User login
SAN DIEGO – A vaccine designed to lower the risk of postoperative Staphylococcus aureus infections is being tested in a phase IIb study of adults who are preparing to undergo elective spinal fusion surgery.
In phase I-II trial results, reported at an annual scientific meeting on infectious diseases, the vaccine proved to be well tolerated, and it induced durable functional antibody responses.
The phase IIb trial aims to enroll 2,600 patients undergoing elective spinal fusion surgery, and is scheduled to end in 2017, said Dr. Buddy Creech of Vanderbilt University in Nashville, Tenn.
“We’re at a point where the disease frequency of Staphylococcus aureus is high enough that we could make the case for universal vaccination,” said Dr. Creech. “If we can prove it in high-risk hosts, I think that’s a win.”
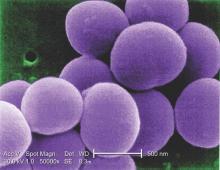
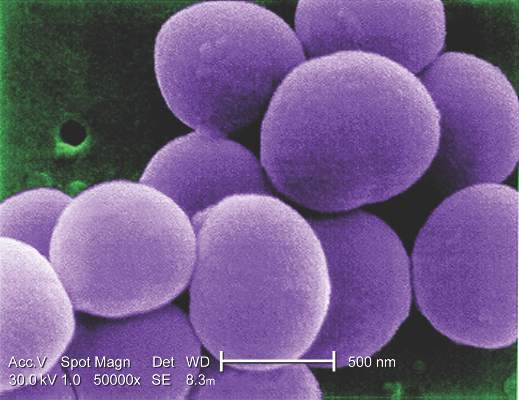
Invasive staphylococcal disease causes more deaths in the United States than AIDS, viral hepatitis, and tuberculosis combined, but the complex virulence factors of S. aureus have eluded vaccine researchers for years. Other investigational vaccines failed in previous large phase III trials. The tetravalent vaccine now being tested targets type 5 and 8 capsular polysaccharides (CP5 and CP8), which are expressed by 95% of hospital-associated S. aureus strains; an adhesion molecule; and an essential recombinant manganese transport protein C.
The vaccine was well tolerated in a phase I study of healthy adults earlier this year. Single doses of vaccine or placebo were administered to 285 healthy adults aged 65-84 years in a randomized, multicenter double-blind trial. Patients averaged 71 years in age, and half were women, Dr. Creech said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Similar rates of mild to moderate adverse events occurred in both groups, and no participants died or had serious vaccine-related side effects, Dr. Creech said.
At 1 month after vaccination, all tetravalent vaccine recipients and 5% of placebo recipients had achieved a predetermined threshold for CP5 opsonophagocytic activity. Likewise, about 92% of vaccinated recipients and 19% of placebo recipients achieved the CP8 opsonophagocytic activity threshold. Further, 84% of vaccinated recipients and none of the placebo recipients had at least a fourfold rise in antibody titers against the adhesion molecule.
In vaccine recipients, an immunoassay found substantial rises in antibodies against all four antigens by day 8; levels peaked around day 15, Dr. Creech said. “There was a decline of antibody titers over the course of the next year, as one would expect with a single-dose vaccine, but they remained over prespecified thresholds,” he added.
“Durability of response is especially important for patients who are scheduling surgeries and others who have a definitive risk period” for infection, he said.
Pfizer is funding the research, and Dr. Creech is a grant investigator for the company. One coauthor is also a grant investigator for Pfizer, nine coauthors are Pfizer employees and shareholders, and three declared no relevant conflicts of interest.
SAN DIEGO – A vaccine designed to lower the risk of postoperative Staphylococcus aureus infections is being tested in a phase IIb study of adults who are preparing to undergo elective spinal fusion surgery.
In phase I-II trial results, reported at an annual scientific meeting on infectious diseases, the vaccine proved to be well tolerated, and it induced durable functional antibody responses.
The phase IIb trial aims to enroll 2,600 patients undergoing elective spinal fusion surgery, and is scheduled to end in 2017, said Dr. Buddy Creech of Vanderbilt University in Nashville, Tenn.
“We’re at a point where the disease frequency of Staphylococcus aureus is high enough that we could make the case for universal vaccination,” said Dr. Creech. “If we can prove it in high-risk hosts, I think that’s a win.”
Invasive staphylococcal disease causes more deaths in the United States than AIDS, viral hepatitis, and tuberculosis combined, but the complex virulence factors of S. aureus have eluded vaccine researchers for years. Other investigational vaccines failed in previous large phase III trials. The tetravalent vaccine now being tested targets type 5 and 8 capsular polysaccharides (CP5 and CP8), which are expressed by 95% of hospital-associated S. aureus strains; an adhesion molecule; and an essential recombinant manganese transport protein C.
The vaccine was well tolerated in a phase I study of healthy adults earlier this year. Single doses of vaccine or placebo were administered to 285 healthy adults aged 65-84 years in a randomized, multicenter double-blind trial. Patients averaged 71 years in age, and half were women, Dr. Creech said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Similar rates of mild to moderate adverse events occurred in both groups, and no participants died or had serious vaccine-related side effects, Dr. Creech said.
At 1 month after vaccination, all tetravalent vaccine recipients and 5% of placebo recipients had achieved a predetermined threshold for CP5 opsonophagocytic activity. Likewise, about 92% of vaccinated recipients and 19% of placebo recipients achieved the CP8 opsonophagocytic activity threshold. Further, 84% of vaccinated recipients and none of the placebo recipients had at least a fourfold rise in antibody titers against the adhesion molecule.
In vaccine recipients, an immunoassay found substantial rises in antibodies against all four antigens by day 8; levels peaked around day 15, Dr. Creech said. “There was a decline of antibody titers over the course of the next year, as one would expect with a single-dose vaccine, but they remained over prespecified thresholds,” he added.
“Durability of response is especially important for patients who are scheduling surgeries and others who have a definitive risk period” for infection, he said.
Pfizer is funding the research, and Dr. Creech is a grant investigator for the company. One coauthor is also a grant investigator for Pfizer, nine coauthors are Pfizer employees and shareholders, and three declared no relevant conflicts of interest.
SAN DIEGO – A vaccine designed to lower the risk of postoperative Staphylococcus aureus infections is being tested in a phase IIb study of adults who are preparing to undergo elective spinal fusion surgery.
In phase I-II trial results, reported at an annual scientific meeting on infectious diseases, the vaccine proved to be well tolerated, and it induced durable functional antibody responses.
The phase IIb trial aims to enroll 2,600 patients undergoing elective spinal fusion surgery, and is scheduled to end in 2017, said Dr. Buddy Creech of Vanderbilt University in Nashville, Tenn.
“We’re at a point where the disease frequency of Staphylococcus aureus is high enough that we could make the case for universal vaccination,” said Dr. Creech. “If we can prove it in high-risk hosts, I think that’s a win.”
Invasive staphylococcal disease causes more deaths in the United States than AIDS, viral hepatitis, and tuberculosis combined, but the complex virulence factors of S. aureus have eluded vaccine researchers for years. Other investigational vaccines failed in previous large phase III trials. The tetravalent vaccine now being tested targets type 5 and 8 capsular polysaccharides (CP5 and CP8), which are expressed by 95% of hospital-associated S. aureus strains; an adhesion molecule; and an essential recombinant manganese transport protein C.
The vaccine was well tolerated in a phase I study of healthy adults earlier this year. Single doses of vaccine or placebo were administered to 285 healthy adults aged 65-84 years in a randomized, multicenter double-blind trial. Patients averaged 71 years in age, and half were women, Dr. Creech said at the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
Similar rates of mild to moderate adverse events occurred in both groups, and no participants died or had serious vaccine-related side effects, Dr. Creech said.
At 1 month after vaccination, all tetravalent vaccine recipients and 5% of placebo recipients had achieved a predetermined threshold for CP5 opsonophagocytic activity. Likewise, about 92% of vaccinated recipients and 19% of placebo recipients achieved the CP8 opsonophagocytic activity threshold. Further, 84% of vaccinated recipients and none of the placebo recipients had at least a fourfold rise in antibody titers against the adhesion molecule.
In vaccine recipients, an immunoassay found substantial rises in antibodies against all four antigens by day 8; levels peaked around day 15, Dr. Creech said. “There was a decline of antibody titers over the course of the next year, as one would expect with a single-dose vaccine, but they remained over prespecified thresholds,” he added.
“Durability of response is especially important for patients who are scheduling surgeries and others who have a definitive risk period” for infection, he said.
Pfizer is funding the research, and Dr. Creech is a grant investigator for the company. One coauthor is also a grant investigator for Pfizer, nine coauthors are Pfizer employees and shareholders, and three declared no relevant conflicts of interest.
AT IDWEEK 2015
Key clinical point: A four-antigen vaccine against Staphylococcus aureus yielded durable functional antibody responses and no major safety signals in a phase I-II trial.
Major finding: There were no vaccine-related serious adverse effects and no signs of dose-related reactogenicity, and researchers observed sustained bacterial killing at month 12.
Data source: A multicenter, randomized, double-blind placebo-controlled trial of 285 healthy adults aged 65-84 years.
Disclosures: Pfizer is funding the research. Dr. Creech is a grant investigator for the company. One coauthor is also a grant investigator for Pfizer, nine coauthors are Pfizer employees and shareholders, and three declared no relevant conflicts of interest.