User login
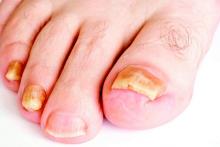
LAS VEGAS – Better penetration through the nail is a key driver behind the increased efficacy of newer topical treatments for onychomycosis, affording a better chance for a clinical and mycologic cure without the potential for toxicity that comes with systemic treatments, according to Dr. David M. Pariser.
At the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, reviewed clinical trial data for 10% topical efinaconazole solution and 5% tavaborole topical solution, newer treatment options for the old problem of nail fungus. “New topical antifungals have improved the challenge of nail penetration,” which had been a key obstacle to efficacy with earlier topical treatments, he said.
Though topical treatments for onychomycosis avoid the risk of systemic side effects and the need for periodic blood tests to check liver function, historically, these treatments have not been very effective, he commented.
Ciclopirox (Penlac), one of the more efficacious treatments, achieves a cure rate of 5.5%-8.5%, and the buildup of the lacquer vehicle requires frequent nail debridement. Because of real or perceived risks, patients may be reluctant to take oral antifungals for onychomycosis, he said, noting that in addition to the potential for liver injury, use of these medications may also be limited by multiple drug-drug interactions.
The two new topical formulations he reviewed have a low molecular weight to allow better penetration through the dense, keratin-rich nail plate, Dr. Pariser said.
One of the topicals, 10% topical efinaconazole solution (Jublia), uses a formulation with low surface tension for good penetration with no surface buildup of vehicle material, achieving better nail penetration than do lacquer-based products, he noted.
A key efinaconazole study assessed clinical improvement in nail appearance in combination with mycologic cure, defined as negative findings on KOH prep exam and negative fungal culture. A complete cure was defined as a “totally clear target toenail and negative KOH/negative fungal culture,” and an “almost complete cure” was defined as mycologic cure, combined with no more than 5% clinically apparent involvement of the target toenail.
After double-blinded randomization into two parallel studies, patients applied either efinaconazole or the vehicle alone once daily at bedtime to the target toenail for 48 weeks. In the studies, 18% and 15% of those in the efinaconazole arm had achieved complete cure 52 weeks after beginning treatment, compared with 3% and 6% of the vehicle arm patients, respectively. However, for a pooled intent-to-treat population, the treatment arm saw a 28% cured or almost-cured rate, compared with 7% of the pooled vehicle-treated patients (P less than .001). Mycologic cure was achieved by 55% and 53% of the patients in the two efinaconazole arms, compared with 17% of the vehicle-only patients in each arm, according to Dr. Pariser.
Adverse events, similar between treatment arms, were mostly mild to moderate and localized, with dermatitis, vesicles, pain, and ingrown toenails the most commonly reported effects.
Tavaborole topical solution, 5% (Kerydin), is a boron-based compound that is highly water soluble, with broad antifungal activity that persists in the presence of keratin. As with efinaconazole, there is no product buildup, so nail debridement is not needed during treatment, Dr. Pariser said.
Two multicenter tavaborole trials compared the active tavaborole solution with a vehicle-only arm in a randomized, double-blind fashion, with product application daily for 48 weeks. The primary efficacy outcome for the trials was complete cure of the target great toenail at week 52. Secondary endpoints were a completely clear or almost clear (10% or less involvement of the target nail) target great toenail, as well as mycologic cure of the nail. Safety was measured by tracking adverse events, and local tolerability, as well as monitoring labs and ECG parameters.
A complete cure for the tavaborole trials required a completely clear nail on clinical exam, as well as negative mycology (negative KOH and negative fungal culture). At 52 weeks, 6.5% and 9.1% of the tavaborole-treated patients saw a complete cure, compared with 0.5% and 1.5% of vehicle-only patients in the two studies. Of those treated with tavaborole, 31.1% and 35.9% achieved mycologic cure, compared with 7.2% and 12.2% of those in the vehicle arm.
The rates of complete or almost complete clearing of the target great toenail for those in the tavaborole arms were 26.1% and 27.5%, compared with 9.3% and 14.6% in the vehicle arms. Predefined treatment success – a combination of mycologic cure and clear or almost clear target great toenail – was seen in 15.3% and 17.9% of the tavaborole-treated patients, compared with 1.5% and 3.9% of the vehicle-only patients (P less than or equal to .001 for all endpoints in both studies).
Treatment-related adverse events were generally mild and similar between the vehicle and treatment arms, with application site exfoliation, erythema, dermatitis, as well as ingrown toenails, the most commonly reported events for both arms.
Dr. Pariser noted that comparing efficacy of the newer agents directly is difficult, since the pivotal clinical trials for each had different designs, entry criteria, clinical assessments, and endpoints.
He disclosed that he is an investigator and consultant for Valeant, which manufactures the 10% topical efinaconazole solution, and an investigator for Anacor Pharmaceuticals, which markets tavaborole.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Better penetration through the nail is a key driver behind the increased efficacy of newer topical treatments for onychomycosis, affording a better chance for a clinical and mycologic cure without the potential for toxicity that comes with systemic treatments, according to Dr. David M. Pariser.
At the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, reviewed clinical trial data for 10% topical efinaconazole solution and 5% tavaborole topical solution, newer treatment options for the old problem of nail fungus. “New topical antifungals have improved the challenge of nail penetration,” which had been a key obstacle to efficacy with earlier topical treatments, he said.
Though topical treatments for onychomycosis avoid the risk of systemic side effects and the need for periodic blood tests to check liver function, historically, these treatments have not been very effective, he commented.
Ciclopirox (Penlac), one of the more efficacious treatments, achieves a cure rate of 5.5%-8.5%, and the buildup of the lacquer vehicle requires frequent nail debridement. Because of real or perceived risks, patients may be reluctant to take oral antifungals for onychomycosis, he said, noting that in addition to the potential for liver injury, use of these medications may also be limited by multiple drug-drug interactions.
The two new topical formulations he reviewed have a low molecular weight to allow better penetration through the dense, keratin-rich nail plate, Dr. Pariser said.
One of the topicals, 10% topical efinaconazole solution (Jublia), uses a formulation with low surface tension for good penetration with no surface buildup of vehicle material, achieving better nail penetration than do lacquer-based products, he noted.
A key efinaconazole study assessed clinical improvement in nail appearance in combination with mycologic cure, defined as negative findings on KOH prep exam and negative fungal culture. A complete cure was defined as a “totally clear target toenail and negative KOH/negative fungal culture,” and an “almost complete cure” was defined as mycologic cure, combined with no more than 5% clinically apparent involvement of the target toenail.
After double-blinded randomization into two parallel studies, patients applied either efinaconazole or the vehicle alone once daily at bedtime to the target toenail for 48 weeks. In the studies, 18% and 15% of those in the efinaconazole arm had achieved complete cure 52 weeks after beginning treatment, compared with 3% and 6% of the vehicle arm patients, respectively. However, for a pooled intent-to-treat population, the treatment arm saw a 28% cured or almost-cured rate, compared with 7% of the pooled vehicle-treated patients (P less than .001). Mycologic cure was achieved by 55% and 53% of the patients in the two efinaconazole arms, compared with 17% of the vehicle-only patients in each arm, according to Dr. Pariser.
Adverse events, similar between treatment arms, were mostly mild to moderate and localized, with dermatitis, vesicles, pain, and ingrown toenails the most commonly reported effects.
Tavaborole topical solution, 5% (Kerydin), is a boron-based compound that is highly water soluble, with broad antifungal activity that persists in the presence of keratin. As with efinaconazole, there is no product buildup, so nail debridement is not needed during treatment, Dr. Pariser said.
Two multicenter tavaborole trials compared the active tavaborole solution with a vehicle-only arm in a randomized, double-blind fashion, with product application daily for 48 weeks. The primary efficacy outcome for the trials was complete cure of the target great toenail at week 52. Secondary endpoints were a completely clear or almost clear (10% or less involvement of the target nail) target great toenail, as well as mycologic cure of the nail. Safety was measured by tracking adverse events, and local tolerability, as well as monitoring labs and ECG parameters.
A complete cure for the tavaborole trials required a completely clear nail on clinical exam, as well as negative mycology (negative KOH and negative fungal culture). At 52 weeks, 6.5% and 9.1% of the tavaborole-treated patients saw a complete cure, compared with 0.5% and 1.5% of vehicle-only patients in the two studies. Of those treated with tavaborole, 31.1% and 35.9% achieved mycologic cure, compared with 7.2% and 12.2% of those in the vehicle arm.
The rates of complete or almost complete clearing of the target great toenail for those in the tavaborole arms were 26.1% and 27.5%, compared with 9.3% and 14.6% in the vehicle arms. Predefined treatment success – a combination of mycologic cure and clear or almost clear target great toenail – was seen in 15.3% and 17.9% of the tavaborole-treated patients, compared with 1.5% and 3.9% of the vehicle-only patients (P less than or equal to .001 for all endpoints in both studies).
Treatment-related adverse events were generally mild and similar between the vehicle and treatment arms, with application site exfoliation, erythema, dermatitis, as well as ingrown toenails, the most commonly reported events for both arms.
Dr. Pariser noted that comparing efficacy of the newer agents directly is difficult, since the pivotal clinical trials for each had different designs, entry criteria, clinical assessments, and endpoints.
He disclosed that he is an investigator and consultant for Valeant, which manufactures the 10% topical efinaconazole solution, and an investigator for Anacor Pharmaceuticals, which markets tavaborole.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
LAS VEGAS – Better penetration through the nail is a key driver behind the increased efficacy of newer topical treatments for onychomycosis, affording a better chance for a clinical and mycologic cure without the potential for toxicity that comes with systemic treatments, according to Dr. David M. Pariser.
At the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar, Dr. Pariser, professor in the department of dermatology at Eastern Virginia Medical School, Norfolk, reviewed clinical trial data for 10% topical efinaconazole solution and 5% tavaborole topical solution, newer treatment options for the old problem of nail fungus. “New topical antifungals have improved the challenge of nail penetration,” which had been a key obstacle to efficacy with earlier topical treatments, he said.
Though topical treatments for onychomycosis avoid the risk of systemic side effects and the need for periodic blood tests to check liver function, historically, these treatments have not been very effective, he commented.
Ciclopirox (Penlac), one of the more efficacious treatments, achieves a cure rate of 5.5%-8.5%, and the buildup of the lacquer vehicle requires frequent nail debridement. Because of real or perceived risks, patients may be reluctant to take oral antifungals for onychomycosis, he said, noting that in addition to the potential for liver injury, use of these medications may also be limited by multiple drug-drug interactions.
The two new topical formulations he reviewed have a low molecular weight to allow better penetration through the dense, keratin-rich nail plate, Dr. Pariser said.
One of the topicals, 10% topical efinaconazole solution (Jublia), uses a formulation with low surface tension for good penetration with no surface buildup of vehicle material, achieving better nail penetration than do lacquer-based products, he noted.
A key efinaconazole study assessed clinical improvement in nail appearance in combination with mycologic cure, defined as negative findings on KOH prep exam and negative fungal culture. A complete cure was defined as a “totally clear target toenail and negative KOH/negative fungal culture,” and an “almost complete cure” was defined as mycologic cure, combined with no more than 5% clinically apparent involvement of the target toenail.
After double-blinded randomization into two parallel studies, patients applied either efinaconazole or the vehicle alone once daily at bedtime to the target toenail for 48 weeks. In the studies, 18% and 15% of those in the efinaconazole arm had achieved complete cure 52 weeks after beginning treatment, compared with 3% and 6% of the vehicle arm patients, respectively. However, for a pooled intent-to-treat population, the treatment arm saw a 28% cured or almost-cured rate, compared with 7% of the pooled vehicle-treated patients (P less than .001). Mycologic cure was achieved by 55% and 53% of the patients in the two efinaconazole arms, compared with 17% of the vehicle-only patients in each arm, according to Dr. Pariser.
Adverse events, similar between treatment arms, were mostly mild to moderate and localized, with dermatitis, vesicles, pain, and ingrown toenails the most commonly reported effects.
Tavaborole topical solution, 5% (Kerydin), is a boron-based compound that is highly water soluble, with broad antifungal activity that persists in the presence of keratin. As with efinaconazole, there is no product buildup, so nail debridement is not needed during treatment, Dr. Pariser said.
Two multicenter tavaborole trials compared the active tavaborole solution with a vehicle-only arm in a randomized, double-blind fashion, with product application daily for 48 weeks. The primary efficacy outcome for the trials was complete cure of the target great toenail at week 52. Secondary endpoints were a completely clear or almost clear (10% or less involvement of the target nail) target great toenail, as well as mycologic cure of the nail. Safety was measured by tracking adverse events, and local tolerability, as well as monitoring labs and ECG parameters.
A complete cure for the tavaborole trials required a completely clear nail on clinical exam, as well as negative mycology (negative KOH and negative fungal culture). At 52 weeks, 6.5% and 9.1% of the tavaborole-treated patients saw a complete cure, compared with 0.5% and 1.5% of vehicle-only patients in the two studies. Of those treated with tavaborole, 31.1% and 35.9% achieved mycologic cure, compared with 7.2% and 12.2% of those in the vehicle arm.
The rates of complete or almost complete clearing of the target great toenail for those in the tavaborole arms were 26.1% and 27.5%, compared with 9.3% and 14.6% in the vehicle arms. Predefined treatment success – a combination of mycologic cure and clear or almost clear target great toenail – was seen in 15.3% and 17.9% of the tavaborole-treated patients, compared with 1.5% and 3.9% of the vehicle-only patients (P less than or equal to .001 for all endpoints in both studies).
Treatment-related adverse events were generally mild and similar between the vehicle and treatment arms, with application site exfoliation, erythema, dermatitis, as well as ingrown toenails, the most commonly reported events for both arms.
Dr. Pariser noted that comparing efficacy of the newer agents directly is difficult, since the pivotal clinical trials for each had different designs, entry criteria, clinical assessments, and endpoints.
He disclosed that he is an investigator and consultant for Valeant, which manufactures the 10% topical efinaconazole solution, and an investigator for Anacor Pharmaceuticals, which markets tavaborole.
SDEF and this news organization are owned by the same parent company.
On Twitter @karioakes
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR