NC | NR | NC | |
| Zinman, 20079 | TZD ± MET; | ||||
| E, 10 μg BID | 16 wk | NC | NC | NC | NC |
| Placebo | NC | NC | NC | NC | |
| Liraglutide (L) | |||||
| Garber, 20094 | None; 52 wk | ||||
| L, 1.2 mg OD | -2 | NR | NR | NR | |
| L, 1.8 mg OD | -4 | NR | NR | NR | |
| Glimepiride, 8 mg OD | -1 | NR | NR | NR | |
| Russell-Jones, 200910 | MET + GLIM; | ||||
| L, 1.8 mg OD | 26 wk | -4 | NR | NR | NR |
| Insulin glargine | +1 | NR | NR | NR | |
| Placebo | -1 | NR | NR | NR | |
| Zinman, 200911 | MET + ROSI; | ||||
| L, 1.2 mg OD | 26 wk | -7 | -11 | -1 | -34 |
| L, 1.8 mg OD | -6 | -9 | -1 | -29 | |
| Placebo | -1 | -4 | -1 | -5 | |
| Buse, 200912 | MET, SU, MET + SU; | ||||
| L, 1.8 mg OD | -3 | -17 | -2 | -36 | |
| E, 10 μg BID | 26 wk | -2 | -15 | -2 | -20 |
| Sitagliptin (Si) | |||||
| Scott, 200717 | Diet/exercise; | ||||
| Si, 25 mg BID | 12 wk | NR | 0 | +1 | -3 |
| Si, 50 mg BIDa | NR | +1 | +1 | 0 | |
| Glipizide, 5 mg OD | NR | 0 | 0 | +3 | |
| Placebo | NR | 0 | 0 | +16 | |
| Saxagliptin (Sa) | |||||
| Hollander, 200922 | TZD; | ||||
| Sa, 2.5 mg OD | 24 wk | NR | +4 | -1 | -1 |
| Sa, 5 mg OD | NR | +9 | +2 | -4 | |
| Placebo | NR | +3 | 0 | -1 | |
| aDose not included in currently approved prescribing information. | |||||
| DPP, dipeptidyl peptidase; GLIM, glimepiride; GLP, glucagon-like peptide; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MET, metformin; NC, no difference between baseline and study end; NR, not reported; OD, once daily; ROSI, rosiglitazone; SBP, systolic blood pressure; SU, sulfonylurea; TZD, thiazolidinedione. | |||||
Favorable safety profile
Overview
In general, GLP-1 agonists and DPP-4 inhibitors are well tolerated. Because of concerns about hypoglycemia with glucose-lowering agents, signs and symptoms of hypoglycemia have been closely monitored in clinical trials. The low incidence and mild to moderate severity of hypoglycemia are important attributes of the GLP-1 agonists and DPP-4 inhibitors.
Other than hypoglycemia, the most common adverse reaction reported in ≥5% of patients and more commonly than with placebo are shown in TABLE 2.33-36 Other medication-specific side effects are seen infrequently but bear mentioning. An increased international normalized ratio, sometimes with bleeding, has been noted with combined use of exenatide and warfarin.33 Immune-related events (eg, urticaria) have been reported with exenatide33 and have been observed to occur more frequently with liraglutide than with comparator agents.34 Peripheral edema is more common when a thiazolidinedione is administered with saxagliptin than with placebo.36
While experience with GLP-1 agonists and DPP-4 inhibitors indicates that they have favorable safety profiles, some concerns have surfaced with 1 or more of these agents during clinical trials or from postmarketing reports. These include gastrointestinal side effects (principally nausea), acute pancreatitis, and hypersensitivity reactions. In addition, new standards recently adopted by the US Food and Drug Administration (FDA) are requiring further investigation of several issues for newly approved glucose-lowering drugs and those in development. These issues are discussed next.
TABLE 2
Most common side effectsa of the GLP-1 agonists and DPP-4 inhibitors33-36
| Agent | Side effects |
|---|---|
| Exenatide | Nausea, vomiting, diarrhea, feeling jittery, dizziness, headache, dyspepsia |
| Liraglutide | Nausea, diarrhea, headache |
| Sitagliptin | Upper respiratory tract infection, nasopharyngitis, headache |
| Saxagliptin | Upper respiratory tract infection, urinary tract infection, headache |
| DPP, dipeptidyl peptidase; GLP, glucagon-like peptide. | |
| aOccurring in ≥5% of patients and more frequently than with placebo. | |
Hypoglycemia
In contrast to other glucose-lowering drugs that stimulate insulin secretion, incretin-based therapies have a glucose-dependent mechanism of action that minimizes the risk of hypoglycemia. Preclinical investigation showed that GLP-1 acts on islet α-cells to strongly inhibit postprandial glucagon secretion.37,38 These observations were subsequently supported by early clinical investigations showing that administration of GLP-1 to healthy volunteers and people with T2DM augmented insulin secretion and decreased glucagon secretion in a glucose-dependent manner.39,40 Similar effects were also observed with a DPP-4 inhibitor.25 At the same time, GLP-1 has been shown not to suppress glucagon secretion at a plasma glucose level <65 mg/dL.41 This mechanism is believed to maintain the counterregulatory hormone response that serves to prevent hypoglycemia.
Accordingly, severe hypoglycemia has not been observed in monotherapy trials of exenatide,6,30 liraglutide,4,42 sitagliptin,17,19 or saxagliptin.21 Mild to mod erate hypoglycemia has been observed in 4% to 9% of patients treated with exenatide monotherapy6,30 and 0% to 12% of patients treated with liraglutide monotherapy4,42; by comparison, the incidence was 24% in patients treated with glimepiride monotherapy.4 Mild to moderate hypoglycemia has been found to be less frequent with sitagliptin and saxagliptin. In monotherapy and combination studies, 0% to 4% of patients treated with sitagliptin and 0% to 2% administered placebo experienced mild to moderate hypoglycemia.17,18,43-45 Mild to moderate hypoglycemia has not been observed in patients treated with saxagliptin monotherapy at doses of 2.5 mg to 40 mg.21 When saxagliptin was added to metformin, hypoglycemia was reported by 5.7% of patients compared with 5.0% of patients who added placebo to metformin.46
It is important to note that the incidence of mild to moderate hypoglycemia is increased in patients treated concomitantly with a GLP-1 agonist or DPP-4 inhibitor and a sulfonylurea. In 1 trial, 14% to 36% of patients treated with a combination of exenatide and a maximally effective dose of a sulfonylurea (glimepiride, glipizide, glyburide, chlorpropamide, tolazamide) reported mild to moderate hypoglycemia compared with 3% of those administered a placebo and a sulfonylurea.47 A trial involving the addition of saxagliptin to glyburide found that 13% to 15% of patients treated daily with glyburide 7.5 mg and saxagliptin 2.5 mg or 5 mg reported mild to moderate hypoglycemia compared with 10% of patients treated with glyburide 10 mg to 15 mg alone.23 For this reason, consideration should be given to reducing the dose of a sulfonylurea or secretagogue when combined with a GLP-1 agonist or DPP-4 inhibitor.33-35
Because of their low incidence of hypoglycemia, the American Diabetes Association/European Association for the Study of Diabetes panel recommends GLP-1 agonists for patients for whom hypoglycemia is particularly undesirable, such as those who perform manual labor, drive a vehicle for a living, or operate heavy or dangerous machinery.48 In fact, the US Federal Aviation Administration lists the GLP-1 agonists and DPP-4 inhibitors as allowable medications for aviators.49 This recommendation is particularly appropriate for the building contractor in Case 1.
Nausea
Transient nausea is the most common GI side effect associated with GLP-1 agonists, occurring in up to 57% of patients treated with exenatide in clinical trials6,30 and 29% treated with liraglutide.4 Diarrhea and vomiting also occurred, although rates were similar to rates with initiation of metformin. The high occurrence of transient nausea in these trials prompted investigators to implement a dose escalation strategy33,34 (see “Patient education and self-management” article in this supplement). Since adoption of this strategy, a comparative trial of exenatide and liraglutide found that nausea occurred in 28% of patients treated with exenatide and in 26% treated with liraglutide.12 Nausea was generally transient, so that by Week 6 of therapy, 16% of patients treated with exenatide and 8% with liraglutide experienced nausea,12 and by Week 26, 9% of exenatide-treated patients and 3% of liraglutide-treated patients continued to experience nausea.12
Of patients treated with the DPP-4 inhibitor sitagliptin, nausea has occurred in 1% to 2% com pared with 1% of those receiving placebo.18,43 In patients treated with saxagliptin, nausea has occurred in 2% to 4% compared with 8% of those receiving placebo.21
Acute pancreatitis
Acute pancreatitis has been observed in clinical trials and/or identified in postmarketing reports involving exenatide,33 liraglutide,34 and sitagliptin.36 Determining whether there is a true association of these agents with acute pancreatitis or this is just coincidental has been difficult, partly because patients with T2DM have a 2.8-fold greater risk of pancreatitis compared with nondiabetic subjects.50 A review of health insurance transactions with 1-year follow-up (June 2005 through June 2008) involving approximately 88,000 patients (exenatide, n=27,996; sitagliptin, n=16,276; approximately equal numbers of matched comparators) showed that the relative risk of pancreatitis was statistically the same with exenatide, sitagliptin, metformin, and glyburide.51
In its ongoing review, the FDA has required the manufacturers of exenatide and sitagliptin to modify product labeling regarding the risk of acute pancreatitis and to conduct additional animal studies.52 As part of the January 2010 approval of liraglutide, the FDA required the manufacturer to perform mechanistic studies in animals and to conduct an epidemiologic evaluation using a large insurance claims database.52 In the interim, exenatide, liraglutide, and sitagliptin should be used cautiously,34,35 if at all, in people with a history of pancreatitis.33 Furthermore, educating patients about the signs and symptoms of pancreatitis, including how to differentiate it from the transient nausea commonly observed with these agents, is critical. Patients at risk of developing acute pancreatitis (eg, due to excess alcohol consumption or gallstones) should not receive an incretin-based therapy. Therapy should be changed if a patient develops acute pancreatitis while using a GLP-1 agonist or DPP-4 inhibitor.
Hypersensitivity
Hypersensitivity reactions have been experienced by some patients treated with exenatide,33 liraglutide,34 sitagliptin,35 or saxagliptin.36 Postmarketing reports have described serious hypersensitivity reactions (anaphylaxis, angioedema) with exenatide.33 In clinical trials, 0.8% of patients treated with liraglutide and 0.4% treated with comparator agents experienced an immunogenic reaction, generally urticaria.34 With sitagliptin, anaphylaxis, angioedema, or exfoliative dermatitis, including Stevens-Johnson syndrome, typically occurs within 3 months but may occur after the first dose. Hypersensitivity events, such as urticaria and facial edema, were shown to occur in 1.5% of patients treated with saxagliptin 2.5 mg, 1.5% of those treated with saxagliptin 5 mg, and 0.4% of those receiving placebo. None of the events necessitated hospitalization or were life-threatening.36 If a hypersensitivity reaction occurs, treatment should be discontinued.
additional safety investigations
The FDA has required additional safety investigations for liraglutide, saxagliptin, and sitagliptin. These investigations will address observations made during preclinical and clinical evaluation, as well as the new standards adopted by the agency in December 2008 regarding cardiovascular safety for all new glucose-lowering agents.53
Thyroid cancer
Rodent studies have suggested that liraglutide in doses many times those utilized in humans is associated with an increased risk of preneoplastic lesions that can lead to C-cell hyperplasia and medullary thyroid cancer, which occurs rarely in humans.52 Thyroid tumors have also been observed in rodents administered native GLP-154 or exenatide33 but not sitagliptin35 or saxagliptin.36 A slight increase in calcitonin, a marker for medullary cancer, which remained well within the normal reference range, was observed during Phase 3 clinical trials in patients treated with liraglutide compared with controls. There were no cases of medullary thyroid cancer in the liraglutide trials, including one that involved more than 2 years of follow-up. Based on this evidence, the FDA determined that the risk of thyroid cancer among humans treated with liraglutide is low. The effects may be due to species-specific differences in GLP-1 receptor expression and action in the thyroid, as 20 months of liraglutide at >60 times the human exposure level did not lead to C-cell hyperplasia in monkeys.55 The FDA has required the manufacturer to conduct additional animal studies, however, and to establish a cancer registry to monitor the annual incidence of medullary thyroid cancer over the next 15 years.52 In addition, the prescribing information for liraglutide carries a boxed warning describing the rodent findings and the risk of medullary thyroid cancer.34 Liraglutide is also contraindicated in patients with a personal or family history of medullary thyroid cancer or in patients with multiple endocrine neoplasia syndrome type 2.34
Cardiovascular risk
The clinical evaluation of liraglutide and saxagliptin was completed prior to December 2008, when the FDA adopted the new cardiovascular safety standards for new antidiabetic drugs. Analyses of data from Phase 2 and 3 clinical trials indicate that liraglutide meets the new 2008 standard for ruling out an unacceptable increase in cardiovascular risk.52 While the overall rates of cardiovascular events were low in preapproval clinical trials, the more stringent criteria for postapproval evaluations were not met. Consequently, the FDA has required postapproval clinical trials of cardiovascular safety with liraglutide52 and saxagliptin.
Summary
The overall safety profiles of GLP-1 agonists and DPP-4 inhibitors are favorable, with a low incidence of hypoglycemia. This attribute, along with their weight and cardiovascular benefits, particularly with the GLP-1 agonists, make them appropriate choices in our 3 patient cases. Ongoing safety investigations with GLP-1 agonists and DPP-4 inhibitors will provide further clarity to the complete safety profiles of these agents.
1. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
2. Holstein A, Plaschke A, Egberts EH. Lower incidence of severe hypoglycaemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev. 2001;17:467-473.
3. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673-2679.
4. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473-481.
5. Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447-1456.
6. Nelson P, Poon T, Guan X, et al. The incretin mimetic exenatide as a monotherapy in patients with type 2 diabetes. Diabetes Technol Ther. 2007;9:317-326.
7. Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083-1091.
8. Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 over-weight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436-447.
9. Zinman B, Hoogwerf BJ, Duran GS, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146:477-485.
10. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52:2046-2055.
11. Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32:1224-1230.
12. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47.
13. Buse JB, Sesti G, Schmidt WE, et al. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33:1300-1303.
14. Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275-286.
15. Russell-Jones D, Shaw J, Brandle M, et al. The once-daily human GLP-1 analog liraglutide reduces body weight in subjects with type 2 diabetes, irrespective of body mass index at baseline. Paper presented at: American Diabetes Association 68th Scientific Session; June 6-10, 2008; San Francisco, CA.
16. DeFronzo RA, Okerson T, Viswanathan P, et al. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943-2952.
17. Scott R, Wu M, Sanchez M, et al. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract. 2007;61:171-180.
18. Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632-2637.
19. Hanefeld M, Herman GA, Wu M, et al. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin. 2007;23:1329-1339.
20. Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733-745.
21. Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:376-386.
22. Hollander P, Li J, Allen E, et al. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab. 2009;94:4810-4819.
23. Chacra AR, Tan GH, Apanovitch A, et al. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract. 2009;63:1395-1406.
24. Horton ES, Silberman C, Davis KL, et al. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33:1759-1765.
25. Deacon CF, Hughes TE, Holst JJ. Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig. Diabetes. 1998;47:764-769.
26. Gutzwiller JP, Drewe J, Goke B, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541-R1544.
27. Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824-830.
28. Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84-90.
29. Ratner RE, Maggs D, Nielsen LL, et al. Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2006;8:419-428.
30. Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448-1460.
31. Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:259-267.
32. Courreges JP, Vilsboll T, Zdravkovic M, et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabet Med. 2008;25:1129-1131.
33. Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
34. Victoza [package insert]. Princeton, NJ: Novo Nordisk Inc; 2010.
35. Januvia [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; February 2, 2010.
36. Onglyza [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2009.
37. Naslund E, Bogefors J, Skogar S, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;277:R910-R916.
38. Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300-1304.
39. Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741-744.
40. Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301-307.
41. Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239-1246.
42. Vilsboll T, Zdravkovic M, Le Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30:1608-1610.
43. Raz I, Hanefeld M, Xu L, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564-2571.
44. Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638-2643.
45. Rosenstock J, Brazg R, Andryuk PJ, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556-1568.
46. DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes on metformin alone. Diabetes Care. 2009;32:1649-1655.
47. Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628-2635.
48. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
49. Guide for aviation medical examiners. Federal Aviation Administration. http://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/pharm/oral_diabetes/index.cfm?print=go. Published 2009. Accessed July 23, 2010.
50. Noel RA, Braun DK, Patterson RE, et al. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2009;32:834-838.
51. Dore DD, Seeger JD, Arnold CK. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin. 2009;25:1019-1027.
52. Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med. 2010;362:774-777.
53. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. US Food and Drug Administration. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guiddances/ucm071627.pdf. Published 2008. Accessed April 14, 2010.
54. Lamari Y, Boissard C, Moukhtar MS, et al. Expression of glucagon-like peptide 1 receptor in a murine C cell line: regulation of calcitonin gene by glucagon-like peptide 1. FEBS Lett. 1996;393:248-252.
55. Knudsen LB, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473-1486.
Pathophysiology of type 2 diabetes mellitus: potential role of incretin-based therapies
Glucose-lowering effects of incretin-based therapies
Safety, tolerability, and nonglycemic effects of incretin-based therapies
- The glucagon-like peptide (GLP)-1 agonists promote a 1 kg to 4 kg weight loss and satiety, while the dipeptidyl peptidase (DPP)-4 inhibitors are usually weight neutral
- The GLP-1 agonists and DPP-4 inhibitors have a favorable safety profile, including rare occurrence of severe hypoglycemia and a low incidence of mild to moderate hypoglycemia
- Mild to moderate nausea associated with GLP-1 agonists generally resolves over 4 to 8 weeks and can be minimized by dose escalation strategies
- Hypersensitivity reactions occur infrequently with DPP-4 inhibitors
- Active surveillance and investigation are ongoing regarding the possible association of GLP-1 agonists and/or DPP-4 inhibitors with acute pancreatitis; thyroid medullary cancer; and with cardiovascular disease
The authors received editorial assistance from the Primary Care Education Consortium and WriteHealth, LLC in the development of this activity and honoraria from the Primary Care Education Consortium. They have disclosed that Dr Campbell is on the advisory board for Daiichi-Sankyo and the speakers bureau for Eli Lilly and Co; Dr Cobble is on the advisory board for Abbott Laboratories, AstraZeneca, and Eli Lilly and Co and speakers bureau for Abbott Laboratories, AstraZeneca/Bristol Myers Squibb, Eli Lilly and Co, GlaxoSmithKline, and Novo Nordisk Inc; Dr Reid is on the advisory board and speakers bureau for Amylin Pharmaceuticals, Medtronic, Novo Nordisk Inc, and sanofi-aventis; and Dr Shomali is on the advisory board for Novo Nordisk Inc and speakers bureau for Amylin Pharmaceuticals, Eli Lilly and Co, sanofi-aventis, and Takeda Pharmaceuticals.
Introduction
The GLP-1 agonists and DPP-4 inhibitors have important benefits beyond lowering glycosylated hemoglobin, fasting plasma glucose, and postprandial glucose. Although an important treatment goal for type 2 diabetes mellitus (T2DM) is to reduce the risk of other diabetes-related diseases, eg, cardiovascular disease, many glucose-lowering agents cause weight gain, thereby adding to the burgeoning problem of obesity and related long-term consequences, as shown in our 3 case studies. In addition, cardiovascular risks associated with thiazolidinediones have become a major concern.
Side effects and their impact on patient tolerability are also important considerations when selecting and titrating therapy. Concerns about hypoglycemia can affect patient adherence to a medication regimen and a patient’s willingness to continue and intensify therapy, especially with insulin and sulfonylureas.1-3 As seen in Cases 1 (metformin-related diarrhea) and 2 (pioglitazone-related edema), patient adherence and willingness to continue therapy can be jeopardized by medication-related side effects.
Given these issues, the use of glucose-lowering medications that reduce related risk factors and have a favorable safety profile is advantageous. The GLP-1 agonists and DPP-4 inhibitors are desirable options, based on these considerations.
Reducing risk
The importance of weight
It is well recognized that weight gain is a major risk factor for T2DM and other disorders. It is also clear that concerns about weight gain adversely affect a patient’s willingness to begin treatment with glucose-lowering medications such as thiazolidinediones (TZDs), insulin, and sulfonylureas.1-3 The other side of the story is probably less appreciated—that is, weight loss can be a significant motivating factor for patients with T2DM that, in our experience, can improve adherence to lifestyle intervention and a medication regimen. In fact, improved quality of life, as assessed by physical and emotional domains, has been reported as a result of liraglutide-associated weight loss.4 In addition, multidimensional assessment of patient satisfaction generally has shown similar improvement with liraglutide 1.2 mg once daily and sitagliptin 100 mg once daily, and significantly greater improvement with liraglutide 1.8 mg once daily.5 Consequently, the ability of GLP-1 agonists to promote weight loss in most patients and of DPP-4 inhibitors to be weight neutral offers important benefits. Weight is an issue in all 3 of our patient cases, especially in Cases 1 and 2, where the patients’ body mass indices (BMIs) are ≥30 kg/m2.
Either as monotherapy or when added to glucose-lowering therapy, twice-daily exenatide or once-daily liraglutide generally promotes a 1 kg to 4 kg weight loss.4,6-11 The addition of exenatide or liraglutide to metformin, a sulfonylurea, or both resulted in a mean 2.9 kg weight loss with exenatide and a 3.2 kg loss with liraglutide after 26 weeks.12 Patients who continued liraglutide for an additional 14 weeks lost an additional 0.4 kg, while patients switched from exenatide to liraglutide lost an additional 0.9 kg.13 The amount of weight lost is greater with a higher baseline BMI.14,15 It is important to note, however, that 16% of patients did not experience any weight loss,14 possibly because no specific caloric restriction was required.14,16 Slight increases to slight decreases in weight have been observed in clinical trials with sitagliptin and saxagliptin.17-23 Comparison of liraglutide with sitagliptin showed a mean weight loss of 2.9 kg and 3.4 kg for liraglutide 1.2 mg and 1.8 mg once daily, respectively, over 26 weeks and a 1.0 kg weight loss with sitagliptin 100 mg once daily.5 Analysis of a large cohort database that followed patients for up to 1 year showed that patients treated with exenatide lost a mean of 3.0 kg, while patients treated with sitagliptin lost 1.1 kg and those treated with insulin gained 0.6 kg.24 Accordingly, the DPP-4 inhibitors are considered weight neutral.
The reason for the difference between GLP-1 agonists and DPP-4 inhibitors with respect to weight remains unclear, but may result from the direct action of GLP-1 agonists on the GLP-1 receptor compared to the indirect action of DPP-4 inhibitors, which slow the clearance of endogenous GLP-1.25 This may explain the ability of GLP-1 agonists—but not DPP-4 inhibitors—to promote satiety and reduce caloric intake.26,27 In a crossover comparison of exenatide with sitagliptin, caloric intake during a standardized meal decreased with exenatide (–134 kcal) and increased with sitagliptin (+130 kcal) (P=.0227).16
The possibility that the nausea associated with GLP-1 agonists but not DPP-4 inhibitors is the reason for the difference in weight effects has been investigated. Analyses have shown, however, that the weight loss observed with GLP-1 agonists is independent of nausea.4,10,28 For example, in a study by Garber et al,4 there was no difference in the amount of weight lost among patients who experienced liraglutide-associated nausea for >7 days, for 1 to 7 days, or not at all. The finding that nausea generally resolved within the first few weeks of liraglutide treatment, while weight loss was maintained over the 52 weeks of the trial, provides further evidence that nausea is not the cause of weight loss (FIGURE 1).4 Similar long-term weight loss has been observed with exenatide: weight loss was achieved through 30 weeks (–3.0 kg; P<.05 vs baseline) of double-blind treatment and maintained during an additional 52 weeks of open-label treatment (–5.3 kg; P<.05 vs baseline).29
FIGURE 1 Change in body weight (kg) over 52 weeks with liraglutide 1.2 mg and 1.8 mg vs glimepiride 8.0 mg4
cardiovascular benefits
The effects of incretin therapy on markers of cardiovascular disease have been assessed in several clinical trials (TABLE 1).4,8-12,17,22,30,31 In general, these trials demonstrate small but significant reductions in systolic blood pressure (1 to 7 mm Hg) with the GLP-1 agonists; diastolic blood pressure, however, is not significantly affected.4,8-12,30,31 There are insufficient data regarding the DPP-4 inhibitors.17,22
Effects on the lipid profile also have been investigated,4,5,8-12,17,22,30,31 but differences among the agents are difficult to assess because of different baseline lipid levels and the limited number of direct comparative studies. In general, clinical studies show that low-density lipoprotein (LDL) cholesterol is reduced by 1 to 17 mg/dL with the GLP-1 agonists and increased by 3 to 9 mg/dL with the DPP-4 inhibitors. Changes in high-density lipoprotein (HDL) cholesterol are generally small for both GLP-1 agonists and DPP-4 inhibitors, ranging from an increase of 5 mg/dL to a decrease of 2 mg/dL. The greatest change in the lipid profile is in the triglyceride level, with a reduction of 12 to 40 mg/dL with the GLP-1 agonists, while the triglyceride changes observed with the DPP-4 inhibitors range from a reduction of 35 mg/dL to an increase of 16 mg/dL.
Two studies have directly compared the effects on the lipid profile of 2 incretin agents over 26 weeks. In the first study, exenatide and liraglutide showed similar changes in LDL cholesterol (–7 vs –8 mg/dL, respectively) and HDL cholesterol (–1 vs –1 mg/dL, respectively) levels, but liraglutide showed a significantly greater reduction in triglyceride levels than exenatide (–36 vs –20 mg/dL, respectively; P=.0485).12 The second study compared liraglutide 1.2 mg and 1.8 mg once daily and sitagliptin 100 mg once daily.5 LDL cholesterol increased by 1 mg/dL in both liraglutide groups and by 2 mg/dL in the sitagliptin group, while there was no change in HDL cholesterol with either dose of liraglutide or sitagliptin. The triglyceride level decreased by 17 mg/dL in the liraglutide 1.2 mg group, 38 mg/dL in the liraglutide 1.8 mg group, and 35 mg/dL in the sitagliptin group. None of the differences between either liraglutide group and sitagliptin were statistically significant.
Effects on the lipid profile appear to be durable. Klonoff et al showed that the effects of exenatide on the lipid profile were sustained over 3.5 years of follow-up (in a 30-week randomized, double-blind trial followed by a 3-year open-label extension).14 In patients younger than 65 years, changes from baseline were as follows: total cholesterol, –10 mg/dL (P=.0056); LDL cholesterol, –11 mg/dL (P=.0012); HDL cholesterol, +8 mg/dL (P<.0001); and triglycerides, –44 mg/dL (P=.0042). Similar changes were observed in those 65 years and older.
The cardiovascular benefits of incretin therapy may extend beyond blood pressure and the lipid profile. An exploratory subanalysis of a randomized controlled trial with liraglutide showed a significant decrease in plasminogen activator inhibitor-1, an inflammatory biomarker, and B-type natriuretic peptide, a marker of left ventricular dysfunction.32 No significant effect on high-sensitivity C-reactive protein, interleukin-6, or tumor necrosis factor-α was observed.
While not appropriate as primary therapy, the effect of the GLP-1 agonists on blood pressure and of the GLP-1 agonists and DPP-4 inhibitors on the lipid profile could be an added benefit for all patients with T2DM because of the strong association between T2DM and cardiovascular disease. This can be seen in all 3 of our cases: Case 1 (hypertriglyceridemia), Case 2 (essential hypertension), and Case 3 (peripheral arterial disease).
TABLE 1
Selected studies with GLP-1 agonists and DPP-4 inhibitors assessing cardiovascular end points4,8-12,17,22,30,31
| Agent/clinical trial | Concomitant treatment; duration (wk) | Change from baseline | |||
|---|---|---|---|---|---|
| Systolic blood pressure (mm Hg) | LDL cholesterol (mg/dL) | HDL cholesterol (mg/dL) | Triglycerides (mg/dL) | ||
| Exenatide (E) | |||||
| Moretto, 200830 | Diet/exercise; | ||||
| E, 5 μg BID | 24 | -4 | NR | NR | NR |
| E, 10 μg BID | -4 | NR | NR | NR | |
| Placebo | 0 | NR | NR | NR | |
| Blonde, 20068 | MET + SU; | ||||
| E, 5 μg BID | 82 wk | -1 | -2 | +5 | -39 |
| E, 10 μg BID | -1 | -2 | +5 | -39 | |
| Nauck, 200731 | MET + SU; | ||||
| E, 10 μg BID | 52 wk | -5 | NC | NR | NC |
| Premix aspart 70/30 BID | +1 | NC | NR | NC | |
| Zinman, 20079 | TZD ± MET; | ||||
| E, 10 μg BID | 16 wk | NC | NC | NC | NC |
| Placebo | NC | NC | NC | NC | |
| Liraglutide (L) | |||||
| Garber, 20094 | None; 52 wk | ||||
| L, 1.2 mg OD | -2 | NR | NR | NR | |
| L, 1.8 mg OD | -4 | NR | NR | NR | |
| Glimepiride, 8 mg OD | -1 | NR | NR | NR | |
| Russell-Jones, 200910 | MET + GLIM; | ||||
| L, 1.8 mg OD | 26 wk | -4 | NR | NR | NR |
| Insulin glargine | +1 | NR | NR | NR | |
| Placebo | -1 | NR | NR | NR | |
| Zinman, 200911 | MET + ROSI; | ||||
| L, 1.2 mg OD | 26 wk | -7 | -11 | -1 | -34 |
| L, 1.8 mg OD | -6 | -9 | -1 | -29 | |
| Placebo | -1 | -4 | -1 | -5 | |
| Buse, 200912 | MET, SU, MET + SU; | ||||
| L, 1.8 mg OD | -3 | -17 | -2 | -36 | |
| E, 10 μg BID | 26 wk | -2 | -15 | -2 | -20 |
| Sitagliptin (Si) | |||||
| Scott, 200717 | Diet/exercise; | ||||
| Si, 25 mg BID | 12 wk | NR | 0 | +1 | -3 |
| Si, 50 mg BIDa | NR | +1 | +1 | 0 | |
| Glipizide, 5 mg OD | NR | 0 | 0 | +3 | |
| Placebo | NR | 0 | 0 | +16 | |
| Saxagliptin (Sa) | |||||
| Hollander, 200922 | TZD; | ||||
| Sa, 2.5 mg OD | 24 wk | NR | +4 | -1 | -1 |
| Sa, 5 mg OD | NR | +9 | +2 | -4 | |
| Placebo | NR | +3 | 0 | -1 | |
| aDose not included in currently approved prescribing information. | |||||
| DPP, dipeptidyl peptidase; GLIM, glimepiride; GLP, glucagon-like peptide; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MET, metformin; NC, no difference between baseline and study end; NR, not reported; OD, once daily; ROSI, rosiglitazone; SBP, systolic blood pressure; SU, sulfonylurea; TZD, thiazolidinedione. | |||||
Favorable safety profile
Overview
In general, GLP-1 agonists and DPP-4 inhibitors are well tolerated. Because of concerns about hypoglycemia with glucose-lowering agents, signs and symptoms of hypoglycemia have been closely monitored in clinical trials. The low incidence and mild to moderate severity of hypoglycemia are important attributes of the GLP-1 agonists and DPP-4 inhibitors.
Other than hypoglycemia, the most common adverse reaction reported in ≥5% of patients and more commonly than with placebo are shown in TABLE 2.33-36 Other medication-specific side effects are seen infrequently but bear mentioning. An increased international normalized ratio, sometimes with bleeding, has been noted with combined use of exenatide and warfarin.33 Immune-related events (eg, urticaria) have been reported with exenatide33 and have been observed to occur more frequently with liraglutide than with comparator agents.34 Peripheral edema is more common when a thiazolidinedione is administered with saxagliptin than with placebo.36
While experience with GLP-1 agonists and DPP-4 inhibitors indicates that they have favorable safety profiles, some concerns have surfaced with 1 or more of these agents during clinical trials or from postmarketing reports. These include gastrointestinal side effects (principally nausea), acute pancreatitis, and hypersensitivity reactions. In addition, new standards recently adopted by the US Food and Drug Administration (FDA) are requiring further investigation of several issues for newly approved glucose-lowering drugs and those in development. These issues are discussed next.
TABLE 2
Most common side effectsa of the GLP-1 agonists and DPP-4 inhibitors33-36
| Agent | Side effects |
|---|---|
| Exenatide | Nausea, vomiting, diarrhea, feeling jittery, dizziness, headache, dyspepsia |
| Liraglutide | Nausea, diarrhea, headache |
| Sitagliptin | Upper respiratory tract infection, nasopharyngitis, headache |
| Saxagliptin | Upper respiratory tract infection, urinary tract infection, headache |
| DPP, dipeptidyl peptidase; GLP, glucagon-like peptide. | |
| aOccurring in ≥5% of patients and more frequently than with placebo. | |
Hypoglycemia
In contrast to other glucose-lowering drugs that stimulate insulin secretion, incretin-based therapies have a glucose-dependent mechanism of action that minimizes the risk of hypoglycemia. Preclinical investigation showed that GLP-1 acts on islet α-cells to strongly inhibit postprandial glucagon secretion.37,38 These observations were subsequently supported by early clinical investigations showing that administration of GLP-1 to healthy volunteers and people with T2DM augmented insulin secretion and decreased glucagon secretion in a glucose-dependent manner.39,40 Similar effects were also observed with a DPP-4 inhibitor.25 At the same time, GLP-1 has been shown not to suppress glucagon secretion at a plasma glucose level <65 mg/dL.41 This mechanism is believed to maintain the counterregulatory hormone response that serves to prevent hypoglycemia.
Accordingly, severe hypoglycemia has not been observed in monotherapy trials of exenatide,6,30 liraglutide,4,42 sitagliptin,17,19 or saxagliptin.21 Mild to mod erate hypoglycemia has been observed in 4% to 9% of patients treated with exenatide monotherapy6,30 and 0% to 12% of patients treated with liraglutide monotherapy4,42; by comparison, the incidence was 24% in patients treated with glimepiride monotherapy.4 Mild to moderate hypoglycemia has been found to be less frequent with sitagliptin and saxagliptin. In monotherapy and combination studies, 0% to 4% of patients treated with sitagliptin and 0% to 2% administered placebo experienced mild to moderate hypoglycemia.17,18,43-45 Mild to moderate hypoglycemia has not been observed in patients treated with saxagliptin monotherapy at doses of 2.5 mg to 40 mg.21 When saxagliptin was added to metformin, hypoglycemia was reported by 5.7% of patients compared with 5.0% of patients who added placebo to metformin.46
It is important to note that the incidence of mild to moderate hypoglycemia is increased in patients treated concomitantly with a GLP-1 agonist or DPP-4 inhibitor and a sulfonylurea. In 1 trial, 14% to 36% of patients treated with a combination of exenatide and a maximally effective dose of a sulfonylurea (glimepiride, glipizide, glyburide, chlorpropamide, tolazamide) reported mild to moderate hypoglycemia compared with 3% of those administered a placebo and a sulfonylurea.47 A trial involving the addition of saxagliptin to glyburide found that 13% to 15% of patients treated daily with glyburide 7.5 mg and saxagliptin 2.5 mg or 5 mg reported mild to moderate hypoglycemia compared with 10% of patients treated with glyburide 10 mg to 15 mg alone.23 For this reason, consideration should be given to reducing the dose of a sulfonylurea or secretagogue when combined with a GLP-1 agonist or DPP-4 inhibitor.33-35
Because of their low incidence of hypoglycemia, the American Diabetes Association/European Association for the Study of Diabetes panel recommends GLP-1 agonists for patients for whom hypoglycemia is particularly undesirable, such as those who perform manual labor, drive a vehicle for a living, or operate heavy or dangerous machinery.48 In fact, the US Federal Aviation Administration lists the GLP-1 agonists and DPP-4 inhibitors as allowable medications for aviators.49 This recommendation is particularly appropriate for the building contractor in Case 1.
Nausea
Transient nausea is the most common GI side effect associated with GLP-1 agonists, occurring in up to 57% of patients treated with exenatide in clinical trials6,30 and 29% treated with liraglutide.4 Diarrhea and vomiting also occurred, although rates were similar to rates with initiation of metformin. The high occurrence of transient nausea in these trials prompted investigators to implement a dose escalation strategy33,34 (see “Patient education and self-management” article in this supplement). Since adoption of this strategy, a comparative trial of exenatide and liraglutide found that nausea occurred in 28% of patients treated with exenatide and in 26% treated with liraglutide.12 Nausea was generally transient, so that by Week 6 of therapy, 16% of patients treated with exenatide and 8% with liraglutide experienced nausea,12 and by Week 26, 9% of exenatide-treated patients and 3% of liraglutide-treated patients continued to experience nausea.12
Of patients treated with the DPP-4 inhibitor sitagliptin, nausea has occurred in 1% to 2% com pared with 1% of those receiving placebo.18,43 In patients treated with saxagliptin, nausea has occurred in 2% to 4% compared with 8% of those receiving placebo.21
Acute pancreatitis
Acute pancreatitis has been observed in clinical trials and/or identified in postmarketing reports involving exenatide,33 liraglutide,34 and sitagliptin.36 Determining whether there is a true association of these agents with acute pancreatitis or this is just coincidental has been difficult, partly because patients with T2DM have a 2.8-fold greater risk of pancreatitis compared with nondiabetic subjects.50 A review of health insurance transactions with 1-year follow-up (June 2005 through June 2008) involving approximately 88,000 patients (exenatide, n=27,996; sitagliptin, n=16,276; approximately equal numbers of matched comparators) showed that the relative risk of pancreatitis was statistically the same with exenatide, sitagliptin, metformin, and glyburide.51
In its ongoing review, the FDA has required the manufacturers of exenatide and sitagliptin to modify product labeling regarding the risk of acute pancreatitis and to conduct additional animal studies.52 As part of the January 2010 approval of liraglutide, the FDA required the manufacturer to perform mechanistic studies in animals and to conduct an epidemiologic evaluation using a large insurance claims database.52 In the interim, exenatide, liraglutide, and sitagliptin should be used cautiously,34,35 if at all, in people with a history of pancreatitis.33 Furthermore, educating patients about the signs and symptoms of pancreatitis, including how to differentiate it from the transient nausea commonly observed with these agents, is critical. Patients at risk of developing acute pancreatitis (eg, due to excess alcohol consumption or gallstones) should not receive an incretin-based therapy. Therapy should be changed if a patient develops acute pancreatitis while using a GLP-1 agonist or DPP-4 inhibitor.
Hypersensitivity
Hypersensitivity reactions have been experienced by some patients treated with exenatide,33 liraglutide,34 sitagliptin,35 or saxagliptin.36 Postmarketing reports have described serious hypersensitivity reactions (anaphylaxis, angioedema) with exenatide.33 In clinical trials, 0.8% of patients treated with liraglutide and 0.4% treated with comparator agents experienced an immunogenic reaction, generally urticaria.34 With sitagliptin, anaphylaxis, angioedema, or exfoliative dermatitis, including Stevens-Johnson syndrome, typically occurs within 3 months but may occur after the first dose. Hypersensitivity events, such as urticaria and facial edema, were shown to occur in 1.5% of patients treated with saxagliptin 2.5 mg, 1.5% of those treated with saxagliptin 5 mg, and 0.4% of those receiving placebo. None of the events necessitated hospitalization or were life-threatening.36 If a hypersensitivity reaction occurs, treatment should be discontinued.
additional safety investigations
The FDA has required additional safety investigations for liraglutide, saxagliptin, and sitagliptin. These investigations will address observations made during preclinical and clinical evaluation, as well as the new standards adopted by the agency in December 2008 regarding cardiovascular safety for all new glucose-lowering agents.53
Thyroid cancer
Rodent studies have suggested that liraglutide in doses many times those utilized in humans is associated with an increased risk of preneoplastic lesions that can lead to C-cell hyperplasia and medullary thyroid cancer, which occurs rarely in humans.52 Thyroid tumors have also been observed in rodents administered native GLP-154 or exenatide33 but not sitagliptin35 or saxagliptin.36 A slight increase in calcitonin, a marker for medullary cancer, which remained well within the normal reference range, was observed during Phase 3 clinical trials in patients treated with liraglutide compared with controls. There were no cases of medullary thyroid cancer in the liraglutide trials, including one that involved more than 2 years of follow-up. Based on this evidence, the FDA determined that the risk of thyroid cancer among humans treated with liraglutide is low. The effects may be due to species-specific differences in GLP-1 receptor expression and action in the thyroid, as 20 months of liraglutide at >60 times the human exposure level did not lead to C-cell hyperplasia in monkeys.55 The FDA has required the manufacturer to conduct additional animal studies, however, and to establish a cancer registry to monitor the annual incidence of medullary thyroid cancer over the next 15 years.52 In addition, the prescribing information for liraglutide carries a boxed warning describing the rodent findings and the risk of medullary thyroid cancer.34 Liraglutide is also contraindicated in patients with a personal or family history of medullary thyroid cancer or in patients with multiple endocrine neoplasia syndrome type 2.34
Cardiovascular risk
The clinical evaluation of liraglutide and saxagliptin was completed prior to December 2008, when the FDA adopted the new cardiovascular safety standards for new antidiabetic drugs. Analyses of data from Phase 2 and 3 clinical trials indicate that liraglutide meets the new 2008 standard for ruling out an unacceptable increase in cardiovascular risk.52 While the overall rates of cardiovascular events were low in preapproval clinical trials, the more stringent criteria for postapproval evaluations were not met. Consequently, the FDA has required postapproval clinical trials of cardiovascular safety with liraglutide52 and saxagliptin.
Summary
The overall safety profiles of GLP-1 agonists and DPP-4 inhibitors are favorable, with a low incidence of hypoglycemia. This attribute, along with their weight and cardiovascular benefits, particularly with the GLP-1 agonists, make them appropriate choices in our 3 patient cases. Ongoing safety investigations with GLP-1 agonists and DPP-4 inhibitors will provide further clarity to the complete safety profiles of these agents.
Pathophysiology of type 2 diabetes mellitus: potential role of incretin-based therapies
Glucose-lowering effects of incretin-based therapies
Safety, tolerability, and nonglycemic effects of incretin-based therapies
- The glucagon-like peptide (GLP)-1 agonists promote a 1 kg to 4 kg weight loss and satiety, while the dipeptidyl peptidase (DPP)-4 inhibitors are usually weight neutral
- The GLP-1 agonists and DPP-4 inhibitors have a favorable safety profile, including rare occurrence of severe hypoglycemia and a low incidence of mild to moderate hypoglycemia
- Mild to moderate nausea associated with GLP-1 agonists generally resolves over 4 to 8 weeks and can be minimized by dose escalation strategies
- Hypersensitivity reactions occur infrequently with DPP-4 inhibitors
- Active surveillance and investigation are ongoing regarding the possible association of GLP-1 agonists and/or DPP-4 inhibitors with acute pancreatitis; thyroid medullary cancer; and with cardiovascular disease
The authors received editorial assistance from the Primary Care Education Consortium and WriteHealth, LLC in the development of this activity and honoraria from the Primary Care Education Consortium. They have disclosed that Dr Campbell is on the advisory board for Daiichi-Sankyo and the speakers bureau for Eli Lilly and Co; Dr Cobble is on the advisory board for Abbott Laboratories, AstraZeneca, and Eli Lilly and Co and speakers bureau for Abbott Laboratories, AstraZeneca/Bristol Myers Squibb, Eli Lilly and Co, GlaxoSmithKline, and Novo Nordisk Inc; Dr Reid is on the advisory board and speakers bureau for Amylin Pharmaceuticals, Medtronic, Novo Nordisk Inc, and sanofi-aventis; and Dr Shomali is on the advisory board for Novo Nordisk Inc and speakers bureau for Amylin Pharmaceuticals, Eli Lilly and Co, sanofi-aventis, and Takeda Pharmaceuticals.
Introduction
The GLP-1 agonists and DPP-4 inhibitors have important benefits beyond lowering glycosylated hemoglobin, fasting plasma glucose, and postprandial glucose. Although an important treatment goal for type 2 diabetes mellitus (T2DM) is to reduce the risk of other diabetes-related diseases, eg, cardiovascular disease, many glucose-lowering agents cause weight gain, thereby adding to the burgeoning problem of obesity and related long-term consequences, as shown in our 3 case studies. In addition, cardiovascular risks associated with thiazolidinediones have become a major concern.
Side effects and their impact on patient tolerability are also important considerations when selecting and titrating therapy. Concerns about hypoglycemia can affect patient adherence to a medication regimen and a patient’s willingness to continue and intensify therapy, especially with insulin and sulfonylureas.1-3 As seen in Cases 1 (metformin-related diarrhea) and 2 (pioglitazone-related edema), patient adherence and willingness to continue therapy can be jeopardized by medication-related side effects.
Given these issues, the use of glucose-lowering medications that reduce related risk factors and have a favorable safety profile is advantageous. The GLP-1 agonists and DPP-4 inhibitors are desirable options, based on these considerations.
Reducing risk
The importance of weight
It is well recognized that weight gain is a major risk factor for T2DM and other disorders. It is also clear that concerns about weight gain adversely affect a patient’s willingness to begin treatment with glucose-lowering medications such as thiazolidinediones (TZDs), insulin, and sulfonylureas.1-3 The other side of the story is probably less appreciated—that is, weight loss can be a significant motivating factor for patients with T2DM that, in our experience, can improve adherence to lifestyle intervention and a medication regimen. In fact, improved quality of life, as assessed by physical and emotional domains, has been reported as a result of liraglutide-associated weight loss.4 In addition, multidimensional assessment of patient satisfaction generally has shown similar improvement with liraglutide 1.2 mg once daily and sitagliptin 100 mg once daily, and significantly greater improvement with liraglutide 1.8 mg once daily.5 Consequently, the ability of GLP-1 agonists to promote weight loss in most patients and of DPP-4 inhibitors to be weight neutral offers important benefits. Weight is an issue in all 3 of our patient cases, especially in Cases 1 and 2, where the patients’ body mass indices (BMIs) are ≥30 kg/m2.
Either as monotherapy or when added to glucose-lowering therapy, twice-daily exenatide or once-daily liraglutide generally promotes a 1 kg to 4 kg weight loss.4,6-11 The addition of exenatide or liraglutide to metformin, a sulfonylurea, or both resulted in a mean 2.9 kg weight loss with exenatide and a 3.2 kg loss with liraglutide after 26 weeks.12 Patients who continued liraglutide for an additional 14 weeks lost an additional 0.4 kg, while patients switched from exenatide to liraglutide lost an additional 0.9 kg.13 The amount of weight lost is greater with a higher baseline BMI.14,15 It is important to note, however, that 16% of patients did not experience any weight loss,14 possibly because no specific caloric restriction was required.14,16 Slight increases to slight decreases in weight have been observed in clinical trials with sitagliptin and saxagliptin.17-23 Comparison of liraglutide with sitagliptin showed a mean weight loss of 2.9 kg and 3.4 kg for liraglutide 1.2 mg and 1.8 mg once daily, respectively, over 26 weeks and a 1.0 kg weight loss with sitagliptin 100 mg once daily.5 Analysis of a large cohort database that followed patients for up to 1 year showed that patients treated with exenatide lost a mean of 3.0 kg, while patients treated with sitagliptin lost 1.1 kg and those treated with insulin gained 0.6 kg.24 Accordingly, the DPP-4 inhibitors are considered weight neutral.
The reason for the difference between GLP-1 agonists and DPP-4 inhibitors with respect to weight remains unclear, but may result from the direct action of GLP-1 agonists on the GLP-1 receptor compared to the indirect action of DPP-4 inhibitors, which slow the clearance of endogenous GLP-1.25 This may explain the ability of GLP-1 agonists—but not DPP-4 inhibitors—to promote satiety and reduce caloric intake.26,27 In a crossover comparison of exenatide with sitagliptin, caloric intake during a standardized meal decreased with exenatide (–134 kcal) and increased with sitagliptin (+130 kcal) (P=.0227).16
The possibility that the nausea associated with GLP-1 agonists but not DPP-4 inhibitors is the reason for the difference in weight effects has been investigated. Analyses have shown, however, that the weight loss observed with GLP-1 agonists is independent of nausea.4,10,28 For example, in a study by Garber et al,4 there was no difference in the amount of weight lost among patients who experienced liraglutide-associated nausea for >7 days, for 1 to 7 days, or not at all. The finding that nausea generally resolved within the first few weeks of liraglutide treatment, while weight loss was maintained over the 52 weeks of the trial, provides further evidence that nausea is not the cause of weight loss (FIGURE 1).4 Similar long-term weight loss has been observed with exenatide: weight loss was achieved through 30 weeks (–3.0 kg; P<.05 vs baseline) of double-blind treatment and maintained during an additional 52 weeks of open-label treatment (–5.3 kg; P<.05 vs baseline).29
FIGURE 1 Change in body weight (kg) over 52 weeks with liraglutide 1.2 mg and 1.8 mg vs glimepiride 8.0 mg4
cardiovascular benefits
The effects of incretin therapy on markers of cardiovascular disease have been assessed in several clinical trials (TABLE 1).4,8-12,17,22,30,31 In general, these trials demonstrate small but significant reductions in systolic blood pressure (1 to 7 mm Hg) with the GLP-1 agonists; diastolic blood pressure, however, is not significantly affected.4,8-12,30,31 There are insufficient data regarding the DPP-4 inhibitors.17,22
Effects on the lipid profile also have been investigated,4,5,8-12,17,22,30,31 but differences among the agents are difficult to assess because of different baseline lipid levels and the limited number of direct comparative studies. In general, clinical studies show that low-density lipoprotein (LDL) cholesterol is reduced by 1 to 17 mg/dL with the GLP-1 agonists and increased by 3 to 9 mg/dL with the DPP-4 inhibitors. Changes in high-density lipoprotein (HDL) cholesterol are generally small for both GLP-1 agonists and DPP-4 inhibitors, ranging from an increase of 5 mg/dL to a decrease of 2 mg/dL. The greatest change in the lipid profile is in the triglyceride level, with a reduction of 12 to 40 mg/dL with the GLP-1 agonists, while the triglyceride changes observed with the DPP-4 inhibitors range from a reduction of 35 mg/dL to an increase of 16 mg/dL.
Two studies have directly compared the effects on the lipid profile of 2 incretin agents over 26 weeks. In the first study, exenatide and liraglutide showed similar changes in LDL cholesterol (–7 vs –8 mg/dL, respectively) and HDL cholesterol (–1 vs –1 mg/dL, respectively) levels, but liraglutide showed a significantly greater reduction in triglyceride levels than exenatide (–36 vs –20 mg/dL, respectively; P=.0485).12 The second study compared liraglutide 1.2 mg and 1.8 mg once daily and sitagliptin 100 mg once daily.5 LDL cholesterol increased by 1 mg/dL in both liraglutide groups and by 2 mg/dL in the sitagliptin group, while there was no change in HDL cholesterol with either dose of liraglutide or sitagliptin. The triglyceride level decreased by 17 mg/dL in the liraglutide 1.2 mg group, 38 mg/dL in the liraglutide 1.8 mg group, and 35 mg/dL in the sitagliptin group. None of the differences between either liraglutide group and sitagliptin were statistically significant.
Effects on the lipid profile appear to be durable. Klonoff et al showed that the effects of exenatide on the lipid profile were sustained over 3.5 years of follow-up (in a 30-week randomized, double-blind trial followed by a 3-year open-label extension).14 In patients younger than 65 years, changes from baseline were as follows: total cholesterol, –10 mg/dL (P=.0056); LDL cholesterol, –11 mg/dL (P=.0012); HDL cholesterol, +8 mg/dL (P<.0001); and triglycerides, –44 mg/dL (P=.0042). Similar changes were observed in those 65 years and older.
The cardiovascular benefits of incretin therapy may extend beyond blood pressure and the lipid profile. An exploratory subanalysis of a randomized controlled trial with liraglutide showed a significant decrease in plasminogen activator inhibitor-1, an inflammatory biomarker, and B-type natriuretic peptide, a marker of left ventricular dysfunction.32 No significant effect on high-sensitivity C-reactive protein, interleukin-6, or tumor necrosis factor-α was observed.
While not appropriate as primary therapy, the effect of the GLP-1 agonists on blood pressure and of the GLP-1 agonists and DPP-4 inhibitors on the lipid profile could be an added benefit for all patients with T2DM because of the strong association between T2DM and cardiovascular disease. This can be seen in all 3 of our cases: Case 1 (hypertriglyceridemia), Case 2 (essential hypertension), and Case 3 (peripheral arterial disease).
TABLE 1
Selected studies with GLP-1 agonists and DPP-4 inhibitors assessing cardiovascular end points4,8-12,17,22,30,31
| Agent/clinical trial | Concomitant treatment; duration (wk) | Change from baseline | |||
|---|---|---|---|---|---|
| Systolic blood pressure (mm Hg) | LDL cholesterol (mg/dL) | HDL cholesterol (mg/dL) | Triglycerides (mg/dL) | ||
| Exenatide (E) | |||||
| Moretto, 200830 | Diet/exercise; | ||||
| E, 5 μg BID | 24 | -4 | NR | NR | NR |
| E, 10 μg BID | -4 | NR | NR | NR | |
| Placebo | 0 | NR | NR | NR | |
| Blonde, 20068 | MET + SU; | ||||
| E, 5 μg BID | 82 wk | -1 | -2 | +5 | -39 |
| E, 10 μg BID | -1 | -2 | +5 | -39 | |
| Nauck, 200731 | MET + SU; | ||||
| E, 10 μg BID | 52 wk | -5 | NC | NR | NC |
| Premix aspart 70/30 BID | +1 | NC | NR | NC | |
| Zinman, 20079 | TZD ± MET; | ||||
| E, 10 μg BID | 16 wk | NC | NC | NC | NC |
| Placebo | NC | NC | NC | NC | |
| Liraglutide (L) | |||||
| Garber, 20094 | None; 52 wk | ||||
| L, 1.2 mg OD | -2 | NR | NR | NR | |
| L, 1.8 mg OD | -4 | NR | NR | NR | |
| Glimepiride, 8 mg OD | -1 | NR | NR | NR | |
| Russell-Jones, 200910 | MET + GLIM; | ||||
| L, 1.8 mg OD | 26 wk | -4 | NR | NR | NR |
| Insulin glargine | +1 | NR | NR | NR | |
| Placebo | -1 | NR | NR | NR | |
| Zinman, 200911 | MET + ROSI; | ||||
| L, 1.2 mg OD | 26 wk | -7 | -11 | -1 | -34 |
| L, 1.8 mg OD | -6 | -9 | -1 | -29 | |
| Placebo | -1 | -4 | -1 | -5 | |
| Buse, 200912 | MET, SU, MET + SU; | ||||
| L, 1.8 mg OD | -3 | -17 | -2 | -36 | |
| E, 10 μg BID | 26 wk | -2 | -15 | -2 | -20 |
| Sitagliptin (Si) | |||||
| Scott, 200717 | Diet/exercise; | ||||
| Si, 25 mg BID | 12 wk | NR | 0 | +1 | -3 |
| Si, 50 mg BIDa | NR | +1 | +1 | 0 | |
| Glipizide, 5 mg OD | NR | 0 | 0 | +3 | |
| Placebo | NR | 0 | 0 | +16 | |
| Saxagliptin (Sa) | |||||
| Hollander, 200922 | TZD; | ||||
| Sa, 2.5 mg OD | 24 wk | NR | +4 | -1 | -1 |
| Sa, 5 mg OD | NR | +9 | +2 | -4 | |
| Placebo | NR | +3 | 0 | -1 | |
| aDose not included in currently approved prescribing information. | |||||
| DPP, dipeptidyl peptidase; GLIM, glimepiride; GLP, glucagon-like peptide; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MET, metformin; NC, no difference between baseline and study end; NR, not reported; OD, once daily; ROSI, rosiglitazone; SBP, systolic blood pressure; SU, sulfonylurea; TZD, thiazolidinedione. | |||||
Favorable safety profile
Overview
In general, GLP-1 agonists and DPP-4 inhibitors are well tolerated. Because of concerns about hypoglycemia with glucose-lowering agents, signs and symptoms of hypoglycemia have been closely monitored in clinical trials. The low incidence and mild to moderate severity of hypoglycemia are important attributes of the GLP-1 agonists and DPP-4 inhibitors.
Other than hypoglycemia, the most common adverse reaction reported in ≥5% of patients and more commonly than with placebo are shown in TABLE 2.33-36 Other medication-specific side effects are seen infrequently but bear mentioning. An increased international normalized ratio, sometimes with bleeding, has been noted with combined use of exenatide and warfarin.33 Immune-related events (eg, urticaria) have been reported with exenatide33 and have been observed to occur more frequently with liraglutide than with comparator agents.34 Peripheral edema is more common when a thiazolidinedione is administered with saxagliptin than with placebo.36
While experience with GLP-1 agonists and DPP-4 inhibitors indicates that they have favorable safety profiles, some concerns have surfaced with 1 or more of these agents during clinical trials or from postmarketing reports. These include gastrointestinal side effects (principally nausea), acute pancreatitis, and hypersensitivity reactions. In addition, new standards recently adopted by the US Food and Drug Administration (FDA) are requiring further investigation of several issues for newly approved glucose-lowering drugs and those in development. These issues are discussed next.
TABLE 2
Most common side effectsa of the GLP-1 agonists and DPP-4 inhibitors33-36
| Agent | Side effects |
|---|---|
| Exenatide | Nausea, vomiting, diarrhea, feeling jittery, dizziness, headache, dyspepsia |
| Liraglutide | Nausea, diarrhea, headache |
| Sitagliptin | Upper respiratory tract infection, nasopharyngitis, headache |
| Saxagliptin | Upper respiratory tract infection, urinary tract infection, headache |
| DPP, dipeptidyl peptidase; GLP, glucagon-like peptide. | |
| aOccurring in ≥5% of patients and more frequently than with placebo. | |
Hypoglycemia
In contrast to other glucose-lowering drugs that stimulate insulin secretion, incretin-based therapies have a glucose-dependent mechanism of action that minimizes the risk of hypoglycemia. Preclinical investigation showed that GLP-1 acts on islet α-cells to strongly inhibit postprandial glucagon secretion.37,38 These observations were subsequently supported by early clinical investigations showing that administration of GLP-1 to healthy volunteers and people with T2DM augmented insulin secretion and decreased glucagon secretion in a glucose-dependent manner.39,40 Similar effects were also observed with a DPP-4 inhibitor.25 At the same time, GLP-1 has been shown not to suppress glucagon secretion at a plasma glucose level <65 mg/dL.41 This mechanism is believed to maintain the counterregulatory hormone response that serves to prevent hypoglycemia.
Accordingly, severe hypoglycemia has not been observed in monotherapy trials of exenatide,6,30 liraglutide,4,42 sitagliptin,17,19 or saxagliptin.21 Mild to mod erate hypoglycemia has been observed in 4% to 9% of patients treated with exenatide monotherapy6,30 and 0% to 12% of patients treated with liraglutide monotherapy4,42; by comparison, the incidence was 24% in patients treated with glimepiride monotherapy.4 Mild to moderate hypoglycemia has been found to be less frequent with sitagliptin and saxagliptin. In monotherapy and combination studies, 0% to 4% of patients treated with sitagliptin and 0% to 2% administered placebo experienced mild to moderate hypoglycemia.17,18,43-45 Mild to moderate hypoglycemia has not been observed in patients treated with saxagliptin monotherapy at doses of 2.5 mg to 40 mg.21 When saxagliptin was added to metformin, hypoglycemia was reported by 5.7% of patients compared with 5.0% of patients who added placebo to metformin.46
It is important to note that the incidence of mild to moderate hypoglycemia is increased in patients treated concomitantly with a GLP-1 agonist or DPP-4 inhibitor and a sulfonylurea. In 1 trial, 14% to 36% of patients treated with a combination of exenatide and a maximally effective dose of a sulfonylurea (glimepiride, glipizide, glyburide, chlorpropamide, tolazamide) reported mild to moderate hypoglycemia compared with 3% of those administered a placebo and a sulfonylurea.47 A trial involving the addition of saxagliptin to glyburide found that 13% to 15% of patients treated daily with glyburide 7.5 mg and saxagliptin 2.5 mg or 5 mg reported mild to moderate hypoglycemia compared with 10% of patients treated with glyburide 10 mg to 15 mg alone.23 For this reason, consideration should be given to reducing the dose of a sulfonylurea or secretagogue when combined with a GLP-1 agonist or DPP-4 inhibitor.33-35
Because of their low incidence of hypoglycemia, the American Diabetes Association/European Association for the Study of Diabetes panel recommends GLP-1 agonists for patients for whom hypoglycemia is particularly undesirable, such as those who perform manual labor, drive a vehicle for a living, or operate heavy or dangerous machinery.48 In fact, the US Federal Aviation Administration lists the GLP-1 agonists and DPP-4 inhibitors as allowable medications for aviators.49 This recommendation is particularly appropriate for the building contractor in Case 1.
Nausea
Transient nausea is the most common GI side effect associated with GLP-1 agonists, occurring in up to 57% of patients treated with exenatide in clinical trials6,30 and 29% treated with liraglutide.4 Diarrhea and vomiting also occurred, although rates were similar to rates with initiation of metformin. The high occurrence of transient nausea in these trials prompted investigators to implement a dose escalation strategy33,34 (see “Patient education and self-management” article in this supplement). Since adoption of this strategy, a comparative trial of exenatide and liraglutide found that nausea occurred in 28% of patients treated with exenatide and in 26% treated with liraglutide.12 Nausea was generally transient, so that by Week 6 of therapy, 16% of patients treated with exenatide and 8% with liraglutide experienced nausea,12 and by Week 26, 9% of exenatide-treated patients and 3% of liraglutide-treated patients continued to experience nausea.12
Of patients treated with the DPP-4 inhibitor sitagliptin, nausea has occurred in 1% to 2% com pared with 1% of those receiving placebo.18,43 In patients treated with saxagliptin, nausea has occurred in 2% to 4% compared with 8% of those receiving placebo.21
Acute pancreatitis
Acute pancreatitis has been observed in clinical trials and/or identified in postmarketing reports involving exenatide,33 liraglutide,34 and sitagliptin.36 Determining whether there is a true association of these agents with acute pancreatitis or this is just coincidental has been difficult, partly because patients with T2DM have a 2.8-fold greater risk of pancreatitis compared with nondiabetic subjects.50 A review of health insurance transactions with 1-year follow-up (June 2005 through June 2008) involving approximately 88,000 patients (exenatide, n=27,996; sitagliptin, n=16,276; approximately equal numbers of matched comparators) showed that the relative risk of pancreatitis was statistically the same with exenatide, sitagliptin, metformin, and glyburide.51
In its ongoing review, the FDA has required the manufacturers of exenatide and sitagliptin to modify product labeling regarding the risk of acute pancreatitis and to conduct additional animal studies.52 As part of the January 2010 approval of liraglutide, the FDA required the manufacturer to perform mechanistic studies in animals and to conduct an epidemiologic evaluation using a large insurance claims database.52 In the interim, exenatide, liraglutide, and sitagliptin should be used cautiously,34,35 if at all, in people with a history of pancreatitis.33 Furthermore, educating patients about the signs and symptoms of pancreatitis, including how to differentiate it from the transient nausea commonly observed with these agents, is critical. Patients at risk of developing acute pancreatitis (eg, due to excess alcohol consumption or gallstones) should not receive an incretin-based therapy. Therapy should be changed if a patient develops acute pancreatitis while using a GLP-1 agonist or DPP-4 inhibitor.
Hypersensitivity
Hypersensitivity reactions have been experienced by some patients treated with exenatide,33 liraglutide,34 sitagliptin,35 or saxagliptin.36 Postmarketing reports have described serious hypersensitivity reactions (anaphylaxis, angioedema) with exenatide.33 In clinical trials, 0.8% of patients treated with liraglutide and 0.4% treated with comparator agents experienced an immunogenic reaction, generally urticaria.34 With sitagliptin, anaphylaxis, angioedema, or exfoliative dermatitis, including Stevens-Johnson syndrome, typically occurs within 3 months but may occur after the first dose. Hypersensitivity events, such as urticaria and facial edema, were shown to occur in 1.5% of patients treated with saxagliptin 2.5 mg, 1.5% of those treated with saxagliptin 5 mg, and 0.4% of those receiving placebo. None of the events necessitated hospitalization or were life-threatening.36 If a hypersensitivity reaction occurs, treatment should be discontinued.
additional safety investigations
The FDA has required additional safety investigations for liraglutide, saxagliptin, and sitagliptin. These investigations will address observations made during preclinical and clinical evaluation, as well as the new standards adopted by the agency in December 2008 regarding cardiovascular safety for all new glucose-lowering agents.53
Thyroid cancer
Rodent studies have suggested that liraglutide in doses many times those utilized in humans is associated with an increased risk of preneoplastic lesions that can lead to C-cell hyperplasia and medullary thyroid cancer, which occurs rarely in humans.52 Thyroid tumors have also been observed in rodents administered native GLP-154 or exenatide33 but not sitagliptin35 or saxagliptin.36 A slight increase in calcitonin, a marker for medullary cancer, which remained well within the normal reference range, was observed during Phase 3 clinical trials in patients treated with liraglutide compared with controls. There were no cases of medullary thyroid cancer in the liraglutide trials, including one that involved more than 2 years of follow-up. Based on this evidence, the FDA determined that the risk of thyroid cancer among humans treated with liraglutide is low. The effects may be due to species-specific differences in GLP-1 receptor expression and action in the thyroid, as 20 months of liraglutide at >60 times the human exposure level did not lead to C-cell hyperplasia in monkeys.55 The FDA has required the manufacturer to conduct additional animal studies, however, and to establish a cancer registry to monitor the annual incidence of medullary thyroid cancer over the next 15 years.52 In addition, the prescribing information for liraglutide carries a boxed warning describing the rodent findings and the risk of medullary thyroid cancer.34 Liraglutide is also contraindicated in patients with a personal or family history of medullary thyroid cancer or in patients with multiple endocrine neoplasia syndrome type 2.34
Cardiovascular risk
The clinical evaluation of liraglutide and saxagliptin was completed prior to December 2008, when the FDA adopted the new cardiovascular safety standards for new antidiabetic drugs. Analyses of data from Phase 2 and 3 clinical trials indicate that liraglutide meets the new 2008 standard for ruling out an unacceptable increase in cardiovascular risk.52 While the overall rates of cardiovascular events were low in preapproval clinical trials, the more stringent criteria for postapproval evaluations were not met. Consequently, the FDA has required postapproval clinical trials of cardiovascular safety with liraglutide52 and saxagliptin.
Summary
The overall safety profiles of GLP-1 agonists and DPP-4 inhibitors are favorable, with a low incidence of hypoglycemia. This attribute, along with their weight and cardiovascular benefits, particularly with the GLP-1 agonists, make them appropriate choices in our 3 patient cases. Ongoing safety investigations with GLP-1 agonists and DPP-4 inhibitors will provide further clarity to the complete safety profiles of these agents.
1. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
2. Holstein A, Plaschke A, Egberts EH. Lower incidence of severe hypoglycaemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev. 2001;17:467-473.
3. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673-2679.
4. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473-481.
5. Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447-1456.
6. Nelson P, Poon T, Guan X, et al. The incretin mimetic exenatide as a monotherapy in patients with type 2 diabetes. Diabetes Technol Ther. 2007;9:317-326.
7. Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083-1091.
8. Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 over-weight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436-447.
9. Zinman B, Hoogwerf BJ, Duran GS, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146:477-485.
10. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52:2046-2055.
11. Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32:1224-1230.
12. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47.
13. Buse JB, Sesti G, Schmidt WE, et al. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33:1300-1303.
14. Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275-286.
15. Russell-Jones D, Shaw J, Brandle M, et al. The once-daily human GLP-1 analog liraglutide reduces body weight in subjects with type 2 diabetes, irrespective of body mass index at baseline. Paper presented at: American Diabetes Association 68th Scientific Session; June 6-10, 2008; San Francisco, CA.
16. DeFronzo RA, Okerson T, Viswanathan P, et al. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943-2952.
17. Scott R, Wu M, Sanchez M, et al. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract. 2007;61:171-180.
18. Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632-2637.
19. Hanefeld M, Herman GA, Wu M, et al. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin. 2007;23:1329-1339.
20. Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733-745.
21. Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:376-386.
22. Hollander P, Li J, Allen E, et al. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab. 2009;94:4810-4819.
23. Chacra AR, Tan GH, Apanovitch A, et al. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract. 2009;63:1395-1406.
24. Horton ES, Silberman C, Davis KL, et al. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33:1759-1765.
25. Deacon CF, Hughes TE, Holst JJ. Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig. Diabetes. 1998;47:764-769.
26. Gutzwiller JP, Drewe J, Goke B, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541-R1544.
27. Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824-830.
28. Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84-90.
29. Ratner RE, Maggs D, Nielsen LL, et al. Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2006;8:419-428.
30. Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448-1460.
31. Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:259-267.
32. Courreges JP, Vilsboll T, Zdravkovic M, et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabet Med. 2008;25:1129-1131.
33. Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
34. Victoza [package insert]. Princeton, NJ: Novo Nordisk Inc; 2010.
35. Januvia [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; February 2, 2010.
36. Onglyza [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2009.
37. Naslund E, Bogefors J, Skogar S, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;277:R910-R916.
38. Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300-1304.
39. Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741-744.
40. Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301-307.
41. Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239-1246.
42. Vilsboll T, Zdravkovic M, Le Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30:1608-1610.
43. Raz I, Hanefeld M, Xu L, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564-2571.
44. Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638-2643.
45. Rosenstock J, Brazg R, Andryuk PJ, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556-1568.
46. DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes on metformin alone. Diabetes Care. 2009;32:1649-1655.
47. Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628-2635.
48. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
49. Guide for aviation medical examiners. Federal Aviation Administration. http://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/pharm/oral_diabetes/index.cfm?print=go. Published 2009. Accessed July 23, 2010.
50. Noel RA, Braun DK, Patterson RE, et al. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2009;32:834-838.
51. Dore DD, Seeger JD, Arnold CK. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin. 2009;25:1019-1027.
52. Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med. 2010;362:774-777.
53. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. US Food and Drug Administration. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guiddances/ucm071627.pdf. Published 2008. Accessed April 14, 2010.
54. Lamari Y, Boissard C, Moukhtar MS, et al. Expression of glucagon-like peptide 1 receptor in a murine C cell line: regulation of calcitonin gene by glucagon-like peptide 1. FEBS Lett. 1996;393:248-252.
55. Knudsen LB, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473-1486.
1. Gangji AS, Cukierman T, Gerstein HC, et al. A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care. 2007;30:389-394.
2. Holstein A, Plaschke A, Egberts EH. Lower incidence of severe hypoglycaemia in patients with type 2 diabetes treated with glimepiride versus glibenclamide. Diabetes Metab Res Rev. 2001;17:467-473.
3. Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673-2679.
4. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet. 2009;373:473-481.
5. Pratley RE, Nauck M, Bailey T, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375:1447-1456.
6. Nelson P, Poon T, Guan X, et al. The incretin mimetic exenatide as a monotherapy in patients with type 2 diabetes. Diabetes Technol Ther. 2007;9:317-326.
7. Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083-1091.
8. Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 over-weight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436-447.
9. Zinman B, Hoogwerf BJ, Duran GS, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146:477-485.
10. Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52:2046-2055.
11. Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care. 2009;32:1224-1230.
12. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009;374:39-47.
13. Buse JB, Sesti G, Schmidt WE, et al. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33:1300-1303.
14. Klonoff DC, Buse JB, Nielsen LL, et al. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008;24:275-286.
15. Russell-Jones D, Shaw J, Brandle M, et al. The once-daily human GLP-1 analog liraglutide reduces body weight in subjects with type 2 diabetes, irrespective of body mass index at baseline. Paper presented at: American Diabetes Association 68th Scientific Session; June 6-10, 2008; San Francisco, CA.
16. DeFronzo RA, Okerson T, Viswanathan P, et al. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin. 2008;24:2943-2952.
17. Scott R, Wu M, Sanchez M, et al. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract. 2007;61:171-180.
18. Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632-2637.
19. Hanefeld M, Herman GA, Wu M, et al. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin. 2007;23:1329-1339.
20. Hermansen K, Kipnes M, Luo E, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733-745.
21. Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:376-386.
22. Hollander P, Li J, Allen E, et al. Saxagliptin added to a thiazolidinedione improves glycemic control in patients with type 2 diabetes and inadequate control on thiazolidinedione alone. J Clin Endocrinol Metab. 2009;94:4810-4819.
23. Chacra AR, Tan GH, Apanovitch A, et al. Saxagliptin added to a submaximal dose of sulphonylurea improves glycaemic control compared with uptitration of sulphonylurea in patients with type 2 diabetes: a randomised controlled trial. Int J Clin Pract. 2009;63:1395-1406.
24. Horton ES, Silberman C, Davis KL, et al. Weight loss, glycemic control, and changes in cardiovascular biomarkers in patients with type 2 diabetes receiving incretin therapies or insulin in a large cohort database. Diabetes Care. 2010;33:1759-1765.
25. Deacon CF, Hughes TE, Holst JJ. Dipeptidyl peptidase IV inhibition potentiates the insulinotropic effect of glucagon-like peptide 1 in the anesthetized pig. Diabetes. 1998;47:764-769.
26. Gutzwiller JP, Drewe J, Goke B, et al. Glucagon-like peptide-1 promotes satiety and reduces food intake in patients with diabetes mellitus type 2. Am J Physiol. 1999;276:R1541-R1544.
27. Zander M, Madsbad S, Madsen JL, et al. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002;359:824-830.
28. Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84-90.
29. Ratner RE, Maggs D, Nielsen LL, et al. Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2006;8:419-428.
30. Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2008;30:1448-1460.
31. Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:259-267.
32. Courreges JP, Vilsboll T, Zdravkovic M, et al. Beneficial effects of once-daily liraglutide, a human glucagon-like peptide-1 analogue, on cardiovascular risk biomarkers in patients with type 2 diabetes. Diabet Med. 2008;25:1129-1131.
33. Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2009.
34. Victoza [package insert]. Princeton, NJ: Novo Nordisk Inc; 2010.
35. Januvia [package insert]. Whitehouse Station, NJ: Merck & Co, Inc; February 2, 2010.
36. Onglyza [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2009.
37. Naslund E, Bogefors J, Skogar S, et al. GLP-1 slows solid gastric emptying and inhibits insulin, glucagon, and PYY release in humans. Am J Physiol. 1999;277:R910-R916.
38. Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2:1300-1304.
39. Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741-744.
40. Nauck MA, Heimesaat MM, Orskov C, et al. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301-307.
41. Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87:1239-1246.
42. Vilsboll T, Zdravkovic M, Le Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30:1608-1610.
43. Raz I, Hanefeld M, Xu L, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564-2571.
44. Charbonnel B, Karasik A, Liu J, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638-2643.
45. Rosenstock J, Brazg R, Andryuk PJ, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556-1568.
46. DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 diabetes on metformin alone. Diabetes Care. 2009;32:1649-1655.
47. Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628-2635.
48. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193-203.
49. Guide for aviation medical examiners. Federal Aviation Administration. http://www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide/pharm/oral_diabetes/index.cfm?print=go. Published 2009. Accessed July 23, 2010.
50. Noel RA, Braun DK, Patterson RE, et al. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: a retrospective cohort study. Diabetes Care. 2009;32:834-838.
51. Dore DD, Seeger JD, Arnold CK. Use of a claims-based active drug safety surveillance system to assess the risk of acute pancreatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin. 2009;25:1019-1027.
52. Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide—the FDA’s review of a new antidiabetic therapy. N Engl J Med. 2010;362:774-777.
53. Guidance for industry: diabetes mellitus—evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. US Food and Drug Administration. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guiddances/ucm071627.pdf. Published 2008. Accessed April 14, 2010.
54. Lamari Y, Boissard C, Moukhtar MS, et al. Expression of glucagon-like peptide 1 receptor in a murine C cell line: regulation of calcitonin gene by glucagon-like peptide 1. FEBS Lett. 1996;393:248-252.
55. Knudsen LB, Madsen LW, Andersen S, et al. Glucagon-like peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010;151:1473-1486.