User login
Evaluating the hospitalized adult patient
Case
A 45-year-old female with moderate persistent asthma is admitted for right lower extremity cellulitis. She has hyponatremia with a sodium of 129 mEq/L and reports a history of longstanding fatigue and lightheadedness on standing. An early morning serum cortisol was 10 mcg/dL, normal per the reference range for the laboratory. Has adrenal insufficiency been excluded in this patient?
Overview
Adrenal insufficiency (AI) is a clinical syndrome characterized by a deficiency of cortisol. Presentation may range from nonspecific symptoms such as fatigue, weight loss, and gastrointestinal concerns to a fulminant adrenal crisis with severe weakness and hypotension (Table 1). The diagnosis of AI is commonly delayed, negatively impacting patients’ quality of life and risking dangerous complications.1,2
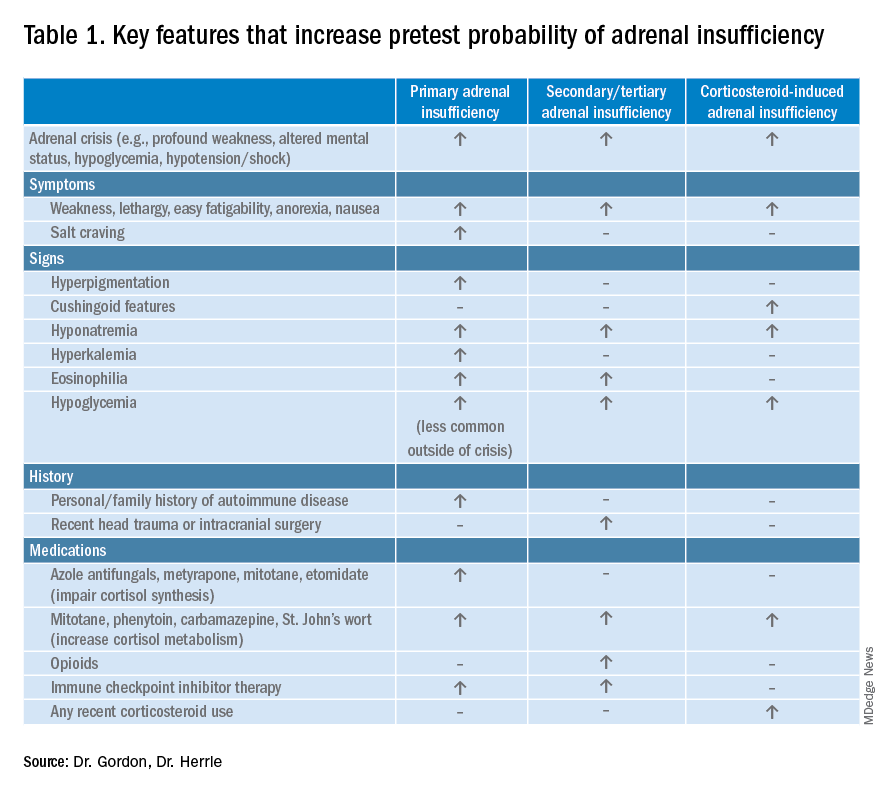
AI can occur due to diseases of the adrenal glands themselves (primary) or impairment of adrenocorticotropin (ACTH) secretion from the pituitary (secondary) or corticotropin-releasing hormone (CRH) secretion from the hypothalamus (tertiary). In the hospital setting, causes of primary AI may include autoimmune disease, infection, metastatic disease, hemorrhage, and adverse medication effects. Secondary and tertiary AI would be of particular concern for patients with traumatic brain injuries or pituitary surgery, but also are seen commonly as a result of adverse medication effects in the hospitalized patient, notably opioids and corticosteroids through suppression the hypothalamic-pituitary-adrenal (HPA) axis and immune checkpoint inhibitors via autoimmune hypophysitis.
Testing for AI in the hospitalized patient presents a host of challenges. Among these are the variability in presentation of different types of AI, high rates of exogenous corticosteroid use, the impact of critical illness on the HPA axis, medical illness altering protein binding of serum cortisol, interfering medications, the variation in assays used by laboratories, and the logistical challenges of obtaining appropriately timed phlebotomy.2,3
Cortisol testing
An intact HPA axis results in ACTH-dependent cortisol release from the adrenal glands. Cortisol secretion exhibits circadian rhythm, with the highest levels in the early morning (6 a.m. to 8 a.m.) and the lowest at night (12 a.m.). It also is pulsatile, which may explain the range of “normal” morning serum cortisol observed in a study of healthy volunteers.3 Note that serum cortisol is equivalent to plasma cortisol in current immunoassays, and will henceforth be called “cortisol” in this paper.3
There are instances when morning cortisol may strongly suggest a diagnosis of AI on its own. A meta-analysis found that morning cortisol of < 5 mcg/dL predicts AI and morning cortisol of > 13 mcg/dL ruled out AI.4 The Endocrine Society of America favors dynamic assessment of adrenal function for most patients.2
Historically, the gold standard for assessing dynamic adrenal function has been the insulin tolerance test (ITT), whereby cortisol is measured after inducing hypoglycemia to a blood glucose < 35 mg/dL. ITT is logistically difficult and poses some risk to the patient. The corticotropin (or cosyntropin) stimulation test (CST), in which a supraphysiologic dose of a synthetic ACTH analog is administered parenterally to a patient and resultant cortisol levels are measured, has been validated against the ITT and is generally preferred.5 CST is used to diagnose primary AI as well as chronic secondary and tertiary AI, given that longstanding lack of ACTH stimulation causes atrophy of the adrenal glands. The sensitivity for secondary and tertiary AI is likely lower than primary AI especially in acute onset of disease.6,7
In performance of the CST a baseline cortisol and ACTH are obtained, with subsequent cortisol testing at 30 and/or 60 minutes after administration of the ACTH analog (Figure 1). Currently, there is no consensus for which time point is preferred, but the 30-minute test is more sensitive for AI and the 60-minute test is more specific.2,7,8
CST is typically performed using a “standard high dose” of 250 mcg of the ACTH analog. There has been interest in the use of a “low-dose” 1 mcg test, which is closer to normal physiologic stimulation of the adrenal glands and may have better sensitivity for early secondary or partial AI. However, the 250-mcg dose is easier to prepare and has fewer technical pitfalls in administration as well as a lower risk for false positive testing. At this point the data do not compellingly favor the use of low-dose CST testing in general practice.2,3,7
Clinical decision making
Diagnostic evaluation should be guided by the likelihood of the disease (i.e., the pretest probability) (Figure 1). Begin with a review of the patient’s signs and symptoms, medical and family history, and medications with special consideration for opioids, exogenous steroids, and immune checkpoint inhibitors (Table 1).
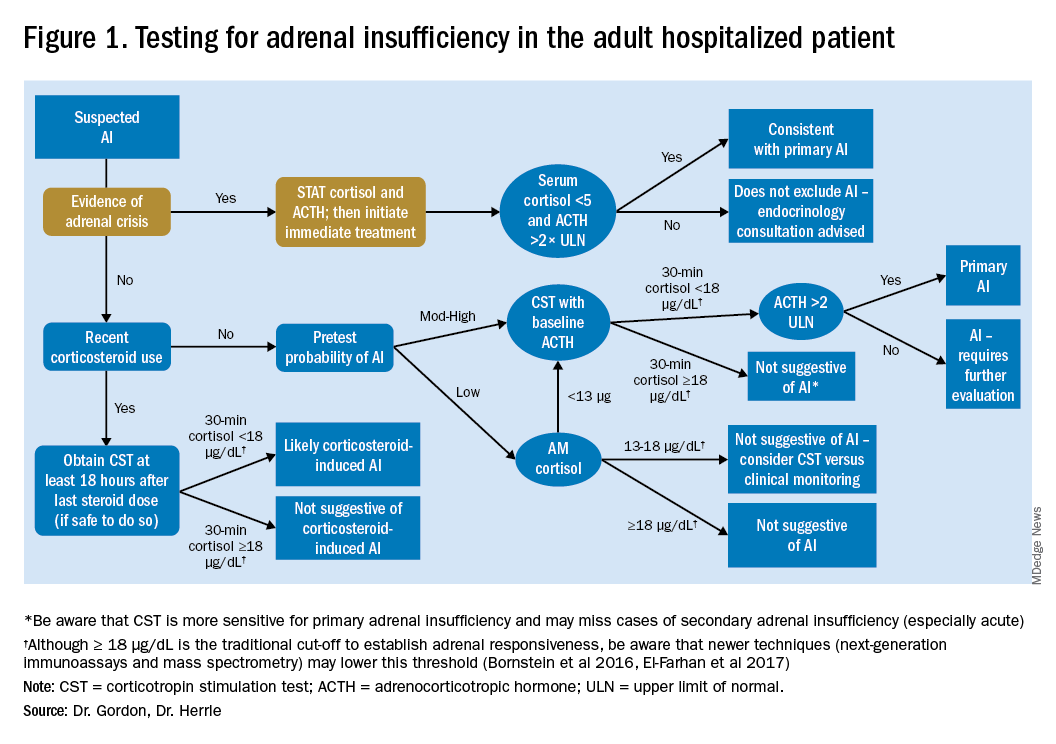
For patients with low pretest probability for AI, morning cortisol and ACTH is a reasonable first test (Figure 1). A cortisol value of 18 mcg/dL or greater does not support AI and no further testing is needed.2 Patients with morning cortisol of 13-18 mcg/dL could be followed clinically or could undergo further testing in the inpatient environment with CST, depending upon the clinical scenario.4 Patients with serum cortisol of <13 mcg/dL warrant CST.
For patients with moderate to high pretest probability for AI, we recommend initial testing with CST. While the results of high-dose CST are not necessarily impacted by time of day, if an a.m. cortisol has not yet been obtained and it is logistically feasible to do so, performing CST in the morning will provide the most useful data for clinical interpretation.
For patients presenting with possible adrenal crisis, it is essential not to delay treatment. In these patients, obtain a cortisol paired with ACTH and initiate treatment immediately. Further testing can be deferred until the time the patient is stable.2
Potential pitfalls
Interpreting cortisol requires awareness of multiple conditions that could directly impact the results.2,3 (Table 2).
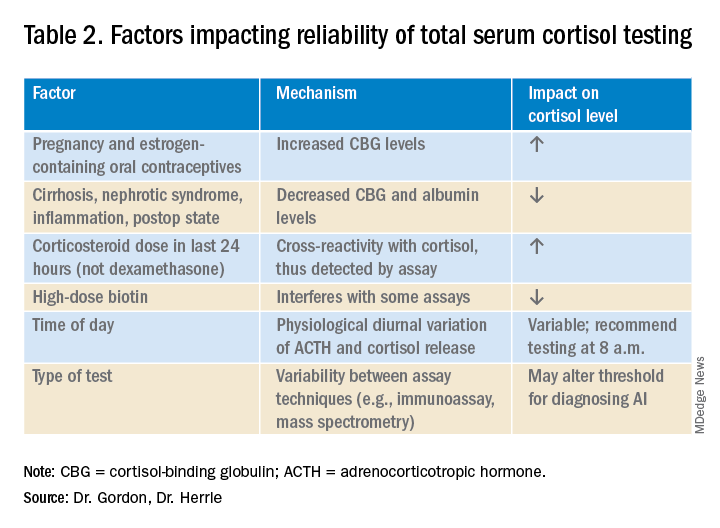
Currently available assays measure “total cortisol,” most of which is protein bound (cortisol-binding globulin as well as albumin). Therefore, conditions that lower serum protein (e.g., nephrotic syndrome, liver disease, inflammation) will lower the measured cortisol. Conversely, conditions that increase serum protein (e.g., estrogen excess in pregnancy and oral contraceptive use) will increase the measured cortisol.2,3
It is also important to recognize that existing immunoassay testing techniques informed the established cut-off for exclusion of AI at 18 mcg/dL. With newer immunoassays and emerging liquid chromatography/tandem mass spectrometry, this cut-off may be lowered; thus the assay should be confirmed with the performing laboratory. There is emerging evidence that serum or plasma free cortisol and salivary cortisol testing for AI may be useful in certain cases, but these techniques are not yet widespread or included in clinical practice guidelines.2,3,7
Population focus: Patients on exogenous steroids
Exogenous corticosteroids suppress the HPA axis via negative inhibition of CRH and ACTH release, often resulting in low endogenous cortisol levels which may or may not reflect true loss of adrenal function. In addition, many corticosteroids will be detected by standard serum cortisol tests that rely on immunoassays. For this reason, cortisol measurement and CST should be done at least 18-24 hours after the last dose of exogenous steroids.
Although the focus has been on higher doses and longer courses of steroids (e.g., chronic use of ≥ 5 mg prednisone daily, or ≥ 20 mg prednisone daily for > 3 weeks), there is increasing evidence that lower doses, shorter courses, and alternate routes (e.g., inhaled, intra-articular) can result in biochemical and clinical evidence of AI.9 Thus, a thorough history and exam should be obtained to determine all recent corticosteroid exposure and cushingoid features.
Application of the data to the case
To effectively assess the patient for adrenal insufficiency, we need additional information. First and foremost, is a description of the patient’s current clinical status. If she is demonstrating evidence of adrenal crisis, treatment should not be delayed for additional testing. If she is stable, a thorough history including use of corticosteroids by any route, pregnancy, oral contraceptives, recent surgery, and liver and kidney disease is essential.
Additional evaluation reveals the patient has been using her fluticasone inhaler daily. No other source of hyponatremia or lightheadedness is identified. The patient’s risk factors of corticosteroid use and unexplained hyponatremia with associated lightheadedness increase her pretest probability of AI and a single morning cortisol of 10 mcg/dL is insufficient to exclude adrenal insufficiency. The appropriate follow-up test is a standard high-dose cosyntropin stimulation test at least 18 hours after her last dose of fluticasone. A cortisol level > 18 mcg/dL at 30 minutes in the absence of other conditions that impact cortisol testing would not be suggestive of AI. A serum cortisol level of < 18 mcg/dL at 30 minutes would raise concern for abnormal adrenal reserve due to chronic corticosteroid therapy and would warrant referral to an endocrinologist.
Bottom line
An isolated serum cortisol is often insufficient to exclude adrenal insufficiency. Hospitalists should be aware of the many factors that impact the interpretation of this test.
Dr. Gordon is assistant professor of medicine at Tufts University, Boston, and a hospitalist at Maine Medical Center, Portland. She is the subspecialty education coordinator of inpatient medicine for the Internal Medicine Residency Program. Dr. Herrle is assistant professor of medicine at Tufts University and a hospitalist at Maine Medical Center. She is the associate director of medical student education for the department of internal medicine at MMC and a medical director for clinical informatics at MaineHealth.
References
1. Bleicken B et al. Delayed diagnosis of adrenal insufficiency is common: A cross-sectional study in 216 patients. Am J Med Sci. 2010;339(6):525-31. doi: 10.1097/MAJ.0b013e3181db6b7a.
2. Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
3. El-Farhan N et al. Measuring cortisol in serum, urine and saliva – Are our assays good enough? Ann Clin Biochem. 2017 May;54(3):308-22. doi: 10.1177/0004563216687335.
4. Kazlauskaite R et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: A metaanalysis. J Clin Endocrinol Metab. 2008;93:4245-53.
5. Wood JB et al. A rapid test of adrenocortical function. Lancet. 1965;191:243-5.
6. Singh Ospina N et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427-34.
7. Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
8. Odom DC et al. A Single, post-ACTH cortisol measurement to screen for adrenal insufficiency in the hospitalized patient. J Hosp Med. 2018;13(8):526-30. doi: 10.12788/jhm.2928.
9. Broersen LHA et al. Adrenal insufficiency in corticosteroids use: Systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(6): 2171-80.
Key points
• In general, random cortisol testing is of limited value and should be avoided.
• Serum cortisol testing in the hospitalized patient is impacted by a variety of patient and disease factors and should be interpreted carefully.
• For patients with low pretest probability of adrenal insufficiency, early morning serum cortisol testing may be sufficient to exclude the diagnosis.
• For patients with moderate to high pretest probability of adrenal insufficiency, standard high-dose (250 mcg) corticotropin stimulation testing is preferred.
Additional reading
Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
Quiz
An 82 y.o. woman with depression is admitted from her long-term care facility with worsening weakness and mild hypoglycemia. Her supine vital signs are stable, but she exhibits a drop in systolic blood pressure of 21 mm Hg upon standing. There is no evidence of infection by history, exam, or initial workup. She is not on chronic corticosteroids by any route.
What would be your initial workup for adrenal insufficiency?
A) Morning serum cortisol and ACTH
B) Insulin tolerance test
C) Corticotropin stimulation test
D) Would not test at this point
Answer: C. Although her symptom of weakness is nonspecific, her hypoglycemia and orthostatic hypotension are concerning enough that she would qualify as moderate to high pretest probability for AI. In this setting, one would acquire a basal serum total cortisol and ACTH then administer the standard high-dose corticotropin stimulation test (250 mcg) followed by repeat serum total cortisol at 30 or 60 minutes.
Evaluating the hospitalized adult patient
Evaluating the hospitalized adult patient
Case
A 45-year-old female with moderate persistent asthma is admitted for right lower extremity cellulitis. She has hyponatremia with a sodium of 129 mEq/L and reports a history of longstanding fatigue and lightheadedness on standing. An early morning serum cortisol was 10 mcg/dL, normal per the reference range for the laboratory. Has adrenal insufficiency been excluded in this patient?
Overview
Adrenal insufficiency (AI) is a clinical syndrome characterized by a deficiency of cortisol. Presentation may range from nonspecific symptoms such as fatigue, weight loss, and gastrointestinal concerns to a fulminant adrenal crisis with severe weakness and hypotension (Table 1). The diagnosis of AI is commonly delayed, negatively impacting patients’ quality of life and risking dangerous complications.1,2

AI can occur due to diseases of the adrenal glands themselves (primary) or impairment of adrenocorticotropin (ACTH) secretion from the pituitary (secondary) or corticotropin-releasing hormone (CRH) secretion from the hypothalamus (tertiary). In the hospital setting, causes of primary AI may include autoimmune disease, infection, metastatic disease, hemorrhage, and adverse medication effects. Secondary and tertiary AI would be of particular concern for patients with traumatic brain injuries or pituitary surgery, but also are seen commonly as a result of adverse medication effects in the hospitalized patient, notably opioids and corticosteroids through suppression the hypothalamic-pituitary-adrenal (HPA) axis and immune checkpoint inhibitors via autoimmune hypophysitis.
Testing for AI in the hospitalized patient presents a host of challenges. Among these are the variability in presentation of different types of AI, high rates of exogenous corticosteroid use, the impact of critical illness on the HPA axis, medical illness altering protein binding of serum cortisol, interfering medications, the variation in assays used by laboratories, and the logistical challenges of obtaining appropriately timed phlebotomy.2,3
Cortisol testing
An intact HPA axis results in ACTH-dependent cortisol release from the adrenal glands. Cortisol secretion exhibits circadian rhythm, with the highest levels in the early morning (6 a.m. to 8 a.m.) and the lowest at night (12 a.m.). It also is pulsatile, which may explain the range of “normal” morning serum cortisol observed in a study of healthy volunteers.3 Note that serum cortisol is equivalent to plasma cortisol in current immunoassays, and will henceforth be called “cortisol” in this paper.3
There are instances when morning cortisol may strongly suggest a diagnosis of AI on its own. A meta-analysis found that morning cortisol of < 5 mcg/dL predicts AI and morning cortisol of > 13 mcg/dL ruled out AI.4 The Endocrine Society of America favors dynamic assessment of adrenal function for most patients.2
Historically, the gold standard for assessing dynamic adrenal function has been the insulin tolerance test (ITT), whereby cortisol is measured after inducing hypoglycemia to a blood glucose < 35 mg/dL. ITT is logistically difficult and poses some risk to the patient. The corticotropin (or cosyntropin) stimulation test (CST), in which a supraphysiologic dose of a synthetic ACTH analog is administered parenterally to a patient and resultant cortisol levels are measured, has been validated against the ITT and is generally preferred.5 CST is used to diagnose primary AI as well as chronic secondary and tertiary AI, given that longstanding lack of ACTH stimulation causes atrophy of the adrenal glands. The sensitivity for secondary and tertiary AI is likely lower than primary AI especially in acute onset of disease.6,7
In performance of the CST a baseline cortisol and ACTH are obtained, with subsequent cortisol testing at 30 and/or 60 minutes after administration of the ACTH analog (Figure 1). Currently, there is no consensus for which time point is preferred, but the 30-minute test is more sensitive for AI and the 60-minute test is more specific.2,7,8
CST is typically performed using a “standard high dose” of 250 mcg of the ACTH analog. There has been interest in the use of a “low-dose” 1 mcg test, which is closer to normal physiologic stimulation of the adrenal glands and may have better sensitivity for early secondary or partial AI. However, the 250-mcg dose is easier to prepare and has fewer technical pitfalls in administration as well as a lower risk for false positive testing. At this point the data do not compellingly favor the use of low-dose CST testing in general practice.2,3,7
Clinical decision making
Diagnostic evaluation should be guided by the likelihood of the disease (i.e., the pretest probability) (Figure 1). Begin with a review of the patient’s signs and symptoms, medical and family history, and medications with special consideration for opioids, exogenous steroids, and immune checkpoint inhibitors (Table 1).

For patients with low pretest probability for AI, morning cortisol and ACTH is a reasonable first test (Figure 1). A cortisol value of 18 mcg/dL or greater does not support AI and no further testing is needed.2 Patients with morning cortisol of 13-18 mcg/dL could be followed clinically or could undergo further testing in the inpatient environment with CST, depending upon the clinical scenario.4 Patients with serum cortisol of <13 mcg/dL warrant CST.
For patients with moderate to high pretest probability for AI, we recommend initial testing with CST. While the results of high-dose CST are not necessarily impacted by time of day, if an a.m. cortisol has not yet been obtained and it is logistically feasible to do so, performing CST in the morning will provide the most useful data for clinical interpretation.
For patients presenting with possible adrenal crisis, it is essential not to delay treatment. In these patients, obtain a cortisol paired with ACTH and initiate treatment immediately. Further testing can be deferred until the time the patient is stable.2
Potential pitfalls
Interpreting cortisol requires awareness of multiple conditions that could directly impact the results.2,3 (Table 2).

Currently available assays measure “total cortisol,” most of which is protein bound (cortisol-binding globulin as well as albumin). Therefore, conditions that lower serum protein (e.g., nephrotic syndrome, liver disease, inflammation) will lower the measured cortisol. Conversely, conditions that increase serum protein (e.g., estrogen excess in pregnancy and oral contraceptive use) will increase the measured cortisol.2,3
It is also important to recognize that existing immunoassay testing techniques informed the established cut-off for exclusion of AI at 18 mcg/dL. With newer immunoassays and emerging liquid chromatography/tandem mass spectrometry, this cut-off may be lowered; thus the assay should be confirmed with the performing laboratory. There is emerging evidence that serum or plasma free cortisol and salivary cortisol testing for AI may be useful in certain cases, but these techniques are not yet widespread or included in clinical practice guidelines.2,3,7
Population focus: Patients on exogenous steroids
Exogenous corticosteroids suppress the HPA axis via negative inhibition of CRH and ACTH release, often resulting in low endogenous cortisol levels which may or may not reflect true loss of adrenal function. In addition, many corticosteroids will be detected by standard serum cortisol tests that rely on immunoassays. For this reason, cortisol measurement and CST should be done at least 18-24 hours after the last dose of exogenous steroids.
Although the focus has been on higher doses and longer courses of steroids (e.g., chronic use of ≥ 5 mg prednisone daily, or ≥ 20 mg prednisone daily for > 3 weeks), there is increasing evidence that lower doses, shorter courses, and alternate routes (e.g., inhaled, intra-articular) can result in biochemical and clinical evidence of AI.9 Thus, a thorough history and exam should be obtained to determine all recent corticosteroid exposure and cushingoid features.
Application of the data to the case
To effectively assess the patient for adrenal insufficiency, we need additional information. First and foremost, is a description of the patient’s current clinical status. If she is demonstrating evidence of adrenal crisis, treatment should not be delayed for additional testing. If she is stable, a thorough history including use of corticosteroids by any route, pregnancy, oral contraceptives, recent surgery, and liver and kidney disease is essential.
Additional evaluation reveals the patient has been using her fluticasone inhaler daily. No other source of hyponatremia or lightheadedness is identified. The patient’s risk factors of corticosteroid use and unexplained hyponatremia with associated lightheadedness increase her pretest probability of AI and a single morning cortisol of 10 mcg/dL is insufficient to exclude adrenal insufficiency. The appropriate follow-up test is a standard high-dose cosyntropin stimulation test at least 18 hours after her last dose of fluticasone. A cortisol level > 18 mcg/dL at 30 minutes in the absence of other conditions that impact cortisol testing would not be suggestive of AI. A serum cortisol level of < 18 mcg/dL at 30 minutes would raise concern for abnormal adrenal reserve due to chronic corticosteroid therapy and would warrant referral to an endocrinologist.
Bottom line
An isolated serum cortisol is often insufficient to exclude adrenal insufficiency. Hospitalists should be aware of the many factors that impact the interpretation of this test.
Dr. Gordon is assistant professor of medicine at Tufts University, Boston, and a hospitalist at Maine Medical Center, Portland. She is the subspecialty education coordinator of inpatient medicine for the Internal Medicine Residency Program. Dr. Herrle is assistant professor of medicine at Tufts University and a hospitalist at Maine Medical Center. She is the associate director of medical student education for the department of internal medicine at MMC and a medical director for clinical informatics at MaineHealth.
References
1. Bleicken B et al. Delayed diagnosis of adrenal insufficiency is common: A cross-sectional study in 216 patients. Am J Med Sci. 2010;339(6):525-31. doi: 10.1097/MAJ.0b013e3181db6b7a.
2. Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
3. El-Farhan N et al. Measuring cortisol in serum, urine and saliva – Are our assays good enough? Ann Clin Biochem. 2017 May;54(3):308-22. doi: 10.1177/0004563216687335.
4. Kazlauskaite R et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: A metaanalysis. J Clin Endocrinol Metab. 2008;93:4245-53.
5. Wood JB et al. A rapid test of adrenocortical function. Lancet. 1965;191:243-5.
6. Singh Ospina N et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427-34.
7. Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
8. Odom DC et al. A Single, post-ACTH cortisol measurement to screen for adrenal insufficiency in the hospitalized patient. J Hosp Med. 2018;13(8):526-30. doi: 10.12788/jhm.2928.
9. Broersen LHA et al. Adrenal insufficiency in corticosteroids use: Systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(6): 2171-80.
Key points
• In general, random cortisol testing is of limited value and should be avoided.
• Serum cortisol testing in the hospitalized patient is impacted by a variety of patient and disease factors and should be interpreted carefully.
• For patients with low pretest probability of adrenal insufficiency, early morning serum cortisol testing may be sufficient to exclude the diagnosis.
• For patients with moderate to high pretest probability of adrenal insufficiency, standard high-dose (250 mcg) corticotropin stimulation testing is preferred.
Additional reading
Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
Quiz
An 82 y.o. woman with depression is admitted from her long-term care facility with worsening weakness and mild hypoglycemia. Her supine vital signs are stable, but she exhibits a drop in systolic blood pressure of 21 mm Hg upon standing. There is no evidence of infection by history, exam, or initial workup. She is not on chronic corticosteroids by any route.
What would be your initial workup for adrenal insufficiency?
A) Morning serum cortisol and ACTH
B) Insulin tolerance test
C) Corticotropin stimulation test
D) Would not test at this point
Answer: C. Although her symptom of weakness is nonspecific, her hypoglycemia and orthostatic hypotension are concerning enough that she would qualify as moderate to high pretest probability for AI. In this setting, one would acquire a basal serum total cortisol and ACTH then administer the standard high-dose corticotropin stimulation test (250 mcg) followed by repeat serum total cortisol at 30 or 60 minutes.
Case
A 45-year-old female with moderate persistent asthma is admitted for right lower extremity cellulitis. She has hyponatremia with a sodium of 129 mEq/L and reports a history of longstanding fatigue and lightheadedness on standing. An early morning serum cortisol was 10 mcg/dL, normal per the reference range for the laboratory. Has adrenal insufficiency been excluded in this patient?
Overview
Adrenal insufficiency (AI) is a clinical syndrome characterized by a deficiency of cortisol. Presentation may range from nonspecific symptoms such as fatigue, weight loss, and gastrointestinal concerns to a fulminant adrenal crisis with severe weakness and hypotension (Table 1). The diagnosis of AI is commonly delayed, negatively impacting patients’ quality of life and risking dangerous complications.1,2

AI can occur due to diseases of the adrenal glands themselves (primary) or impairment of adrenocorticotropin (ACTH) secretion from the pituitary (secondary) or corticotropin-releasing hormone (CRH) secretion from the hypothalamus (tertiary). In the hospital setting, causes of primary AI may include autoimmune disease, infection, metastatic disease, hemorrhage, and adverse medication effects. Secondary and tertiary AI would be of particular concern for patients with traumatic brain injuries or pituitary surgery, but also are seen commonly as a result of adverse medication effects in the hospitalized patient, notably opioids and corticosteroids through suppression the hypothalamic-pituitary-adrenal (HPA) axis and immune checkpoint inhibitors via autoimmune hypophysitis.
Testing for AI in the hospitalized patient presents a host of challenges. Among these are the variability in presentation of different types of AI, high rates of exogenous corticosteroid use, the impact of critical illness on the HPA axis, medical illness altering protein binding of serum cortisol, interfering medications, the variation in assays used by laboratories, and the logistical challenges of obtaining appropriately timed phlebotomy.2,3
Cortisol testing
An intact HPA axis results in ACTH-dependent cortisol release from the adrenal glands. Cortisol secretion exhibits circadian rhythm, with the highest levels in the early morning (6 a.m. to 8 a.m.) and the lowest at night (12 a.m.). It also is pulsatile, which may explain the range of “normal” morning serum cortisol observed in a study of healthy volunteers.3 Note that serum cortisol is equivalent to plasma cortisol in current immunoassays, and will henceforth be called “cortisol” in this paper.3
There are instances when morning cortisol may strongly suggest a diagnosis of AI on its own. A meta-analysis found that morning cortisol of < 5 mcg/dL predicts AI and morning cortisol of > 13 mcg/dL ruled out AI.4 The Endocrine Society of America favors dynamic assessment of adrenal function for most patients.2
Historically, the gold standard for assessing dynamic adrenal function has been the insulin tolerance test (ITT), whereby cortisol is measured after inducing hypoglycemia to a blood glucose < 35 mg/dL. ITT is logistically difficult and poses some risk to the patient. The corticotropin (or cosyntropin) stimulation test (CST), in which a supraphysiologic dose of a synthetic ACTH analog is administered parenterally to a patient and resultant cortisol levels are measured, has been validated against the ITT and is generally preferred.5 CST is used to diagnose primary AI as well as chronic secondary and tertiary AI, given that longstanding lack of ACTH stimulation causes atrophy of the adrenal glands. The sensitivity for secondary and tertiary AI is likely lower than primary AI especially in acute onset of disease.6,7
In performance of the CST a baseline cortisol and ACTH are obtained, with subsequent cortisol testing at 30 and/or 60 minutes after administration of the ACTH analog (Figure 1). Currently, there is no consensus for which time point is preferred, but the 30-minute test is more sensitive for AI and the 60-minute test is more specific.2,7,8
CST is typically performed using a “standard high dose” of 250 mcg of the ACTH analog. There has been interest in the use of a “low-dose” 1 mcg test, which is closer to normal physiologic stimulation of the adrenal glands and may have better sensitivity for early secondary or partial AI. However, the 250-mcg dose is easier to prepare and has fewer technical pitfalls in administration as well as a lower risk for false positive testing. At this point the data do not compellingly favor the use of low-dose CST testing in general practice.2,3,7
Clinical decision making
Diagnostic evaluation should be guided by the likelihood of the disease (i.e., the pretest probability) (Figure 1). Begin with a review of the patient’s signs and symptoms, medical and family history, and medications with special consideration for opioids, exogenous steroids, and immune checkpoint inhibitors (Table 1).

For patients with low pretest probability for AI, morning cortisol and ACTH is a reasonable first test (Figure 1). A cortisol value of 18 mcg/dL or greater does not support AI and no further testing is needed.2 Patients with morning cortisol of 13-18 mcg/dL could be followed clinically or could undergo further testing in the inpatient environment with CST, depending upon the clinical scenario.4 Patients with serum cortisol of <13 mcg/dL warrant CST.
For patients with moderate to high pretest probability for AI, we recommend initial testing with CST. While the results of high-dose CST are not necessarily impacted by time of day, if an a.m. cortisol has not yet been obtained and it is logistically feasible to do so, performing CST in the morning will provide the most useful data for clinical interpretation.
For patients presenting with possible adrenal crisis, it is essential not to delay treatment. In these patients, obtain a cortisol paired with ACTH and initiate treatment immediately. Further testing can be deferred until the time the patient is stable.2
Potential pitfalls
Interpreting cortisol requires awareness of multiple conditions that could directly impact the results.2,3 (Table 2).

Currently available assays measure “total cortisol,” most of which is protein bound (cortisol-binding globulin as well as albumin). Therefore, conditions that lower serum protein (e.g., nephrotic syndrome, liver disease, inflammation) will lower the measured cortisol. Conversely, conditions that increase serum protein (e.g., estrogen excess in pregnancy and oral contraceptive use) will increase the measured cortisol.2,3
It is also important to recognize that existing immunoassay testing techniques informed the established cut-off for exclusion of AI at 18 mcg/dL. With newer immunoassays and emerging liquid chromatography/tandem mass spectrometry, this cut-off may be lowered; thus the assay should be confirmed with the performing laboratory. There is emerging evidence that serum or plasma free cortisol and salivary cortisol testing for AI may be useful in certain cases, but these techniques are not yet widespread or included in clinical practice guidelines.2,3,7
Population focus: Patients on exogenous steroids
Exogenous corticosteroids suppress the HPA axis via negative inhibition of CRH and ACTH release, often resulting in low endogenous cortisol levels which may or may not reflect true loss of adrenal function. In addition, many corticosteroids will be detected by standard serum cortisol tests that rely on immunoassays. For this reason, cortisol measurement and CST should be done at least 18-24 hours after the last dose of exogenous steroids.
Although the focus has been on higher doses and longer courses of steroids (e.g., chronic use of ≥ 5 mg prednisone daily, or ≥ 20 mg prednisone daily for > 3 weeks), there is increasing evidence that lower doses, shorter courses, and alternate routes (e.g., inhaled, intra-articular) can result in biochemical and clinical evidence of AI.9 Thus, a thorough history and exam should be obtained to determine all recent corticosteroid exposure and cushingoid features.
Application of the data to the case
To effectively assess the patient for adrenal insufficiency, we need additional information. First and foremost, is a description of the patient’s current clinical status. If she is demonstrating evidence of adrenal crisis, treatment should not be delayed for additional testing. If she is stable, a thorough history including use of corticosteroids by any route, pregnancy, oral contraceptives, recent surgery, and liver and kidney disease is essential.
Additional evaluation reveals the patient has been using her fluticasone inhaler daily. No other source of hyponatremia or lightheadedness is identified. The patient’s risk factors of corticosteroid use and unexplained hyponatremia with associated lightheadedness increase her pretest probability of AI and a single morning cortisol of 10 mcg/dL is insufficient to exclude adrenal insufficiency. The appropriate follow-up test is a standard high-dose cosyntropin stimulation test at least 18 hours after her last dose of fluticasone. A cortisol level > 18 mcg/dL at 30 minutes in the absence of other conditions that impact cortisol testing would not be suggestive of AI. A serum cortisol level of < 18 mcg/dL at 30 minutes would raise concern for abnormal adrenal reserve due to chronic corticosteroid therapy and would warrant referral to an endocrinologist.
Bottom line
An isolated serum cortisol is often insufficient to exclude adrenal insufficiency. Hospitalists should be aware of the many factors that impact the interpretation of this test.
Dr. Gordon is assistant professor of medicine at Tufts University, Boston, and a hospitalist at Maine Medical Center, Portland. She is the subspecialty education coordinator of inpatient medicine for the Internal Medicine Residency Program. Dr. Herrle is assistant professor of medicine at Tufts University and a hospitalist at Maine Medical Center. She is the associate director of medical student education for the department of internal medicine at MMC and a medical director for clinical informatics at MaineHealth.
References
1. Bleicken B et al. Delayed diagnosis of adrenal insufficiency is common: A cross-sectional study in 216 patients. Am J Med Sci. 2010;339(6):525-31. doi: 10.1097/MAJ.0b013e3181db6b7a.
2. Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
3. El-Farhan N et al. Measuring cortisol in serum, urine and saliva – Are our assays good enough? Ann Clin Biochem. 2017 May;54(3):308-22. doi: 10.1177/0004563216687335.
4. Kazlauskaite R et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: A metaanalysis. J Clin Endocrinol Metab. 2008;93:4245-53.
5. Wood JB et al. A rapid test of adrenocortical function. Lancet. 1965;191:243-5.
6. Singh Ospina N et al. ACTH stimulation tests for the diagnosis of adrenal insufficiency: systematic review and meta-analysis. J Clin Endocrinol Metab. 2016;101(2):427-34.
7. Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
8. Odom DC et al. A Single, post-ACTH cortisol measurement to screen for adrenal insufficiency in the hospitalized patient. J Hosp Med. 2018;13(8):526-30. doi: 10.12788/jhm.2928.
9. Broersen LHA et al. Adrenal insufficiency in corticosteroids use: Systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(6): 2171-80.
Key points
• In general, random cortisol testing is of limited value and should be avoided.
• Serum cortisol testing in the hospitalized patient is impacted by a variety of patient and disease factors and should be interpreted carefully.
• For patients with low pretest probability of adrenal insufficiency, early morning serum cortisol testing may be sufficient to exclude the diagnosis.
• For patients with moderate to high pretest probability of adrenal insufficiency, standard high-dose (250 mcg) corticotropin stimulation testing is preferred.
Additional reading
Bornstein SR et al. Diagnosis and treatment of primary adrenal insufficiency: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016 Feb;101(2):364-89.
Burgos N et al. Pitfalls in the interpretation of the cosyntropin stimulation test for the diagnosis of adrenal insufficiency. Curr Opin Endocrinol Diabetes Obes. 2019;26(3):139-45.
Quiz
An 82 y.o. woman with depression is admitted from her long-term care facility with worsening weakness and mild hypoglycemia. Her supine vital signs are stable, but she exhibits a drop in systolic blood pressure of 21 mm Hg upon standing. There is no evidence of infection by history, exam, or initial workup. She is not on chronic corticosteroids by any route.
What would be your initial workup for adrenal insufficiency?
A) Morning serum cortisol and ACTH
B) Insulin tolerance test
C) Corticotropin stimulation test
D) Would not test at this point
Answer: C. Although her symptom of weakness is nonspecific, her hypoglycemia and orthostatic hypotension are concerning enough that she would qualify as moderate to high pretest probability for AI. In this setting, one would acquire a basal serum total cortisol and ACTH then administer the standard high-dose corticotropin stimulation test (250 mcg) followed by repeat serum total cortisol at 30 or 60 minutes.

