User login
Internal carotid artery dissection (ICAD) is an uncommon cause of stroke that typically occurs in the setting of (often minor) trauma but can also occur spontaneously. Patients with ICAD typically present with ipsilateral head, face, or neck pain. In approximately half of ICAD cases, an acute partial, painful Horner syndrome is present on examination. Although computed tomography angiography (CTA) is currently the imaging study of choice, magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) and Doppler ultrasound can also be used. Management options include thrombolysis, antiplatelet or anticoagulation therapy, and endovascular or surgical intervention.
Case
A 56-year-old man with a history of migraines presented to the ED with a chief complaint of a 4-day history of right-sided headache. He stated that the pain felt different from his usual migraines and was located behind his right eye. Prior to presentation at the ED, the patient had initially visited an urgent care facility for evaluation. The physician who evaluated the patient at the urgent care facility noted the patient’s left eye appeared dilated and referred him to the ED for evaluation.
The patient further stated that the day prior to presentation, one of his friends had also remarked that the patient’s left eye appeared to be enlarged. The patient denied any visual disturbances, focal weakness, nausea, vomiting, neck pain, or stiffness. His medical history was significant for paroxysmal atrial flutter and hypertension. Regarding medications, the patient was taking dronedarone, clonazepam, omeprazole, and metoprolol.
On physical examination, the patient’s vital signs were: blood pressure, 162/109 mm Hg; heart rate, 85 beats/minute and regular; respiratory rate, 18 breaths/minute; and temperature, 98.6°F. Oxygen saturation was 98% on room air. The patient appeared to be in no acute distress. Ocular examination revealed a dilated left pupil of approximately 5 to 6 mm, and a right pupil measuring approximately 3 mm. Both pupils reacted to light, and the extraocular muscles were intact. The patient’s face appeared symmetrical and had intact sensation. He had normal speech, midline tongue, and good bilateral shoulder shrug. The neck examination revealed normal range of motion with full flexion, without jugular vein distention, lymphadenopathy, or palpable thyroid. The cardiovascular, lung, and abdominal examinations were all normal. The neurological examination showed the patient to be awake, alert, and oriented to person, place, and time. He exhibited 5/5 motor strength in all four extremities, normal gait, and normal finger-to-nose performance; his reflexes were 2+ and symmetrical.
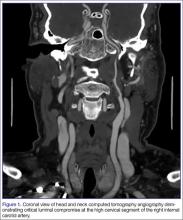
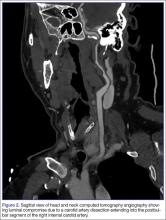
The emergency physician (EP) ordered a stat noncontrast CT scan of the head, complete blood count (CBC), and basic metabolic panel (BMP). The CT scan of the head was interpreted by the radiologist as “no acute intracranial abnormality,” and the CBC and the BMP were normal. Since the EP was concerned about a carotid artery aneurysm or dissection, he ordered a CTA of the head and neck (Figures 1 and 2). The CTA was read as:
Long segment dissection of postbulbar cervical segment of right internal carotid artery, very likely extending into the intracranial segment with critical stenosis at the junction of the high cervical segment and proximal petrous segment. Preserved runoff circulation to the anterior and middle cerebral arteries with robust capacity for collateral support in the context of patent anterior and posterior communicating arteries.
Based on the radiology report, the EP consulted with vascular surgery services, and ordered a Doppler study of the ICAs. The Doppler study demonstrated “arterial thrombus in the right distal extracranial internal carotid artery with hemodynamics suggestive of a distal occlusion/significant obstruction.” The patient was started on an intravenous (IV) heparin drip and admitted to the hospital. Three days later he experienced sudden onset of left arm weakness. An emergent CTA of the head and neck revealed an embolic occlusion of mid-to-distal M1 segment of the right middle cerebral artery. The patient was immediately evaluated by interventional radiology for possible clot removal; however, based on his rapid neurological improvement, he was instead treated medically with aspirin and clopidogrel and continued to show significant neurological improvement. He was discharged home on hospital day 8 on both antiplatelet agents with minimal neurological deficit.
Discussion
Even though ICAD accounts for only 1% to 2% of all strokes, it is responsible for 10% to 25% of strokes in young and middle-aged adults.1-3 The peak incidence for ICAD is in the fifth decade, and it affects men and women equally.4 The extracranial portion of the ICA is the most commonly affected vessel (>90%); dissections of the intracranial portion are associated with greater neurological deficits and have a poorer prognosis.2,5 Cerebral ischemia resulting from a dissection of the extracranial ICA may occur days to weeks after the onset of local symptoms such as head or neck pain, Horner syndrome, or tinnitus.5
In ICAD, a tear in the artery wall causes blood to enter the tunica media of the vessel, forming an intramural hematoma.2,6 This may result in either stenosis of the lumen of the vessel from the enlarging hematoma or an outward aneurysmal dilatation of the vessel that compresses surrounding structures.2,6 Subsequent cerebral ischemia is the result of either arterial embolism or hemodynamic compromise from vessel stenosis.2,3
Causes
Dissections often occur secondary to trauma, though the severity of the trauma may be quite minor.2,6 Seemingly trivial mechanisms that have been associated with dissections include nose blowing, coughing, sudden neck turning, and prolonged telephone conversations. Other known causes are motor vehicle accidents and chiropractic maneuvers. Inherited connective tissue disorders, including Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, and osteogenesis imperfecta, are associated with spontaneous ICAD.2,6
Symptoms
The most common presenting symptom of carotid dissection in approximately two-thirds of patients is ipsilateral head, face, or neck pain,2,6 typically described as sharp, constant, and nonthrobbing. Patients report a subjective bruit in 21% to 39% of cases.7 An acute partial painful Horner syndrome is strongly associated with ICAD, but is present in fewer than half of such patients.6 It is characterized by miosis and ptosis and is the result of compression of the ascending sympathetic fibers that travel alongside the ICA within the carotid sheath. Anhidrosis is not present because the fibers for sweat function in the face travel along the external carotid artery.2
Differential Diagnosis
While there is overlap between strokes caused by ICAD versus plaque, some features can help guide the clinician. Symptomatic carotid disease will frequently present with a history of one or more transient ischemic attacks characterized by focal neurological dysfunction or transient monocular blindness—typically within the previous 6 months.8 This history is not usually present in patients with ICAD. Secondly, pain is a much more prominent symptom in ICAD compared to patients with severe carotid atherosclerosis. The history of trauma, even minor, should make dissection higher on the differential diagnosis. Fortunately, the imaging studies to evaluate for these two diseases are the same.
Assessing Pupillary Asymmetry and Ptosis
Careful attention must be paid to assessing a patient for pupillary asymmetry. In a patient with anisocoria, determining the abnormal pupil may require examination of the patient in both bright and dark lighting conditions. The first step is to examine the patient’s pupils under normal lighting conditions. The next step is to assess each pupil’s response to shining a bright light in each eye. The abnormal pupil is the pupil that does not respond well or at all to bright light shone directly in the eye. If the anisocoria is greatest in bright light, the larger pupil is the abnormal pupil. When the anisocoria is greater in dark conditions, the smaller pupil is the abnormal pupil. In this case, the patient’s abnormal pupil was incorrectly diagnosed as the contralateral larger pupil (ie, left)—highlighting the importance of performing a complete pupillary examination in all patients presenting with neurological symptoms.9
Furthermore, as demonstrated in this case, ptosis in a patient with Horner syndrome caused by an ICAD can be subtle. The ptosis is the result of paralysis of Müeller’s muscle, which is innervated by the sympathetic pathway. The levator palpebrae superioris, which causes the more profound ptosis seen in third nerve palsies, is unaffected.10
Imaging Studies
Once the diagnosis of ICAD is suspected, appropriate vascular imaging must be obtained. Digital subtraction angiography has historically been the gold standard for vascular imaging of the neck vessels, but it has largely been replaced by less invasive and more readily available imaging modalities such as CTA and MRI/MRA.11
Computed Tomography Angiography. This is a widely available, rapid imaging choice and has a sensitivity of 80% to 95% in the detection of ICAD.7 It has a greater ability than MRI to identify dissection features such as intimal flaps, pseudoaneurysms, and high-grade stenosis versus occlusion. One of its disadvantages is the need for iodinated contrast, which can limit the ability to obtain the test in those with renal disease or patients with true allergies to IV contrast material. In addition, a mural hematoma can be mistaken for a noncalcified atherosclerotic plaque in the vessel lumen.6
Magnetic Resonance Imaging and Magnetic Resonance Angiography. Both MRI and MRA are also frequently used to diagnose ICAD. The intramural hematoma displays a hyperdense signal on T1-weighted images and has a characteristic crescent shape adjacent to the lumen.11 Magnetic resonance imaging studies are also sensitive in detecting cerebral ischemia resulting from the dissection. However, the sensitivity of MRI/MRA is highest 2 days after the dissection has occurred.2
Doppler Ultrasound. This is another imaging modality used to detect ICAD—one that is noninvasive, less expensive, requires no contrast material, and is widely available. Limitations of Doppler ultrasound include the inability to scan the distal ICA and a lower sensitivity in detecting dissections that cause low-grade stenosis.2 It is more commonly used for follow-up monitoring of dissections.
Management
There are several options for managing ICAD. In patients with unstable lesions, progressing neurological deficit, or further strokes, endovascular stenting has been shown to have a technical success rate of 99% and a procedural complication rate of 1.3%.12 Similarly, if the patient exhibits symptoms of cerebral ischemia, severe narrowing of the arterial lumen, or an unstable plaque, IV heparin is frequently used, followed by warfarin.13 However, since the majority of carotid and vertebral artery dissections heal spontaneously,14 antithrombotic therapies, including aspirin, clopidogrel or warfarin, are often prescribed to prevent thromboembolic complications.
Conclusion
Diagnosing ICAD requires knowledge of the typical history and presenting features of the disease. Careful attention to the ocular examination must be undertaken in any patient presenting with headache or face or neck pain, because the findings can be subtle. In a patient in whom ICAD is suspected, imaging with CTA or MRI/MRA should be performed. Early consultation with vascular surgery services can help determine the most appropriate treatment strategy.
1. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
2. Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81(956):383-388.
3. Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29(12):2646-2648.
4. Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330(6):393-397.
5. Biousse V, D’Anglejan-Chatillon J, Touboul PJ, Amarenco P, Bousser MG. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. 1995;26(2):235-239.
6. Kasravi N, Leung A, Silver I, Burneo JG. Dissection of the internal carotid artery causing Horner syndrome and palsy of cranial nerve XII. CMAJ. 2010;182(9):E373-E377.
1. Borgman CJ. Horner syndrome secondary to internal carotid artery dissection after a short-distance endurance run: a case study and review. J Optom. 2012;5:209-216.
2. Mohler ER III, Fairman RM. Management of symptomatic carotid atherosclerotic disease. UpToDate Web site. http://www.uptodate.com/contents/management-of-symptomatic-carotid-atherosclerotic-disease. Updated February 24, 2016. Accessed May 6, 2016.
3. Mann J. Anisocoria guidemap. Life in the Fastlane Web site. http://lifeinthefastlane.com/resources/jeff-manns-em-guidemaps/anisocoria-guidemap/. Accessed March 15, 2016.
10. Kedar S, Biousse V, Newman NJ. Horner syndrome. UpToDate Web site. http://www.uptodate.com/contents/horner-syndrome. Updated July 14, 2015. Accessed May 6, 2016.
11. Vertinsky AT, Schwartz NE, Fischbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
12. Pham MH, Rahme RJ, Arnaout O, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 2011;68(4):856-866.
13. Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
14. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
Internal carotid artery dissection (ICAD) is an uncommon cause of stroke that typically occurs in the setting of (often minor) trauma but can also occur spontaneously. Patients with ICAD typically present with ipsilateral head, face, or neck pain. In approximately half of ICAD cases, an acute partial, painful Horner syndrome is present on examination. Although computed tomography angiography (CTA) is currently the imaging study of choice, magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) and Doppler ultrasound can also be used. Management options include thrombolysis, antiplatelet or anticoagulation therapy, and endovascular or surgical intervention.
Case
A 56-year-old man with a history of migraines presented to the ED with a chief complaint of a 4-day history of right-sided headache. He stated that the pain felt different from his usual migraines and was located behind his right eye. Prior to presentation at the ED, the patient had initially visited an urgent care facility for evaluation. The physician who evaluated the patient at the urgent care facility noted the patient’s left eye appeared dilated and referred him to the ED for evaluation.
The patient further stated that the day prior to presentation, one of his friends had also remarked that the patient’s left eye appeared to be enlarged. The patient denied any visual disturbances, focal weakness, nausea, vomiting, neck pain, or stiffness. His medical history was significant for paroxysmal atrial flutter and hypertension. Regarding medications, the patient was taking dronedarone, clonazepam, omeprazole, and metoprolol.
On physical examination, the patient’s vital signs were: blood pressure, 162/109 mm Hg; heart rate, 85 beats/minute and regular; respiratory rate, 18 breaths/minute; and temperature, 98.6°F. Oxygen saturation was 98% on room air. The patient appeared to be in no acute distress. Ocular examination revealed a dilated left pupil of approximately 5 to 6 mm, and a right pupil measuring approximately 3 mm. Both pupils reacted to light, and the extraocular muscles were intact. The patient’s face appeared symmetrical and had intact sensation. He had normal speech, midline tongue, and good bilateral shoulder shrug. The neck examination revealed normal range of motion with full flexion, without jugular vein distention, lymphadenopathy, or palpable thyroid. The cardiovascular, lung, and abdominal examinations were all normal. The neurological examination showed the patient to be awake, alert, and oriented to person, place, and time. He exhibited 5/5 motor strength in all four extremities, normal gait, and normal finger-to-nose performance; his reflexes were 2+ and symmetrical.
The emergency physician (EP) ordered a stat noncontrast CT scan of the head, complete blood count (CBC), and basic metabolic panel (BMP). The CT scan of the head was interpreted by the radiologist as “no acute intracranial abnormality,” and the CBC and the BMP were normal. Since the EP was concerned about a carotid artery aneurysm or dissection, he ordered a CTA of the head and neck (Figures 1 and 2). The CTA was read as:
Long segment dissection of postbulbar cervical segment of right internal carotid artery, very likely extending into the intracranial segment with critical stenosis at the junction of the high cervical segment and proximal petrous segment. Preserved runoff circulation to the anterior and middle cerebral arteries with robust capacity for collateral support in the context of patent anterior and posterior communicating arteries.
Based on the radiology report, the EP consulted with vascular surgery services, and ordered a Doppler study of the ICAs. The Doppler study demonstrated “arterial thrombus in the right distal extracranial internal carotid artery with hemodynamics suggestive of a distal occlusion/significant obstruction.” The patient was started on an intravenous (IV) heparin drip and admitted to the hospital. Three days later he experienced sudden onset of left arm weakness. An emergent CTA of the head and neck revealed an embolic occlusion of mid-to-distal M1 segment of the right middle cerebral artery. The patient was immediately evaluated by interventional radiology for possible clot removal; however, based on his rapid neurological improvement, he was instead treated medically with aspirin and clopidogrel and continued to show significant neurological improvement. He was discharged home on hospital day 8 on both antiplatelet agents with minimal neurological deficit.
Discussion
Even though ICAD accounts for only 1% to 2% of all strokes, it is responsible for 10% to 25% of strokes in young and middle-aged adults.1-3 The peak incidence for ICAD is in the fifth decade, and it affects men and women equally.4 The extracranial portion of the ICA is the most commonly affected vessel (>90%); dissections of the intracranial portion are associated with greater neurological deficits and have a poorer prognosis.2,5 Cerebral ischemia resulting from a dissection of the extracranial ICA may occur days to weeks after the onset of local symptoms such as head or neck pain, Horner syndrome, or tinnitus.5
In ICAD, a tear in the artery wall causes blood to enter the tunica media of the vessel, forming an intramural hematoma.2,6 This may result in either stenosis of the lumen of the vessel from the enlarging hematoma or an outward aneurysmal dilatation of the vessel that compresses surrounding structures.2,6 Subsequent cerebral ischemia is the result of either arterial embolism or hemodynamic compromise from vessel stenosis.2,3
Causes
Dissections often occur secondary to trauma, though the severity of the trauma may be quite minor.2,6 Seemingly trivial mechanisms that have been associated with dissections include nose blowing, coughing, sudden neck turning, and prolonged telephone conversations. Other known causes are motor vehicle accidents and chiropractic maneuvers. Inherited connective tissue disorders, including Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, and osteogenesis imperfecta, are associated with spontaneous ICAD.2,6
Symptoms
The most common presenting symptom of carotid dissection in approximately two-thirds of patients is ipsilateral head, face, or neck pain,2,6 typically described as sharp, constant, and nonthrobbing. Patients report a subjective bruit in 21% to 39% of cases.7 An acute partial painful Horner syndrome is strongly associated with ICAD, but is present in fewer than half of such patients.6 It is characterized by miosis and ptosis and is the result of compression of the ascending sympathetic fibers that travel alongside the ICA within the carotid sheath. Anhidrosis is not present because the fibers for sweat function in the face travel along the external carotid artery.2
Differential Diagnosis
While there is overlap between strokes caused by ICAD versus plaque, some features can help guide the clinician. Symptomatic carotid disease will frequently present with a history of one or more transient ischemic attacks characterized by focal neurological dysfunction or transient monocular blindness—typically within the previous 6 months.8 This history is not usually present in patients with ICAD. Secondly, pain is a much more prominent symptom in ICAD compared to patients with severe carotid atherosclerosis. The history of trauma, even minor, should make dissection higher on the differential diagnosis. Fortunately, the imaging studies to evaluate for these two diseases are the same.
Assessing Pupillary Asymmetry and Ptosis
Careful attention must be paid to assessing a patient for pupillary asymmetry. In a patient with anisocoria, determining the abnormal pupil may require examination of the patient in both bright and dark lighting conditions. The first step is to examine the patient’s pupils under normal lighting conditions. The next step is to assess each pupil’s response to shining a bright light in each eye. The abnormal pupil is the pupil that does not respond well or at all to bright light shone directly in the eye. If the anisocoria is greatest in bright light, the larger pupil is the abnormal pupil. When the anisocoria is greater in dark conditions, the smaller pupil is the abnormal pupil. In this case, the patient’s abnormal pupil was incorrectly diagnosed as the contralateral larger pupil (ie, left)—highlighting the importance of performing a complete pupillary examination in all patients presenting with neurological symptoms.9
Furthermore, as demonstrated in this case, ptosis in a patient with Horner syndrome caused by an ICAD can be subtle. The ptosis is the result of paralysis of Müeller’s muscle, which is innervated by the sympathetic pathway. The levator palpebrae superioris, which causes the more profound ptosis seen in third nerve palsies, is unaffected.10
Imaging Studies
Once the diagnosis of ICAD is suspected, appropriate vascular imaging must be obtained. Digital subtraction angiography has historically been the gold standard for vascular imaging of the neck vessels, but it has largely been replaced by less invasive and more readily available imaging modalities such as CTA and MRI/MRA.11
Computed Tomography Angiography. This is a widely available, rapid imaging choice and has a sensitivity of 80% to 95% in the detection of ICAD.7 It has a greater ability than MRI to identify dissection features such as intimal flaps, pseudoaneurysms, and high-grade stenosis versus occlusion. One of its disadvantages is the need for iodinated contrast, which can limit the ability to obtain the test in those with renal disease or patients with true allergies to IV contrast material. In addition, a mural hematoma can be mistaken for a noncalcified atherosclerotic plaque in the vessel lumen.6
Magnetic Resonance Imaging and Magnetic Resonance Angiography. Both MRI and MRA are also frequently used to diagnose ICAD. The intramural hematoma displays a hyperdense signal on T1-weighted images and has a characteristic crescent shape adjacent to the lumen.11 Magnetic resonance imaging studies are also sensitive in detecting cerebral ischemia resulting from the dissection. However, the sensitivity of MRI/MRA is highest 2 days after the dissection has occurred.2
Doppler Ultrasound. This is another imaging modality used to detect ICAD—one that is noninvasive, less expensive, requires no contrast material, and is widely available. Limitations of Doppler ultrasound include the inability to scan the distal ICA and a lower sensitivity in detecting dissections that cause low-grade stenosis.2 It is more commonly used for follow-up monitoring of dissections.
Management
There are several options for managing ICAD. In patients with unstable lesions, progressing neurological deficit, or further strokes, endovascular stenting has been shown to have a technical success rate of 99% and a procedural complication rate of 1.3%.12 Similarly, if the patient exhibits symptoms of cerebral ischemia, severe narrowing of the arterial lumen, or an unstable plaque, IV heparin is frequently used, followed by warfarin.13 However, since the majority of carotid and vertebral artery dissections heal spontaneously,14 antithrombotic therapies, including aspirin, clopidogrel or warfarin, are often prescribed to prevent thromboembolic complications.
Conclusion
Diagnosing ICAD requires knowledge of the typical history and presenting features of the disease. Careful attention to the ocular examination must be undertaken in any patient presenting with headache or face or neck pain, because the findings can be subtle. In a patient in whom ICAD is suspected, imaging with CTA or MRI/MRA should be performed. Early consultation with vascular surgery services can help determine the most appropriate treatment strategy.
Internal carotid artery dissection (ICAD) is an uncommon cause of stroke that typically occurs in the setting of (often minor) trauma but can also occur spontaneously. Patients with ICAD typically present with ipsilateral head, face, or neck pain. In approximately half of ICAD cases, an acute partial, painful Horner syndrome is present on examination. Although computed tomography angiography (CTA) is currently the imaging study of choice, magnetic resonance imaging/magnetic resonance angiography (MRI/MRA) and Doppler ultrasound can also be used. Management options include thrombolysis, antiplatelet or anticoagulation therapy, and endovascular or surgical intervention.
Case
A 56-year-old man with a history of migraines presented to the ED with a chief complaint of a 4-day history of right-sided headache. He stated that the pain felt different from his usual migraines and was located behind his right eye. Prior to presentation at the ED, the patient had initially visited an urgent care facility for evaluation. The physician who evaluated the patient at the urgent care facility noted the patient’s left eye appeared dilated and referred him to the ED for evaluation.
The patient further stated that the day prior to presentation, one of his friends had also remarked that the patient’s left eye appeared to be enlarged. The patient denied any visual disturbances, focal weakness, nausea, vomiting, neck pain, or stiffness. His medical history was significant for paroxysmal atrial flutter and hypertension. Regarding medications, the patient was taking dronedarone, clonazepam, omeprazole, and metoprolol.
On physical examination, the patient’s vital signs were: blood pressure, 162/109 mm Hg; heart rate, 85 beats/minute and regular; respiratory rate, 18 breaths/minute; and temperature, 98.6°F. Oxygen saturation was 98% on room air. The patient appeared to be in no acute distress. Ocular examination revealed a dilated left pupil of approximately 5 to 6 mm, and a right pupil measuring approximately 3 mm. Both pupils reacted to light, and the extraocular muscles were intact. The patient’s face appeared symmetrical and had intact sensation. He had normal speech, midline tongue, and good bilateral shoulder shrug. The neck examination revealed normal range of motion with full flexion, without jugular vein distention, lymphadenopathy, or palpable thyroid. The cardiovascular, lung, and abdominal examinations were all normal. The neurological examination showed the patient to be awake, alert, and oriented to person, place, and time. He exhibited 5/5 motor strength in all four extremities, normal gait, and normal finger-to-nose performance; his reflexes were 2+ and symmetrical.
The emergency physician (EP) ordered a stat noncontrast CT scan of the head, complete blood count (CBC), and basic metabolic panel (BMP). The CT scan of the head was interpreted by the radiologist as “no acute intracranial abnormality,” and the CBC and the BMP were normal. Since the EP was concerned about a carotid artery aneurysm or dissection, he ordered a CTA of the head and neck (Figures 1 and 2). The CTA was read as:
Long segment dissection of postbulbar cervical segment of right internal carotid artery, very likely extending into the intracranial segment with critical stenosis at the junction of the high cervical segment and proximal petrous segment. Preserved runoff circulation to the anterior and middle cerebral arteries with robust capacity for collateral support in the context of patent anterior and posterior communicating arteries.
Based on the radiology report, the EP consulted with vascular surgery services, and ordered a Doppler study of the ICAs. The Doppler study demonstrated “arterial thrombus in the right distal extracranial internal carotid artery with hemodynamics suggestive of a distal occlusion/significant obstruction.” The patient was started on an intravenous (IV) heparin drip and admitted to the hospital. Three days later he experienced sudden onset of left arm weakness. An emergent CTA of the head and neck revealed an embolic occlusion of mid-to-distal M1 segment of the right middle cerebral artery. The patient was immediately evaluated by interventional radiology for possible clot removal; however, based on his rapid neurological improvement, he was instead treated medically with aspirin and clopidogrel and continued to show significant neurological improvement. He was discharged home on hospital day 8 on both antiplatelet agents with minimal neurological deficit.
Discussion
Even though ICAD accounts for only 1% to 2% of all strokes, it is responsible for 10% to 25% of strokes in young and middle-aged adults.1-3 The peak incidence for ICAD is in the fifth decade, and it affects men and women equally.4 The extracranial portion of the ICA is the most commonly affected vessel (>90%); dissections of the intracranial portion are associated with greater neurological deficits and have a poorer prognosis.2,5 Cerebral ischemia resulting from a dissection of the extracranial ICA may occur days to weeks after the onset of local symptoms such as head or neck pain, Horner syndrome, or tinnitus.5
In ICAD, a tear in the artery wall causes blood to enter the tunica media of the vessel, forming an intramural hematoma.2,6 This may result in either stenosis of the lumen of the vessel from the enlarging hematoma or an outward aneurysmal dilatation of the vessel that compresses surrounding structures.2,6 Subsequent cerebral ischemia is the result of either arterial embolism or hemodynamic compromise from vessel stenosis.2,3
Causes
Dissections often occur secondary to trauma, though the severity of the trauma may be quite minor.2,6 Seemingly trivial mechanisms that have been associated with dissections include nose blowing, coughing, sudden neck turning, and prolonged telephone conversations. Other known causes are motor vehicle accidents and chiropractic maneuvers. Inherited connective tissue disorders, including Ehlers-Danlos syndrome, Marfan syndrome, fibromuscular dysplasia, and osteogenesis imperfecta, are associated with spontaneous ICAD.2,6
Symptoms
The most common presenting symptom of carotid dissection in approximately two-thirds of patients is ipsilateral head, face, or neck pain,2,6 typically described as sharp, constant, and nonthrobbing. Patients report a subjective bruit in 21% to 39% of cases.7 An acute partial painful Horner syndrome is strongly associated with ICAD, but is present in fewer than half of such patients.6 It is characterized by miosis and ptosis and is the result of compression of the ascending sympathetic fibers that travel alongside the ICA within the carotid sheath. Anhidrosis is not present because the fibers for sweat function in the face travel along the external carotid artery.2
Differential Diagnosis
While there is overlap between strokes caused by ICAD versus plaque, some features can help guide the clinician. Symptomatic carotid disease will frequently present with a history of one or more transient ischemic attacks characterized by focal neurological dysfunction or transient monocular blindness—typically within the previous 6 months.8 This history is not usually present in patients with ICAD. Secondly, pain is a much more prominent symptom in ICAD compared to patients with severe carotid atherosclerosis. The history of trauma, even minor, should make dissection higher on the differential diagnosis. Fortunately, the imaging studies to evaluate for these two diseases are the same.
Assessing Pupillary Asymmetry and Ptosis
Careful attention must be paid to assessing a patient for pupillary asymmetry. In a patient with anisocoria, determining the abnormal pupil may require examination of the patient in both bright and dark lighting conditions. The first step is to examine the patient’s pupils under normal lighting conditions. The next step is to assess each pupil’s response to shining a bright light in each eye. The abnormal pupil is the pupil that does not respond well or at all to bright light shone directly in the eye. If the anisocoria is greatest in bright light, the larger pupil is the abnormal pupil. When the anisocoria is greater in dark conditions, the smaller pupil is the abnormal pupil. In this case, the patient’s abnormal pupil was incorrectly diagnosed as the contralateral larger pupil (ie, left)—highlighting the importance of performing a complete pupillary examination in all patients presenting with neurological symptoms.9
Furthermore, as demonstrated in this case, ptosis in a patient with Horner syndrome caused by an ICAD can be subtle. The ptosis is the result of paralysis of Müeller’s muscle, which is innervated by the sympathetic pathway. The levator palpebrae superioris, which causes the more profound ptosis seen in third nerve palsies, is unaffected.10
Imaging Studies
Once the diagnosis of ICAD is suspected, appropriate vascular imaging must be obtained. Digital subtraction angiography has historically been the gold standard for vascular imaging of the neck vessels, but it has largely been replaced by less invasive and more readily available imaging modalities such as CTA and MRI/MRA.11
Computed Tomography Angiography. This is a widely available, rapid imaging choice and has a sensitivity of 80% to 95% in the detection of ICAD.7 It has a greater ability than MRI to identify dissection features such as intimal flaps, pseudoaneurysms, and high-grade stenosis versus occlusion. One of its disadvantages is the need for iodinated contrast, which can limit the ability to obtain the test in those with renal disease or patients with true allergies to IV contrast material. In addition, a mural hematoma can be mistaken for a noncalcified atherosclerotic plaque in the vessel lumen.6
Magnetic Resonance Imaging and Magnetic Resonance Angiography. Both MRI and MRA are also frequently used to diagnose ICAD. The intramural hematoma displays a hyperdense signal on T1-weighted images and has a characteristic crescent shape adjacent to the lumen.11 Magnetic resonance imaging studies are also sensitive in detecting cerebral ischemia resulting from the dissection. However, the sensitivity of MRI/MRA is highest 2 days after the dissection has occurred.2
Doppler Ultrasound. This is another imaging modality used to detect ICAD—one that is noninvasive, less expensive, requires no contrast material, and is widely available. Limitations of Doppler ultrasound include the inability to scan the distal ICA and a lower sensitivity in detecting dissections that cause low-grade stenosis.2 It is more commonly used for follow-up monitoring of dissections.
Management
There are several options for managing ICAD. In patients with unstable lesions, progressing neurological deficit, or further strokes, endovascular stenting has been shown to have a technical success rate of 99% and a procedural complication rate of 1.3%.12 Similarly, if the patient exhibits symptoms of cerebral ischemia, severe narrowing of the arterial lumen, or an unstable plaque, IV heparin is frequently used, followed by warfarin.13 However, since the majority of carotid and vertebral artery dissections heal spontaneously,14 antithrombotic therapies, including aspirin, clopidogrel or warfarin, are often prescribed to prevent thromboembolic complications.
Conclusion
Diagnosing ICAD requires knowledge of the typical history and presenting features of the disease. Careful attention to the ocular examination must be undertaken in any patient presenting with headache or face or neck pain, because the findings can be subtle. In a patient in whom ICAD is suspected, imaging with CTA or MRI/MRA should be performed. Early consultation with vascular surgery services can help determine the most appropriate treatment strategy.
1. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
2. Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81(956):383-388.
3. Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29(12):2646-2648.
4. Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330(6):393-397.
5. Biousse V, D’Anglejan-Chatillon J, Touboul PJ, Amarenco P, Bousser MG. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. 1995;26(2):235-239.
6. Kasravi N, Leung A, Silver I, Burneo JG. Dissection of the internal carotid artery causing Horner syndrome and palsy of cranial nerve XII. CMAJ. 2010;182(9):E373-E377.
1. Borgman CJ. Horner syndrome secondary to internal carotid artery dissection after a short-distance endurance run: a case study and review. J Optom. 2012;5:209-216.
2. Mohler ER III, Fairman RM. Management of symptomatic carotid atherosclerotic disease. UpToDate Web site. http://www.uptodate.com/contents/management-of-symptomatic-carotid-atherosclerotic-disease. Updated February 24, 2016. Accessed May 6, 2016.
3. Mann J. Anisocoria guidemap. Life in the Fastlane Web site. http://lifeinthefastlane.com/resources/jeff-manns-em-guidemaps/anisocoria-guidemap/. Accessed March 15, 2016.
10. Kedar S, Biousse V, Newman NJ. Horner syndrome. UpToDate Web site. http://www.uptodate.com/contents/horner-syndrome. Updated July 14, 2015. Accessed May 6, 2016.
11. Vertinsky AT, Schwartz NE, Fischbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
12. Pham MH, Rahme RJ, Arnaout O, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 2011;68(4):856-866.
13. Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
14. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.
1. CADISS trial investigators, Markus HS, Hayter E, et al. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): a randomised trial. Lancet Neurol. 2015;14(4):361-367.
2. Thanvi B, Munshi SK, Dawson SL, Robinson TG. Carotid and vertebral artery dissection syndromes. Postgrad Med J. 2005;81(956):383-388.
3. Lucas C, Moulin T, Deplanque D, Tatu L, Chavot D. Stroke patterns of internal carotid artery dissection in 40 patients. Stroke. 1998;29(12):2646-2648.
4. Schievink WI, Mokri B, O’Fallon WM. Recurrent spontaneous cervical-artery dissection. N Engl J Med. 1994;330(6):393-397.
5. Biousse V, D’Anglejan-Chatillon J, Touboul PJ, Amarenco P, Bousser MG. Time course of symptoms in extracranial carotid artery dissections. A series of 80 patients. Stroke. 1995;26(2):235-239.
6. Kasravi N, Leung A, Silver I, Burneo JG. Dissection of the internal carotid artery causing Horner syndrome and palsy of cranial nerve XII. CMAJ. 2010;182(9):E373-E377.
1. Borgman CJ. Horner syndrome secondary to internal carotid artery dissection after a short-distance endurance run: a case study and review. J Optom. 2012;5:209-216.
2. Mohler ER III, Fairman RM. Management of symptomatic carotid atherosclerotic disease. UpToDate Web site. http://www.uptodate.com/contents/management-of-symptomatic-carotid-atherosclerotic-disease. Updated February 24, 2016. Accessed May 6, 2016.
3. Mann J. Anisocoria guidemap. Life in the Fastlane Web site. http://lifeinthefastlane.com/resources/jeff-manns-em-guidemaps/anisocoria-guidemap/. Accessed March 15, 2016.
10. Kedar S, Biousse V, Newman NJ. Horner syndrome. UpToDate Web site. http://www.uptodate.com/contents/horner-syndrome. Updated July 14, 2015. Accessed May 6, 2016.
11. Vertinsky AT, Schwartz NE, Fischbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29(9):1753-1760.
12. Pham MH, Rahme RJ, Arnaout O, et al. Endovascular stenting of extracranial carotid and vertebral artery dissections: a systematic review of the literature. Neurosurgery. 2011;68(4):856-866.
13. Caplan LR. Dissections of brain-supplying arteries. Nat Clin Pract Neurol. 2008;4(1):34-42.
14. Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344(12):898-906.