User login
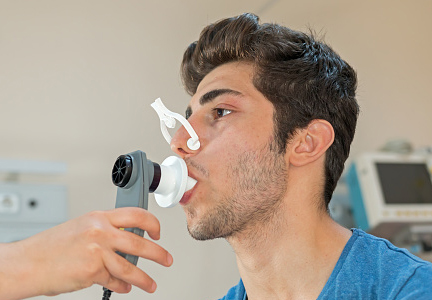
A 41-year-old woman presented with intermittent shortness of breath that worsened with exposure to cold air and cigarette smoke. She said her symptoms got better when she used albuterol, which had been prescribed after an emergency department visit during a worsening episode.
The patient was severely obese (body mass index 48 kg/m2) and had bilateral expiratory wheezes but no other significant findings. Based on the clinical presentation, we suspected she had asthma.
To establish the diagnosis and assess the severity of her condition, we questioned her further about her symptoms, and this information increased our suspicion of asthma. Is spirometry also indicated?
SPIROMETRY’S ROLE IN DIAGNOSING ASTHMA
Asthma is a chronic inflammatory condition of the airways characterized by recurrent or persistent symptoms with evidence of variable airflow obstruction or hyperresponsiveness to certain stimuli.1 The clinical diagnosis is based on episodic symptoms of chest tightness, wheezing, shortness of breath, or cough, but we cannot reliably diagnose asthma based on symptoms alone.
Spirometry provides an objective measure of obstruction, which adds to the reliability of the diagnosis. Therefore, it should be done in all patients in whom asthma is suspected.
Spirometry provides another diagnostic measure by quantifying whether airway obstruction reverses after the patient is given a dose of a bronchodilator. Although the exact criteria for reversibility of obstruction are unclear, the American Thoracic Society defines it as an increase in the forced expiratory volume in 1 second (FEV1) of 12% or more from baseline and an absolute increase of 200 mL or more. It can also be an increase of more than 200 mL in the forced vital capacity (FVC).2,3
Spirometry can also be used to evaluate or rule out other causes of chronic shortness of breath and common asthma mimics.
Failure to perform spirometry can result in a false diagnosis of asthma in patients who do not have it, or in a missed diagnosis in patients who do.4,5 Either situation often leads to inappropriate use of medications, exposure of patients to side effects, delays in appropriate diagnosis, and ongoing morbidity.
Despite the evidence in its favor, spirometry is underused. In a 2012 Canadian study, only 42.7% of 465,866 patients with newly diagnosed asthma had any spirometry testing performed within 1 year before or 2.5 years after the diagnosis.6 Similarly, in a 2015 US study, only 47.6% of 134,208 patients had spirometry performed within 1 year of diagnosis.7 Interestingly, this study found that the use of spirometry actually decreased after publication of guidelines from the National Asthma Education and Prevention Program1 that recommended spirometry.
CASE CONTINUED
Her baseline values were normal; her FEV1/FVC ratio was 73.67% (lower limit of normal 72.62%) and thus was not significant for airway obstruction. However, after 4 puffs of an inhaled short-acting beta agonist, her FEV1 increased by 15% from baseline (from 1.98 L/second before to 2.25 L/second after), a clinically significant response (defined as ≥ 12% from baseline and an absolute increase of at least 200 mL1–3). Had we not included bronchodilator testing, given the absence of underlying baseline obstruction, her shortness of breath could have been attributed to other causes, resulting in a missed asthma diagnosis.
Nevertheless, postbronchodilator measurements should not be performed in all patients with normal baseline results unless asthma is strongly suspected on clinical grounds. In one study, only 3% of 1,394 patients with normal baseline results showed improvement with a bronchodilator.8 In this patient population, bronchodilator testing would add both time and cost with little benefit.
Our patient’s reversibility of obstruction helped confirm the diagnosis of asthma. Absence of reversibility, however, does not rule out asthma, because spirometry results, like clinical symptoms of asthma, can vary. If clinical suspicion remains high and spirometry does not show clinically significant reversibility, then bronchoprovocation testing (most commonly with methacholine) could be done.
Although a positive methacholine challenge test can help identify asthma in patients with atypical symptoms or normal baseline test results, conditions other than asthma can also cause positive results. The sensitivity of methacholine challenge has been reported to be as high as 96%, while its specificity averages less than 80%.9 Given its high negative predictive value, the test can help rule out asthma, as negative results are rarely falsely negative.
SPIROMETRY’S ROLE IN ASSESSING ASTHMA SEVERITY AND CONTROL
Once the diagnosis of asthma is established, its severity and control need to be assessed to guide therapy. This is typically done by ascertaining how often the patient experiences asthma symptoms, how often the patient uses short-acting beta agonists (ranging from days per month to multiple times a day), and how often he or she has nighttime symptoms. The most severe symptom or most abnormal response is used to categorize asthma as intermittent or persistent, with severity ranging from mild to severe.
Symptoms are not always effective measures of asthma control, and subjective measures of symptoms often do not correlate with asthma severity, resulting in underestimation of the degree of airway obstruction.10,11 A review of 500 patients with an established asthma diagnosis found that in 110 patients with self-reported control of symptoms that included use of short-acting beta agonists no more than once per day, no night awakenings in the past week, and no missed school or work in the past 3 months, only 61 (55%) had an FEV1 above 80% of predicted.12 Further, neither the FEV1 nor FEV1/FVC ratio was shown to have a direct relationship with subjective measures of disease severity or control.
These observations highlight the need to use the objective findings from spirometry to assess asthma control and severity. Relying on the clinical symptoms alone likely underestimates the severity of asthma, especially in patients who are “poor perceivers” of symptoms. This can lead to undertreatment or an inappropriate step-down in therapy.
Current guidelines recommend repeating spirometry once therapy has brought the disease under control to establish a true baseline of airway function.1–3 Spirometry should be repeated again during any prolonged loss of asthma control and at 1- to 2-year intervals in patients with well-controlled disease as a means to monitor disease progression by measuring changes in airway function over time.
ROLE IN PREDICTING EXACERBATIONS
Current questionnaire-based assessments of breathing symptoms focus on disease severity and control, not on the risk of exacerbation. Although it may seem intuitive that patients who have the most severe disease are at highest risk of exacerbations, many patients with “mild” disease and “good” control experience exacerbations that require expensive emergency department visits. Nearly half of all the money spent on direct medical care for asthma is for urgent outpatient clinic and emergency department visits and hospitalizations.13
Using the FEV1, either by itself or in combination with other diagnostic tools such as questionnaires, has been shown to be superior to the clinical history alone in identifying patients at high risk of acute exacerbations.14,15 In addition to improving patient care and quality of life, spirometry could substantially reduce costs of care.
BOTTOM LINE
Although asthma remains a clinical diagnosis based on episodic symptoms consistent with airflow obstruction, symptoms alone cannot reliably be used to diagnose the disease or assess its severity and control.
Spirometry, including FEV1 and FVC, is an important objective measure to help with the diagnosis and should be done in all patients in whom asthma is suspected, both at the time of diagnosis and at intervals to assess disease progression. Spirometry also provides data to help assess the severity of asthma, which often does not correlate with clinical perception of symptoms, and it can be a predictive tool to identify patients at high risk for exacerbation, a common cause of emergency room visits and hospitalizations.
Some patients perceive spirometry as cumbersome and do not want to do it or cannot do it—spirometry takes quite a bit of effort and coordination while following directions. Also, it is not always easy to do, as patients with severe obstruction have a hard time maximally exhaling. Nevertheless, testing is safe, with few risks or adverse outcomes and can be easily performed in primary care settings and subspecialty clinics.
- National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007.
- Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med 1995; 152:1107–1136.
- Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement; asthma control and exacerbations. Am J Respir Crit Care Med 2009 Jul 1;180:59–99.
- van Schayck CP, van Der Heijden FM, van Den Boom G, Tirimanna PR, van Herwaarden CL. Underdiagnosis of asthma: is the doctor or the patient to blame? The DIMCA project. Thorax 2000; 55:562–565.
- Joyce DP, Chapman KR, Keston S. Prior diagnosis and treatment of patients with normal results of methacholine challenge and unexplained respiratory symptoms. Chest 1996; 109:697–701.
- Gershon AS, Victor JC, Guan J, Aaron SD, To T. Pulmonary function testing in the diagnosis of asthma: a population study. Chest 2012; 141:1190–1196.
- Sokol KS, Sharma G, Lin YL, Goldblum RM. Choosing Wisely: adherence by physicians to recommended use of spirometry in the diagnosis and management of adult asthma. Am J Med 2015; 128:502–508.
- Hagewald MJ, Townsend RG, Abbott JT, Crapo RO. Bronchodilator response in patients with normal baseline spirometry. Respir Care 2012; 57:1564–1570.
- Yurdakul AS, Dursun B, Canbakan S, Cakaloglu A, Capan N. The assessment of validity of different asthma diagnostic tools in adults. J Asthma 2005; 42:843–846.
- Stahl E. Correlation between objective measures of airway calibre and clinical symptoms in asthma: a systematic review of clinical studies. Respir Med 2000; 94:735–741.
- Teeter JG, Bleecker ER. Relationships between airway obstructions and respiratory symptoms in adult asthmatics. Chest 1998; 113:272–277.
- Cowie RL, Underwood MF, Field SK. Asthma symptoms do not predict spirometry. Can Respir J 2007; 14:339–342.
- Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. Assessing the economic impact. J Allergy Clin Immunol 2001; 107:3–8.
- Osborne ML, Pedula KL, O’Hollaren M, et al. Assessing future need for acute care in adult asthmatics. The profile of asthma risk study: a prospective health maintenance organization-based study. Chest 2007; 132:1151–1161.
- Kitch BT, Paltiel AD, Kuntz KM, et al. A single measure of FEV1 is associated with risk of asthma attacks in long-term follow-up. Chest 2004; 126:1875–1882.
A 41-year-old woman presented with intermittent shortness of breath that worsened with exposure to cold air and cigarette smoke. She said her symptoms got better when she used albuterol, which had been prescribed after an emergency department visit during a worsening episode.
The patient was severely obese (body mass index 48 kg/m2) and had bilateral expiratory wheezes but no other significant findings. Based on the clinical presentation, we suspected she had asthma.
To establish the diagnosis and assess the severity of her condition, we questioned her further about her symptoms, and this information increased our suspicion of asthma. Is spirometry also indicated?
SPIROMETRY’S ROLE IN DIAGNOSING ASTHMA
Asthma is a chronic inflammatory condition of the airways characterized by recurrent or persistent symptoms with evidence of variable airflow obstruction or hyperresponsiveness to certain stimuli.1 The clinical diagnosis is based on episodic symptoms of chest tightness, wheezing, shortness of breath, or cough, but we cannot reliably diagnose asthma based on symptoms alone.
Spirometry provides an objective measure of obstruction, which adds to the reliability of the diagnosis. Therefore, it should be done in all patients in whom asthma is suspected.
Spirometry provides another diagnostic measure by quantifying whether airway obstruction reverses after the patient is given a dose of a bronchodilator. Although the exact criteria for reversibility of obstruction are unclear, the American Thoracic Society defines it as an increase in the forced expiratory volume in 1 second (FEV1) of 12% or more from baseline and an absolute increase of 200 mL or more. It can also be an increase of more than 200 mL in the forced vital capacity (FVC).2,3
Spirometry can also be used to evaluate or rule out other causes of chronic shortness of breath and common asthma mimics.
Failure to perform spirometry can result in a false diagnosis of asthma in patients who do not have it, or in a missed diagnosis in patients who do.4,5 Either situation often leads to inappropriate use of medications, exposure of patients to side effects, delays in appropriate diagnosis, and ongoing morbidity.
Despite the evidence in its favor, spirometry is underused. In a 2012 Canadian study, only 42.7% of 465,866 patients with newly diagnosed asthma had any spirometry testing performed within 1 year before or 2.5 years after the diagnosis.6 Similarly, in a 2015 US study, only 47.6% of 134,208 patients had spirometry performed within 1 year of diagnosis.7 Interestingly, this study found that the use of spirometry actually decreased after publication of guidelines from the National Asthma Education and Prevention Program1 that recommended spirometry.
CASE CONTINUED
Her baseline values were normal; her FEV1/FVC ratio was 73.67% (lower limit of normal 72.62%) and thus was not significant for airway obstruction. However, after 4 puffs of an inhaled short-acting beta agonist, her FEV1 increased by 15% from baseline (from 1.98 L/second before to 2.25 L/second after), a clinically significant response (defined as ≥ 12% from baseline and an absolute increase of at least 200 mL1–3). Had we not included bronchodilator testing, given the absence of underlying baseline obstruction, her shortness of breath could have been attributed to other causes, resulting in a missed asthma diagnosis.
Nevertheless, postbronchodilator measurements should not be performed in all patients with normal baseline results unless asthma is strongly suspected on clinical grounds. In one study, only 3% of 1,394 patients with normal baseline results showed improvement with a bronchodilator.8 In this patient population, bronchodilator testing would add both time and cost with little benefit.
Our patient’s reversibility of obstruction helped confirm the diagnosis of asthma. Absence of reversibility, however, does not rule out asthma, because spirometry results, like clinical symptoms of asthma, can vary. If clinical suspicion remains high and spirometry does not show clinically significant reversibility, then bronchoprovocation testing (most commonly with methacholine) could be done.
Although a positive methacholine challenge test can help identify asthma in patients with atypical symptoms or normal baseline test results, conditions other than asthma can also cause positive results. The sensitivity of methacholine challenge has been reported to be as high as 96%, while its specificity averages less than 80%.9 Given its high negative predictive value, the test can help rule out asthma, as negative results are rarely falsely negative.
SPIROMETRY’S ROLE IN ASSESSING ASTHMA SEVERITY AND CONTROL
Once the diagnosis of asthma is established, its severity and control need to be assessed to guide therapy. This is typically done by ascertaining how often the patient experiences asthma symptoms, how often the patient uses short-acting beta agonists (ranging from days per month to multiple times a day), and how often he or she has nighttime symptoms. The most severe symptom or most abnormal response is used to categorize asthma as intermittent or persistent, with severity ranging from mild to severe.
Symptoms are not always effective measures of asthma control, and subjective measures of symptoms often do not correlate with asthma severity, resulting in underestimation of the degree of airway obstruction.10,11 A review of 500 patients with an established asthma diagnosis found that in 110 patients with self-reported control of symptoms that included use of short-acting beta agonists no more than once per day, no night awakenings in the past week, and no missed school or work in the past 3 months, only 61 (55%) had an FEV1 above 80% of predicted.12 Further, neither the FEV1 nor FEV1/FVC ratio was shown to have a direct relationship with subjective measures of disease severity or control.
These observations highlight the need to use the objective findings from spirometry to assess asthma control and severity. Relying on the clinical symptoms alone likely underestimates the severity of asthma, especially in patients who are “poor perceivers” of symptoms. This can lead to undertreatment or an inappropriate step-down in therapy.
Current guidelines recommend repeating spirometry once therapy has brought the disease under control to establish a true baseline of airway function.1–3 Spirometry should be repeated again during any prolonged loss of asthma control and at 1- to 2-year intervals in patients with well-controlled disease as a means to monitor disease progression by measuring changes in airway function over time.
ROLE IN PREDICTING EXACERBATIONS
Current questionnaire-based assessments of breathing symptoms focus on disease severity and control, not on the risk of exacerbation. Although it may seem intuitive that patients who have the most severe disease are at highest risk of exacerbations, many patients with “mild” disease and “good” control experience exacerbations that require expensive emergency department visits. Nearly half of all the money spent on direct medical care for asthma is for urgent outpatient clinic and emergency department visits and hospitalizations.13
Using the FEV1, either by itself or in combination with other diagnostic tools such as questionnaires, has been shown to be superior to the clinical history alone in identifying patients at high risk of acute exacerbations.14,15 In addition to improving patient care and quality of life, spirometry could substantially reduce costs of care.
BOTTOM LINE
Although asthma remains a clinical diagnosis based on episodic symptoms consistent with airflow obstruction, symptoms alone cannot reliably be used to diagnose the disease or assess its severity and control.
Spirometry, including FEV1 and FVC, is an important objective measure to help with the diagnosis and should be done in all patients in whom asthma is suspected, both at the time of diagnosis and at intervals to assess disease progression. Spirometry also provides data to help assess the severity of asthma, which often does not correlate with clinical perception of symptoms, and it can be a predictive tool to identify patients at high risk for exacerbation, a common cause of emergency room visits and hospitalizations.
Some patients perceive spirometry as cumbersome and do not want to do it or cannot do it—spirometry takes quite a bit of effort and coordination while following directions. Also, it is not always easy to do, as patients with severe obstruction have a hard time maximally exhaling. Nevertheless, testing is safe, with few risks or adverse outcomes and can be easily performed in primary care settings and subspecialty clinics.
A 41-year-old woman presented with intermittent shortness of breath that worsened with exposure to cold air and cigarette smoke. She said her symptoms got better when she used albuterol, which had been prescribed after an emergency department visit during a worsening episode.
The patient was severely obese (body mass index 48 kg/m2) and had bilateral expiratory wheezes but no other significant findings. Based on the clinical presentation, we suspected she had asthma.
To establish the diagnosis and assess the severity of her condition, we questioned her further about her symptoms, and this information increased our suspicion of asthma. Is spirometry also indicated?
SPIROMETRY’S ROLE IN DIAGNOSING ASTHMA
Asthma is a chronic inflammatory condition of the airways characterized by recurrent or persistent symptoms with evidence of variable airflow obstruction or hyperresponsiveness to certain stimuli.1 The clinical diagnosis is based on episodic symptoms of chest tightness, wheezing, shortness of breath, or cough, but we cannot reliably diagnose asthma based on symptoms alone.
Spirometry provides an objective measure of obstruction, which adds to the reliability of the diagnosis. Therefore, it should be done in all patients in whom asthma is suspected.
Spirometry provides another diagnostic measure by quantifying whether airway obstruction reverses after the patient is given a dose of a bronchodilator. Although the exact criteria for reversibility of obstruction are unclear, the American Thoracic Society defines it as an increase in the forced expiratory volume in 1 second (FEV1) of 12% or more from baseline and an absolute increase of 200 mL or more. It can also be an increase of more than 200 mL in the forced vital capacity (FVC).2,3
Spirometry can also be used to evaluate or rule out other causes of chronic shortness of breath and common asthma mimics.
Failure to perform spirometry can result in a false diagnosis of asthma in patients who do not have it, or in a missed diagnosis in patients who do.4,5 Either situation often leads to inappropriate use of medications, exposure of patients to side effects, delays in appropriate diagnosis, and ongoing morbidity.
Despite the evidence in its favor, spirometry is underused. In a 2012 Canadian study, only 42.7% of 465,866 patients with newly diagnosed asthma had any spirometry testing performed within 1 year before or 2.5 years after the diagnosis.6 Similarly, in a 2015 US study, only 47.6% of 134,208 patients had spirometry performed within 1 year of diagnosis.7 Interestingly, this study found that the use of spirometry actually decreased after publication of guidelines from the National Asthma Education and Prevention Program1 that recommended spirometry.
CASE CONTINUED
Her baseline values were normal; her FEV1/FVC ratio was 73.67% (lower limit of normal 72.62%) and thus was not significant for airway obstruction. However, after 4 puffs of an inhaled short-acting beta agonist, her FEV1 increased by 15% from baseline (from 1.98 L/second before to 2.25 L/second after), a clinically significant response (defined as ≥ 12% from baseline and an absolute increase of at least 200 mL1–3). Had we not included bronchodilator testing, given the absence of underlying baseline obstruction, her shortness of breath could have been attributed to other causes, resulting in a missed asthma diagnosis.
Nevertheless, postbronchodilator measurements should not be performed in all patients with normal baseline results unless asthma is strongly suspected on clinical grounds. In one study, only 3% of 1,394 patients with normal baseline results showed improvement with a bronchodilator.8 In this patient population, bronchodilator testing would add both time and cost with little benefit.
Our patient’s reversibility of obstruction helped confirm the diagnosis of asthma. Absence of reversibility, however, does not rule out asthma, because spirometry results, like clinical symptoms of asthma, can vary. If clinical suspicion remains high and spirometry does not show clinically significant reversibility, then bronchoprovocation testing (most commonly with methacholine) could be done.
Although a positive methacholine challenge test can help identify asthma in patients with atypical symptoms or normal baseline test results, conditions other than asthma can also cause positive results. The sensitivity of methacholine challenge has been reported to be as high as 96%, while its specificity averages less than 80%.9 Given its high negative predictive value, the test can help rule out asthma, as negative results are rarely falsely negative.
SPIROMETRY’S ROLE IN ASSESSING ASTHMA SEVERITY AND CONTROL
Once the diagnosis of asthma is established, its severity and control need to be assessed to guide therapy. This is typically done by ascertaining how often the patient experiences asthma symptoms, how often the patient uses short-acting beta agonists (ranging from days per month to multiple times a day), and how often he or she has nighttime symptoms. The most severe symptom or most abnormal response is used to categorize asthma as intermittent or persistent, with severity ranging from mild to severe.
Symptoms are not always effective measures of asthma control, and subjective measures of symptoms often do not correlate with asthma severity, resulting in underestimation of the degree of airway obstruction.10,11 A review of 500 patients with an established asthma diagnosis found that in 110 patients with self-reported control of symptoms that included use of short-acting beta agonists no more than once per day, no night awakenings in the past week, and no missed school or work in the past 3 months, only 61 (55%) had an FEV1 above 80% of predicted.12 Further, neither the FEV1 nor FEV1/FVC ratio was shown to have a direct relationship with subjective measures of disease severity or control.
These observations highlight the need to use the objective findings from spirometry to assess asthma control and severity. Relying on the clinical symptoms alone likely underestimates the severity of asthma, especially in patients who are “poor perceivers” of symptoms. This can lead to undertreatment or an inappropriate step-down in therapy.
Current guidelines recommend repeating spirometry once therapy has brought the disease under control to establish a true baseline of airway function.1–3 Spirometry should be repeated again during any prolonged loss of asthma control and at 1- to 2-year intervals in patients with well-controlled disease as a means to monitor disease progression by measuring changes in airway function over time.
ROLE IN PREDICTING EXACERBATIONS
Current questionnaire-based assessments of breathing symptoms focus on disease severity and control, not on the risk of exacerbation. Although it may seem intuitive that patients who have the most severe disease are at highest risk of exacerbations, many patients with “mild” disease and “good” control experience exacerbations that require expensive emergency department visits. Nearly half of all the money spent on direct medical care for asthma is for urgent outpatient clinic and emergency department visits and hospitalizations.13
Using the FEV1, either by itself or in combination with other diagnostic tools such as questionnaires, has been shown to be superior to the clinical history alone in identifying patients at high risk of acute exacerbations.14,15 In addition to improving patient care and quality of life, spirometry could substantially reduce costs of care.
BOTTOM LINE
Although asthma remains a clinical diagnosis based on episodic symptoms consistent with airflow obstruction, symptoms alone cannot reliably be used to diagnose the disease or assess its severity and control.
Spirometry, including FEV1 and FVC, is an important objective measure to help with the diagnosis and should be done in all patients in whom asthma is suspected, both at the time of diagnosis and at intervals to assess disease progression. Spirometry also provides data to help assess the severity of asthma, which often does not correlate with clinical perception of symptoms, and it can be a predictive tool to identify patients at high risk for exacerbation, a common cause of emergency room visits and hospitalizations.
Some patients perceive spirometry as cumbersome and do not want to do it or cannot do it—spirometry takes quite a bit of effort and coordination while following directions. Also, it is not always easy to do, as patients with severe obstruction have a hard time maximally exhaling. Nevertheless, testing is safe, with few risks or adverse outcomes and can be easily performed in primary care settings and subspecialty clinics.
- National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007.
- Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med 1995; 152:1107–1136.
- Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement; asthma control and exacerbations. Am J Respir Crit Care Med 2009 Jul 1;180:59–99.
- van Schayck CP, van Der Heijden FM, van Den Boom G, Tirimanna PR, van Herwaarden CL. Underdiagnosis of asthma: is the doctor or the patient to blame? The DIMCA project. Thorax 2000; 55:562–565.
- Joyce DP, Chapman KR, Keston S. Prior diagnosis and treatment of patients with normal results of methacholine challenge and unexplained respiratory symptoms. Chest 1996; 109:697–701.
- Gershon AS, Victor JC, Guan J, Aaron SD, To T. Pulmonary function testing in the diagnosis of asthma: a population study. Chest 2012; 141:1190–1196.
- Sokol KS, Sharma G, Lin YL, Goldblum RM. Choosing Wisely: adherence by physicians to recommended use of spirometry in the diagnosis and management of adult asthma. Am J Med 2015; 128:502–508.
- Hagewald MJ, Townsend RG, Abbott JT, Crapo RO. Bronchodilator response in patients with normal baseline spirometry. Respir Care 2012; 57:1564–1570.
- Yurdakul AS, Dursun B, Canbakan S, Cakaloglu A, Capan N. The assessment of validity of different asthma diagnostic tools in adults. J Asthma 2005; 42:843–846.
- Stahl E. Correlation between objective measures of airway calibre and clinical symptoms in asthma: a systematic review of clinical studies. Respir Med 2000; 94:735–741.
- Teeter JG, Bleecker ER. Relationships between airway obstructions and respiratory symptoms in adult asthmatics. Chest 1998; 113:272–277.
- Cowie RL, Underwood MF, Field SK. Asthma symptoms do not predict spirometry. Can Respir J 2007; 14:339–342.
- Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. Assessing the economic impact. J Allergy Clin Immunol 2001; 107:3–8.
- Osborne ML, Pedula KL, O’Hollaren M, et al. Assessing future need for acute care in adult asthmatics. The profile of asthma risk study: a prospective health maintenance organization-based study. Chest 2007; 132:1151–1161.
- Kitch BT, Paltiel AD, Kuntz KM, et al. A single measure of FEV1 is associated with risk of asthma attacks in long-term follow-up. Chest 2004; 126:1875–1882.
- National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007.
- Standardization of spirometry, 1994 update. American Thoracic Society. Am J Respir Crit Care Med 1995; 152:1107–1136.
- Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement; asthma control and exacerbations. Am J Respir Crit Care Med 2009 Jul 1;180:59–99.
- van Schayck CP, van Der Heijden FM, van Den Boom G, Tirimanna PR, van Herwaarden CL. Underdiagnosis of asthma: is the doctor or the patient to blame? The DIMCA project. Thorax 2000; 55:562–565.
- Joyce DP, Chapman KR, Keston S. Prior diagnosis and treatment of patients with normal results of methacholine challenge and unexplained respiratory symptoms. Chest 1996; 109:697–701.
- Gershon AS, Victor JC, Guan J, Aaron SD, To T. Pulmonary function testing in the diagnosis of asthma: a population study. Chest 2012; 141:1190–1196.
- Sokol KS, Sharma G, Lin YL, Goldblum RM. Choosing Wisely: adherence by physicians to recommended use of spirometry in the diagnosis and management of adult asthma. Am J Med 2015; 128:502–508.
- Hagewald MJ, Townsend RG, Abbott JT, Crapo RO. Bronchodilator response in patients with normal baseline spirometry. Respir Care 2012; 57:1564–1570.
- Yurdakul AS, Dursun B, Canbakan S, Cakaloglu A, Capan N. The assessment of validity of different asthma diagnostic tools in adults. J Asthma 2005; 42:843–846.
- Stahl E. Correlation between objective measures of airway calibre and clinical symptoms in asthma: a systematic review of clinical studies. Respir Med 2000; 94:735–741.
- Teeter JG, Bleecker ER. Relationships between airway obstructions and respiratory symptoms in adult asthmatics. Chest 1998; 113:272–277.
- Cowie RL, Underwood MF, Field SK. Asthma symptoms do not predict spirometry. Can Respir J 2007; 14:339–342.
- Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. Assessing the economic impact. J Allergy Clin Immunol 2001; 107:3–8.
- Osborne ML, Pedula KL, O’Hollaren M, et al. Assessing future need for acute care in adult asthmatics. The profile of asthma risk study: a prospective health maintenance organization-based study. Chest 2007; 132:1151–1161.
- Kitch BT, Paltiel AD, Kuntz KM, et al. A single measure of FEV1 is associated with risk of asthma attacks in long-term follow-up. Chest 2004; 126:1875–1882.