User login
PARIS – The in a 127-patient, randomized, placebo-controlled trial, Sonja Stander, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
She used her speaker’s platform not only to present key outcomes of the phase 2 study, but also to explain some of the underappreciated aspects of prurigo nodularis and how the results shed new light on the pathogenesis of the disease.
“There is a knowledge gap regarding this disease,” observed Dr. Stander, professor of dermatology at the University of Munster (Germany).
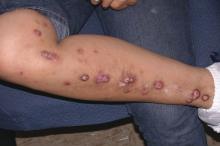
Prurigo nodularis is a chronic skin condition characterized by numerous intensely pruritic, symmetrically distributed, erosive nodular lesions. As Dr. Stander and her fellow members of the EADV Task Force Pruritus recently reported, prurigo nodularis should be considered as one of the clinical manifestations of chronic prurigo, hallmarks of which include neuronal sensitization to itch and development of an itch-scratch cycle (J Eur Acad Dermatol Venereol. 2018 Jul;32[7]:1059-65). At present, there is no approved treatment for prurigo nodularis in the United States or Europe.
Despite its name, prurigo nodularis is not just about the itch. Additional symptoms were frequently reported by participants in the serlopitant study. Indeed, while 97% of participants reported experiencing devilish pruritus, 52% reported a burning sensation on involved skin, 41% said the lesions were painful, and 36% indicated they were plagued by a stinging sensation. All of these disturbing sensations were significantly reduced with 8 weeks of serlopitant in the trial.
The clinical implications of these baseline findings are clear: “We have to ask not only about itch, but also about pain and burning and stinging, which are really an issue with these patients,” the dermatologist said.
Dr. Stander noted that prurigo nodularis has a large negative impact on quality of life as reflected in study participants’ mean baseline Dermatology Life Quality Index score of 61. And it’s a disease that sticks around: At enrollment, 38% of subjects had a 1- to 5-year history of prurigo nodularis, 20% had had the disease for 5-10 years, and fully 29% had had prurigo nodularis for longer than 10 years.
“This is not a chronic relapsing disease. These patients had prurigo nodularis all the time,” Dr. Stander said.
Consistent with the findings of other studies, half of patients with prurigo nodularis had an atopic predisposition as defined by an Erlangen Atopy Questionnaire score of 10 or higher.
Patients in the phase 2 study were randomized to either a 15-mg loading dose of oral serlopitant followed by 5 mg/day for 8 weeks or to placebo. All four of the most common manifestations of prurigo nodularis – itching, burning, pain, and stinging – were reduced to a significantly greater extent in the serlopitant group than with placebo. For example, the proportion of serlopitant-treated patients who rated their itching as none or mild went from zero at baseline to 54% at week 8, as compared with 26% among placebo-treated controls. After 8 weeks, 46% of serlopitant-treated patients rated their itching as moderate, severe, or very severe, as did 71% of controls.
Similarly, the proportion of patients in the serlopitant group who reported no or only a mild burning sensation climbed from 41% at baseline to 73% after 8 weeks. In the control group, the proportion improved from 36% to 47% over the course of 8 weeks. At week 8, 27% of patients on serlopitant characterized themselves as experiencing a moderate, severe, or very severe burning sensation; the rate was twice as high in controls.
Although the stinging sensation associated with prurigo nodularis was significantly reduced by serlopitant, the effect was less robust than with the other common sensory expressions of the disease. The proportion of patients with no or mild stinging increased by an absolute 23% after 8 weeks on serlopitant and by 6% with placebo. At week 8, 22% of patients treated with the neurokinin-1 receptor antagonist complained of a moderate to very severe stinging sensation, as did 40% of controls.
As a neurokinin-1 receptor antagonist, serlopitant inhibits substance P. The drug’s efficacy suggests that substance P plays a role in inducing the itch and other sensory symptoms of prurigo nodularis, according to Dr. Stander.
Based upon the favorable phase 2 results, multiple phase 3 clinical trials of serlopitant for prurigo nodularis are underway in the United States and elsewhere, including a year-long safety study.
Serlopitant is being developed by Menlo Therapeutics. Dr. Stander reported receiving research funding from and serving as a consultant to the company.
PARIS – The in a 127-patient, randomized, placebo-controlled trial, Sonja Stander, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
She used her speaker’s platform not only to present key outcomes of the phase 2 study, but also to explain some of the underappreciated aspects of prurigo nodularis and how the results shed new light on the pathogenesis of the disease.
“There is a knowledge gap regarding this disease,” observed Dr. Stander, professor of dermatology at the University of Munster (Germany).
Prurigo nodularis is a chronic skin condition characterized by numerous intensely pruritic, symmetrically distributed, erosive nodular lesions. As Dr. Stander and her fellow members of the EADV Task Force Pruritus recently reported, prurigo nodularis should be considered as one of the clinical manifestations of chronic prurigo, hallmarks of which include neuronal sensitization to itch and development of an itch-scratch cycle (J Eur Acad Dermatol Venereol. 2018 Jul;32[7]:1059-65). At present, there is no approved treatment for prurigo nodularis in the United States or Europe.
Despite its name, prurigo nodularis is not just about the itch. Additional symptoms were frequently reported by participants in the serlopitant study. Indeed, while 97% of participants reported experiencing devilish pruritus, 52% reported a burning sensation on involved skin, 41% said the lesions were painful, and 36% indicated they were plagued by a stinging sensation. All of these disturbing sensations were significantly reduced with 8 weeks of serlopitant in the trial.
The clinical implications of these baseline findings are clear: “We have to ask not only about itch, but also about pain and burning and stinging, which are really an issue with these patients,” the dermatologist said.
Dr. Stander noted that prurigo nodularis has a large negative impact on quality of life as reflected in study participants’ mean baseline Dermatology Life Quality Index score of 61. And it’s a disease that sticks around: At enrollment, 38% of subjects had a 1- to 5-year history of prurigo nodularis, 20% had had the disease for 5-10 years, and fully 29% had had prurigo nodularis for longer than 10 years.
“This is not a chronic relapsing disease. These patients had prurigo nodularis all the time,” Dr. Stander said.
Consistent with the findings of other studies, half of patients with prurigo nodularis had an atopic predisposition as defined by an Erlangen Atopy Questionnaire score of 10 or higher.
Patients in the phase 2 study were randomized to either a 15-mg loading dose of oral serlopitant followed by 5 mg/day for 8 weeks or to placebo. All four of the most common manifestations of prurigo nodularis – itching, burning, pain, and stinging – were reduced to a significantly greater extent in the serlopitant group than with placebo. For example, the proportion of serlopitant-treated patients who rated their itching as none or mild went from zero at baseline to 54% at week 8, as compared with 26% among placebo-treated controls. After 8 weeks, 46% of serlopitant-treated patients rated their itching as moderate, severe, or very severe, as did 71% of controls.
Similarly, the proportion of patients in the serlopitant group who reported no or only a mild burning sensation climbed from 41% at baseline to 73% after 8 weeks. In the control group, the proportion improved from 36% to 47% over the course of 8 weeks. At week 8, 27% of patients on serlopitant characterized themselves as experiencing a moderate, severe, or very severe burning sensation; the rate was twice as high in controls.
Although the stinging sensation associated with prurigo nodularis was significantly reduced by serlopitant, the effect was less robust than with the other common sensory expressions of the disease. The proportion of patients with no or mild stinging increased by an absolute 23% after 8 weeks on serlopitant and by 6% with placebo. At week 8, 22% of patients treated with the neurokinin-1 receptor antagonist complained of a moderate to very severe stinging sensation, as did 40% of controls.
As a neurokinin-1 receptor antagonist, serlopitant inhibits substance P. The drug’s efficacy suggests that substance P plays a role in inducing the itch and other sensory symptoms of prurigo nodularis, according to Dr. Stander.
Based upon the favorable phase 2 results, multiple phase 3 clinical trials of serlopitant for prurigo nodularis are underway in the United States and elsewhere, including a year-long safety study.
Serlopitant is being developed by Menlo Therapeutics. Dr. Stander reported receiving research funding from and serving as a consultant to the company.
PARIS – The in a 127-patient, randomized, placebo-controlled trial, Sonja Stander, MD, said at the annual congress of the European Academy of Dermatology and Venereology.
She used her speaker’s platform not only to present key outcomes of the phase 2 study, but also to explain some of the underappreciated aspects of prurigo nodularis and how the results shed new light on the pathogenesis of the disease.
“There is a knowledge gap regarding this disease,” observed Dr. Stander, professor of dermatology at the University of Munster (Germany).
Prurigo nodularis is a chronic skin condition characterized by numerous intensely pruritic, symmetrically distributed, erosive nodular lesions. As Dr. Stander and her fellow members of the EADV Task Force Pruritus recently reported, prurigo nodularis should be considered as one of the clinical manifestations of chronic prurigo, hallmarks of which include neuronal sensitization to itch and development of an itch-scratch cycle (J Eur Acad Dermatol Venereol. 2018 Jul;32[7]:1059-65). At present, there is no approved treatment for prurigo nodularis in the United States or Europe.
Despite its name, prurigo nodularis is not just about the itch. Additional symptoms were frequently reported by participants in the serlopitant study. Indeed, while 97% of participants reported experiencing devilish pruritus, 52% reported a burning sensation on involved skin, 41% said the lesions were painful, and 36% indicated they were plagued by a stinging sensation. All of these disturbing sensations were significantly reduced with 8 weeks of serlopitant in the trial.
The clinical implications of these baseline findings are clear: “We have to ask not only about itch, but also about pain and burning and stinging, which are really an issue with these patients,” the dermatologist said.
Dr. Stander noted that prurigo nodularis has a large negative impact on quality of life as reflected in study participants’ mean baseline Dermatology Life Quality Index score of 61. And it’s a disease that sticks around: At enrollment, 38% of subjects had a 1- to 5-year history of prurigo nodularis, 20% had had the disease for 5-10 years, and fully 29% had had prurigo nodularis for longer than 10 years.
“This is not a chronic relapsing disease. These patients had prurigo nodularis all the time,” Dr. Stander said.
Consistent with the findings of other studies, half of patients with prurigo nodularis had an atopic predisposition as defined by an Erlangen Atopy Questionnaire score of 10 or higher.
Patients in the phase 2 study were randomized to either a 15-mg loading dose of oral serlopitant followed by 5 mg/day for 8 weeks or to placebo. All four of the most common manifestations of prurigo nodularis – itching, burning, pain, and stinging – were reduced to a significantly greater extent in the serlopitant group than with placebo. For example, the proportion of serlopitant-treated patients who rated their itching as none or mild went from zero at baseline to 54% at week 8, as compared with 26% among placebo-treated controls. After 8 weeks, 46% of serlopitant-treated patients rated their itching as moderate, severe, or very severe, as did 71% of controls.
Similarly, the proportion of patients in the serlopitant group who reported no or only a mild burning sensation climbed from 41% at baseline to 73% after 8 weeks. In the control group, the proportion improved from 36% to 47% over the course of 8 weeks. At week 8, 27% of patients on serlopitant characterized themselves as experiencing a moderate, severe, or very severe burning sensation; the rate was twice as high in controls.
Although the stinging sensation associated with prurigo nodularis was significantly reduced by serlopitant, the effect was less robust than with the other common sensory expressions of the disease. The proportion of patients with no or mild stinging increased by an absolute 23% after 8 weeks on serlopitant and by 6% with placebo. At week 8, 22% of patients treated with the neurokinin-1 receptor antagonist complained of a moderate to very severe stinging sensation, as did 40% of controls.
As a neurokinin-1 receptor antagonist, serlopitant inhibits substance P. The drug’s efficacy suggests that substance P plays a role in inducing the itch and other sensory symptoms of prurigo nodularis, according to Dr. Stander.
Based upon the favorable phase 2 results, multiple phase 3 clinical trials of serlopitant for prurigo nodularis are underway in the United States and elsewhere, including a year-long safety study.
Serlopitant is being developed by Menlo Therapeutics. Dr. Stander reported receiving research funding from and serving as a consultant to the company.
REPORTING FROM THE EADV CONGRESS
Key clinical point: Serlopitant decreases the disruptive itch, burning, pain, and stinging of prurigo nodularis.
Major finding: The proportion of prurigo nodularis patients who rated their itching as none or mild went from zero at baseline to 54% after 8 weeks on oral serlopitant.
Study details: This 8-week, randomized, double-blind phase 2 study included 127 patients with prurigo nodularis who were randomized to oral serlopitant or placebo.
Disclosures: Serlopitant is being developed by Menlo Therapeutics. The presenter reported receiving research funding from and serving as a consultant to the company.