User login
Atrophic Areas on the Axillary and Anogenital Anatomy
Atrophic Areas on the Axillary and Anogenital Anatomy
Discussion
A diagnosis of lichen sclerosus (LS) was made based on clinical and dermoscopic features, followed by confirmation with histology. The patient’s presentation included typical signs and symptoms of LS: itching, burning, intermittent bleeding, perianal hemorrhage, fusion of the clitoral head, and fissures. Other presentations can include dyspareunia, erosions, and excoriations; however, these symptoms and signs were not reported or seen in this patient.
LS typically affects the anogenital region and has 2 peak incidences: in preadolescent teens and during the fifth to sixth decade of life.1 This patient presented with a case of extragenital LS, which is less common than the classic presentation of LS that affects the genitals. This variant’s epidemiology differs, as it is less common in children and more common in postmenopausal women.2 Extragenital LS presents as white, atrophic plaques with a predilection for sites including the trunk, breasts, upper arms, and sites of physical trauma, with symptoms of dryness and pruritus. Over time, the papules can coalesce and form ivory, scar-like papules or plaques with a wrinkled surface. In advanced stages, telangiectasia or follicular plugging can be present, along with flattening of the dermal-epidermal junction. This flat interface is fragile and can result in bullae that may become hemorrhagic.
Cutaneous squamous cell carcinoma (SCC) may infrequently arise from LS, similar to other chronic inflammatory dermatoses.3 Lichen planus is typically not associated with an increased risk of SCC, except in the oral and hypertrophic variants. However, LS may be considered a premalignant process, and many vulvar SCC cases are noted to have adjacent LS lesions.3
Autoimmune and genetic factors contribute to the pathogenesis of LS. Extracellular matrix protein 1 (ECM1) binds molecules of the basement membrane zone and dermis, contributing to the structure and integrity of skin. Autoantibodies against ECM1 and other antigens of the basement membrane zone, including BP180 and BP320, were found in LS.2 HLA-DQ7 major histocompatibility complex class II antigens have been associated with LS.1
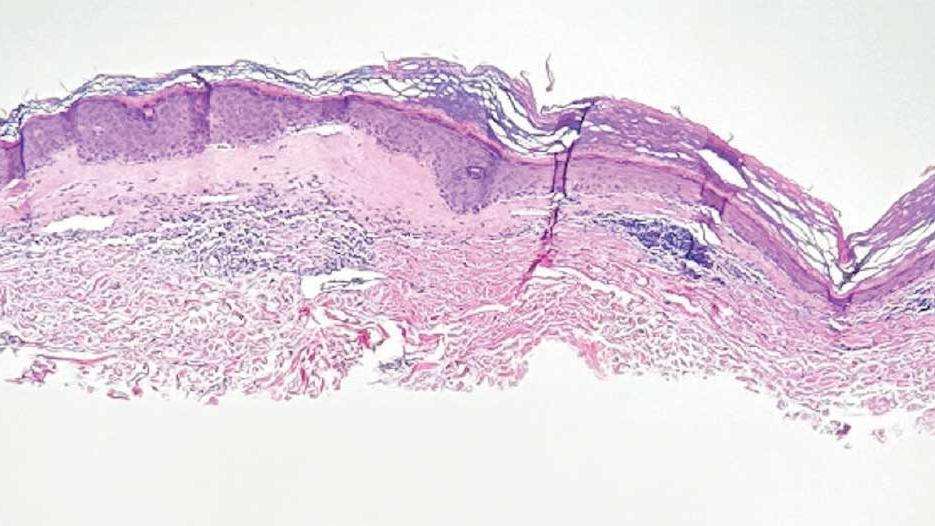
On histologic examination, the epidermis of LS is atrophic with hyperkeratosis. The dermis shows homogenization and sclerosis of superficial collagen with a band-like lymphocytic infiltrate below the sclerosis. The basal layer is thickened, showing basal cell vacuolization and hydropic degeneration.4
First-line treatment for genital and extragenital variants of LS is high-potency topical steroids for 3 months or until the skin texture and color resolve (ie, clobetasol 0.05% cream or ointment). The second-line treatment is a topical calcineurin inhibitor. These treatments are used for management. They are not cures for LS, as relapse is possible after the initial treatment course is completed. Adverse effects of high potency topical steroids are skin burning, skin atrophy, and fragility, telangiectasia. The adverse effects of topical calcineurin inhibitors are stinging and burning on application.
Other Diagnostic Considerations
Inverse psoriasis (IP) is a variant of psoriasis that presents as erythematous, well-demarcated plaques with minimal scale in intertriginous areas and flexural surfaces. Localized dermatophyte, candidal, or bacterial infections can trigger IP.5 It occurs in about 3% to 7% of patients with plaque psoriasis and is thought to form due to koebnerization via mechanical friction of flexural zones.6 The patient described in this case did not have IP because IP would be more likely to present as a well-demarcated erythematous plaque rather than a patch.
Histologically, IP shows regular psoriasiform acanthosis and hypogranulosis of the epidermis, Munro microabscess, spongiform pustules of Kogoj, dilated tortuous dermal vessels, and thinning of the suprapapillary plates.5
Lichen planus pigmentosus-inversus (LPPI) is also known as lichen planus pigmentosus—intertriginous variant. This variant of lichen planus pigmentosus presents as multiple gray to dark brown macules and patches with poorly defined borders in a linear distribution limited to intertriginous areas, flexural surfaces, or following the lines of Blaschko.7 About 20% of cases present with frontal fibrosing alopecia. It is most common in individuals with intermediate and darker skin pigmentation, has a higher prevalence in females, and typically occurs within the third and fifth decades of life. Friction is a common trigger of LPPI.7 A diagnosis of LPPI is incorrect because the lesions would present as gray to dark brown macules, as opposed to the shiny white atrophic thin papules with surrounding pink and purple patches seen in this case.
Histologically, while both LS and LPPI share band-like lymphocytic infiltrate and basal cell vacuolization, findings in the dermis differ. LPPI shows melanophages and prominent melanin incontinence, while LS shows homogenization and sclerosis of superficial collagen.1,8 LPPI also shows absence of compensatory keratinocyte proliferation.
Morphea is an inflammatory disease that affects the dermis and subcutaneous fat, resulting in sclerosis that appears scarlike. Its prevalence increases with age and has a 4:1 prevalence in females, with the plaque type being the most common variant. 9 The typical presentation of plaque-type morphea is an insidious onset of asymptomatic, slightly elevated, erythematous or violaceous, slightly edematous plaques with centrifugal expansion. The center of the plaque may become sclerotic and indurated, acquiring a shiny white color with a peripheral “lilac” ring. Trunk and upper extremity involvement is common. Morphea is associated with increased antisingle-stranded DNA, antitopoisomerase IIa, antiphospholipid, antifibrillin-1, and antihistone antibodies. Triggers of morphea are believed to be localized insults to the skin, including mechanical trauma, injections, vaccinations, and irradiation.9 This answer is incorrect because the patient’s lesions were pruritic and had genital involvement, which are not typical of morphea. Morphea can be differentiated with based on symptoms (lack of pruritus, pain, burning), morphology of lesions (induration versus atrophy), dermoscopy (fibrotic beams with less scale and hemorrhage vs keratotic follicular plugs), and histopathology (depth of inflammation in superficial and deep dermis).
Histology of morphea can differ based on the stage, whether the lesion is sampled in the inflammatory margin or central sclerosis, and the depth of affected skin. At the inflammatory margin, vascular changes, including endothelial swelling and edema, are present, as well as CD4+ T cells, eosinophils, plasma cells, and mast cells surrounding smaller blood vessels. In late stages, the inflammatory infiltrate is no longer present, the epidermis appears regular, and there is a flattened dermal-epidermal junction. Distinct features include homogenous collagen bundles that replace many dermal structures, with atrophic eccrine glands that appear “trapped” in the thickened dermis, and homogenized and hyalinized subcutis.9
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma and presents as annular, erythematous or hypopigmented patches and plaques with fine scale and tumors on the buttocks and sun-protected areas of the limbs and trunk. Lesions can appear with prominent poikiloderma or atrophic or lichenified skin.10 It is most common in males of African descent aged 50 to 55 years. The etiology is largely unknown but believed to be multifactorial. This answer is incorrect because the lesions in this patient appeared more atrophic, were less well demarcated, and lacked the scale that would be present in MF.
On histology, both LS and MF show band-like lymphocytic infiltrate, however MF lacks the homogenization and sclerosis of superficial collagen that is present in the dermis of LS. Also, MF demonstrates epidermotropism of atypical lymphocytes forming Pautrier microabscess.10
Primary Care Role
Primary care physicians can diagnose and treat LS. Referral to dermatology is not mandatory. Note that topical steroids can be used daily for up to 12 weeks. In LS, early treatment is associated with improved outcomes and minimizes the risk of irreversible skin changes.11 Follow-up during the treatment period is recommended to monitor subjective and objective response to treatment. Follow-up after the initial treatment is recommended since LS is typically chronic, can relapse, and SCC can infrequently arise from LS lesions.11
- Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW. Lichen sclerosus: an autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019;15:1429-1439. doi:10.7150/ijbs.34613
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389/fmed.2023.1106318
- Kuraitis D, Murina A. Squamous cell carcinoma arising in chronic inflammatory dermatoses. Cutis. 2024;113:29-34. doi:10.12788/cutis.0914
- Gaertner E, Elstein W. Lichen planus pigmentosus-inversus: case report and review of an unusual entity. Dermatol Online J. 2012;18:11.
- Micali G, Verzì AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019;12:953-959. doi:10.2147/CCID.S189000
- Syed ZU, Khachemoune A. Inverse psoriasis: case presentation and review. Am J Clin Dermatol. 2011;12:143-146. doi:10.2165/11532060-000000000-00000
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514. doi:10.1111/ijd.13806
- Vinay K, Kumar S, Bishnoi A, et al. A clinico-demographic study of 344 patients with lichen planus pigmentosus seen in a tertiary care center in India over an 8-year period. Int J Dermatol. 2020;59:245-252. doi:10.1111/ijd.14540
- Papara C, De Luca DA, Bieber K, et al. Morphea: the 2023 update. Front Med (Lausanne). 2023;10:1108623. doi:10.3389/fmed.2023.1108623
- Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides. Cri t Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151(10):1061-1067. doi:10.1001/jamadermatol.2015.0643
Discussion
A diagnosis of lichen sclerosus (LS) was made based on clinical and dermoscopic features, followed by confirmation with histology. The patient’s presentation included typical signs and symptoms of LS: itching, burning, intermittent bleeding, perianal hemorrhage, fusion of the clitoral head, and fissures. Other presentations can include dyspareunia, erosions, and excoriations; however, these symptoms and signs were not reported or seen in this patient.
LS typically affects the anogenital region and has 2 peak incidences: in preadolescent teens and during the fifth to sixth decade of life.1 This patient presented with a case of extragenital LS, which is less common than the classic presentation of LS that affects the genitals. This variant’s epidemiology differs, as it is less common in children and more common in postmenopausal women.2 Extragenital LS presents as white, atrophic plaques with a predilection for sites including the trunk, breasts, upper arms, and sites of physical trauma, with symptoms of dryness and pruritus. Over time, the papules can coalesce and form ivory, scar-like papules or plaques with a wrinkled surface. In advanced stages, telangiectasia or follicular plugging can be present, along with flattening of the dermal-epidermal junction. This flat interface is fragile and can result in bullae that may become hemorrhagic.
Cutaneous squamous cell carcinoma (SCC) may infrequently arise from LS, similar to other chronic inflammatory dermatoses.3 Lichen planus is typically not associated with an increased risk of SCC, except in the oral and hypertrophic variants. However, LS may be considered a premalignant process, and many vulvar SCC cases are noted to have adjacent LS lesions.3
Autoimmune and genetic factors contribute to the pathogenesis of LS. Extracellular matrix protein 1 (ECM1) binds molecules of the basement membrane zone and dermis, contributing to the structure and integrity of skin. Autoantibodies against ECM1 and other antigens of the basement membrane zone, including BP180 and BP320, were found in LS.2 HLA-DQ7 major histocompatibility complex class II antigens have been associated with LS.1
On histologic examination, the epidermis of LS is atrophic with hyperkeratosis. The dermis shows homogenization and sclerosis of superficial collagen with a band-like lymphocytic infiltrate below the sclerosis. The basal layer is thickened, showing basal cell vacuolization and hydropic degeneration.4
First-line treatment for genital and extragenital variants of LS is high-potency topical steroids for 3 months or until the skin texture and color resolve (ie, clobetasol 0.05% cream or ointment). The second-line treatment is a topical calcineurin inhibitor. These treatments are used for management. They are not cures for LS, as relapse is possible after the initial treatment course is completed. Adverse effects of high potency topical steroids are skin burning, skin atrophy, and fragility, telangiectasia. The adverse effects of topical calcineurin inhibitors are stinging and burning on application.
Other Diagnostic Considerations
Inverse psoriasis (IP) is a variant of psoriasis that presents as erythematous, well-demarcated plaques with minimal scale in intertriginous areas and flexural surfaces. Localized dermatophyte, candidal, or bacterial infections can trigger IP.5 It occurs in about 3% to 7% of patients with plaque psoriasis and is thought to form due to koebnerization via mechanical friction of flexural zones.6 The patient described in this case did not have IP because IP would be more likely to present as a well-demarcated erythematous plaque rather than a patch.
Histologically, IP shows regular psoriasiform acanthosis and hypogranulosis of the epidermis, Munro microabscess, spongiform pustules of Kogoj, dilated tortuous dermal vessels, and thinning of the suprapapillary plates.5
Lichen planus pigmentosus-inversus (LPPI) is also known as lichen planus pigmentosus—intertriginous variant. This variant of lichen planus pigmentosus presents as multiple gray to dark brown macules and patches with poorly defined borders in a linear distribution limited to intertriginous areas, flexural surfaces, or following the lines of Blaschko.7 About 20% of cases present with frontal fibrosing alopecia. It is most common in individuals with intermediate and darker skin pigmentation, has a higher prevalence in females, and typically occurs within the third and fifth decades of life. Friction is a common trigger of LPPI.7 A diagnosis of LPPI is incorrect because the lesions would present as gray to dark brown macules, as opposed to the shiny white atrophic thin papules with surrounding pink and purple patches seen in this case.
Histologically, while both LS and LPPI share band-like lymphocytic infiltrate and basal cell vacuolization, findings in the dermis differ. LPPI shows melanophages and prominent melanin incontinence, while LS shows homogenization and sclerosis of superficial collagen.1,8 LPPI also shows absence of compensatory keratinocyte proliferation.
Morphea is an inflammatory disease that affects the dermis and subcutaneous fat, resulting in sclerosis that appears scarlike. Its prevalence increases with age and has a 4:1 prevalence in females, with the plaque type being the most common variant. 9 The typical presentation of plaque-type morphea is an insidious onset of asymptomatic, slightly elevated, erythematous or violaceous, slightly edematous plaques with centrifugal expansion. The center of the plaque may become sclerotic and indurated, acquiring a shiny white color with a peripheral “lilac” ring. Trunk and upper extremity involvement is common. Morphea is associated with increased antisingle-stranded DNA, antitopoisomerase IIa, antiphospholipid, antifibrillin-1, and antihistone antibodies. Triggers of morphea are believed to be localized insults to the skin, including mechanical trauma, injections, vaccinations, and irradiation.9 This answer is incorrect because the patient’s lesions were pruritic and had genital involvement, which are not typical of morphea. Morphea can be differentiated with based on symptoms (lack of pruritus, pain, burning), morphology of lesions (induration versus atrophy), dermoscopy (fibrotic beams with less scale and hemorrhage vs keratotic follicular plugs), and histopathology (depth of inflammation in superficial and deep dermis).
Histology of morphea can differ based on the stage, whether the lesion is sampled in the inflammatory margin or central sclerosis, and the depth of affected skin. At the inflammatory margin, vascular changes, including endothelial swelling and edema, are present, as well as CD4+ T cells, eosinophils, plasma cells, and mast cells surrounding smaller blood vessels. In late stages, the inflammatory infiltrate is no longer present, the epidermis appears regular, and there is a flattened dermal-epidermal junction. Distinct features include homogenous collagen bundles that replace many dermal structures, with atrophic eccrine glands that appear “trapped” in the thickened dermis, and homogenized and hyalinized subcutis.9
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma and presents as annular, erythematous or hypopigmented patches and plaques with fine scale and tumors on the buttocks and sun-protected areas of the limbs and trunk. Lesions can appear with prominent poikiloderma or atrophic or lichenified skin.10 It is most common in males of African descent aged 50 to 55 years. The etiology is largely unknown but believed to be multifactorial. This answer is incorrect because the lesions in this patient appeared more atrophic, were less well demarcated, and lacked the scale that would be present in MF.
On histology, both LS and MF show band-like lymphocytic infiltrate, however MF lacks the homogenization and sclerosis of superficial collagen that is present in the dermis of LS. Also, MF demonstrates epidermotropism of atypical lymphocytes forming Pautrier microabscess.10
Primary Care Role
Primary care physicians can diagnose and treat LS. Referral to dermatology is not mandatory. Note that topical steroids can be used daily for up to 12 weeks. In LS, early treatment is associated with improved outcomes and minimizes the risk of irreversible skin changes.11 Follow-up during the treatment period is recommended to monitor subjective and objective response to treatment. Follow-up after the initial treatment is recommended since LS is typically chronic, can relapse, and SCC can infrequently arise from LS lesions.11
Discussion
A diagnosis of lichen sclerosus (LS) was made based on clinical and dermoscopic features, followed by confirmation with histology. The patient’s presentation included typical signs and symptoms of LS: itching, burning, intermittent bleeding, perianal hemorrhage, fusion of the clitoral head, and fissures. Other presentations can include dyspareunia, erosions, and excoriations; however, these symptoms and signs were not reported or seen in this patient.
LS typically affects the anogenital region and has 2 peak incidences: in preadolescent teens and during the fifth to sixth decade of life.1 This patient presented with a case of extragenital LS, which is less common than the classic presentation of LS that affects the genitals. This variant’s epidemiology differs, as it is less common in children and more common in postmenopausal women.2 Extragenital LS presents as white, atrophic plaques with a predilection for sites including the trunk, breasts, upper arms, and sites of physical trauma, with symptoms of dryness and pruritus. Over time, the papules can coalesce and form ivory, scar-like papules or plaques with a wrinkled surface. In advanced stages, telangiectasia or follicular plugging can be present, along with flattening of the dermal-epidermal junction. This flat interface is fragile and can result in bullae that may become hemorrhagic.
Cutaneous squamous cell carcinoma (SCC) may infrequently arise from LS, similar to other chronic inflammatory dermatoses.3 Lichen planus is typically not associated with an increased risk of SCC, except in the oral and hypertrophic variants. However, LS may be considered a premalignant process, and many vulvar SCC cases are noted to have adjacent LS lesions.3
Autoimmune and genetic factors contribute to the pathogenesis of LS. Extracellular matrix protein 1 (ECM1) binds molecules of the basement membrane zone and dermis, contributing to the structure and integrity of skin. Autoantibodies against ECM1 and other antigens of the basement membrane zone, including BP180 and BP320, were found in LS.2 HLA-DQ7 major histocompatibility complex class II antigens have been associated with LS.1
On histologic examination, the epidermis of LS is atrophic with hyperkeratosis. The dermis shows homogenization and sclerosis of superficial collagen with a band-like lymphocytic infiltrate below the sclerosis. The basal layer is thickened, showing basal cell vacuolization and hydropic degeneration.4
First-line treatment for genital and extragenital variants of LS is high-potency topical steroids for 3 months or until the skin texture and color resolve (ie, clobetasol 0.05% cream or ointment). The second-line treatment is a topical calcineurin inhibitor. These treatments are used for management. They are not cures for LS, as relapse is possible after the initial treatment course is completed. Adverse effects of high potency topical steroids are skin burning, skin atrophy, and fragility, telangiectasia. The adverse effects of topical calcineurin inhibitors are stinging and burning on application.
Other Diagnostic Considerations
Inverse psoriasis (IP) is a variant of psoriasis that presents as erythematous, well-demarcated plaques with minimal scale in intertriginous areas and flexural surfaces. Localized dermatophyte, candidal, or bacterial infections can trigger IP.5 It occurs in about 3% to 7% of patients with plaque psoriasis and is thought to form due to koebnerization via mechanical friction of flexural zones.6 The patient described in this case did not have IP because IP would be more likely to present as a well-demarcated erythematous plaque rather than a patch.
Histologically, IP shows regular psoriasiform acanthosis and hypogranulosis of the epidermis, Munro microabscess, spongiform pustules of Kogoj, dilated tortuous dermal vessels, and thinning of the suprapapillary plates.5
Lichen planus pigmentosus-inversus (LPPI) is also known as lichen planus pigmentosus—intertriginous variant. This variant of lichen planus pigmentosus presents as multiple gray to dark brown macules and patches with poorly defined borders in a linear distribution limited to intertriginous areas, flexural surfaces, or following the lines of Blaschko.7 About 20% of cases present with frontal fibrosing alopecia. It is most common in individuals with intermediate and darker skin pigmentation, has a higher prevalence in females, and typically occurs within the third and fifth decades of life. Friction is a common trigger of LPPI.7 A diagnosis of LPPI is incorrect because the lesions would present as gray to dark brown macules, as opposed to the shiny white atrophic thin papules with surrounding pink and purple patches seen in this case.
Histologically, while both LS and LPPI share band-like lymphocytic infiltrate and basal cell vacuolization, findings in the dermis differ. LPPI shows melanophages and prominent melanin incontinence, while LS shows homogenization and sclerosis of superficial collagen.1,8 LPPI also shows absence of compensatory keratinocyte proliferation.
Morphea is an inflammatory disease that affects the dermis and subcutaneous fat, resulting in sclerosis that appears scarlike. Its prevalence increases with age and has a 4:1 prevalence in females, with the plaque type being the most common variant. 9 The typical presentation of plaque-type morphea is an insidious onset of asymptomatic, slightly elevated, erythematous or violaceous, slightly edematous plaques with centrifugal expansion. The center of the plaque may become sclerotic and indurated, acquiring a shiny white color with a peripheral “lilac” ring. Trunk and upper extremity involvement is common. Morphea is associated with increased antisingle-stranded DNA, antitopoisomerase IIa, antiphospholipid, antifibrillin-1, and antihistone antibodies. Triggers of morphea are believed to be localized insults to the skin, including mechanical trauma, injections, vaccinations, and irradiation.9 This answer is incorrect because the patient’s lesions were pruritic and had genital involvement, which are not typical of morphea. Morphea can be differentiated with based on symptoms (lack of pruritus, pain, burning), morphology of lesions (induration versus atrophy), dermoscopy (fibrotic beams with less scale and hemorrhage vs keratotic follicular plugs), and histopathology (depth of inflammation in superficial and deep dermis).
Histology of morphea can differ based on the stage, whether the lesion is sampled in the inflammatory margin or central sclerosis, and the depth of affected skin. At the inflammatory margin, vascular changes, including endothelial swelling and edema, are present, as well as CD4+ T cells, eosinophils, plasma cells, and mast cells surrounding smaller blood vessels. In late stages, the inflammatory infiltrate is no longer present, the epidermis appears regular, and there is a flattened dermal-epidermal junction. Distinct features include homogenous collagen bundles that replace many dermal structures, with atrophic eccrine glands that appear “trapped” in the thickened dermis, and homogenized and hyalinized subcutis.9
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma and presents as annular, erythematous or hypopigmented patches and plaques with fine scale and tumors on the buttocks and sun-protected areas of the limbs and trunk. Lesions can appear with prominent poikiloderma or atrophic or lichenified skin.10 It is most common in males of African descent aged 50 to 55 years. The etiology is largely unknown but believed to be multifactorial. This answer is incorrect because the lesions in this patient appeared more atrophic, were less well demarcated, and lacked the scale that would be present in MF.
On histology, both LS and MF show band-like lymphocytic infiltrate, however MF lacks the homogenization and sclerosis of superficial collagen that is present in the dermis of LS. Also, MF demonstrates epidermotropism of atypical lymphocytes forming Pautrier microabscess.10
Primary Care Role
Primary care physicians can diagnose and treat LS. Referral to dermatology is not mandatory. Note that topical steroids can be used daily for up to 12 weeks. In LS, early treatment is associated with improved outcomes and minimizes the risk of irreversible skin changes.11 Follow-up during the treatment period is recommended to monitor subjective and objective response to treatment. Follow-up after the initial treatment is recommended since LS is typically chronic, can relapse, and SCC can infrequently arise from LS lesions.11
- Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW. Lichen sclerosus: an autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019;15:1429-1439. doi:10.7150/ijbs.34613
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389/fmed.2023.1106318
- Kuraitis D, Murina A. Squamous cell carcinoma arising in chronic inflammatory dermatoses. Cutis. 2024;113:29-34. doi:10.12788/cutis.0914
- Gaertner E, Elstein W. Lichen planus pigmentosus-inversus: case report and review of an unusual entity. Dermatol Online J. 2012;18:11.
- Micali G, Verzì AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019;12:953-959. doi:10.2147/CCID.S189000
- Syed ZU, Khachemoune A. Inverse psoriasis: case presentation and review. Am J Clin Dermatol. 2011;12:143-146. doi:10.2165/11532060-000000000-00000
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514. doi:10.1111/ijd.13806
- Vinay K, Kumar S, Bishnoi A, et al. A clinico-demographic study of 344 patients with lichen planus pigmentosus seen in a tertiary care center in India over an 8-year period. Int J Dermatol. 2020;59:245-252. doi:10.1111/ijd.14540
- Papara C, De Luca DA, Bieber K, et al. Morphea: the 2023 update. Front Med (Lausanne). 2023;10:1108623. doi:10.3389/fmed.2023.1108623
- Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides. Cri t Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151(10):1061-1067. doi:10.1001/jamadermatol.2015.0643
- Tran DA, Tan X, Macri CJ, Goldstein AT, Fu SW. Lichen sclerosus: an autoimmunopathogenic and genomic enigma with emerging genetic and immune targets. Int J Biol Sci. 2019;15:1429-1439. doi:10.7150/ijbs.34613
- De Luca DA, Papara C, Vorobyev A, et al. Lichen sclerosus: the 2023 update. Front Med (Lausanne). 2023;10:1106318. doi:10.3389/fmed.2023.1106318
- Kuraitis D, Murina A. Squamous cell carcinoma arising in chronic inflammatory dermatoses. Cutis. 2024;113:29-34. doi:10.12788/cutis.0914
- Gaertner E, Elstein W. Lichen planus pigmentosus-inversus: case report and review of an unusual entity. Dermatol Online J. 2012;18:11.
- Micali G, Verzì AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019;12:953-959. doi:10.2147/CCID.S189000
- Syed ZU, Khachemoune A. Inverse psoriasis: case presentation and review. Am J Clin Dermatol. 2011;12:143-146. doi:10.2165/11532060-000000000-00000
- Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, et al. Lichen planus pigmentosus and its variants: review and update. Int J Dermatol. 2018;57:505-514. doi:10.1111/ijd.13806
- Vinay K, Kumar S, Bishnoi A, et al. A clinico-demographic study of 344 patients with lichen planus pigmentosus seen in a tertiary care center in India over an 8-year period. Int J Dermatol. 2020;59:245-252. doi:10.1111/ijd.14540
- Papara C, De Luca DA, Bieber K, et al. Morphea: the 2023 update. Front Med (Lausanne). 2023;10:1108623. doi:10.3389/fmed.2023.1108623
- Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides. Cri t Rev Oncol Hematol. 2008;65:172-182. doi:10.1016/j.critrevonc.2007.08.004
- Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol. 2015;151(10):1061-1067. doi:10.1001/jamadermatol.2015.0643
Atrophic Areas on the Axillary and Anogenital Anatomy
Atrophic Areas on the Axillary and Anogenital Anatomy
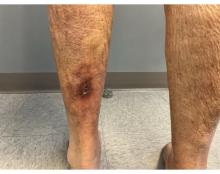
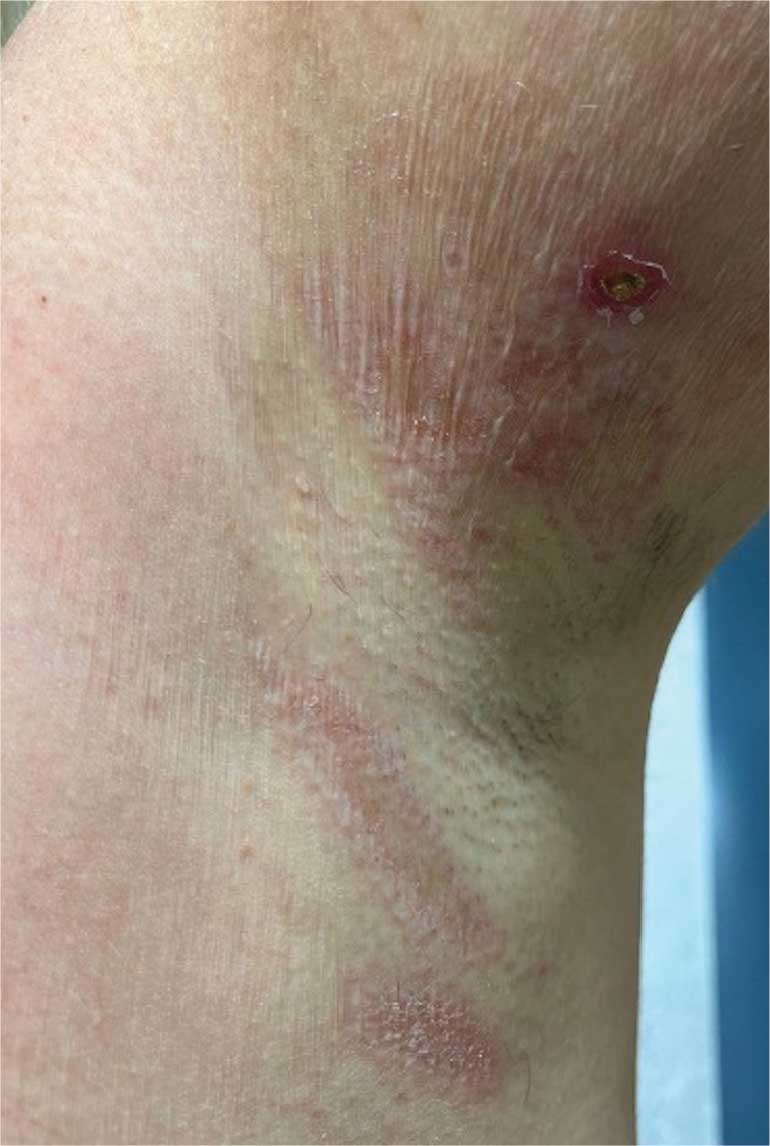
A 62-year-old woman presented for a fullbody skin examination and was found to have a rash in her axillae and inframammary regions. The rash was intermittently pruritic, and the patient felt that the inframammary rash had started from contact with brassiere underwires. She had no oral lesions but noted intermittent burning and itching of the vaginal folds and intermittent bleeding near her anus. Physical examination revealed confluent, shiny, white, atrophic, thin papules with surrounding pink and purple patches on bilateral axillae, bilateral inframammary folds, bilateral inner thighs, and on the clitoral hood and labia minora. There was also an hourglass-shaped erythematous patch involving the vagina and anus. A small fissure was noted perianally, and small hemorrhage was noted on the clitoral head, with fusion of the clitoral head and superior labia minora (Figures 1 and 2).
lesion from punch biopsy of the patient’s left axilla.
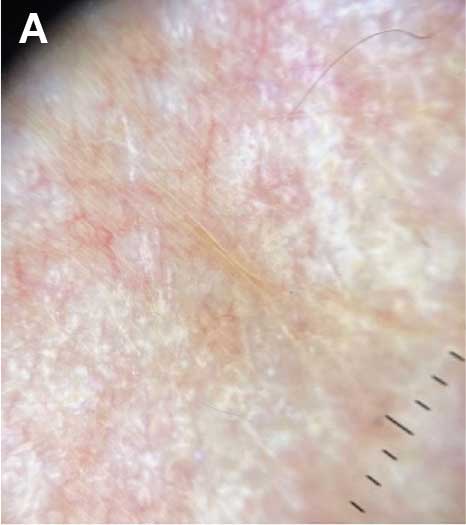
sclerosus plaque showing bright white grouped dots
on a pink background with follicular plugging and linear
branching vessels.
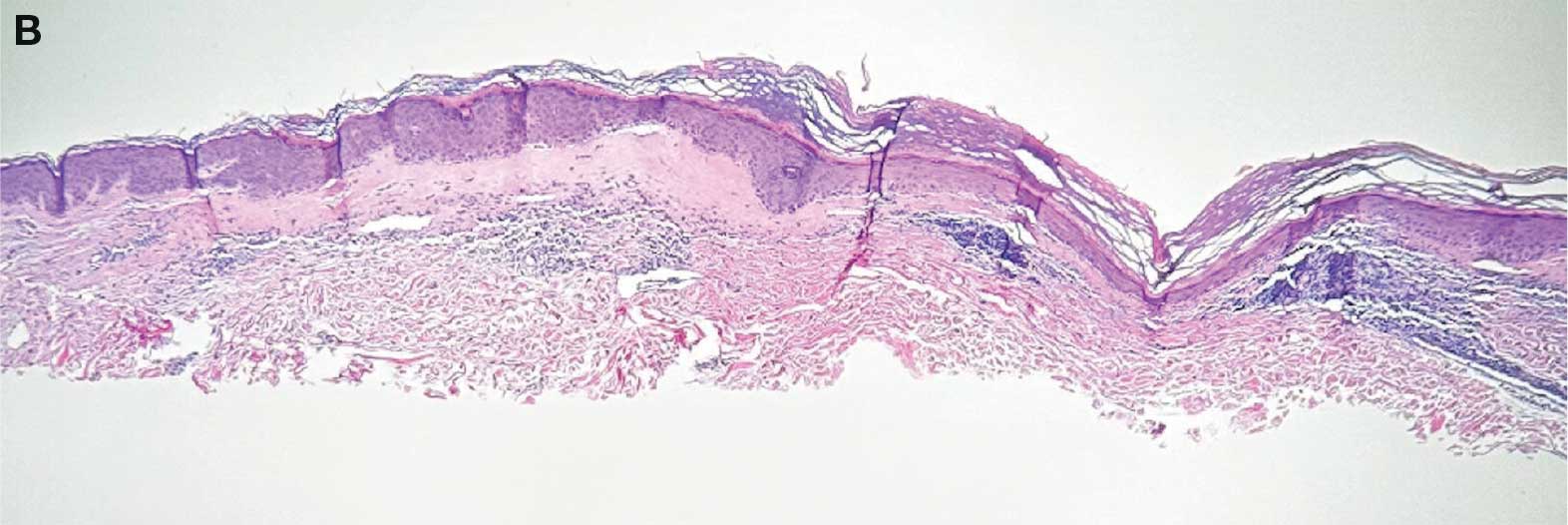
showing a compact corneal layer with a pale papillary
dermis and an underlying lymphocytic infiltrate. These
findings give the “red, white, and blue” appearance.
Low power 20× magnification.
nsbp;
Understanding of Hidradenitis Suppurativa Pathophysiology Advancing
NEW YORK, NY — , according to two investigators intimately involved in much of the recent progress.
“Success is being achieved by targeting multiple inflammatory axes in HS, and therapeutics are evolving rapidly,” reported James G. Krueger, MD, PhD, head of the Laboratory of Investigative Dermatology, Rockefeller University, New York, NY.
The activity of targeted anti-inflammatory therapies — bimekizumab just joined adalimumab and secukinumab as a third approved biologic for HS — is not news, but the degree to which inflammation is upregulated systemically, not just at areas of skin involvement, has changed the conceptualization of HS.
HS Is a Systemic Inflammatory Disease
Relative to psoriasis, for which there are many parallels, “HS is hugely more inflammatory in the systemic circulation,” Krueger said at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024. Yet, HS is also more complex involving additional pathways that appear to include dysbiosis. The concept of follicular occlusion, once a common explanation for HS, has been left far behind.
“Unlike psoriasis, which we can treat really well by inhibiting a single pathway target, HS is just not that simple,” Krueger said. Although largely an inflammatory process, the cascade of inflammatory factors for specific manifestations, such as tunnels, means that optimal therapy in one case might have little benefit in another.
The relatively new evidence that HS activity is not confined to lesional skin might be the most important recent step toward new strategies to target disease. These studies were performed by Kristina Navrazhina, MD, PhD, now a resident in dermatology at the Icahn School of Medicine at Mount Sinai, New York. She received her PhD while studying HS activity in non-lesional skin. Her work has led her to conclude that the best chance for better outcomes in HS is early diagnosis and treatment. Although this is generally true of any pathology, the changes in the HS phenotype once fistulae form includes a poor response to conventional therapies.
In fact, based on her work in evaluating HS activity in non-lesional skin, Navrazhina has shown that “many patients with modest lesions already have advanced disease.” Consistent with the premise that HS is a deeply systemic inflammatory process, nodules, considered an early manifestation, turn out to be “the tip of the iceberg.”
Non-Lesional HS Skin Is Inflamed
When she has employed RNA sequencing based on tape strip sampling from completely normal skin away from nodules, interleukin (IL)-17 and a broad array of other inflammatory markers were found to be upregulated. When she performed ultrasound to look for disease activity under the normal skin, she has often found tunnels already formed. Doppler ultrasound showed some of these tunnels were actively draining.
This might provide a partial explanation for why therapies are not always effective even when clinical signs of disease are modest.
“Are we missing the opportunity for intervening?” Navrazhina asked, noting that early intervention has been limited traditionally by extremely long diagnostic delays. Citing the literature, Navrazhina said the average delay is 7 years for HS versus 1 year for psoriasis. Patients often cycle through 3 or 4 providers before the diagnosis is made, she said.
Awakening first-line clinicians to the signs and symptoms of HS, whether in the emergency room or primary care, is a critical message because of the incrementally difficult task to control disease once fistulae have formed.
Krueger made the same appeal. For the neutrophilic inflammation that characterizes nodules, targeted therapies are often effective, but he agreed that available therapies are generally far less so once tunnels form.
Role Seen for Bacteria in HS Pathogenesis
One reason might be an interaction between anaerobic bacteria and the keratinocytes that form the tunnel walls, according to Krueger. Although HS is not typically considered an infectious disease, he reported that the interaction of these bacteria with keratinocytes is associated with expression of approximately 1000 inflammatory gene products. The process of tunnel formation is traced to how factors recruited by upregulated inflammation, such as chemokines, coordinate.
He described recent work pursing novel strategies such as highly targeted antibiotics or inhibitors of complement factor C5a, which has been proposed as a biomarker for HS, to intervene in preventing or reversing HS tunnels.
While this work progresses, one of the most Important unmet needs in HS is an accepted measure of clinically meaningful improvement in advanced disease, particularly the impact of therapy on HS tunnels, according to Krueger.
“There is no measure of tunnel activity that the FDA accepts in evaluating drugs,” he noted, which will be essential for approving therapies that offer this benefit.
A phase 3 trials program for one of the promising drugs, sonelokimab, was announced early in 2024. A nanobody that targets IL-17A/A, IL-17A/F, and IL-17F/F, the small size of this molecule permits exceptional tissue penetration while the broad anti-IL-17 activity has a high degree of theoretical potential in late-stage HS, according to Krueger.
There are numerous pieces of the HS puzzle that are still missing, but both Krueger and Navrazhina are enthusiastic about new targets and opportunities for disease control that are stemming from a better understanding of the underlying pathophysiology. Not least, both indicated that testing for inflammatory phenotypes will allow for individualized therapeutic choices with a maximum likelihood of response, particularly if earlier diagnosis permits earlier treatment.
“Due to the heterogeneity of HS, it is hard to know who will respond to which treatment or which treatment should be started first,” Navrazhina said. She thinks that early measures of the inflammatory profile in nodules or even non-lesional skin might provide that guidance.
Both Krueger and Navrazhina reported no financial relationships relevant to this work.
A version of this article appeared on Medscape.com.
NEW YORK, NY — , according to two investigators intimately involved in much of the recent progress.
“Success is being achieved by targeting multiple inflammatory axes in HS, and therapeutics are evolving rapidly,” reported James G. Krueger, MD, PhD, head of the Laboratory of Investigative Dermatology, Rockefeller University, New York, NY.
The activity of targeted anti-inflammatory therapies — bimekizumab just joined adalimumab and secukinumab as a third approved biologic for HS — is not news, but the degree to which inflammation is upregulated systemically, not just at areas of skin involvement, has changed the conceptualization of HS.
HS Is a Systemic Inflammatory Disease
Relative to psoriasis, for which there are many parallels, “HS is hugely more inflammatory in the systemic circulation,” Krueger said at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024. Yet, HS is also more complex involving additional pathways that appear to include dysbiosis. The concept of follicular occlusion, once a common explanation for HS, has been left far behind.
“Unlike psoriasis, which we can treat really well by inhibiting a single pathway target, HS is just not that simple,” Krueger said. Although largely an inflammatory process, the cascade of inflammatory factors for specific manifestations, such as tunnels, means that optimal therapy in one case might have little benefit in another.
The relatively new evidence that HS activity is not confined to lesional skin might be the most important recent step toward new strategies to target disease. These studies were performed by Kristina Navrazhina, MD, PhD, now a resident in dermatology at the Icahn School of Medicine at Mount Sinai, New York. She received her PhD while studying HS activity in non-lesional skin. Her work has led her to conclude that the best chance for better outcomes in HS is early diagnosis and treatment. Although this is generally true of any pathology, the changes in the HS phenotype once fistulae form includes a poor response to conventional therapies.
In fact, based on her work in evaluating HS activity in non-lesional skin, Navrazhina has shown that “many patients with modest lesions already have advanced disease.” Consistent with the premise that HS is a deeply systemic inflammatory process, nodules, considered an early manifestation, turn out to be “the tip of the iceberg.”
Non-Lesional HS Skin Is Inflamed
When she has employed RNA sequencing based on tape strip sampling from completely normal skin away from nodules, interleukin (IL)-17 and a broad array of other inflammatory markers were found to be upregulated. When she performed ultrasound to look for disease activity under the normal skin, she has often found tunnels already formed. Doppler ultrasound showed some of these tunnels were actively draining.
This might provide a partial explanation for why therapies are not always effective even when clinical signs of disease are modest.
“Are we missing the opportunity for intervening?” Navrazhina asked, noting that early intervention has been limited traditionally by extremely long diagnostic delays. Citing the literature, Navrazhina said the average delay is 7 years for HS versus 1 year for psoriasis. Patients often cycle through 3 or 4 providers before the diagnosis is made, she said.
Awakening first-line clinicians to the signs and symptoms of HS, whether in the emergency room or primary care, is a critical message because of the incrementally difficult task to control disease once fistulae have formed.
Krueger made the same appeal. For the neutrophilic inflammation that characterizes nodules, targeted therapies are often effective, but he agreed that available therapies are generally far less so once tunnels form.
Role Seen for Bacteria in HS Pathogenesis
One reason might be an interaction between anaerobic bacteria and the keratinocytes that form the tunnel walls, according to Krueger. Although HS is not typically considered an infectious disease, he reported that the interaction of these bacteria with keratinocytes is associated with expression of approximately 1000 inflammatory gene products. The process of tunnel formation is traced to how factors recruited by upregulated inflammation, such as chemokines, coordinate.
He described recent work pursing novel strategies such as highly targeted antibiotics or inhibitors of complement factor C5a, which has been proposed as a biomarker for HS, to intervene in preventing or reversing HS tunnels.
While this work progresses, one of the most Important unmet needs in HS is an accepted measure of clinically meaningful improvement in advanced disease, particularly the impact of therapy on HS tunnels, according to Krueger.
“There is no measure of tunnel activity that the FDA accepts in evaluating drugs,” he noted, which will be essential for approving therapies that offer this benefit.
A phase 3 trials program for one of the promising drugs, sonelokimab, was announced early in 2024. A nanobody that targets IL-17A/A, IL-17A/F, and IL-17F/F, the small size of this molecule permits exceptional tissue penetration while the broad anti-IL-17 activity has a high degree of theoretical potential in late-stage HS, according to Krueger.
There are numerous pieces of the HS puzzle that are still missing, but both Krueger and Navrazhina are enthusiastic about new targets and opportunities for disease control that are stemming from a better understanding of the underlying pathophysiology. Not least, both indicated that testing for inflammatory phenotypes will allow for individualized therapeutic choices with a maximum likelihood of response, particularly if earlier diagnosis permits earlier treatment.
“Due to the heterogeneity of HS, it is hard to know who will respond to which treatment or which treatment should be started first,” Navrazhina said. She thinks that early measures of the inflammatory profile in nodules or even non-lesional skin might provide that guidance.
Both Krueger and Navrazhina reported no financial relationships relevant to this work.
A version of this article appeared on Medscape.com.
NEW YORK, NY — , according to two investigators intimately involved in much of the recent progress.
“Success is being achieved by targeting multiple inflammatory axes in HS, and therapeutics are evolving rapidly,” reported James G. Krueger, MD, PhD, head of the Laboratory of Investigative Dermatology, Rockefeller University, New York, NY.
The activity of targeted anti-inflammatory therapies — bimekizumab just joined adalimumab and secukinumab as a third approved biologic for HS — is not news, but the degree to which inflammation is upregulated systemically, not just at areas of skin involvement, has changed the conceptualization of HS.
HS Is a Systemic Inflammatory Disease
Relative to psoriasis, for which there are many parallels, “HS is hugely more inflammatory in the systemic circulation,” Krueger said at the 27th Annual Winter Symposium — Advances in Medical and Surgical Dermatology (MSWS) 2024. Yet, HS is also more complex involving additional pathways that appear to include dysbiosis. The concept of follicular occlusion, once a common explanation for HS, has been left far behind.
“Unlike psoriasis, which we can treat really well by inhibiting a single pathway target, HS is just not that simple,” Krueger said. Although largely an inflammatory process, the cascade of inflammatory factors for specific manifestations, such as tunnels, means that optimal therapy in one case might have little benefit in another.
The relatively new evidence that HS activity is not confined to lesional skin might be the most important recent step toward new strategies to target disease. These studies were performed by Kristina Navrazhina, MD, PhD, now a resident in dermatology at the Icahn School of Medicine at Mount Sinai, New York. She received her PhD while studying HS activity in non-lesional skin. Her work has led her to conclude that the best chance for better outcomes in HS is early diagnosis and treatment. Although this is generally true of any pathology, the changes in the HS phenotype once fistulae form includes a poor response to conventional therapies.
In fact, based on her work in evaluating HS activity in non-lesional skin, Navrazhina has shown that “many patients with modest lesions already have advanced disease.” Consistent with the premise that HS is a deeply systemic inflammatory process, nodules, considered an early manifestation, turn out to be “the tip of the iceberg.”
Non-Lesional HS Skin Is Inflamed
When she has employed RNA sequencing based on tape strip sampling from completely normal skin away from nodules, interleukin (IL)-17 and a broad array of other inflammatory markers were found to be upregulated. When she performed ultrasound to look for disease activity under the normal skin, she has often found tunnels already formed. Doppler ultrasound showed some of these tunnels were actively draining.
This might provide a partial explanation for why therapies are not always effective even when clinical signs of disease are modest.
“Are we missing the opportunity for intervening?” Navrazhina asked, noting that early intervention has been limited traditionally by extremely long diagnostic delays. Citing the literature, Navrazhina said the average delay is 7 years for HS versus 1 year for psoriasis. Patients often cycle through 3 or 4 providers before the diagnosis is made, she said.
Awakening first-line clinicians to the signs and symptoms of HS, whether in the emergency room or primary care, is a critical message because of the incrementally difficult task to control disease once fistulae have formed.
Krueger made the same appeal. For the neutrophilic inflammation that characterizes nodules, targeted therapies are often effective, but he agreed that available therapies are generally far less so once tunnels form.
Role Seen for Bacteria in HS Pathogenesis
One reason might be an interaction between anaerobic bacteria and the keratinocytes that form the tunnel walls, according to Krueger. Although HS is not typically considered an infectious disease, he reported that the interaction of these bacteria with keratinocytes is associated with expression of approximately 1000 inflammatory gene products. The process of tunnel formation is traced to how factors recruited by upregulated inflammation, such as chemokines, coordinate.
He described recent work pursing novel strategies such as highly targeted antibiotics or inhibitors of complement factor C5a, which has been proposed as a biomarker for HS, to intervene in preventing or reversing HS tunnels.
While this work progresses, one of the most Important unmet needs in HS is an accepted measure of clinically meaningful improvement in advanced disease, particularly the impact of therapy on HS tunnels, according to Krueger.
“There is no measure of tunnel activity that the FDA accepts in evaluating drugs,” he noted, which will be essential for approving therapies that offer this benefit.
A phase 3 trials program for one of the promising drugs, sonelokimab, was announced early in 2024. A nanobody that targets IL-17A/A, IL-17A/F, and IL-17F/F, the small size of this molecule permits exceptional tissue penetration while the broad anti-IL-17 activity has a high degree of theoretical potential in late-stage HS, according to Krueger.
There are numerous pieces of the HS puzzle that are still missing, but both Krueger and Navrazhina are enthusiastic about new targets and opportunities for disease control that are stemming from a better understanding of the underlying pathophysiology. Not least, both indicated that testing for inflammatory phenotypes will allow for individualized therapeutic choices with a maximum likelihood of response, particularly if earlier diagnosis permits earlier treatment.
“Due to the heterogeneity of HS, it is hard to know who will respond to which treatment or which treatment should be started first,” Navrazhina said. She thinks that early measures of the inflammatory profile in nodules or even non-lesional skin might provide that guidance.
Both Krueger and Navrazhina reported no financial relationships relevant to this work.
A version of this article appeared on Medscape.com.
An 81-Year-Old White Woman Presented With a 2-Week History of a Painful Lesion on Her Left Calf
Calciphylaxis, also known as calcific uremic arteriolopathy, is a rare condition most commonly observed in patients with end-stage renal disease (ESRD). Because of the non-healing nature of the wounds and need for frequent hospitalizations, there is a significant risk of sepsis with a 1-year mortality rate greater than 50%.
Beyond ESRD, calciphylaxis is also associated with obesity, diabetes, hypoalbuminemia, autoimmune conditions, hepatic disease, malignancies, and dialysis. Rates in patients on dialysis have been increasing, ranging from 1% to 4%. Certain medications have also been implicated in the development of calciphylaxis, including warfarin, steroids, calcium-based phosphate binders, vitamin D, and iron. There is also an association with White individuals and more cases have been reported in females.
Pathophysiology of this condition includes calcification of the medial layer of arterioles and small arteries near the skin. Damage to vessel endothelium and formation of microthrombi contribute to the ischemia, which results in necrosis and ulceration of the skin. Elevated calcium and phosphate have been associated with these findings; however, these lab abnormalities alone are typically not enough to cause calciphylaxis. Vascular calcification inhibitors such as fetuin-A, osteoprotegerin, and matrix G1a protein may play a role in pathogenesis, with individuals lacking these factors potentially being at a greater risk. Specifically, matrix G1a protein is dependent on vitamin K dependent carboxylation, which may elucidate why warfarin has been implicated in the development of calciphylaxis because of interference with this pathway.
Upon presentation, patients will have painful ischemic plaques on the skin or painful subcutaneous nodules. Long-standing lesions may have a necrotic eschar or secondary infection, or may be associated with livedo reticularis. Areas with a greater concentration of adipose tissue such as the abdomen, thighs, and buttocks are most commonly affected, but lesions may appear anywhere. A biopsy may be done, but a clinical diagnosis is often sufficient as biopsies carry risks of prolonged healing and infection.
The differential diagnosis includes warfarin skin necrosis, cholesterol embolization, vasculitis, antiphospholipid syndrome, and cellulitis. Although this is a cutaneous manifestation, calciphylaxis is indicative of a systemic problem and requires multidisciplinary intervention.
Patients who present with calciphylaxis require a complete metabolic panel, liver function tests, coagulation studies, and albumin tests. Depending on the presentation, imaging studies such as nuclear medicine scans may be used if extensive soft tissue involvement is suspected.
Clinical management includes carefully avoiding electrolyte imbalances, initiating dialysis if necessary, discontinuing potentially offending supplements and medications, and administering proper wound care and pain management. Debridement of necrotic tissue may be necessary and should be initiated early as this has been associated with a 6-month increase in survival. Physicians should have a low threshold for starting antibiotics if secondary infection is suspected, but prophylaxis is not recommended. Sodium thiosulfate has been used off-label, but the mechanism of action is unknown and some meta-analyses indicate this treatment is not significantly associated with improvement of skin lesions. Interventions such as hyperbaric oxygen have also been used, but there is still more research to be done on these modalities.
The case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, Fort Lauderdale, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Kodumudi V et al. Adv Ther. 2020 Dec;37(12):4797-4807. doi: 10.1007/s12325-020-01504-w.
Seethapathy H et al. Adv Chronic Kidney Dis. 2019 Nov;26(6):484-490. doi: 10.1053/j.ackd.2019.09.005.
Turek M et al. Am J Case Rep. 2021 Jun 7:22:e930026. doi: 10.12659/AJCR.930026.
Wen W at al. JAMA Netw Open. 2023;6(4):e2310068. doi:10.1001/jamanetworkopen.2023.10068.
Westphal SG, Plumb T. Calciphylaxis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK519020/.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a rare condition most commonly observed in patients with end-stage renal disease (ESRD). Because of the non-healing nature of the wounds and need for frequent hospitalizations, there is a significant risk of sepsis with a 1-year mortality rate greater than 50%.
Beyond ESRD, calciphylaxis is also associated with obesity, diabetes, hypoalbuminemia, autoimmune conditions, hepatic disease, malignancies, and dialysis. Rates in patients on dialysis have been increasing, ranging from 1% to 4%. Certain medications have also been implicated in the development of calciphylaxis, including warfarin, steroids, calcium-based phosphate binders, vitamin D, and iron. There is also an association with White individuals and more cases have been reported in females.
Pathophysiology of this condition includes calcification of the medial layer of arterioles and small arteries near the skin. Damage to vessel endothelium and formation of microthrombi contribute to the ischemia, which results in necrosis and ulceration of the skin. Elevated calcium and phosphate have been associated with these findings; however, these lab abnormalities alone are typically not enough to cause calciphylaxis. Vascular calcification inhibitors such as fetuin-A, osteoprotegerin, and matrix G1a protein may play a role in pathogenesis, with individuals lacking these factors potentially being at a greater risk. Specifically, matrix G1a protein is dependent on vitamin K dependent carboxylation, which may elucidate why warfarin has been implicated in the development of calciphylaxis because of interference with this pathway.
Upon presentation, patients will have painful ischemic plaques on the skin or painful subcutaneous nodules. Long-standing lesions may have a necrotic eschar or secondary infection, or may be associated with livedo reticularis. Areas with a greater concentration of adipose tissue such as the abdomen, thighs, and buttocks are most commonly affected, but lesions may appear anywhere. A biopsy may be done, but a clinical diagnosis is often sufficient as biopsies carry risks of prolonged healing and infection.
The differential diagnosis includes warfarin skin necrosis, cholesterol embolization, vasculitis, antiphospholipid syndrome, and cellulitis. Although this is a cutaneous manifestation, calciphylaxis is indicative of a systemic problem and requires multidisciplinary intervention.
Patients who present with calciphylaxis require a complete metabolic panel, liver function tests, coagulation studies, and albumin tests. Depending on the presentation, imaging studies such as nuclear medicine scans may be used if extensive soft tissue involvement is suspected.
Clinical management includes carefully avoiding electrolyte imbalances, initiating dialysis if necessary, discontinuing potentially offending supplements and medications, and administering proper wound care and pain management. Debridement of necrotic tissue may be necessary and should be initiated early as this has been associated with a 6-month increase in survival. Physicians should have a low threshold for starting antibiotics if secondary infection is suspected, but prophylaxis is not recommended. Sodium thiosulfate has been used off-label, but the mechanism of action is unknown and some meta-analyses indicate this treatment is not significantly associated with improvement of skin lesions. Interventions such as hyperbaric oxygen have also been used, but there is still more research to be done on these modalities.
The case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, Fort Lauderdale, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Kodumudi V et al. Adv Ther. 2020 Dec;37(12):4797-4807. doi: 10.1007/s12325-020-01504-w.
Seethapathy H et al. Adv Chronic Kidney Dis. 2019 Nov;26(6):484-490. doi: 10.1053/j.ackd.2019.09.005.
Turek M et al. Am J Case Rep. 2021 Jun 7:22:e930026. doi: 10.12659/AJCR.930026.
Wen W at al. JAMA Netw Open. 2023;6(4):e2310068. doi:10.1001/jamanetworkopen.2023.10068.
Westphal SG, Plumb T. Calciphylaxis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK519020/.
Calciphylaxis, also known as calcific uremic arteriolopathy, is a rare condition most commonly observed in patients with end-stage renal disease (ESRD). Because of the non-healing nature of the wounds and need for frequent hospitalizations, there is a significant risk of sepsis with a 1-year mortality rate greater than 50%.
Beyond ESRD, calciphylaxis is also associated with obesity, diabetes, hypoalbuminemia, autoimmune conditions, hepatic disease, malignancies, and dialysis. Rates in patients on dialysis have been increasing, ranging from 1% to 4%. Certain medications have also been implicated in the development of calciphylaxis, including warfarin, steroids, calcium-based phosphate binders, vitamin D, and iron. There is also an association with White individuals and more cases have been reported in females.
Pathophysiology of this condition includes calcification of the medial layer of arterioles and small arteries near the skin. Damage to vessel endothelium and formation of microthrombi contribute to the ischemia, which results in necrosis and ulceration of the skin. Elevated calcium and phosphate have been associated with these findings; however, these lab abnormalities alone are typically not enough to cause calciphylaxis. Vascular calcification inhibitors such as fetuin-A, osteoprotegerin, and matrix G1a protein may play a role in pathogenesis, with individuals lacking these factors potentially being at a greater risk. Specifically, matrix G1a protein is dependent on vitamin K dependent carboxylation, which may elucidate why warfarin has been implicated in the development of calciphylaxis because of interference with this pathway.
Upon presentation, patients will have painful ischemic plaques on the skin or painful subcutaneous nodules. Long-standing lesions may have a necrotic eschar or secondary infection, or may be associated with livedo reticularis. Areas with a greater concentration of adipose tissue such as the abdomen, thighs, and buttocks are most commonly affected, but lesions may appear anywhere. A biopsy may be done, but a clinical diagnosis is often sufficient as biopsies carry risks of prolonged healing and infection.
The differential diagnosis includes warfarin skin necrosis, cholesterol embolization, vasculitis, antiphospholipid syndrome, and cellulitis. Although this is a cutaneous manifestation, calciphylaxis is indicative of a systemic problem and requires multidisciplinary intervention.
Patients who present with calciphylaxis require a complete metabolic panel, liver function tests, coagulation studies, and albumin tests. Depending on the presentation, imaging studies such as nuclear medicine scans may be used if extensive soft tissue involvement is suspected.
Clinical management includes carefully avoiding electrolyte imbalances, initiating dialysis if necessary, discontinuing potentially offending supplements and medications, and administering proper wound care and pain management. Debridement of necrotic tissue may be necessary and should be initiated early as this has been associated with a 6-month increase in survival. Physicians should have a low threshold for starting antibiotics if secondary infection is suspected, but prophylaxis is not recommended. Sodium thiosulfate has been used off-label, but the mechanism of action is unknown and some meta-analyses indicate this treatment is not significantly associated with improvement of skin lesions. Interventions such as hyperbaric oxygen have also been used, but there is still more research to be done on these modalities.
The case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, Fort Lauderdale, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Kodumudi V et al. Adv Ther. 2020 Dec;37(12):4797-4807. doi: 10.1007/s12325-020-01504-w.
Seethapathy H et al. Adv Chronic Kidney Dis. 2019 Nov;26(6):484-490. doi: 10.1053/j.ackd.2019.09.005.
Turek M et al. Am J Case Rep. 2021 Jun 7:22:e930026. doi: 10.12659/AJCR.930026.
Wen W at al. JAMA Netw Open. 2023;6(4):e2310068. doi:10.1001/jamanetworkopen.2023.10068.
Westphal SG, Plumb T. Calciphylaxis. [Updated 2023 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: www.ncbi.nlm.nih.gov/books/NBK519020/.
An 81-year-old White woman with a medical history significant for end stage renal disease (ESRD) on dialysis, diabetes, and a cerebrovascular accident presented with a 2-week history of a very painful lesion on her left calf. Upon physical exam, she was also noted to have tender subcutaneous nodules on her left anterolateral thigh that had been present for several weeks.
What's your diagnosis?
Geriatric Dermatology: Q&A With Daniel C. Butler, MD
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Daniel C. Butler, MD, is associate professor of dermatology and director of the new Inflammatory and Aging Skin Research Program in the Division of Dermatology at the University of Arizona College of Medicine, Tucson, Arizona. Before returning to Arizona, where he had attended medical school, Butler practiced and was a researcher at the University of California, San Francisco, and its geriatric dermatology clinic. He is a co-founder and continues to co-lead the American Academy of Dermatology (AAD) Geriatric Dermatology Expert Resource Group (ERG).
Butler’s interest in geriatric dermatology is rooted in his experience growing up with four grandparents and witnessing their wisdom, relationships, moments with loved ones, and other unique and desirable parts of growing old. “When I looked later at how aging was perceived in dermatology, I found it was a lot about ‘antiaging,’” he told this news organization. “I thought there was a needed voice in dermatology for healthy aging, for all the desirable things that only growing old can provide, along with all the incredible ‘antiaging’ things we can do.”
In interviews, Butler spoke about research priorities in geriatric dermatology, how the “4M” model of geriatrics should be applied within dermatology, how dermatologists can best work with older complex patients, and more. The conversation was edited for clarity and length.
What is geriatric dermatology? It is described by the AAD’s Geriatric Dermatology ERG as “an emerging subspecialty.” Yet it’s also viewed more broadly. Please speak about its various identities and meanings and its importance for dermatology.
If you’re a Mohs surgeon, you’re seeing a strong majority of over 65 patients. And in various specialty clinics, such as inflammatory skin disease, geriatric dermatology pertains to you. In many ways, it can be viewed as a mindset.
From a framework standpoint, and as a field, geriatric dermatology is a basic science initiative, a clinical initiative, an educational initiative, and an advocacy initiative. The goal is to be able to influence, grow, and learn in each of these categories for our older patients. This is happening: Research in this field has progressed, and education has progressed, which has driven some progress in clinical care.
How has research progressed in the basic science of aging skin? What are key questions for dermatology?
There has been a lot of basic science research on aging skin and on how an aging immune system, for instance, is reflected in conditions such as bullous pemphigoid, atopic dermatitis (AD), and chronic itch. But aging involves more than immunosenescence. I think of aging skin as a three-headed monster that involves changes in the skin barrier and the microbiome as well. But is there a primary piece of aging in the skin? What comes first or influences the other? More research on these questions can potentially influence our treatments.
With respect to the immune system, what we’re finding in the skin is that age-related change is not a decline in the immune system per se, but rather aberrance in response. Parts of the system tend to become overactive, with a skew toward overexpression of type 2 inflammation. This can be problematic, driving conditions such as chronic itch.
With respect to the skin barrier, we lose essential fatty acids, and we lose a lot of our recovery ability and our ability to respond quickly to environmental stressors. But are barrier changes triggering the immune system? Or is it the other way around?
The microbiome, which is a big focus of research, involves similar chicken-and-egg discussions. Is it the microbiome that changes and alters the barrier, which then entices the immune system? Which one happens first? We have a lot to learn, and there’s probably not one answer for every patient.
Please speak about research more broadly. What questions and issues need to be answered and addressed to improve the dermatologic care of older adults?
In general, research in dermatology is very disease-specific and not particularly conducive to looking at the larger demographic populations. We have a huge opportunity, therefore, to break the mold and grow geriatric dermatology as an area of population-based research — so that geriatric dermatology research encompasses not only the melanoma researcher who’s trying to understand how aging influences the melanocytes but also the epidemiologic researcher looking at how our diagnoses and coding and prescription practices are different in the 65-plus age group.
Clinically speaking, researchers want to better understand how aging influences the clinical presentations of our diseases. And there’s research to be done on best practices. For example, what are the best practices for treating basal cell carcinomas in patients with mild cognitive impairment? How should we consider the use of topicals in a patient who has severe arthritis or who lives alone? And then how should we teach practical approaches to help providers meet people where they are?
Looking at it from a healthcare system standpoint, there are many care delivery and access issues — practical pieces — to research, and we’re getting a lot better with this. We’re also advocating not only for more inclusion of older adults in clinical trials of treatments but also for the use of evaluations and outcomes that are relevant and important for older adults.
One piece of good news is that we’re seeing safer treatment options with tremendous efficacy that target known pathways for diseases like AD and chronic itch that affect older adults. Again, now we must find ways to improve access to these novel, safe options.
Our research program at the University of Arizona College of Medicine, which we’re just getting off the ground, aims to be dual-sided, looking both at the basic science of aging skin and at access and care delivery issues, such as how to ensure that patients on Medicare have access to medications that are at least on par with others with private insurance.
What are the most common dermatologic problems experienced by older adults?
Based on my experience and on research that we expect to be published soon, it’s absolutely nonmelanoma skin cancers, precancers like actinic keratoses — and on the inflammatory disease side, itch, AD, and psoriasis. Of course, also common are the age-related changes to the skin that we put in the benign category, such as solar lentigines.
How does age influence dermatologic diseases from a pathophysiological and clinical standpoint?
Diseases overall are very similar and respond to the same treatments, but age in and of itself does influence little pieces. For example, there is more crossover in the presentation of psoriasis and AD in older adults, leading to delays in the diagnosis of psoriasis.
With AD, we’ve found that itch is the predominant symptom for older adults rather than the red rash. We see higher or more severe itch scores in older adults with AD with less visual changes on the skin than in younger cohorts. And rash occurs in different locations than in young patients. Older adults typically present with it on their chest, back, and across the trunk, rather than in folded areas. They’re also more likely to get it on their legs in a nummular pattern as opposed to the more traditional flexural area presentation.
What unique considerations need to be made in treating older adults? How should the 4M model of geriatrics be applied to dermatologic care?
Our care model pushes us to be very algorithmic, but at the end of the day, what’s really important are the 4Ms: Mobility, medication, mentation, and “what matters most.” As you’re having your shared decision-making conversations with your patients and their families, these should be your priorities.
A patient with physical limitations, for instance, may not be able to apply a topical cream twice a day all over the body. They may have comorbidities and treatments for these comorbidities that may conflict with medications you’re considering.
And then mentation is so important. For a long time, we used antihistamines for older adults, but this has been proven to be bad for their mentation and risky in other ways. We need to be sure we’re prioritizing their ability to be clear mentally when we’re prescribing medications and even when we’re considering surgical approaches. Do they show capacity for that procedure or treatment, and how will they respond to that treatment later on?
Using the 4M model to drive conversations is a way to get all of us to connect to the patient and learn about what’s most important for them. In many ways, geriatrics is about taking a step back from your specialist skills and thinking about how you would want a family member treated.
We want to avoid treating just the lesion or the pathologic diagnosis. We want to avoid the “conveyor belt” from a biopsy to Mohs. I have 95-year-olds who say, “Heck yeah, if Mohs is the best treatment, that’s what I want.” And I have 70-year-olds who say, “I think I’ll go with another option,” and that’s the right decision for them. It’s having the conversation that matters.
In practice, given time constraints and other confines, how can dermatologists best work with more complex older patients? What are your practical tips?
People talk about having 45-minute “golden year” conversations with their older patients, but it doesn’t have to be this way. In pursuing geriatric dermatology, I decided early on that I wanted to make sure it was practical, so I’ve focused on maximizing shorter visits and on embracing the concept that relationships can be developed over time. Each time we meet with someone, we’re building equity to have bigger conversations later on.
I can have a 15-minute conversation about whether my patient may want to have Mohs surgery, for instance, or escalate treatment to a systemic agent for their chronic inflammatory disease. If that time isn’t enough, I can encourage further thought about treatment options, acknowledge that decisions aren’t necessarily easy, and schedule a follow-up or offer to call the patient after clinic to continue the conversation.
Sometimes, when I’m at an impasse and my patient is unsure how to proceed, I’ll use clear metrics relevant to older adults — sleep, activity level, and caregiver burden — to help my patient. If someone is not sleeping because of their lesion — if they’re so itchy or their inflammatory disease is uncontrolled, for instance — I’ll point out that the side effects of not sleeping are worse than the medications or surgery we’d pursue. If someone removes themselves from an activity due to their skin condition, that’s a red flag. And if the caregiver in the room is overwhelmed or frustrated by having to put cream on twice a day, I’ll use this to advance treatment.
What resources are available for dermatologists interested in improving their geriatric dermatology skills or advancing the area?
For those interested in investigating these issues or improving their practices, the AAD’s Geriatric Dermatology ERG is always welcoming of new members. The ERG will have an all-inclusive meeting at the 2025 annual AAD meeting in March.
The AAD also has educational modules on geriatric dermatology that were recently published as an initiative of our ERG. More information is available on the website. Also valuable is the ElderDerm conference hosted by the George Washington University School of Medicine and Health Sciences, Washington, DC; the second such conference takes place in May 2025.
Butler reported that he had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Cutaneous Lupus Associated with Greater Risk for Atherosclerotic Cardiovascular Disease
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
than with psoriasis.
METHODOLOGY:
- A retrospective matched longitudinal study compared the incidence and prevalence of ASCVD of 8138 individuals with CLE; 24,675 with SLE; 192,577 with psoriasis; and 81,380 control individuals.
- The disease-free control population was matched in a 10:1 ratio to the CLE population on the basis of age, sex, insurance type, and enrollment duration.
- Prevalent ASCVD was defined as coronary artery disease, prior myocardial infarction, or cerebrovascular accident, with ASCVD incidence assessed by number of hospitalizations over 3 years.
TAKEAWAY:
- Persons with CLE had higher ASCVD risk than control individuals (odds ratio [OR], 1.72; P < .001), similar to those with SLE (OR, 2.41; P < .001) but unlike those with psoriasis (OR, 1.03; P = .48).
- ASCVD incidence at 3 years was 24.8 per 1000 person-years for SLE, 15.2 per 1000 person-years for CLE, 14.0 per 1000 person-years for psoriasis, and 10.3 per 1000 person-years for controls.
- Multivariable Cox proportional regression modeling showed ASCVD risk was highest in those with SLE (hazard ratio [HR], 2.23; P < .001) vs CLE (HR, 1.32; P < .001) and psoriasis (HR, 1.06; P = .09).
- ASCVD prevalence was higher in individuals with CLE receiving systemic therapy (2.7%) than in those receiving no therapy (1.6%), suggesting a potential link between disease severity and CVD risk.
IN PRACTICE:
“Persons with CLE are at higher risk for ASCVD, and guidelines for the evaluation and management of ASCVD may improve their quality of care,” the authors wrote.
SOURCE:
The study was led by Henry W. Chen, MD, Department of Dermatology, University of Texas Southwestern Medical Center, Dallas. It was published online on December 4, 2024, in JAMA Dermatology.
LIMITATIONS:
The study was limited by its relatively young population (median age, 49 years) and the exclusion of adults aged > 65 years on Medicare insurance plans. The database lacked race and ethnicity data, and the analysis was restricted to a shorter 3-year period. The study could not fully evaluate detailed risk factors such as blood pressure levels, cholesterol measurements, or glycemic control, nor could it accurately assess smoking status.
DISCLOSURES:
The research was supported by the Department of Dermatology at the University of Texas Southwestern Medical Center and a grant from the National Institutes of Health. Several authors reported receiving grants or personal fees from various pharmaceutical companies. One author reported being a deputy editor for diversity, equity, and inclusion at JAMA Cardiology. Additional disclosures are noted in the original article.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Study Addresses Lichen Planus Prevalence, Treatment
TOPLINE:
METHODOLOGY:
- To evaluate the prevalence of LP, researchers analyzed 566,851 eligible patients from the Explorys database, comprising electronic medical records from over 40 healthcare networks and 53 million patients across the United States.
- They also assessed treatment plans separately among 1998 newly diagnosed patients with LP between October 2015 and January 2020, who required at least one dermatology encounter within the first year following diagnosis.
- The primary outcome was overall prevalence of LP in the United States, including prevalence across specific age, sex, and racial subgroups. Additionally, dermatologist-prescribed treatments for non-oral LP were also reported.
TAKEAWAY:
- Overall, there were 1098 cases of LP (median age, 66 years; 74% women); the crude prevalence of LP was 0.19% and the age- and sex-standardized overall prevalence was 0.15%. Prevalence in women was 1.77 times higher than in men.
- Asian patients showed the highest standardized prevalence (0.2%), followed by Black patients (0.16). Prevalence increased with age, ranging from 0.04% among those aged 18-29 years to 0.26% among those aged 60-69 years and 0.33% among those aged 70-79 years.
IN PRACTICE:
“LP is a fairly common disease, which disproportionately affects women and individuals older than 60 years of age,” the authors wrote. “Future research to help identify patients who may need systemic treatment and determine appropriate treatments for patients with LP to limit sequelae is important as no medication is currently FDA approved for LP.”
SOURCE:
The study was led by Natalia Pelet Del Toro, MD, Department of Dermatology, Northwell Health, New Hyde Park, New York, and was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The absence of a precise diagnosis code for non-oral LP introduces potential misclassification risks. Additionally, the study design did not allow for the establishment of disease severity levels, limiting the ability to correlate treatment choices with disease severity.
DISCLOSURES:
The study did not receive any funding. Two authors reported to have received advisory fees, grants, and/or honoraria from several pharmaceutical companies. Pelet Del Toro and another author did not declare any conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- To evaluate the prevalence of LP, researchers analyzed 566,851 eligible patients from the Explorys database, comprising electronic medical records from over 40 healthcare networks and 53 million patients across the United States.
- They also assessed treatment plans separately among 1998 newly diagnosed patients with LP between October 2015 and January 2020, who required at least one dermatology encounter within the first year following diagnosis.
- The primary outcome was overall prevalence of LP in the United States, including prevalence across specific age, sex, and racial subgroups. Additionally, dermatologist-prescribed treatments for non-oral LP were also reported.
TAKEAWAY:
- Overall, there were 1098 cases of LP (median age, 66 years; 74% women); the crude prevalence of LP was 0.19% and the age- and sex-standardized overall prevalence was 0.15%. Prevalence in women was 1.77 times higher than in men.
- Asian patients showed the highest standardized prevalence (0.2%), followed by Black patients (0.16). Prevalence increased with age, ranging from 0.04% among those aged 18-29 years to 0.26% among those aged 60-69 years and 0.33% among those aged 70-79 years.
IN PRACTICE:
“LP is a fairly common disease, which disproportionately affects women and individuals older than 60 years of age,” the authors wrote. “Future research to help identify patients who may need systemic treatment and determine appropriate treatments for patients with LP to limit sequelae is important as no medication is currently FDA approved for LP.”
SOURCE:
The study was led by Natalia Pelet Del Toro, MD, Department of Dermatology, Northwell Health, New Hyde Park, New York, and was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The absence of a precise diagnosis code for non-oral LP introduces potential misclassification risks. Additionally, the study design did not allow for the establishment of disease severity levels, limiting the ability to correlate treatment choices with disease severity.
DISCLOSURES:
The study did not receive any funding. Two authors reported to have received advisory fees, grants, and/or honoraria from several pharmaceutical companies. Pelet Del Toro and another author did not declare any conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- To evaluate the prevalence of LP, researchers analyzed 566,851 eligible patients from the Explorys database, comprising electronic medical records from over 40 healthcare networks and 53 million patients across the United States.
- They also assessed treatment plans separately among 1998 newly diagnosed patients with LP between October 2015 and January 2020, who required at least one dermatology encounter within the first year following diagnosis.
- The primary outcome was overall prevalence of LP in the United States, including prevalence across specific age, sex, and racial subgroups. Additionally, dermatologist-prescribed treatments for non-oral LP were also reported.
TAKEAWAY:
- Overall, there were 1098 cases of LP (median age, 66 years; 74% women); the crude prevalence of LP was 0.19% and the age- and sex-standardized overall prevalence was 0.15%. Prevalence in women was 1.77 times higher than in men.
- Asian patients showed the highest standardized prevalence (0.2%), followed by Black patients (0.16). Prevalence increased with age, ranging from 0.04% among those aged 18-29 years to 0.26% among those aged 60-69 years and 0.33% among those aged 70-79 years.
IN PRACTICE:
“LP is a fairly common disease, which disproportionately affects women and individuals older than 60 years of age,” the authors wrote. “Future research to help identify patients who may need systemic treatment and determine appropriate treatments for patients with LP to limit sequelae is important as no medication is currently FDA approved for LP.”
SOURCE:
The study was led by Natalia Pelet Del Toro, MD, Department of Dermatology, Northwell Health, New Hyde Park, New York, and was published online in The Journal of the American Academy of Dermatology.
LIMITATIONS:
The absence of a precise diagnosis code for non-oral LP introduces potential misclassification risks. Additionally, the study design did not allow for the establishment of disease severity levels, limiting the ability to correlate treatment choices with disease severity.
DISCLOSURES:
The study did not receive any funding. Two authors reported to have received advisory fees, grants, and/or honoraria from several pharmaceutical companies. Pelet Del Toro and another author did not declare any conflict of interests.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Study Finds Different Survival Rates for Hidradenitis Suppurativa Treatments in Children
results from a small single-center study showed.
A previous study found that overall drug survival of adalimumab and infliximab in adults with HS at 12 and 24 months was 56.3% and 30.5%, and 58.3% and 48.6%, respectively. “They also found that older age, longer disease duration, higher body mass index (BMI), and surgery during treatment are associated with increased drug survival,” Robyn Guo, a third-year medical student at Duke University, Durham, North Carolina, told this news organization following the annual Symposium on Hidradenitis Suppurativa Advances, where the study was presented during an oral abstract session. “To our knowledge, the drug survival of biologic therapies in pediatric HS patients has not been previously investigated.”
Adalimumab and infliximab are tumor necrosis factor blockers approved for multiple indications; adalimumab is approved for treating moderate to severe HS in patients aged 12 years or older. Infliximab is not approved for HS but is used to treat the disease.
To determine the drug survival of adalimumab and infliximab in pediatric patients with HS and whether patient comorbidities and HS lesion location are associated with length of biologic survival in pediatric patients with HS, Guo and colleagues used Kaplan-Meier survival curves to calculate biologic survival at 12 and 24 months following biologic initiation and Cox proportional hazards regression to analyze potential factors associated with biologic survival. The study population included 49 pediatric patients in the adalimumab cohort and 11 in the infliximab cohort.
The researchers found that drug survival for adalimumab was 90.6% at 12 months (95% CI, 83.0%-98.8%) and 78.3% at 24 months (95% CI, 67.7%-90.6%), while drug survival for infliximab was 54.5% at 12 months (95% CI, 31.8%-93.6%) and 36.4% at 24 months, an overall difference that reached statistical significance (P = .0009). “Our data suggests that adalimumab survival is significantly higher than infliximab survival in pediatric HS patients,” Guo said.
On univariate Cox regression analysis, gluteal HS lesions were associated with shorter adalimumab survival, and obesity was associated with longer infliximab survival.
The researchers acknowledged certain limitations of their study, including the small sample size and that unadjusted Cox regression analysis did not account for baseline HS severity, biologic therapy dosing, and concomitant medication use. Also, there were patients in both cohorts who were not biologic-naive: Two in the adalimumab cohort were previously treated with infliximab, and five patients in the infliximab cohort were previously treated with adalimumab.
“We plan on conducting further analysis using adjusted Cox regression analysis to account for baseline disease severity measured by Hurley stage, BMI, medication dosing, and concomitant medication use,” Guo said.
The researchers reported having no financial disclosures.
A version of this article appeared on Medscape.com.
results from a small single-center study showed.
A previous study found that overall drug survival of adalimumab and infliximab in adults with HS at 12 and 24 months was 56.3% and 30.5%, and 58.3% and 48.6%, respectively. “They also found that older age, longer disease duration, higher body mass index (BMI), and surgery during treatment are associated with increased drug survival,” Robyn Guo, a third-year medical student at Duke University, Durham, North Carolina, told this news organization following the annual Symposium on Hidradenitis Suppurativa Advances, where the study was presented during an oral abstract session. “To our knowledge, the drug survival of biologic therapies in pediatric HS patients has not been previously investigated.”
Adalimumab and infliximab are tumor necrosis factor blockers approved for multiple indications; adalimumab is approved for treating moderate to severe HS in patients aged 12 years or older. Infliximab is not approved for HS but is used to treat the disease.
To determine the drug survival of adalimumab and infliximab in pediatric patients with HS and whether patient comorbidities and HS lesion location are associated with length of biologic survival in pediatric patients with HS, Guo and colleagues used Kaplan-Meier survival curves to calculate biologic survival at 12 and 24 months following biologic initiation and Cox proportional hazards regression to analyze potential factors associated with biologic survival. The study population included 49 pediatric patients in the adalimumab cohort and 11 in the infliximab cohort.
The researchers found that drug survival for adalimumab was 90.6% at 12 months (95% CI, 83.0%-98.8%) and 78.3% at 24 months (95% CI, 67.7%-90.6%), while drug survival for infliximab was 54.5% at 12 months (95% CI, 31.8%-93.6%) and 36.4% at 24 months, an overall difference that reached statistical significance (P = .0009). “Our data suggests that adalimumab survival is significantly higher than infliximab survival in pediatric HS patients,” Guo said.
On univariate Cox regression analysis, gluteal HS lesions were associated with shorter adalimumab survival, and obesity was associated with longer infliximab survival.
The researchers acknowledged certain limitations of their study, including the small sample size and that unadjusted Cox regression analysis did not account for baseline HS severity, biologic therapy dosing, and concomitant medication use. Also, there were patients in both cohorts who were not biologic-naive: Two in the adalimumab cohort were previously treated with infliximab, and five patients in the infliximab cohort were previously treated with adalimumab.
“We plan on conducting further analysis using adjusted Cox regression analysis to account for baseline disease severity measured by Hurley stage, BMI, medication dosing, and concomitant medication use,” Guo said.
The researchers reported having no financial disclosures.
A version of this article appeared on Medscape.com.
results from a small single-center study showed.
A previous study found that overall drug survival of adalimumab and infliximab in adults with HS at 12 and 24 months was 56.3% and 30.5%, and 58.3% and 48.6%, respectively. “They also found that older age, longer disease duration, higher body mass index (BMI), and surgery during treatment are associated with increased drug survival,” Robyn Guo, a third-year medical student at Duke University, Durham, North Carolina, told this news organization following the annual Symposium on Hidradenitis Suppurativa Advances, where the study was presented during an oral abstract session. “To our knowledge, the drug survival of biologic therapies in pediatric HS patients has not been previously investigated.”
Adalimumab and infliximab are tumor necrosis factor blockers approved for multiple indications; adalimumab is approved for treating moderate to severe HS in patients aged 12 years or older. Infliximab is not approved for HS but is used to treat the disease.
To determine the drug survival of adalimumab and infliximab in pediatric patients with HS and whether patient comorbidities and HS lesion location are associated with length of biologic survival in pediatric patients with HS, Guo and colleagues used Kaplan-Meier survival curves to calculate biologic survival at 12 and 24 months following biologic initiation and Cox proportional hazards regression to analyze potential factors associated with biologic survival. The study population included 49 pediatric patients in the adalimumab cohort and 11 in the infliximab cohort.
The researchers found that drug survival for adalimumab was 90.6% at 12 months (95% CI, 83.0%-98.8%) and 78.3% at 24 months (95% CI, 67.7%-90.6%), while drug survival for infliximab was 54.5% at 12 months (95% CI, 31.8%-93.6%) and 36.4% at 24 months, an overall difference that reached statistical significance (P = .0009). “Our data suggests that adalimumab survival is significantly higher than infliximab survival in pediatric HS patients,” Guo said.
On univariate Cox regression analysis, gluteal HS lesions were associated with shorter adalimumab survival, and obesity was associated with longer infliximab survival.
The researchers acknowledged certain limitations of their study, including the small sample size and that unadjusted Cox regression analysis did not account for baseline HS severity, biologic therapy dosing, and concomitant medication use. Also, there were patients in both cohorts who were not biologic-naive: Two in the adalimumab cohort were previously treated with infliximab, and five patients in the infliximab cohort were previously treated with adalimumab.
“We plan on conducting further analysis using adjusted Cox regression analysis to account for baseline disease severity measured by Hurley stage, BMI, medication dosing, and concomitant medication use,” Guo said.
The researchers reported having no financial disclosures.
A version of this article appeared on Medscape.com.
FROM SDPA 24
FDA Approves Bimekizumab For Treating Hidradenitis Suppurativa
.
Approval was based on results from two phase 3 studies, BE HEARD I and BE HEARD II, which found that bimekizumab improved the signs and symptoms of disease compared with placebo at week 16 and were sustained to week 48, according to a press release from UCB, the drug’s manufacturer. In both trials, a higher proportion of patients treated with bimekizumab achieved Hidradenitis Suppurativa Clinical Response (HiSCR) scores of 50 and 75 compared with those who received placebo.
The company noted that bimekizumab (Bimzelx) is the first and only approved medicine designed to selectively inhibit IL-17F in addition to IL-17A. According to the prescribing information, the recommended dosing for patients with HS is 320 mg administered by subcutaneous injection at week 0, 2, 4, 6, 8, 10, 12, 14, and 16, then every 4 weeks thereafter.
“The approval of bimekizumab for moderate-to-severe HS is tremendous news for people living with HS” and the clinicians who care for them, Jennifer L. Hsiao, MD, director of the HS clinic at the University of Southern California, Los Angeles, told this news organization.
“It is exciting that we already have two-year trial data for bimekizumab in HS and can see that bimekizumab raises the bar in terms of depth and durability of response that we can expect to see in our patients,” she added. “Given the limited treatment options for HS at this time, the addition of bimekizumab to our treatment armamentarium is a huge step forward for the HS community.”
This development marks the fifth approved indication for bimekizumab since it was first approved in October 2023 for the treatment of moderate to severe plaque psoriasis, followed by approvals for active psoriatic arthritis, nonradiographic axial spondyloarthritis, and active ankylosing spondylitis in September 2024.
According to the prescribing information, certain adverse reactions have been observed with bimekizumab, including suicidal ideation and behavior, infections, liver biochemical abnormalities, and inflammatory bowel disease. A pregnancy exposure registry has been established that monitors pregnancy outcomes in women exposed to bimekizumab. For information, clinicians or patients can contact the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases Study at 1-877-311- 8972 or visit MotherToBaby Pregnancy Studies.
Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, Sanofi, and UCB; a speaker for AbbVie, Galderma, Novartis, Sanofi Regeneron, and UCB; and an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
.
Approval was based on results from two phase 3 studies, BE HEARD I and BE HEARD II, which found that bimekizumab improved the signs and symptoms of disease compared with placebo at week 16 and were sustained to week 48, according to a press release from UCB, the drug’s manufacturer. In both trials, a higher proportion of patients treated with bimekizumab achieved Hidradenitis Suppurativa Clinical Response (HiSCR) scores of 50 and 75 compared with those who received placebo.
The company noted that bimekizumab (Bimzelx) is the first and only approved medicine designed to selectively inhibit IL-17F in addition to IL-17A. According to the prescribing information, the recommended dosing for patients with HS is 320 mg administered by subcutaneous injection at week 0, 2, 4, 6, 8, 10, 12, 14, and 16, then every 4 weeks thereafter.
“The approval of bimekizumab for moderate-to-severe HS is tremendous news for people living with HS” and the clinicians who care for them, Jennifer L. Hsiao, MD, director of the HS clinic at the University of Southern California, Los Angeles, told this news organization.
“It is exciting that we already have two-year trial data for bimekizumab in HS and can see that bimekizumab raises the bar in terms of depth and durability of response that we can expect to see in our patients,” she added. “Given the limited treatment options for HS at this time, the addition of bimekizumab to our treatment armamentarium is a huge step forward for the HS community.”
This development marks the fifth approved indication for bimekizumab since it was first approved in October 2023 for the treatment of moderate to severe plaque psoriasis, followed by approvals for active psoriatic arthritis, nonradiographic axial spondyloarthritis, and active ankylosing spondylitis in September 2024.
According to the prescribing information, certain adverse reactions have been observed with bimekizumab, including suicidal ideation and behavior, infections, liver biochemical abnormalities, and inflammatory bowel disease. A pregnancy exposure registry has been established that monitors pregnancy outcomes in women exposed to bimekizumab. For information, clinicians or patients can contact the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases Study at 1-877-311- 8972 or visit MotherToBaby Pregnancy Studies.
Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, Sanofi, and UCB; a speaker for AbbVie, Galderma, Novartis, Sanofi Regeneron, and UCB; and an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
.
Approval was based on results from two phase 3 studies, BE HEARD I and BE HEARD II, which found that bimekizumab improved the signs and symptoms of disease compared with placebo at week 16 and were sustained to week 48, according to a press release from UCB, the drug’s manufacturer. In both trials, a higher proportion of patients treated with bimekizumab achieved Hidradenitis Suppurativa Clinical Response (HiSCR) scores of 50 and 75 compared with those who received placebo.
The company noted that bimekizumab (Bimzelx) is the first and only approved medicine designed to selectively inhibit IL-17F in addition to IL-17A. According to the prescribing information, the recommended dosing for patients with HS is 320 mg administered by subcutaneous injection at week 0, 2, 4, 6, 8, 10, 12, 14, and 16, then every 4 weeks thereafter.
“The approval of bimekizumab for moderate-to-severe HS is tremendous news for people living with HS” and the clinicians who care for them, Jennifer L. Hsiao, MD, director of the HS clinic at the University of Southern California, Los Angeles, told this news organization.
“It is exciting that we already have two-year trial data for bimekizumab in HS and can see that bimekizumab raises the bar in terms of depth and durability of response that we can expect to see in our patients,” she added. “Given the limited treatment options for HS at this time, the addition of bimekizumab to our treatment armamentarium is a huge step forward for the HS community.”
This development marks the fifth approved indication for bimekizumab since it was first approved in October 2023 for the treatment of moderate to severe plaque psoriasis, followed by approvals for active psoriatic arthritis, nonradiographic axial spondyloarthritis, and active ankylosing spondylitis in September 2024.
According to the prescribing information, certain adverse reactions have been observed with bimekizumab, including suicidal ideation and behavior, infections, liver biochemical abnormalities, and inflammatory bowel disease. A pregnancy exposure registry has been established that monitors pregnancy outcomes in women exposed to bimekizumab. For information, clinicians or patients can contact the Organization of Teratology Information Specialists (OTIS) Autoimmune Diseases Study at 1-877-311- 8972 or visit MotherToBaby Pregnancy Studies.
Hsiao disclosed that she is a member of the board of directors for the HS Foundation and has served as a consultant for AbbVie, Aclaris, Boehringer Ingelheim, Incyte, Novartis, Sanofi, and UCB; a speaker for AbbVie, Galderma, Novartis, Sanofi Regeneron, and UCB; and an investigator for Amgen, Boehringer Ingelheim, and Incyte.
A version of this article first appeared on Medscape.com.
Dupilumab Beneficial When Antihistamines Fall Short for Chronic Spontaneous Urticaria
based on data from 151 individuals.
“Approximately 50% of patients with chronic spontaneous urticaria do not respond to antihistamines,” said Thomas B. Casale, MD, professor of internal medicine at the University of South Florida, Tampa, in an interview. Omalizumab, the only biologic approved for this condition, is not effective in all patients, and additional treatment options are needed, added Casale, the lead author who presented the new data, at the American College of Allergy, Asthma & Immunology (ACAAI) 2024 Annual Scientific Meeting.
Dupilumab (Dupixent), a fully human monoclonal antibody that targets the interleukin (IL)–4 and IL-13 pathways, is currently approved in the United States for the treatment of several allergy and dermatology indications, including atopic dermatitis, severe asthma exacerbations, chronic rhinosinusitis with nasal polyps, and prurigo nodularis.
In the study, known as LIBERTY-CSU CUPID Study C, researchers randomized 74 patients with CSU aged 6 years or older to add-on dupilumab subcutaneously every 2 weeks and 77 to placebo. (Patients were omalizumab-naive and had symptomatic CSU, despite treatment with up to four times the approved dose of standard-of-care H1-antihistamines.) Dupilumab doses were 300 mg for adults and adolescents weighing ≥ 60 kg or 200 mg for adolescents weighing < 60 kg and children weighing ≥ 30 kg.
The primary outcomes were Itch Severity Score over 7 days (ISS7; range, 0-21) and Urticaria Activity Score over 7 days (UAS7; range, 0-42).
Over the 24-week study period, patients in the dupilumab group showed significantly greater change from baseline than those in the placebo group on both measures, with least squares mean changes of 8.6 vs 6.1 for ISS7 and 15.9 vs 11.2 for UAS7 (P = .02 for both).
In addition, at 24 weeks, significantly more patients in the dupilumab group than in the placebo group achieved well-controlled disease based on a UAS of 6 or lower (41% vs 23%; P = .005). Significantly more dupilumab-treated patients also achieved a complete response (defined as a UAS of 0), compared with placebo-treated patients (30% vs 18%; P = .02).
Overall rates of treatment-emergent adverse events were 53% for both groups, and safety data were mainly consistent with dupilumab’s known safety profile, the researchers wrote.
The findings were not surprising, as a previous related study, LIBERTY-CSU CUPID Study A, showed that dupilumab was effective for CSU, Casale told this news organization. “This replicate study confirms the previous study and provides evidence for regulatory approval.”
If approved by the Food and Drug Administration (FDA), “dupilumab will provide another therapeutic option for patients with chronic urticaria unresponsive to antihistamines,” Casale commented.
No new safety signals occurred, and the ability to self-administer the medication at home provides an advantage for patients, he added. As for additional research, “analysis of patient characteristics and potential biomarkers that would predict responsiveness is needed.”
More Research Needed to Fine-Tune Management
An unmet need persists for patients with CSU whose disease is not adequately controlled by higher-dose H1-antihistamines, Robert G. Micheletti, MD, associate professor of dermatology and medicine at the University of Pennsylvania, Philadelphia, said in an interview. “It is important not only to identify effective add-on therapies for these patients but also to generate data to support insurance coverage and drug access,” said Micheletti, who was not involved in the study.
Also referring to the similar findings reported in the LIBERTY-CSU CUPID Study A, Micheletti said, “as in the earlier study, the results demonstrate significantly reduced itch and urticaria in treated patients compared to placebo.”
“While many providers currently prescribe dupilumab off-label for refractory CSU, FDA approval would improve access to the drug for patients who need it and provide an alternative for patients who may not be good candidates for omalizumab,” he added. However, more research is needed to define optimal management of patients with CSU with inadequate response to omalizumab.
“The LIBERTY-CSU CUPID Study B showed a small improvement in itch severity and urticaria activity among such patients receiving dupilumab,” he noted. “Future work should aim to identify biomarkers and other features predictive of response to various therapies.”
Study B involved patients with CSU who were uncontrolled on standard-of-care antihistamines and refractory or intolerant to omalizumab, according to Regeneron.
On November 15, after the ACAAI meeting had ended, the company announced that the FDA had accepted the resubmission of an application for approval of dupilumab for the treatment of CSU in adults and pediatric patients aged 12 years or older not adequately controlled with H1-antihistamines.
The study was supported and sponsored by Sanofi and Regeneron Pharmaceuticals. Casale disclosed serving as a consultant for ALK, ARS Pharma, AstraZeneca, Bryn Pharma, Celgene, Eli Lilly, Genentech, GSK, Jasper, Novartis, Regeneron, and Sanofi and as a speaker for Genentech and Regeneron. Micheletti had no relevant financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
based on data from 151 individuals.
“Approximately 50% of patients with chronic spontaneous urticaria do not respond to antihistamines,” said Thomas B. Casale, MD, professor of internal medicine at the University of South Florida, Tampa, in an interview. Omalizumab, the only biologic approved for this condition, is not effective in all patients, and additional treatment options are needed, added Casale, the lead author who presented the new data, at the American College of Allergy, Asthma & Immunology (ACAAI) 2024 Annual Scientific Meeting.
Dupilumab (Dupixent), a fully human monoclonal antibody that targets the interleukin (IL)–4 and IL-13 pathways, is currently approved in the United States for the treatment of several allergy and dermatology indications, including atopic dermatitis, severe asthma exacerbations, chronic rhinosinusitis with nasal polyps, and prurigo nodularis.
In the study, known as LIBERTY-CSU CUPID Study C, researchers randomized 74 patients with CSU aged 6 years or older to add-on dupilumab subcutaneously every 2 weeks and 77 to placebo. (Patients were omalizumab-naive and had symptomatic CSU, despite treatment with up to four times the approved dose of standard-of-care H1-antihistamines.) Dupilumab doses were 300 mg for adults and adolescents weighing ≥ 60 kg or 200 mg for adolescents weighing < 60 kg and children weighing ≥ 30 kg.
The primary outcomes were Itch Severity Score over 7 days (ISS7; range, 0-21) and Urticaria Activity Score over 7 days (UAS7; range, 0-42).
Over the 24-week study period, patients in the dupilumab group showed significantly greater change from baseline than those in the placebo group on both measures, with least squares mean changes of 8.6 vs 6.1 for ISS7 and 15.9 vs 11.2 for UAS7 (P = .02 for both).
In addition, at 24 weeks, significantly more patients in the dupilumab group than in the placebo group achieved well-controlled disease based on a UAS of 6 or lower (41% vs 23%; P = .005). Significantly more dupilumab-treated patients also achieved a complete response (defined as a UAS of 0), compared with placebo-treated patients (30% vs 18%; P = .02).
Overall rates of treatment-emergent adverse events were 53% for both groups, and safety data were mainly consistent with dupilumab’s known safety profile, the researchers wrote.
The findings were not surprising, as a previous related study, LIBERTY-CSU CUPID Study A, showed that dupilumab was effective for CSU, Casale told this news organization. “This replicate study confirms the previous study and provides evidence for regulatory approval.”
If approved by the Food and Drug Administration (FDA), “dupilumab will provide another therapeutic option for patients with chronic urticaria unresponsive to antihistamines,” Casale commented.
No new safety signals occurred, and the ability to self-administer the medication at home provides an advantage for patients, he added. As for additional research, “analysis of patient characteristics and potential biomarkers that would predict responsiveness is needed.”
More Research Needed to Fine-Tune Management
An unmet need persists for patients with CSU whose disease is not adequately controlled by higher-dose H1-antihistamines, Robert G. Micheletti, MD, associate professor of dermatology and medicine at the University of Pennsylvania, Philadelphia, said in an interview. “It is important not only to identify effective add-on therapies for these patients but also to generate data to support insurance coverage and drug access,” said Micheletti, who was not involved in the study.
Also referring to the similar findings reported in the LIBERTY-CSU CUPID Study A, Micheletti said, “as in the earlier study, the results demonstrate significantly reduced itch and urticaria in treated patients compared to placebo.”
“While many providers currently prescribe dupilumab off-label for refractory CSU, FDA approval would improve access to the drug for patients who need it and provide an alternative for patients who may not be good candidates for omalizumab,” he added. However, more research is needed to define optimal management of patients with CSU with inadequate response to omalizumab.
“The LIBERTY-CSU CUPID Study B showed a small improvement in itch severity and urticaria activity among such patients receiving dupilumab,” he noted. “Future work should aim to identify biomarkers and other features predictive of response to various therapies.”
Study B involved patients with CSU who were uncontrolled on standard-of-care antihistamines and refractory or intolerant to omalizumab, according to Regeneron.
On November 15, after the ACAAI meeting had ended, the company announced that the FDA had accepted the resubmission of an application for approval of dupilumab for the treatment of CSU in adults and pediatric patients aged 12 years or older not adequately controlled with H1-antihistamines.
The study was supported and sponsored by Sanofi and Regeneron Pharmaceuticals. Casale disclosed serving as a consultant for ALK, ARS Pharma, AstraZeneca, Bryn Pharma, Celgene, Eli Lilly, Genentech, GSK, Jasper, Novartis, Regeneron, and Sanofi and as a speaker for Genentech and Regeneron. Micheletti had no relevant financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
based on data from 151 individuals.
“Approximately 50% of patients with chronic spontaneous urticaria do not respond to antihistamines,” said Thomas B. Casale, MD, professor of internal medicine at the University of South Florida, Tampa, in an interview. Omalizumab, the only biologic approved for this condition, is not effective in all patients, and additional treatment options are needed, added Casale, the lead author who presented the new data, at the American College of Allergy, Asthma & Immunology (ACAAI) 2024 Annual Scientific Meeting.
Dupilumab (Dupixent), a fully human monoclonal antibody that targets the interleukin (IL)–4 and IL-13 pathways, is currently approved in the United States for the treatment of several allergy and dermatology indications, including atopic dermatitis, severe asthma exacerbations, chronic rhinosinusitis with nasal polyps, and prurigo nodularis.
In the study, known as LIBERTY-CSU CUPID Study C, researchers randomized 74 patients with CSU aged 6 years or older to add-on dupilumab subcutaneously every 2 weeks and 77 to placebo. (Patients were omalizumab-naive and had symptomatic CSU, despite treatment with up to four times the approved dose of standard-of-care H1-antihistamines.) Dupilumab doses were 300 mg for adults and adolescents weighing ≥ 60 kg or 200 mg for adolescents weighing < 60 kg and children weighing ≥ 30 kg.
The primary outcomes were Itch Severity Score over 7 days (ISS7; range, 0-21) and Urticaria Activity Score over 7 days (UAS7; range, 0-42).
Over the 24-week study period, patients in the dupilumab group showed significantly greater change from baseline than those in the placebo group on both measures, with least squares mean changes of 8.6 vs 6.1 for ISS7 and 15.9 vs 11.2 for UAS7 (P = .02 for both).
In addition, at 24 weeks, significantly more patients in the dupilumab group than in the placebo group achieved well-controlled disease based on a UAS of 6 or lower (41% vs 23%; P = .005). Significantly more dupilumab-treated patients also achieved a complete response (defined as a UAS of 0), compared with placebo-treated patients (30% vs 18%; P = .02).
Overall rates of treatment-emergent adverse events were 53% for both groups, and safety data were mainly consistent with dupilumab’s known safety profile, the researchers wrote.
The findings were not surprising, as a previous related study, LIBERTY-CSU CUPID Study A, showed that dupilumab was effective for CSU, Casale told this news organization. “This replicate study confirms the previous study and provides evidence for regulatory approval.”
If approved by the Food and Drug Administration (FDA), “dupilumab will provide another therapeutic option for patients with chronic urticaria unresponsive to antihistamines,” Casale commented.
No new safety signals occurred, and the ability to self-administer the medication at home provides an advantage for patients, he added. As for additional research, “analysis of patient characteristics and potential biomarkers that would predict responsiveness is needed.”
More Research Needed to Fine-Tune Management
An unmet need persists for patients with CSU whose disease is not adequately controlled by higher-dose H1-antihistamines, Robert G. Micheletti, MD, associate professor of dermatology and medicine at the University of Pennsylvania, Philadelphia, said in an interview. “It is important not only to identify effective add-on therapies for these patients but also to generate data to support insurance coverage and drug access,” said Micheletti, who was not involved in the study.
Also referring to the similar findings reported in the LIBERTY-CSU CUPID Study A, Micheletti said, “as in the earlier study, the results demonstrate significantly reduced itch and urticaria in treated patients compared to placebo.”
“While many providers currently prescribe dupilumab off-label for refractory CSU, FDA approval would improve access to the drug for patients who need it and provide an alternative for patients who may not be good candidates for omalizumab,” he added. However, more research is needed to define optimal management of patients with CSU with inadequate response to omalizumab.
“The LIBERTY-CSU CUPID Study B showed a small improvement in itch severity and urticaria activity among such patients receiving dupilumab,” he noted. “Future work should aim to identify biomarkers and other features predictive of response to various therapies.”
Study B involved patients with CSU who were uncontrolled on standard-of-care antihistamines and refractory or intolerant to omalizumab, according to Regeneron.
On November 15, after the ACAAI meeting had ended, the company announced that the FDA had accepted the resubmission of an application for approval of dupilumab for the treatment of CSU in adults and pediatric patients aged 12 years or older not adequately controlled with H1-antihistamines.
The study was supported and sponsored by Sanofi and Regeneron Pharmaceuticals. Casale disclosed serving as a consultant for ALK, ARS Pharma, AstraZeneca, Bryn Pharma, Celgene, Eli Lilly, Genentech, GSK, Jasper, Novartis, Regeneron, and Sanofi and as a speaker for Genentech and Regeneron. Micheletti had no relevant financial conflicts to disclose.
A version of this article first appeared on Medscape.com.
FROM ACAAI 2024
Ob.Gyn. Says Collaboration with Dermatologists Essential for Managing Vulvar Dermatoses
— and she believes collaboration with dermatologists is essential, especially for complex cases in what she calls a neglected, data-poor area of medicine.
She also recommends that dermatologists have a good understanding of the vestibule, “one of the most important structures in vulvar medicine,” and that they become equipped to recognize generalized and localized causes of vulvar pain and/or itch.
“The problem is, we don’t talk about [vulvovaginal pain and itch] ... it’s taboo and we’re not taught about it in medical school,” Cigna, assistant professor of obstetrics and gynecology at The George Washington University (GWU), Washington, DC, said in a grand rounds lecture held recently at the GWU School of Medicine and Health Sciences Department of Dermatology.
“There are dermatologists who don’t have much training in vulvar dermatology, and a lot of gyns don’t get as much training” as they should, she said in an interview after the lecture. “So who’s looking at people’s vulvar skin and figuring out what’s going on and giving them effective treatments and evidence-based education?”
Cigna and dermatologist Emily Murphy, MD, will be co-directors of a joint ob.gyn-dermatology Vulvar Dermatology Clinic at GWU that will be launched in 2025, with monthly clinics for particularly challenging cases where the etiology is unclear or treatment is ineffective. “We want to collaborate in a more systematic way and put our heads together and think creatively about what will improve patient care,” Cigna said in the interview.
Dermatologists have valuable expertise in the immunology and genetic factors involved in skin disorders, Cigna said. Moreover, Murphy, assistant professor of dermatology and director of the Vulvar Health Program at GWU, said in an interview, dermatologists “are comfortable in going to off-label systemic medications that ob.gyns may not use that often” and bring to the table expertise in various types of procedures.
Murphy recently trained with Melissa Mauskar, MD, associate of dermatology and obstetrics and gynecology at the University of Texas Southwestern, Dallas, and founder and director of the Gynecologic Dermatology Clinic there. “It’s so important for dermatologists to be involved. It just takes some extra training that residents aren’t getting right now,” said Murphy, a member of the newly formed Vulvar Dermatoses Research Consortium.
In her grand rounds lecture, Cigna offered pearls to dermatologists for approaching a history and exam and covered highlights of the diagnosis and treatment of various problems, from vulvar Candida infections and lichen simplex chronicus to vulvar lichen sclerosus (LS), vulvar lichen planus (LP), vulvar Crohn’s disease, pudendal neuralgia, and pelvic floor muscle spasm, as well as the role of mast cell proliferation in vulvar issues.
Approaching the History and Exam
A comprehensive history covers the start, duration, and location of pain and/or itching as well as a detailed timeline (such as timing of potential causes, including injuries or births) and symptoms (such as burning, cutting, aching, and stinging). The question of whether pain “is on the outside, at the entrance, or deeper inside” is “crucial, especially for those in dermatology,” Cigna emphasized.
“And if you’re seeing a patient for a vulvar condition, please ask them about sex. Ask, is this affecting your sexual or intimate life with your partner because this can also give you a clue about what’s going on and how you can help them,” she told the audience of dermatologists.
Queries about trauma history (physical and emotional/verbal), competitive sports (such as daily cycling, equestrian, and heavy weight lifting), endometriosis/gynecologic surgery, connective tissue disorders (such as Ehler-Danlos syndrome), and irritable bowel syndrome are all potentially important to consider. It is important to ask about anxiety, depression, and obsessive-compulsive disorder, which do not cause — but are highly associated with — vulvar dermatoses, she said.
A surprisingly large number of people with vulvovaginal issues are being diagnosed with Ehler-Danlos syndrome, so “I’m always asking, are you hypermobile because this might be affecting the musculoskeletal system, which might be affecting the pelvis,” Cigna said. “Anything that affects the pelvis can affect the vulva as well.”
The pelvic examination should be “offered” rather than assumed to be part of the exam, as part of a trauma-informed approach that is crucial for earning trust, she advised. “Just saying, ‘we’re going to talk, and then I can offer you an exam if you like’…patients like it. It helps them feel safer and more open.”
Many diagnoses are differentiated by eliciting pain on the anterior vs the posterior half of the vulvar vestibule — the part of the vulva that lies between the labia minora and is composed of nonkeratinized tissue with embryonic origins in the endoderm. “If you touch on the keratinized skin (of the vulva) and they don’t have pain, but on the vestibule they do have pain, and there is no pain inside the vagina, this suggests there is a vestibular problem,” said Cigna.
Pain/tenderness isolated to the posterior half of the vestibule suggests a muscular cause, and pain in both the posterior and anterior parts of the vestibule suggests a cause that is more systemic or diffuse, which could be a result of a hormonal issue such as one related to oral contraceptives or decreased testosterone, or a nerve-related process.
Cigna uses gentle swipes of a Q-tip moistened with water or gel to examine the vulva rather than a poke or touch, with the exception being the posterior vestibule, which overlies muscle insertion sites. “Make sure to get a baseline in remote areas such as the inner thigh, and always distinguish between ‘scratchy/sensitive’ sensations and pain,” she said, noting the value of having the patient hold a mirror on her inner thigh.
Causes of Vulvar Itch: Infectious and Noninfectious
With vulvar candidiasis, a common infectious cause of vulvar itch, “you have to ask if they’re also itching on the inside because if you treat them with a topical and you don’t treat the vaginal yeast infection that may be co-occurring, they’ll keep reseeding their vulvar skin,” Cigna said, “and it will never be fully treated.”
Candida albicans is the most common cause of vulvar or vulvovaginal candidiasis, and resistance to antifungals has been rising. Non-albicans Candida “tends to have even higher resistance rates,” she said. Ordering a sensitivity panel along with the culture is helpful, but “comprehensive vaginal biome” panels are generally not useful. “It’s hard to correlate the information clinically,” she said, “and there’s not always a lot of information about susceptibilities, which is what I really like to know.”
Cigna’s treatments for vaginal infections include miconazole, terconazole, and fluconazole (and occasionally, itraconazole or voriconazole — a “decision we don’t take lightly”). Vulvar treatments include nystatin ointment, clotrimazole cream, and miconazole cream. Often, optimal treatment involves addressing “both inside and out,” she said, noting the importance of also killing yeast in undergarment fabric.
“In my experience, Diflucan [oral fluconazole] doesn’t treat persistent vulvar cutaneous skin yeast well, so while I might try Diflucan, I typically use something topical as well,” she said. “And with vaginal yeast, we do use boric acid from time to time, especially for non-albicans species because it tends to be a little more effective.”
Noninfectious causes of vulvar itch include allergic, neuropathic, and muscular causes, as well as autoimmune dermatoses and mast cell activation syndrome. Well known in dermatology are acute contact dermatitis and lichen simplex chronicus — both characterized by induration, thickening, and a “puffy” erythematous appearance, and worsening of pruritus at night. What may be less appreciated is the long list of implicated allergens , including Always menstrual pads made of a plastic-containing “dry weave” material, Cigna said. There are at least several cotton-only, low-preservative feminine products available on the market, she noted.
Common Autoimmune Vulvar Dermatoses: LS and LP
Vulvar LS has traditionally been thought to affect mainly prepubertal and postmenopausal women, but the autoimmune condition is now known to affect more reproductive-age people with vulvas than previously appreciated, Cigna said.
And notably, in an observational web-based study of premenopausal women (aged 18-50 years) with biopsy-confirmed vulvar LS, the leading symptom was not itch but dyspareunia and tearing with intercourse. This means “we’re missing people,” said Cigna, an author of the study. “We think the reason we’re not seeing itch as commonly in this population is that itch is likely mediated by the low estrogen state of pre- and postmenopausal people.”
Vulvar LS also occurs in pregnancy, with symptoms that are either stable or decrease during pregnancy and increase in the postpartum period, as demonstrated in a recently published online survey.
Patients with vulvar LS can present with hypopigmentation, lichenification, and scarring and architectural changes, the latter of which can involve clitoral phimosis, labial resorption, and narrowing of the introitus. (The vaginal mucosa is unaffected.) The presentation can be subtle, especially in premenopausal women, and differentiation between LS, vitiligo, and yeast is sometimes necessary.
A timely biopsy-driven definitive diagnosis is important because vulvar LS increases the risk for cancer if it’s not adequately treated and because long-term steroid use can affect the accuracy of pathology reports. “We really care about keeping this disease in remission as much as possible,” Cigna said. Experts in the field recommend long-term maintenance therapy with a mid-ultra-potent steroid one to three times/week or an alternative. “I’ve just started using ruxolitinib cream, a Janus kinase (JAK) inhibitor, and tacrolimus, a calcineurin inhibitor,” she said.
With vulvar LP, based on current evidence, the risk for malignant transformation is low, but “it crosses into the vagina and can cause vaginal adhesions, so if you’re diagnosing someone with lichen planus, you need to make sure you’re talking with them about dilators, and if you’re not comfortable, send them to [gyn],” she said.
The use of vulvoscopy is important for one’s ability to see the fine Wickham’s striae that often characterize vulvar LP, she noted. Medical treatments for vulvar LP include topical calcineurin inhibitors, high-potency steroids, and JAK inhibitors.
Surgical treatment of vulvar granuloma fissuratum caused by vulvar LS is possible (when the patient is in complete remission, to prevent koebnerization), with daily post-op application of clobetasol and retraction of tissues, noted Cigna, the author of a study on vulvar lysis of adhesions.
With both LS and LP, Cigna said, “don’t forget (consideration of) hormones” as an adjunctive treatment, especially in postmenopausal women. “Patients in a low hormone state will have more flares.”
Vulvar Crohn’s
“We all have to know how to look for this,” Cigna said. “Unilateral or asymmetric swelling is classic, but don’t rule out the diagnosis if you see symmetric swelling.” Patients also typically have linear “knife-like” fissures or ulcerations, the vulva “is very indurated,” and “swelling is so intense, the patients are miserable,” she said.
Vulvar Crohn’s disease may precede intestinal disease in 20%-30% of patients, so referral to a gastroenterologist — and ideally subsequent collaboration — is important, as vulvar manifestations are treated with systemic medications typical for Crohn’s.
A biopsy is required for diagnosis, and the pathologist should be advised to look for lichenified squamous mucosa with the Touton giant cell reaction. “Vulvar Crohn’s is a rare enough disorder that if you don’t have an experienced or informed pathologist looking at your specimen, they may miss it because they won’t be looking for it,” Cigna added in the interview. “You should be really clear about what you’re looking for.”
Neuropathic Itch, Pelvic Floor Muscle Spasm
Patients with pudendal neuralgia — caused by an injured, entrapped, or irritated pudendal nerve (originating from S2-S4) — typically present with chronic vulvar and pelvic pain that is often unprovoked and worsens with sitting. Itching upon touch is often another symptom, and some patients describe a foreign body sensation. The cause is often trauma (such as an accident or childbirth-related) as opposed to myofascial irritation, Cigna explained in her lecture.
“Your exam will be largely normal, with no skin findings, so patients will get sent away if you don’t know to look for pudendal neuralgia by pressing on the pudendal nerve or doing (or referring for) a diagnostic nerve block,” Cigna added in the interview.
Persistent genital arousal disorder (PGAD) is “more global” in that it can also originate not only from the pudendal nerve but also from nerve roots higher in the spine or even from the brain. “People feel a sense of arousal, but some describe it as an itch,” Cigna said in her lecture, referring to a 2021 consensus document on PGAD/genito-pelvic dysesthesia by the International Society for the Study of Women’s Sexual Health as a valuable resource for understanding and managing the condition.
Diagnosis and treatment usually start with a pudendal nerve block with a combination of steroid and anesthetic. If this does not relieve arousal/itching, the next step may be an MRI to look higher in the spine.
Pelvic Floor Muscle Spasm
Vulvar pain, skin itching, and irritation can be symptoms of pelvic floor muscle spasm. “Oftentimes people come to me and say, ‘I have a dermatologic problem,’” Cigna said. “The skin may look red and erythematous, but it’s probably more likely a muscle problem when you’re not finding anything, and no amount of steroid will help the itch go away when the problem lies underneath.”
Co-occurring symptoms can include vaginal dryness, clitoral pain, urethral discomfort, bladder pain/irritation, increased urgency, constipation, and anal fissures. The first-line treatment approach is pelvic floor therapy.
“Pelvic floor therapy is not just for incontinence. It’s also for pain and discomfort from muscles,” she said, noting that most patients with vulvar disorders are referred for pelvic floor therapy. “Almost all of them end up having pelvic floor dysfunction because the pelvic floor muscles spasm whenever there’s pain or inflammation.”
A Cautionary Word on Vulvodynia, and a Mast Cell Paradigm to Explore
Vulvodynia is defined as persistent pain of at least 3 months’ duration with no clear cause. “These are the patients with no skin findings,” Cigna said. But in most cases, she said, careful investigation identifies causes that are musculoskeletal, hormonal, or nerve-related.
“It’s a term that’s thrown around a lot — it’s kind of a catchall. Yet it should be a small minority of patients who truly have a diagnosis of vulvodynia,” she said.
In the early stages of investigation is the idea that mast cell proliferation and mast cell activation may play a role in some cases of vulvar and vestibular pain and itching. “We see that some patients with vulvodynia and vestibulodynia have mast cells that are increased in number in the epithelium and beneath the epithelium, and nerve staining shows an increased number of nerve endings traveling into the epithelium,” Cigna said.
“We do diagnose some people clinically” based on urticaria and other symptoms suggestive of mast cell proliferation/activation (such as flushing, abdominal cramping, diarrhea, hypotensive syncope or near syncope, and tachycardia), and “then we send them to the allergist for testing,” Cigna said.
Cigna and Murphy have no relevant financial disclosures.
A version of this article appeared on Medscape.com.
— and she believes collaboration with dermatologists is essential, especially for complex cases in what she calls a neglected, data-poor area of medicine.
She also recommends that dermatologists have a good understanding of the vestibule, “one of the most important structures in vulvar medicine,” and that they become equipped to recognize generalized and localized causes of vulvar pain and/or itch.
“The problem is, we don’t talk about [vulvovaginal pain and itch] ... it’s taboo and we’re not taught about it in medical school,” Cigna, assistant professor of obstetrics and gynecology at The George Washington University (GWU), Washington, DC, said in a grand rounds lecture held recently at the GWU School of Medicine and Health Sciences Department of Dermatology.
“There are dermatologists who don’t have much training in vulvar dermatology, and a lot of gyns don’t get as much training” as they should, she said in an interview after the lecture. “So who’s looking at people’s vulvar skin and figuring out what’s going on and giving them effective treatments and evidence-based education?”
Cigna and dermatologist Emily Murphy, MD, will be co-directors of a joint ob.gyn-dermatology Vulvar Dermatology Clinic at GWU that will be launched in 2025, with monthly clinics for particularly challenging cases where the etiology is unclear or treatment is ineffective. “We want to collaborate in a more systematic way and put our heads together and think creatively about what will improve patient care,” Cigna said in the interview.
Dermatologists have valuable expertise in the immunology and genetic factors involved in skin disorders, Cigna said. Moreover, Murphy, assistant professor of dermatology and director of the Vulvar Health Program at GWU, said in an interview, dermatologists “are comfortable in going to off-label systemic medications that ob.gyns may not use that often” and bring to the table expertise in various types of procedures.
Murphy recently trained with Melissa Mauskar, MD, associate of dermatology and obstetrics and gynecology at the University of Texas Southwestern, Dallas, and founder and director of the Gynecologic Dermatology Clinic there. “It’s so important for dermatologists to be involved. It just takes some extra training that residents aren’t getting right now,” said Murphy, a member of the newly formed Vulvar Dermatoses Research Consortium.
In her grand rounds lecture, Cigna offered pearls to dermatologists for approaching a history and exam and covered highlights of the diagnosis and treatment of various problems, from vulvar Candida infections and lichen simplex chronicus to vulvar lichen sclerosus (LS), vulvar lichen planus (LP), vulvar Crohn’s disease, pudendal neuralgia, and pelvic floor muscle spasm, as well as the role of mast cell proliferation in vulvar issues.
Approaching the History and Exam
A comprehensive history covers the start, duration, and location of pain and/or itching as well as a detailed timeline (such as timing of potential causes, including injuries or births) and symptoms (such as burning, cutting, aching, and stinging). The question of whether pain “is on the outside, at the entrance, or deeper inside” is “crucial, especially for those in dermatology,” Cigna emphasized.
“And if you’re seeing a patient for a vulvar condition, please ask them about sex. Ask, is this affecting your sexual or intimate life with your partner because this can also give you a clue about what’s going on and how you can help them,” she told the audience of dermatologists.
Queries about trauma history (physical and emotional/verbal), competitive sports (such as daily cycling, equestrian, and heavy weight lifting), endometriosis/gynecologic surgery, connective tissue disorders (such as Ehler-Danlos syndrome), and irritable bowel syndrome are all potentially important to consider. It is important to ask about anxiety, depression, and obsessive-compulsive disorder, which do not cause — but are highly associated with — vulvar dermatoses, she said.
A surprisingly large number of people with vulvovaginal issues are being diagnosed with Ehler-Danlos syndrome, so “I’m always asking, are you hypermobile because this might be affecting the musculoskeletal system, which might be affecting the pelvis,” Cigna said. “Anything that affects the pelvis can affect the vulva as well.”
The pelvic examination should be “offered” rather than assumed to be part of the exam, as part of a trauma-informed approach that is crucial for earning trust, she advised. “Just saying, ‘we’re going to talk, and then I can offer you an exam if you like’…patients like it. It helps them feel safer and more open.”
Many diagnoses are differentiated by eliciting pain on the anterior vs the posterior half of the vulvar vestibule — the part of the vulva that lies between the labia minora and is composed of nonkeratinized tissue with embryonic origins in the endoderm. “If you touch on the keratinized skin (of the vulva) and they don’t have pain, but on the vestibule they do have pain, and there is no pain inside the vagina, this suggests there is a vestibular problem,” said Cigna.
Pain/tenderness isolated to the posterior half of the vestibule suggests a muscular cause, and pain in both the posterior and anterior parts of the vestibule suggests a cause that is more systemic or diffuse, which could be a result of a hormonal issue such as one related to oral contraceptives or decreased testosterone, or a nerve-related process.
Cigna uses gentle swipes of a Q-tip moistened with water or gel to examine the vulva rather than a poke or touch, with the exception being the posterior vestibule, which overlies muscle insertion sites. “Make sure to get a baseline in remote areas such as the inner thigh, and always distinguish between ‘scratchy/sensitive’ sensations and pain,” she said, noting the value of having the patient hold a mirror on her inner thigh.
Causes of Vulvar Itch: Infectious and Noninfectious
With vulvar candidiasis, a common infectious cause of vulvar itch, “you have to ask if they’re also itching on the inside because if you treat them with a topical and you don’t treat the vaginal yeast infection that may be co-occurring, they’ll keep reseeding their vulvar skin,” Cigna said, “and it will never be fully treated.”
Candida albicans is the most common cause of vulvar or vulvovaginal candidiasis, and resistance to antifungals has been rising. Non-albicans Candida “tends to have even higher resistance rates,” she said. Ordering a sensitivity panel along with the culture is helpful, but “comprehensive vaginal biome” panels are generally not useful. “It’s hard to correlate the information clinically,” she said, “and there’s not always a lot of information about susceptibilities, which is what I really like to know.”
Cigna’s treatments for vaginal infections include miconazole, terconazole, and fluconazole (and occasionally, itraconazole or voriconazole — a “decision we don’t take lightly”). Vulvar treatments include nystatin ointment, clotrimazole cream, and miconazole cream. Often, optimal treatment involves addressing “both inside and out,” she said, noting the importance of also killing yeast in undergarment fabric.
“In my experience, Diflucan [oral fluconazole] doesn’t treat persistent vulvar cutaneous skin yeast well, so while I might try Diflucan, I typically use something topical as well,” she said. “And with vaginal yeast, we do use boric acid from time to time, especially for non-albicans species because it tends to be a little more effective.”
Noninfectious causes of vulvar itch include allergic, neuropathic, and muscular causes, as well as autoimmune dermatoses and mast cell activation syndrome. Well known in dermatology are acute contact dermatitis and lichen simplex chronicus — both characterized by induration, thickening, and a “puffy” erythematous appearance, and worsening of pruritus at night. What may be less appreciated is the long list of implicated allergens , including Always menstrual pads made of a plastic-containing “dry weave” material, Cigna said. There are at least several cotton-only, low-preservative feminine products available on the market, she noted.
Common Autoimmune Vulvar Dermatoses: LS and LP
Vulvar LS has traditionally been thought to affect mainly prepubertal and postmenopausal women, but the autoimmune condition is now known to affect more reproductive-age people with vulvas than previously appreciated, Cigna said.
And notably, in an observational web-based study of premenopausal women (aged 18-50 years) with biopsy-confirmed vulvar LS, the leading symptom was not itch but dyspareunia and tearing with intercourse. This means “we’re missing people,” said Cigna, an author of the study. “We think the reason we’re not seeing itch as commonly in this population is that itch is likely mediated by the low estrogen state of pre- and postmenopausal people.”
Vulvar LS also occurs in pregnancy, with symptoms that are either stable or decrease during pregnancy and increase in the postpartum period, as demonstrated in a recently published online survey.
Patients with vulvar LS can present with hypopigmentation, lichenification, and scarring and architectural changes, the latter of which can involve clitoral phimosis, labial resorption, and narrowing of the introitus. (The vaginal mucosa is unaffected.) The presentation can be subtle, especially in premenopausal women, and differentiation between LS, vitiligo, and yeast is sometimes necessary.
A timely biopsy-driven definitive diagnosis is important because vulvar LS increases the risk for cancer if it’s not adequately treated and because long-term steroid use can affect the accuracy of pathology reports. “We really care about keeping this disease in remission as much as possible,” Cigna said. Experts in the field recommend long-term maintenance therapy with a mid-ultra-potent steroid one to three times/week or an alternative. “I’ve just started using ruxolitinib cream, a Janus kinase (JAK) inhibitor, and tacrolimus, a calcineurin inhibitor,” she said.
With vulvar LP, based on current evidence, the risk for malignant transformation is low, but “it crosses into the vagina and can cause vaginal adhesions, so if you’re diagnosing someone with lichen planus, you need to make sure you’re talking with them about dilators, and if you’re not comfortable, send them to [gyn],” she said.
The use of vulvoscopy is important for one’s ability to see the fine Wickham’s striae that often characterize vulvar LP, she noted. Medical treatments for vulvar LP include topical calcineurin inhibitors, high-potency steroids, and JAK inhibitors.
Surgical treatment of vulvar granuloma fissuratum caused by vulvar LS is possible (when the patient is in complete remission, to prevent koebnerization), with daily post-op application of clobetasol and retraction of tissues, noted Cigna, the author of a study on vulvar lysis of adhesions.
With both LS and LP, Cigna said, “don’t forget (consideration of) hormones” as an adjunctive treatment, especially in postmenopausal women. “Patients in a low hormone state will have more flares.”
Vulvar Crohn’s
“We all have to know how to look for this,” Cigna said. “Unilateral or asymmetric swelling is classic, but don’t rule out the diagnosis if you see symmetric swelling.” Patients also typically have linear “knife-like” fissures or ulcerations, the vulva “is very indurated,” and “swelling is so intense, the patients are miserable,” she said.
Vulvar Crohn’s disease may precede intestinal disease in 20%-30% of patients, so referral to a gastroenterologist — and ideally subsequent collaboration — is important, as vulvar manifestations are treated with systemic medications typical for Crohn’s.
A biopsy is required for diagnosis, and the pathologist should be advised to look for lichenified squamous mucosa with the Touton giant cell reaction. “Vulvar Crohn’s is a rare enough disorder that if you don’t have an experienced or informed pathologist looking at your specimen, they may miss it because they won’t be looking for it,” Cigna added in the interview. “You should be really clear about what you’re looking for.”
Neuropathic Itch, Pelvic Floor Muscle Spasm
Patients with pudendal neuralgia — caused by an injured, entrapped, or irritated pudendal nerve (originating from S2-S4) — typically present with chronic vulvar and pelvic pain that is often unprovoked and worsens with sitting. Itching upon touch is often another symptom, and some patients describe a foreign body sensation. The cause is often trauma (such as an accident or childbirth-related) as opposed to myofascial irritation, Cigna explained in her lecture.
“Your exam will be largely normal, with no skin findings, so patients will get sent away if you don’t know to look for pudendal neuralgia by pressing on the pudendal nerve or doing (or referring for) a diagnostic nerve block,” Cigna added in the interview.
Persistent genital arousal disorder (PGAD) is “more global” in that it can also originate not only from the pudendal nerve but also from nerve roots higher in the spine or even from the brain. “People feel a sense of arousal, but some describe it as an itch,” Cigna said in her lecture, referring to a 2021 consensus document on PGAD/genito-pelvic dysesthesia by the International Society for the Study of Women’s Sexual Health as a valuable resource for understanding and managing the condition.
Diagnosis and treatment usually start with a pudendal nerve block with a combination of steroid and anesthetic. If this does not relieve arousal/itching, the next step may be an MRI to look higher in the spine.
Pelvic Floor Muscle Spasm
Vulvar pain, skin itching, and irritation can be symptoms of pelvic floor muscle spasm. “Oftentimes people come to me and say, ‘I have a dermatologic problem,’” Cigna said. “The skin may look red and erythematous, but it’s probably more likely a muscle problem when you’re not finding anything, and no amount of steroid will help the itch go away when the problem lies underneath.”
Co-occurring symptoms can include vaginal dryness, clitoral pain, urethral discomfort, bladder pain/irritation, increased urgency, constipation, and anal fissures. The first-line treatment approach is pelvic floor therapy.
“Pelvic floor therapy is not just for incontinence. It’s also for pain and discomfort from muscles,” she said, noting that most patients with vulvar disorders are referred for pelvic floor therapy. “Almost all of them end up having pelvic floor dysfunction because the pelvic floor muscles spasm whenever there’s pain or inflammation.”
A Cautionary Word on Vulvodynia, and a Mast Cell Paradigm to Explore
Vulvodynia is defined as persistent pain of at least 3 months’ duration with no clear cause. “These are the patients with no skin findings,” Cigna said. But in most cases, she said, careful investigation identifies causes that are musculoskeletal, hormonal, or nerve-related.
“It’s a term that’s thrown around a lot — it’s kind of a catchall. Yet it should be a small minority of patients who truly have a diagnosis of vulvodynia,” she said.
In the early stages of investigation is the idea that mast cell proliferation and mast cell activation may play a role in some cases of vulvar and vestibular pain and itching. “We see that some patients with vulvodynia and vestibulodynia have mast cells that are increased in number in the epithelium and beneath the epithelium, and nerve staining shows an increased number of nerve endings traveling into the epithelium,” Cigna said.
“We do diagnose some people clinically” based on urticaria and other symptoms suggestive of mast cell proliferation/activation (such as flushing, abdominal cramping, diarrhea, hypotensive syncope or near syncope, and tachycardia), and “then we send them to the allergist for testing,” Cigna said.
Cigna and Murphy have no relevant financial disclosures.
A version of this article appeared on Medscape.com.
— and she believes collaboration with dermatologists is essential, especially for complex cases in what she calls a neglected, data-poor area of medicine.
She also recommends that dermatologists have a good understanding of the vestibule, “one of the most important structures in vulvar medicine,” and that they become equipped to recognize generalized and localized causes of vulvar pain and/or itch.
“The problem is, we don’t talk about [vulvovaginal pain and itch] ... it’s taboo and we’re not taught about it in medical school,” Cigna, assistant professor of obstetrics and gynecology at The George Washington University (GWU), Washington, DC, said in a grand rounds lecture held recently at the GWU School of Medicine and Health Sciences Department of Dermatology.
“There are dermatologists who don’t have much training in vulvar dermatology, and a lot of gyns don’t get as much training” as they should, she said in an interview after the lecture. “So who’s looking at people’s vulvar skin and figuring out what’s going on and giving them effective treatments and evidence-based education?”
Cigna and dermatologist Emily Murphy, MD, will be co-directors of a joint ob.gyn-dermatology Vulvar Dermatology Clinic at GWU that will be launched in 2025, with monthly clinics for particularly challenging cases where the etiology is unclear or treatment is ineffective. “We want to collaborate in a more systematic way and put our heads together and think creatively about what will improve patient care,” Cigna said in the interview.
Dermatologists have valuable expertise in the immunology and genetic factors involved in skin disorders, Cigna said. Moreover, Murphy, assistant professor of dermatology and director of the Vulvar Health Program at GWU, said in an interview, dermatologists “are comfortable in going to off-label systemic medications that ob.gyns may not use that often” and bring to the table expertise in various types of procedures.
Murphy recently trained with Melissa Mauskar, MD, associate of dermatology and obstetrics and gynecology at the University of Texas Southwestern, Dallas, and founder and director of the Gynecologic Dermatology Clinic there. “It’s so important for dermatologists to be involved. It just takes some extra training that residents aren’t getting right now,” said Murphy, a member of the newly formed Vulvar Dermatoses Research Consortium.
In her grand rounds lecture, Cigna offered pearls to dermatologists for approaching a history and exam and covered highlights of the diagnosis and treatment of various problems, from vulvar Candida infections and lichen simplex chronicus to vulvar lichen sclerosus (LS), vulvar lichen planus (LP), vulvar Crohn’s disease, pudendal neuralgia, and pelvic floor muscle spasm, as well as the role of mast cell proliferation in vulvar issues.
Approaching the History and Exam
A comprehensive history covers the start, duration, and location of pain and/or itching as well as a detailed timeline (such as timing of potential causes, including injuries or births) and symptoms (such as burning, cutting, aching, and stinging). The question of whether pain “is on the outside, at the entrance, or deeper inside” is “crucial, especially for those in dermatology,” Cigna emphasized.
“And if you’re seeing a patient for a vulvar condition, please ask them about sex. Ask, is this affecting your sexual or intimate life with your partner because this can also give you a clue about what’s going on and how you can help them,” she told the audience of dermatologists.
Queries about trauma history (physical and emotional/verbal), competitive sports (such as daily cycling, equestrian, and heavy weight lifting), endometriosis/gynecologic surgery, connective tissue disorders (such as Ehler-Danlos syndrome), and irritable bowel syndrome are all potentially important to consider. It is important to ask about anxiety, depression, and obsessive-compulsive disorder, which do not cause — but are highly associated with — vulvar dermatoses, she said.
A surprisingly large number of people with vulvovaginal issues are being diagnosed with Ehler-Danlos syndrome, so “I’m always asking, are you hypermobile because this might be affecting the musculoskeletal system, which might be affecting the pelvis,” Cigna said. “Anything that affects the pelvis can affect the vulva as well.”
The pelvic examination should be “offered” rather than assumed to be part of the exam, as part of a trauma-informed approach that is crucial for earning trust, she advised. “Just saying, ‘we’re going to talk, and then I can offer you an exam if you like’…patients like it. It helps them feel safer and more open.”
Many diagnoses are differentiated by eliciting pain on the anterior vs the posterior half of the vulvar vestibule — the part of the vulva that lies between the labia minora and is composed of nonkeratinized tissue with embryonic origins in the endoderm. “If you touch on the keratinized skin (of the vulva) and they don’t have pain, but on the vestibule they do have pain, and there is no pain inside the vagina, this suggests there is a vestibular problem,” said Cigna.
Pain/tenderness isolated to the posterior half of the vestibule suggests a muscular cause, and pain in both the posterior and anterior parts of the vestibule suggests a cause that is more systemic or diffuse, which could be a result of a hormonal issue such as one related to oral contraceptives or decreased testosterone, or a nerve-related process.
Cigna uses gentle swipes of a Q-tip moistened with water or gel to examine the vulva rather than a poke or touch, with the exception being the posterior vestibule, which overlies muscle insertion sites. “Make sure to get a baseline in remote areas such as the inner thigh, and always distinguish between ‘scratchy/sensitive’ sensations and pain,” she said, noting the value of having the patient hold a mirror on her inner thigh.
Causes of Vulvar Itch: Infectious and Noninfectious
With vulvar candidiasis, a common infectious cause of vulvar itch, “you have to ask if they’re also itching on the inside because if you treat them with a topical and you don’t treat the vaginal yeast infection that may be co-occurring, they’ll keep reseeding their vulvar skin,” Cigna said, “and it will never be fully treated.”
Candida albicans is the most common cause of vulvar or vulvovaginal candidiasis, and resistance to antifungals has been rising. Non-albicans Candida “tends to have even higher resistance rates,” she said. Ordering a sensitivity panel along with the culture is helpful, but “comprehensive vaginal biome” panels are generally not useful. “It’s hard to correlate the information clinically,” she said, “and there’s not always a lot of information about susceptibilities, which is what I really like to know.”
Cigna’s treatments for vaginal infections include miconazole, terconazole, and fluconazole (and occasionally, itraconazole or voriconazole — a “decision we don’t take lightly”). Vulvar treatments include nystatin ointment, clotrimazole cream, and miconazole cream. Often, optimal treatment involves addressing “both inside and out,” she said, noting the importance of also killing yeast in undergarment fabric.
“In my experience, Diflucan [oral fluconazole] doesn’t treat persistent vulvar cutaneous skin yeast well, so while I might try Diflucan, I typically use something topical as well,” she said. “And with vaginal yeast, we do use boric acid from time to time, especially for non-albicans species because it tends to be a little more effective.”
Noninfectious causes of vulvar itch include allergic, neuropathic, and muscular causes, as well as autoimmune dermatoses and mast cell activation syndrome. Well known in dermatology are acute contact dermatitis and lichen simplex chronicus — both characterized by induration, thickening, and a “puffy” erythematous appearance, and worsening of pruritus at night. What may be less appreciated is the long list of implicated allergens , including Always menstrual pads made of a plastic-containing “dry weave” material, Cigna said. There are at least several cotton-only, low-preservative feminine products available on the market, she noted.
Common Autoimmune Vulvar Dermatoses: LS and LP
Vulvar LS has traditionally been thought to affect mainly prepubertal and postmenopausal women, but the autoimmune condition is now known to affect more reproductive-age people with vulvas than previously appreciated, Cigna said.
And notably, in an observational web-based study of premenopausal women (aged 18-50 years) with biopsy-confirmed vulvar LS, the leading symptom was not itch but dyspareunia and tearing with intercourse. This means “we’re missing people,” said Cigna, an author of the study. “We think the reason we’re not seeing itch as commonly in this population is that itch is likely mediated by the low estrogen state of pre- and postmenopausal people.”
Vulvar LS also occurs in pregnancy, with symptoms that are either stable or decrease during pregnancy and increase in the postpartum period, as demonstrated in a recently published online survey.
Patients with vulvar LS can present with hypopigmentation, lichenification, and scarring and architectural changes, the latter of which can involve clitoral phimosis, labial resorption, and narrowing of the introitus. (The vaginal mucosa is unaffected.) The presentation can be subtle, especially in premenopausal women, and differentiation between LS, vitiligo, and yeast is sometimes necessary.
A timely biopsy-driven definitive diagnosis is important because vulvar LS increases the risk for cancer if it’s not adequately treated and because long-term steroid use can affect the accuracy of pathology reports. “We really care about keeping this disease in remission as much as possible,” Cigna said. Experts in the field recommend long-term maintenance therapy with a mid-ultra-potent steroid one to three times/week or an alternative. “I’ve just started using ruxolitinib cream, a Janus kinase (JAK) inhibitor, and tacrolimus, a calcineurin inhibitor,” she said.
With vulvar LP, based on current evidence, the risk for malignant transformation is low, but “it crosses into the vagina and can cause vaginal adhesions, so if you’re diagnosing someone with lichen planus, you need to make sure you’re talking with them about dilators, and if you’re not comfortable, send them to [gyn],” she said.
The use of vulvoscopy is important for one’s ability to see the fine Wickham’s striae that often characterize vulvar LP, she noted. Medical treatments for vulvar LP include topical calcineurin inhibitors, high-potency steroids, and JAK inhibitors.
Surgical treatment of vulvar granuloma fissuratum caused by vulvar LS is possible (when the patient is in complete remission, to prevent koebnerization), with daily post-op application of clobetasol and retraction of tissues, noted Cigna, the author of a study on vulvar lysis of adhesions.
With both LS and LP, Cigna said, “don’t forget (consideration of) hormones” as an adjunctive treatment, especially in postmenopausal women. “Patients in a low hormone state will have more flares.”
Vulvar Crohn’s
“We all have to know how to look for this,” Cigna said. “Unilateral or asymmetric swelling is classic, but don’t rule out the diagnosis if you see symmetric swelling.” Patients also typically have linear “knife-like” fissures or ulcerations, the vulva “is very indurated,” and “swelling is so intense, the patients are miserable,” she said.
Vulvar Crohn’s disease may precede intestinal disease in 20%-30% of patients, so referral to a gastroenterologist — and ideally subsequent collaboration — is important, as vulvar manifestations are treated with systemic medications typical for Crohn’s.
A biopsy is required for diagnosis, and the pathologist should be advised to look for lichenified squamous mucosa with the Touton giant cell reaction. “Vulvar Crohn’s is a rare enough disorder that if you don’t have an experienced or informed pathologist looking at your specimen, they may miss it because they won’t be looking for it,” Cigna added in the interview. “You should be really clear about what you’re looking for.”
Neuropathic Itch, Pelvic Floor Muscle Spasm
Patients with pudendal neuralgia — caused by an injured, entrapped, or irritated pudendal nerve (originating from S2-S4) — typically present with chronic vulvar and pelvic pain that is often unprovoked and worsens with sitting. Itching upon touch is often another symptom, and some patients describe a foreign body sensation. The cause is often trauma (such as an accident or childbirth-related) as opposed to myofascial irritation, Cigna explained in her lecture.
“Your exam will be largely normal, with no skin findings, so patients will get sent away if you don’t know to look for pudendal neuralgia by pressing on the pudendal nerve or doing (or referring for) a diagnostic nerve block,” Cigna added in the interview.
Persistent genital arousal disorder (PGAD) is “more global” in that it can also originate not only from the pudendal nerve but also from nerve roots higher in the spine or even from the brain. “People feel a sense of arousal, but some describe it as an itch,” Cigna said in her lecture, referring to a 2021 consensus document on PGAD/genito-pelvic dysesthesia by the International Society for the Study of Women’s Sexual Health as a valuable resource for understanding and managing the condition.
Diagnosis and treatment usually start with a pudendal nerve block with a combination of steroid and anesthetic. If this does not relieve arousal/itching, the next step may be an MRI to look higher in the spine.
Pelvic Floor Muscle Spasm
Vulvar pain, skin itching, and irritation can be symptoms of pelvic floor muscle spasm. “Oftentimes people come to me and say, ‘I have a dermatologic problem,’” Cigna said. “The skin may look red and erythematous, but it’s probably more likely a muscle problem when you’re not finding anything, and no amount of steroid will help the itch go away when the problem lies underneath.”
Co-occurring symptoms can include vaginal dryness, clitoral pain, urethral discomfort, bladder pain/irritation, increased urgency, constipation, and anal fissures. The first-line treatment approach is pelvic floor therapy.
“Pelvic floor therapy is not just for incontinence. It’s also for pain and discomfort from muscles,” she said, noting that most patients with vulvar disorders are referred for pelvic floor therapy. “Almost all of them end up having pelvic floor dysfunction because the pelvic floor muscles spasm whenever there’s pain or inflammation.”
A Cautionary Word on Vulvodynia, and a Mast Cell Paradigm to Explore
Vulvodynia is defined as persistent pain of at least 3 months’ duration with no clear cause. “These are the patients with no skin findings,” Cigna said. But in most cases, she said, careful investigation identifies causes that are musculoskeletal, hormonal, or nerve-related.
“It’s a term that’s thrown around a lot — it’s kind of a catchall. Yet it should be a small minority of patients who truly have a diagnosis of vulvodynia,” she said.
In the early stages of investigation is the idea that mast cell proliferation and mast cell activation may play a role in some cases of vulvar and vestibular pain and itching. “We see that some patients with vulvodynia and vestibulodynia have mast cells that are increased in number in the epithelium and beneath the epithelium, and nerve staining shows an increased number of nerve endings traveling into the epithelium,” Cigna said.
“We do diagnose some people clinically” based on urticaria and other symptoms suggestive of mast cell proliferation/activation (such as flushing, abdominal cramping, diarrhea, hypotensive syncope or near syncope, and tachycardia), and “then we send them to the allergist for testing,” Cigna said.
Cigna and Murphy have no relevant financial disclosures.
A version of this article appeared on Medscape.com.