User login
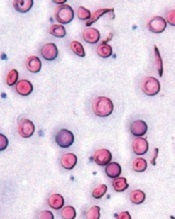
Image courtesy of the
University of Michigan
Activating the antioxidant regulator Nrf2 may slow the progression of sickle cell disease (SCD), according to preclinical research published in JCI Insight.
Investigators found the severity of hemolytic anemia, vascular inflammation, and lung injury increased with age in mice with SCD.
However, activating Nrf2 in young animals had a prophylactic effect, reducing the severity of these adverse effects and improving survival.
To uncover these findings, Solomon Ofori-Acquah, PhD, of the University of Pittsburgh in Pennsylvania, and his colleagues conducted a 10-month longitudinal observational study of mice with SCD.
The team found that, in mice with homozygous SCD (SS), there was a link between intravascular hemolysis, vascular inflammation, lung injury, and early death.
Mice as young as 2 months showed exacerbation of intravascular hemolysis. And additional investigation linked worsening intravascular hemolysis and oxidative stress to the release of VE-cadherin and progressive lung damage in aging SS mice.
The investigators knew that Nrf2 regulates the expression of genes that protect against the effects of intravascular hemolysis. So they decided to see if activating Nrf2 in young mice with SCD would slow the disease progression that occurs with age.
The team took SS mice that were about a month old and randomized them to receive 3H-1, 2-dithiole-3-thione (D3T) or a DMSO vehicle for 3 months or longer.
Treatment with D3T stabilized the concentration of hemoglobin, increased white blood cell counts, increased reticulocyte counts (though not significantly), kept HO-1 levels stable, increased levels of NQO1 and ferritin, and impeded the progression of endothelial dysfunction.
The investigators also looked at the role of Nrf2 in nonhematopoietic tissues and were surprised to find that Nrf2 deficiency in nonhematopoietic tissues exacerbated anemia and caused premature pulmonary edema in mice with SCD.
The team said this suggests a dominant protective role for nonhematopoietic Nrf2 against tissue damage in both erythroid and nonerythroid tissues in SCD.
And, when taken together, the results of this research indicate that activating Nrf2 can impede the onset of the severe adult phenotype of SCD in mice. ![]()

Image courtesy of the
University of Michigan
Activating the antioxidant regulator Nrf2 may slow the progression of sickle cell disease (SCD), according to preclinical research published in JCI Insight.
Investigators found the severity of hemolytic anemia, vascular inflammation, and lung injury increased with age in mice with SCD.
However, activating Nrf2 in young animals had a prophylactic effect, reducing the severity of these adverse effects and improving survival.
To uncover these findings, Solomon Ofori-Acquah, PhD, of the University of Pittsburgh in Pennsylvania, and his colleagues conducted a 10-month longitudinal observational study of mice with SCD.
The team found that, in mice with homozygous SCD (SS), there was a link between intravascular hemolysis, vascular inflammation, lung injury, and early death.
Mice as young as 2 months showed exacerbation of intravascular hemolysis. And additional investigation linked worsening intravascular hemolysis and oxidative stress to the release of VE-cadherin and progressive lung damage in aging SS mice.
The investigators knew that Nrf2 regulates the expression of genes that protect against the effects of intravascular hemolysis. So they decided to see if activating Nrf2 in young mice with SCD would slow the disease progression that occurs with age.
The team took SS mice that were about a month old and randomized them to receive 3H-1, 2-dithiole-3-thione (D3T) or a DMSO vehicle for 3 months or longer.
Treatment with D3T stabilized the concentration of hemoglobin, increased white blood cell counts, increased reticulocyte counts (though not significantly), kept HO-1 levels stable, increased levels of NQO1 and ferritin, and impeded the progression of endothelial dysfunction.
The investigators also looked at the role of Nrf2 in nonhematopoietic tissues and were surprised to find that Nrf2 deficiency in nonhematopoietic tissues exacerbated anemia and caused premature pulmonary edema in mice with SCD.
The team said this suggests a dominant protective role for nonhematopoietic Nrf2 against tissue damage in both erythroid and nonerythroid tissues in SCD.
And, when taken together, the results of this research indicate that activating Nrf2 can impede the onset of the severe adult phenotype of SCD in mice. ![]()

Image courtesy of the
University of Michigan
Activating the antioxidant regulator Nrf2 may slow the progression of sickle cell disease (SCD), according to preclinical research published in JCI Insight.
Investigators found the severity of hemolytic anemia, vascular inflammation, and lung injury increased with age in mice with SCD.
However, activating Nrf2 in young animals had a prophylactic effect, reducing the severity of these adverse effects and improving survival.
To uncover these findings, Solomon Ofori-Acquah, PhD, of the University of Pittsburgh in Pennsylvania, and his colleagues conducted a 10-month longitudinal observational study of mice with SCD.
The team found that, in mice with homozygous SCD (SS), there was a link between intravascular hemolysis, vascular inflammation, lung injury, and early death.
Mice as young as 2 months showed exacerbation of intravascular hemolysis. And additional investigation linked worsening intravascular hemolysis and oxidative stress to the release of VE-cadherin and progressive lung damage in aging SS mice.
The investigators knew that Nrf2 regulates the expression of genes that protect against the effects of intravascular hemolysis. So they decided to see if activating Nrf2 in young mice with SCD would slow the disease progression that occurs with age.
The team took SS mice that were about a month old and randomized them to receive 3H-1, 2-dithiole-3-thione (D3T) or a DMSO vehicle for 3 months or longer.
Treatment with D3T stabilized the concentration of hemoglobin, increased white blood cell counts, increased reticulocyte counts (though not significantly), kept HO-1 levels stable, increased levels of NQO1 and ferritin, and impeded the progression of endothelial dysfunction.
The investigators also looked at the role of Nrf2 in nonhematopoietic tissues and were surprised to find that Nrf2 deficiency in nonhematopoietic tissues exacerbated anemia and caused premature pulmonary edema in mice with SCD.
The team said this suggests a dominant protective role for nonhematopoietic Nrf2 against tissue damage in both erythroid and nonerythroid tissues in SCD.
And, when taken together, the results of this research indicate that activating Nrf2 can impede the onset of the severe adult phenotype of SCD in mice. ![]()