User login
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
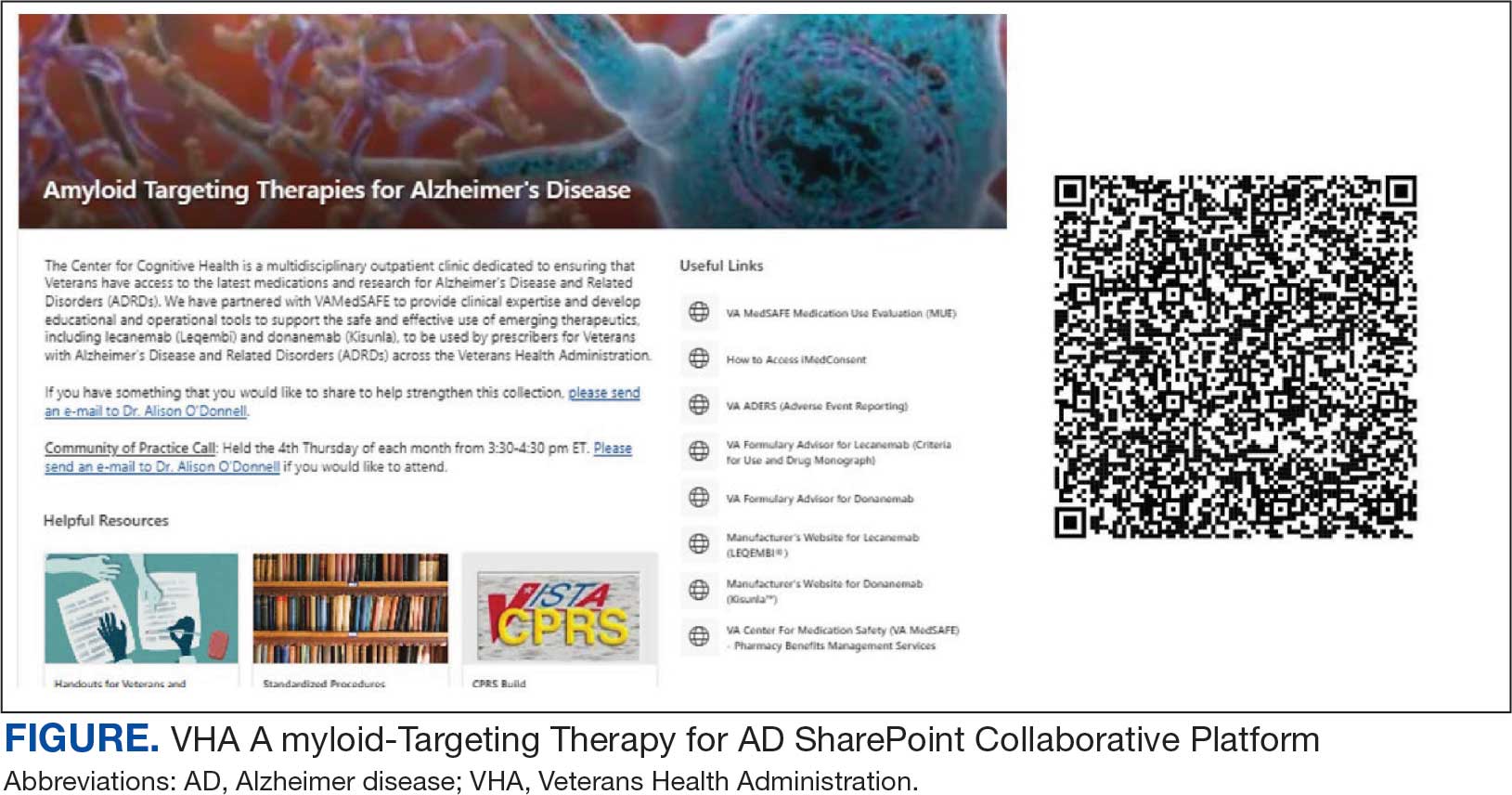
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.
Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.
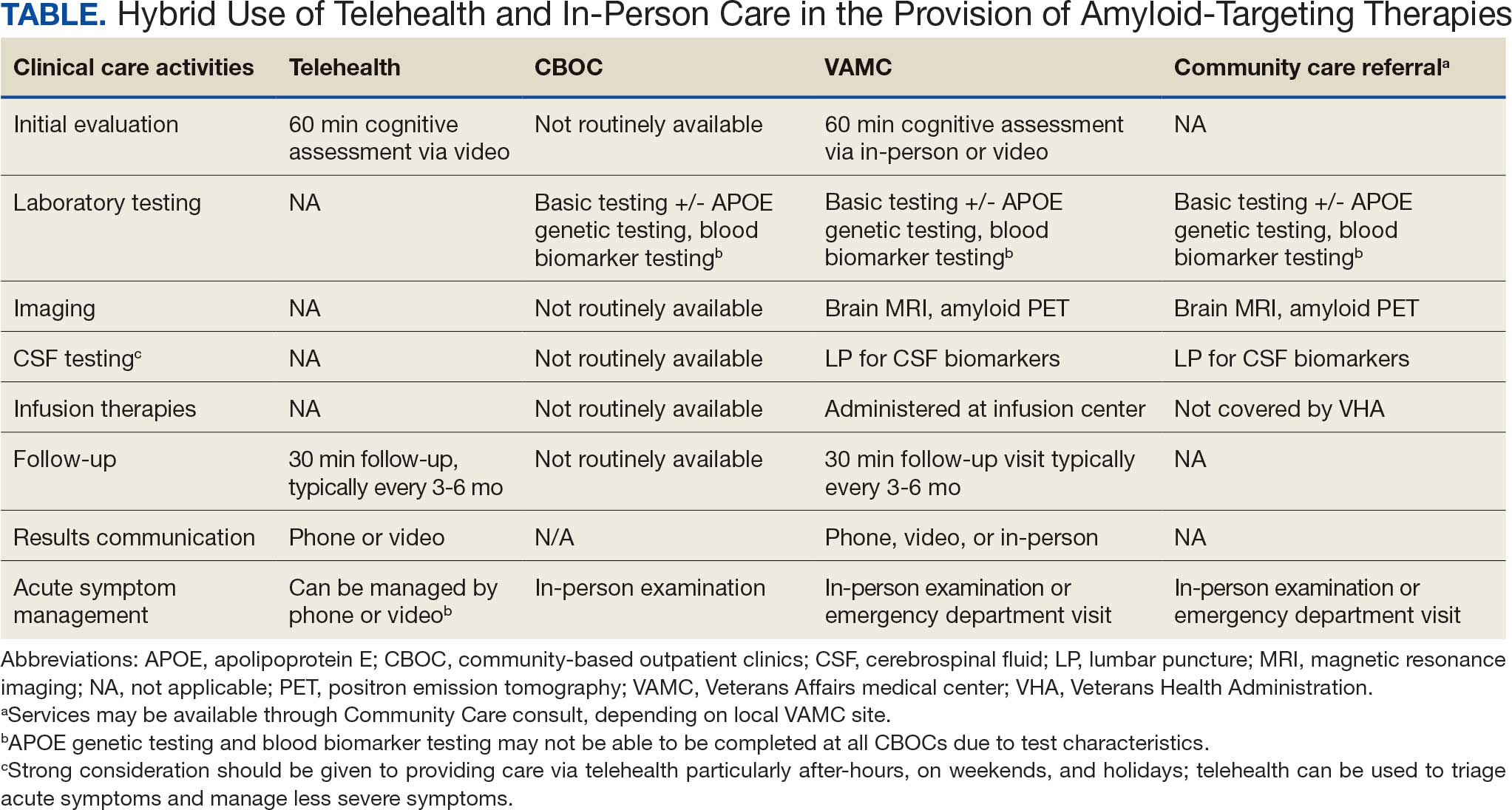
The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.

Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.

The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
The Veterans Health Administration (VHA) is the largest US integrated health care system, providing health care to > 9 million veterans annually. Dementia affects > 7.2 million Americans, and an estimated 450,000 veterans live with Alzheimer disease (AD).1,2 Compared with the general population, veterans have a higher burden of chronic medical conditions and are disproportionately affected by AD due to exposure to military-related risk factors (eg, traumatic brain injury and posttraumatic stress disorder) and the high prevalence of nonmilitary risk factors, such as cardiovascular disease. The VHA is a pioneer in dementia care, having established a Dementia System of Care to provide primary and specialty care to veterans with dementia. The VHA also is leading the way in implementing the Institute for Healthcare Improvement Age-Friendly Health Systems (AFHS) framework for providing goal-concordant care in > 100 VHA medical centers. The VHA aims to be the largest AFHS in the country.
AD profoundly affects individuals and their families. The progressive nature of the most common form of dementia diminishes the quality of life for patients as well as their care partners in an ongoing fashion, often leading to emotional, physical, and financial strain. Costs for health and long-term care for people living with AD and other dementias were projected at $360 billion in 2024, largely due to the need for nursing home care.1 Although several oral medications are available, their capacity to effectively mitigate the negative effects of AD is limited. Cholinesterase inhibitors and memantine may offer temporary symptomatic relief, but they do not alter disease progression.3 The use of these agents is relatively low, with about one-third of patients diagnosed with AD receiving these medications.4
Amyloid-Targeting Therapies
Recent advancements in biologics, particularly amyloid-targeting therapies, such as lecanemab and donanemab, offer new hope for managing AD. Older adults treated with these medications show less decline on measures of cognition and function than those receiving a placebo at 18 months.5,6 However, accessing and using these medications is challenging.
Use of amyloid-targeting therapies poses challenges. The medications are expensive, potentially placing a financial burden on patients, families, and health care systems.7 Determining initial eligibility for treatment requires a battery of cognitive assessments, laboratory tests, advanced radiologic studies (eg, magnetic resonance imaging [MRI] of the brain and amyloid positron emission tomography [PET] scans), and possible cerebrospinal fluid (CSF) testing. Frequent ongoing assessments are necessary to monitor safety and efficacy. These treatments carry substantial risks, particularly amyloid-related imaging abnormalities (ARIA) such as cerebral edema, microhemorrhages, and superficial siderosis. Therefore, follow-up assessments typically occur around months 2, 3, 4, and 7, depending on which medication is selected. Finally, at present, both agents must be intravenous (IV)-administered in a monitored clinical setting, which requires additional coordination, transportation, and cost.
Ongoing evaluations and in-person administration particularly affect patients and care partners with limitations regarding transportation, time off work, and navigating complex health care systems.8 VHA clinicians at sites that have implemented or are interested in implementing amyloid-targeting therapy programs endorse similar challenges when implementing these therapies in their US Department of Veterans Affairs (VA) medical centers (VAMCs).9
The VHA was one of the first health care systems to use amyloid-targeting therapies, covering the cost of lecanemab and donanemab, in addition to costs associated with concomitant evaluation and testing. However, given the safety concerns with this novel class of medications, the VHA National Formulary Committee developed criteria for use and recommended the VA Center for Medication Safety (VAMedSAFE) conduct a mandatory real-time medication use evaluation (MUE). VAMedSAFE developed the MUE to monitor the safe and appropriate use of amyloid-targeting therapy for AD. Two authors (AJO, SMH) partnered with VAMedSAFE through the VA Pittsburgh Healthcare System Technology Enhancing Cognition and Health–Geriatric Research, Education, and Clinical Center (TECH-GRECC) to provide clinical expertise, substantive feedback for the development of the MUE, and guidance for VHA sites starting amyloid targeting-therapy programs. We started a VHA Amyloid-Targeting Therapy for AD SharePoint collaborative platform and VHA AD Therapeutics Community of Practice (CoP) for shared learning (Figure). The private SharePoint platform houses an array of implementation materials for VAMCs starting programs: key documents and links; educational materials; sample guidelines; note templates; and electronic health record screenshots. The CoP allows VHAs to share best practices and discuss challenges.

Even with these advantages, we found that ensuring the safe and appropriate use of amyloid-targeting therapies did not overcome the barriers associated with their complexity. This was especially true for veterans living in rural areas. Only 4 VAMCs had administered amyloid-targeting therapies in the first year they were available. Preliminary data demonstrated that 27 (84%) of 32 veterans who initiated lecanemab in the VHA between October 2023 and September 2024 resided in urban areas.10 To address the underutilization of amyloid-targeting therapy, we propose leveraging the strengths of VHA telehealth to facilitate expansion of access to these medications for veterans with early AD. Telehealth may substantially increase access to evaluation for veterans with early dementia and, when medically appropriate, to receive amyloid-targeting therapies by reducing transportation needs and mitigating costs while ensuring appropriate monitoring through ongoing clinical assessments.
Using Telehealth
The VHA is a pioneer in telehealth, with programs dating back to 2003.11 Between October 1, 2018, and September 30, 2019, the VHA served > 900,000 veterans through the provision of > 2.6 million episodes of care via telehealth.12 The COVID-19 pandemic further cemented the role of telemedicine as an essential component of health care. Telehealth has demonstrated success in the assessment and management of individuals living with dementia. At the VHA, the GRECC-Connect Project is a partnership between 9 urban GRECC sites that seek to provide consultative geriatric and dementia care to rural veterans through telehealth.13 Additional evidence supports the potential to leverage telehealth to effectively communicate results of amyloid PET scans.14
This approach is not without limitations such as the digital divide, or the gap that separates technology-enabled individuals and those unprepared to adopt technology due to limited digital literacy levels or access to needed hardware, software, and connectivity. The VHA has taken steps to address these digital divide barriers by broadly providing tools—such as tablets and broadband connectivity—to veterans. Specifically, the VHA has instituted digital divide consults to determine whether telehealth could be a potential solution for appropriate veterans and to provide an iPad (if eligible) to connect with VA clinicians. Complementary to the digital divide consult, a VHA-specific telehealth preparedness assessment tool is under development and being tested by 2 authors (JF, SMH). This telehealth preparedness assessment tool is designed to aid in the seamless integration of telehealth services with the support of tailored education materials specific to gaps in digital literacy that a veteran might experience.
Building on these initiatives, there is an opportunity to expand access to amyloid-targeting therapies, regardless of distance to large VAMCs, by leveraging telehealth as an alternative method of connecting patients with specialty care. Specifically, a hybrid approach could be used to accomplish the myriad initial and follow-up tasks involved in the provision of amyloid-targeting therapies (Table). Not all VHA facilities possess the specialty expertise to prescribe these medications, and local clinicians may not have sufficient knowledge and clinical support to prescribe and monitor these therapies.

The first step is identifying local and regional subject matter experts, followed by the development and expansion of these networks. The National TeleNeurology Program is a good example of a national telehealth program that leverages technology to bring specialty services to rural areas with limited access to care. Although amyloid-targeting therapies often require more complex logistics, such as laboratory tests and imaging, these initial hurdles can be overcome through localized services and collaboration between VAMCs.
While treatment and imaging will most likely need to occur at a VAMC, most basic laboratory studies can be performed at community-based outpatient clinics (CBOCs). Some CBOCs may not be able to process more specialized laboratory tests such as apolipoprotein E genetic testing. Samples for these tests can be collected and processed at VAMCs, which usually have contracts with outside laboratories capable of performing these studies. Most, although not all, VAMCs offer advanced imaging, including MRI of the brain and amyloid PETs. VAMCs without those modalities may need to coordinate with other regional VAMCs. Additionally, a pilot program is already underway whereby VAMCs without the ability to quantify the amount of amyloid on PETs are able to leverage technology and collaborations with other VAMCs to obtain these data.
Once the initial phases of evaluation and care are completed, telemedicine can be leveraged for follow-up and ongoing management. Interdisciplinary teams can help facilitate care related to amyloid-targeting therapies, including the close monitoring of veterans for development of ARIA.15 To achieve this monitoring, specialty clinic teams prescribing amyloid-targeting therapies, which may be geographically distant, need to coordinate with local primary care clinical teams and emergency clinicians. All of these health care team members, along with neurologists and neurosurgeons, should be involved in the development and implementation of protocols in the event that patients present to their local primary or specialty care clinics or emergency department with ARIA symptoms.
If amyloid-targeting therapies are to be provided along with other emerging treatments for rural veterans, telehealth must be part of the solution. There is a pressing need to explore innovative evaluation and delivery models for these therapies, particularly as we expect additional diagnostics and therapeutics to be available in the future. With the advent of commercially available blood tests (ie, blood biomarkers) for AD, there is hope for a transition away from PETs and CSF testing given their cost, limited access, and invasiveness for diagnosis and monitoring of AD. These advances will increase the utility of telehealth to help rural veterans access amyloid-targeting therapies.
Additionally, administering the drug at home or at local clinics, supported by a dedicated health care team or home health agency, could further improve accessibility. Telehealth can be leveraged in this scenario, allowing specialty clinics and specialists to connect with patients and clinicians based out of local clinics or even home health agencies. In this scenario, specialists can provide hands-on care guidance and oversight even though they may be geographically distant from care recipients. Transitioning from IV administration to subcutaneous formulations would further enhance convenience and reduce barriers; these formulations may be available soon.16 Addressing logistical challenges to care and access through technology-based solutions will require coordinated efforts and continued VHA investment.
Conclusions
The VHA has a large population of veterans with dementia, and the costs to care for these veterans will only increase. While the current benefits of amyloid-targeting therapies are modest, now is the time to establish care processes that will support future innovations in amyloid-targeting therapies and other treatments and diagnostics. We are developing better ways to detect AD using clinical decision support tools, improving care pathways and the management of AD, and leveraging telehealth to improve access. The VA is conducting research to investigate whether a cognitive screening and laboratory evaluation that includes a telehealth preparedness assessment will be feasible and effective for improving the detection of AD and access to treatment, and we plan to publish the results.
The lessons learned can be extended to non-VHA care settings to help achieve potential benefits for other patients with early AD. Emerging therapies have the potential to improve the quality of life for both patients and care partners, adding life to years and not just years to life. Policymakers and payors must prioritize research funding to evaluate the safety and efficacy of these approaches to the delivery of health services, ensuring that emerging therapies are accessible for all individuals affected by AD.
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
- Alzheimer’s Association. 2025 Alzheimer’s disease facts and figures. Alzheimers Dement. 2025;21(4):e70235. doi:10.1002/alz.70235
- US Department of Veterans Affairs. Statistical Projections of Alzheimer’s Dementia for VA Patients, VA Enrollees, and US Veterans. December 18, 2020. Accessed November 2, 2025. https://www.va.gov/GERIATRICS/docs/VHA_ALZHEIMERS_DEMENTIA_Statistical_Projections_FY21_and_FY33_sgc121820.pdf
- Casey DA, Antimisiaris D, O’Brien J. Drugs for Alzheimer’s disease: are they effective? P T. 2010;35(4):208-211.
- Barthold D, Joyce G, Ferido P, et al. Pharmaceutical treatment for Alzheimer’s disease and related dementias: utilization and disparities. J Alzheimers Dis. 2020;76(2):579-589. doi:10.3233/JAD-200133
- Sims JR, Zimmer JA, Evans CD, et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512-527. doi:10.1001/jama.2023.13239
- van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388(1):9-21. doi:10.1056/NEJMoa2212948
- Tanne JH. Lecanemab: US Veterans Health Administration will cover cost of new Alzheimer’s drug. BMJ. 2023;380:p628. doi:10.1136/bmj.p628
- Nadeau SE. Lecanemab questions. Neurology. 2024;102(7):e209320. doi:10.1212/WNL.0000000000209320 9. O’Donnell AJ, Fortunato AT, Spitznogle BL, et al. Implementation of lecanemab for Alzheimer’s disease: facilitators and barriers. Presented at: American Geriatrics Society 2025 Annual Scientific Meeting, Chicago. May 2025.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- O’Donnell AJ, Zhao X, Parr A, et al. Use of lecanemab for Alzheimer’s disease within the Veteran’s Health Foundation: early findings. Abstract presented at: Alzheimer’s Association International Conference 2025; July 27, 2025; Toronto, Canada.
- Hopp F, Whitten P, Subramanian U, et al. Perspectives from the Veterans Health Administration about opportunities and barriers in telemedicine. J Telemed Telecare. 2006;12(8):404-409. doi:10.1258/135763306779378717
- VA reports significant increase in veteran use of telehealth services. News release. US Department of Veterans Affairs. November 22, 2019. Accessed November 19, 2025. https://news.va.gov/press-room/va-reports-significant-increase-in-veteran-use-of-telehealth-services/
- Powers BB, Homer MC, Morone N, et al. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
- Erickson CM, Chin NA, Rosario HL, et al. Feasibility of virtual Alzheimer’s biomarker disclosure: findings from an observational cohort. Alzheimers Dement (N Y). 2023;9(3):e12413. doi:10.1002/trc2.12413
- Turk KW, Knobel MD, Nothern A, et al. An interprofessional team for disease-modifying therapy in Alzheimer disease implementation. Neurol Clin Pract. 2024;14(6):e200346. doi:10.1212/CPJ.0000000000200346
- FDA accepts LEQEMBI® (lecanemab-irmb) biologics license application for subcutaneous maintenance dosing for the treatment of early Alzheimer’s disease. News release. Elsai US. January 13, 2025. Accessed November 2, 2025. https://media-us.eisai.com/2025-01-13-FDA-Accepts-LEQEMBI-R-lecanemab-irmb-Biologics-License-Application-for-Subcutaneous-Maintenance-Dosing-for-the-Treatment-of-Early-Alzheimers-Disease
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
Can Telehealth Improve Access to Amyloid-Targeting Therapies for Veterans Living With Alzheimer Disease?
Whole Health(y) Aging With Gerofit: The Development of a Pilot Wellness Program for Older Veterans
Whole Health(y) Aging With Gerofit: The Development of a Pilot Wellness Program for Older Veterans
About half of the > 9 million veterans served by the Veterans Health Administration (VHA) are aged ≥ 65 years.1 Veterans are at a higher risk for comorbidities, which may contribute to increased health care costs, mobility limitations and disability, poor quality of life, and mortality. 2-5 Programs and policies that promote health maintenance, independent living, and quality of life are needed among older veterans. To support veterans’ overall health and well-being, the VHA has shifted to whole health, a patient-centered care model.6
The whole health paradigm employs personalized, proactive, and patient-driven care, emphasizing complementary and integrative health practices, and prioritizing health promotion and disease prevention over disease treatment.7 The veteran is empowered to decide “what matters to [me],” reflect on life and health, and define mission, aspiration, and purpose. This approach gives veterans a more active and direct role in their care, distinguishing it from traditional care models. In turn, it helps reduce the burden on clinicians and fosters a more collaborative environment in which both the clinician and veteran work together to shape the care process.7 Veterans utilize the Circle of Health to identify skills and support needed to implement changes in self-care. The Circle of Health includes 8 self-care components: moving the body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind.6 This process drives the creation of a personal health plan, creating opportunities for individuals to engage in well-being programs that matter to them and help them meet their goals.
Gerofit is a VHA best practice and whole health outpatient exercise program for veterans aged ≥65 years.8 Gerofit has focused primarily on exercise within the moving the body self-care component.9 A longitudinal study followed 691 Gerofit participants across 6 US Department of Veterans Affairs (VA) medical centers who on average were 73 years old, had 16 different medical conditions, and took 10 medications. Most were obese and had a mean gait speed of 1.04 m/s, suggesting functional impairment.10 Prior studies have shown that Gerofit participation is associated with a range of health benefits. Two studies reported improvements in psychological well-being and sustained gains in endurance, strength, and flexibility following early Gerofit program participation. 11,12 A 10-year analysis of 115 veterans found that long-term Gerofit participation reduced mortality risk, while another study of 452 veterans showed decreased medication use following 1 year in the program.13,14
The VHA whole health model comprises 3 components: (1) The Pathway, (2) well-being programs, and (3) whole health clinical care.6 The Pathway engages veterans in identifying personal health goals, while well-being programs offer selfcare and skill-building activities. Traditional clinical settings often focus primarily on the third component due to time and resource constraints. The Gerofit platform addresses all 3 components. Its existing infrastructure, including a supportive community and dedicated facilities, provides a setting for implementing The Pathway and well-being programs. The Gerofit structure allows for the time and continuity necessary for these components, which are often limited during standard clinical visits.
By expanding the Gerofit exercise regimen to include additional wellness activities, it can holistically support older veterans. Research supports this integrative approach. For example, a 2020 study found that incorporating a holistic health program into an existing exercise program within a church setting led to improved physical activity and overall health among women participants.15 This article describes the integration of Whole Health(y) Aging with Gerofit (WHAG), a pilot program in Baltimore, Maryland, that integrates whole health components into the established Gerofit framework to enhance the overall well-being of participating veterans (Figure 1).

WHOLE HEALTH(Y) AGING WITH GEROFIT
Gerofit enrollment has been described elsewhere in detail.16 Patients aged ≥ 65 years are eligible to participate with clinician approval if they are medically stable. Following VHA clinician referral and primary care approval, veterans completed a telephone visit to determine eligibility and discuss their exercise history, goals, and preferences. Veterans dependent in activities of daily living and those with cognitive impairment, unstable angina, active proliferative diabetic retinopathy, oxygen dependence, frank incontinence, active open wounds, active substance abuse, volatile behavioral issues, or who are experiencing homelessness are not eligible for Gerofit.
The exercise physiologist identified veteran barriers and incentives to participation and assisted with a plan to maximize SMART goals (specific, measurable, achievable, relevant, and time-bound). Veterans then completed an assessment visit, either in person or virtually, depending on the selected programming. Functional assessments conducted by trained Gerofit exercise physiologists include testing of lower and upper body strength and submaximal endurance.9,17,18 Participation in Gerofit is voluntary and not time limited.
Prior to these newly expanded offerings, veterans could only enroll in a personalized, structured exercise program. Based on feedback from Gerofit participants indicating areas of interest, WHAG was developed to provide additional wellness offerings aligned with other Circle of Health components.6 This included virtual group nutrition education and cooking interventions with optional fresh produce delivery; wellness classes, the Companion Dog Fostering & Adoption program, and Gerofit in the Mind, which included mindfulness classes and relaxation seminars (Figure 1). Programs were virtual (except dog fostering and adoption) and rotated throughout the year. Not all programs are offered simultaneously.
Attendance, completion of selected questions from the individual Personal Health Inventory (PHI) Short Form, measured physical function, self-reported physical activity levels, physical and mental health status, and program satisfaction were measured for all WHAG subprograms.18 Selected questions from the PHI Short Form use a 5-point Likert scale to rate the following whole health components: physical activity; sleep, relaxation, and recovery; healthy eating habits; and positive outlook, healthy relationships, and caring for mental health. Physical function was assessed using 30-second arm curls (upper body strength), 30-second chair stands (lower body strength), and the 2-minute step test (virtual) or 6-minute walk test (in person) (submaximal cardiovascular endurance).
Self-reported physical activity was assessed by asking frequency (days per week) and duration (minutes per session) of cardiovascular and strength exercises to calculate total minutes per week. Physical and mental health status was assessed using the Patient Reported Outcomes Measurement Information System (PROMIS) Global Health Scale.19 Demographic data included sex, race and ethnicity, and age at baseline visit. Mean (SD) was calculated for continuous variables and presented unless otherwise specified, and frequencies were calculated for categorical variables. Subsequent reports will describe additional assessments and detailed outcomes unique to individual programs.
Overview
Veterans chose the programs that best suited their needs without limitations.7 Staff provided guidance on newly available programs based on an individual’s specified goals. Gerofit staff assisted veterans with development of individualized personal health plans, monitoring progress towards their goals, supporting program participation, and connecting veterans with additional whole health resources.
Gerofit Exercise Group. Exercise was designed to address the Moving the Body component of whole health. Veterans could elect to schedule 1-hour, 3-times-weekly in-person gym appointments, participate in 3-times-weekly livestreamed virtual group exercise classes through VA Video Connect, or receive a self-directed at-home exercise plan.
Gerofit Learning Opportunities for Wellness Classes. These virtual health education sessions addressed the personal development component of whole health and were designed to increase self-efficacy and empower veterans to take an active role in their health care. Topics focused broadly on issues related to healthy aging (eg, importance of sleep, goal setting, self-care, and comorbidity education). Veterans could participate in any classes of interest, which were led by health care professionals and offered twice monthly. Sessions encouraged participant questions and peer interaction.
Nutrition. Improving dietary quality is a frequently reported goal of Gerofit participants. WHAG incorporated multiple strategies to assist veterans in meeting these goals. For example, through a partnership with Therapeutic Alternative of Maryland Farm, Gerofit provided veterans free, locally grown fresh produce. This initiative addressed barriers to healthy eating by improving access to fresh produce, which has been shown to influence cooking frequency and diet quality.20-22 Participation in nutrition classes was not required. In 2021, veterans received produce weekly; however, many reported excess quantities. Beginning in 2022, veterans could select both produce items and quantities desired.
In addition, a registered dietitian led a 14-week virtual nutrition education program guided by the social cognitive theory framework and focused on self-regulation skills such as goal setting, overcoming barriers, and identifying triggers.23 Prior research highlighted low health literacy as a common barrier among older veterans, which informed several key components of the curriculum.24 These included how to read and interpret nutrition labels, define balanced meals and snacks, and understand the classification of various food groups such as fats, carbohydrates, and proteins. The online program curriculum included an instructor guide and participant materials for each individual lesson, including an educational handout on the specific week’s topic, applied activity (group or individual), and recipes related to the produce shares. Structured group discussion promoted camaraderie and recipe sharing, and additional instruction on produce preparation and storage.
Reported lack of self-efficacy and knowledge regarding produce preparation prompted a 5-week virtual cooking series, led by a medical student and supervised by a registered dietitian. Sessions combined brief nutrition education with live cooking demonstrations adapted from the VA Healthy Teaching Kitchen curriculum. Recipes emphasized low-cost, commonly found food items. The Healthy Teaching Kitchen modifications focused on Dietary Approaches to Stop Hypertension diets, diabetes, and the importance of protein for older adults. Participants were allowed time to discuss recipes and food preparation tips, and other household members were allowed to observe.
Dog Fostering and Adoption. Veterans could foster or adopt a rescue dog through a partnership with local rescue groups. This program allowed participating veterans to have a companion, which addressed the surroundings, moving the body, and spirit and soul whole health components. The Companion Dog Fostering and Adoption Program and results on physical function and daily physical activity from the first 3 months were recently published. Positive effects on physical activity, physical function, and quality of life were observed at 3 months as compared to baseline in veterans who received a companion dog.25
Gerofit in the Garden. Veterans could opt to receive an EarthBox containing soil and seedlings for 1 vegetable and 1 herb. The boxes are designed to fit on a small tabletop, regardless of home type or availability of backyard. In-person instruction for veterans on care and maintenance was provided by a farm employee with experience in gardening and farming practices.
Gerofit in the Mind. Online relaxation seminars were offered twice monthly for 4 months. Led by a certified sound health guide, sessions incorporated sound baths, crystal bowls, Tibetan bowls, tuning forks, and breath work. Virtual mindfulness classes led by a certified yoga instructor were offered weekly for 1 month. Veterans could drop in and participate based on their availability. Classes were designed to introduce veterans to the practice of mindfulness, improve mood, and lower stress and anxiety.
Pilot Program Outcomes
Sixteen male veterans participated in WHAG. Participants were 62% Black, with a mean age of 76 years. Veterans collaborated with Gerofit staff to develop personal health plans, which ultimately guided program participation (Figure 2).

Five participants enrolled in 1 WHAG program, 11 enrolled in 2, and 8 enrolled in ≥ 3 (Table 1). Sixteen veterans completed baseline testing and 12 completed 3-month follow-up assessments (Table 2). At baseline, participants were below the reference range for physical functioning and physical activity levels. After 3 months, improvements were observed in endurance self-reported physical activity, and strength with many values in the reference range. However, physical and mental global health scores did not change.
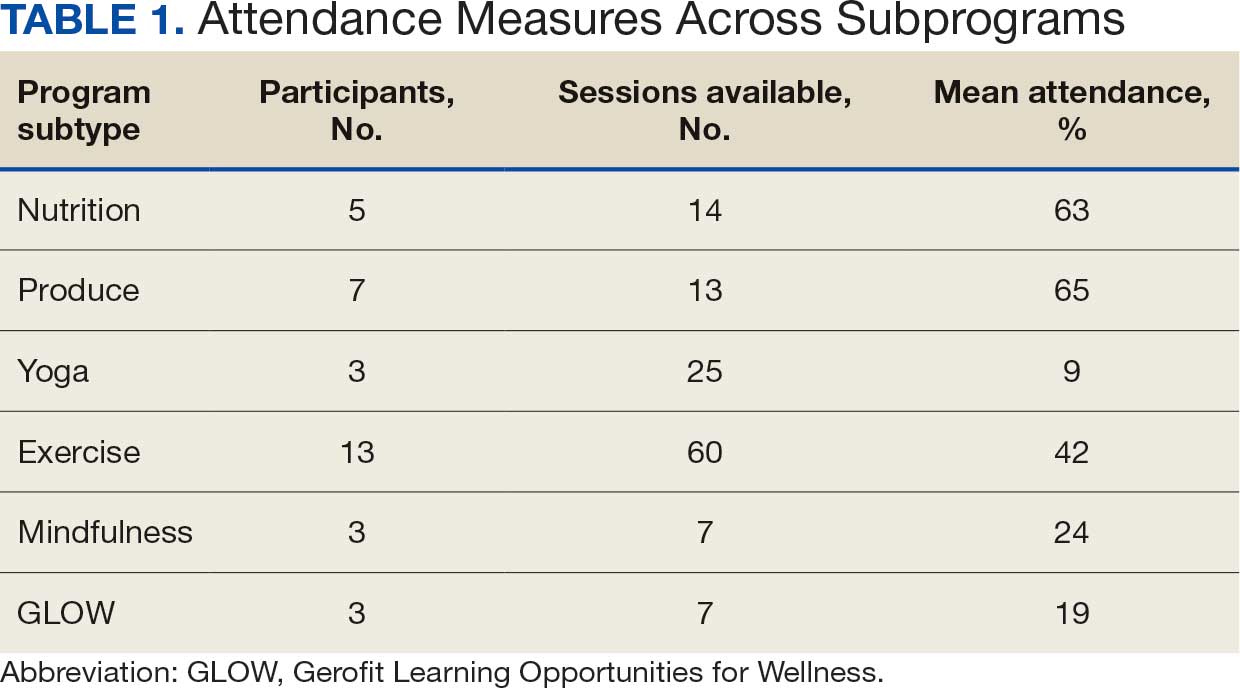

Ten veterans completed the PHI Short Form. Veterans most frequently identified multiple areas they wished to improve, including moving the body (n = 10), recharge (n = 10), food and drink (n = 9), and power of the mind (n = 7). Baseline self-ratings on each whole health component, along with follow-up ratings at the program’s conclusion, are presented in Figure 3. Some participants aimed to maintain current levels rather than seek improvement. At the 3-month mark, most veterans perceived themselves as improving in ≥1 health component.

Discussion Programs that target holistic wellness are needed to ensure the health of a rapidly aging population. The WHAG pilot program is an example of a comprehensive, patient-centered wellness program that supports participants in defining personal wellness goals to promote healthy aging. Gerofit addresses the continuum by beginning with goal-oriented discussions with veterans to guide program participation and support desired outcomes.
Gerofit provided a strong pre-existing framework of virtual social support and physical infrastructure for the addition of WHAG. Gerofit staff were responsible for recruitment and engagement, program oversight, and outcome data collection. Additionally, VHA facilities provide physical space for in-person and virtual programming. Integrating WHAG into Gerofit allows veterans to prioritize “what matters” and engage with peers in a nontraditional way, such as the dog fostering and adoption program provides veterans with an opportunity to increase physical activity levels and improve mental and physical health through the human-animal bond.25
By providing virtual options, WHAG enhances access to health care in medically underserved areas. WHAG also improves the veteran experience with the VA, building on Gerofit’s track record of high patient satisfaction, strong adherence, high retention, and consistent consults for veterans to join.10 The program allows veterans to be at the forefront of their VHA care, choosing to participate in the various offerings based on their personal preferences.
In this population of older veterans from Baltimore, Maryland, the majority of whom reside in disadvantaged areas, we observed that the programs with the highest participation were related to diet, stress reduction, and physical activity. These 3 areas align with common barriers faced by individuals in underserved communities. Many of these communities are food deserts, lack space or resources for gardening, and have limited or unsafe access to opportunities for physical activity, making gyms or even neighborhood exercise difficult to access.26-28 Offering produce delivery and virtual nutrition classes may potentially alleviate this barrier by providing economic stability by increasing access to healthy foods paired with nutrition education to promote use of free, fresh food. Teaching older adults with impaired mobility how to overcome barriers to consuming a healthy diet may improve their dietary intake.23,29,30 Future evaluations aim to examine how these various nutrition programs impact dietary intake and how changes in dietary intake may impact functional outcomes among this group.
Group classes provide opportunities for social connection and mutual support, both of which are powerful motivators for older adults. Frequent contact with others may help reduce the risk of depression, loneliness, and social isolation.28 Routine contact with staff allows for observation of short-term changes in behavior and mood, giving staff the chance to follow up when needed. The addition of these new programs gives participants more opportunities to engage with Gerofit staff and fellow veterans beyond traditional exercise sessions. This WHAG model could expand to other Gerofit sites; however, future whole health programs should take into account the unique needs and barriers specific to each location. Doing so will help ensure offerings align with participant preferences. Programs should be thoughtfully selected and designed to directly address local challenges to promote optimal engagement and support the greatest potential for success.
CONCLUSIONS
Programs that promote and support functional independence in older adults are needed, particularly given the rapidly growing and aging population. Identifying comprehensive strategies that promote healthy aging is likely to be beneficial not only for chronic disease management and social engagement but may also promote functional independence and reduce the risk of further functional decline.
- US Department of Veterans Affairs. Veterans Health Administration– About VHA. Veterans Health Administration. 2023. Accessed December 4, 2025. https://www.va.gov/health/aboutvha.asp
- Nelson KM. The burden of obesity among a national probability sample of veterans. J Gen Intern Med. 2006;21:915- 919. doi:10.1111/j.1525-1497.2006.00526.x
- Koepsell TD, Forsberg CW, Littman AJ. Obesity, overweight, and weight control practices in U.S. veterans. Prev Med. 2009;48:267-271. doi:10.1016/j.ypmed.2009.01.008
- Das SR, Kinsinger LS, Yancy WS Jr, et al. Obesity prevalence among veterans at Veterans Affairs medical facilities. Am J Prev Med. 2005;28:291-294. doi:10.1016/j.amepre.2004.12.007
- Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252-3257. doi:10.1001/archinte.160.21.3252
- Bokhour BG, Haun JN, Hyde J, et al. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295-300. doi:10.1097/MLR.0000000000001316
- Marchand WR, Beckstrom J, Nazarenko E, et al. The Veterans Health Administration whole health model of care: early implementation and utilization at a large healthcare system. Mil Med. 2020;185:2150-2157. doi:10.1093/milmed/usaa198
- Shulkin D, Elnahal S, Maddock E, Shaheen M. Best Care Everywhere by VA Professionals Across the Nation. US Dept of Veterans Affairs; 2017.
- Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit Program. J Am Geriatr Soc. 2018;66:1009-1016. doi:10.1111/jgs.15276
- Cowper PA, Morey MC, Bearon LB, et al. The impact of supervised exercise on the psychological well-being and health status of older veterans. J Appl Gerontol. 1991;10:469-485. doi:10.1177/073346489101000408
- Pepin MJ, Valencia WM, Bettger JP, et al. Impact of supervised exercise on one-year medication use in older veterans with multiple morbidities. Gerontol Geriatr Med. 2020;6:2333721420956751. doi:10.1177/073346489101000408
- Morey MC, Pieper CF, Sullivan RJ Jr, et al. Fiveyear performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44:1226-1231. doi:10.1111/j.1532-5415.1996.tb01374.x
- Morey MC, Pieper CF, Crowley GM, et al. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50:1929-1933. doi:10.1046/j.1532-5415.2002.50602.x
- Jorna M, Ball K, Salmon J. Effects of a holistic health program on women’s physical activity and mental and spiritual health. J Sci Med Sport. 2006;9:395-401. doi:10.1016/j.jsams.2006.06.011
- Jennings SC, Manning KM, Bettger JP, et al. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol Geriatr Med. 2020;6:2333721420980313. doi:10.1177/2333721420980313
- Morey MC, Crowley GM, Robbins MS, et al. The Gerofit program: a VA innovation. South Med J. 1994;87:S83-87.
- Addison O, Serra MC, Katzel L, et al. Mobility improvements are found in older veterans after 6 months of Gerofit regardless of BMI classification. J Aging Phys Act. 2019;27:848-854. doi:10.1123/japa.2018-0317
- Veterans Health Administration Office of Patient Centered Care and Cultural Transformation. Making your plan— whole health. November 14, 2023. Accessed December 4, 2025. https://www.va.gov/WHOLEHEALTH/phi.asp
- Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the Patient-Reported Outcomes Measurement Information System (PROMIS) global items. Qual Life Res. 2009;18:873-880. doi:10.1007/s11136-009-9496-9
- Aktary ML, Caron-Roy S, Sajobi T, et al. Impact of a farmers’ market nutrition coupon programme on diet quality and psychosocial well-being among low-income adults: protocol for a randomised controlled trial and a longitudinal qualitative investigation. BMJ Open. 2020;10:e035143. doi:10.1136/bmjopen-2019-035143
- Afshin A, Penalvo JL, Del Gobbo L, et al. The prospective impact of food pricing on improving dietary consumption: a systematic review and meta-analysis. PLoS One. 2017;12:e0172277. doi:10.1371/journal.pone.0172277
- Singleton CR, Kessee N, Chatman C, et al. Racial/ ethnic differences in the shopping behaviors and fruit and vegetable consumption of farmers’ market incentive program users in Illinois. Ethn Dis. 2020;30:109. doi:10.18865/ed.30.1.109
- Cassatt S, Giffuni J, Ortmeyer H, et al. A pilot study to evaluate the development and implementation of a virtual nutrition education program in older veterans. Abstract presented at: American Heart Association Epidemiology and Prevention/Lifestyle and Cardiometabolic Health 2022 Scientific Sessions; March 1-4, 2022; Chicago, IL. https:// www.ahajournals.org/doi/10.1161/circ.145.suppl_1.P002
- Parker EA, Perez WJ, Phipps B, et al. Dietary quality and perceived barriers to weight loss among older overweight veterans with dysmobility. Int J Environ Res Public Health. 2022;19:9153. doi:10.3390/ijerph19159153
- Ortmeyer HK, Giffuni J, Etchberger D, et al. The role of companion dogs in the VA Maryland Health Care System Whole Health(y) GeroFit Program. Animals (Basel). 2023;13:19. doi:10.3390/ani13193047
- Milaneschi Y, Tanaka T, Ferrucci L. Nutritional determinants of mobility. Curr Opin Clin Nutr Metab Care. 2010;13:625- 629.
- Lane JM, Davis BA. Food, physical activity, and health deserts in Alabama: the spatial link between healthy eating, exercise, and socioeconomic factors. GeoJournal. 2022;87:5229-5249.
- Komatsu H, Yagasaki K, Saito Y, et al. Regular group exercise contributes to balanced health in older adults in Japan: a qualitative study. BMC Geriatr. 2017;17:190. doi:10.1186/s12877-017-0584-3
- Komatsu H, Yagasaki K, Saito Y, et al. Regular group exercise contributes to balanced health in older adults in Japan: a qualitative study. BMC Geriatr. 2017;17:190. doi:10.1186/s12877-017-0584-3
- Wolfson JA, Ramsing R, Richardson CR, et al. Barriers to healthy food access: associations with household income and cooking behavior. Prev Med Rep. 2019;13:298-305. doi:10.1016/j.pmedr.2019.01.023
About half of the > 9 million veterans served by the Veterans Health Administration (VHA) are aged ≥ 65 years.1 Veterans are at a higher risk for comorbidities, which may contribute to increased health care costs, mobility limitations and disability, poor quality of life, and mortality. 2-5 Programs and policies that promote health maintenance, independent living, and quality of life are needed among older veterans. To support veterans’ overall health and well-being, the VHA has shifted to whole health, a patient-centered care model.6
The whole health paradigm employs personalized, proactive, and patient-driven care, emphasizing complementary and integrative health practices, and prioritizing health promotion and disease prevention over disease treatment.7 The veteran is empowered to decide “what matters to [me],” reflect on life and health, and define mission, aspiration, and purpose. This approach gives veterans a more active and direct role in their care, distinguishing it from traditional care models. In turn, it helps reduce the burden on clinicians and fosters a more collaborative environment in which both the clinician and veteran work together to shape the care process.7 Veterans utilize the Circle of Health to identify skills and support needed to implement changes in self-care. The Circle of Health includes 8 self-care components: moving the body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind.6 This process drives the creation of a personal health plan, creating opportunities for individuals to engage in well-being programs that matter to them and help them meet their goals.
Gerofit is a VHA best practice and whole health outpatient exercise program for veterans aged ≥65 years.8 Gerofit has focused primarily on exercise within the moving the body self-care component.9 A longitudinal study followed 691 Gerofit participants across 6 US Department of Veterans Affairs (VA) medical centers who on average were 73 years old, had 16 different medical conditions, and took 10 medications. Most were obese and had a mean gait speed of 1.04 m/s, suggesting functional impairment.10 Prior studies have shown that Gerofit participation is associated with a range of health benefits. Two studies reported improvements in psychological well-being and sustained gains in endurance, strength, and flexibility following early Gerofit program participation. 11,12 A 10-year analysis of 115 veterans found that long-term Gerofit participation reduced mortality risk, while another study of 452 veterans showed decreased medication use following 1 year in the program.13,14
The VHA whole health model comprises 3 components: (1) The Pathway, (2) well-being programs, and (3) whole health clinical care.6 The Pathway engages veterans in identifying personal health goals, while well-being programs offer selfcare and skill-building activities. Traditional clinical settings often focus primarily on the third component due to time and resource constraints. The Gerofit platform addresses all 3 components. Its existing infrastructure, including a supportive community and dedicated facilities, provides a setting for implementing The Pathway and well-being programs. The Gerofit structure allows for the time and continuity necessary for these components, which are often limited during standard clinical visits.
By expanding the Gerofit exercise regimen to include additional wellness activities, it can holistically support older veterans. Research supports this integrative approach. For example, a 2020 study found that incorporating a holistic health program into an existing exercise program within a church setting led to improved physical activity and overall health among women participants.15 This article describes the integration of Whole Health(y) Aging with Gerofit (WHAG), a pilot program in Baltimore, Maryland, that integrates whole health components into the established Gerofit framework to enhance the overall well-being of participating veterans (Figure 1).

WHOLE HEALTH(Y) AGING WITH GEROFIT
Gerofit enrollment has been described elsewhere in detail.16 Patients aged ≥ 65 years are eligible to participate with clinician approval if they are medically stable. Following VHA clinician referral and primary care approval, veterans completed a telephone visit to determine eligibility and discuss their exercise history, goals, and preferences. Veterans dependent in activities of daily living and those with cognitive impairment, unstable angina, active proliferative diabetic retinopathy, oxygen dependence, frank incontinence, active open wounds, active substance abuse, volatile behavioral issues, or who are experiencing homelessness are not eligible for Gerofit.
The exercise physiologist identified veteran barriers and incentives to participation and assisted with a plan to maximize SMART goals (specific, measurable, achievable, relevant, and time-bound). Veterans then completed an assessment visit, either in person or virtually, depending on the selected programming. Functional assessments conducted by trained Gerofit exercise physiologists include testing of lower and upper body strength and submaximal endurance.9,17,18 Participation in Gerofit is voluntary and not time limited.
Prior to these newly expanded offerings, veterans could only enroll in a personalized, structured exercise program. Based on feedback from Gerofit participants indicating areas of interest, WHAG was developed to provide additional wellness offerings aligned with other Circle of Health components.6 This included virtual group nutrition education and cooking interventions with optional fresh produce delivery; wellness classes, the Companion Dog Fostering & Adoption program, and Gerofit in the Mind, which included mindfulness classes and relaxation seminars (Figure 1). Programs were virtual (except dog fostering and adoption) and rotated throughout the year. Not all programs are offered simultaneously.
Attendance, completion of selected questions from the individual Personal Health Inventory (PHI) Short Form, measured physical function, self-reported physical activity levels, physical and mental health status, and program satisfaction were measured for all WHAG subprograms.18 Selected questions from the PHI Short Form use a 5-point Likert scale to rate the following whole health components: physical activity; sleep, relaxation, and recovery; healthy eating habits; and positive outlook, healthy relationships, and caring for mental health. Physical function was assessed using 30-second arm curls (upper body strength), 30-second chair stands (lower body strength), and the 2-minute step test (virtual) or 6-minute walk test (in person) (submaximal cardiovascular endurance).
Self-reported physical activity was assessed by asking frequency (days per week) and duration (minutes per session) of cardiovascular and strength exercises to calculate total minutes per week. Physical and mental health status was assessed using the Patient Reported Outcomes Measurement Information System (PROMIS) Global Health Scale.19 Demographic data included sex, race and ethnicity, and age at baseline visit. Mean (SD) was calculated for continuous variables and presented unless otherwise specified, and frequencies were calculated for categorical variables. Subsequent reports will describe additional assessments and detailed outcomes unique to individual programs.
Overview
Veterans chose the programs that best suited their needs without limitations.7 Staff provided guidance on newly available programs based on an individual’s specified goals. Gerofit staff assisted veterans with development of individualized personal health plans, monitoring progress towards their goals, supporting program participation, and connecting veterans with additional whole health resources.
Gerofit Exercise Group. Exercise was designed to address the Moving the Body component of whole health. Veterans could elect to schedule 1-hour, 3-times-weekly in-person gym appointments, participate in 3-times-weekly livestreamed virtual group exercise classes through VA Video Connect, or receive a self-directed at-home exercise plan.
Gerofit Learning Opportunities for Wellness Classes. These virtual health education sessions addressed the personal development component of whole health and were designed to increase self-efficacy and empower veterans to take an active role in their health care. Topics focused broadly on issues related to healthy aging (eg, importance of sleep, goal setting, self-care, and comorbidity education). Veterans could participate in any classes of interest, which were led by health care professionals and offered twice monthly. Sessions encouraged participant questions and peer interaction.
Nutrition. Improving dietary quality is a frequently reported goal of Gerofit participants. WHAG incorporated multiple strategies to assist veterans in meeting these goals. For example, through a partnership with Therapeutic Alternative of Maryland Farm, Gerofit provided veterans free, locally grown fresh produce. This initiative addressed barriers to healthy eating by improving access to fresh produce, which has been shown to influence cooking frequency and diet quality.20-22 Participation in nutrition classes was not required. In 2021, veterans received produce weekly; however, many reported excess quantities. Beginning in 2022, veterans could select both produce items and quantities desired.
In addition, a registered dietitian led a 14-week virtual nutrition education program guided by the social cognitive theory framework and focused on self-regulation skills such as goal setting, overcoming barriers, and identifying triggers.23 Prior research highlighted low health literacy as a common barrier among older veterans, which informed several key components of the curriculum.24 These included how to read and interpret nutrition labels, define balanced meals and snacks, and understand the classification of various food groups such as fats, carbohydrates, and proteins. The online program curriculum included an instructor guide and participant materials for each individual lesson, including an educational handout on the specific week’s topic, applied activity (group or individual), and recipes related to the produce shares. Structured group discussion promoted camaraderie and recipe sharing, and additional instruction on produce preparation and storage.
Reported lack of self-efficacy and knowledge regarding produce preparation prompted a 5-week virtual cooking series, led by a medical student and supervised by a registered dietitian. Sessions combined brief nutrition education with live cooking demonstrations adapted from the VA Healthy Teaching Kitchen curriculum. Recipes emphasized low-cost, commonly found food items. The Healthy Teaching Kitchen modifications focused on Dietary Approaches to Stop Hypertension diets, diabetes, and the importance of protein for older adults. Participants were allowed time to discuss recipes and food preparation tips, and other household members were allowed to observe.
Dog Fostering and Adoption. Veterans could foster or adopt a rescue dog through a partnership with local rescue groups. This program allowed participating veterans to have a companion, which addressed the surroundings, moving the body, and spirit and soul whole health components. The Companion Dog Fostering and Adoption Program and results on physical function and daily physical activity from the first 3 months were recently published. Positive effects on physical activity, physical function, and quality of life were observed at 3 months as compared to baseline in veterans who received a companion dog.25
Gerofit in the Garden. Veterans could opt to receive an EarthBox containing soil and seedlings for 1 vegetable and 1 herb. The boxes are designed to fit on a small tabletop, regardless of home type or availability of backyard. In-person instruction for veterans on care and maintenance was provided by a farm employee with experience in gardening and farming practices.
Gerofit in the Mind. Online relaxation seminars were offered twice monthly for 4 months. Led by a certified sound health guide, sessions incorporated sound baths, crystal bowls, Tibetan bowls, tuning forks, and breath work. Virtual mindfulness classes led by a certified yoga instructor were offered weekly for 1 month. Veterans could drop in and participate based on their availability. Classes were designed to introduce veterans to the practice of mindfulness, improve mood, and lower stress and anxiety.
Pilot Program Outcomes
Sixteen male veterans participated in WHAG. Participants were 62% Black, with a mean age of 76 years. Veterans collaborated with Gerofit staff to develop personal health plans, which ultimately guided program participation (Figure 2).

Five participants enrolled in 1 WHAG program, 11 enrolled in 2, and 8 enrolled in ≥ 3 (Table 1). Sixteen veterans completed baseline testing and 12 completed 3-month follow-up assessments (Table 2). At baseline, participants were below the reference range for physical functioning and physical activity levels. After 3 months, improvements were observed in endurance self-reported physical activity, and strength with many values in the reference range. However, physical and mental global health scores did not change.


Ten veterans completed the PHI Short Form. Veterans most frequently identified multiple areas they wished to improve, including moving the body (n = 10), recharge (n = 10), food and drink (n = 9), and power of the mind (n = 7). Baseline self-ratings on each whole health component, along with follow-up ratings at the program’s conclusion, are presented in Figure 3. Some participants aimed to maintain current levels rather than seek improvement. At the 3-month mark, most veterans perceived themselves as improving in ≥1 health component.

Discussion Programs that target holistic wellness are needed to ensure the health of a rapidly aging population. The WHAG pilot program is an example of a comprehensive, patient-centered wellness program that supports participants in defining personal wellness goals to promote healthy aging. Gerofit addresses the continuum by beginning with goal-oriented discussions with veterans to guide program participation and support desired outcomes.
Gerofit provided a strong pre-existing framework of virtual social support and physical infrastructure for the addition of WHAG. Gerofit staff were responsible for recruitment and engagement, program oversight, and outcome data collection. Additionally, VHA facilities provide physical space for in-person and virtual programming. Integrating WHAG into Gerofit allows veterans to prioritize “what matters” and engage with peers in a nontraditional way, such as the dog fostering and adoption program provides veterans with an opportunity to increase physical activity levels and improve mental and physical health through the human-animal bond.25
By providing virtual options, WHAG enhances access to health care in medically underserved areas. WHAG also improves the veteran experience with the VA, building on Gerofit’s track record of high patient satisfaction, strong adherence, high retention, and consistent consults for veterans to join.10 The program allows veterans to be at the forefront of their VHA care, choosing to participate in the various offerings based on their personal preferences.
In this population of older veterans from Baltimore, Maryland, the majority of whom reside in disadvantaged areas, we observed that the programs with the highest participation were related to diet, stress reduction, and physical activity. These 3 areas align with common barriers faced by individuals in underserved communities. Many of these communities are food deserts, lack space or resources for gardening, and have limited or unsafe access to opportunities for physical activity, making gyms or even neighborhood exercise difficult to access.26-28 Offering produce delivery and virtual nutrition classes may potentially alleviate this barrier by providing economic stability by increasing access to healthy foods paired with nutrition education to promote use of free, fresh food. Teaching older adults with impaired mobility how to overcome barriers to consuming a healthy diet may improve their dietary intake.23,29,30 Future evaluations aim to examine how these various nutrition programs impact dietary intake and how changes in dietary intake may impact functional outcomes among this group.
Group classes provide opportunities for social connection and mutual support, both of which are powerful motivators for older adults. Frequent contact with others may help reduce the risk of depression, loneliness, and social isolation.28 Routine contact with staff allows for observation of short-term changes in behavior and mood, giving staff the chance to follow up when needed. The addition of these new programs gives participants more opportunities to engage with Gerofit staff and fellow veterans beyond traditional exercise sessions. This WHAG model could expand to other Gerofit sites; however, future whole health programs should take into account the unique needs and barriers specific to each location. Doing so will help ensure offerings align with participant preferences. Programs should be thoughtfully selected and designed to directly address local challenges to promote optimal engagement and support the greatest potential for success.
CONCLUSIONS
Programs that promote and support functional independence in older adults are needed, particularly given the rapidly growing and aging population. Identifying comprehensive strategies that promote healthy aging is likely to be beneficial not only for chronic disease management and social engagement but may also promote functional independence and reduce the risk of further functional decline.
About half of the > 9 million veterans served by the Veterans Health Administration (VHA) are aged ≥ 65 years.1 Veterans are at a higher risk for comorbidities, which may contribute to increased health care costs, mobility limitations and disability, poor quality of life, and mortality. 2-5 Programs and policies that promote health maintenance, independent living, and quality of life are needed among older veterans. To support veterans’ overall health and well-being, the VHA has shifted to whole health, a patient-centered care model.6
The whole health paradigm employs personalized, proactive, and patient-driven care, emphasizing complementary and integrative health practices, and prioritizing health promotion and disease prevention over disease treatment.7 The veteran is empowered to decide “what matters to [me],” reflect on life and health, and define mission, aspiration, and purpose. This approach gives veterans a more active and direct role in their care, distinguishing it from traditional care models. In turn, it helps reduce the burden on clinicians and fosters a more collaborative environment in which both the clinician and veteran work together to shape the care process.7 Veterans utilize the Circle of Health to identify skills and support needed to implement changes in self-care. The Circle of Health includes 8 self-care components: moving the body; surroundings; personal development; food and drink; recharge; family, friends, and coworkers; spirit and soul; and power of the mind.6 This process drives the creation of a personal health plan, creating opportunities for individuals to engage in well-being programs that matter to them and help them meet their goals.
Gerofit is a VHA best practice and whole health outpatient exercise program for veterans aged ≥65 years.8 Gerofit has focused primarily on exercise within the moving the body self-care component.9 A longitudinal study followed 691 Gerofit participants across 6 US Department of Veterans Affairs (VA) medical centers who on average were 73 years old, had 16 different medical conditions, and took 10 medications. Most were obese and had a mean gait speed of 1.04 m/s, suggesting functional impairment.10 Prior studies have shown that Gerofit participation is associated with a range of health benefits. Two studies reported improvements in psychological well-being and sustained gains in endurance, strength, and flexibility following early Gerofit program participation. 11,12 A 10-year analysis of 115 veterans found that long-term Gerofit participation reduced mortality risk, while another study of 452 veterans showed decreased medication use following 1 year in the program.13,14
The VHA whole health model comprises 3 components: (1) The Pathway, (2) well-being programs, and (3) whole health clinical care.6 The Pathway engages veterans in identifying personal health goals, while well-being programs offer selfcare and skill-building activities. Traditional clinical settings often focus primarily on the third component due to time and resource constraints. The Gerofit platform addresses all 3 components. Its existing infrastructure, including a supportive community and dedicated facilities, provides a setting for implementing The Pathway and well-being programs. The Gerofit structure allows for the time and continuity necessary for these components, which are often limited during standard clinical visits.
By expanding the Gerofit exercise regimen to include additional wellness activities, it can holistically support older veterans. Research supports this integrative approach. For example, a 2020 study found that incorporating a holistic health program into an existing exercise program within a church setting led to improved physical activity and overall health among women participants.15 This article describes the integration of Whole Health(y) Aging with Gerofit (WHAG), a pilot program in Baltimore, Maryland, that integrates whole health components into the established Gerofit framework to enhance the overall well-being of participating veterans (Figure 1).

WHOLE HEALTH(Y) AGING WITH GEROFIT
Gerofit enrollment has been described elsewhere in detail.16 Patients aged ≥ 65 years are eligible to participate with clinician approval if they are medically stable. Following VHA clinician referral and primary care approval, veterans completed a telephone visit to determine eligibility and discuss their exercise history, goals, and preferences. Veterans dependent in activities of daily living and those with cognitive impairment, unstable angina, active proliferative diabetic retinopathy, oxygen dependence, frank incontinence, active open wounds, active substance abuse, volatile behavioral issues, or who are experiencing homelessness are not eligible for Gerofit.
The exercise physiologist identified veteran barriers and incentives to participation and assisted with a plan to maximize SMART goals (specific, measurable, achievable, relevant, and time-bound). Veterans then completed an assessment visit, either in person or virtually, depending on the selected programming. Functional assessments conducted by trained Gerofit exercise physiologists include testing of lower and upper body strength and submaximal endurance.9,17,18 Participation in Gerofit is voluntary and not time limited.
Prior to these newly expanded offerings, veterans could only enroll in a personalized, structured exercise program. Based on feedback from Gerofit participants indicating areas of interest, WHAG was developed to provide additional wellness offerings aligned with other Circle of Health components.6 This included virtual group nutrition education and cooking interventions with optional fresh produce delivery; wellness classes, the Companion Dog Fostering & Adoption program, and Gerofit in the Mind, which included mindfulness classes and relaxation seminars (Figure 1). Programs were virtual (except dog fostering and adoption) and rotated throughout the year. Not all programs are offered simultaneously.
Attendance, completion of selected questions from the individual Personal Health Inventory (PHI) Short Form, measured physical function, self-reported physical activity levels, physical and mental health status, and program satisfaction were measured for all WHAG subprograms.18 Selected questions from the PHI Short Form use a 5-point Likert scale to rate the following whole health components: physical activity; sleep, relaxation, and recovery; healthy eating habits; and positive outlook, healthy relationships, and caring for mental health. Physical function was assessed using 30-second arm curls (upper body strength), 30-second chair stands (lower body strength), and the 2-minute step test (virtual) or 6-minute walk test (in person) (submaximal cardiovascular endurance).
Self-reported physical activity was assessed by asking frequency (days per week) and duration (minutes per session) of cardiovascular and strength exercises to calculate total minutes per week. Physical and mental health status was assessed using the Patient Reported Outcomes Measurement Information System (PROMIS) Global Health Scale.19 Demographic data included sex, race and ethnicity, and age at baseline visit. Mean (SD) was calculated for continuous variables and presented unless otherwise specified, and frequencies were calculated for categorical variables. Subsequent reports will describe additional assessments and detailed outcomes unique to individual programs.
Overview
Veterans chose the programs that best suited their needs without limitations.7 Staff provided guidance on newly available programs based on an individual’s specified goals. Gerofit staff assisted veterans with development of individualized personal health plans, monitoring progress towards their goals, supporting program participation, and connecting veterans with additional whole health resources.
Gerofit Exercise Group. Exercise was designed to address the Moving the Body component of whole health. Veterans could elect to schedule 1-hour, 3-times-weekly in-person gym appointments, participate in 3-times-weekly livestreamed virtual group exercise classes through VA Video Connect, or receive a self-directed at-home exercise plan.
Gerofit Learning Opportunities for Wellness Classes. These virtual health education sessions addressed the personal development component of whole health and were designed to increase self-efficacy and empower veterans to take an active role in their health care. Topics focused broadly on issues related to healthy aging (eg, importance of sleep, goal setting, self-care, and comorbidity education). Veterans could participate in any classes of interest, which were led by health care professionals and offered twice monthly. Sessions encouraged participant questions and peer interaction.
Nutrition. Improving dietary quality is a frequently reported goal of Gerofit participants. WHAG incorporated multiple strategies to assist veterans in meeting these goals. For example, through a partnership with Therapeutic Alternative of Maryland Farm, Gerofit provided veterans free, locally grown fresh produce. This initiative addressed barriers to healthy eating by improving access to fresh produce, which has been shown to influence cooking frequency and diet quality.20-22 Participation in nutrition classes was not required. In 2021, veterans received produce weekly; however, many reported excess quantities. Beginning in 2022, veterans could select both produce items and quantities desired.
In addition, a registered dietitian led a 14-week virtual nutrition education program guided by the social cognitive theory framework and focused on self-regulation skills such as goal setting, overcoming barriers, and identifying triggers.23 Prior research highlighted low health literacy as a common barrier among older veterans, which informed several key components of the curriculum.24 These included how to read and interpret nutrition labels, define balanced meals and snacks, and understand the classification of various food groups such as fats, carbohydrates, and proteins. The online program curriculum included an instructor guide and participant materials for each individual lesson, including an educational handout on the specific week’s topic, applied activity (group or individual), and recipes related to the produce shares. Structured group discussion promoted camaraderie and recipe sharing, and additional instruction on produce preparation and storage.
Reported lack of self-efficacy and knowledge regarding produce preparation prompted a 5-week virtual cooking series, led by a medical student and supervised by a registered dietitian. Sessions combined brief nutrition education with live cooking demonstrations adapted from the VA Healthy Teaching Kitchen curriculum. Recipes emphasized low-cost, commonly found food items. The Healthy Teaching Kitchen modifications focused on Dietary Approaches to Stop Hypertension diets, diabetes, and the importance of protein for older adults. Participants were allowed time to discuss recipes and food preparation tips, and other household members were allowed to observe.
Dog Fostering and Adoption. Veterans could foster or adopt a rescue dog through a partnership with local rescue groups. This program allowed participating veterans to have a companion, which addressed the surroundings, moving the body, and spirit and soul whole health components. The Companion Dog Fostering and Adoption Program and results on physical function and daily physical activity from the first 3 months were recently published. Positive effects on physical activity, physical function, and quality of life were observed at 3 months as compared to baseline in veterans who received a companion dog.25
Gerofit in the Garden. Veterans could opt to receive an EarthBox containing soil and seedlings for 1 vegetable and 1 herb. The boxes are designed to fit on a small tabletop, regardless of home type or availability of backyard. In-person instruction for veterans on care and maintenance was provided by a farm employee with experience in gardening and farming practices.
Gerofit in the Mind. Online relaxation seminars were offered twice monthly for 4 months. Led by a certified sound health guide, sessions incorporated sound baths, crystal bowls, Tibetan bowls, tuning forks, and breath work. Virtual mindfulness classes led by a certified yoga instructor were offered weekly for 1 month. Veterans could drop in and participate based on their availability. Classes were designed to introduce veterans to the practice of mindfulness, improve mood, and lower stress and anxiety.
Pilot Program Outcomes
Sixteen male veterans participated in WHAG. Participants were 62% Black, with a mean age of 76 years. Veterans collaborated with Gerofit staff to develop personal health plans, which ultimately guided program participation (Figure 2).

Five participants enrolled in 1 WHAG program, 11 enrolled in 2, and 8 enrolled in ≥ 3 (Table 1). Sixteen veterans completed baseline testing and 12 completed 3-month follow-up assessments (Table 2). At baseline, participants were below the reference range for physical functioning and physical activity levels. After 3 months, improvements were observed in endurance self-reported physical activity, and strength with many values in the reference range. However, physical and mental global health scores did not change.


Ten veterans completed the PHI Short Form. Veterans most frequently identified multiple areas they wished to improve, including moving the body (n = 10), recharge (n = 10), food and drink (n = 9), and power of the mind (n = 7). Baseline self-ratings on each whole health component, along with follow-up ratings at the program’s conclusion, are presented in Figure 3. Some participants aimed to maintain current levels rather than seek improvement. At the 3-month mark, most veterans perceived themselves as improving in ≥1 health component.

Discussion Programs that target holistic wellness are needed to ensure the health of a rapidly aging population. The WHAG pilot program is an example of a comprehensive, patient-centered wellness program that supports participants in defining personal wellness goals to promote healthy aging. Gerofit addresses the continuum by beginning with goal-oriented discussions with veterans to guide program participation and support desired outcomes.
Gerofit provided a strong pre-existing framework of virtual social support and physical infrastructure for the addition of WHAG. Gerofit staff were responsible for recruitment and engagement, program oversight, and outcome data collection. Additionally, VHA facilities provide physical space for in-person and virtual programming. Integrating WHAG into Gerofit allows veterans to prioritize “what matters” and engage with peers in a nontraditional way, such as the dog fostering and adoption program provides veterans with an opportunity to increase physical activity levels and improve mental and physical health through the human-animal bond.25
By providing virtual options, WHAG enhances access to health care in medically underserved areas. WHAG also improves the veteran experience with the VA, building on Gerofit’s track record of high patient satisfaction, strong adherence, high retention, and consistent consults for veterans to join.10 The program allows veterans to be at the forefront of their VHA care, choosing to participate in the various offerings based on their personal preferences.
In this population of older veterans from Baltimore, Maryland, the majority of whom reside in disadvantaged areas, we observed that the programs with the highest participation were related to diet, stress reduction, and physical activity. These 3 areas align with common barriers faced by individuals in underserved communities. Many of these communities are food deserts, lack space or resources for gardening, and have limited or unsafe access to opportunities for physical activity, making gyms or even neighborhood exercise difficult to access.26-28 Offering produce delivery and virtual nutrition classes may potentially alleviate this barrier by providing economic stability by increasing access to healthy foods paired with nutrition education to promote use of free, fresh food. Teaching older adults with impaired mobility how to overcome barriers to consuming a healthy diet may improve their dietary intake.23,29,30 Future evaluations aim to examine how these various nutrition programs impact dietary intake and how changes in dietary intake may impact functional outcomes among this group.
Group classes provide opportunities for social connection and mutual support, both of which are powerful motivators for older adults. Frequent contact with others may help reduce the risk of depression, loneliness, and social isolation.28 Routine contact with staff allows for observation of short-term changes in behavior and mood, giving staff the chance to follow up when needed. The addition of these new programs gives participants more opportunities to engage with Gerofit staff and fellow veterans beyond traditional exercise sessions. This WHAG model could expand to other Gerofit sites; however, future whole health programs should take into account the unique needs and barriers specific to each location. Doing so will help ensure offerings align with participant preferences. Programs should be thoughtfully selected and designed to directly address local challenges to promote optimal engagement and support the greatest potential for success.
CONCLUSIONS
Programs that promote and support functional independence in older adults are needed, particularly given the rapidly growing and aging population. Identifying comprehensive strategies that promote healthy aging is likely to be beneficial not only for chronic disease management and social engagement but may also promote functional independence and reduce the risk of further functional decline.
- US Department of Veterans Affairs. Veterans Health Administration– About VHA. Veterans Health Administration. 2023. Accessed December 4, 2025. https://www.va.gov/health/aboutvha.asp
- Nelson KM. The burden of obesity among a national probability sample of veterans. J Gen Intern Med. 2006;21:915- 919. doi:10.1111/j.1525-1497.2006.00526.x
- Koepsell TD, Forsberg CW, Littman AJ. Obesity, overweight, and weight control practices in U.S. veterans. Prev Med. 2009;48:267-271. doi:10.1016/j.ypmed.2009.01.008
- Das SR, Kinsinger LS, Yancy WS Jr, et al. Obesity prevalence among veterans at Veterans Affairs medical facilities. Am J Prev Med. 2005;28:291-294. doi:10.1016/j.amepre.2004.12.007
- Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252-3257. doi:10.1001/archinte.160.21.3252
- Bokhour BG, Haun JN, Hyde J, et al. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295-300. doi:10.1097/MLR.0000000000001316
- Marchand WR, Beckstrom J, Nazarenko E, et al. The Veterans Health Administration whole health model of care: early implementation and utilization at a large healthcare system. Mil Med. 2020;185:2150-2157. doi:10.1093/milmed/usaa198
- Shulkin D, Elnahal S, Maddock E, Shaheen M. Best Care Everywhere by VA Professionals Across the Nation. US Dept of Veterans Affairs; 2017.
- Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit Program. J Am Geriatr Soc. 2018;66:1009-1016. doi:10.1111/jgs.15276
- Cowper PA, Morey MC, Bearon LB, et al. The impact of supervised exercise on the psychological well-being and health status of older veterans. J Appl Gerontol. 1991;10:469-485. doi:10.1177/073346489101000408
- Pepin MJ, Valencia WM, Bettger JP, et al. Impact of supervised exercise on one-year medication use in older veterans with multiple morbidities. Gerontol Geriatr Med. 2020;6:2333721420956751. doi:10.1177/073346489101000408
- Morey MC, Pieper CF, Sullivan RJ Jr, et al. Fiveyear performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44:1226-1231. doi:10.1111/j.1532-5415.1996.tb01374.x
- Morey MC, Pieper CF, Crowley GM, et al. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50:1929-1933. doi:10.1046/j.1532-5415.2002.50602.x
- Jorna M, Ball K, Salmon J. Effects of a holistic health program on women’s physical activity and mental and spiritual health. J Sci Med Sport. 2006;9:395-401. doi:10.1016/j.jsams.2006.06.011
- Jennings SC, Manning KM, Bettger JP, et al. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol Geriatr Med. 2020;6:2333721420980313. doi:10.1177/2333721420980313
- Morey MC, Crowley GM, Robbins MS, et al. The Gerofit program: a VA innovation. South Med J. 1994;87:S83-87.
- Addison O, Serra MC, Katzel L, et al. Mobility improvements are found in older veterans after 6 months of Gerofit regardless of BMI classification. J Aging Phys Act. 2019;27:848-854. doi:10.1123/japa.2018-0317
- Veterans Health Administration Office of Patient Centered Care and Cultural Transformation. Making your plan— whole health. November 14, 2023. Accessed December 4, 2025. https://www.va.gov/WHOLEHEALTH/phi.asp
- Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the Patient-Reported Outcomes Measurement Information System (PROMIS) global items. Qual Life Res. 2009;18:873-880. doi:10.1007/s11136-009-9496-9
- Aktary ML, Caron-Roy S, Sajobi T, et al. Impact of a farmers’ market nutrition coupon programme on diet quality and psychosocial well-being among low-income adults: protocol for a randomised controlled trial and a longitudinal qualitative investigation. BMJ Open. 2020;10:e035143. doi:10.1136/bmjopen-2019-035143
- Afshin A, Penalvo JL, Del Gobbo L, et al. The prospective impact of food pricing on improving dietary consumption: a systematic review and meta-analysis. PLoS One. 2017;12:e0172277. doi:10.1371/journal.pone.0172277
- Singleton CR, Kessee N, Chatman C, et al. Racial/ ethnic differences in the shopping behaviors and fruit and vegetable consumption of farmers’ market incentive program users in Illinois. Ethn Dis. 2020;30:109. doi:10.18865/ed.30.1.109
- Cassatt S, Giffuni J, Ortmeyer H, et al. A pilot study to evaluate the development and implementation of a virtual nutrition education program in older veterans. Abstract presented at: American Heart Association Epidemiology and Prevention/Lifestyle and Cardiometabolic Health 2022 Scientific Sessions; March 1-4, 2022; Chicago, IL. https:// www.ahajournals.org/doi/10.1161/circ.145.suppl_1.P002
- Parker EA, Perez WJ, Phipps B, et al. Dietary quality and perceived barriers to weight loss among older overweight veterans with dysmobility. Int J Environ Res Public Health. 2022;19:9153. doi:10.3390/ijerph19159153
- Ortmeyer HK, Giffuni J, Etchberger D, et al. The role of companion dogs in the VA Maryland Health Care System Whole Health(y) GeroFit Program. Animals (Basel). 2023;13:19. doi:10.3390/ani13193047
- Milaneschi Y, Tanaka T, Ferrucci L. Nutritional determinants of mobility. Curr Opin Clin Nutr Metab Care. 2010;13:625- 629.
- Lane JM, Davis BA. Food, physical activity, and health deserts in Alabama: the spatial link between healthy eating, exercise, and socioeconomic factors. GeoJournal. 2022;87:5229-5249.
- Komatsu H, Yagasaki K, Saito Y, et al. Regular group exercise contributes to balanced health in older adults in Japan: a qualitative study. BMC Geriatr. 2017;17:190. doi:10.1186/s12877-017-0584-3
- Komatsu H, Yagasaki K, Saito Y, et al. Regular group exercise contributes to balanced health in older adults in Japan: a qualitative study. BMC Geriatr. 2017;17:190. doi:10.1186/s12877-017-0584-3
- Wolfson JA, Ramsing R, Richardson CR, et al. Barriers to healthy food access: associations with household income and cooking behavior. Prev Med Rep. 2019;13:298-305. doi:10.1016/j.pmedr.2019.01.023
- US Department of Veterans Affairs. Veterans Health Administration– About VHA. Veterans Health Administration. 2023. Accessed December 4, 2025. https://www.va.gov/health/aboutvha.asp
- Nelson KM. The burden of obesity among a national probability sample of veterans. J Gen Intern Med. 2006;21:915- 919. doi:10.1111/j.1525-1497.2006.00526.x
- Koepsell TD, Forsberg CW, Littman AJ. Obesity, overweight, and weight control practices in U.S. veterans. Prev Med. 2009;48:267-271. doi:10.1016/j.ypmed.2009.01.008
- Das SR, Kinsinger LS, Yancy WS Jr, et al. Obesity prevalence among veterans at Veterans Affairs medical facilities. Am J Prev Med. 2005;28:291-294. doi:10.1016/j.amepre.2004.12.007
- Agha Z, Lofgren RP, VanRuiswyk JV, et al. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160:3252-3257. doi:10.1001/archinte.160.21.3252
- Bokhour BG, Haun JN, Hyde J, et al. Transforming the Veterans Affairs to a whole health system of care: time for action and research. Med Care. 2020;58:295-300. doi:10.1097/MLR.0000000000001316
- Marchand WR, Beckstrom J, Nazarenko E, et al. The Veterans Health Administration whole health model of care: early implementation and utilization at a large healthcare system. Mil Med. 2020;185:2150-2157. doi:10.1093/milmed/usaa198
- Shulkin D, Elnahal S, Maddock E, Shaheen M. Best Care Everywhere by VA Professionals Across the Nation. US Dept of Veterans Affairs; 2017.
- Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit Program. J Am Geriatr Soc. 2018;66:1009-1016. doi:10.1111/jgs.15276
- Cowper PA, Morey MC, Bearon LB, et al. The impact of supervised exercise on the psychological well-being and health status of older veterans. J Appl Gerontol. 1991;10:469-485. doi:10.1177/073346489101000408
- Pepin MJ, Valencia WM, Bettger JP, et al. Impact of supervised exercise on one-year medication use in older veterans with multiple morbidities. Gerontol Geriatr Med. 2020;6:2333721420956751. doi:10.1177/073346489101000408
- Morey MC, Pieper CF, Sullivan RJ Jr, et al. Fiveyear performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44:1226-1231. doi:10.1111/j.1532-5415.1996.tb01374.x
- Morey MC, Pieper CF, Crowley GM, et al. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50:1929-1933. doi:10.1046/j.1532-5415.2002.50602.x
- Jorna M, Ball K, Salmon J. Effects of a holistic health program on women’s physical activity and mental and spiritual health. J Sci Med Sport. 2006;9:395-401. doi:10.1016/j.jsams.2006.06.011
- Jennings SC, Manning KM, Bettger JP, et al. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol Geriatr Med. 2020;6:2333721420980313. doi:10.1177/2333721420980313
- Morey MC, Crowley GM, Robbins MS, et al. The Gerofit program: a VA innovation. South Med J. 1994;87:S83-87.
- Addison O, Serra MC, Katzel L, et al. Mobility improvements are found in older veterans after 6 months of Gerofit regardless of BMI classification. J Aging Phys Act. 2019;27:848-854. doi:10.1123/japa.2018-0317
- Veterans Health Administration Office of Patient Centered Care and Cultural Transformation. Making your plan— whole health. November 14, 2023. Accessed December 4, 2025. https://www.va.gov/WHOLEHEALTH/phi.asp
- Hays RD, Bjorner JB, Revicki DA, et al. Development of physical and mental health summary scores from the Patient-Reported Outcomes Measurement Information System (PROMIS) global items. Qual Life Res. 2009;18:873-880. doi:10.1007/s11136-009-9496-9
- Aktary ML, Caron-Roy S, Sajobi T, et al. Impact of a farmers’ market nutrition coupon programme on diet quality and psychosocial well-being among low-income adults: protocol for a randomised controlled trial and a longitudinal qualitative investigation. BMJ Open. 2020;10:e035143. doi:10.1136/bmjopen-2019-035143
- Afshin A, Penalvo JL, Del Gobbo L, et al. The prospective impact of food pricing on improving dietary consumption: a systematic review and meta-analysis. PLoS One. 2017;12:e0172277. doi:10.1371/journal.pone.0172277
- Singleton CR, Kessee N, Chatman C, et al. Racial/ ethnic differences in the shopping behaviors and fruit and vegetable consumption of farmers’ market incentive program users in Illinois. Ethn Dis. 2020;30:109. doi:10.18865/ed.30.1.109
- Cassatt S, Giffuni J, Ortmeyer H, et al. A pilot study to evaluate the development and implementation of a virtual nutrition education program in older veterans. Abstract presented at: American Heart Association Epidemiology and Prevention/Lifestyle and Cardiometabolic Health 2022 Scientific Sessions; March 1-4, 2022; Chicago, IL. https:// www.ahajournals.org/doi/10.1161/circ.145.suppl_1.P002
- Parker EA, Perez WJ, Phipps B, et al. Dietary quality and perceived barriers to weight loss among older overweight veterans with dysmobility. Int J Environ Res Public Health. 2022;19:9153. doi:10.3390/ijerph19159153
- Ortmeyer HK, Giffuni J, Etchberger D, et al. The role of companion dogs in the VA Maryland Health Care System Whole Health(y) GeroFit Program. Animals (Basel). 2023;13:19. doi:10.3390/ani13193047
- Milaneschi Y, Tanaka T, Ferrucci L. Nutritional determinants of mobility. Curr Opin Clin Nutr Metab Care. 2010;13:625- 629.
- Lane JM, Davis BA. Food, physical activity, and health deserts in Alabama: the spatial link between healthy eating, exercise, and socioeconomic factors. GeoJournal. 2022;87:5229-5249.
- Komatsu H, Yagasaki K, Saito Y, et al. Regular group exercise contributes to balanced health in older adults in Japan: a qualitative study. BMC Geriatr. 2017;17:190. doi:10.1186/s12877-017-0584-3
- Komatsu H, Yagasaki K, Saito Y, et al. Regular group exercise contributes to balanced health in older adults in Japan: a qualitative study. BMC Geriatr. 2017;17:190. doi:10.1186/s12877-017-0584-3
- Wolfson JA, Ramsing R, Richardson CR, et al. Barriers to healthy food access: associations with household income and cooking behavior. Prev Med Rep. 2019;13:298-305. doi:10.1016/j.pmedr.2019.01.023
Whole Health(y) Aging With Gerofit: The Development of a Pilot Wellness Program for Older Veterans
Whole Health(y) Aging With Gerofit: The Development of a Pilot Wellness Program for Older Veterans
VA Centenarian Program Expands, Honors Veterans at Key Life Milestones
The Centenarian Program at the US Department of Veterans Affairs (VA) has expanded to begin honoring veterans for special occasions, such as birthdays, as well as veterans with very limited life expectancy.
Initially launched in 2020 as a special initiative that awarded commemorative coins to American heroes aged 100 years, the program recognizes each individual’s service to the country.
“This program symbolizes the commitment that we promise to our veterans,” said Center for Development and Civic Engagement (CDCE) Chief Dennis Montgomery in West Palm Beach, Florida. “They are never forgotten. No matter the years since time of service, the VA will always honor and remind them of the gratitude we proudly hold in our hearts for their bravery and sacrifice.”
Eligible veterans receive a personalized letter from the VA Secretary, a commemorative coin, and public recognition from their local VA facility, which often includes a celebration. To be eligible, veterans must be enrolled and receiving care through the VA health care systems.
Coins are customized for each veteran with unique attributes, including the veteran’s name, branch of service, military occupational specialty, and years of service.
“I originally learned of the program in 2022, and I explored the possibilities to expand the reach of active engagement from the Center of Development and Civic Engagement and the VA Secretary’s office,” said Saraswathy Battar, MD, a geriatrician at the Thomas H. Corey VA Medical Center (VAMC) Community Living Center in West Palm Beach, who oversaw the program until her retirement in November 2025. The program is currently administered by the office of VA Secretary Douglas Collins.
Between August 2022 and October 15, 2025, 1182 centenarian veterans and 285 special recognition honorees received commemorative coins.
“As our local veteran population grows within the centenarian coin eligibility criteria,” Montgomery said, “I know this program will continue to grow as well and gain more popularity to honor our veterans as they have earned and deserved.”
The VA conducts outreach through local health care professionals to identify veterans eligible for the program. This allows for veterans admitted to the VA to be identified, leading to activation of the ceremony process.
If veterans are in declining health, they become eligible to receive recognition at age 95, Montgomery said.
The Centenarian Program at the US Department of Veterans Affairs (VA) has expanded to begin honoring veterans for special occasions, such as birthdays, as well as veterans with very limited life expectancy.
Initially launched in 2020 as a special initiative that awarded commemorative coins to American heroes aged 100 years, the program recognizes each individual’s service to the country.
“This program symbolizes the commitment that we promise to our veterans,” said Center for Development and Civic Engagement (CDCE) Chief Dennis Montgomery in West Palm Beach, Florida. “They are never forgotten. No matter the years since time of service, the VA will always honor and remind them of the gratitude we proudly hold in our hearts for their bravery and sacrifice.”
Eligible veterans receive a personalized letter from the VA Secretary, a commemorative coin, and public recognition from their local VA facility, which often includes a celebration. To be eligible, veterans must be enrolled and receiving care through the VA health care systems.
Coins are customized for each veteran with unique attributes, including the veteran’s name, branch of service, military occupational specialty, and years of service.
“I originally learned of the program in 2022, and I explored the possibilities to expand the reach of active engagement from the Center of Development and Civic Engagement and the VA Secretary’s office,” said Saraswathy Battar, MD, a geriatrician at the Thomas H. Corey VA Medical Center (VAMC) Community Living Center in West Palm Beach, who oversaw the program until her retirement in November 2025. The program is currently administered by the office of VA Secretary Douglas Collins.
Between August 2022 and October 15, 2025, 1182 centenarian veterans and 285 special recognition honorees received commemorative coins.
“As our local veteran population grows within the centenarian coin eligibility criteria,” Montgomery said, “I know this program will continue to grow as well and gain more popularity to honor our veterans as they have earned and deserved.”
The VA conducts outreach through local health care professionals to identify veterans eligible for the program. This allows for veterans admitted to the VA to be identified, leading to activation of the ceremony process.
If veterans are in declining health, they become eligible to receive recognition at age 95, Montgomery said.
The Centenarian Program at the US Department of Veterans Affairs (VA) has expanded to begin honoring veterans for special occasions, such as birthdays, as well as veterans with very limited life expectancy.
Initially launched in 2020 as a special initiative that awarded commemorative coins to American heroes aged 100 years, the program recognizes each individual’s service to the country.
“This program symbolizes the commitment that we promise to our veterans,” said Center for Development and Civic Engagement (CDCE) Chief Dennis Montgomery in West Palm Beach, Florida. “They are never forgotten. No matter the years since time of service, the VA will always honor and remind them of the gratitude we proudly hold in our hearts for their bravery and sacrifice.”
Eligible veterans receive a personalized letter from the VA Secretary, a commemorative coin, and public recognition from their local VA facility, which often includes a celebration. To be eligible, veterans must be enrolled and receiving care through the VA health care systems.
Coins are customized for each veteran with unique attributes, including the veteran’s name, branch of service, military occupational specialty, and years of service.
“I originally learned of the program in 2022, and I explored the possibilities to expand the reach of active engagement from the Center of Development and Civic Engagement and the VA Secretary’s office,” said Saraswathy Battar, MD, a geriatrician at the Thomas H. Corey VA Medical Center (VAMC) Community Living Center in West Palm Beach, who oversaw the program until her retirement in November 2025. The program is currently administered by the office of VA Secretary Douglas Collins.
Between August 2022 and October 15, 2025, 1182 centenarian veterans and 285 special recognition honorees received commemorative coins.
“As our local veteran population grows within the centenarian coin eligibility criteria,” Montgomery said, “I know this program will continue to grow as well and gain more popularity to honor our veterans as they have earned and deserved.”
The VA conducts outreach through local health care professionals to identify veterans eligible for the program. This allows for veterans admitted to the VA to be identified, leading to activation of the ceremony process.
If veterans are in declining health, they become eligible to receive recognition at age 95, Montgomery said.
Factors Influencing Outcomes of a Telehealth-Based Physical Activity Program in Older Veterans Postdischarge
Factors Influencing Outcomes of a Telehealth-Based Physical Activity Program in Older Veterans Postdischarge
Deconditioning among hospitalized older adults contributes to significant decline in posthospitalization functional ability, physical performance, and physical activity.1-10 Previous hospital-to-home interventions have targeted improving function and physical activity, including recent programs leveraging home telehealth as a feasible and potentially effective mode of delivering in-home exercise and rehabilitation.11-14 However, pilot interventions have shown mixed effectiveness.11,12,14 This study expands on a previously published intervention describing a pilot home telehealth program for veterans posthospital discharge that demonstrated significant 6-month improvement in physical activity as well as trends in physical function improvement, including among those with cognitive impairment.15 Factors that contribute to improved outcomes are the focus of the present study.
Key factors underlying the complexity of hospital-to-home transitions include hospitalization elements (ie, reason for admission and length of stay), associated posthospital syndromes (ie, postdischarge falls, medication changes, cognitive impairment, and pain), and postdischarge health care application (ie, physical therapy and hospital readmission).16-18 These factors may be associated with postdischarge functional ability, physical performance, and physical activity, but their direct influence on intervention outcomes is unclear (Figure 1).5,7,9,16-20 The objective of this study was to examine the influence of hospitalization, posthospital syndrome, and postdischarge health care application factors on outcomes of a US Department of Veterans Affairs (VA) Video Connect (VVC) intervention to enhance function and physical activity in older adults posthospital discharge.
health care application factors on physical activity, functional ability, and
physical performance intervention outcomes.
Methods
The previous analysis reported on patient characteristics, program feasibility, and preliminary outcomes.13,15 The current study reports on relationships between hospitalization, posthospital syndrome, and postdischarge health care application factors and change in key outcomes, namely postdischarge self-reported functional ability, physical performance, and physical activity from baseline to endpoint.
Participants provided written informed consent. The protocol and consent forms were approved by the VA Ann Arbor Healthcare System (VAAAHS) Research and Development Committee, and the project was registered on clinicaltrials.gov (NCT04045054).
Intervention
The pilot program targeted older adults following recent hospital discharge from VAAAHS. Participants were eligible if they were aged ≥ 50 years, had been discharged following an inpatient stay in the past 1 to 2 weeks, evaluated by physical therapy during hospitalization with stated rehabilitation goals on discharge, and followed by a VAAAHS primary care physician. Participants were either recruited during hospital admission or shortly after discharge.13
An experienced physical activity trainer (PAT) supported the progression of participants’ rehabilitation goals via a home exercise program and coached the patient and caregiver to optimize functional ability, physical performance, and physical activity. The PAT was a nonlicensed research assistant with extensive experience in applying standard physical activity enhancement protocols (eg, increased walking) to older adults with comorbidities. Participation in the program lasted about 6 months. Initiation of the PAT program was delayed if the patient was already receiving postdischarge home-based or outpatient physical therapy. The PAT contacted the patient weekly via VVC for the first 6 weeks, then monthly for a total of 6 months. Each contact included information on optimal walking form, injury prevention, program progression, and ways to incorporate sit-to-stand transitions, nonsitting behavior, and walking into daily routines. The initial VVC contact lasted about 60 minutes and subsequent sessions lasted about 30 minutes.13
Demographic characteristics were self-reported by participants and included age, sex, race, years of education, and marital status. Clinical characteristics were obtained from each participant’s electronic health record (EHR), including copay status, index hospitalization length of stay, admission diagnosis, and postsurgery status (postsurgery vs nonpostsurgery). Intervention adherence was tracked as the number of PAT sessions attended.
Posthospital Syndrome Factors
Participant falls (categorized as those who reported a fall vs those who did not) and medication changes (number of changes reported, including new medication, discontinued medication, dose changes, medication changes, or changes in medication schedule) were reported by participants or caregivers during each VVC contact. Participants completed the Montreal Cognitive Assessment (MoCA) at baseline, and were dichotomized into 2 groups: no cognitive impairment (MoCA score ≥ 26) and mild to moderate cognitive impairment (MoCA score 10-25).13,21
Participants rated how much pain interfered with their normal daily activities since the previous VVC session on a 5-point Likert scale (1, not at all; to 5, extremely).22 Similar to prior research, participants were placed into 2 groups based on their mean pain interference score (individuals with scores from 1.0 to 2.0 in 1 group, and individuals with > 2.0 in another).23-25 Participants were separated into a no or mild pain interference group and a moderate to severe pain interference group. Hospital readmissions (VA and non-VA) and postdischarge physical therapy outcomes were obtained from the participant’s EHR, including primary care visits.
Outcomes
Outcomes were collected at baseline (posthospital discharge) and 6 months postenrollment.
Self-Reported Functional Ability. This measure is provided by participants or caregivers and measured by the Katz Index of Independence in Activities of Daily Living (ADL), Lawton and Brody Instrumental ADL Scale (IADL), Nagi Disability Model, and Rosow-Breslau Scale. The Katz ADL assesses the ability to complete 6 self-care activities and awards 1 point for independence and 0 if the individual is dependent (total score range, 0-6).26 The Lawton and Brody IADL measures an individual’s independence in 8 instrumental ADLs; it awards 1 point for independence and 0 if the individual is dependent (total score range, 0-8).27 The Nagi Disability Model evaluates an individual’s difficulty performing 5 tasks (total score range, 0-5) and tallies the number of items with a response other than “no difficulty at all” (higher total score indicates greater difficulty). 28 The Rosow-Breslau Scale is a 3-item measure of mobility disability; individual responses are 0 (no help) and 1 (requires help or unable); higher total score (range, 0-3) indicates greater disability.29
Physical Performance. Measured using the Short Physical Performance Battery (SPPB), which evaluates standing balance, sit to stand, and walking performance. Scores range from 0 to 4 on the balance, gait speed, and chair stand tests, for a total composite score between 0 and 12 (higher score indicates better performance).30
Physical Activity. Measured using actigraphy, namely a physical activity monitor adherent to the thigh (activ-PAL3TM, PAL Technologies Ltd., Glasgow, UK).31 Participants were instructed to wear the activPal for ≥ 1 week. Participants with a minimum of 5 days of wear were included in this analysis.
Data Analyses
Analyses were performed using SPSS software version 29.0.32 Continuous variables were summarized using mean (SD) or median and IQR using the weighted average method; categorical variables were summarized using frequencies and percentages. Baseline scores on outcome variables were compared by categorical hospitalization, posthospital syndrome, and postdischarge health care application factor variables using Mann-Whitney U tests. The differences between outcome variables from baseline to endpoint were then calculated to produce change scores. Relationships between the number of PAT sessions attended and baseline outcomes and outcome change scores were estimated using Spearman correlations. Relationships between categorical factors (hospitalization, posthospital syndrome, and postdischarge health care application) and outcome variable change scores (which were normally distributed) were examined using Mann-Whitney U tests. Relationships with continuous hospitalization (length of stay) and posthospital syndrome factors (medication changes) were estimated using Spearman correlations. Effect sizes (ES) were estimated with Cohen d; small (d = 0.2), medium (d = 0.5), or large (d ≥ 0.8). Missing data were handled using pairwise deletion.33 Therefore, sample sizes were reported for each analysis. For all statistical tests, P < .05 was considered significant.
Results
Twenty-four individuals completed the pilot intervention.15 Mean (SD) age was 73.6 (8.1) years (range, 64-93 years) and participants were predominantly White males (Table 1). Eight participants had a high school education only and 13 had more than a high school education. Diagnoses at admission included 9 patients with orthopedic/musculoskeletal conditions (6 were for joint replacement), 6 patients with vascular/pulmonary conditions, and 4 with gastrointestinal/renal/urological conditions. Of the 11 postsurgery participants, 7 were orthopedic, 4 were gastrointestinal, and 1 was peripheral vascular.
Baseline outcome scores did not differ significantly between groups, except individuals with moderate to severe pain interference reported a significantly lower IADL score (median [IQR] 4 [2-7]) than individuals with mild or moderate pain interference (median [IQR] 8 [7-8]; P = .02) (Table 2). The mean (SD) number of PAT sessions attended was 9.3 (3.7) (range, 3-19). There were no significant relationships between number of sessions attended and any baseline outcome variables or outcome change scores.
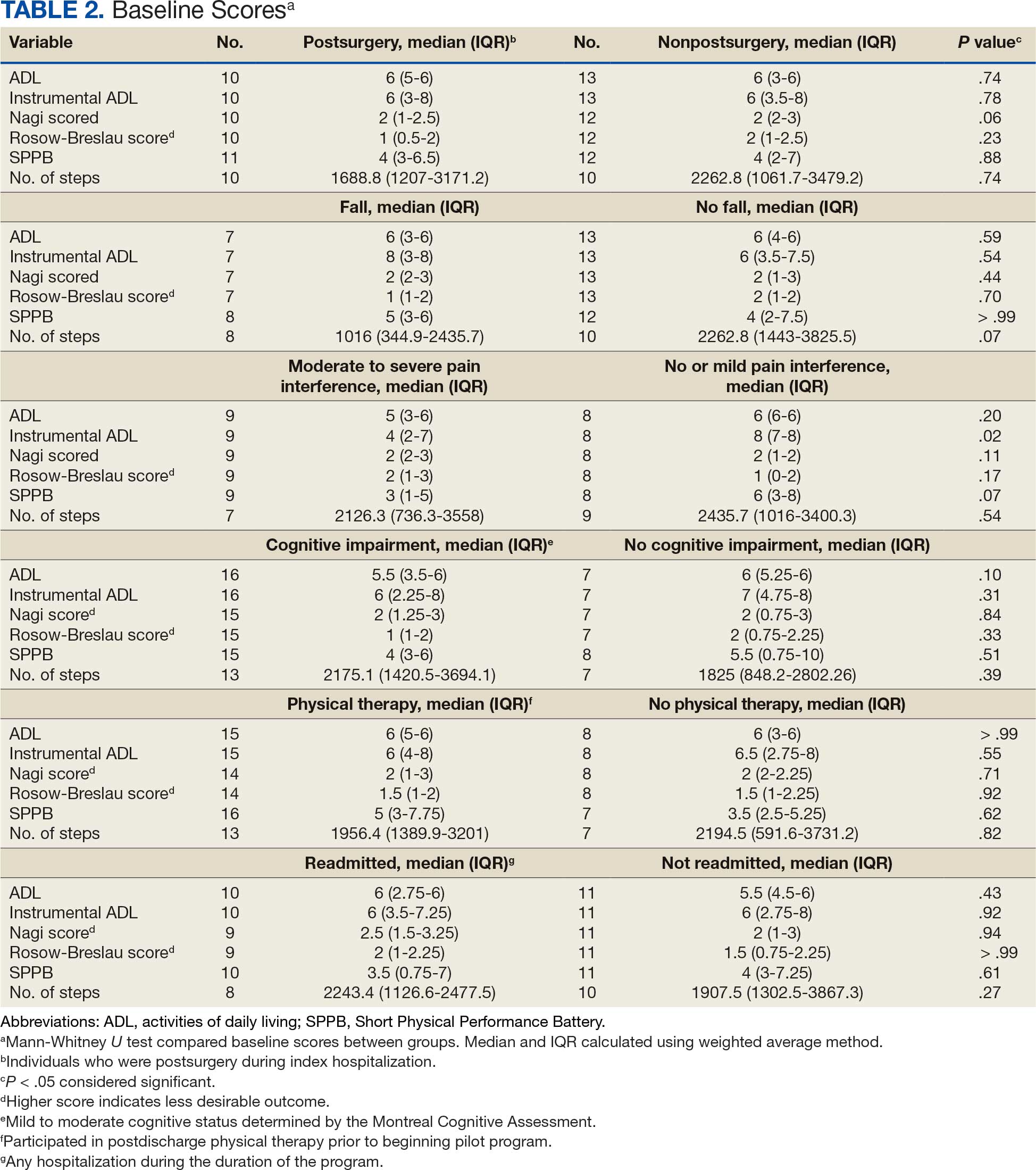
Hospitalization Factors
Participants who were postsurgery tended to have greater improvement than individuals who were nonpostsurgery in ADLs (median [IQR] 0 [0-1.5]; ES, 0.6; P = .10) and SPPB (median [IQR] 2 [1.5-9]; ES, 0.9; P = .07), but the improvements were not statistically significant (Table 3). Mean (SD) length of stay of the index hospitalization was 6.7 (6.1) days. Longer length of stay was significantly correlated with an increase in Nagi score (ρ, 0.45; 95% CI, 0.01-0.75). There were no other significant or trending relationships between length of stay and outcome variables.
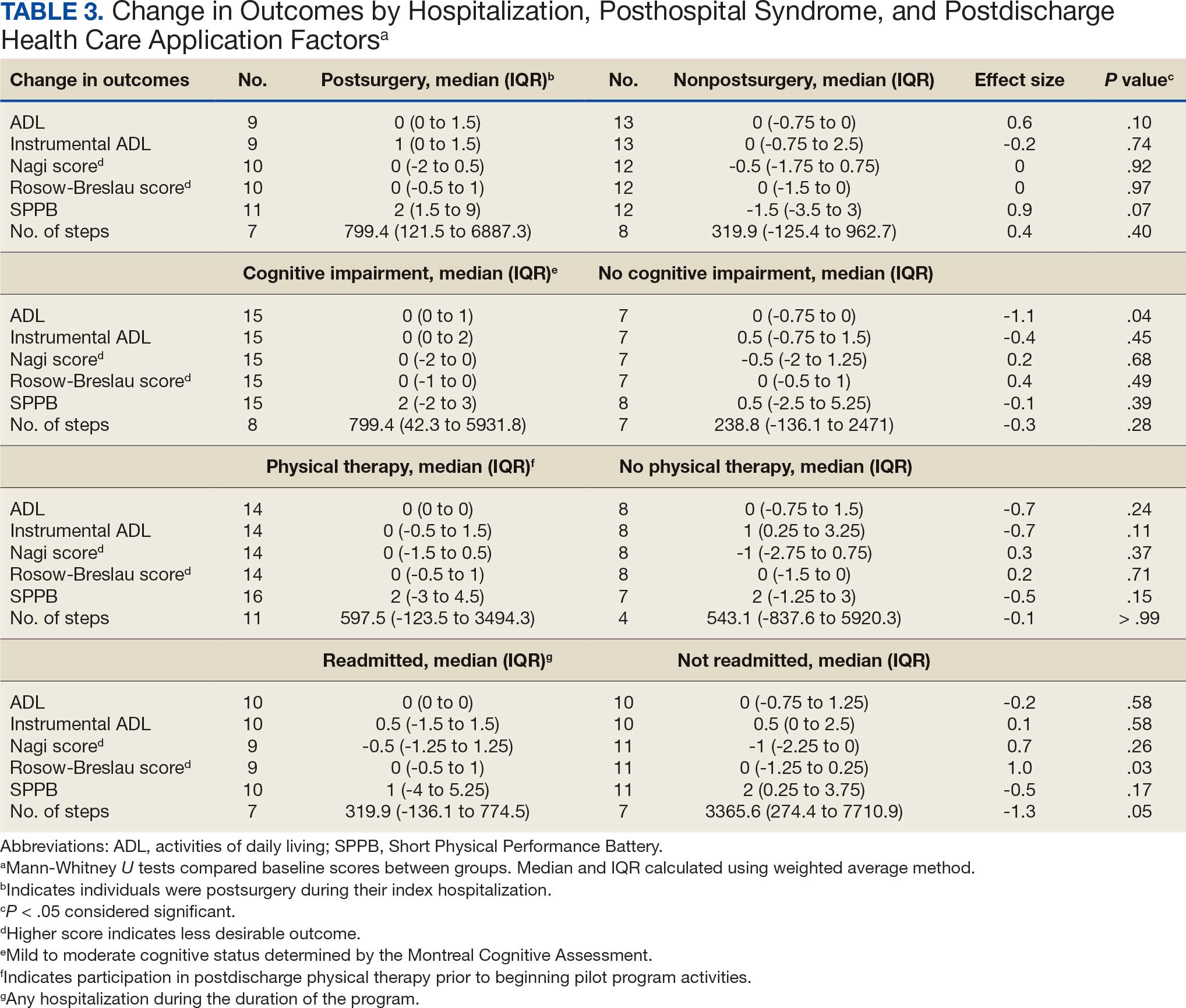
Posthospital Syndrome Factors
The 16 participants with mild to moderate cognitive impairment had less improvement in ADLs (median [IQR] 0 [0-1]) than the 8 participants with no impairment (median [IQR] 0 [-0.75 to 0]; ES, -1.1; P = .04). Change in outcome variables from baseline to endpoint did not significantly differ between the 8 patients who reported a fall compared with the 13 who did not, nor were any trends observed. Change in outcome variables from baseline to endpoint also did not significantly differ between the 8 participants who reported no or mild pain interference compared with the 10 patients with moderate to severe pain interference, nor were any trends observed. Mean (SD) number of medication changes was 2.5 (1.6). Higher number of medication changes was significantly correlated with a decrease in Rosow-Breslau score (ρ, -0.47; 95% CI, -0.76 to -0.02). There were no other significant or trending relationships between number of medication changes and outcome variables.
Postdischarge Health Care Application Factors
The 16 participants who attended posthospital physical therapy trended towards less improvement in IADLs (median [IQR] 0 [-0.5 to 1.5]; ES, -0.7; P = .11) and SPPB (median [IQR] 2 [-3.0 to 4.5]; ES, -0.5; P = .15) than the 8 patients with no postdischarge physical therapy. Eleven participants were readmitted, while 13 had no readmissions in their medical records between baseline and endpoint. Participants with ≥ 1 readmission experienced a greater increase in Rosow-Breslau score (median [IQR] 0 [-0.5 to 1.0]) than those not readmitted (median [IQR] 0 [-1.25 to 0.25]; ES, 1.0; P = .03). Borderline greater improvement in number of steps was found in those not readmitted (median [IQR] 3365.6 [274.4-7710.9]) compared with those readmitted (median [IQR] 319.9 [-136.1 to 774.5]; ES, -1.3; P = .05). Patients who were readmitted also tended to have lower and not statistically significant improvements in SPPB (median [IQR] 1 [-4.0 to 5.3]) compared with those not readmitted (median [IQR] 2 [0.3-3.8]; ES, -0.5; P = .17) (Table 3).
Discussion
This study examined the association between hospitalization, posthospital syndrome, and postdischarge health care use in patients undergoing a VVC-based intervention following hospital discharge. Participants who had no or mild cognitive impairment, no readmissions, higher medication changes, and a shorter hospital length of stay tended to experience lower disability, including in mobility and ADLs. This suggests individuals who are less clinically complex may be more likely to benefit from this type of virtual rehabilitation program. These findings are consistent with clinical experiences; home-based programs to improve physical activity posthospital discharge can be challenging for those who were medically ill (and did not undergo a specific surgical procedure), cognitively impaired, and become acutely ill and trigger hospital readmission. 15 For example, the sample in this study had higher rates of falls, pain, and readmissions compared to previous research.2,3,34-39
The importance of posthospital syndrome in the context of recovery of function and health at home following hospitalization is well documented.16-18 The potential impact of posthospital syndrome on physical activity-focused interventions is less understood. In our analysis, participants with mild or moderate cognitive impairment tended to become more dependent in their ADLs, while those with no cognitive impairment tended to become more independent in their ADLs. This functional decline over time is perhaps expected in persons with cognitive impairment, but the significant difference with a large ES warrants further consideration on how to tailor interventions to better promote functional recovery in these individuals.40,41 While some cognitive decline may not be preventable, this finding supports the need to promote healthy cognitive aging, identify declines in cognition, and work to mitigate additional decline. Programs specifically designed to promote function and physical activity in older adults with cognitive impairment are needed, especially during care transitions.41-43
While participants reported that falls and pain interference did not have a significant impact on change in outcomes between baseline and endpoint, these areas need further investigation. Falls and pain have been associated with function and physical activity in older adults.42-46 Pain is common, yet underappreciated during older adult hospital-to-home transitions.11,12,45,46 There is a need for more comprehensive assessment of pain (including pain intensity) and qualitative research.
Hospitalization and postdischarge health care application factors may have a significant impact on home-telehealth physical activity intervention success. Individuals who were postsurgery tended to have greater improvements in ADLs and physical performance. Most postsurgery participants had joint replacement surgery. Postsurgery status may not be modifiable, but it is important to note expected differences in recovery between medical and surgical admissions and the need to tailor care based on admission diagnosis. Those with a longer length of hospital stay may be considered at higher risk of suboptimal outcomes postdischarge, which indicates an opportunity for targeting resources and support, in addition to efforts of reducing length of stay where possible.47
Readmissions were significantly related to a change in Rosow-Breslau mobility disability score. This may indicate the detrimental impact a readmission can have on increasing mobility and physical activity postdischarge, or the potential of this pilot program to impact readmissions by increasing mobility and physical activity, contrary to prior physical exercise interventions.5,7,9,48 With 5% to 79% of readmissions considered preventable, continued efforts and program dissemination and implementation to address preventable readmissions are warranted.49 Individuals with postdischarge physical therapy (prior to beginning the pilot program) tended to demonstrate less improvement in disability and physical performance. This relationship needs further investigation; the 2 groups did not appear to have significant differences at baseline, albeit with a small sample size. It is possible they experienced initial improvements with postdischarge physical therapy and plateaued or had little further reserve to improve upon entering the VVC program.
Strengths and Limitations
This pilot program provided evaluative data on the use of VVC to enhance function and physical activity in older adults posthospital discharge. It included individual (eg, fall, pain, cognitive impairment) and health service (eg, readmission, physical therapy) level factors as predictors of function and physical activity posthospitalization.5,7,9,15-19
The results of this pilot project stem from a small sample lacking diversity in terms of race, ethnicity, and sex. There was some variation in baseline and endpoints between participants, and when hospitalization, posthospital syndrome, and postdischarge health care application factors were collected. The majority of participants were recruited within a month postdischarge, and the program lasted about 6 months. Data collection was attempted at regular PAT contacts, but there was some variation in when visits occurred based on participant availability and preference. Some participants had missing data, which was handled using pairwise deletion.33 Larger studies are needed to confirm the findings of this study, particularly the trends that did not reach statistical significance. Home health services other than physical therapy (eg, nursing, occupational therapy) were not fully accounted for and should be considered in future research.
Conclusions
In patients undergoing a 6-month pilot VVC-based physical activity intervention posthospital discharge, improvements in mobility and disability were most likely in those who had no cognitive impairment and were not readmitted. Larger sample and qualitative investigations are necessary to optimize outcomes for patients who meet these clinical profiles.
- Liebzeit D, Bratzke L, Boltz M, Purvis S, King B. Getting back to normal: a grounded theory study of function in post-hospitalized older adults. Gerontologist. 2020;60:704-714. doi:10.1093/geront/gnz057
- Ponzetto M, Zanocchi M, Maero B, et al. Post-hospitalization mortality in the elderly. Arch Gerontol Geriatr. 2003;36:83-91. doi:10.1016/s0167-4943(02)00061-4
- Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6:e26951. doi:10.1371/journal.pone.0026951
- Ponzetto M, Maero B, Maina P, et al. Risk factors for early and late mortality in hospitalized older patients: the continuing importance of functional status. J Gerontol A Biol Sci Med Sci. 2003;58:1049-1054. doi:10.1093/gerona/58.11.m1049
- Huang HT, Chang CM, Liu LF, Lin HS, Chen CH. Trajectories and predictors of functional decline of hospitalised older patients. J Clin Nurs. 2013;22:1322-1331. doi:10.1111/jocn.12055
- Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171- 2179. doi:10.1111/j.1532-5415.2008.02023.x
- Helvik AS, Selbæk G, Engedal K. Functional decline in older adults one year after hospitalization. Arch Gerontol Geriatr. 2013;57:305-310. doi:10.1016/j.archger.2013.05.008
- Zaslavsky O, Zisberg A, Shadmi E. Impact of functional change before and during hospitalization on functional recovery 1 month following hospitalization. J Gerontol Biol Sci Med Sci. 2015;70:381-386. doi:10.1093/gerona/glu168
- Chen CC, Wang C, Huang GH. Functional trajectory 6 months posthospitalization: a cohort study of older hospitalized patients in Taiwan. Nurs Res. 2008;57:93-100. doi:10.1097/01.NNR.0000313485.18670.e2
- Kleinpell RM, Fletcher K, Jennings BM. Reducing functional decline in hospitalized elderly. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008. Accessed September 3, 2025. http://www.ncbi.nlm.nih.gov/books/NBK2629/
- Liebzeit D, Rutkowski R, Arbaje AI, Fields B, Werner NE. A scoping review of interventions for older adults transitioning from hospital to home. J Am Geriatr Soc. 2021;69:2950-2962. doi:10.1111/jgs.17323
- Hladkowicz E, Dumitrascu F, Auais M, et al. Evaluations of postoperative transitions in care for older adults: a scoping review. BMC Geriatr. 2022;22:329. doi:10.1186/s12877-022-02989-6
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA Video Connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: baseline assessment. BMC Geriatr. 2021;21:502. doi:10.1186/s12877-021-02454-w
- Dawson R, Oliveira JS, Kwok WS, et al. Exercise interventions delivered through telehealth to improve physical functioning for older adults with frailty, cognitive, or mobility disability: a systematic review and meta-analysis. Telemed J E Health. 2024;30:940-950. doi:10.1089/tmj.2023.0177
- Liebzeit D, Phillips KK, Hogikyan RV, Cigolle CT, Alexander NB. A pilot home-telehealth program to enhance functional ability, physical performance, and physical activity in older adult veterans post-hospital discharge. Res Gerontol Nurs. 2024;17:271-279. doi:10.3928/19404921-20241105-01
- Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100-102. doi:10.1056/NEJMp1212324
- Caraballo C, Dharmarajan K, Krumholz HM. Post hospital syndrome: is the stress of hospitalization causing harm? Rev Esp Cardiol (Engl Ed). 2019;72:896-898. doi:10.1016/j.rec.2019.04.010
- Rawal S, Kwan JL, Razak F, et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern Med. 2019;179:38- 45. doi:10.1001/jamainternmed.2018.5100
- Dutzi I, Schwenk M, Kirchner M, Jooss E, Bauer JM, Hauer K. Influence of cognitive impairment on rehabilitation received and its mediating effect on functional recovery. J Alzheimers Dis. 2021;84:745-756. doi:10.3233/JAD-210620
- Uriz-Otano F, Uriz-Otano JI, Malafarina V. Factors associated with short-term functional recovery in elderly people with a hip fracture. Influence ofcognitiveimpairment. JAmMedDirAssoc. 2015;16:215-220. doi:10.1016/j.jamda.2014.09.009
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. doi:10.1111/j.1532-5415.2005.53221.x
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483.
- White RS, Jiang J, Hall CB, et al. Higher perceived stress scale scores are associated with higher pain intensity and pain interference levels in older adults. J Am Geriatr Soc. 2014;62:2350-2356. doi:10.1111/jgs.13135
- Blyth FM, March LM, Brnabic AJ, et al. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127-134. doi:10.1016/s0304-3959(00)00355-9
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain. 2004;110:361-368. doi:10.1016/j.pain.2004.04.017
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi:10.1001/jama.1963.03060120024016
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
- Alexander NB, Guire KE, Thelen DG, et al. Self-reported walking ability predicts functional mobility performance in frail older adults. J Am Geriatr Soc. 2000;48:1408-1413. doi:10.1111/j.1532-5415.2000.tb02630.x
- Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556-559. doi:10.1093/geronj/21.4.556
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. doi:10.1093/geronj/49.2.m85
- Chan CS, Slaughter SE, Jones CA, Ickert C, Wagg AS. Measuring activity performance of older adults using the activPAL: a rapid review. Healthcare (Basel). 2017;5:94. doi:10.3390/healthcare5040094
- IBM SPSS software. IBM Corp; 2019. Accessed September 3, 2025. https://www.ibm.com/spss
- Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64:402-406. doi:10.4097/kjae.2013.64.5.402
- Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365:2287-2295. doi:10.1056/NEJMsa1101942
- Bogaisky M, Dezieck L. Early hospital readmission of nursing home residents and community-dwelling elderly adults discharged from the geriatrics service of an urban teaching hospital: patterns and risk factors. J Am Geriatr Soc. 2015;63:548-552. doi:10.1111/jgs.13317
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428. doi:10.1056/NEJMsa0803563
- Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9:277-282. doi:10.1002/jhm.2152
- Mahoney J, Sager M, Dunham NC, Johnson J. Risk of falls after hospital discharge. J Am Geriatr Soc. 1994;42:269- 274. doi:10.1111/j.1532-5415.1994.tb01750.x
- Hoffman GJ, Liu H, Alexander NB, Tinetti M, Braun TM, Min LC. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276. doi:10.1001/jamanetworkopen.2019.4276
- Gill DP, Hubbard RA, Koepsell TD, et al. Differences in rate of functional decline across three dementia types. Alzheimers Dement. 2013;9:S63-S71. doi:10.1016/j.jalz.2012.10.010
- Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment–the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167-173. doi:10.1159/000154929
- Patti A, Zangla D, Sahin FN, et al. Physical exercise and prevention of falls. Effects of a Pilates training method compared with a general physical activity program. Medicine (Baltimore). 2021;100:e25289. doi:10.1097/MD.0000000000025289
- Nagarkar A, Kulkarni S. Association between daily activities and fall in older adults: an analysis of longitudinal ageing study in India (2017-18). BMC Geriatr. 2022;22:203. doi:10.1186/s12877-022-02879-x
- Ek S, Rizzuto D, Xu W, Calderón-Larrañaga A, Welmer AK. Predictors for functional decline after an injurious fall: a population-based cohort study. Aging Clin Exp Res. 2021;33:2183-2190. doi:10.1007/s40520-020-01747-1
- Dagnino APA, Campos MM. Chronic pain in the elderly: mechanisms and perspectives. Front Hum Neurosci. 2022;16:736688. doi:10.3389/fnhum.2022.736688
- Ritchie CS, Patel K, Boscardin J, et al. Impact of persistent pain on function, cognition, and well-being of older adults. J Am Geriatr Soc. 2023;71:26-35. doi:10.1111/jgs.18125
- Han TS, Murray P, Robin J, et al. Evaluation of the association of length of stay in hospital and outcomes. Int J Qual Health Care. 2022;34:mzab160. doi:10.1093/intqhc/ mzab160
- Lærum-Onsager E, Molin M, Olsen CF, et al. Effect of nutritional and physical exercise intervention on hospital readmission for patients aged 65 or older: a systematic review and meta-analysis of randomized controlled trials. Int J Behav Nutr Phys Act. 2021;18:62. doi:10.1186/s12966-021-01123-w
- Van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391-E402. doi:10.1503/cmaj.101860
Deconditioning among hospitalized older adults contributes to significant decline in posthospitalization functional ability, physical performance, and physical activity.1-10 Previous hospital-to-home interventions have targeted improving function and physical activity, including recent programs leveraging home telehealth as a feasible and potentially effective mode of delivering in-home exercise and rehabilitation.11-14 However, pilot interventions have shown mixed effectiveness.11,12,14 This study expands on a previously published intervention describing a pilot home telehealth program for veterans posthospital discharge that demonstrated significant 6-month improvement in physical activity as well as trends in physical function improvement, including among those with cognitive impairment.15 Factors that contribute to improved outcomes are the focus of the present study.
Key factors underlying the complexity of hospital-to-home transitions include hospitalization elements (ie, reason for admission and length of stay), associated posthospital syndromes (ie, postdischarge falls, medication changes, cognitive impairment, and pain), and postdischarge health care application (ie, physical therapy and hospital readmission).16-18 These factors may be associated with postdischarge functional ability, physical performance, and physical activity, but their direct influence on intervention outcomes is unclear (Figure 1).5,7,9,16-20 The objective of this study was to examine the influence of hospitalization, posthospital syndrome, and postdischarge health care application factors on outcomes of a US Department of Veterans Affairs (VA) Video Connect (VVC) intervention to enhance function and physical activity in older adults posthospital discharge.

health care application factors on physical activity, functional ability, and
physical performance intervention outcomes.
Methods
The previous analysis reported on patient characteristics, program feasibility, and preliminary outcomes.13,15 The current study reports on relationships between hospitalization, posthospital syndrome, and postdischarge health care application factors and change in key outcomes, namely postdischarge self-reported functional ability, physical performance, and physical activity from baseline to endpoint.
Participants provided written informed consent. The protocol and consent forms were approved by the VA Ann Arbor Healthcare System (VAAAHS) Research and Development Committee, and the project was registered on clinicaltrials.gov (NCT04045054).
Intervention
The pilot program targeted older adults following recent hospital discharge from VAAAHS. Participants were eligible if they were aged ≥ 50 years, had been discharged following an inpatient stay in the past 1 to 2 weeks, evaluated by physical therapy during hospitalization with stated rehabilitation goals on discharge, and followed by a VAAAHS primary care physician. Participants were either recruited during hospital admission or shortly after discharge.13
An experienced physical activity trainer (PAT) supported the progression of participants’ rehabilitation goals via a home exercise program and coached the patient and caregiver to optimize functional ability, physical performance, and physical activity. The PAT was a nonlicensed research assistant with extensive experience in applying standard physical activity enhancement protocols (eg, increased walking) to older adults with comorbidities. Participation in the program lasted about 6 months. Initiation of the PAT program was delayed if the patient was already receiving postdischarge home-based or outpatient physical therapy. The PAT contacted the patient weekly via VVC for the first 6 weeks, then monthly for a total of 6 months. Each contact included information on optimal walking form, injury prevention, program progression, and ways to incorporate sit-to-stand transitions, nonsitting behavior, and walking into daily routines. The initial VVC contact lasted about 60 minutes and subsequent sessions lasted about 30 minutes.13
Demographic characteristics were self-reported by participants and included age, sex, race, years of education, and marital status. Clinical characteristics were obtained from each participant’s electronic health record (EHR), including copay status, index hospitalization length of stay, admission diagnosis, and postsurgery status (postsurgery vs nonpostsurgery). Intervention adherence was tracked as the number of PAT sessions attended.
Posthospital Syndrome Factors
Participant falls (categorized as those who reported a fall vs those who did not) and medication changes (number of changes reported, including new medication, discontinued medication, dose changes, medication changes, or changes in medication schedule) were reported by participants or caregivers during each VVC contact. Participants completed the Montreal Cognitive Assessment (MoCA) at baseline, and were dichotomized into 2 groups: no cognitive impairment (MoCA score ≥ 26) and mild to moderate cognitive impairment (MoCA score 10-25).13,21
Participants rated how much pain interfered with their normal daily activities since the previous VVC session on a 5-point Likert scale (1, not at all; to 5, extremely).22 Similar to prior research, participants were placed into 2 groups based on their mean pain interference score (individuals with scores from 1.0 to 2.0 in 1 group, and individuals with > 2.0 in another).23-25 Participants were separated into a no or mild pain interference group and a moderate to severe pain interference group. Hospital readmissions (VA and non-VA) and postdischarge physical therapy outcomes were obtained from the participant’s EHR, including primary care visits.
Outcomes
Outcomes were collected at baseline (posthospital discharge) and 6 months postenrollment.
Self-Reported Functional Ability. This measure is provided by participants or caregivers and measured by the Katz Index of Independence in Activities of Daily Living (ADL), Lawton and Brody Instrumental ADL Scale (IADL), Nagi Disability Model, and Rosow-Breslau Scale. The Katz ADL assesses the ability to complete 6 self-care activities and awards 1 point for independence and 0 if the individual is dependent (total score range, 0-6).26 The Lawton and Brody IADL measures an individual’s independence in 8 instrumental ADLs; it awards 1 point for independence and 0 if the individual is dependent (total score range, 0-8).27 The Nagi Disability Model evaluates an individual’s difficulty performing 5 tasks (total score range, 0-5) and tallies the number of items with a response other than “no difficulty at all” (higher total score indicates greater difficulty). 28 The Rosow-Breslau Scale is a 3-item measure of mobility disability; individual responses are 0 (no help) and 1 (requires help or unable); higher total score (range, 0-3) indicates greater disability.29
Physical Performance. Measured using the Short Physical Performance Battery (SPPB), which evaluates standing balance, sit to stand, and walking performance. Scores range from 0 to 4 on the balance, gait speed, and chair stand tests, for a total composite score between 0 and 12 (higher score indicates better performance).30
Physical Activity. Measured using actigraphy, namely a physical activity monitor adherent to the thigh (activ-PAL3TM, PAL Technologies Ltd., Glasgow, UK).31 Participants were instructed to wear the activPal for ≥ 1 week. Participants with a minimum of 5 days of wear were included in this analysis.
Data Analyses
Analyses were performed using SPSS software version 29.0.32 Continuous variables were summarized using mean (SD) or median and IQR using the weighted average method; categorical variables were summarized using frequencies and percentages. Baseline scores on outcome variables were compared by categorical hospitalization, posthospital syndrome, and postdischarge health care application factor variables using Mann-Whitney U tests. The differences between outcome variables from baseline to endpoint were then calculated to produce change scores. Relationships between the number of PAT sessions attended and baseline outcomes and outcome change scores were estimated using Spearman correlations. Relationships between categorical factors (hospitalization, posthospital syndrome, and postdischarge health care application) and outcome variable change scores (which were normally distributed) were examined using Mann-Whitney U tests. Relationships with continuous hospitalization (length of stay) and posthospital syndrome factors (medication changes) were estimated using Spearman correlations. Effect sizes (ES) were estimated with Cohen d; small (d = 0.2), medium (d = 0.5), or large (d ≥ 0.8). Missing data were handled using pairwise deletion.33 Therefore, sample sizes were reported for each analysis. For all statistical tests, P < .05 was considered significant.
Results
Twenty-four individuals completed the pilot intervention.15 Mean (SD) age was 73.6 (8.1) years (range, 64-93 years) and participants were predominantly White males (Table 1). Eight participants had a high school education only and 13 had more than a high school education. Diagnoses at admission included 9 patients with orthopedic/musculoskeletal conditions (6 were for joint replacement), 6 patients with vascular/pulmonary conditions, and 4 with gastrointestinal/renal/urological conditions. Of the 11 postsurgery participants, 7 were orthopedic, 4 were gastrointestinal, and 1 was peripheral vascular.

Baseline outcome scores did not differ significantly between groups, except individuals with moderate to severe pain interference reported a significantly lower IADL score (median [IQR] 4 [2-7]) than individuals with mild or moderate pain interference (median [IQR] 8 [7-8]; P = .02) (Table 2). The mean (SD) number of PAT sessions attended was 9.3 (3.7) (range, 3-19). There were no significant relationships between number of sessions attended and any baseline outcome variables or outcome change scores.

Hospitalization Factors
Participants who were postsurgery tended to have greater improvement than individuals who were nonpostsurgery in ADLs (median [IQR] 0 [0-1.5]; ES, 0.6; P = .10) and SPPB (median [IQR] 2 [1.5-9]; ES, 0.9; P = .07), but the improvements were not statistically significant (Table 3). Mean (SD) length of stay of the index hospitalization was 6.7 (6.1) days. Longer length of stay was significantly correlated with an increase in Nagi score (ρ, 0.45; 95% CI, 0.01-0.75). There were no other significant or trending relationships between length of stay and outcome variables.

Posthospital Syndrome Factors
The 16 participants with mild to moderate cognitive impairment had less improvement in ADLs (median [IQR] 0 [0-1]) than the 8 participants with no impairment (median [IQR] 0 [-0.75 to 0]; ES, -1.1; P = .04). Change in outcome variables from baseline to endpoint did not significantly differ between the 8 patients who reported a fall compared with the 13 who did not, nor were any trends observed. Change in outcome variables from baseline to endpoint also did not significantly differ between the 8 participants who reported no or mild pain interference compared with the 10 patients with moderate to severe pain interference, nor were any trends observed. Mean (SD) number of medication changes was 2.5 (1.6). Higher number of medication changes was significantly correlated with a decrease in Rosow-Breslau score (ρ, -0.47; 95% CI, -0.76 to -0.02). There were no other significant or trending relationships between number of medication changes and outcome variables.
Postdischarge Health Care Application Factors
The 16 participants who attended posthospital physical therapy trended towards less improvement in IADLs (median [IQR] 0 [-0.5 to 1.5]; ES, -0.7; P = .11) and SPPB (median [IQR] 2 [-3.0 to 4.5]; ES, -0.5; P = .15) than the 8 patients with no postdischarge physical therapy. Eleven participants were readmitted, while 13 had no readmissions in their medical records between baseline and endpoint. Participants with ≥ 1 readmission experienced a greater increase in Rosow-Breslau score (median [IQR] 0 [-0.5 to 1.0]) than those not readmitted (median [IQR] 0 [-1.25 to 0.25]; ES, 1.0; P = .03). Borderline greater improvement in number of steps was found in those not readmitted (median [IQR] 3365.6 [274.4-7710.9]) compared with those readmitted (median [IQR] 319.9 [-136.1 to 774.5]; ES, -1.3; P = .05). Patients who were readmitted also tended to have lower and not statistically significant improvements in SPPB (median [IQR] 1 [-4.0 to 5.3]) compared with those not readmitted (median [IQR] 2 [0.3-3.8]; ES, -0.5; P = .17) (Table 3).
Discussion
This study examined the association between hospitalization, posthospital syndrome, and postdischarge health care use in patients undergoing a VVC-based intervention following hospital discharge. Participants who had no or mild cognitive impairment, no readmissions, higher medication changes, and a shorter hospital length of stay tended to experience lower disability, including in mobility and ADLs. This suggests individuals who are less clinically complex may be more likely to benefit from this type of virtual rehabilitation program. These findings are consistent with clinical experiences; home-based programs to improve physical activity posthospital discharge can be challenging for those who were medically ill (and did not undergo a specific surgical procedure), cognitively impaired, and become acutely ill and trigger hospital readmission. 15 For example, the sample in this study had higher rates of falls, pain, and readmissions compared to previous research.2,3,34-39
The importance of posthospital syndrome in the context of recovery of function and health at home following hospitalization is well documented.16-18 The potential impact of posthospital syndrome on physical activity-focused interventions is less understood. In our analysis, participants with mild or moderate cognitive impairment tended to become more dependent in their ADLs, while those with no cognitive impairment tended to become more independent in their ADLs. This functional decline over time is perhaps expected in persons with cognitive impairment, but the significant difference with a large ES warrants further consideration on how to tailor interventions to better promote functional recovery in these individuals.40,41 While some cognitive decline may not be preventable, this finding supports the need to promote healthy cognitive aging, identify declines in cognition, and work to mitigate additional decline. Programs specifically designed to promote function and physical activity in older adults with cognitive impairment are needed, especially during care transitions.41-43
While participants reported that falls and pain interference did not have a significant impact on change in outcomes between baseline and endpoint, these areas need further investigation. Falls and pain have been associated with function and physical activity in older adults.42-46 Pain is common, yet underappreciated during older adult hospital-to-home transitions.11,12,45,46 There is a need for more comprehensive assessment of pain (including pain intensity) and qualitative research.
Hospitalization and postdischarge health care application factors may have a significant impact on home-telehealth physical activity intervention success. Individuals who were postsurgery tended to have greater improvements in ADLs and physical performance. Most postsurgery participants had joint replacement surgery. Postsurgery status may not be modifiable, but it is important to note expected differences in recovery between medical and surgical admissions and the need to tailor care based on admission diagnosis. Those with a longer length of hospital stay may be considered at higher risk of suboptimal outcomes postdischarge, which indicates an opportunity for targeting resources and support, in addition to efforts of reducing length of stay where possible.47
Readmissions were significantly related to a change in Rosow-Breslau mobility disability score. This may indicate the detrimental impact a readmission can have on increasing mobility and physical activity postdischarge, or the potential of this pilot program to impact readmissions by increasing mobility and physical activity, contrary to prior physical exercise interventions.5,7,9,48 With 5% to 79% of readmissions considered preventable, continued efforts and program dissemination and implementation to address preventable readmissions are warranted.49 Individuals with postdischarge physical therapy (prior to beginning the pilot program) tended to demonstrate less improvement in disability and physical performance. This relationship needs further investigation; the 2 groups did not appear to have significant differences at baseline, albeit with a small sample size. It is possible they experienced initial improvements with postdischarge physical therapy and plateaued or had little further reserve to improve upon entering the VVC program.
Strengths and Limitations
This pilot program provided evaluative data on the use of VVC to enhance function and physical activity in older adults posthospital discharge. It included individual (eg, fall, pain, cognitive impairment) and health service (eg, readmission, physical therapy) level factors as predictors of function and physical activity posthospitalization.5,7,9,15-19
The results of this pilot project stem from a small sample lacking diversity in terms of race, ethnicity, and sex. There was some variation in baseline and endpoints between participants, and when hospitalization, posthospital syndrome, and postdischarge health care application factors were collected. The majority of participants were recruited within a month postdischarge, and the program lasted about 6 months. Data collection was attempted at regular PAT contacts, but there was some variation in when visits occurred based on participant availability and preference. Some participants had missing data, which was handled using pairwise deletion.33 Larger studies are needed to confirm the findings of this study, particularly the trends that did not reach statistical significance. Home health services other than physical therapy (eg, nursing, occupational therapy) were not fully accounted for and should be considered in future research.
Conclusions
In patients undergoing a 6-month pilot VVC-based physical activity intervention posthospital discharge, improvements in mobility and disability were most likely in those who had no cognitive impairment and were not readmitted. Larger sample and qualitative investigations are necessary to optimize outcomes for patients who meet these clinical profiles.
Deconditioning among hospitalized older adults contributes to significant decline in posthospitalization functional ability, physical performance, and physical activity.1-10 Previous hospital-to-home interventions have targeted improving function and physical activity, including recent programs leveraging home telehealth as a feasible and potentially effective mode of delivering in-home exercise and rehabilitation.11-14 However, pilot interventions have shown mixed effectiveness.11,12,14 This study expands on a previously published intervention describing a pilot home telehealth program for veterans posthospital discharge that demonstrated significant 6-month improvement in physical activity as well as trends in physical function improvement, including among those with cognitive impairment.15 Factors that contribute to improved outcomes are the focus of the present study.
Key factors underlying the complexity of hospital-to-home transitions include hospitalization elements (ie, reason for admission and length of stay), associated posthospital syndromes (ie, postdischarge falls, medication changes, cognitive impairment, and pain), and postdischarge health care application (ie, physical therapy and hospital readmission).16-18 These factors may be associated with postdischarge functional ability, physical performance, and physical activity, but their direct influence on intervention outcomes is unclear (Figure 1).5,7,9,16-20 The objective of this study was to examine the influence of hospitalization, posthospital syndrome, and postdischarge health care application factors on outcomes of a US Department of Veterans Affairs (VA) Video Connect (VVC) intervention to enhance function and physical activity in older adults posthospital discharge.

health care application factors on physical activity, functional ability, and
physical performance intervention outcomes.
Methods
The previous analysis reported on patient characteristics, program feasibility, and preliminary outcomes.13,15 The current study reports on relationships between hospitalization, posthospital syndrome, and postdischarge health care application factors and change in key outcomes, namely postdischarge self-reported functional ability, physical performance, and physical activity from baseline to endpoint.
Participants provided written informed consent. The protocol and consent forms were approved by the VA Ann Arbor Healthcare System (VAAAHS) Research and Development Committee, and the project was registered on clinicaltrials.gov (NCT04045054).
Intervention
The pilot program targeted older adults following recent hospital discharge from VAAAHS. Participants were eligible if they were aged ≥ 50 years, had been discharged following an inpatient stay in the past 1 to 2 weeks, evaluated by physical therapy during hospitalization with stated rehabilitation goals on discharge, and followed by a VAAAHS primary care physician. Participants were either recruited during hospital admission or shortly after discharge.13
An experienced physical activity trainer (PAT) supported the progression of participants’ rehabilitation goals via a home exercise program and coached the patient and caregiver to optimize functional ability, physical performance, and physical activity. The PAT was a nonlicensed research assistant with extensive experience in applying standard physical activity enhancement protocols (eg, increased walking) to older adults with comorbidities. Participation in the program lasted about 6 months. Initiation of the PAT program was delayed if the patient was already receiving postdischarge home-based or outpatient physical therapy. The PAT contacted the patient weekly via VVC for the first 6 weeks, then monthly for a total of 6 months. Each contact included information on optimal walking form, injury prevention, program progression, and ways to incorporate sit-to-stand transitions, nonsitting behavior, and walking into daily routines. The initial VVC contact lasted about 60 minutes and subsequent sessions lasted about 30 minutes.13
Demographic characteristics were self-reported by participants and included age, sex, race, years of education, and marital status. Clinical characteristics were obtained from each participant’s electronic health record (EHR), including copay status, index hospitalization length of stay, admission diagnosis, and postsurgery status (postsurgery vs nonpostsurgery). Intervention adherence was tracked as the number of PAT sessions attended.
Posthospital Syndrome Factors
Participant falls (categorized as those who reported a fall vs those who did not) and medication changes (number of changes reported, including new medication, discontinued medication, dose changes, medication changes, or changes in medication schedule) were reported by participants or caregivers during each VVC contact. Participants completed the Montreal Cognitive Assessment (MoCA) at baseline, and were dichotomized into 2 groups: no cognitive impairment (MoCA score ≥ 26) and mild to moderate cognitive impairment (MoCA score 10-25).13,21
Participants rated how much pain interfered with their normal daily activities since the previous VVC session on a 5-point Likert scale (1, not at all; to 5, extremely).22 Similar to prior research, participants were placed into 2 groups based on their mean pain interference score (individuals with scores from 1.0 to 2.0 in 1 group, and individuals with > 2.0 in another).23-25 Participants were separated into a no or mild pain interference group and a moderate to severe pain interference group. Hospital readmissions (VA and non-VA) and postdischarge physical therapy outcomes were obtained from the participant’s EHR, including primary care visits.
Outcomes
Outcomes were collected at baseline (posthospital discharge) and 6 months postenrollment.
Self-Reported Functional Ability. This measure is provided by participants or caregivers and measured by the Katz Index of Independence in Activities of Daily Living (ADL), Lawton and Brody Instrumental ADL Scale (IADL), Nagi Disability Model, and Rosow-Breslau Scale. The Katz ADL assesses the ability to complete 6 self-care activities and awards 1 point for independence and 0 if the individual is dependent (total score range, 0-6).26 The Lawton and Brody IADL measures an individual’s independence in 8 instrumental ADLs; it awards 1 point for independence and 0 if the individual is dependent (total score range, 0-8).27 The Nagi Disability Model evaluates an individual’s difficulty performing 5 tasks (total score range, 0-5) and tallies the number of items with a response other than “no difficulty at all” (higher total score indicates greater difficulty). 28 The Rosow-Breslau Scale is a 3-item measure of mobility disability; individual responses are 0 (no help) and 1 (requires help or unable); higher total score (range, 0-3) indicates greater disability.29
Physical Performance. Measured using the Short Physical Performance Battery (SPPB), which evaluates standing balance, sit to stand, and walking performance. Scores range from 0 to 4 on the balance, gait speed, and chair stand tests, for a total composite score between 0 and 12 (higher score indicates better performance).30
Physical Activity. Measured using actigraphy, namely a physical activity monitor adherent to the thigh (activ-PAL3TM, PAL Technologies Ltd., Glasgow, UK).31 Participants were instructed to wear the activPal for ≥ 1 week. Participants with a minimum of 5 days of wear were included in this analysis.
Data Analyses
Analyses were performed using SPSS software version 29.0.32 Continuous variables were summarized using mean (SD) or median and IQR using the weighted average method; categorical variables were summarized using frequencies and percentages. Baseline scores on outcome variables were compared by categorical hospitalization, posthospital syndrome, and postdischarge health care application factor variables using Mann-Whitney U tests. The differences between outcome variables from baseline to endpoint were then calculated to produce change scores. Relationships between the number of PAT sessions attended and baseline outcomes and outcome change scores were estimated using Spearman correlations. Relationships between categorical factors (hospitalization, posthospital syndrome, and postdischarge health care application) and outcome variable change scores (which were normally distributed) were examined using Mann-Whitney U tests. Relationships with continuous hospitalization (length of stay) and posthospital syndrome factors (medication changes) were estimated using Spearman correlations. Effect sizes (ES) were estimated with Cohen d; small (d = 0.2), medium (d = 0.5), or large (d ≥ 0.8). Missing data were handled using pairwise deletion.33 Therefore, sample sizes were reported for each analysis. For all statistical tests, P < .05 was considered significant.
Results
Twenty-four individuals completed the pilot intervention.15 Mean (SD) age was 73.6 (8.1) years (range, 64-93 years) and participants were predominantly White males (Table 1). Eight participants had a high school education only and 13 had more than a high school education. Diagnoses at admission included 9 patients with orthopedic/musculoskeletal conditions (6 were for joint replacement), 6 patients with vascular/pulmonary conditions, and 4 with gastrointestinal/renal/urological conditions. Of the 11 postsurgery participants, 7 were orthopedic, 4 were gastrointestinal, and 1 was peripheral vascular.

Baseline outcome scores did not differ significantly between groups, except individuals with moderate to severe pain interference reported a significantly lower IADL score (median [IQR] 4 [2-7]) than individuals with mild or moderate pain interference (median [IQR] 8 [7-8]; P = .02) (Table 2). The mean (SD) number of PAT sessions attended was 9.3 (3.7) (range, 3-19). There were no significant relationships between number of sessions attended and any baseline outcome variables or outcome change scores.

Hospitalization Factors
Participants who were postsurgery tended to have greater improvement than individuals who were nonpostsurgery in ADLs (median [IQR] 0 [0-1.5]; ES, 0.6; P = .10) and SPPB (median [IQR] 2 [1.5-9]; ES, 0.9; P = .07), but the improvements were not statistically significant (Table 3). Mean (SD) length of stay of the index hospitalization was 6.7 (6.1) days. Longer length of stay was significantly correlated with an increase in Nagi score (ρ, 0.45; 95% CI, 0.01-0.75). There were no other significant or trending relationships between length of stay and outcome variables.

Posthospital Syndrome Factors
The 16 participants with mild to moderate cognitive impairment had less improvement in ADLs (median [IQR] 0 [0-1]) than the 8 participants with no impairment (median [IQR] 0 [-0.75 to 0]; ES, -1.1; P = .04). Change in outcome variables from baseline to endpoint did not significantly differ between the 8 patients who reported a fall compared with the 13 who did not, nor were any trends observed. Change in outcome variables from baseline to endpoint also did not significantly differ between the 8 participants who reported no or mild pain interference compared with the 10 patients with moderate to severe pain interference, nor were any trends observed. Mean (SD) number of medication changes was 2.5 (1.6). Higher number of medication changes was significantly correlated with a decrease in Rosow-Breslau score (ρ, -0.47; 95% CI, -0.76 to -0.02). There were no other significant or trending relationships between number of medication changes and outcome variables.
Postdischarge Health Care Application Factors
The 16 participants who attended posthospital physical therapy trended towards less improvement in IADLs (median [IQR] 0 [-0.5 to 1.5]; ES, -0.7; P = .11) and SPPB (median [IQR] 2 [-3.0 to 4.5]; ES, -0.5; P = .15) than the 8 patients with no postdischarge physical therapy. Eleven participants were readmitted, while 13 had no readmissions in their medical records between baseline and endpoint. Participants with ≥ 1 readmission experienced a greater increase in Rosow-Breslau score (median [IQR] 0 [-0.5 to 1.0]) than those not readmitted (median [IQR] 0 [-1.25 to 0.25]; ES, 1.0; P = .03). Borderline greater improvement in number of steps was found in those not readmitted (median [IQR] 3365.6 [274.4-7710.9]) compared with those readmitted (median [IQR] 319.9 [-136.1 to 774.5]; ES, -1.3; P = .05). Patients who were readmitted also tended to have lower and not statistically significant improvements in SPPB (median [IQR] 1 [-4.0 to 5.3]) compared with those not readmitted (median [IQR] 2 [0.3-3.8]; ES, -0.5; P = .17) (Table 3).
Discussion
This study examined the association between hospitalization, posthospital syndrome, and postdischarge health care use in patients undergoing a VVC-based intervention following hospital discharge. Participants who had no or mild cognitive impairment, no readmissions, higher medication changes, and a shorter hospital length of stay tended to experience lower disability, including in mobility and ADLs. This suggests individuals who are less clinically complex may be more likely to benefit from this type of virtual rehabilitation program. These findings are consistent with clinical experiences; home-based programs to improve physical activity posthospital discharge can be challenging for those who were medically ill (and did not undergo a specific surgical procedure), cognitively impaired, and become acutely ill and trigger hospital readmission. 15 For example, the sample in this study had higher rates of falls, pain, and readmissions compared to previous research.2,3,34-39
The importance of posthospital syndrome in the context of recovery of function and health at home following hospitalization is well documented.16-18 The potential impact of posthospital syndrome on physical activity-focused interventions is less understood. In our analysis, participants with mild or moderate cognitive impairment tended to become more dependent in their ADLs, while those with no cognitive impairment tended to become more independent in their ADLs. This functional decline over time is perhaps expected in persons with cognitive impairment, but the significant difference with a large ES warrants further consideration on how to tailor interventions to better promote functional recovery in these individuals.40,41 While some cognitive decline may not be preventable, this finding supports the need to promote healthy cognitive aging, identify declines in cognition, and work to mitigate additional decline. Programs specifically designed to promote function and physical activity in older adults with cognitive impairment are needed, especially during care transitions.41-43
While participants reported that falls and pain interference did not have a significant impact on change in outcomes between baseline and endpoint, these areas need further investigation. Falls and pain have been associated with function and physical activity in older adults.42-46 Pain is common, yet underappreciated during older adult hospital-to-home transitions.11,12,45,46 There is a need for more comprehensive assessment of pain (including pain intensity) and qualitative research.
Hospitalization and postdischarge health care application factors may have a significant impact on home-telehealth physical activity intervention success. Individuals who were postsurgery tended to have greater improvements in ADLs and physical performance. Most postsurgery participants had joint replacement surgery. Postsurgery status may not be modifiable, but it is important to note expected differences in recovery between medical and surgical admissions and the need to tailor care based on admission diagnosis. Those with a longer length of hospital stay may be considered at higher risk of suboptimal outcomes postdischarge, which indicates an opportunity for targeting resources and support, in addition to efforts of reducing length of stay where possible.47
Readmissions were significantly related to a change in Rosow-Breslau mobility disability score. This may indicate the detrimental impact a readmission can have on increasing mobility and physical activity postdischarge, or the potential of this pilot program to impact readmissions by increasing mobility and physical activity, contrary to prior physical exercise interventions.5,7,9,48 With 5% to 79% of readmissions considered preventable, continued efforts and program dissemination and implementation to address preventable readmissions are warranted.49 Individuals with postdischarge physical therapy (prior to beginning the pilot program) tended to demonstrate less improvement in disability and physical performance. This relationship needs further investigation; the 2 groups did not appear to have significant differences at baseline, albeit with a small sample size. It is possible they experienced initial improvements with postdischarge physical therapy and plateaued or had little further reserve to improve upon entering the VVC program.
Strengths and Limitations
This pilot program provided evaluative data on the use of VVC to enhance function and physical activity in older adults posthospital discharge. It included individual (eg, fall, pain, cognitive impairment) and health service (eg, readmission, physical therapy) level factors as predictors of function and physical activity posthospitalization.5,7,9,15-19
The results of this pilot project stem from a small sample lacking diversity in terms of race, ethnicity, and sex. There was some variation in baseline and endpoints between participants, and when hospitalization, posthospital syndrome, and postdischarge health care application factors were collected. The majority of participants were recruited within a month postdischarge, and the program lasted about 6 months. Data collection was attempted at regular PAT contacts, but there was some variation in when visits occurred based on participant availability and preference. Some participants had missing data, which was handled using pairwise deletion.33 Larger studies are needed to confirm the findings of this study, particularly the trends that did not reach statistical significance. Home health services other than physical therapy (eg, nursing, occupational therapy) were not fully accounted for and should be considered in future research.
Conclusions
In patients undergoing a 6-month pilot VVC-based physical activity intervention posthospital discharge, improvements in mobility and disability were most likely in those who had no cognitive impairment and were not readmitted. Larger sample and qualitative investigations are necessary to optimize outcomes for patients who meet these clinical profiles.
- Liebzeit D, Bratzke L, Boltz M, Purvis S, King B. Getting back to normal: a grounded theory study of function in post-hospitalized older adults. Gerontologist. 2020;60:704-714. doi:10.1093/geront/gnz057
- Ponzetto M, Zanocchi M, Maero B, et al. Post-hospitalization mortality in the elderly. Arch Gerontol Geriatr. 2003;36:83-91. doi:10.1016/s0167-4943(02)00061-4
- Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6:e26951. doi:10.1371/journal.pone.0026951
- Ponzetto M, Maero B, Maina P, et al. Risk factors for early and late mortality in hospitalized older patients: the continuing importance of functional status. J Gerontol A Biol Sci Med Sci. 2003;58:1049-1054. doi:10.1093/gerona/58.11.m1049
- Huang HT, Chang CM, Liu LF, Lin HS, Chen CH. Trajectories and predictors of functional decline of hospitalised older patients. J Clin Nurs. 2013;22:1322-1331. doi:10.1111/jocn.12055
- Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171- 2179. doi:10.1111/j.1532-5415.2008.02023.x
- Helvik AS, Selbæk G, Engedal K. Functional decline in older adults one year after hospitalization. Arch Gerontol Geriatr. 2013;57:305-310. doi:10.1016/j.archger.2013.05.008
- Zaslavsky O, Zisberg A, Shadmi E. Impact of functional change before and during hospitalization on functional recovery 1 month following hospitalization. J Gerontol Biol Sci Med Sci. 2015;70:381-386. doi:10.1093/gerona/glu168
- Chen CC, Wang C, Huang GH. Functional trajectory 6 months posthospitalization: a cohort study of older hospitalized patients in Taiwan. Nurs Res. 2008;57:93-100. doi:10.1097/01.NNR.0000313485.18670.e2
- Kleinpell RM, Fletcher K, Jennings BM. Reducing functional decline in hospitalized elderly. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008. Accessed September 3, 2025. http://www.ncbi.nlm.nih.gov/books/NBK2629/
- Liebzeit D, Rutkowski R, Arbaje AI, Fields B, Werner NE. A scoping review of interventions for older adults transitioning from hospital to home. J Am Geriatr Soc. 2021;69:2950-2962. doi:10.1111/jgs.17323
- Hladkowicz E, Dumitrascu F, Auais M, et al. Evaluations of postoperative transitions in care for older adults: a scoping review. BMC Geriatr. 2022;22:329. doi:10.1186/s12877-022-02989-6
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA Video Connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: baseline assessment. BMC Geriatr. 2021;21:502. doi:10.1186/s12877-021-02454-w
- Dawson R, Oliveira JS, Kwok WS, et al. Exercise interventions delivered through telehealth to improve physical functioning for older adults with frailty, cognitive, or mobility disability: a systematic review and meta-analysis. Telemed J E Health. 2024;30:940-950. doi:10.1089/tmj.2023.0177
- Liebzeit D, Phillips KK, Hogikyan RV, Cigolle CT, Alexander NB. A pilot home-telehealth program to enhance functional ability, physical performance, and physical activity in older adult veterans post-hospital discharge. Res Gerontol Nurs. 2024;17:271-279. doi:10.3928/19404921-20241105-01
- Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100-102. doi:10.1056/NEJMp1212324
- Caraballo C, Dharmarajan K, Krumholz HM. Post hospital syndrome: is the stress of hospitalization causing harm? Rev Esp Cardiol (Engl Ed). 2019;72:896-898. doi:10.1016/j.rec.2019.04.010
- Rawal S, Kwan JL, Razak F, et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern Med. 2019;179:38- 45. doi:10.1001/jamainternmed.2018.5100
- Dutzi I, Schwenk M, Kirchner M, Jooss E, Bauer JM, Hauer K. Influence of cognitive impairment on rehabilitation received and its mediating effect on functional recovery. J Alzheimers Dis. 2021;84:745-756. doi:10.3233/JAD-210620
- Uriz-Otano F, Uriz-Otano JI, Malafarina V. Factors associated with short-term functional recovery in elderly people with a hip fracture. Influence ofcognitiveimpairment. JAmMedDirAssoc. 2015;16:215-220. doi:10.1016/j.jamda.2014.09.009
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. doi:10.1111/j.1532-5415.2005.53221.x
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483.
- White RS, Jiang J, Hall CB, et al. Higher perceived stress scale scores are associated with higher pain intensity and pain interference levels in older adults. J Am Geriatr Soc. 2014;62:2350-2356. doi:10.1111/jgs.13135
- Blyth FM, March LM, Brnabic AJ, et al. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127-134. doi:10.1016/s0304-3959(00)00355-9
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain. 2004;110:361-368. doi:10.1016/j.pain.2004.04.017
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi:10.1001/jama.1963.03060120024016
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
- Alexander NB, Guire KE, Thelen DG, et al. Self-reported walking ability predicts functional mobility performance in frail older adults. J Am Geriatr Soc. 2000;48:1408-1413. doi:10.1111/j.1532-5415.2000.tb02630.x
- Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556-559. doi:10.1093/geronj/21.4.556
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. doi:10.1093/geronj/49.2.m85
- Chan CS, Slaughter SE, Jones CA, Ickert C, Wagg AS. Measuring activity performance of older adults using the activPAL: a rapid review. Healthcare (Basel). 2017;5:94. doi:10.3390/healthcare5040094
- IBM SPSS software. IBM Corp; 2019. Accessed September 3, 2025. https://www.ibm.com/spss
- Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64:402-406. doi:10.4097/kjae.2013.64.5.402
- Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365:2287-2295. doi:10.1056/NEJMsa1101942
- Bogaisky M, Dezieck L. Early hospital readmission of nursing home residents and community-dwelling elderly adults discharged from the geriatrics service of an urban teaching hospital: patterns and risk factors. J Am Geriatr Soc. 2015;63:548-552. doi:10.1111/jgs.13317
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428. doi:10.1056/NEJMsa0803563
- Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9:277-282. doi:10.1002/jhm.2152
- Mahoney J, Sager M, Dunham NC, Johnson J. Risk of falls after hospital discharge. J Am Geriatr Soc. 1994;42:269- 274. doi:10.1111/j.1532-5415.1994.tb01750.x
- Hoffman GJ, Liu H, Alexander NB, Tinetti M, Braun TM, Min LC. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276. doi:10.1001/jamanetworkopen.2019.4276
- Gill DP, Hubbard RA, Koepsell TD, et al. Differences in rate of functional decline across three dementia types. Alzheimers Dement. 2013;9:S63-S71. doi:10.1016/j.jalz.2012.10.010
- Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment–the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167-173. doi:10.1159/000154929
- Patti A, Zangla D, Sahin FN, et al. Physical exercise and prevention of falls. Effects of a Pilates training method compared with a general physical activity program. Medicine (Baltimore). 2021;100:e25289. doi:10.1097/MD.0000000000025289
- Nagarkar A, Kulkarni S. Association between daily activities and fall in older adults: an analysis of longitudinal ageing study in India (2017-18). BMC Geriatr. 2022;22:203. doi:10.1186/s12877-022-02879-x
- Ek S, Rizzuto D, Xu W, Calderón-Larrañaga A, Welmer AK. Predictors for functional decline after an injurious fall: a population-based cohort study. Aging Clin Exp Res. 2021;33:2183-2190. doi:10.1007/s40520-020-01747-1
- Dagnino APA, Campos MM. Chronic pain in the elderly: mechanisms and perspectives. Front Hum Neurosci. 2022;16:736688. doi:10.3389/fnhum.2022.736688
- Ritchie CS, Patel K, Boscardin J, et al. Impact of persistent pain on function, cognition, and well-being of older adults. J Am Geriatr Soc. 2023;71:26-35. doi:10.1111/jgs.18125
- Han TS, Murray P, Robin J, et al. Evaluation of the association of length of stay in hospital and outcomes. Int J Qual Health Care. 2022;34:mzab160. doi:10.1093/intqhc/ mzab160
- Lærum-Onsager E, Molin M, Olsen CF, et al. Effect of nutritional and physical exercise intervention on hospital readmission for patients aged 65 or older: a systematic review and meta-analysis of randomized controlled trials. Int J Behav Nutr Phys Act. 2021;18:62. doi:10.1186/s12966-021-01123-w
- Van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391-E402. doi:10.1503/cmaj.101860
- Liebzeit D, Bratzke L, Boltz M, Purvis S, King B. Getting back to normal: a grounded theory study of function in post-hospitalized older adults. Gerontologist. 2020;60:704-714. doi:10.1093/geront/gnz057
- Ponzetto M, Zanocchi M, Maero B, et al. Post-hospitalization mortality in the elderly. Arch Gerontol Geriatr. 2003;36:83-91. doi:10.1016/s0167-4943(02)00061-4
- Buurman BM, Hoogerduijn JG, de Haan RJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6:e26951. doi:10.1371/journal.pone.0026951
- Ponzetto M, Maero B, Maina P, et al. Risk factors for early and late mortality in hospitalized older patients: the continuing importance of functional status. J Gerontol A Biol Sci Med Sci. 2003;58:1049-1054. doi:10.1093/gerona/58.11.m1049
- Huang HT, Chang CM, Liu LF, Lin HS, Chen CH. Trajectories and predictors of functional decline of hospitalised older patients. J Clin Nurs. 2013;22:1322-1331. doi:10.1111/jocn.12055
- Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of activities of daily living in older adults after hospitalization for acute medical illness. J Am Geriatr Soc. 2008;56:2171- 2179. doi:10.1111/j.1532-5415.2008.02023.x
- Helvik AS, Selbæk G, Engedal K. Functional decline in older adults one year after hospitalization. Arch Gerontol Geriatr. 2013;57:305-310. doi:10.1016/j.archger.2013.05.008
- Zaslavsky O, Zisberg A, Shadmi E. Impact of functional change before and during hospitalization on functional recovery 1 month following hospitalization. J Gerontol Biol Sci Med Sci. 2015;70:381-386. doi:10.1093/gerona/glu168
- Chen CC, Wang C, Huang GH. Functional trajectory 6 months posthospitalization: a cohort study of older hospitalized patients in Taiwan. Nurs Res. 2008;57:93-100. doi:10.1097/01.NNR.0000313485.18670.e2
- Kleinpell RM, Fletcher K, Jennings BM. Reducing functional decline in hospitalized elderly. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses. Agency for Healthcare Research and Quality (US); 2008. Accessed September 3, 2025. http://www.ncbi.nlm.nih.gov/books/NBK2629/
- Liebzeit D, Rutkowski R, Arbaje AI, Fields B, Werner NE. A scoping review of interventions for older adults transitioning from hospital to home. J Am Geriatr Soc. 2021;69:2950-2962. doi:10.1111/jgs.17323
- Hladkowicz E, Dumitrascu F, Auais M, et al. Evaluations of postoperative transitions in care for older adults: a scoping review. BMC Geriatr. 2022;22:329. doi:10.1186/s12877-022-02989-6
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA Video Connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: baseline assessment. BMC Geriatr. 2021;21:502. doi:10.1186/s12877-021-02454-w
- Dawson R, Oliveira JS, Kwok WS, et al. Exercise interventions delivered through telehealth to improve physical functioning for older adults with frailty, cognitive, or mobility disability: a systematic review and meta-analysis. Telemed J E Health. 2024;30:940-950. doi:10.1089/tmj.2023.0177
- Liebzeit D, Phillips KK, Hogikyan RV, Cigolle CT, Alexander NB. A pilot home-telehealth program to enhance functional ability, physical performance, and physical activity in older adult veterans post-hospital discharge. Res Gerontol Nurs. 2024;17:271-279. doi:10.3928/19404921-20241105-01
- Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100-102. doi:10.1056/NEJMp1212324
- Caraballo C, Dharmarajan K, Krumholz HM. Post hospital syndrome: is the stress of hospitalization causing harm? Rev Esp Cardiol (Engl Ed). 2019;72:896-898. doi:10.1016/j.rec.2019.04.010
- Rawal S, Kwan JL, Razak F, et al. Association of the trauma of hospitalization with 30-day readmission or emergency department visit. JAMA Intern Med. 2019;179:38- 45. doi:10.1001/jamainternmed.2018.5100
- Dutzi I, Schwenk M, Kirchner M, Jooss E, Bauer JM, Hauer K. Influence of cognitive impairment on rehabilitation received and its mediating effect on functional recovery. J Alzheimers Dis. 2021;84:745-756. doi:10.3233/JAD-210620
- Uriz-Otano F, Uriz-Otano JI, Malafarina V. Factors associated with short-term functional recovery in elderly people with a hip fracture. Influence ofcognitiveimpairment. JAmMedDirAssoc. 2015;16:215-220. doi:10.1016/j.jamda.2014.09.009
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. doi:10.1111/j.1532-5415.2005.53221.x
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473-483.
- White RS, Jiang J, Hall CB, et al. Higher perceived stress scale scores are associated with higher pain intensity and pain interference levels in older adults. J Am Geriatr Soc. 2014;62:2350-2356. doi:10.1111/jgs.13135
- Blyth FM, March LM, Brnabic AJ, et al. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127-134. doi:10.1016/s0304-3959(00)00355-9
- Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP). Pain. 2004;110:361-368. doi:10.1016/j.pain.2004.04.017
- Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914-919. doi:10.1001/jama.1963.03060120024016
- Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
- Alexander NB, Guire KE, Thelen DG, et al. Self-reported walking ability predicts functional mobility performance in frail older adults. J Am Geriatr Soc. 2000;48:1408-1413. doi:10.1111/j.1532-5415.2000.tb02630.x
- Rosow I, Breslau N. A Guttman health scale for the aged. J Gerontol. 1966;21:556-559. doi:10.1093/geronj/21.4.556
- Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85-M94. doi:10.1093/geronj/49.2.m85
- Chan CS, Slaughter SE, Jones CA, Ickert C, Wagg AS. Measuring activity performance of older adults using the activPAL: a rapid review. Healthcare (Basel). 2017;5:94. doi:10.3390/healthcare5040094
- IBM SPSS software. IBM Corp; 2019. Accessed September 3, 2025. https://www.ibm.com/spss
- Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64:402-406. doi:10.4097/kjae.2013.64.5.402
- Epstein AM, Jha AK, Orav EJ. The relationship between hospital admission rates and rehospitalizations. N Engl J Med. 2011;365:2287-2295. doi:10.1056/NEJMsa1101942
- Bogaisky M, Dezieck L. Early hospital readmission of nursing home residents and community-dwelling elderly adults discharged from the geriatrics service of an urban teaching hospital: patterns and risk factors. J Am Geriatr Soc. 2015;63:548-552. doi:10.1111/jgs.13317
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418-1428. doi:10.1056/NEJMsa0803563
- Hoyer EH, Needham DM, Atanelov L, Knox B, Friedman M, Brotman DJ. Association of impaired functional status at hospital discharge and subsequent rehospitalization. J Hosp Med. 2014;9:277-282. doi:10.1002/jhm.2152
- Mahoney J, Sager M, Dunham NC, Johnson J. Risk of falls after hospital discharge. J Am Geriatr Soc. 1994;42:269- 274. doi:10.1111/j.1532-5415.1994.tb01750.x
- Hoffman GJ, Liu H, Alexander NB, Tinetti M, Braun TM, Min LC. Posthospital fall injuries and 30-day readmissions in adults 65 years and older. JAMA Netw Open. 2019;2:e194276. doi:10.1001/jamanetworkopen.2019.4276
- Gill DP, Hubbard RA, Koepsell TD, et al. Differences in rate of functional decline across three dementia types. Alzheimers Dement. 2013;9:S63-S71. doi:10.1016/j.jalz.2012.10.010
- Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment–the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167-173. doi:10.1159/000154929
- Patti A, Zangla D, Sahin FN, et al. Physical exercise and prevention of falls. Effects of a Pilates training method compared with a general physical activity program. Medicine (Baltimore). 2021;100:e25289. doi:10.1097/MD.0000000000025289
- Nagarkar A, Kulkarni S. Association between daily activities and fall in older adults: an analysis of longitudinal ageing study in India (2017-18). BMC Geriatr. 2022;22:203. doi:10.1186/s12877-022-02879-x
- Ek S, Rizzuto D, Xu W, Calderón-Larrañaga A, Welmer AK. Predictors for functional decline after an injurious fall: a population-based cohort study. Aging Clin Exp Res. 2021;33:2183-2190. doi:10.1007/s40520-020-01747-1
- Dagnino APA, Campos MM. Chronic pain in the elderly: mechanisms and perspectives. Front Hum Neurosci. 2022;16:736688. doi:10.3389/fnhum.2022.736688
- Ritchie CS, Patel K, Boscardin J, et al. Impact of persistent pain on function, cognition, and well-being of older adults. J Am Geriatr Soc. 2023;71:26-35. doi:10.1111/jgs.18125
- Han TS, Murray P, Robin J, et al. Evaluation of the association of length of stay in hospital and outcomes. Int J Qual Health Care. 2022;34:mzab160. doi:10.1093/intqhc/ mzab160
- Lærum-Onsager E, Molin M, Olsen CF, et al. Effect of nutritional and physical exercise intervention on hospital readmission for patients aged 65 or older: a systematic review and meta-analysis of randomized controlled trials. Int J Behav Nutr Phys Act. 2021;18:62. doi:10.1186/s12966-021-01123-w
- Van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183:E391-E402. doi:10.1503/cmaj.101860
Factors Influencing Outcomes of a Telehealth-Based Physical Activity Program in Older Veterans Postdischarge
Factors Influencing Outcomes of a Telehealth-Based Physical Activity Program in Older Veterans Postdischarge
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
Primary care practitioners (PCPs) in the US Department of Veterans Affairs (VA) provide care for patients with higher rates of many diseases—diabetes, heart disease, cancer, chronic obstructive pulmonary disease (COPD), and stroke—compared to the nonveteran population. 1 Due to the medical complexities of these diseases, they are often misdiagnosed or not diagnosed at all.
COPD is hiding in plain sight, impacting quality of life and burdening US health care systems.2 Research has yielded new treatments and evidence-based guidelines; however, COPD remains underdiagnosed. Only 13 million of the estimated 79 million US adults with COPD aged 20 to 79 years have been formally diagnosed.3 By the time patients are diagnosed, the disease is often advanced, and therapies are less effective. In 2 large studies of patients with COPD symptoms, later diagnosis was associated with worse outcomes.4,5
Veterans have a higher prevalence of COPD (8%-19%) than nonveterans (6%), likely due to higher rates of smoking and service-related exposures, especially among veterans of post-9/11 conflicts.6,7 Veterans do not always report symptoms and PCPs may not ask about symptoms, leading to underdiagnosis.8 The combination of high likelihood and underdetection of COPD presents a challenge and a target for VA quality improvement (QI).
The US Preventive Services Task Force (USPSTF) recommends against screening asymptomatic patients for COPD. However, both the USPSTF and the Global Initiative for Chronic Obstructive Lung Disease Report advocate for active case finding in primary care clinics to determine whether high-risk patients, such as smokers, experience COPD symptoms and warrant spirometry. 9,10 To make early COPD diagnoses, clinicians may use questionnaires alone or in combination with handheld peak expiratory flow rate measurements.11,12 Formal spirometry, considered the gold standard for COPD diagnosis, is ordered for patients who report COPD symptoms (ie, shortness of breath with exertion) or who have both COPD symptoms and reduced peak flow rates.
A systematic review and meta-analysis found that while the combination of questionnaires and peak flows was the more effective strategy overall, questionnaires alone were also valuable for identifying patients with possible COPD.13 Implementation of either screening method in primary care practices would be challenging. In a simulation study that applied chronic disease and preventive care guidelines to hypothetical patient panels, the time required for PCPs to provide guideline-recommended chronic and preventive care in addition to acute care far exceeded 8 hours per day, even in team-based settings.14 Overburdened PCPs are therefore unlikely to accept additional tasks like COPD case finding.
Why don’t patients report their pulmonary symptoms? Patients may not recognize the symptoms as evidence of COPD. Others may be afraid of a COPD diagnosis or the stigma that is associated with it.15 Perhaps they believe COPD treatment is ineffective because of lung damage from smoking. Some patients may not want to know if they have COPD, while others reduce activity levels to avoid symptoms.16
QUALITY IMPROVEMENT PROJECT
Given the high prevalence of COPD among veterans and the potential for underdiagnosis, VA Northeast Ohio Healthcare System (VANEOHS) internal medicine residents and faculty assessed the state of COPD diagnosis in its primary care clinic with a QI project in 2022. Patients in the clinic between August 1, 2015, and November 30, 2022, with an International Classification of Diseases-10 (ICD-10) COPD diagnosis code (J44) in the electronic health record were included. Of 157 included patients, 105 patients who had prior spirometry testing were excluded. Of the 52 patients with diagnosed COPD and no spirometry testing, 30 patients had computed tomography (CT) findings consistent with COPD (ie, airway thickening, emphysema, air trapping) that was performed for CT lung cancer screening (LCS).17 Twenty-three of these 30 patients were contacted by phone. All 23 were ever smokers and 13 reported COPD symptoms. The PCPs of the symptomatic patients were then contacted. Spirometry was ordered for all 13 patients and completed by 7. Three spirometry tests confirmed the COPD diagnosis. One PCP initiated inhaler therapy for a patient with newly diagnosed COPD.
All 11 PCPs of symptomatic patients were interviewed (many had > 1 symptomatic patient). They reported being unaware of patients’ COPD symptoms because the patients did not mention them, noting that screening for COPD was not a priority.
Role of Lung Cancer Screening
VA PCPs use electronic health record clinical reminders to track tests, consults, chronic disease education, cancer screenings, and routine health maintenance. A clinical reminder already exists (based on USPSTF recommendations) for LCS for patients aged 50 to 80 years who have a smoking history of 20 pack years. Patients who meet these criteria would also be considered high risk for COPD.
The VANEOHS QI project suggests that previously undiagnosed patients with findings of COPD on LCS may also have symptoms of COPD. Therefore, we wondered whether the LCS clinical reminder could serve a second purpose by prompting PCPs to ask veterans who meet LCS criteria about their COPD symptoms.
In 2022, about 13 million patients were eligible for LCS.18 Patients who qualify for LCS are at high risk for other cardiopulmonary disorders, such as COPD and coronary artery disease. Lung cancer is detected in only 1% of patients screened with CT at baseline. However, more often LCS yields evidence of additional cardiopulmonary disorders, such as emphysema or coronary artery calcifications. The International Early Lung Cancer Program (I-ELCAP) and the National Lung Cancer Screening Trial (NLST), which included > 79,000 patients, found evidence of emphysema on CT imaging in 24% and 31% of cases, respectively.19,20 In both cohorts, > 80% of patients with emphysema on CT imaging had no prior history of COPD.
In a 2022 article summarizing the potential impact of CT LCS on COPD diagnosis, Mulshine et al suggest that detection of emphysema on CT LCS provides “earlier recognition for PCPs to identify patients who would benefit from detailed symptom screening to prompt spirometry for COPD detection” and additional motivation for tobacco cessation.21 The VANEOHS QI project was developed and implemented prior to I-ELCAP or NLST reporting results but reinforces the value of CT LCS for COPD diagnosis.
Early diagnosis of COPD remains challenging because PCPs do not ask, patients do not tell, and symptoms can easily be dismissed. However, earlier diagnosis of COPD in symptomatic patients improves outcomes.3,4 To bridge this gap, VA PCPs and primary care patient aligned care teams (PACTs) need to commit to probing high-risk patients for COPD symptoms and ordering spirometry for those who are symptomatic. To accomplish this task, primary care teams need help.
The VANEOHS QI project confirmed that some patients with evidence of COPD on CT have symptoms of COPD that they did not share with their PCPs and suggests that LCS can be used as a dual action case finding method to screen both for lung cancer and COPD. We propose that patients who are eligible for LCS should also be probed for COPD symptoms at their clinic visits; for symptomatic patients, spirometry should be ordered, and COPD evidence-based management should be initiated when spirometry results are consistent with COPD. Annual probing for COPD symptoms could be considered in asymptomatic patients with ongoing tobacco use or emphysema on CT, since they may develop symptoms in the future. This new case-finding method bypasses the need for time-prohibitive questionnaires or peak flow measurements.
Future Opportunities
VA PCPs juggle many priorities and despite the simplicity of this new case finding COPD method, it may be unintentionally overlooked. PCPs often run out of time or may forget to ask patients about COPD symptoms when ordering LCS.
Future innovations to increase COPD diagnosis could include the creation of a yearly VA clinical reminder linked to the tobacco use reminder that has check boxes asking about symptoms of COPD in current and prior smokers. If patients have COPD symptoms, the reminder can prompt the ordering of spirometry. Similar reminders could be implemented to identify veterans with exposures to burn pits or other military environmental exposures who may have COPD symptoms. Another possible way to increase COPD diagnosis would be a partnership between primary care and the VA LCS program where patients receiving screening are asked about COPD symptoms during their LCS interviews and PACTs are alerted to order spirometry for symptomatic patients.
Elusive no longer! We can pull the veil back on COPD diagnosis and identify patients with possible COPD earlier in their course using their eligibility for LCS as a yearly reminder to probe them for symptoms. While not all patients who undergo LCS—even those with evidence of COPD on CT—will have COPD symptoms, symptoms may develop over time. LCS provides the possibility of 2 diagnoses from 1 test. This is an opportunity we cannot afford to miss.
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
- Betancourt JA, Granados PS, Pacheco GJ, et al. Exploring health outcomes for U.S. veterans compared to non-veterans from 2003 to 2019. Healthcare (Basel). 2021;9(5):604. doi:10.3390/healthcare90506064
- Bamonti PM, Fischer I, Moye J, Poghosyan H, Pietrzak RH. Obstructive respiratory disease in U.S. veterans: prevalence, characteristics, and health burden. J Psychiatr Res. 2024;176:140-147. doi:10.1016/j.jpsychires.2024.05.053
- Criner RN, Han MK. COPD care in the 21st century: a public health priority. Respir Care. 2018;63(5):591-600. doi:10.4187/respcare.06276
- Larsson K, Janson C, Ställberg B, et al. Impact of COPD diagnosis timing on clinical and economic outcomes: the ARCTIC observational cohort study. Int J Chron Obstruct Pulmon Dis. 2019;14:995-1008. doi:10.2147/COPD.S195382
- Kostikas K, Price D, Gutzwiller FS, et al. Clinical impact and healthcare resource utilization associated with early versus late COPD diagnosis in patients from UK CPRD Database. Int J Chron Obstruct Pulmon Dis. 2020;15:1729- 1738. doi:10.2147/COPD.S255414
- Bamonti PM, Robinson SA, Wan ES, Moy ML. Improving physiological, physical, and psychological health outcomes: a narrative review in US veterans with COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:1269-1283. doi:10.2147/COPD.S339323
- Savitz DA, Woskie SR, Bello A, et al. Deployment to military bases with open burn pits and respiratory and cardiovascular disease. JAMA Netw Open. 2024;7(4):e247629. doi:10.1001/jamanetworkopen.2024.7629
- Murphy DE, Chaudhry Z, Almoosa KF, Panos RJ. High prevalence of chronic obstructive pulmonary disease among veterans in the urban midwest. Mil Med. 2011;176(5):552-560. doi:10.7205/milmed-d-10-00377
- Guirguis-Blake JM, Senger CA, Webber EM, Mularski RA, Whitlock EP. Screening for chronic obstructive pulmonary disease: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2016;315(13):1378-1393. doi:10.1001/jama.2016.2654
- Capriotti T, Tomy R, Morales M. COPD updates: 2023 GOLD Report for primary care providers. Clinical Advisor. May 9, 2023. Accessed May 14, 2025. https://www.clinicaladvisor.com/features/copd-updates-2023-gold-report-primary-care/
- Leidy NK, Martinez FJ, Malley KG, et al. Can CAPTURE be used to identify undiagnosed patients with mild- to- moderate COPD likely to benefit from treatment? Int J Chron Obstruct Pulmon Dis. 2018;13:1901-1912. doi:10.2147/COPD.S152226
- Jithoo A, Enright PL, Burney P, et al. Case-finding options for COPD: results from the burden of obstructive lung disease study. Eur Respir J. 2013;41(3):548-555. doi:10.1183/09031936.00132011
- Haroon SM, Jordan RE, O’Beirne-Elliman J, Adab P. Effectiveness of case finding strategies for COPD in primary care: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15056. doi:10.1038/npjpcrm.2015.56
- Porter J, Boyd C, Skandari MR, Laiteerapong N. Revisiting the time needed to provide adult primary care. J Gen Intern Med. 2023;38(1)147-155. doi:10.1007/s11606-022-07707-x
- Woo S, Zhou W, Larson JL. Stigma experiences in people with chronic obstructive pulmonary disease: an integrative review. Int J Chron Obstruct Pulmon Dis. 2021;16:1647- 1659. doi:10.2147/COPD.S306874
- Aaron SD, Montes de Oca M, Celli B, et al. Early diagnosis and treatment of COPD: the costs and benefits of case finding. Am J Respir Crit Care Med. 2024;209(8):928-937. doi:10.1164/rccm.202311-2120PP
- Kwon A, Lee C, Arafah A, Klein M, Namboodiri S, Lee C. Increasing chronic obstructive pulmonary disease (COPD) diagnosis with pulmonary function testing for patients with chest imaging evidence of COPD. Poster presented at: Society of General Internal Medicine Midwest Regional Meeting; October 19-20, 2023; Chicago, IL.
- Henderson LM, Su I, Rivera MP, et al. Prevalence of lung cancer screening in the US, 2022. JAMA Netw Open. 2024;7(3):e243190. doi:10.1001/jamanetworkopen.2024.3190
- Steiger D, Siddiqi MF, Yip R, Yankelevitz DF, Henschke CI; I-ELCAP investigators. The importance of low-dose CT screening to identify emphysema in asymptomatic participants with and without a prior diagnosis of COPD. Clin Imaging. 2021;78:136-141. doi:10.1016/j.clinimag.2021.03.012
- Pinsky PF, Lynch DA, Gierada DS. Incidental findings on low-dose CT scan lung cancer screenings and deaths from respiratory diseases. Chest. 2022;161(4):1092-1100. doi:10.1016/j.chest.2021.11.015
- Mulshine JL, Aldigé CR, Ambrose LF, et al. Emphysema detection in the course of lung cancer screening: optimizing a rare opportunity to impact population health. Ann Am Thorac Soc. 2023;20(4):499- 503. doi:10.1513/AnnalsATS.202207-631PS
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
The Use of Lung Cancer Screening to Increase Chronic Obstructive Pulmonary Disease Diagnosis in Veterans Affairs Primary Care
Utilization and Cost of Veterans Health Administration Referrals to Community Care-Based Physical Therapy
Utilization and Cost of Veterans Health Administration Referrals to Community Care-Based Physical Therapy
The Veterans Health Administration (VHA) is the largest US integrated health system, providing care to veterans through VHA and non-VHA practitioners and facilities.1,2 Providing high-quality, timely, and veteran-centric care remains a priority for the VHA. Legislative efforts have expanded opportunities for eligible veterans to receive care in the community purchased by VHA, known as community care (CC).1 The Veterans Access, Choice, and Accountability Act of 2014 came in response to reports of long wait times and drive times for patients.3-5 The MISSION Act of 2018 expanded access to CC by streamlining it and broadening eligibility criteria, especially for veterans in rural communities who often experience more barriers in accessing care than veterans living in urban communities.1,6-10 Since the implementation of the Choice and MISSION Acts, > 2.7 million veterans have received care through community practitioners within the VHA CC network.11
Background
Increased access to CC could benefit veterans living in rural communities by increasing care options and circumventing challenges to accessing VHA care (ie, geographic, transportation, and distance barriers, practitioner and specialist shortages, and hospital closures). 5,9,10,12,13 However, health care system deficits in rural areas could also limit CC effectiveness for veterans living in those communities. 3 Other challenges posed by using CC include care coordination, information sharing, care continuity, delayed payments to CC practitioners, and mixed findings regarding CC quality.5,8,13,14 VHA practitioners are specifically trained to meet the multifaceted needs unique to veterans’ health and subculture, training CC practitioners may not receive.5,15
CC offers services for primary care and a broad range of specialties, including rehabilitation services such as physical therapy (PT).6 PT is used for the effective treatment of various conditions veterans experience and promote wellbeing and independence.16 US Department of Veterans Affairs (VA) databases reveal a high prevalence of veterans receiving PT services through CC; PT is one of the most frequently used CC outpatient specialty services by veterans living in rural communities.14,17
Telerehabitltation Enterprisewide Initiative
VHA has greatly invested in delivering care virtually, especially for veterans living in rural communities.18 In 2017, the VHA Office of Rural Health funded the Telerehabilitation Enterprise-Wide Initiative (TR-EWI) in partnership with the Physical Medicine and Rehabilitation Services national program office to increase access to specialized rehabilitation services for veterans living in rural communities by leveraging telehealth technologies.18-21 This alternative mode of health care delivery allows clinicians to overcome access barriers by delivering rehabilitation therapies directly to veterans' homes or nearby community-based outpatient clinics. TR-EWI was conceived as a hub-and-spoke model, where rehabilitation expertise at the hub was virtually delivered to spoke sites that did not have in-house expertise. In subsequent years, the TR-EWI also evolved to provide targeted telerehabilitation programs within rural-serving community-based outpatient clinics, including PT as a predominant service.19,20
As TR-EWI progressed—and in conjunction with the uptake of telehealth across VHA during the COVID-19 pandemic—there has been increased focus on PT telerehabilitation, especially for the 4.6 million veterans in rural communities.18,22,23 Because health care delivery system deficits in rural areas could limit the effective use of CC, many TR-EWI sites hope to reduce their CC referrals by providing telehealth PT services to veterans who might otherwise need to be referred to CC. This strategy aligns with VHA goals of providing high-quality and timely care. To better understand opportunities for programs like TR-EWI to provide rehabilitation services for veterans and reduce care sent to the community, research that examines CC referral trends for PT over time is warranted.
This study examines CC from a rehabilitation perspective with a focus on CC referral trends for PT, specifically for Veterans Integrated Service Networks (VISNs) where TREWI sites are located. The study’s objectives were to describe rehabilitation PT services being referred to CC and examine associated CC costs for PT services. Two research questions guided the study. First, what are the utilization trends for CC PT referrals from fiscal year (FY) 2019 to FY 2022? Secondly, what is the cost breakdown of CC for PT referrals from FY 2020 to FY 2022?
Methods
This study was conducted by a multidisciplinary team comprised of public health, disability, rehabilitation counseling, and PT professionals. It was deemed a quality improvement project under VA guidance and followed the SQUIRE guidelines for quality improvement reporting.24,25 The study used the VA Common Operating Platform (Palantir) to obtain individual-level CC referral data from the HealthShare Referral Manager (HSRM) database and consult data from the Computerized Patient Record System. Palantir is used to store and integrate VA data derived from the VA Corporate Data Warehouse and VHA Support Service Center. Referrals are authorizations for care to be delivered by a CC practitioner.
TR-EWI is comprised of 7 sites: VISN 2, VISN 4, VISN 8, VISN 12, VISN 15, VISN 19, and VISN 22. Each site provides telerehabilitation services with an emphasis on reaching veterans living in rural communities. We joined the referrals and consults cubes in Palantir to extract PT referrals for FY 2019 to FY 2022 for the 7 VISNs with TR-EWI sites and obtain referral-specific information and demographic characteristics. 26 Data were extracted in October 2022.
The VHA Community Care Referral Dashboard (CC Dashboard) provided nonindividual level CC cost data.27 The CC Dashboard provides insights into the costs of CC services for VHA enrollees by category of care, standardized episode of care, and eligibility. Data are based on nationallevel HSRM referrals that are not suspended or linked to a canceled or discontinued consult. Data were aggregated by VISN. The dashboard only includes referrals dating back to FY 2020; therefore, PT data from FY 2020 through FY 2022 for VISNs with TR-EWI sites were collected. Data were extracted in December 2022.
This study examined CC referrals, station name, eligibility types, clinical diagnoses (International Classification of Diseases, Tenth Revision codes), and demographic information in the Palantir dataset. Six eligibility criteria can qualify a veteran to receive CC.28 Within clinical diagnoses, the variable of interest was the provisional diagnosis. Patient demographics included age, gender, and rurality of residence, as determined by the Rural-Urban Commuting Area system.29,30 Rural and highly rural categories were combined for analysis. For the CC cost dataset, this study examined CC referrals, referral cost, and eligibility type.
Analysis
For the first research question, we examined referral data from FY 2019 to FY 2022 using the Palantir dataset, performed descriptive statistical analysis for all variables, and analyzed data to identify trends. Descriptive statistics were completed using IBM SPSS Statistics for Windows Version 29.0.0.0.
A qualitative analysis of provisional diagnosis data revealed what is being referred to CC for PT. A preliminary overview of provisional diagnosis data was conducted to familiarize coders with the data. We developed a coding framework to categorize diagnoses based on anatomical location, body structure, and clinical areas of interest. Data were reviewed individually and grouped into categories within the coding framework before meeting as a team to achieve group consensus on categorization. We then totaled the frequency of occurrence for provisional diagnoses within each category. Qualitative analyses were completed using Microsoft Excel.
For the second research question, the study used the CC cost dataset to examine the cost breakdown of CC PT referrals from FY 2020 to FY 2022. We calculated the number and cost of PT referrals across eligibility groups for each FY and VISN. Data were analyzed using SPSS to identify cost trends.
Results
There were 344,406 referrals to CC for PT from FY 2019 to FY 2022 for the 7 VISNs analyzed (Table 1). Of these, 22.5% were from FY 2019, 19.1% from FY 2020, 28.2% from FY 2021, and 30.3% from FY 2022. VISN 8 and VISN 22 reported the most overall PT referrals, with VISN 8 comprising 22.2% and VISN 22 comprising 18.1% of all referrals. VISN 2 reported the least overall referrals (3.7%). VISN 4 and VISN 12 had decreases in referrals over time. VISN 2 and VISN 15 had decreases in referrals from FY 2019 to FY 2021 and slight increases from FY 2021 to FY 2022. VISN 19 and VISN 22 both saw slight increases from FY 2019 to FY 2020 and substantial increases from FY 2020 to FY 2022, with FY 2022 accounting for 40.0% and 42.3% of all referrals for VISN 19 and VISN 20, respectively (Figure 1).
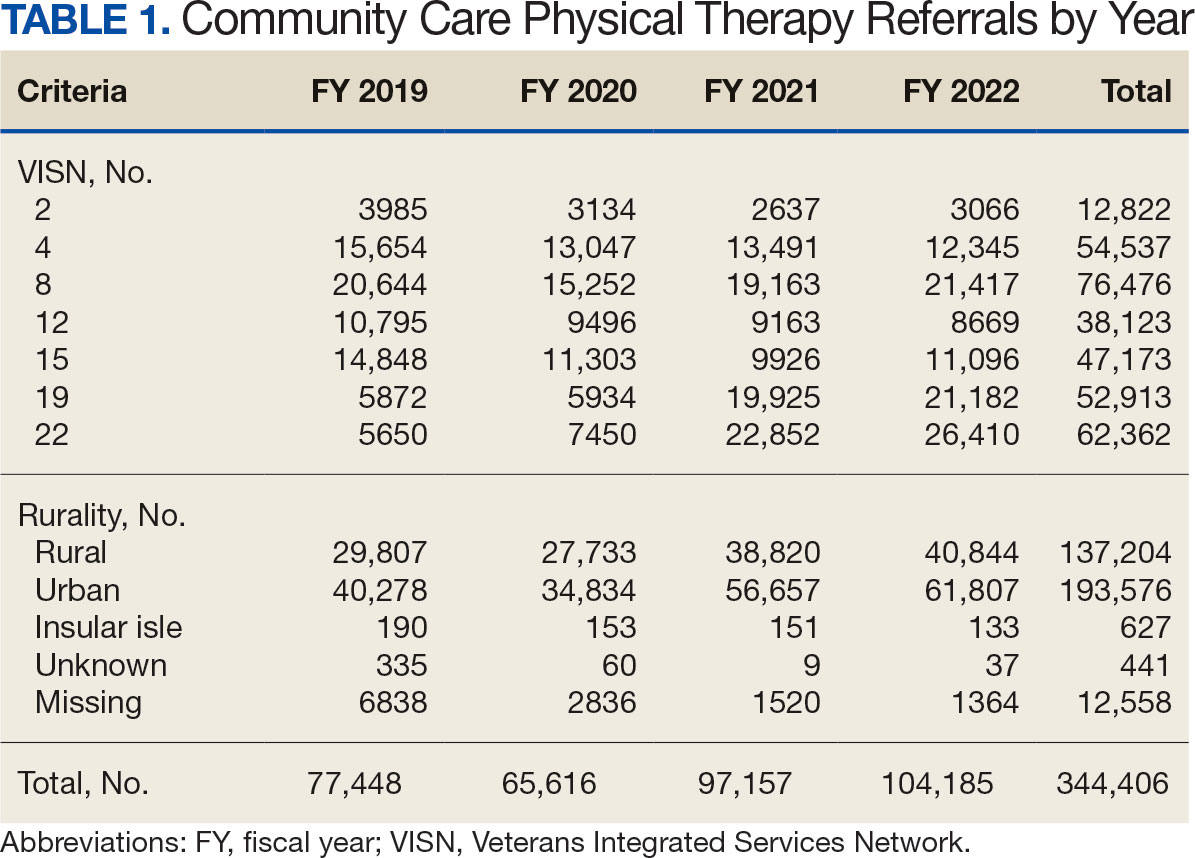
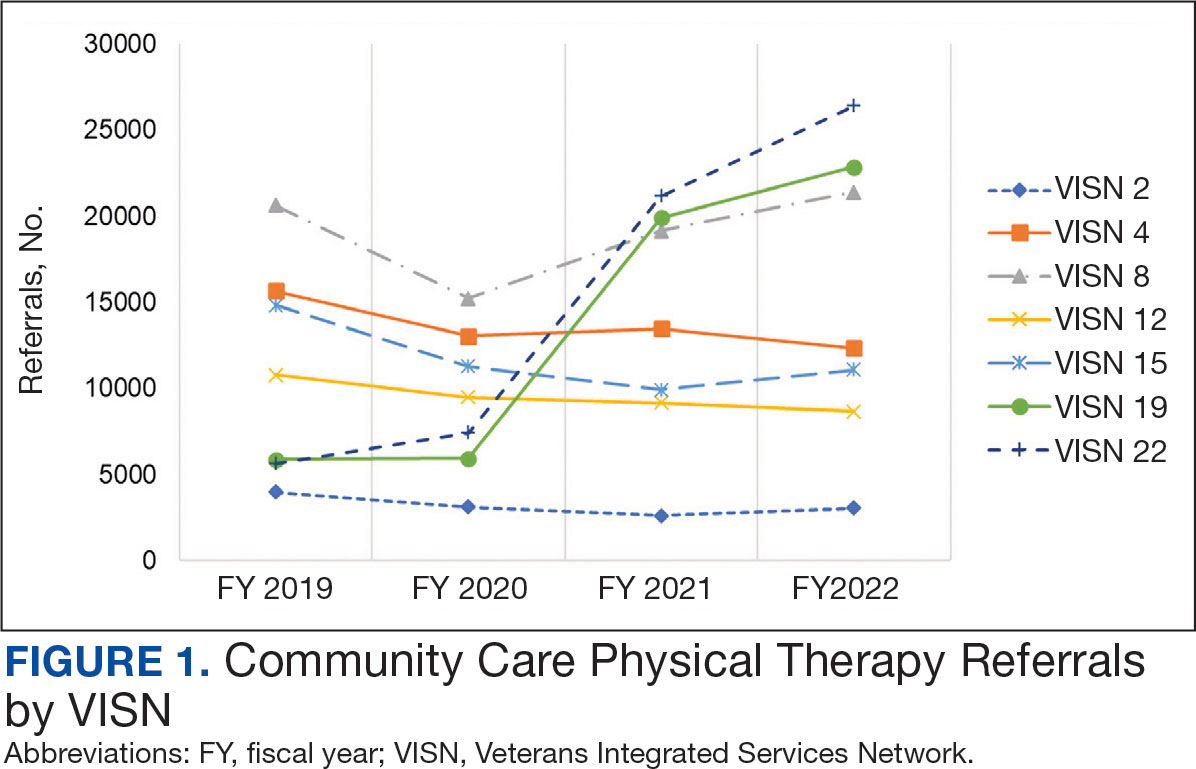
For FY 2019 and FY 2020, VISN 8 had the highest percentage of referrals (26.7% and 23.2%, respectively), whereas VISN 22 was among the lowest (7.3% and 11.4%, respectively). However, for FY 2021 and FY 2022, VISN 22 reported the highest percentage of referrals (23.5% and 25.3%, respectively) compared to all other VISNs. VISN 2 consistently reported the lowest percentage of referrals across all years.
There were 56 stations analyzed across the 7 VISNs (Appendix 1). Nine stations each accounted for ≥ 3.0% of the total PT referrals and only 2 stations accounted for > 5.0% of referrals. Orlando, Florida (6.0%), Philadelphia, Pennsylvania (5.2%), Tampa, Florida (4.9%), Aurora, Colorado (4.9%), and Gainesville, Florida (4.4%) reported the top 5 highest referrals, with 3 being from VISN 8 (Orlando, Tampa, Gainesville). Stations with the lowest reported referrals were all in VISN 2 in New York: The Bronx, (0%), New York Harbor (0%), Hudson Valley (0.1%) and Finger Lakes (0.2%).
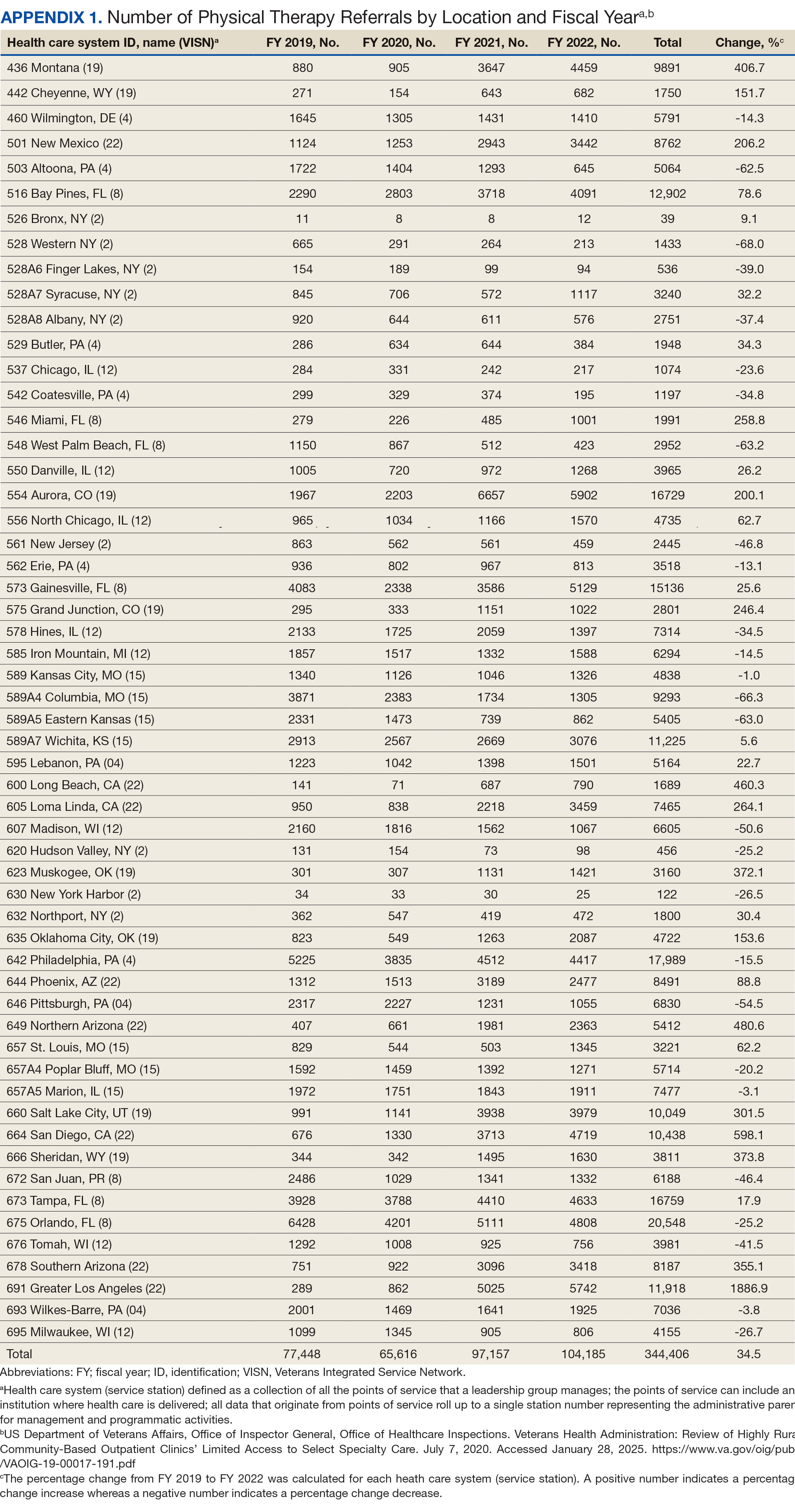
Rurality
Urban stations comprised 56.2% and rural stations comprised 39.8% of PT CC referrals, while 0.2% of referrals were from insular isle US territories: Guam, American Samoa, Northern Marianas, and the Virgin Islands. The sample had missing or unknown data for 3.8% of referrals. FY 2022 had the largest difference in rural and urban referrals. Additionally, there was an overall trend of more referrals over time for rural and urban, with a large increase in rural (+40.0%) and urban (+62.7%) referrals from FY 2020 to FY 2021 and a modest increase from FY 2021 to FY 2022 (+5.2% for rural and +9.1% for urban). There was a decrease in rural (-7.0%) and urban (-3.5%) referrals from FY 2019 to FY 2020 (Figure 2).
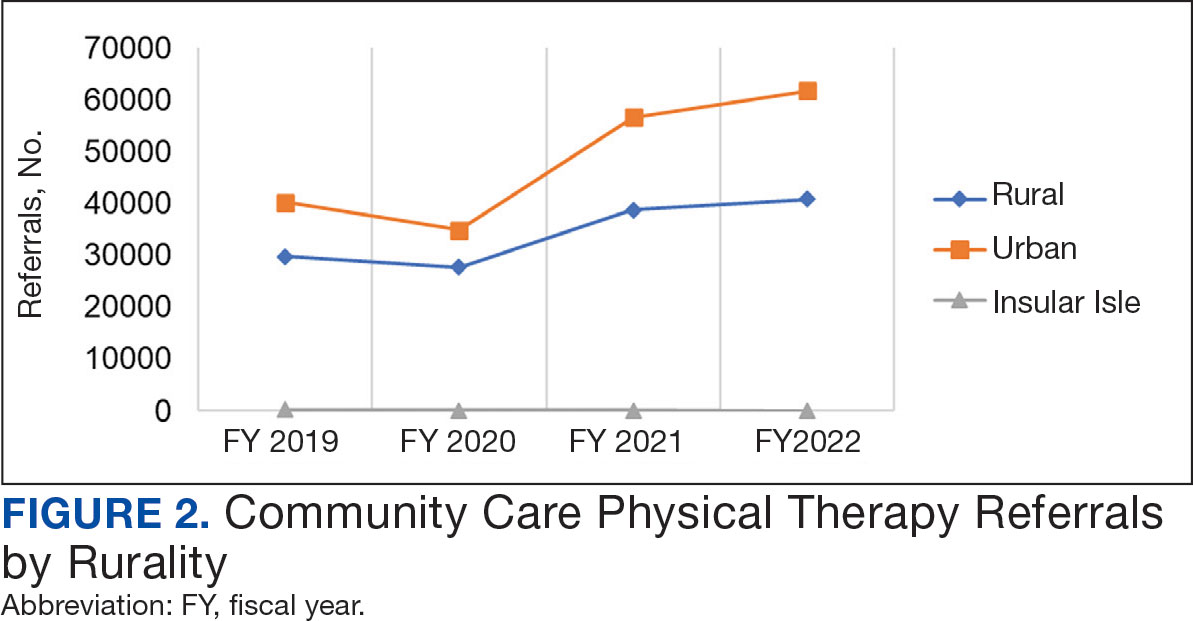
There were differences in referrals by rurality and VISN (Table 2). VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals, whereas VISN 4, VISN 8, and VISN 22 reported more urban than rural referrals. VISN 2 reported similar numbers for both, with slightly more urban than rural referrals. When reviewing trends over time for each FY, VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals and VISN 4, VISN 8, and VISN 22 had more urban than rural referrals. In FY 2019 and FY 2020, VISN 2 reported slightly more urban than rural referrals but almost the same number of referrals in FY 2021 and FY 2022 (Appendix 2).
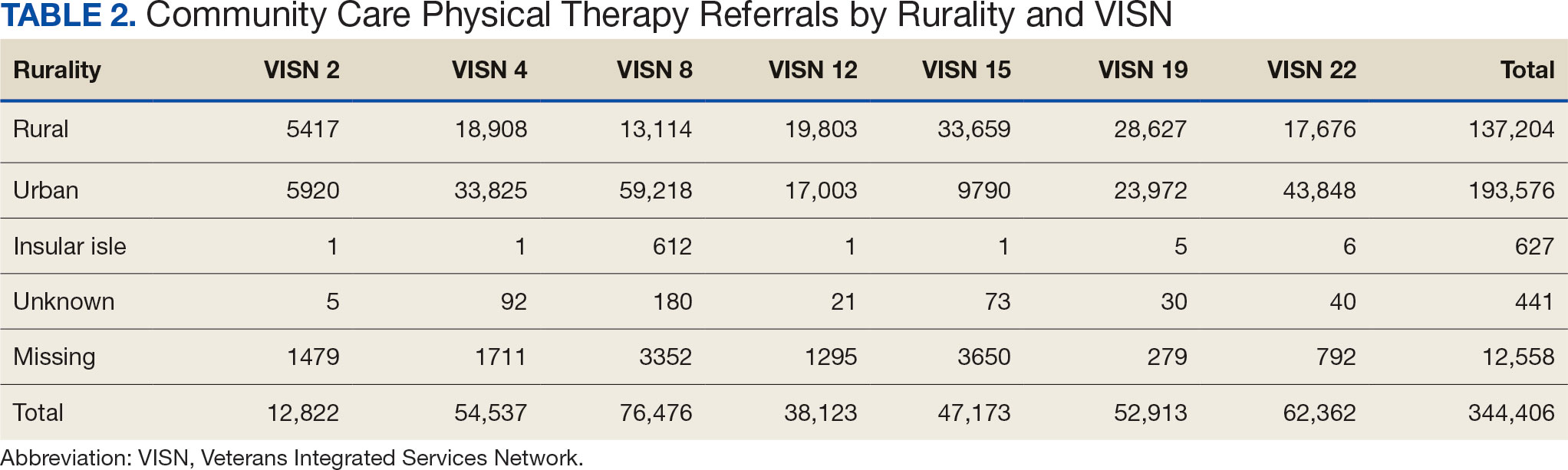
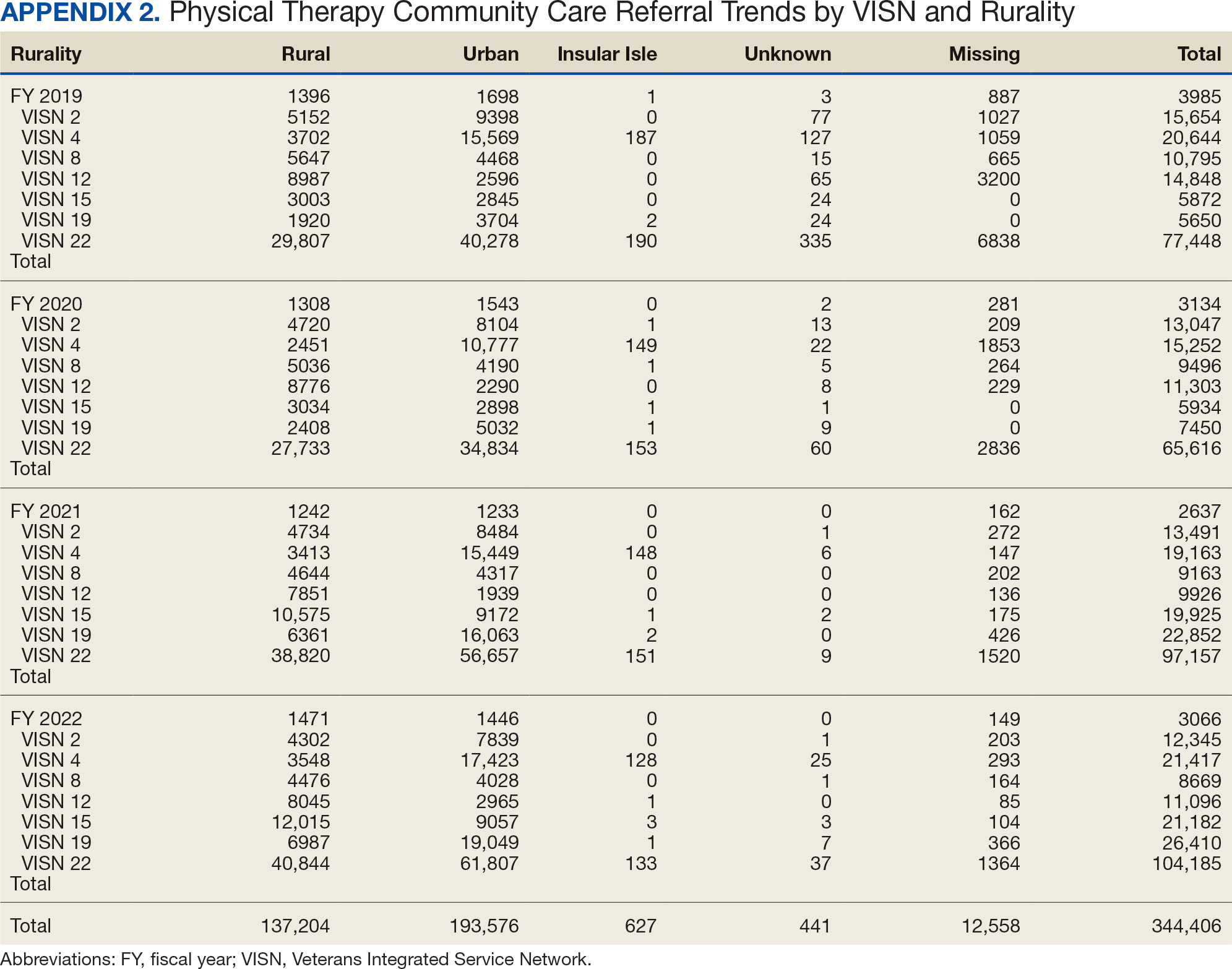
Demographics
The mean (SD) age was 61.2 (15.8) years (range, 20-105). Most PT CC referrals were for veterans aged 70 to 79 years (26.9%), followed by 60 to 69 years (20.7%), and 50 to 59 years (16.4%) (Appendix 3). Trends were consistent across VISNs. There was less of a difference between rural and urban referral percentages as the population aged. Veterans aged < 49 years residing in more urban areas accounted for more referrals to CC compared to their rural counterparts. This difference was less apparent in the 70 to 79 years and 80 to 89 years age brackets.
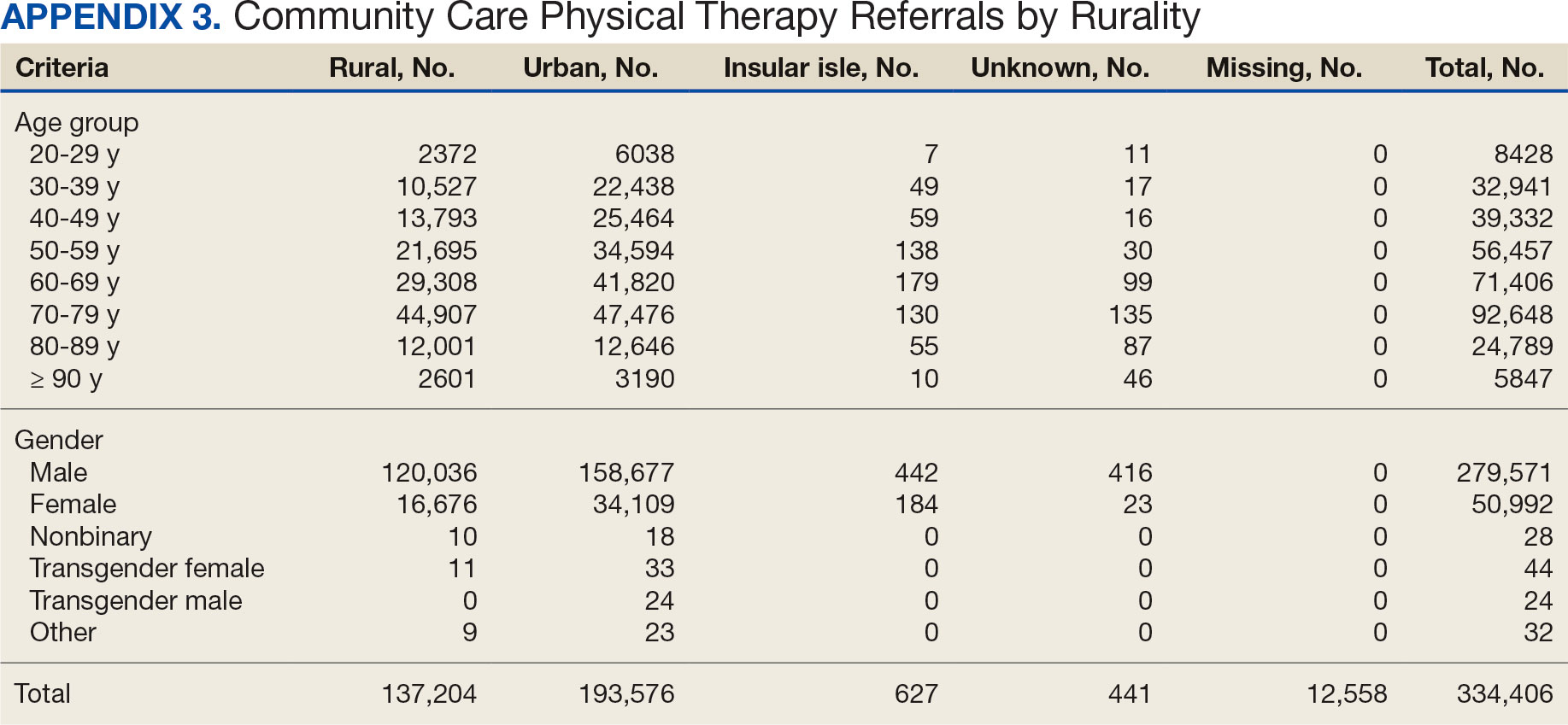
Most PT CC referrals (81.2%) were male and 14.8% were female. About 3.6% of referral data were missing sex information, and there was a smaller difference between male veterans living in rural communities and male veterans living in urban communities compared with female veterans. A total of 42.9% of male veterans resided in rural areas compared to 56.8% in urban areas; 32.7% of female veterans resided in rural areas compared to 66.9% in urban areas (Appendix 3).
Other Criteria
Of the 334,406 referrals, 114,983 (34.4%) had eligibility data, mostly from FY 2021 and FY 2022 (Table 3). Available eligibility data were likely affected by the MISSION Act and new regulations for reporting CC eligibility. Distance (33.4%) was the most common eligibility criteria, followed by timeliness of care (28.8%), and best medical interest (19.8%); 40.4% were rural and 59.5% were urban. Distance (55.4%) was most common for rural veterans, while timeliness of care (39.7%) was most common for urban veterans. For both groups, the second most common eligibility reason was best medical interest (Appendix 4).
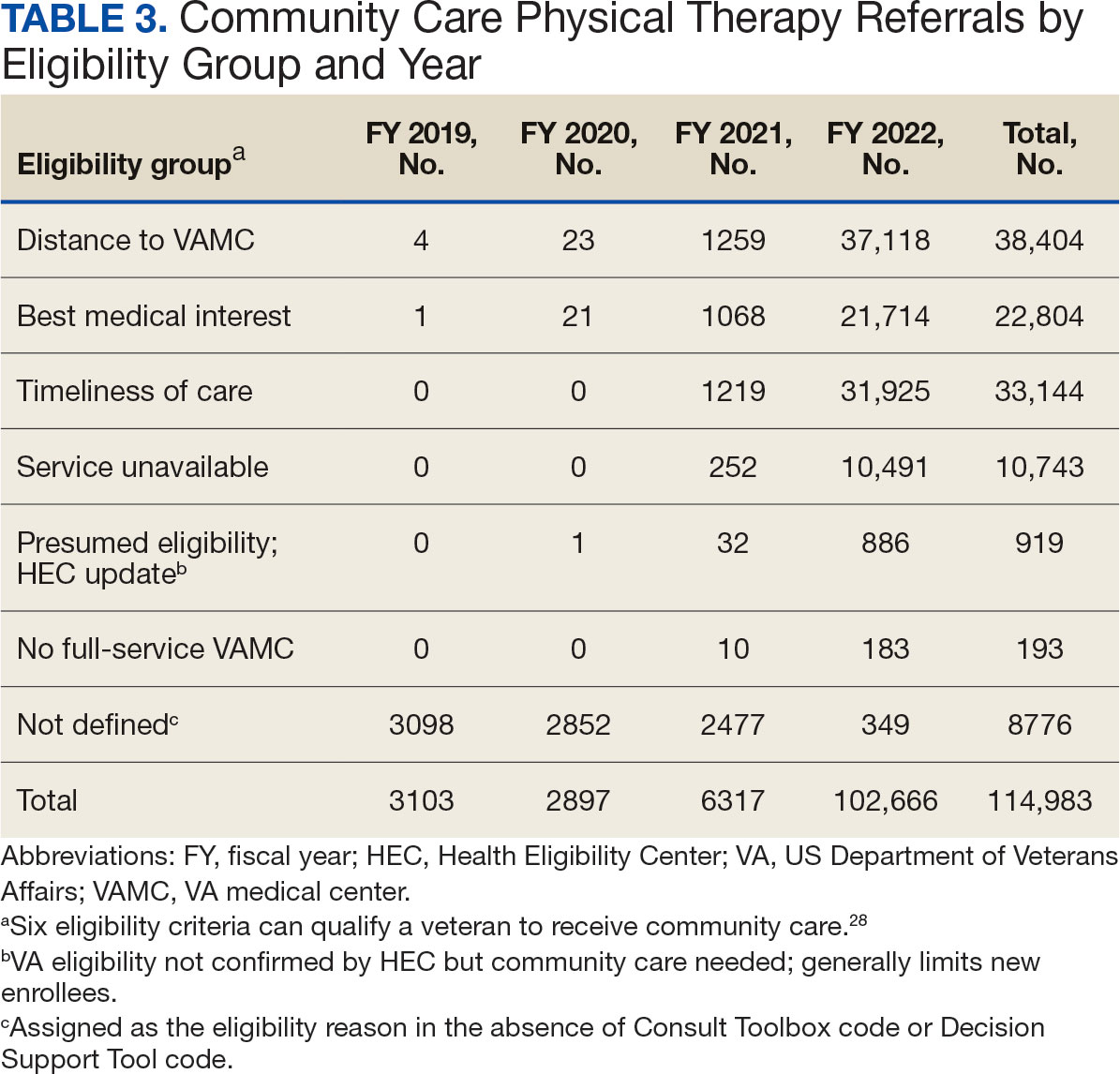
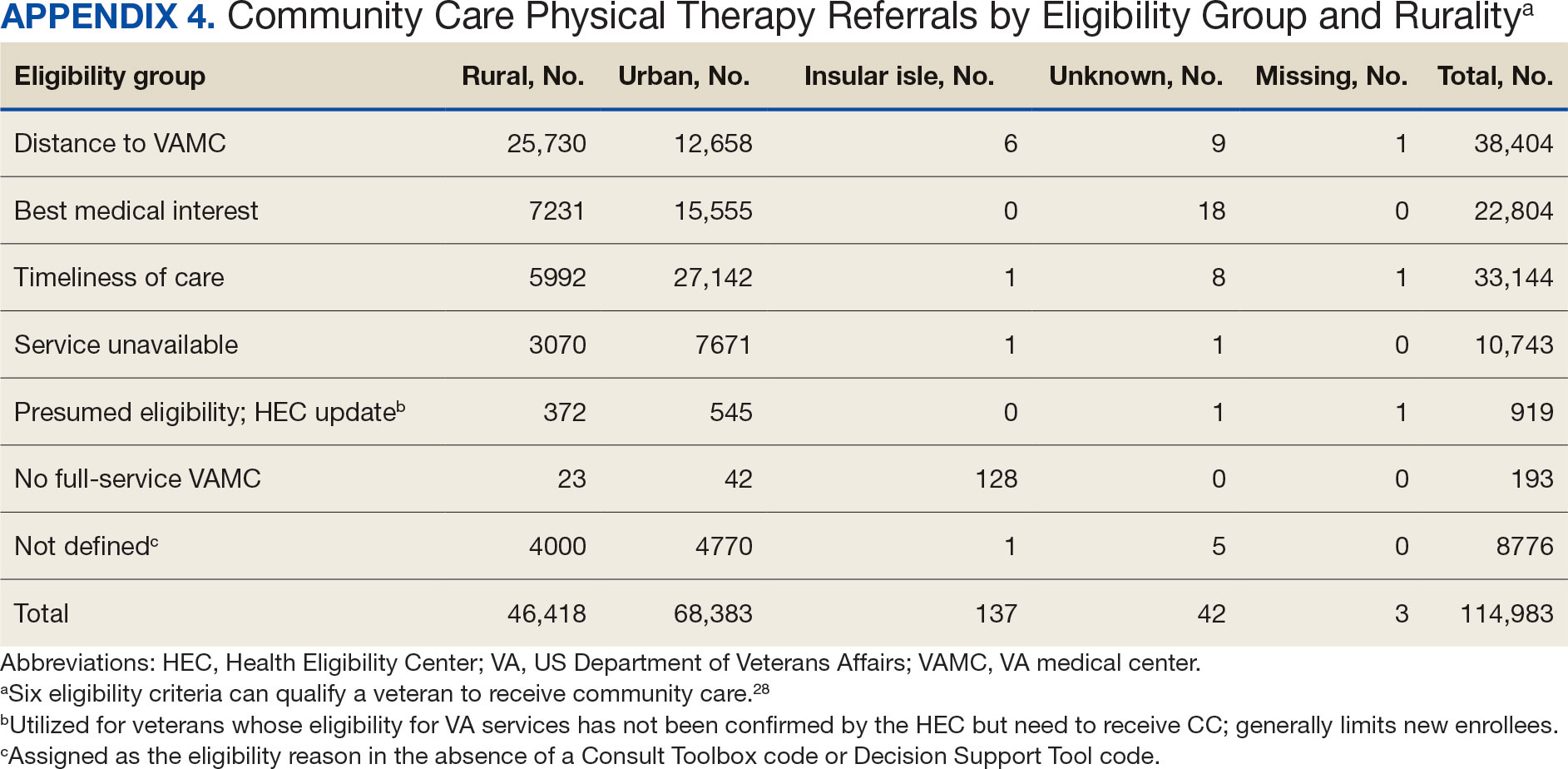
Bone, joint, or soft tissue disorders were common diagnoses, with 25.2% located in the lower back, 14.7% in the shoulder, and 12.8% in the knee (Appendix 5). Amputations of the upper and lower limbs, fractures, cancer-related diagnoses, integumentary system disorders, thoracic and abdominal injuries and disorders, and other medical and mental health conditions each accounted for < 1% of the total diagnoses.
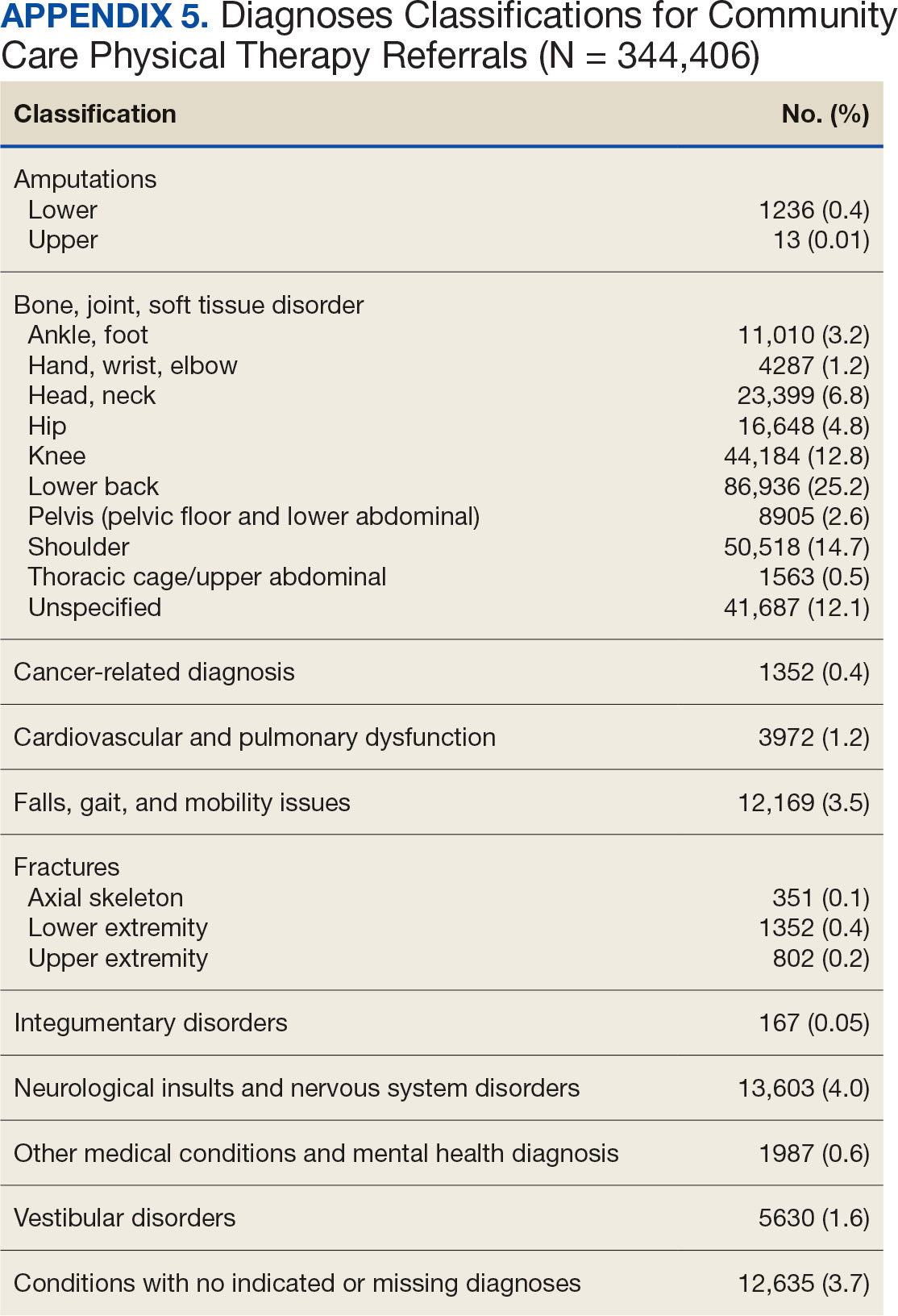
Costs
At time of analysis, the CC Dashboard had cost data available for 200,204 CC PT referrals from FY 2020 to FY 2022. The difference in referral numbers for the 2 datasets is likely attributed to several factors: CC cost data is exclusively from the HSRM, whereas Palantir includes other data sources; how VA cleans data pulled into Palantir; how the CC Dashboard algorithm populates data; and variances based on timing of reporting and/or if referrals are eventually canceled.
The total cost of PT CC referrals from FY 2020 to FY 2022 in selected VISNs was about $220,615,399 (Appendix 6). Appendix 7 details the methodology for determining the average standardized episode- of-care cost by VISN and how referral costs are calculated. Data show a continuous increase in total estimated cost from $46.8 million in FY 2020 to $92.1 million in FY 2022. From FY 2020 to FY 2022, aggregate costs ranged from $6,758,053 in VISN 2 to $47,209,162 in VISN 8 (Figure 3). The total referral cost for PT was highest at VISN 4 in FY 2020 ($10,447,140) and highest at VISN 22 in FY 2021 ($18,835,657) and FY 2022 ($22,962,438) (Figure 4). For referral costs from FY 2020 to FY 2022, distance accounted for $75,561,948 (34.3%), timeliness of care accounted for $60,413,496 (27.3%), and best medical interest accounted for $46,291,390 (21.0%) (Table 4).
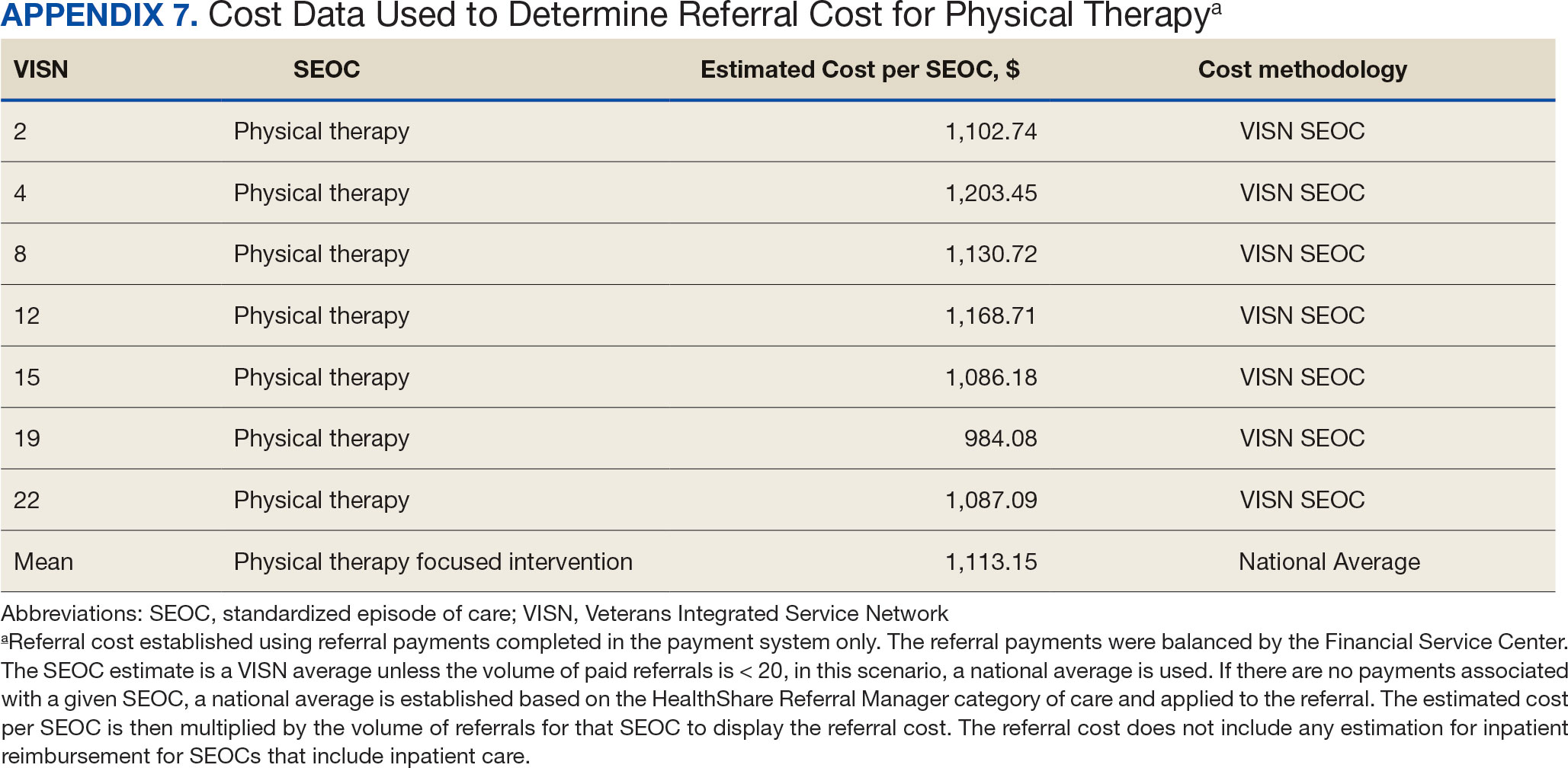
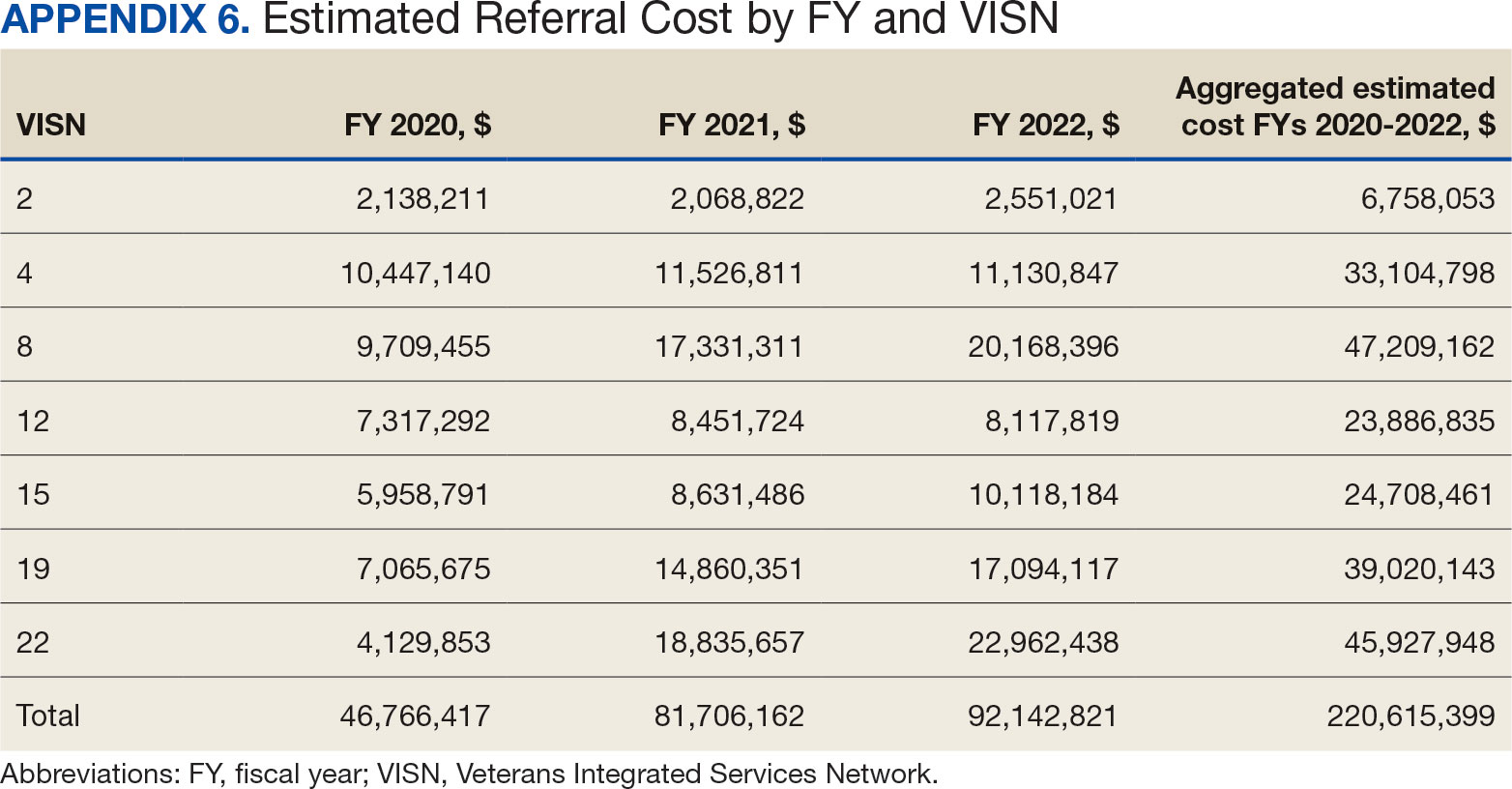
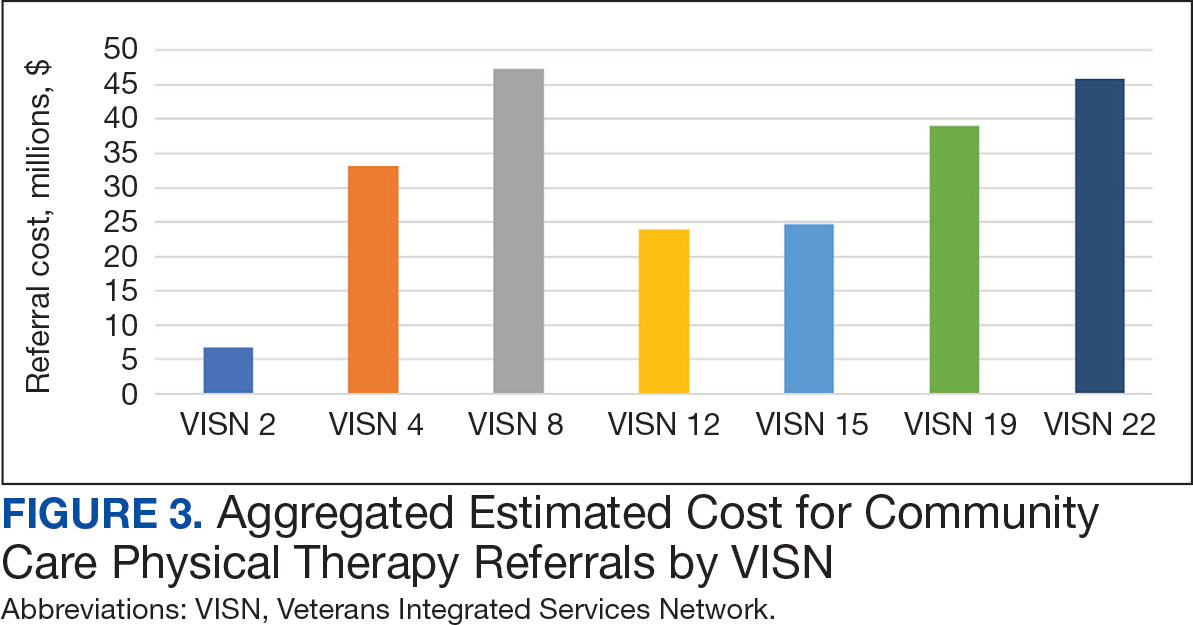
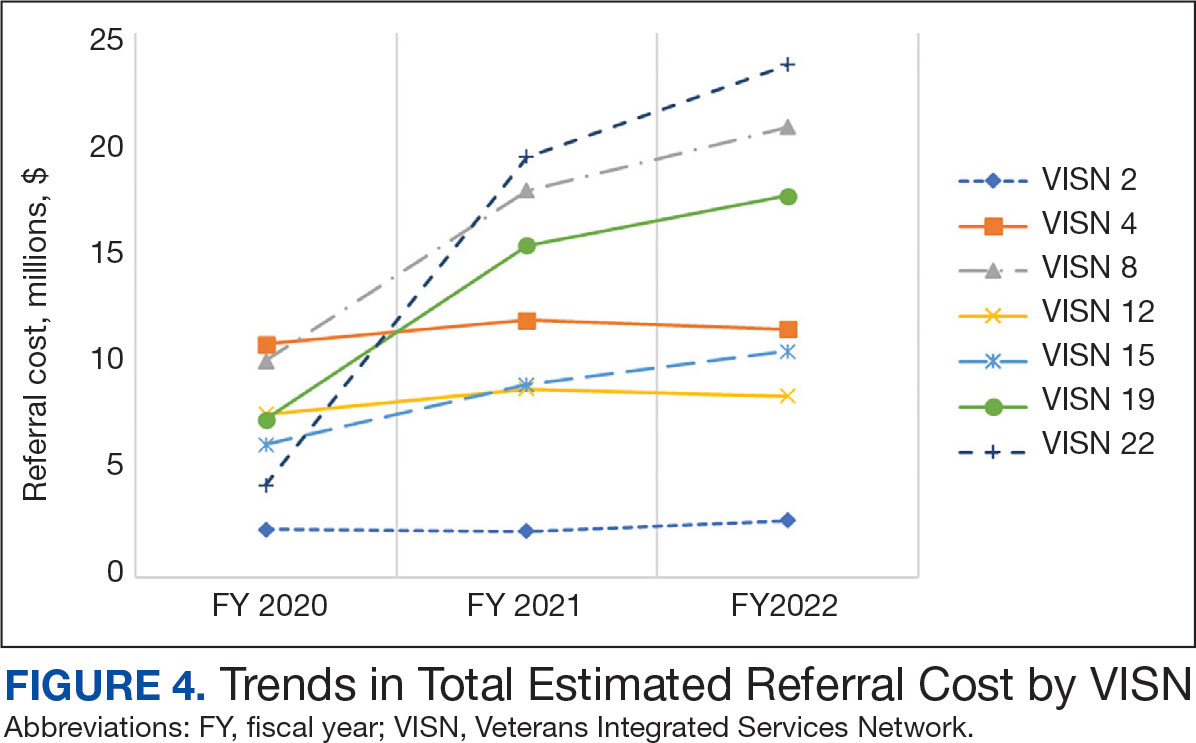
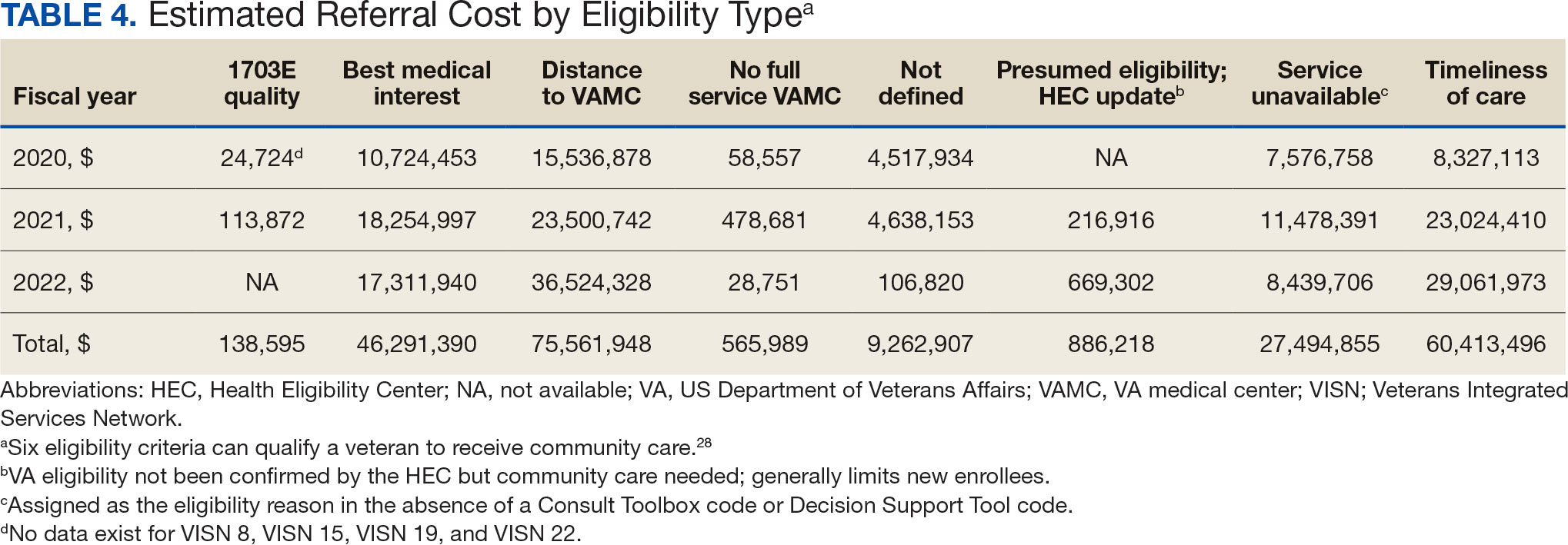
Overall costs were primarily driven by specific VISNs within each eligibility type (Appendix 8; Figure 5). VISN 19, VISN 22, and VISN 15 accounted for the highest referral costs for distance; VISN 22, VISN 8, and VISN 19 accounted for the secondhighest referral cost, timeliness of care; and VISN 4, VISN 8, and VISN 12 accounted for the third-highest referral cost, best medical interest (Figure 5). VISN 2, VISN 4, VISN 12, VISN 15, and VISN 22 had service unavailable as an eligibility type with 1 of the top 3 associated referral costs, which was higher in cost than timeliness of care for VISN 2, VISN 4, VISN 12, and VISN 15.
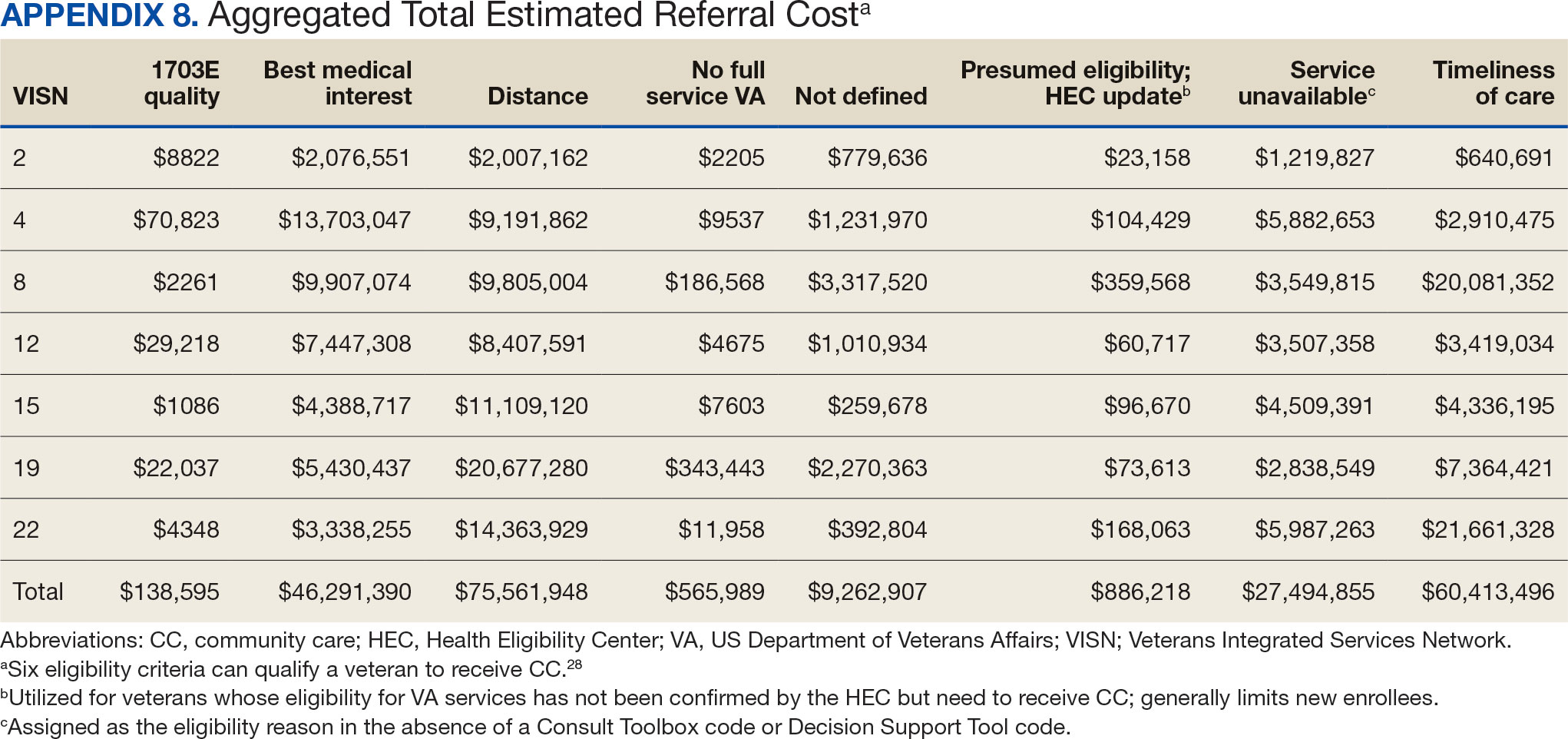
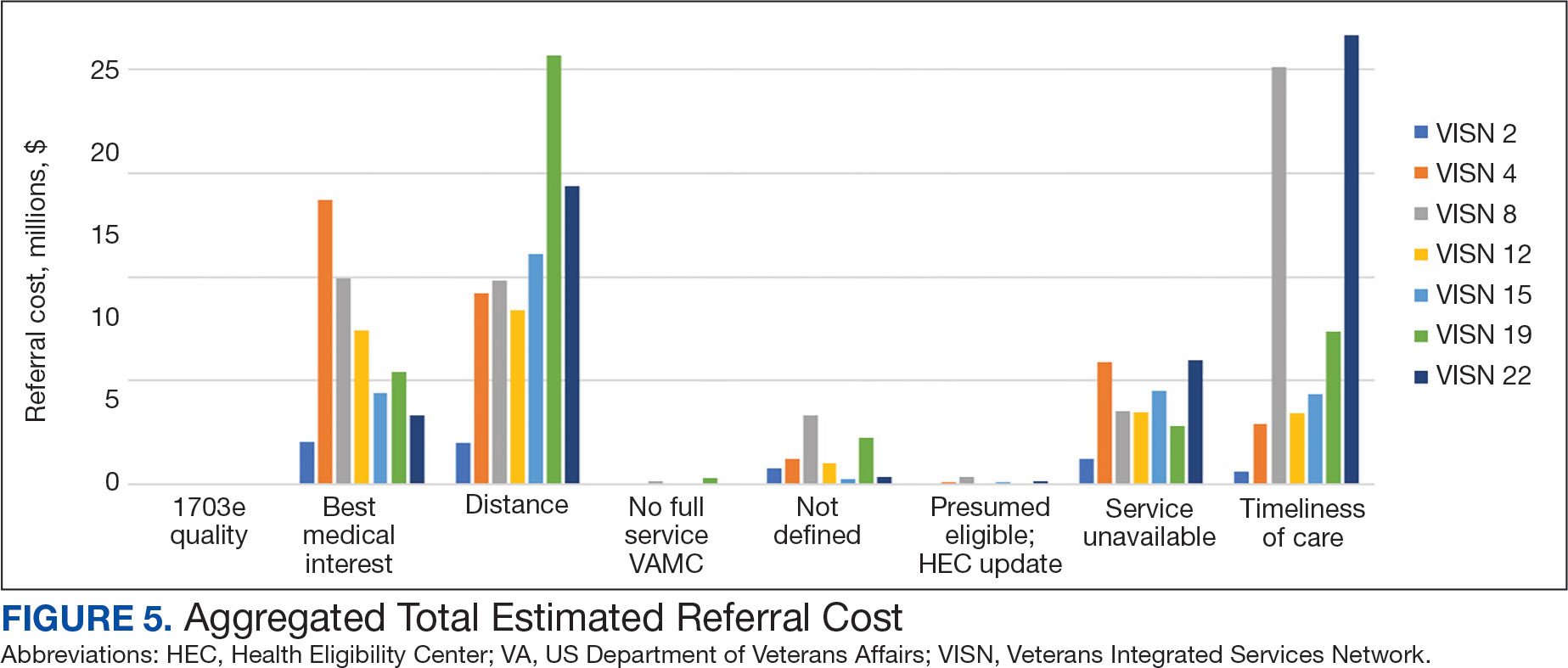
Discussion
This study examines the referral of rehabilitation PT services to CC, evaluates CC costs for PT services, and analyzes utilization and cost trends among veterans within the VHA. Utilization data demonstrated a decrease in referrals from FY 2019 to FY 2020 and increases in referrals from FY 2020 to FY 2022 for most variables of interest, with cost data exhibiting similar trends. Results highlight the need for further investigation to address variations in PT referrals and costs across VISNs and eligibility reasons for CC referral.
Results demonstrated a noteworthy increase in PT CC referrals over time. The largest increase occurred from FY 2020 to FY 2021, with a smaller increase from FY 2021 to FY 2022. During this period, total enrollee numbers decreased by 3.0% across the 7 VISNs included in this analysis and by 1.6% across all VISNs, a trend that illustrates an overall decrease in enrollees as CC use increased. Results align with the implementation of the MISSION Act of 2018, which further expanded veterans’ options to use CC.1,6,7 Results also align with the onset of the COVID-19 pandemic, which disrupted care access for many veterans, placed a larger emphasis on the use of telehealth, and increased opportunities to stay within the VA for care by rapidly shifting to telehealth and leveraging telerehabilitation investments and initiatives (such as TR-EWI).20,31
VISN 8, VISN 19, and VISN 22, accounted for more than half of PT referrals. These VISNs had higher enrollee counts compared to the other VISNs.32 VISN 8 consistently had high levels of referrals, whereas VISN 19 and VISN 22 saw dramatic increases in FY 2021 and FY 2022. In contrast, VISN 4 and VISN 12 gradually decreased referrals during the study. VISN 2 had the lowest referral numbers during the study period, and all stations with the lowest individual referral numbers were located within VISN 2. Of the VISNs included in this study, VISN 2 had the second lowest number of enrollees (324,042).32 Reasons for increases and decreases over time could not be determined based on data collected in this study.
There were more urban than rural PT CC referrals; however, both exhibited an increase in referrals over time. This is consistent with population trends showing that most VHA patients (62.6%) and veterans (75.9%) reside in urban areas, which could explain some of the trends in this study.33 Some VISNs have larger urban catchment areas (eg, VISN 8 and VISN 22), and some have larger rural catchment areas (eg, VISN 15 and VISN 19), which could partially explain the rural-urban differences by VISN.32 Rural-urban referral trends might also reflect existing health care delivery system deficits in rural areas and known challenges associated with accessing health care for veterans living in rural communities.8,9
This study found larger differences in rural and urban PT CC referrals for younger age groups, with more than twice as many urban referrals in veterans aged 20 to 29 years and aged 30 to 39 years, and roughly 1.8 times as many urban referrals in veterans aged 40 to 49 years. However, there were similar numbers of rural and urban referrals in those aged 70 to 79 years and aged 80 to 89 years. These trends are consistent with data showing veterans residing in rural communities are older than their urban counterparts.23,34 Data suggest that older veteran populations might seek PT at higher rates than younger veteran populations. Moreover, data suggest there could be differences in PT-seeking rates for younger veteran populations who reside in rural vs urban areas. Additional research is needed to understand these trends.
Distance and timeliness of care were the predominant reasons for referral among eligibility groups, which is consistent with the MISSION Act goals.1,6,7 The most common eligibility reason for rural referrals was distance; timeliness of care was most common for urban referrals. This finding is expected, as veterans living in rural communities are farther away from VHA facilities and have longer drive times, whereas veterans living in urban communities might live closer, yet experience longer wait times due to services and/or appointment availability. Best medical interest accounted for almost 20% of referrals, which does not provide detailed insights into why those veterans were referred to CC.
The top PT diagnoses referred to CC were related to bone, joint, or soft tissue disorders of the lower back, shoulder, and knee. This suggests that musculoskeletal-related issues are prevalent among veterans seeking PT care, which is consistent with research that found > 50% of veterans receiving VHA care have musculoskeletal disorders.35 The probability of experiencing musculoskeletal problems increases with age, as does the need for PT services. Amputations and fractures accounted for < 1% of CC referrals, which is consistent with the historic provision of VHA clinical specialized care to conditions prevalent among veterans. It may also represent VHA efforts to internally provide care for complex conditions requiring more extensive interdisciplinary coordination.
The total cost of referrals over time was about $221 million. VISN 8 accounted for the highest overall cost; VISN 2 had the lowest, mirroring referral utilization trends and aligning with VISN enrollee numbers. VISN 19 and VISN 22 reported large cost increases from FY 2020 to FY 2021. Total referral costs increased by $34.9 million from FY 2020 to FY 2021, which may be due to health care inflation (2.9% during FY 2019 to FY 2022), increased awareness of CC services, or increased VHA wait times.36 Additionally, there were limitations in care provided across health care systems during the COVID-19 pandemic, including the VA.5 The increase from FY 2020 to FY 2021 may reflect a rebound from restrictions in appointments across VA, CC, and the private sector.
While the increase in total referral cost may be partly attributed to inflation, the cost effectiveness and efficiency of referring veterans to CC vs keeping veterans within VHA care is an ongoing debate.5 Examining and addressing cost drivers within the top eligibility types and their respective VISNs is necessary to determine resource allocation and improve quality of care. This study found that best medical interest and unavailable services accounted for 33.4% of the total cost of CC referrals, highlighting the need for policies that strengthen in-house competencies and recruit personnel to provide PT services currently unavailable within the VA.
Future Directions
The VHA should explore opportunities for in-house care, especially for services appropriate for telehealth.18,20,37 Data indicated a smaller cost increase from FY 2021 to FY 2022 compared to the relatively large increase from FY 2020 to FY 2021. The increased telehealth usage across VHA by TR-EWI and non—TR-EWI sites within selected VISNs may have contributed to limiting the increase in CC costs. Future studies should investigate contextual factors of increased telehealth usage, which would offer guidance for implementation to optimize the integration of telehealth with PT rehabilitation provided in-house. Additionally, future studies can examine potential limitations experienced during PT telehealth visits, such as the inability to conduct hands-on assessments, challenges in viewing the quality of patient movement, ensuring patient safety in the remote environment, and the lack of PT equipment in homes for telehealth visits, and how these challenges are being addressed.38,39 Research is also needed to understand tradeoffs of CC vs VHA care and the potential and cost benefits of keeping veterans within VHA using programs like TR-EWI.5 Veterans living in rural communities may especially benefit from this as expanding telehealth options can provide access to PT care that may not be readily available, enabling them to stay connected and engaged in their care.18,40
Future studies could examine contributory factors to rising costs, such as demographic shifts, changes in PT service utilization, and policy. Researchers might also consider qualitative studies with clinicians and veterans within each VISN, which may provide insights into how local factors impact PT referral to the community.
Limitations
Due to its descriptive nature, this study can only speculate about factors influencing trends. Limitations include the inability to link the Palantir and CC Dashboard datasets for cost comparisons and potential data change over time on Palantir due to platform updates. The focus on VISNs with TREWI sites limited generalizability and this study did not compare CC PT vs VHA PT. Finally, there may have been cost drivers not identified in this study.
Conclusions
This descriptive study provides insights into the utilization and cost of PT CC referrals for selected VISNs. Cost trends underscore the financial commitment to providing PT services to veterans. Understanding what factors are driving this cost is necessary for VHA to optimally provide and manage the rehabilitation resources needed to serve veterans through traditional in-person care, telehealth, and CC options while ensuring timely, highquality care.
- Congressional Budget Office. The Veterans Community Care Program: Background and Early Effects. October 26, 2021. Accessed September 23, 2024. https://www.cbo.gov/publication/57257
- US Dept of Veterans Affairs. Providing Health Care for Veterans. Updated September 10, 2024. Accessed September 23, 2024. https://www.va.gov/health/
- Davila H, Rosen AK, Beilstein-Wedel E, Shwartz M, Chatelain LJ, Gurewich D. Rural veterans’ experiences with outpatient care in the Veterans Health Administration versus community care. Med Care. 2021;59(Suppl 3):S286-S291. doi:10.1097/MLR.0000000000001552
- Vanneman ME, Wagner TH, Shwartz M, et al. Veterans’ experiences with outpatient care: comparing the Veterans Affairs system with community-based care. Health Aff (Millwood). 2020;39(8):1368-1376. doi:10.1377/hlthaff.2019.01375
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10(3):9.
- Feyman Y, Legler A, Griffith KN. Appointment wait time data for primary & specialty care in veterans health administration facilities vs. community medical centers. Data Brief. 2021;36:107134. doi:10.1016/j.dib.2021.107134
- Kelley AT, Greenstone CL, Kirsh SR. Defining access and the role of community care in the Veterans Health Administration. J Gen Intern Med. 2020;35(5):1584-1585. doi:10.1007/s11606-019-05358-z
- Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(Suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
- US Dept of Veterans Affairs, Office of Rural Health. Rural Veterans: Rural Veteran Health Care Challenges. Updated May 14, 2024. Accessed September 23, 2024. https:// www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Ohl ME, Carrell M, Thurman A, et al. “Availability of healthcare providers for rural veterans eligible for purchased care under the veterans choice act.” BMC Health Serv Res. 2018;18(1):315. doi:10.1186/s12913-018-3108-8
- Mattocks KM, Cunningham KJ, Greenstone C, Atkins D, Rosen AK, Upton M. Innovations in community care programs, policies, and research. Med Care. 2021;59(Suppl 3):S229-S231. doi:10.1097/MLR.0000000000001550
- Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52 Suppl 1(Suppl 1):459-480. doi:10.1111/1475-6773.12633
- Patzel M, Barnes C, Ramalingam N, et al. Jumping through hoops: community care clinician and staff experiences providing primary care to rural veterans. J Gen Intern Med. 2023;38(Suppl 3):821-828. doi:10.1007/s11606-023-08126-2
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Campbell P, Pope R, Simas V, Canetti E, Schram B, Orr R. The effects of early physiotherapy treatment on musculoskeletal injury outcomes in military personnel: a narrative review. Int J Environ Res Public Health. 2022;19(20):13416. doi:10.3390/ijerph192013416
- Gurewich D, Shwartz M, Beilstein-Wedel E, Davila H, Rosen AK. Did access to care improve since passage of the veterans choice act? Differences between rural and urban veterans. Med Care. 2021;59(Suppl 3):S270-S278. doi:10.1097/MLR.0000000000001490
- Myers US, Birks A, Grubaugh AL, Axon RN. Flattening the curve by getting ahead of it: how the VA healthcare system is leveraging telehealth to provide continued access to care for rural veterans. J Rural Health. 2021;37(1):194-196. doi:10.1111/jrh.12449
- Hale-Gallardo JL, Kreider CM, Jia H, et al. Telerehabilitation for rural veterans: a qualitative assessment of barriers and facilitators to implementation. J Multidiscip Healthc. 2020;13:559-570. doi:10.2147/JMDH.S247267
- Kreider CM, Hale-Gallardo J, Kramer JC, et al. Providers’ shift to telerehabilitation at the U.S. Veterans Health Administration during COVID-19: practical applications. Front Public Health. 2022;10:831762. doi:10.3389/fpubh.2022.831762
- Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019;36(3):122-128.
- US Dept of Veterans Affairs, Office of Rural Health. VHA Office of Rural Health. Updated August 30, 2024. Accessed September 23, 2024. https://www.ruralhealth.va.gov/index.asp
- National Center for Veterans Analysis and Statistics. Rural Veterans: 2021-2023. April 2023. Accessed September 23, 2024. https://www.datahub.va.gov/stories/s/Rural-Veterans-FY2021-2023/kkh2-eymp/
- U.S. Department of Veterans Affairs, Office of Research & Development. Program Guide: 1200.21, VHA Operations Activities That May Constitute Research. January 9, 2019. https://www.research.va.gov/resources/policies/ProgramGuide-1200-21-VHA-Operations-Activities.pdf
- Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. J Nurs Care Qual. 2016;31(1):1-8. doi:10.1097/NCQ.0000000000000153
- US Dept of Veterans Affairs. Veterans Health Administration: Veterans Integrated Service Networks (VISNs). Updated January 29, 2024. Accessed September 23, 2024. https://www.va.gov/HEALTH/visns.asp
- Stomberg C, Frost A, Becker C, Stang H, Windschitl M, Carrier E. Community Care referral dashboard [Data dashboard]. https://app.powerbigov.us/groups/me/reports/090d22a7-0e1f-4cc5-bea8-0a1b87aa0bd9/ReportSectionacfd03cdebd76ffca9ec [Source not verified]
- US Dept of Veterans Affairs. Eligibility for community care outside VA. Updated May 30, 2024. Accessed September 23, 2024. https://www.va.gov/COMMUNITYCARE/programs/veterans/General_Care.asp
- US Department of Veterans Affairs, Office of Rural Health. How to define rurality fact sheet. Updated December 2023. Accessed January 28, 2025. https://www.ruralhealth.va.gov/docs/ORH_RuralityFactSheet_508.pdf
- Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed September 23, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx
- Gurewich D, Beilstein-Wedel E, Shwartz M, Davila H, Rosen AK. Disparities in wait times for care among US veterans by race and ethnici t y. JAMA Netw Open. 2023;6(1):e2252061. doi:10.1001/jamanetworkopen.2022.52061
- U.S. Department of Veterans Affairs, VA Office of Rural Health, Veterans Rural Health Resource Center-Gainesville, GeoSpatial Outcomes Division. VA and Community Healthcare, and VHA Rurality web map application. Published 2023. https://portal.vhagis.inv.vaec.va.gov/arcgis/apps/webappbuilder/index.html [source not verified]
- Chartbook on Healthcare for Veterans: National Healthcare Quality and Disparities Report. Agency for Healthcare Research and Quality; November 2020. Accessed September 23, 2024. https://www.ahrq.gov/research/findings/nhqrdr/chartbooks/veterans/index.html
- Lum HD, Nearing K, Pimentel CB, Levy CR, Hung WW. Anywhere to anywhere: use of telehealth to increase health care access for older, rural veterans. Public Policy Aging Rep. 2020;30(1):12-18. doi:10.1093/ppar/prz030
- Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157(8):1696-1703. doi:10.1097/j.pain.0000000000000567
- US Inflation Calculator. Health Care Inflation in the United States (1948-2024). Accessed September 23, 2024. https://www.usinflationcalculator.com/inflation/health-care-inflation-in-the-united-states/
- Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638. doi:10.1177/0269215516645148
- Elor A, Conde S, Powel l M, Robbins A, Chen NN, Kurniawan S. Physical therapist impressions of telehealth and virtual reality needs amidst a pandemic. Front Virtual Real. 2022;3. doi:10.3389/frvir.2022.915332
- Lee AC, Harada N. Telehealth as a means of health care delivery for physical therapist practice. Phys Ther. 2012;92(3):463-468. doi:10.2522/ptj.20110100
- Hynes DM, Edwards S, Hickok A, et al. Veterans’ use of Veterans Health Administration primary care in an era of expanding choice. Med Care. 2021;59(Suppl 3):S292- S300. doi:10.1097/MLR.0000000000001554
The Veterans Health Administration (VHA) is the largest US integrated health system, providing care to veterans through VHA and non-VHA practitioners and facilities.1,2 Providing high-quality, timely, and veteran-centric care remains a priority for the VHA. Legislative efforts have expanded opportunities for eligible veterans to receive care in the community purchased by VHA, known as community care (CC).1 The Veterans Access, Choice, and Accountability Act of 2014 came in response to reports of long wait times and drive times for patients.3-5 The MISSION Act of 2018 expanded access to CC by streamlining it and broadening eligibility criteria, especially for veterans in rural communities who often experience more barriers in accessing care than veterans living in urban communities.1,6-10 Since the implementation of the Choice and MISSION Acts, > 2.7 million veterans have received care through community practitioners within the VHA CC network.11
Background
Increased access to CC could benefit veterans living in rural communities by increasing care options and circumventing challenges to accessing VHA care (ie, geographic, transportation, and distance barriers, practitioner and specialist shortages, and hospital closures). 5,9,10,12,13 However, health care system deficits in rural areas could also limit CC effectiveness for veterans living in those communities. 3 Other challenges posed by using CC include care coordination, information sharing, care continuity, delayed payments to CC practitioners, and mixed findings regarding CC quality.5,8,13,14 VHA practitioners are specifically trained to meet the multifaceted needs unique to veterans’ health and subculture, training CC practitioners may not receive.5,15
CC offers services for primary care and a broad range of specialties, including rehabilitation services such as physical therapy (PT).6 PT is used for the effective treatment of various conditions veterans experience and promote wellbeing and independence.16 US Department of Veterans Affairs (VA) databases reveal a high prevalence of veterans receiving PT services through CC; PT is one of the most frequently used CC outpatient specialty services by veterans living in rural communities.14,17
Telerehabitltation Enterprisewide Initiative
VHA has greatly invested in delivering care virtually, especially for veterans living in rural communities.18 In 2017, the VHA Office of Rural Health funded the Telerehabilitation Enterprise-Wide Initiative (TR-EWI) in partnership with the Physical Medicine and Rehabilitation Services national program office to increase access to specialized rehabilitation services for veterans living in rural communities by leveraging telehealth technologies.18-21 This alternative mode of health care delivery allows clinicians to overcome access barriers by delivering rehabilitation therapies directly to veterans' homes or nearby community-based outpatient clinics. TR-EWI was conceived as a hub-and-spoke model, where rehabilitation expertise at the hub was virtually delivered to spoke sites that did not have in-house expertise. In subsequent years, the TR-EWI also evolved to provide targeted telerehabilitation programs within rural-serving community-based outpatient clinics, including PT as a predominant service.19,20
As TR-EWI progressed—and in conjunction with the uptake of telehealth across VHA during the COVID-19 pandemic—there has been increased focus on PT telerehabilitation, especially for the 4.6 million veterans in rural communities.18,22,23 Because health care delivery system deficits in rural areas could limit the effective use of CC, many TR-EWI sites hope to reduce their CC referrals by providing telehealth PT services to veterans who might otherwise need to be referred to CC. This strategy aligns with VHA goals of providing high-quality and timely care. To better understand opportunities for programs like TR-EWI to provide rehabilitation services for veterans and reduce care sent to the community, research that examines CC referral trends for PT over time is warranted.
This study examines CC from a rehabilitation perspective with a focus on CC referral trends for PT, specifically for Veterans Integrated Service Networks (VISNs) where TREWI sites are located. The study’s objectives were to describe rehabilitation PT services being referred to CC and examine associated CC costs for PT services. Two research questions guided the study. First, what are the utilization trends for CC PT referrals from fiscal year (FY) 2019 to FY 2022? Secondly, what is the cost breakdown of CC for PT referrals from FY 2020 to FY 2022?
Methods
This study was conducted by a multidisciplinary team comprised of public health, disability, rehabilitation counseling, and PT professionals. It was deemed a quality improvement project under VA guidance and followed the SQUIRE guidelines for quality improvement reporting.24,25 The study used the VA Common Operating Platform (Palantir) to obtain individual-level CC referral data from the HealthShare Referral Manager (HSRM) database and consult data from the Computerized Patient Record System. Palantir is used to store and integrate VA data derived from the VA Corporate Data Warehouse and VHA Support Service Center. Referrals are authorizations for care to be delivered by a CC practitioner.
TR-EWI is comprised of 7 sites: VISN 2, VISN 4, VISN 8, VISN 12, VISN 15, VISN 19, and VISN 22. Each site provides telerehabilitation services with an emphasis on reaching veterans living in rural communities. We joined the referrals and consults cubes in Palantir to extract PT referrals for FY 2019 to FY 2022 for the 7 VISNs with TR-EWI sites and obtain referral-specific information and demographic characteristics. 26 Data were extracted in October 2022.
The VHA Community Care Referral Dashboard (CC Dashboard) provided nonindividual level CC cost data.27 The CC Dashboard provides insights into the costs of CC services for VHA enrollees by category of care, standardized episode of care, and eligibility. Data are based on nationallevel HSRM referrals that are not suspended or linked to a canceled or discontinued consult. Data were aggregated by VISN. The dashboard only includes referrals dating back to FY 2020; therefore, PT data from FY 2020 through FY 2022 for VISNs with TR-EWI sites were collected. Data were extracted in December 2022.
This study examined CC referrals, station name, eligibility types, clinical diagnoses (International Classification of Diseases, Tenth Revision codes), and demographic information in the Palantir dataset. Six eligibility criteria can qualify a veteran to receive CC.28 Within clinical diagnoses, the variable of interest was the provisional diagnosis. Patient demographics included age, gender, and rurality of residence, as determined by the Rural-Urban Commuting Area system.29,30 Rural and highly rural categories were combined for analysis. For the CC cost dataset, this study examined CC referrals, referral cost, and eligibility type.
Analysis
For the first research question, we examined referral data from FY 2019 to FY 2022 using the Palantir dataset, performed descriptive statistical analysis for all variables, and analyzed data to identify trends. Descriptive statistics were completed using IBM SPSS Statistics for Windows Version 29.0.0.0.
A qualitative analysis of provisional diagnosis data revealed what is being referred to CC for PT. A preliminary overview of provisional diagnosis data was conducted to familiarize coders with the data. We developed a coding framework to categorize diagnoses based on anatomical location, body structure, and clinical areas of interest. Data were reviewed individually and grouped into categories within the coding framework before meeting as a team to achieve group consensus on categorization. We then totaled the frequency of occurrence for provisional diagnoses within each category. Qualitative analyses were completed using Microsoft Excel.
For the second research question, the study used the CC cost dataset to examine the cost breakdown of CC PT referrals from FY 2020 to FY 2022. We calculated the number and cost of PT referrals across eligibility groups for each FY and VISN. Data were analyzed using SPSS to identify cost trends.
Results
There were 344,406 referrals to CC for PT from FY 2019 to FY 2022 for the 7 VISNs analyzed (Table 1). Of these, 22.5% were from FY 2019, 19.1% from FY 2020, 28.2% from FY 2021, and 30.3% from FY 2022. VISN 8 and VISN 22 reported the most overall PT referrals, with VISN 8 comprising 22.2% and VISN 22 comprising 18.1% of all referrals. VISN 2 reported the least overall referrals (3.7%). VISN 4 and VISN 12 had decreases in referrals over time. VISN 2 and VISN 15 had decreases in referrals from FY 2019 to FY 2021 and slight increases from FY 2021 to FY 2022. VISN 19 and VISN 22 both saw slight increases from FY 2019 to FY 2020 and substantial increases from FY 2020 to FY 2022, with FY 2022 accounting for 40.0% and 42.3% of all referrals for VISN 19 and VISN 20, respectively (Figure 1).


For FY 2019 and FY 2020, VISN 8 had the highest percentage of referrals (26.7% and 23.2%, respectively), whereas VISN 22 was among the lowest (7.3% and 11.4%, respectively). However, for FY 2021 and FY 2022, VISN 22 reported the highest percentage of referrals (23.5% and 25.3%, respectively) compared to all other VISNs. VISN 2 consistently reported the lowest percentage of referrals across all years.
There were 56 stations analyzed across the 7 VISNs (Appendix 1). Nine stations each accounted for ≥ 3.0% of the total PT referrals and only 2 stations accounted for > 5.0% of referrals. Orlando, Florida (6.0%), Philadelphia, Pennsylvania (5.2%), Tampa, Florida (4.9%), Aurora, Colorado (4.9%), and Gainesville, Florida (4.4%) reported the top 5 highest referrals, with 3 being from VISN 8 (Orlando, Tampa, Gainesville). Stations with the lowest reported referrals were all in VISN 2 in New York: The Bronx, (0%), New York Harbor (0%), Hudson Valley (0.1%) and Finger Lakes (0.2%).

Rurality
Urban stations comprised 56.2% and rural stations comprised 39.8% of PT CC referrals, while 0.2% of referrals were from insular isle US territories: Guam, American Samoa, Northern Marianas, and the Virgin Islands. The sample had missing or unknown data for 3.8% of referrals. FY 2022 had the largest difference in rural and urban referrals. Additionally, there was an overall trend of more referrals over time for rural and urban, with a large increase in rural (+40.0%) and urban (+62.7%) referrals from FY 2020 to FY 2021 and a modest increase from FY 2021 to FY 2022 (+5.2% for rural and +9.1% for urban). There was a decrease in rural (-7.0%) and urban (-3.5%) referrals from FY 2019 to FY 2020 (Figure 2).

There were differences in referrals by rurality and VISN (Table 2). VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals, whereas VISN 4, VISN 8, and VISN 22 reported more urban than rural referrals. VISN 2 reported similar numbers for both, with slightly more urban than rural referrals. When reviewing trends over time for each FY, VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals and VISN 4, VISN 8, and VISN 22 had more urban than rural referrals. In FY 2019 and FY 2020, VISN 2 reported slightly more urban than rural referrals but almost the same number of referrals in FY 2021 and FY 2022 (Appendix 2).


Demographics
The mean (SD) age was 61.2 (15.8) years (range, 20-105). Most PT CC referrals were for veterans aged 70 to 79 years (26.9%), followed by 60 to 69 years (20.7%), and 50 to 59 years (16.4%) (Appendix 3). Trends were consistent across VISNs. There was less of a difference between rural and urban referral percentages as the population aged. Veterans aged < 49 years residing in more urban areas accounted for more referrals to CC compared to their rural counterparts. This difference was less apparent in the 70 to 79 years and 80 to 89 years age brackets.

Most PT CC referrals (81.2%) were male and 14.8% were female. About 3.6% of referral data were missing sex information, and there was a smaller difference between male veterans living in rural communities and male veterans living in urban communities compared with female veterans. A total of 42.9% of male veterans resided in rural areas compared to 56.8% in urban areas; 32.7% of female veterans resided in rural areas compared to 66.9% in urban areas (Appendix 3).
Other Criteria
Of the 334,406 referrals, 114,983 (34.4%) had eligibility data, mostly from FY 2021 and FY 2022 (Table 3). Available eligibility data were likely affected by the MISSION Act and new regulations for reporting CC eligibility. Distance (33.4%) was the most common eligibility criteria, followed by timeliness of care (28.8%), and best medical interest (19.8%); 40.4% were rural and 59.5% were urban. Distance (55.4%) was most common for rural veterans, while timeliness of care (39.7%) was most common for urban veterans. For both groups, the second most common eligibility reason was best medical interest (Appendix 4).


Bone, joint, or soft tissue disorders were common diagnoses, with 25.2% located in the lower back, 14.7% in the shoulder, and 12.8% in the knee (Appendix 5). Amputations of the upper and lower limbs, fractures, cancer-related diagnoses, integumentary system disorders, thoracic and abdominal injuries and disorders, and other medical and mental health conditions each accounted for < 1% of the total diagnoses.

Costs
At time of analysis, the CC Dashboard had cost data available for 200,204 CC PT referrals from FY 2020 to FY 2022. The difference in referral numbers for the 2 datasets is likely attributed to several factors: CC cost data is exclusively from the HSRM, whereas Palantir includes other data sources; how VA cleans data pulled into Palantir; how the CC Dashboard algorithm populates data; and variances based on timing of reporting and/or if referrals are eventually canceled.
The total cost of PT CC referrals from FY 2020 to FY 2022 in selected VISNs was about $220,615,399 (Appendix 6). Appendix 7 details the methodology for determining the average standardized episode- of-care cost by VISN and how referral costs are calculated. Data show a continuous increase in total estimated cost from $46.8 million in FY 2020 to $92.1 million in FY 2022. From FY 2020 to FY 2022, aggregate costs ranged from $6,758,053 in VISN 2 to $47,209,162 in VISN 8 (Figure 3). The total referral cost for PT was highest at VISN 4 in FY 2020 ($10,447,140) and highest at VISN 22 in FY 2021 ($18,835,657) and FY 2022 ($22,962,438) (Figure 4). For referral costs from FY 2020 to FY 2022, distance accounted for $75,561,948 (34.3%), timeliness of care accounted for $60,413,496 (27.3%), and best medical interest accounted for $46,291,390 (21.0%) (Table 4).





Overall costs were primarily driven by specific VISNs within each eligibility type (Appendix 8; Figure 5). VISN 19, VISN 22, and VISN 15 accounted for the highest referral costs for distance; VISN 22, VISN 8, and VISN 19 accounted for the secondhighest referral cost, timeliness of care; and VISN 4, VISN 8, and VISN 12 accounted for the third-highest referral cost, best medical interest (Figure 5). VISN 2, VISN 4, VISN 12, VISN 15, and VISN 22 had service unavailable as an eligibility type with 1 of the top 3 associated referral costs, which was higher in cost than timeliness of care for VISN 2, VISN 4, VISN 12, and VISN 15.


Discussion
This study examines the referral of rehabilitation PT services to CC, evaluates CC costs for PT services, and analyzes utilization and cost trends among veterans within the VHA. Utilization data demonstrated a decrease in referrals from FY 2019 to FY 2020 and increases in referrals from FY 2020 to FY 2022 for most variables of interest, with cost data exhibiting similar trends. Results highlight the need for further investigation to address variations in PT referrals and costs across VISNs and eligibility reasons for CC referral.
Results demonstrated a noteworthy increase in PT CC referrals over time. The largest increase occurred from FY 2020 to FY 2021, with a smaller increase from FY 2021 to FY 2022. During this period, total enrollee numbers decreased by 3.0% across the 7 VISNs included in this analysis and by 1.6% across all VISNs, a trend that illustrates an overall decrease in enrollees as CC use increased. Results align with the implementation of the MISSION Act of 2018, which further expanded veterans’ options to use CC.1,6,7 Results also align with the onset of the COVID-19 pandemic, which disrupted care access for many veterans, placed a larger emphasis on the use of telehealth, and increased opportunities to stay within the VA for care by rapidly shifting to telehealth and leveraging telerehabilitation investments and initiatives (such as TR-EWI).20,31
VISN 8, VISN 19, and VISN 22, accounted for more than half of PT referrals. These VISNs had higher enrollee counts compared to the other VISNs.32 VISN 8 consistently had high levels of referrals, whereas VISN 19 and VISN 22 saw dramatic increases in FY 2021 and FY 2022. In contrast, VISN 4 and VISN 12 gradually decreased referrals during the study. VISN 2 had the lowest referral numbers during the study period, and all stations with the lowest individual referral numbers were located within VISN 2. Of the VISNs included in this study, VISN 2 had the second lowest number of enrollees (324,042).32 Reasons for increases and decreases over time could not be determined based on data collected in this study.
There were more urban than rural PT CC referrals; however, both exhibited an increase in referrals over time. This is consistent with population trends showing that most VHA patients (62.6%) and veterans (75.9%) reside in urban areas, which could explain some of the trends in this study.33 Some VISNs have larger urban catchment areas (eg, VISN 8 and VISN 22), and some have larger rural catchment areas (eg, VISN 15 and VISN 19), which could partially explain the rural-urban differences by VISN.32 Rural-urban referral trends might also reflect existing health care delivery system deficits in rural areas and known challenges associated with accessing health care for veterans living in rural communities.8,9
This study found larger differences in rural and urban PT CC referrals for younger age groups, with more than twice as many urban referrals in veterans aged 20 to 29 years and aged 30 to 39 years, and roughly 1.8 times as many urban referrals in veterans aged 40 to 49 years. However, there were similar numbers of rural and urban referrals in those aged 70 to 79 years and aged 80 to 89 years. These trends are consistent with data showing veterans residing in rural communities are older than their urban counterparts.23,34 Data suggest that older veteran populations might seek PT at higher rates than younger veteran populations. Moreover, data suggest there could be differences in PT-seeking rates for younger veteran populations who reside in rural vs urban areas. Additional research is needed to understand these trends.
Distance and timeliness of care were the predominant reasons for referral among eligibility groups, which is consistent with the MISSION Act goals.1,6,7 The most common eligibility reason for rural referrals was distance; timeliness of care was most common for urban referrals. This finding is expected, as veterans living in rural communities are farther away from VHA facilities and have longer drive times, whereas veterans living in urban communities might live closer, yet experience longer wait times due to services and/or appointment availability. Best medical interest accounted for almost 20% of referrals, which does not provide detailed insights into why those veterans were referred to CC.
The top PT diagnoses referred to CC were related to bone, joint, or soft tissue disorders of the lower back, shoulder, and knee. This suggests that musculoskeletal-related issues are prevalent among veterans seeking PT care, which is consistent with research that found > 50% of veterans receiving VHA care have musculoskeletal disorders.35 The probability of experiencing musculoskeletal problems increases with age, as does the need for PT services. Amputations and fractures accounted for < 1% of CC referrals, which is consistent with the historic provision of VHA clinical specialized care to conditions prevalent among veterans. It may also represent VHA efforts to internally provide care for complex conditions requiring more extensive interdisciplinary coordination.
The total cost of referrals over time was about $221 million. VISN 8 accounted for the highest overall cost; VISN 2 had the lowest, mirroring referral utilization trends and aligning with VISN enrollee numbers. VISN 19 and VISN 22 reported large cost increases from FY 2020 to FY 2021. Total referral costs increased by $34.9 million from FY 2020 to FY 2021, which may be due to health care inflation (2.9% during FY 2019 to FY 2022), increased awareness of CC services, or increased VHA wait times.36 Additionally, there were limitations in care provided across health care systems during the COVID-19 pandemic, including the VA.5 The increase from FY 2020 to FY 2021 may reflect a rebound from restrictions in appointments across VA, CC, and the private sector.
While the increase in total referral cost may be partly attributed to inflation, the cost effectiveness and efficiency of referring veterans to CC vs keeping veterans within VHA care is an ongoing debate.5 Examining and addressing cost drivers within the top eligibility types and their respective VISNs is necessary to determine resource allocation and improve quality of care. This study found that best medical interest and unavailable services accounted for 33.4% of the total cost of CC referrals, highlighting the need for policies that strengthen in-house competencies and recruit personnel to provide PT services currently unavailable within the VA.
Future Directions
The VHA should explore opportunities for in-house care, especially for services appropriate for telehealth.18,20,37 Data indicated a smaller cost increase from FY 2021 to FY 2022 compared to the relatively large increase from FY 2020 to FY 2021. The increased telehealth usage across VHA by TR-EWI and non—TR-EWI sites within selected VISNs may have contributed to limiting the increase in CC costs. Future studies should investigate contextual factors of increased telehealth usage, which would offer guidance for implementation to optimize the integration of telehealth with PT rehabilitation provided in-house. Additionally, future studies can examine potential limitations experienced during PT telehealth visits, such as the inability to conduct hands-on assessments, challenges in viewing the quality of patient movement, ensuring patient safety in the remote environment, and the lack of PT equipment in homes for telehealth visits, and how these challenges are being addressed.38,39 Research is also needed to understand tradeoffs of CC vs VHA care and the potential and cost benefits of keeping veterans within VHA using programs like TR-EWI.5 Veterans living in rural communities may especially benefit from this as expanding telehealth options can provide access to PT care that may not be readily available, enabling them to stay connected and engaged in their care.18,40
Future studies could examine contributory factors to rising costs, such as demographic shifts, changes in PT service utilization, and policy. Researchers might also consider qualitative studies with clinicians and veterans within each VISN, which may provide insights into how local factors impact PT referral to the community.
Limitations
Due to its descriptive nature, this study can only speculate about factors influencing trends. Limitations include the inability to link the Palantir and CC Dashboard datasets for cost comparisons and potential data change over time on Palantir due to platform updates. The focus on VISNs with TREWI sites limited generalizability and this study did not compare CC PT vs VHA PT. Finally, there may have been cost drivers not identified in this study.
Conclusions
This descriptive study provides insights into the utilization and cost of PT CC referrals for selected VISNs. Cost trends underscore the financial commitment to providing PT services to veterans. Understanding what factors are driving this cost is necessary for VHA to optimally provide and manage the rehabilitation resources needed to serve veterans through traditional in-person care, telehealth, and CC options while ensuring timely, highquality care.
The Veterans Health Administration (VHA) is the largest US integrated health system, providing care to veterans through VHA and non-VHA practitioners and facilities.1,2 Providing high-quality, timely, and veteran-centric care remains a priority for the VHA. Legislative efforts have expanded opportunities for eligible veterans to receive care in the community purchased by VHA, known as community care (CC).1 The Veterans Access, Choice, and Accountability Act of 2014 came in response to reports of long wait times and drive times for patients.3-5 The MISSION Act of 2018 expanded access to CC by streamlining it and broadening eligibility criteria, especially for veterans in rural communities who often experience more barriers in accessing care than veterans living in urban communities.1,6-10 Since the implementation of the Choice and MISSION Acts, > 2.7 million veterans have received care through community practitioners within the VHA CC network.11
Background
Increased access to CC could benefit veterans living in rural communities by increasing care options and circumventing challenges to accessing VHA care (ie, geographic, transportation, and distance barriers, practitioner and specialist shortages, and hospital closures). 5,9,10,12,13 However, health care system deficits in rural areas could also limit CC effectiveness for veterans living in those communities. 3 Other challenges posed by using CC include care coordination, information sharing, care continuity, delayed payments to CC practitioners, and mixed findings regarding CC quality.5,8,13,14 VHA practitioners are specifically trained to meet the multifaceted needs unique to veterans’ health and subculture, training CC practitioners may not receive.5,15
CC offers services for primary care and a broad range of specialties, including rehabilitation services such as physical therapy (PT).6 PT is used for the effective treatment of various conditions veterans experience and promote wellbeing and independence.16 US Department of Veterans Affairs (VA) databases reveal a high prevalence of veterans receiving PT services through CC; PT is one of the most frequently used CC outpatient specialty services by veterans living in rural communities.14,17
Telerehabitltation Enterprisewide Initiative
VHA has greatly invested in delivering care virtually, especially for veterans living in rural communities.18 In 2017, the VHA Office of Rural Health funded the Telerehabilitation Enterprise-Wide Initiative (TR-EWI) in partnership with the Physical Medicine and Rehabilitation Services national program office to increase access to specialized rehabilitation services for veterans living in rural communities by leveraging telehealth technologies.18-21 This alternative mode of health care delivery allows clinicians to overcome access barriers by delivering rehabilitation therapies directly to veterans' homes or nearby community-based outpatient clinics. TR-EWI was conceived as a hub-and-spoke model, where rehabilitation expertise at the hub was virtually delivered to spoke sites that did not have in-house expertise. In subsequent years, the TR-EWI also evolved to provide targeted telerehabilitation programs within rural-serving community-based outpatient clinics, including PT as a predominant service.19,20
As TR-EWI progressed—and in conjunction with the uptake of telehealth across VHA during the COVID-19 pandemic—there has been increased focus on PT telerehabilitation, especially for the 4.6 million veterans in rural communities.18,22,23 Because health care delivery system deficits in rural areas could limit the effective use of CC, many TR-EWI sites hope to reduce their CC referrals by providing telehealth PT services to veterans who might otherwise need to be referred to CC. This strategy aligns with VHA goals of providing high-quality and timely care. To better understand opportunities for programs like TR-EWI to provide rehabilitation services for veterans and reduce care sent to the community, research that examines CC referral trends for PT over time is warranted.
This study examines CC from a rehabilitation perspective with a focus on CC referral trends for PT, specifically for Veterans Integrated Service Networks (VISNs) where TREWI sites are located. The study’s objectives were to describe rehabilitation PT services being referred to CC and examine associated CC costs for PT services. Two research questions guided the study. First, what are the utilization trends for CC PT referrals from fiscal year (FY) 2019 to FY 2022? Secondly, what is the cost breakdown of CC for PT referrals from FY 2020 to FY 2022?
Methods
This study was conducted by a multidisciplinary team comprised of public health, disability, rehabilitation counseling, and PT professionals. It was deemed a quality improvement project under VA guidance and followed the SQUIRE guidelines for quality improvement reporting.24,25 The study used the VA Common Operating Platform (Palantir) to obtain individual-level CC referral data from the HealthShare Referral Manager (HSRM) database and consult data from the Computerized Patient Record System. Palantir is used to store and integrate VA data derived from the VA Corporate Data Warehouse and VHA Support Service Center. Referrals are authorizations for care to be delivered by a CC practitioner.
TR-EWI is comprised of 7 sites: VISN 2, VISN 4, VISN 8, VISN 12, VISN 15, VISN 19, and VISN 22. Each site provides telerehabilitation services with an emphasis on reaching veterans living in rural communities. We joined the referrals and consults cubes in Palantir to extract PT referrals for FY 2019 to FY 2022 for the 7 VISNs with TR-EWI sites and obtain referral-specific information and demographic characteristics. 26 Data were extracted in October 2022.
The VHA Community Care Referral Dashboard (CC Dashboard) provided nonindividual level CC cost data.27 The CC Dashboard provides insights into the costs of CC services for VHA enrollees by category of care, standardized episode of care, and eligibility. Data are based on nationallevel HSRM referrals that are not suspended or linked to a canceled or discontinued consult. Data were aggregated by VISN. The dashboard only includes referrals dating back to FY 2020; therefore, PT data from FY 2020 through FY 2022 for VISNs with TR-EWI sites were collected. Data were extracted in December 2022.
This study examined CC referrals, station name, eligibility types, clinical diagnoses (International Classification of Diseases, Tenth Revision codes), and demographic information in the Palantir dataset. Six eligibility criteria can qualify a veteran to receive CC.28 Within clinical diagnoses, the variable of interest was the provisional diagnosis. Patient demographics included age, gender, and rurality of residence, as determined by the Rural-Urban Commuting Area system.29,30 Rural and highly rural categories were combined for analysis. For the CC cost dataset, this study examined CC referrals, referral cost, and eligibility type.
Analysis
For the first research question, we examined referral data from FY 2019 to FY 2022 using the Palantir dataset, performed descriptive statistical analysis for all variables, and analyzed data to identify trends. Descriptive statistics were completed using IBM SPSS Statistics for Windows Version 29.0.0.0.
A qualitative analysis of provisional diagnosis data revealed what is being referred to CC for PT. A preliminary overview of provisional diagnosis data was conducted to familiarize coders with the data. We developed a coding framework to categorize diagnoses based on anatomical location, body structure, and clinical areas of interest. Data were reviewed individually and grouped into categories within the coding framework before meeting as a team to achieve group consensus on categorization. We then totaled the frequency of occurrence for provisional diagnoses within each category. Qualitative analyses were completed using Microsoft Excel.
For the second research question, the study used the CC cost dataset to examine the cost breakdown of CC PT referrals from FY 2020 to FY 2022. We calculated the number and cost of PT referrals across eligibility groups for each FY and VISN. Data were analyzed using SPSS to identify cost trends.
Results
There were 344,406 referrals to CC for PT from FY 2019 to FY 2022 for the 7 VISNs analyzed (Table 1). Of these, 22.5% were from FY 2019, 19.1% from FY 2020, 28.2% from FY 2021, and 30.3% from FY 2022. VISN 8 and VISN 22 reported the most overall PT referrals, with VISN 8 comprising 22.2% and VISN 22 comprising 18.1% of all referrals. VISN 2 reported the least overall referrals (3.7%). VISN 4 and VISN 12 had decreases in referrals over time. VISN 2 and VISN 15 had decreases in referrals from FY 2019 to FY 2021 and slight increases from FY 2021 to FY 2022. VISN 19 and VISN 22 both saw slight increases from FY 2019 to FY 2020 and substantial increases from FY 2020 to FY 2022, with FY 2022 accounting for 40.0% and 42.3% of all referrals for VISN 19 and VISN 20, respectively (Figure 1).


For FY 2019 and FY 2020, VISN 8 had the highest percentage of referrals (26.7% and 23.2%, respectively), whereas VISN 22 was among the lowest (7.3% and 11.4%, respectively). However, for FY 2021 and FY 2022, VISN 22 reported the highest percentage of referrals (23.5% and 25.3%, respectively) compared to all other VISNs. VISN 2 consistently reported the lowest percentage of referrals across all years.
There were 56 stations analyzed across the 7 VISNs (Appendix 1). Nine stations each accounted for ≥ 3.0% of the total PT referrals and only 2 stations accounted for > 5.0% of referrals. Orlando, Florida (6.0%), Philadelphia, Pennsylvania (5.2%), Tampa, Florida (4.9%), Aurora, Colorado (4.9%), and Gainesville, Florida (4.4%) reported the top 5 highest referrals, with 3 being from VISN 8 (Orlando, Tampa, Gainesville). Stations with the lowest reported referrals were all in VISN 2 in New York: The Bronx, (0%), New York Harbor (0%), Hudson Valley (0.1%) and Finger Lakes (0.2%).

Rurality
Urban stations comprised 56.2% and rural stations comprised 39.8% of PT CC referrals, while 0.2% of referrals were from insular isle US territories: Guam, American Samoa, Northern Marianas, and the Virgin Islands. The sample had missing or unknown data for 3.8% of referrals. FY 2022 had the largest difference in rural and urban referrals. Additionally, there was an overall trend of more referrals over time for rural and urban, with a large increase in rural (+40.0%) and urban (+62.7%) referrals from FY 2020 to FY 2021 and a modest increase from FY 2021 to FY 2022 (+5.2% for rural and +9.1% for urban). There was a decrease in rural (-7.0%) and urban (-3.5%) referrals from FY 2019 to FY 2020 (Figure 2).

There were differences in referrals by rurality and VISN (Table 2). VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals, whereas VISN 4, VISN 8, and VISN 22 reported more urban than rural referrals. VISN 2 reported similar numbers for both, with slightly more urban than rural referrals. When reviewing trends over time for each FY, VISN 12, VISN 15, and VISN 19 reported more rural than urban referrals and VISN 4, VISN 8, and VISN 22 had more urban than rural referrals. In FY 2019 and FY 2020, VISN 2 reported slightly more urban than rural referrals but almost the same number of referrals in FY 2021 and FY 2022 (Appendix 2).


Demographics
The mean (SD) age was 61.2 (15.8) years (range, 20-105). Most PT CC referrals were for veterans aged 70 to 79 years (26.9%), followed by 60 to 69 years (20.7%), and 50 to 59 years (16.4%) (Appendix 3). Trends were consistent across VISNs. There was less of a difference between rural and urban referral percentages as the population aged. Veterans aged < 49 years residing in more urban areas accounted for more referrals to CC compared to their rural counterparts. This difference was less apparent in the 70 to 79 years and 80 to 89 years age brackets.

Most PT CC referrals (81.2%) were male and 14.8% were female. About 3.6% of referral data were missing sex information, and there was a smaller difference between male veterans living in rural communities and male veterans living in urban communities compared with female veterans. A total of 42.9% of male veterans resided in rural areas compared to 56.8% in urban areas; 32.7% of female veterans resided in rural areas compared to 66.9% in urban areas (Appendix 3).
Other Criteria
Of the 334,406 referrals, 114,983 (34.4%) had eligibility data, mostly from FY 2021 and FY 2022 (Table 3). Available eligibility data were likely affected by the MISSION Act and new regulations for reporting CC eligibility. Distance (33.4%) was the most common eligibility criteria, followed by timeliness of care (28.8%), and best medical interest (19.8%); 40.4% were rural and 59.5% were urban. Distance (55.4%) was most common for rural veterans, while timeliness of care (39.7%) was most common for urban veterans. For both groups, the second most common eligibility reason was best medical interest (Appendix 4).


Bone, joint, or soft tissue disorders were common diagnoses, with 25.2% located in the lower back, 14.7% in the shoulder, and 12.8% in the knee (Appendix 5). Amputations of the upper and lower limbs, fractures, cancer-related diagnoses, integumentary system disorders, thoracic and abdominal injuries and disorders, and other medical and mental health conditions each accounted for < 1% of the total diagnoses.

Costs
At time of analysis, the CC Dashboard had cost data available for 200,204 CC PT referrals from FY 2020 to FY 2022. The difference in referral numbers for the 2 datasets is likely attributed to several factors: CC cost data is exclusively from the HSRM, whereas Palantir includes other data sources; how VA cleans data pulled into Palantir; how the CC Dashboard algorithm populates data; and variances based on timing of reporting and/or if referrals are eventually canceled.
The total cost of PT CC referrals from FY 2020 to FY 2022 in selected VISNs was about $220,615,399 (Appendix 6). Appendix 7 details the methodology for determining the average standardized episode- of-care cost by VISN and how referral costs are calculated. Data show a continuous increase in total estimated cost from $46.8 million in FY 2020 to $92.1 million in FY 2022. From FY 2020 to FY 2022, aggregate costs ranged from $6,758,053 in VISN 2 to $47,209,162 in VISN 8 (Figure 3). The total referral cost for PT was highest at VISN 4 in FY 2020 ($10,447,140) and highest at VISN 22 in FY 2021 ($18,835,657) and FY 2022 ($22,962,438) (Figure 4). For referral costs from FY 2020 to FY 2022, distance accounted for $75,561,948 (34.3%), timeliness of care accounted for $60,413,496 (27.3%), and best medical interest accounted for $46,291,390 (21.0%) (Table 4).





Overall costs were primarily driven by specific VISNs within each eligibility type (Appendix 8; Figure 5). VISN 19, VISN 22, and VISN 15 accounted for the highest referral costs for distance; VISN 22, VISN 8, and VISN 19 accounted for the secondhighest referral cost, timeliness of care; and VISN 4, VISN 8, and VISN 12 accounted for the third-highest referral cost, best medical interest (Figure 5). VISN 2, VISN 4, VISN 12, VISN 15, and VISN 22 had service unavailable as an eligibility type with 1 of the top 3 associated referral costs, which was higher in cost than timeliness of care for VISN 2, VISN 4, VISN 12, and VISN 15.


Discussion
This study examines the referral of rehabilitation PT services to CC, evaluates CC costs for PT services, and analyzes utilization and cost trends among veterans within the VHA. Utilization data demonstrated a decrease in referrals from FY 2019 to FY 2020 and increases in referrals from FY 2020 to FY 2022 for most variables of interest, with cost data exhibiting similar trends. Results highlight the need for further investigation to address variations in PT referrals and costs across VISNs and eligibility reasons for CC referral.
Results demonstrated a noteworthy increase in PT CC referrals over time. The largest increase occurred from FY 2020 to FY 2021, with a smaller increase from FY 2021 to FY 2022. During this period, total enrollee numbers decreased by 3.0% across the 7 VISNs included in this analysis and by 1.6% across all VISNs, a trend that illustrates an overall decrease in enrollees as CC use increased. Results align with the implementation of the MISSION Act of 2018, which further expanded veterans’ options to use CC.1,6,7 Results also align with the onset of the COVID-19 pandemic, which disrupted care access for many veterans, placed a larger emphasis on the use of telehealth, and increased opportunities to stay within the VA for care by rapidly shifting to telehealth and leveraging telerehabilitation investments and initiatives (such as TR-EWI).20,31
VISN 8, VISN 19, and VISN 22, accounted for more than half of PT referrals. These VISNs had higher enrollee counts compared to the other VISNs.32 VISN 8 consistently had high levels of referrals, whereas VISN 19 and VISN 22 saw dramatic increases in FY 2021 and FY 2022. In contrast, VISN 4 and VISN 12 gradually decreased referrals during the study. VISN 2 had the lowest referral numbers during the study period, and all stations with the lowest individual referral numbers were located within VISN 2. Of the VISNs included in this study, VISN 2 had the second lowest number of enrollees (324,042).32 Reasons for increases and decreases over time could not be determined based on data collected in this study.
There were more urban than rural PT CC referrals; however, both exhibited an increase in referrals over time. This is consistent with population trends showing that most VHA patients (62.6%) and veterans (75.9%) reside in urban areas, which could explain some of the trends in this study.33 Some VISNs have larger urban catchment areas (eg, VISN 8 and VISN 22), and some have larger rural catchment areas (eg, VISN 15 and VISN 19), which could partially explain the rural-urban differences by VISN.32 Rural-urban referral trends might also reflect existing health care delivery system deficits in rural areas and known challenges associated with accessing health care for veterans living in rural communities.8,9
This study found larger differences in rural and urban PT CC referrals for younger age groups, with more than twice as many urban referrals in veterans aged 20 to 29 years and aged 30 to 39 years, and roughly 1.8 times as many urban referrals in veterans aged 40 to 49 years. However, there were similar numbers of rural and urban referrals in those aged 70 to 79 years and aged 80 to 89 years. These trends are consistent with data showing veterans residing in rural communities are older than their urban counterparts.23,34 Data suggest that older veteran populations might seek PT at higher rates than younger veteran populations. Moreover, data suggest there could be differences in PT-seeking rates for younger veteran populations who reside in rural vs urban areas. Additional research is needed to understand these trends.
Distance and timeliness of care were the predominant reasons for referral among eligibility groups, which is consistent with the MISSION Act goals.1,6,7 The most common eligibility reason for rural referrals was distance; timeliness of care was most common for urban referrals. This finding is expected, as veterans living in rural communities are farther away from VHA facilities and have longer drive times, whereas veterans living in urban communities might live closer, yet experience longer wait times due to services and/or appointment availability. Best medical interest accounted for almost 20% of referrals, which does not provide detailed insights into why those veterans were referred to CC.
The top PT diagnoses referred to CC were related to bone, joint, or soft tissue disorders of the lower back, shoulder, and knee. This suggests that musculoskeletal-related issues are prevalent among veterans seeking PT care, which is consistent with research that found > 50% of veterans receiving VHA care have musculoskeletal disorders.35 The probability of experiencing musculoskeletal problems increases with age, as does the need for PT services. Amputations and fractures accounted for < 1% of CC referrals, which is consistent with the historic provision of VHA clinical specialized care to conditions prevalent among veterans. It may also represent VHA efforts to internally provide care for complex conditions requiring more extensive interdisciplinary coordination.
The total cost of referrals over time was about $221 million. VISN 8 accounted for the highest overall cost; VISN 2 had the lowest, mirroring referral utilization trends and aligning with VISN enrollee numbers. VISN 19 and VISN 22 reported large cost increases from FY 2020 to FY 2021. Total referral costs increased by $34.9 million from FY 2020 to FY 2021, which may be due to health care inflation (2.9% during FY 2019 to FY 2022), increased awareness of CC services, or increased VHA wait times.36 Additionally, there were limitations in care provided across health care systems during the COVID-19 pandemic, including the VA.5 The increase from FY 2020 to FY 2021 may reflect a rebound from restrictions in appointments across VA, CC, and the private sector.
While the increase in total referral cost may be partly attributed to inflation, the cost effectiveness and efficiency of referring veterans to CC vs keeping veterans within VHA care is an ongoing debate.5 Examining and addressing cost drivers within the top eligibility types and their respective VISNs is necessary to determine resource allocation and improve quality of care. This study found that best medical interest and unavailable services accounted for 33.4% of the total cost of CC referrals, highlighting the need for policies that strengthen in-house competencies and recruit personnel to provide PT services currently unavailable within the VA.
Future Directions
The VHA should explore opportunities for in-house care, especially for services appropriate for telehealth.18,20,37 Data indicated a smaller cost increase from FY 2021 to FY 2022 compared to the relatively large increase from FY 2020 to FY 2021. The increased telehealth usage across VHA by TR-EWI and non—TR-EWI sites within selected VISNs may have contributed to limiting the increase in CC costs. Future studies should investigate contextual factors of increased telehealth usage, which would offer guidance for implementation to optimize the integration of telehealth with PT rehabilitation provided in-house. Additionally, future studies can examine potential limitations experienced during PT telehealth visits, such as the inability to conduct hands-on assessments, challenges in viewing the quality of patient movement, ensuring patient safety in the remote environment, and the lack of PT equipment in homes for telehealth visits, and how these challenges are being addressed.38,39 Research is also needed to understand tradeoffs of CC vs VHA care and the potential and cost benefits of keeping veterans within VHA using programs like TR-EWI.5 Veterans living in rural communities may especially benefit from this as expanding telehealth options can provide access to PT care that may not be readily available, enabling them to stay connected and engaged in their care.18,40
Future studies could examine contributory factors to rising costs, such as demographic shifts, changes in PT service utilization, and policy. Researchers might also consider qualitative studies with clinicians and veterans within each VISN, which may provide insights into how local factors impact PT referral to the community.
Limitations
Due to its descriptive nature, this study can only speculate about factors influencing trends. Limitations include the inability to link the Palantir and CC Dashboard datasets for cost comparisons and potential data change over time on Palantir due to platform updates. The focus on VISNs with TREWI sites limited generalizability and this study did not compare CC PT vs VHA PT. Finally, there may have been cost drivers not identified in this study.
Conclusions
This descriptive study provides insights into the utilization and cost of PT CC referrals for selected VISNs. Cost trends underscore the financial commitment to providing PT services to veterans. Understanding what factors are driving this cost is necessary for VHA to optimally provide and manage the rehabilitation resources needed to serve veterans through traditional in-person care, telehealth, and CC options while ensuring timely, highquality care.
- Congressional Budget Office. The Veterans Community Care Program: Background and Early Effects. October 26, 2021. Accessed September 23, 2024. https://www.cbo.gov/publication/57257
- US Dept of Veterans Affairs. Providing Health Care for Veterans. Updated September 10, 2024. Accessed September 23, 2024. https://www.va.gov/health/
- Davila H, Rosen AK, Beilstein-Wedel E, Shwartz M, Chatelain LJ, Gurewich D. Rural veterans’ experiences with outpatient care in the Veterans Health Administration versus community care. Med Care. 2021;59(Suppl 3):S286-S291. doi:10.1097/MLR.0000000000001552
- Vanneman ME, Wagner TH, Shwartz M, et al. Veterans’ experiences with outpatient care: comparing the Veterans Affairs system with community-based care. Health Aff (Millwood). 2020;39(8):1368-1376. doi:10.1377/hlthaff.2019.01375
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10(3):9.
- Feyman Y, Legler A, Griffith KN. Appointment wait time data for primary & specialty care in veterans health administration facilities vs. community medical centers. Data Brief. 2021;36:107134. doi:10.1016/j.dib.2021.107134
- Kelley AT, Greenstone CL, Kirsh SR. Defining access and the role of community care in the Veterans Health Administration. J Gen Intern Med. 2020;35(5):1584-1585. doi:10.1007/s11606-019-05358-z
- Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(Suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
- US Dept of Veterans Affairs, Office of Rural Health. Rural Veterans: Rural Veteran Health Care Challenges. Updated May 14, 2024. Accessed September 23, 2024. https:// www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Ohl ME, Carrell M, Thurman A, et al. “Availability of healthcare providers for rural veterans eligible for purchased care under the veterans choice act.” BMC Health Serv Res. 2018;18(1):315. doi:10.1186/s12913-018-3108-8
- Mattocks KM, Cunningham KJ, Greenstone C, Atkins D, Rosen AK, Upton M. Innovations in community care programs, policies, and research. Med Care. 2021;59(Suppl 3):S229-S231. doi:10.1097/MLR.0000000000001550
- Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52 Suppl 1(Suppl 1):459-480. doi:10.1111/1475-6773.12633
- Patzel M, Barnes C, Ramalingam N, et al. Jumping through hoops: community care clinician and staff experiences providing primary care to rural veterans. J Gen Intern Med. 2023;38(Suppl 3):821-828. doi:10.1007/s11606-023-08126-2
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Campbell P, Pope R, Simas V, Canetti E, Schram B, Orr R. The effects of early physiotherapy treatment on musculoskeletal injury outcomes in military personnel: a narrative review. Int J Environ Res Public Health. 2022;19(20):13416. doi:10.3390/ijerph192013416
- Gurewich D, Shwartz M, Beilstein-Wedel E, Davila H, Rosen AK. Did access to care improve since passage of the veterans choice act? Differences between rural and urban veterans. Med Care. 2021;59(Suppl 3):S270-S278. doi:10.1097/MLR.0000000000001490
- Myers US, Birks A, Grubaugh AL, Axon RN. Flattening the curve by getting ahead of it: how the VA healthcare system is leveraging telehealth to provide continued access to care for rural veterans. J Rural Health. 2021;37(1):194-196. doi:10.1111/jrh.12449
- Hale-Gallardo JL, Kreider CM, Jia H, et al. Telerehabilitation for rural veterans: a qualitative assessment of barriers and facilitators to implementation. J Multidiscip Healthc. 2020;13:559-570. doi:10.2147/JMDH.S247267
- Kreider CM, Hale-Gallardo J, Kramer JC, et al. Providers’ shift to telerehabilitation at the U.S. Veterans Health Administration during COVID-19: practical applications. Front Public Health. 2022;10:831762. doi:10.3389/fpubh.2022.831762
- Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019;36(3):122-128.
- US Dept of Veterans Affairs, Office of Rural Health. VHA Office of Rural Health. Updated August 30, 2024. Accessed September 23, 2024. https://www.ruralhealth.va.gov/index.asp
- National Center for Veterans Analysis and Statistics. Rural Veterans: 2021-2023. April 2023. Accessed September 23, 2024. https://www.datahub.va.gov/stories/s/Rural-Veterans-FY2021-2023/kkh2-eymp/
- U.S. Department of Veterans Affairs, Office of Research & Development. Program Guide: 1200.21, VHA Operations Activities That May Constitute Research. January 9, 2019. https://www.research.va.gov/resources/policies/ProgramGuide-1200-21-VHA-Operations-Activities.pdf
- Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. J Nurs Care Qual. 2016;31(1):1-8. doi:10.1097/NCQ.0000000000000153
- US Dept of Veterans Affairs. Veterans Health Administration: Veterans Integrated Service Networks (VISNs). Updated January 29, 2024. Accessed September 23, 2024. https://www.va.gov/HEALTH/visns.asp
- Stomberg C, Frost A, Becker C, Stang H, Windschitl M, Carrier E. Community Care referral dashboard [Data dashboard]. https://app.powerbigov.us/groups/me/reports/090d22a7-0e1f-4cc5-bea8-0a1b87aa0bd9/ReportSectionacfd03cdebd76ffca9ec [Source not verified]
- US Dept of Veterans Affairs. Eligibility for community care outside VA. Updated May 30, 2024. Accessed September 23, 2024. https://www.va.gov/COMMUNITYCARE/programs/veterans/General_Care.asp
- US Department of Veterans Affairs, Office of Rural Health. How to define rurality fact sheet. Updated December 2023. Accessed January 28, 2025. https://www.ruralhealth.va.gov/docs/ORH_RuralityFactSheet_508.pdf
- Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed September 23, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx
- Gurewich D, Beilstein-Wedel E, Shwartz M, Davila H, Rosen AK. Disparities in wait times for care among US veterans by race and ethnici t y. JAMA Netw Open. 2023;6(1):e2252061. doi:10.1001/jamanetworkopen.2022.52061
- U.S. Department of Veterans Affairs, VA Office of Rural Health, Veterans Rural Health Resource Center-Gainesville, GeoSpatial Outcomes Division. VA and Community Healthcare, and VHA Rurality web map application. Published 2023. https://portal.vhagis.inv.vaec.va.gov/arcgis/apps/webappbuilder/index.html [source not verified]
- Chartbook on Healthcare for Veterans: National Healthcare Quality and Disparities Report. Agency for Healthcare Research and Quality; November 2020. Accessed September 23, 2024. https://www.ahrq.gov/research/findings/nhqrdr/chartbooks/veterans/index.html
- Lum HD, Nearing K, Pimentel CB, Levy CR, Hung WW. Anywhere to anywhere: use of telehealth to increase health care access for older, rural veterans. Public Policy Aging Rep. 2020;30(1):12-18. doi:10.1093/ppar/prz030
- Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157(8):1696-1703. doi:10.1097/j.pain.0000000000000567
- US Inflation Calculator. Health Care Inflation in the United States (1948-2024). Accessed September 23, 2024. https://www.usinflationcalculator.com/inflation/health-care-inflation-in-the-united-states/
- Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638. doi:10.1177/0269215516645148
- Elor A, Conde S, Powel l M, Robbins A, Chen NN, Kurniawan S. Physical therapist impressions of telehealth and virtual reality needs amidst a pandemic. Front Virtual Real. 2022;3. doi:10.3389/frvir.2022.915332
- Lee AC, Harada N. Telehealth as a means of health care delivery for physical therapist practice. Phys Ther. 2012;92(3):463-468. doi:10.2522/ptj.20110100
- Hynes DM, Edwards S, Hickok A, et al. Veterans’ use of Veterans Health Administration primary care in an era of expanding choice. Med Care. 2021;59(Suppl 3):S292- S300. doi:10.1097/MLR.0000000000001554
- Congressional Budget Office. The Veterans Community Care Program: Background and Early Effects. October 26, 2021. Accessed September 23, 2024. https://www.cbo.gov/publication/57257
- US Dept of Veterans Affairs. Providing Health Care for Veterans. Updated September 10, 2024. Accessed September 23, 2024. https://www.va.gov/health/
- Davila H, Rosen AK, Beilstein-Wedel E, Shwartz M, Chatelain LJ, Gurewich D. Rural veterans’ experiences with outpatient care in the Veterans Health Administration versus community care. Med Care. 2021;59(Suppl 3):S286-S291. doi:10.1097/MLR.0000000000001552
- Vanneman ME, Wagner TH, Shwartz M, et al. Veterans’ experiences with outpatient care: comparing the Veterans Affairs system with community-based care. Health Aff (Millwood). 2020;39(8):1368-1376. doi:10.1377/hlthaff.2019.01375
- Rasmussen P, Farmer CM. The promise and challenges of VA community care: veterans’ issues in focus. Rand Health Q. 2023;10(3):9.
- Feyman Y, Legler A, Griffith KN. Appointment wait time data for primary & specialty care in veterans health administration facilities vs. community medical centers. Data Brief. 2021;36:107134. doi:10.1016/j.dib.2021.107134
- Kelley AT, Greenstone CL, Kirsh SR. Defining access and the role of community care in the Veterans Health Administration. J Gen Intern Med. 2020;35(5):1584-1585. doi:10.1007/s11606-019-05358-z
- Garvin LA, Pugatch M, Gurewich D, Pendergast JN, Miller CJ. Interorganizational care coordination of rural veterans by Veterans Affairs and community care programs: a systematic review. Med Care. 2021;59(Suppl 3):S259-S269. doi:10.1097/MLR.0000000000001542
- US Dept of Veterans Affairs, Office of Rural Health. Rural Veterans: Rural Veteran Health Care Challenges. Updated May 14, 2024. Accessed September 23, 2024. https:// www.ruralhealth.va.gov/aboutus/ruralvets.asp
- Ohl ME, Carrell M, Thurman A, et al. “Availability of healthcare providers for rural veterans eligible for purchased care under the veterans choice act.” BMC Health Serv Res. 2018;18(1):315. doi:10.1186/s12913-018-3108-8
- Mattocks KM, Cunningham KJ, Greenstone C, Atkins D, Rosen AK, Upton M. Innovations in community care programs, policies, and research. Med Care. 2021;59(Suppl 3):S229-S231. doi:10.1097/MLR.0000000000001550
- Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52 Suppl 1(Suppl 1):459-480. doi:10.1111/1475-6773.12633
- Patzel M, Barnes C, Ramalingam N, et al. Jumping through hoops: community care clinician and staff experiences providing primary care to rural veterans. J Gen Intern Med. 2023;38(Suppl 3):821-828. doi:10.1007/s11606-023-08126-2
- Mattocks KM, Kroll-Desrosiers A, Kinney R, Elwy AR, Cunningham KJ, Mengeling MA. Understanding VA’s use of and relationships with community care providers under the MISSION Act. Med Care. 2021;59(Suppl 3):S252-S258. doi:10.1097/MLR.0000000000001545
- Olenick M, Flowers M, Diaz VJ. US veterans and their unique issues: enhancing health care professional awareness. Adv Med Educ Pract. 2015;6:635-639. doi:10.2147/AMEP.S89479
- Campbell P, Pope R, Simas V, Canetti E, Schram B, Orr R. The effects of early physiotherapy treatment on musculoskeletal injury outcomes in military personnel: a narrative review. Int J Environ Res Public Health. 2022;19(20):13416. doi:10.3390/ijerph192013416
- Gurewich D, Shwartz M, Beilstein-Wedel E, Davila H, Rosen AK. Did access to care improve since passage of the veterans choice act? Differences between rural and urban veterans. Med Care. 2021;59(Suppl 3):S270-S278. doi:10.1097/MLR.0000000000001490
- Myers US, Birks A, Grubaugh AL, Axon RN. Flattening the curve by getting ahead of it: how the VA healthcare system is leveraging telehealth to provide continued access to care for rural veterans. J Rural Health. 2021;37(1):194-196. doi:10.1111/jrh.12449
- Hale-Gallardo JL, Kreider CM, Jia H, et al. Telerehabilitation for rural veterans: a qualitative assessment of barriers and facilitators to implementation. J Multidiscip Healthc. 2020;13:559-570. doi:10.2147/JMDH.S247267
- Kreider CM, Hale-Gallardo J, Kramer JC, et al. Providers’ shift to telerehabilitation at the U.S. Veterans Health Administration during COVID-19: practical applications. Front Public Health. 2022;10:831762. doi:10.3389/fpubh.2022.831762
- Cowper-Ripley DC, Jia H, Wang X, et al. Trends in VA telerehabilitation patients and encounters over time and by rurality. Fed Pract. 2019;36(3):122-128.
- US Dept of Veterans Affairs, Office of Rural Health. VHA Office of Rural Health. Updated August 30, 2024. Accessed September 23, 2024. https://www.ruralhealth.va.gov/index.asp
- National Center for Veterans Analysis and Statistics. Rural Veterans: 2021-2023. April 2023. Accessed September 23, 2024. https://www.datahub.va.gov/stories/s/Rural-Veterans-FY2021-2023/kkh2-eymp/
- U.S. Department of Veterans Affairs, Office of Research & Development. Program Guide: 1200.21, VHA Operations Activities That May Constitute Research. January 9, 2019. https://www.research.va.gov/resources/policies/ProgramGuide-1200-21-VHA-Operations-Activities.pdf
- Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. J Nurs Care Qual. 2016;31(1):1-8. doi:10.1097/NCQ.0000000000000153
- US Dept of Veterans Affairs. Veterans Health Administration: Veterans Integrated Service Networks (VISNs). Updated January 29, 2024. Accessed September 23, 2024. https://www.va.gov/HEALTH/visns.asp
- Stomberg C, Frost A, Becker C, Stang H, Windschitl M, Carrier E. Community Care referral dashboard [Data dashboard]. https://app.powerbigov.us/groups/me/reports/090d22a7-0e1f-4cc5-bea8-0a1b87aa0bd9/ReportSectionacfd03cdebd76ffca9ec [Source not verified]
- US Dept of Veterans Affairs. Eligibility for community care outside VA. Updated May 30, 2024. Accessed September 23, 2024. https://www.va.gov/COMMUNITYCARE/programs/veterans/General_Care.asp
- US Department of Veterans Affairs, Office of Rural Health. How to define rurality fact sheet. Updated December 2023. Accessed January 28, 2025. https://www.ruralhealth.va.gov/docs/ORH_RuralityFactSheet_508.pdf
- Rural-Urban Commuting Area Codes. Economic Research Service, US Dept of Agriculture. Updated September 25, 2023. Accessed September 23, 2024. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx
- Gurewich D, Beilstein-Wedel E, Shwartz M, Davila H, Rosen AK. Disparities in wait times for care among US veterans by race and ethnici t y. JAMA Netw Open. 2023;6(1):e2252061. doi:10.1001/jamanetworkopen.2022.52061
- U.S. Department of Veterans Affairs, VA Office of Rural Health, Veterans Rural Health Resource Center-Gainesville, GeoSpatial Outcomes Division. VA and Community Healthcare, and VHA Rurality web map application. Published 2023. https://portal.vhagis.inv.vaec.va.gov/arcgis/apps/webappbuilder/index.html [source not verified]
- Chartbook on Healthcare for Veterans: National Healthcare Quality and Disparities Report. Agency for Healthcare Research and Quality; November 2020. Accessed September 23, 2024. https://www.ahrq.gov/research/findings/nhqrdr/chartbooks/veterans/index.html
- Lum HD, Nearing K, Pimentel CB, Levy CR, Hung WW. Anywhere to anywhere: use of telehealth to increase health care access for older, rural veterans. Public Policy Aging Rep. 2020;30(1):12-18. doi:10.1093/ppar/prz030
- Goulet JL, Kerns RD, Bair M, et al. The musculoskeletal diagnosis cohort: examining pain and pain care among veterans. Pain. 2016;157(8):1696-1703. doi:10.1097/j.pain.0000000000000567
- US Inflation Calculator. Health Care Inflation in the United States (1948-2024). Accessed September 23, 2024. https://www.usinflationcalculator.com/inflation/health-care-inflation-in-the-united-states/
- Cottrell MA, Galea OA, O’Leary SP, Hill AJ, Russell TG. Real-time telerehabilitation for the treatment of musculoskeletal conditions is effective and comparable to standard practice: a systematic review and meta-analysis. Clin Rehabil. 2017;31(5):625-638. doi:10.1177/0269215516645148
- Elor A, Conde S, Powel l M, Robbins A, Chen NN, Kurniawan S. Physical therapist impressions of telehealth and virtual reality needs amidst a pandemic. Front Virtual Real. 2022;3. doi:10.3389/frvir.2022.915332
- Lee AC, Harada N. Telehealth as a means of health care delivery for physical therapist practice. Phys Ther. 2012;92(3):463-468. doi:10.2522/ptj.20110100
- Hynes DM, Edwards S, Hickok A, et al. Veterans’ use of Veterans Health Administration primary care in an era of expanding choice. Med Care. 2021;59(Suppl 3):S292- S300. doi:10.1097/MLR.0000000000001554
Utilization and Cost of Veterans Health Administration Referrals to Community Care-Based Physical Therapy
Utilization and Cost of Veterans Health Administration Referrals to Community Care-Based Physical Therapy
Impact of 3 Months of Supervised Exercise on Function by Arthritis Status
Impact of 3 Months of Supervised Exercise on Function by Arthritis Status
About half of US adults aged ≥ 65 years report arthritis, and of those, 44% have an arthritis-attributable activity limitation.1,2 Arthritis is a significant health issue for veterans, with veterans reporting higher rates of disability compared with the civilian population.3
Osteoarthritis (OA) is the most common type of arthritis.4 Among individuals aged ≥ 40 years, the incidence of OA is nearly twice as high among veterans compared with civilians and is a leading cause of separation from military service and disability.5,6 OA pain and disability have been shown to be associated with increases in health care and medication use, including opioids, nonsteroidal anti-inflammatory medications, and muscle relaxants.7,8 Because OA is chronic and has no cure, safe and effective management strategies—such as exercise— are critical to minimize pain and maintain physical function.9
Exercise can reduce pain and disability associated with OA and is a first-line recommendation in guidelines for the treatment of knee and hip OA.9 Given the limited exercise and high levels of physical inactivity among veterans with OA, there is a need to identify opportunities that support veterans with OA engaging in regular exercise.
Gerofit, an outpatient clinical exercise program available at 30 Veterans Health Administration (VHA) sites, may provide an opportunity for older veterans with arthritis to engage in exercise.10 Gerofit is specifically designed for veterans aged ≥ 65 years. It is not disease-specific and supports older veterans with multiple chronic conditions, including OA. Veterans aged ≥ 65 years with a referral from a VA clinician are eligible for Gerofit. Those who are unable to perform activities of daily living; unable to independently function without assistance; have a history of unstable angina, proliferative diabetic retinopathy, oxygen dependence, volatile behavioral issues, or are unable to work successfully in a group environment/setting; experience active substance abuse, homelessness, or uncontrolled incontinence; and have open wounds that cannot be appropriately dressed are excluded from Gerofit. Exercise sessions are held 3 times per week and last from 60 to 90 minutes. Sessions are supervised by Gerofit staff and include personalized exercise prescriptions based on functional assessments. Exercise prescriptions include aerobic, resistance, and balance/flexibility components and are modified by the Gerofit program staff as needed. Gerofit adopts a functional fitness approach and includes individual progression as appropriate according to evidence-based guidelines, using the Borg ratings of perceived exertion. 11 Assessments are performed at baseline, 3 months, 6 months, and annually thereafter. Clinical staff conduct all assessments, including physical function testing, and record them in a database. Assessments are reviewed with the veteran to chart progress and identify future goals or needs. Veterans perform personalized self-paced exercises in the Gerofit group setting. Exercise prescriptions are continuously modified to meet individualized needs and goals. Veterans may participate continuously with no end date.
Participation in supervised exercise is associated with improved physical function and individuals with arthritis can improve function even though their baseline functional status is lower than individuals without arthritis. 12 In this analysis, we examine the impact of exercise on the status and location of arthritis (upper body, lower body, or both). Lower body arthritis is more common than upper body arthritis and lower extremity function is associated with increased ability to perform activities of daily living, resulting in independence among older adults.13,14 We also include upper body strength measures to capture important functional movements such as reaching and pulling.15 Among those who participate in Gerofit, the greatest gains in physical function occur during the initial 3 months, which tend to be sustained over 12 months.16 For this reason, this study focused on the initial 3 months of the program.
Older adults with arthritis may have pain and functional limitations that exceed those of the general older adult population. Exercise programs for older adults that do not specifically target arthritis but are able to improve physical function among those with arthritis could potentially increase access to exercise for older adults living with arthritis. Therefore, the purpose of this study was to determine whether change in physical function with participation in Gerofit for 3 months varies by arthritis status, including no arthritis, any arthritis, lower body arthritis, or both upper and lower body arthritis compared with no arthritis.
Methods
This is a secondary analysis of previously collected data from 10 VHA Gerofit sites (Ann Arbor, Baltimore, Greater Los Angeles, Canandaigua, Cincinnati, Miami, Honolulu, Denver, Durham, and Pittsburgh) from 2002 to 2019. Implementation data regarding the consistency of the program delivery at Gerofit expansion sites have been previously published.16 Although the delivery of Gerofit transitioned to telehealth due to COVID-19, data for this analysis were collected from in-person exercise sessions prior to the pandemic.17 Data were collected for clinical purposes. This project was part of the Gerofit quality improvement initiative and was reviewed and approved by the Durham Institutional Review Board as quality improvement.
Participants in Gerofit who completed baseline and 3-month assessments were included to analyze the effects of exercise on physical function. At each of the time points, physical functional assessments included: (1) usual gait speed (> 10 meters [m/s], or 10- meter walk test [10MWT]); (2) lower body strength (chair stands [number completed in 30 seconds]); (3) upper body strength (number of arm curls [5-lb for females/8-lb for males] completed in 30 seconds); and (4) 6-minute walk distance [6MWD] in meters to measure aerobic endurance). These measures have been validated in older adults.18-21 Arm curls were added to the physical function assessments after the 10MWT, chair stands, and 6MWD; therefore, fewer participants had data for this measure. Participants self-reported at baseline on 45 common medical conditions, including arthritis or rheumatism (both upper body and lower body were offered as choices). Self-reporting has been shown to be an acceptable method of identifying arthritis in adults.22
Descriptive statistics at baseline were calculated for all participants. One-way analysis of variance and X2 tests were used to determine differences in baseline characteristics across arthritis status. The primary outcomes were changes in physical function measures from baseline to 3 months by arthritis status. Arthritis status was defined as: any arthritis, which includes individuals who reported upper body arthritis, lower body arthritis, or both; and arthritis status individuals reporting either upper body arthritis, lower body arthritis, or both. Categories of arthritis for arthritis status were mutually exclusive. Two separate linear models were constructed for each of the 4 physical function measures, with change from baseline to 3 months as the outcome (dependent variable) and arthritis status, age, and body mass index (BMI) as predictors (independent variables). The first model compared any arthritis with no arthritis and the second model compared arthritis status (both upper and lower body arthritis vs lower body arthritis) with no arthritis. These models were used to obtain mean changes and 95% CIs in physical function and to test for differences in the change in physical function measures by arthritis status. Statistical analyses were performed using R software, version 4.0.3.
Results
Baseline and 3-month data were available for 737 Gerofit participants and included in the analysis. The mean (SD) age was 73.5 (7.1) years. A total of 707 participants were male (95.9%) and 322 (43.6%) reported some arthritis, with arthritis in both the upper and lower body being reported by 168 participants (52.2%) (Table 1). There were no differences in age, sex, or race for those with any arthritis compared with those with no arthritis, but BMI was significantly higher in those reporting any arthritis compared with no arthritis. For the baseline functional measures, statistically significant differences were observed between those with no arthritis and those reporting any arthritis for the 10MWT (P = .001), chair stands (P = .046), and 6MWD (P = .001), but not for arm curls (P = .77), with those with no arthritis performing better.
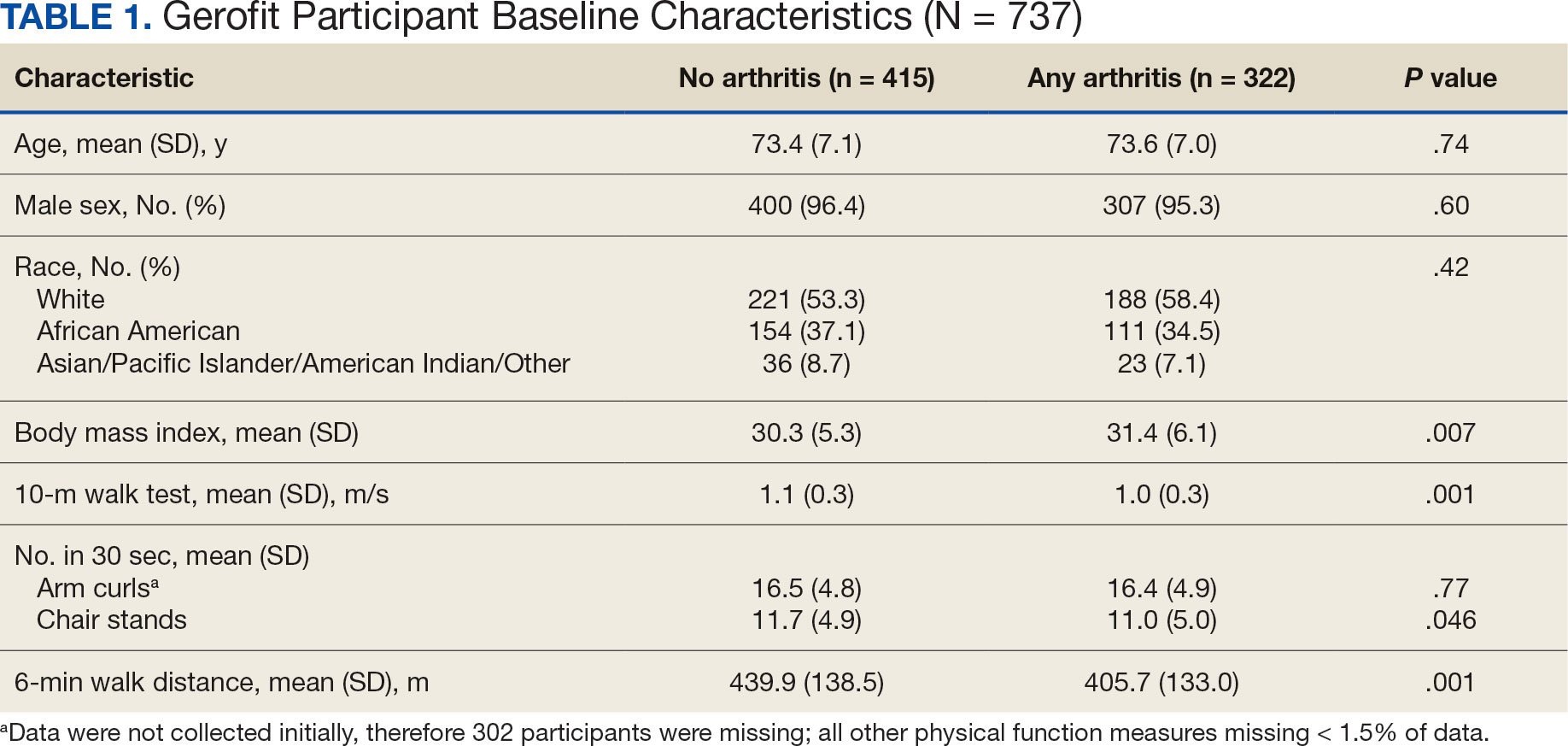
All 4 arthritis status groups showed improvements in each of the physical function measures over 3 months. For the 10MWT the mean change (95% CI) in gait speed (m/s) was 0.06 (0.04-0.08) for patients with no arthritis, 0.07 (0.05- 0.08) for any arthritis, 0.07 (0.04-0.11) for lower body arthritis, and 0.07 (0.04- 0.09) for both lower and upper body arthritis. For the number of arm curls in 30 seconds the mean change (95% CI) was 2.3 (1.8-2.8) for patients with no arthritis, 2.1 (1.5-2.6) for any arthritis, 2.0 (1.1-3.0) for lower body arthritis, and 1.9 (1.1-2.7) for both lower and upper body arthritis. For the number of chair stands in 30 seconds the mean change (95% CI) was 2.1 (1.7-2.4) for patients with no arthritis, 2.2 (1.8-2.6) for any arthritis, 2.3 (1.6-2.9), for lower body arthritis, and 2.0 (1.5-2.5) for both lower and upper body arthritis. For the 6MWD distance in meters the mean change (95% CI) was 21.5 (15.5-27.4) for patients with no arthritis, 28.6 (21.9-35.3) for any arthritis, 30.4 (19.5-41.3) for lower body arthritis, and 28.6 (19.2-38.0) for both lower and upper body arthritis (Figure).
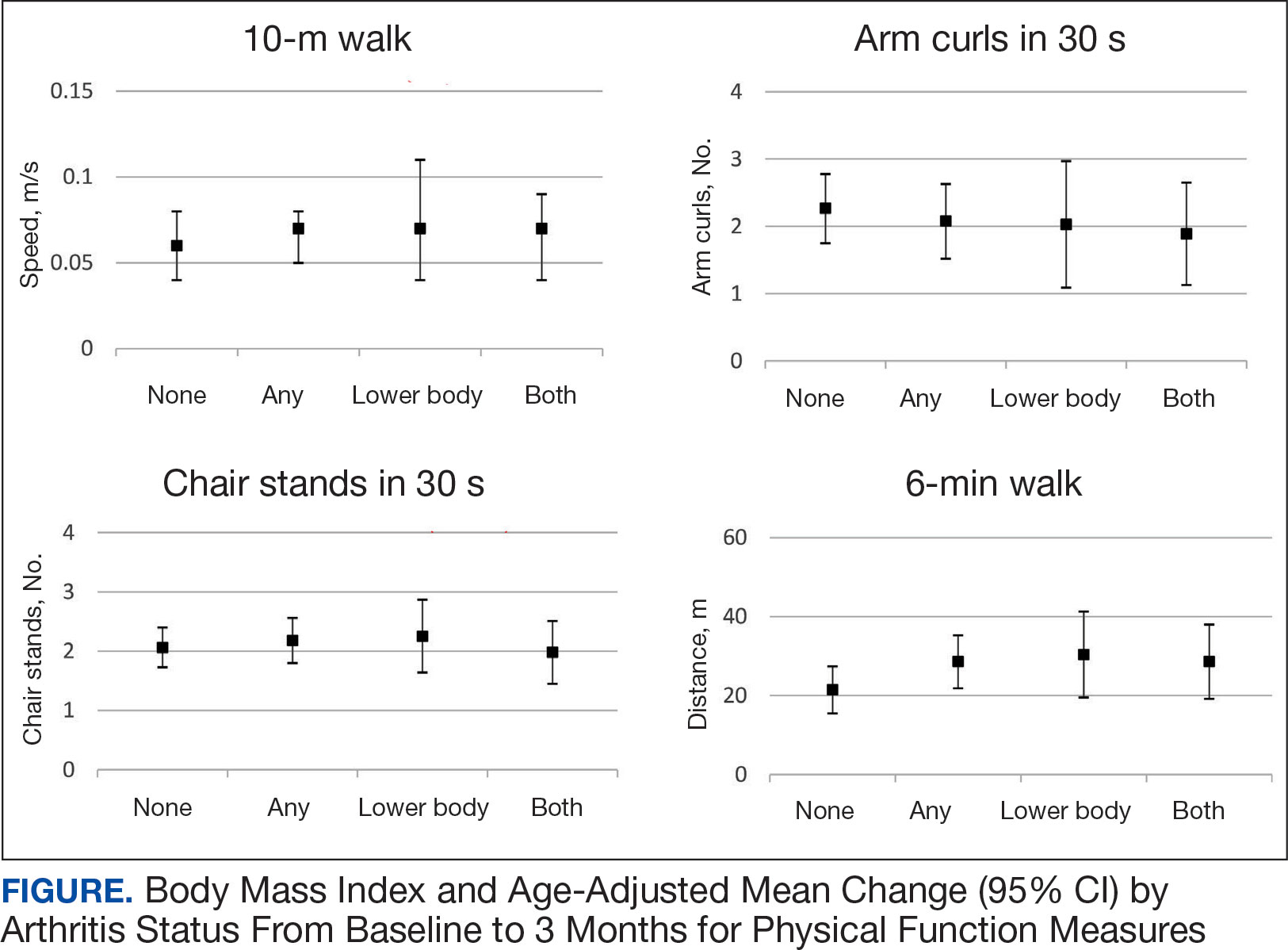
We used 2 models to measure the change from baseline to 3 months for each of the arthritis groups. Model 1 compared any arthritis vs no arthritis and model 2 compared lower body arthritis and both upper and lower body arthritis vs no arthritis for each physical function measure (Table 2). There were no statistically significant differences in 3-month change in physical function for any of the physical function measures between arthritis groups after adjusting for age and BMI.
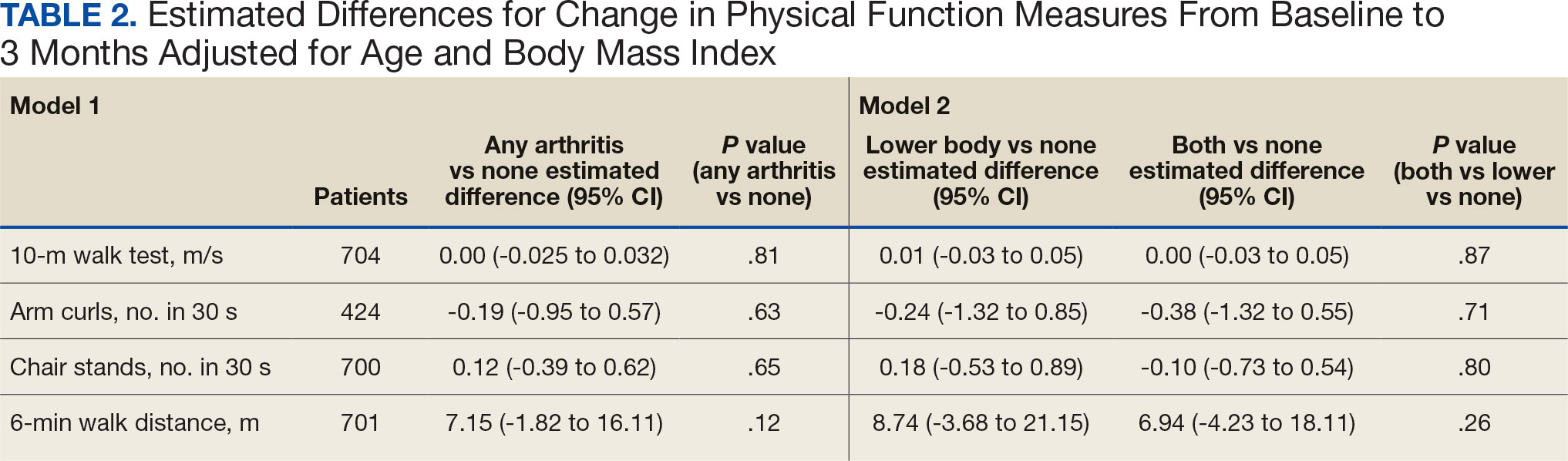
Discussion
Participation in Gerofit was associated with functional gains among all participants over 3 months, regardless of arthritis status. Older veterans reporting any arthritis had significantly lower physical function scores upon enrollment into Gerofit compared with those veterans reporting no arthritis. However, compared with individuals who reported no arthritis, individuals who reported arthritis (any arthritis, lower body arthritis only, or both lower and upper body arthritis) experienced similar improvements (ie, no statistically significant differences in mean change from baseline to follow-up among those with and without arthritis). This study suggests that progressive, multicomponent exercise programs for older adults may be beneficial for those with arthritis.
Involvement of multiple sites of arthritis is associated with moderate to severe functional limitations as well as lower healthrelated quality of life.23 While it has been found that individuals with arthritis can improve function with supervised exercise, even though their baseline functional status is lower than individuals without arthritis, it was not clear whether individuals with multiple joint involvement also would benefit.12 The results of this study suggest that these individuals can improve across various domains of physical function despite variation in arthritis location and status. As incidence of arthritis increases with age, targeting older adults for exercise programs such as Gerofit may improve functional limitations and health-related quality of life associated with arthritis.2
We evaluated physical function using multiple measures to assess upper (arm curls) and lower (chair stands, 10MWT) extremity physical function and aerobic endurance (6MWD). Participants in this study reached clinically meaningful changes with 3 months of participation in Gerofit for most of the physical function measures. Gerofit participants had a mean gait speed improvement of 0.05 to 0.07 m/s compared with 0.10 to 0.30 m/s, which was reported previously. 24,25 In this study, nearly all groups achieved the clinically important improvements in the chair stand in 30 seconds (2.0 to 2.6) and the 6MWD (21.8 to 59.1 m) that have been reported in the literature.24-26
The Osteoarthritis Research Society International recommends the chair stand and 6MWD performance-based tests for individuals with hip and knee arthritis because they align with patient-reported outcomes and represent the types of activities relevant to this population.27 The findings of this study suggest that improvement in these physical function measures with participation in exercise align with data from arthritis-specific exercise programs designed for wide implementation. Hughes and colleagues reported improvements in the 6MWD after the 8-week Fit and Strong exercise intervention, which included walking and lower body resistance training.28 The Arthritis Foundation’s Walk With Ease program is a 6-week walking program that has shown improvements in chair stands and gait speed.29 Another Arthritis Foundation program, People with Arthritis Can Exercise, is an 8-week course consisting of a variety of resistance, aerobic, and balance activities. This program has been associated with increases in chair stands but not gait speed or 6MWD.30,31
This study found that participation in a VHA outpatient clinical supervised exercise program results in improvements in physical function that can be realized by older adults regardless of arthritis burden. Gerofit programs typically require 1.5 to 2.0 dedicated full-time equivalent employees to run the program effectively and additional administrative support, depending on size of the program.32 The cost savings generated by the program include reductions in hospitalization rates, emergency department visits, days in hospital, and medication use and provide a compelling argument for the program’s financial viability to health care systems through long-term savings and improved health outcomes for older adults.33-36
While evidenced-based arthritis programs exist, this study illustrates that an exercise program without a focus on arthritis also improves physical function, potentially reducing the risk of disability related to arthritis. The clinical implication for these findings is that arthritis-specific exercise programs may not be needed to achieve functional improvements in individuals with arthritis. This is critical for under-resourced or exercise- limited health care systems or communities. Therefore, if exercise programming is limited, or arthritis-specific programs and interventions are not available, nonspecific exercise programs will also be beneficial to individuals with arthritis. Thus, individuals with arthritis should be encouraged to participate in any available exercise programming to achieve improvements in physical function. In addition, many older adults have multiple comorbidities, most of which improve with participation in exercise. 37 Disease-specific exercise programs can offer tailored exercises and coaching related to common barriers in participation, such as joint pain for arthritis.31 It is unclear whether these additional programmatic components are associated with greater improvements in outcomes, such as physical function. More research is needed to explore the benefits of disease-specific tailored exercise programs compared with general exercise programs.
Strengths and Limitations
This study demonstrated the effect of participation in a clinical, supervised exercise program in a real-world setting. It suggests that even exercise programs not specifically targeted for arthritis populations can improve physical function among those with arthritis.
As a VHA clinical supervised exercise program, Gerofit may not be generalizable to all older adults or other exercise programs. In addition, this analysis only included a veteran population that was > 95% male and may not be generalizable to other populations. Arthritis status was defined by self-report and not verified in the health record. However, this approach has been shown to be acceptable in this setting and the most common type of arthritis in this population (OA) is a painful musculoskeletal condition associated with functional limitations.4,22,38,39 Self-reported arthritis or rheumatism is associated with functional limitations.1 Therefore, it is unlikely that the results would differ for physician-diagnosed or radiographically defined OA. Additionally, the study did not have data on the total number of joints with arthritis or arthritis severity but rather used upper body, lower body, and both upper and lower body arthritis as a proxy for arthritis status. While our models were adjusted for age and BMI, 2 known confounding factors for the association between arthritis and physical function, there are other potential confounding factors that were not included in the models. 40,41 Finally, this study only included individuals with completed baseline and 3-month follow-up assessments, and the individuals who participated for longer or shorter periods may have had different physical function outcomes than individuals included in this study.
Conclusions
Participation in 3 months VHA Gerofit outpatient supervised exercise programs can improve physical function for all older adults, regardless of arthritis status. These programs may increase access to exercise programming that is beneficial for common conditions affecting older adults, such as arthritis.
- Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults- -United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421-426.
- Theis KA, Murphy LB, Guglielmo D, et al. Prevalence of arthritis and arthritis-attributable activity limitation—United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2021;70:1401-1407. doi:10.15585/mmwr.mm7040a2
- Murphy LB, Helmick CG, Allen KD, et al. Arthritis among veterans—United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2014;63:999-1003.
- Park J, Mendy A, Vieira ER. Various types of arthritis in the United States: prevalence and age-related trends from 1999 to 2014. Am J Public Health. 2018;108:256-258.
- Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis Rheum. 2011;63:2974-2982. doi:10.1002/art.30498
- Patzkowski JC, Rivera JC, Ficke JR, Wenke JC. The changing face of disability in the US Army: the Operation Enduring Freedom and Operation Iraqi Freedom effect. J Am Acad Orthop Surg. 2012;20(suppl 1):S23-S30. doi:10.5435/JAAOS-20-08-S23
- Rivera JC, Amuan ME, Morris RM, Johnson AE, Pugh MJ. Arthritis, comorbidities, and care utilization in veterans of Operations Enduring and Iraqi Freedom. J Orthop Res. 2017;35:682-687. doi:10.1002/jor.23323
- Singh JA, Nelson DB, Fink HA, Nichol KL. Health-related quality of life predicts future health care utilization and mortality in veterans with self-reported physician-diagnosed arthritis: the Veterans Arthritis Quality of Life Study. Semin Arthritis Rheum. 2005;34:755- 765. doi:10.1016/j.semarthrit.2004.08.001
- Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712. doi:10.1016/j.semarthrit.2013.11.012
- Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit Program: a VA innovation. South Med J. 1994;87:S83-S87.
- Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20:873-899. doi:10.1080/026404102320761787
- Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44:1226-1231. doi:10.1111/j.1532-5415.1996.tb01374.x
- Allen KD, Gol ight ly YM. State of the evidence. Curr Opin Rheumatol. 2015;27:276-283. doi:10.1097/BOR.0000000000000161
- den Ouden MEM, Schuurmans MJ, Arts IEMA, van der Schouw YT. Association between physical performance characteristics and independence in activities of daily living in middle-aged and elderly men. Geriatr Gerontol Int. 2013;13:274-280. doi:10.1111/j.1447-0594.2012.00890.x
- Daly M, Vidt ME, Eggebeen JD, et al. Upper extremity muscle volumes and functional strength after resistance training in older adults. J Aging Phys Act. 2013;21:186-207. doi:10.1123/japa.21.2.186
- Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit Program. J Am Geriatr Soc. 2018;66:1009-1016. doi:10.1111/jgs.15276
- Jennings SC, Manning KM, Bettger JP, et al. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol Geriatr Med. 2020;6:2333721420980313. doi:10.1177/ 2333721420980313
- Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314-322. doi:10.1046/j.1532-5415.2003.51104.x
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community residing older adults. Res Q Exerc Sport. 1999;70:113- 119. doi:10.1080/02701367.1999.10608028
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129-161. doi:10.1123/japa.7.2.129
- Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837-841. doi:10.1016/s0003-9993(99)90236-8
- Peeters GGME, Alshurafa M, Schaap L, de Vet HCW. Diagnostic accuracy of self-reported arthritis in the general adult population is acceptable. J Clin Epidemiol. 2015;68:452-459. doi:10.1016/j.jclinepi.2014.09.019
- Cuperus N, Vliet Vlieland TPM, Mahler EAM, Kersten CC, Hoogeboom TJ, van den Ende CHM. The clinical burden of generalized osteoarthritis represented by self-reported health-related quality of life and activity limitations: a cross-sectional study. Rheumatol Int. 2015;35:871-877. doi:10.1007/s00296-014-3149-1
- Coleman G, Dobson F, Hinman RS, Bennell K, White DK. Measures of physical performance. Arthritis Care Res (Hoboken). 2020;72(suppl 10):452-485. doi:10.1002/acr.24373
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743-749. doi:10.1111/j.1532-5415.2006.00701.x
- Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther. 2011;41:319-327. doi:10.2519/jospt.2011.3515
- Dobson F, Hinman R, Roos EM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1042- 1052. doi:10.1016/j.joca.2013.05.002
- Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217-228. doi:10.1093/geront/44.2.217
- Callahan LF, Shreffler JH, Altpeter M, et al. Evaluation of group and self-directed formats of the Arthritis Foundation's Walk With Ease Program. Arthritis Care Res (Hoboken). 2011;63:1098-1107. doi:10.1002/acr.20490
- Boutaugh ML. Arthritis Foundation community-based physical activity programs: effectiveness and implementation issues. Arthritis Rheum. 2003;49:463-470. doi:10.1002/art.11050
- Callahan LF, Mielenz T, Freburger J, et al. A randomized controlled trial of the People with Arthritis Can Exercise Program: symptoms, function, physical activity, and psychosocial outcomes. Arthritis Rheum. 2008;59:92-101. doi:10.1002/art.23239
- Hall KS, Jennings SC, Pearson MP. Outpatient care models: the Gerofit model of care for exercise promotion in older adults. In: Malone ML, Boltz M, Macias Tejada J, White H, eds. Geriatrics Models of Care. Springer; 2024:205-213. doi:10.1007/978-3-031-56204-4_21
- Pepin MJ, Valencia WM, Bettger JP, et al. Impact of supervised exercise on one-year medication use in older veterans with multiple morbidities. Gerontol Geriatr Med. 2020;6:2333721420956751. doi:10.1177/ 2333721420956751
- Abbate L, Li J, Veazie P, et al. Does Gerofit exercise reduce veterans’ use of emergency department and inpatient care? Innov Aging. 2020;4(suppl 1):771. doi:10.1093/geroni/igaa057.2786
- Morey MC, Pieper CF, Crowley GM, Sullivan RJ Jr, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50:1929-1933. doi:10.1046/j.1532-5415.2002.50602.x
- Manning KM, Hall KS, Sloane R, et al. Longitudinal analysis of physical function in older adults: the effects of physical inactivity and exercise training. Aging Cell. 2024;23:e13987. doi:10.1111/acel.13987
- Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85(7 suppl 3):S31-S42; quiz S3-S4. doi:10.1016/j.apmr.2004.03.010
- Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009; 57:1556-1561. doi:10.1111/j.1532-5415.2009.02388.x
- Katz JN, Wright EA, Baron JA, Losina E. Development and validation of an index of musculoskeletal functional limitations. BMC Musculoskelet Disord. 2009;10:62. doi:10.1186/1471-2474-10-62
- Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30:184-195. doi:10.1016/j.joca.2021.04.020
- Riebe D, Blissmer BJ, Greaney ML, Ewing Garber C, Lees FD, Clark PG. The relationship between obesity, physical activity, and physical function in older adults. J Aging Health. 2009;21:1159-1178. doi:10.1177/0898264309350076
About half of US adults aged ≥ 65 years report arthritis, and of those, 44% have an arthritis-attributable activity limitation.1,2 Arthritis is a significant health issue for veterans, with veterans reporting higher rates of disability compared with the civilian population.3
Osteoarthritis (OA) is the most common type of arthritis.4 Among individuals aged ≥ 40 years, the incidence of OA is nearly twice as high among veterans compared with civilians and is a leading cause of separation from military service and disability.5,6 OA pain and disability have been shown to be associated with increases in health care and medication use, including opioids, nonsteroidal anti-inflammatory medications, and muscle relaxants.7,8 Because OA is chronic and has no cure, safe and effective management strategies—such as exercise— are critical to minimize pain and maintain physical function.9
Exercise can reduce pain and disability associated with OA and is a first-line recommendation in guidelines for the treatment of knee and hip OA.9 Given the limited exercise and high levels of physical inactivity among veterans with OA, there is a need to identify opportunities that support veterans with OA engaging in regular exercise.
Gerofit, an outpatient clinical exercise program available at 30 Veterans Health Administration (VHA) sites, may provide an opportunity for older veterans with arthritis to engage in exercise.10 Gerofit is specifically designed for veterans aged ≥ 65 years. It is not disease-specific and supports older veterans with multiple chronic conditions, including OA. Veterans aged ≥ 65 years with a referral from a VA clinician are eligible for Gerofit. Those who are unable to perform activities of daily living; unable to independently function without assistance; have a history of unstable angina, proliferative diabetic retinopathy, oxygen dependence, volatile behavioral issues, or are unable to work successfully in a group environment/setting; experience active substance abuse, homelessness, or uncontrolled incontinence; and have open wounds that cannot be appropriately dressed are excluded from Gerofit. Exercise sessions are held 3 times per week and last from 60 to 90 minutes. Sessions are supervised by Gerofit staff and include personalized exercise prescriptions based on functional assessments. Exercise prescriptions include aerobic, resistance, and balance/flexibility components and are modified by the Gerofit program staff as needed. Gerofit adopts a functional fitness approach and includes individual progression as appropriate according to evidence-based guidelines, using the Borg ratings of perceived exertion. 11 Assessments are performed at baseline, 3 months, 6 months, and annually thereafter. Clinical staff conduct all assessments, including physical function testing, and record them in a database. Assessments are reviewed with the veteran to chart progress and identify future goals or needs. Veterans perform personalized self-paced exercises in the Gerofit group setting. Exercise prescriptions are continuously modified to meet individualized needs and goals. Veterans may participate continuously with no end date.
Participation in supervised exercise is associated with improved physical function and individuals with arthritis can improve function even though their baseline functional status is lower than individuals without arthritis. 12 In this analysis, we examine the impact of exercise on the status and location of arthritis (upper body, lower body, or both). Lower body arthritis is more common than upper body arthritis and lower extremity function is associated with increased ability to perform activities of daily living, resulting in independence among older adults.13,14 We also include upper body strength measures to capture important functional movements such as reaching and pulling.15 Among those who participate in Gerofit, the greatest gains in physical function occur during the initial 3 months, which tend to be sustained over 12 months.16 For this reason, this study focused on the initial 3 months of the program.
Older adults with arthritis may have pain and functional limitations that exceed those of the general older adult population. Exercise programs for older adults that do not specifically target arthritis but are able to improve physical function among those with arthritis could potentially increase access to exercise for older adults living with arthritis. Therefore, the purpose of this study was to determine whether change in physical function with participation in Gerofit for 3 months varies by arthritis status, including no arthritis, any arthritis, lower body arthritis, or both upper and lower body arthritis compared with no arthritis.
Methods
This is a secondary analysis of previously collected data from 10 VHA Gerofit sites (Ann Arbor, Baltimore, Greater Los Angeles, Canandaigua, Cincinnati, Miami, Honolulu, Denver, Durham, and Pittsburgh) from 2002 to 2019. Implementation data regarding the consistency of the program delivery at Gerofit expansion sites have been previously published.16 Although the delivery of Gerofit transitioned to telehealth due to COVID-19, data for this analysis were collected from in-person exercise sessions prior to the pandemic.17 Data were collected for clinical purposes. This project was part of the Gerofit quality improvement initiative and was reviewed and approved by the Durham Institutional Review Board as quality improvement.
Participants in Gerofit who completed baseline and 3-month assessments were included to analyze the effects of exercise on physical function. At each of the time points, physical functional assessments included: (1) usual gait speed (> 10 meters [m/s], or 10- meter walk test [10MWT]); (2) lower body strength (chair stands [number completed in 30 seconds]); (3) upper body strength (number of arm curls [5-lb for females/8-lb for males] completed in 30 seconds); and (4) 6-minute walk distance [6MWD] in meters to measure aerobic endurance). These measures have been validated in older adults.18-21 Arm curls were added to the physical function assessments after the 10MWT, chair stands, and 6MWD; therefore, fewer participants had data for this measure. Participants self-reported at baseline on 45 common medical conditions, including arthritis or rheumatism (both upper body and lower body were offered as choices). Self-reporting has been shown to be an acceptable method of identifying arthritis in adults.22
Descriptive statistics at baseline were calculated for all participants. One-way analysis of variance and X2 tests were used to determine differences in baseline characteristics across arthritis status. The primary outcomes were changes in physical function measures from baseline to 3 months by arthritis status. Arthritis status was defined as: any arthritis, which includes individuals who reported upper body arthritis, lower body arthritis, or both; and arthritis status individuals reporting either upper body arthritis, lower body arthritis, or both. Categories of arthritis for arthritis status were mutually exclusive. Two separate linear models were constructed for each of the 4 physical function measures, with change from baseline to 3 months as the outcome (dependent variable) and arthritis status, age, and body mass index (BMI) as predictors (independent variables). The first model compared any arthritis with no arthritis and the second model compared arthritis status (both upper and lower body arthritis vs lower body arthritis) with no arthritis. These models were used to obtain mean changes and 95% CIs in physical function and to test for differences in the change in physical function measures by arthritis status. Statistical analyses were performed using R software, version 4.0.3.
Results
Baseline and 3-month data were available for 737 Gerofit participants and included in the analysis. The mean (SD) age was 73.5 (7.1) years. A total of 707 participants were male (95.9%) and 322 (43.6%) reported some arthritis, with arthritis in both the upper and lower body being reported by 168 participants (52.2%) (Table 1). There were no differences in age, sex, or race for those with any arthritis compared with those with no arthritis, but BMI was significantly higher in those reporting any arthritis compared with no arthritis. For the baseline functional measures, statistically significant differences were observed between those with no arthritis and those reporting any arthritis for the 10MWT (P = .001), chair stands (P = .046), and 6MWD (P = .001), but not for arm curls (P = .77), with those with no arthritis performing better.

All 4 arthritis status groups showed improvements in each of the physical function measures over 3 months. For the 10MWT the mean change (95% CI) in gait speed (m/s) was 0.06 (0.04-0.08) for patients with no arthritis, 0.07 (0.05- 0.08) for any arthritis, 0.07 (0.04-0.11) for lower body arthritis, and 0.07 (0.04- 0.09) for both lower and upper body arthritis. For the number of arm curls in 30 seconds the mean change (95% CI) was 2.3 (1.8-2.8) for patients with no arthritis, 2.1 (1.5-2.6) for any arthritis, 2.0 (1.1-3.0) for lower body arthritis, and 1.9 (1.1-2.7) for both lower and upper body arthritis. For the number of chair stands in 30 seconds the mean change (95% CI) was 2.1 (1.7-2.4) for patients with no arthritis, 2.2 (1.8-2.6) for any arthritis, 2.3 (1.6-2.9), for lower body arthritis, and 2.0 (1.5-2.5) for both lower and upper body arthritis. For the 6MWD distance in meters the mean change (95% CI) was 21.5 (15.5-27.4) for patients with no arthritis, 28.6 (21.9-35.3) for any arthritis, 30.4 (19.5-41.3) for lower body arthritis, and 28.6 (19.2-38.0) for both lower and upper body arthritis (Figure).

We used 2 models to measure the change from baseline to 3 months for each of the arthritis groups. Model 1 compared any arthritis vs no arthritis and model 2 compared lower body arthritis and both upper and lower body arthritis vs no arthritis for each physical function measure (Table 2). There were no statistically significant differences in 3-month change in physical function for any of the physical function measures between arthritis groups after adjusting for age and BMI.

Discussion
Participation in Gerofit was associated with functional gains among all participants over 3 months, regardless of arthritis status. Older veterans reporting any arthritis had significantly lower physical function scores upon enrollment into Gerofit compared with those veterans reporting no arthritis. However, compared with individuals who reported no arthritis, individuals who reported arthritis (any arthritis, lower body arthritis only, or both lower and upper body arthritis) experienced similar improvements (ie, no statistically significant differences in mean change from baseline to follow-up among those with and without arthritis). This study suggests that progressive, multicomponent exercise programs for older adults may be beneficial for those with arthritis.
Involvement of multiple sites of arthritis is associated with moderate to severe functional limitations as well as lower healthrelated quality of life.23 While it has been found that individuals with arthritis can improve function with supervised exercise, even though their baseline functional status is lower than individuals without arthritis, it was not clear whether individuals with multiple joint involvement also would benefit.12 The results of this study suggest that these individuals can improve across various domains of physical function despite variation in arthritis location and status. As incidence of arthritis increases with age, targeting older adults for exercise programs such as Gerofit may improve functional limitations and health-related quality of life associated with arthritis.2
We evaluated physical function using multiple measures to assess upper (arm curls) and lower (chair stands, 10MWT) extremity physical function and aerobic endurance (6MWD). Participants in this study reached clinically meaningful changes with 3 months of participation in Gerofit for most of the physical function measures. Gerofit participants had a mean gait speed improvement of 0.05 to 0.07 m/s compared with 0.10 to 0.30 m/s, which was reported previously. 24,25 In this study, nearly all groups achieved the clinically important improvements in the chair stand in 30 seconds (2.0 to 2.6) and the 6MWD (21.8 to 59.1 m) that have been reported in the literature.24-26
The Osteoarthritis Research Society International recommends the chair stand and 6MWD performance-based tests for individuals with hip and knee arthritis because they align with patient-reported outcomes and represent the types of activities relevant to this population.27 The findings of this study suggest that improvement in these physical function measures with participation in exercise align with data from arthritis-specific exercise programs designed for wide implementation. Hughes and colleagues reported improvements in the 6MWD after the 8-week Fit and Strong exercise intervention, which included walking and lower body resistance training.28 The Arthritis Foundation’s Walk With Ease program is a 6-week walking program that has shown improvements in chair stands and gait speed.29 Another Arthritis Foundation program, People with Arthritis Can Exercise, is an 8-week course consisting of a variety of resistance, aerobic, and balance activities. This program has been associated with increases in chair stands but not gait speed or 6MWD.30,31
This study found that participation in a VHA outpatient clinical supervised exercise program results in improvements in physical function that can be realized by older adults regardless of arthritis burden. Gerofit programs typically require 1.5 to 2.0 dedicated full-time equivalent employees to run the program effectively and additional administrative support, depending on size of the program.32 The cost savings generated by the program include reductions in hospitalization rates, emergency department visits, days in hospital, and medication use and provide a compelling argument for the program’s financial viability to health care systems through long-term savings and improved health outcomes for older adults.33-36
While evidenced-based arthritis programs exist, this study illustrates that an exercise program without a focus on arthritis also improves physical function, potentially reducing the risk of disability related to arthritis. The clinical implication for these findings is that arthritis-specific exercise programs may not be needed to achieve functional improvements in individuals with arthritis. This is critical for under-resourced or exercise- limited health care systems or communities. Therefore, if exercise programming is limited, or arthritis-specific programs and interventions are not available, nonspecific exercise programs will also be beneficial to individuals with arthritis. Thus, individuals with arthritis should be encouraged to participate in any available exercise programming to achieve improvements in physical function. In addition, many older adults have multiple comorbidities, most of which improve with participation in exercise. 37 Disease-specific exercise programs can offer tailored exercises and coaching related to common barriers in participation, such as joint pain for arthritis.31 It is unclear whether these additional programmatic components are associated with greater improvements in outcomes, such as physical function. More research is needed to explore the benefits of disease-specific tailored exercise programs compared with general exercise programs.
Strengths and Limitations
This study demonstrated the effect of participation in a clinical, supervised exercise program in a real-world setting. It suggests that even exercise programs not specifically targeted for arthritis populations can improve physical function among those with arthritis.
As a VHA clinical supervised exercise program, Gerofit may not be generalizable to all older adults or other exercise programs. In addition, this analysis only included a veteran population that was > 95% male and may not be generalizable to other populations. Arthritis status was defined by self-report and not verified in the health record. However, this approach has been shown to be acceptable in this setting and the most common type of arthritis in this population (OA) is a painful musculoskeletal condition associated with functional limitations.4,22,38,39 Self-reported arthritis or rheumatism is associated with functional limitations.1 Therefore, it is unlikely that the results would differ for physician-diagnosed or radiographically defined OA. Additionally, the study did not have data on the total number of joints with arthritis or arthritis severity but rather used upper body, lower body, and both upper and lower body arthritis as a proxy for arthritis status. While our models were adjusted for age and BMI, 2 known confounding factors for the association between arthritis and physical function, there are other potential confounding factors that were not included in the models. 40,41 Finally, this study only included individuals with completed baseline and 3-month follow-up assessments, and the individuals who participated for longer or shorter periods may have had different physical function outcomes than individuals included in this study.
Conclusions
Participation in 3 months VHA Gerofit outpatient supervised exercise programs can improve physical function for all older adults, regardless of arthritis status. These programs may increase access to exercise programming that is beneficial for common conditions affecting older adults, such as arthritis.
About half of US adults aged ≥ 65 years report arthritis, and of those, 44% have an arthritis-attributable activity limitation.1,2 Arthritis is a significant health issue for veterans, with veterans reporting higher rates of disability compared with the civilian population.3
Osteoarthritis (OA) is the most common type of arthritis.4 Among individuals aged ≥ 40 years, the incidence of OA is nearly twice as high among veterans compared with civilians and is a leading cause of separation from military service and disability.5,6 OA pain and disability have been shown to be associated with increases in health care and medication use, including opioids, nonsteroidal anti-inflammatory medications, and muscle relaxants.7,8 Because OA is chronic and has no cure, safe and effective management strategies—such as exercise— are critical to minimize pain and maintain physical function.9
Exercise can reduce pain and disability associated with OA and is a first-line recommendation in guidelines for the treatment of knee and hip OA.9 Given the limited exercise and high levels of physical inactivity among veterans with OA, there is a need to identify opportunities that support veterans with OA engaging in regular exercise.
Gerofit, an outpatient clinical exercise program available at 30 Veterans Health Administration (VHA) sites, may provide an opportunity for older veterans with arthritis to engage in exercise.10 Gerofit is specifically designed for veterans aged ≥ 65 years. It is not disease-specific and supports older veterans with multiple chronic conditions, including OA. Veterans aged ≥ 65 years with a referral from a VA clinician are eligible for Gerofit. Those who are unable to perform activities of daily living; unable to independently function without assistance; have a history of unstable angina, proliferative diabetic retinopathy, oxygen dependence, volatile behavioral issues, or are unable to work successfully in a group environment/setting; experience active substance abuse, homelessness, or uncontrolled incontinence; and have open wounds that cannot be appropriately dressed are excluded from Gerofit. Exercise sessions are held 3 times per week and last from 60 to 90 minutes. Sessions are supervised by Gerofit staff and include personalized exercise prescriptions based on functional assessments. Exercise prescriptions include aerobic, resistance, and balance/flexibility components and are modified by the Gerofit program staff as needed. Gerofit adopts a functional fitness approach and includes individual progression as appropriate according to evidence-based guidelines, using the Borg ratings of perceived exertion. 11 Assessments are performed at baseline, 3 months, 6 months, and annually thereafter. Clinical staff conduct all assessments, including physical function testing, and record them in a database. Assessments are reviewed with the veteran to chart progress and identify future goals or needs. Veterans perform personalized self-paced exercises in the Gerofit group setting. Exercise prescriptions are continuously modified to meet individualized needs and goals. Veterans may participate continuously with no end date.
Participation in supervised exercise is associated with improved physical function and individuals with arthritis can improve function even though their baseline functional status is lower than individuals without arthritis. 12 In this analysis, we examine the impact of exercise on the status and location of arthritis (upper body, lower body, or both). Lower body arthritis is more common than upper body arthritis and lower extremity function is associated with increased ability to perform activities of daily living, resulting in independence among older adults.13,14 We also include upper body strength measures to capture important functional movements such as reaching and pulling.15 Among those who participate in Gerofit, the greatest gains in physical function occur during the initial 3 months, which tend to be sustained over 12 months.16 For this reason, this study focused on the initial 3 months of the program.
Older adults with arthritis may have pain and functional limitations that exceed those of the general older adult population. Exercise programs for older adults that do not specifically target arthritis but are able to improve physical function among those with arthritis could potentially increase access to exercise for older adults living with arthritis. Therefore, the purpose of this study was to determine whether change in physical function with participation in Gerofit for 3 months varies by arthritis status, including no arthritis, any arthritis, lower body arthritis, or both upper and lower body arthritis compared with no arthritis.
Methods
This is a secondary analysis of previously collected data from 10 VHA Gerofit sites (Ann Arbor, Baltimore, Greater Los Angeles, Canandaigua, Cincinnati, Miami, Honolulu, Denver, Durham, and Pittsburgh) from 2002 to 2019. Implementation data regarding the consistency of the program delivery at Gerofit expansion sites have been previously published.16 Although the delivery of Gerofit transitioned to telehealth due to COVID-19, data for this analysis were collected from in-person exercise sessions prior to the pandemic.17 Data were collected for clinical purposes. This project was part of the Gerofit quality improvement initiative and was reviewed and approved by the Durham Institutional Review Board as quality improvement.
Participants in Gerofit who completed baseline and 3-month assessments were included to analyze the effects of exercise on physical function. At each of the time points, physical functional assessments included: (1) usual gait speed (> 10 meters [m/s], or 10- meter walk test [10MWT]); (2) lower body strength (chair stands [number completed in 30 seconds]); (3) upper body strength (number of arm curls [5-lb for females/8-lb for males] completed in 30 seconds); and (4) 6-minute walk distance [6MWD] in meters to measure aerobic endurance). These measures have been validated in older adults.18-21 Arm curls were added to the physical function assessments after the 10MWT, chair stands, and 6MWD; therefore, fewer participants had data for this measure. Participants self-reported at baseline on 45 common medical conditions, including arthritis or rheumatism (both upper body and lower body were offered as choices). Self-reporting has been shown to be an acceptable method of identifying arthritis in adults.22
Descriptive statistics at baseline were calculated for all participants. One-way analysis of variance and X2 tests were used to determine differences in baseline characteristics across arthritis status. The primary outcomes were changes in physical function measures from baseline to 3 months by arthritis status. Arthritis status was defined as: any arthritis, which includes individuals who reported upper body arthritis, lower body arthritis, or both; and arthritis status individuals reporting either upper body arthritis, lower body arthritis, or both. Categories of arthritis for arthritis status were mutually exclusive. Two separate linear models were constructed for each of the 4 physical function measures, with change from baseline to 3 months as the outcome (dependent variable) and arthritis status, age, and body mass index (BMI) as predictors (independent variables). The first model compared any arthritis with no arthritis and the second model compared arthritis status (both upper and lower body arthritis vs lower body arthritis) with no arthritis. These models were used to obtain mean changes and 95% CIs in physical function and to test for differences in the change in physical function measures by arthritis status. Statistical analyses were performed using R software, version 4.0.3.
Results
Baseline and 3-month data were available for 737 Gerofit participants and included in the analysis. The mean (SD) age was 73.5 (7.1) years. A total of 707 participants were male (95.9%) and 322 (43.6%) reported some arthritis, with arthritis in both the upper and lower body being reported by 168 participants (52.2%) (Table 1). There were no differences in age, sex, or race for those with any arthritis compared with those with no arthritis, but BMI was significantly higher in those reporting any arthritis compared with no arthritis. For the baseline functional measures, statistically significant differences were observed between those with no arthritis and those reporting any arthritis for the 10MWT (P = .001), chair stands (P = .046), and 6MWD (P = .001), but not for arm curls (P = .77), with those with no arthritis performing better.

All 4 arthritis status groups showed improvements in each of the physical function measures over 3 months. For the 10MWT the mean change (95% CI) in gait speed (m/s) was 0.06 (0.04-0.08) for patients with no arthritis, 0.07 (0.05- 0.08) for any arthritis, 0.07 (0.04-0.11) for lower body arthritis, and 0.07 (0.04- 0.09) for both lower and upper body arthritis. For the number of arm curls in 30 seconds the mean change (95% CI) was 2.3 (1.8-2.8) for patients with no arthritis, 2.1 (1.5-2.6) for any arthritis, 2.0 (1.1-3.0) for lower body arthritis, and 1.9 (1.1-2.7) for both lower and upper body arthritis. For the number of chair stands in 30 seconds the mean change (95% CI) was 2.1 (1.7-2.4) for patients with no arthritis, 2.2 (1.8-2.6) for any arthritis, 2.3 (1.6-2.9), for lower body arthritis, and 2.0 (1.5-2.5) for both lower and upper body arthritis. For the 6MWD distance in meters the mean change (95% CI) was 21.5 (15.5-27.4) for patients with no arthritis, 28.6 (21.9-35.3) for any arthritis, 30.4 (19.5-41.3) for lower body arthritis, and 28.6 (19.2-38.0) for both lower and upper body arthritis (Figure).

We used 2 models to measure the change from baseline to 3 months for each of the arthritis groups. Model 1 compared any arthritis vs no arthritis and model 2 compared lower body arthritis and both upper and lower body arthritis vs no arthritis for each physical function measure (Table 2). There were no statistically significant differences in 3-month change in physical function for any of the physical function measures between arthritis groups after adjusting for age and BMI.

Discussion
Participation in Gerofit was associated with functional gains among all participants over 3 months, regardless of arthritis status. Older veterans reporting any arthritis had significantly lower physical function scores upon enrollment into Gerofit compared with those veterans reporting no arthritis. However, compared with individuals who reported no arthritis, individuals who reported arthritis (any arthritis, lower body arthritis only, or both lower and upper body arthritis) experienced similar improvements (ie, no statistically significant differences in mean change from baseline to follow-up among those with and without arthritis). This study suggests that progressive, multicomponent exercise programs for older adults may be beneficial for those with arthritis.
Involvement of multiple sites of arthritis is associated with moderate to severe functional limitations as well as lower healthrelated quality of life.23 While it has been found that individuals with arthritis can improve function with supervised exercise, even though their baseline functional status is lower than individuals without arthritis, it was not clear whether individuals with multiple joint involvement also would benefit.12 The results of this study suggest that these individuals can improve across various domains of physical function despite variation in arthritis location and status. As incidence of arthritis increases with age, targeting older adults for exercise programs such as Gerofit may improve functional limitations and health-related quality of life associated with arthritis.2
We evaluated physical function using multiple measures to assess upper (arm curls) and lower (chair stands, 10MWT) extremity physical function and aerobic endurance (6MWD). Participants in this study reached clinically meaningful changes with 3 months of participation in Gerofit for most of the physical function measures. Gerofit participants had a mean gait speed improvement of 0.05 to 0.07 m/s compared with 0.10 to 0.30 m/s, which was reported previously. 24,25 In this study, nearly all groups achieved the clinically important improvements in the chair stand in 30 seconds (2.0 to 2.6) and the 6MWD (21.8 to 59.1 m) that have been reported in the literature.24-26
The Osteoarthritis Research Society International recommends the chair stand and 6MWD performance-based tests for individuals with hip and knee arthritis because they align with patient-reported outcomes and represent the types of activities relevant to this population.27 The findings of this study suggest that improvement in these physical function measures with participation in exercise align with data from arthritis-specific exercise programs designed for wide implementation. Hughes and colleagues reported improvements in the 6MWD after the 8-week Fit and Strong exercise intervention, which included walking and lower body resistance training.28 The Arthritis Foundation’s Walk With Ease program is a 6-week walking program that has shown improvements in chair stands and gait speed.29 Another Arthritis Foundation program, People with Arthritis Can Exercise, is an 8-week course consisting of a variety of resistance, aerobic, and balance activities. This program has been associated with increases in chair stands but not gait speed or 6MWD.30,31
This study found that participation in a VHA outpatient clinical supervised exercise program results in improvements in physical function that can be realized by older adults regardless of arthritis burden. Gerofit programs typically require 1.5 to 2.0 dedicated full-time equivalent employees to run the program effectively and additional administrative support, depending on size of the program.32 The cost savings generated by the program include reductions in hospitalization rates, emergency department visits, days in hospital, and medication use and provide a compelling argument for the program’s financial viability to health care systems through long-term savings and improved health outcomes for older adults.33-36
While evidenced-based arthritis programs exist, this study illustrates that an exercise program without a focus on arthritis also improves physical function, potentially reducing the risk of disability related to arthritis. The clinical implication for these findings is that arthritis-specific exercise programs may not be needed to achieve functional improvements in individuals with arthritis. This is critical for under-resourced or exercise- limited health care systems or communities. Therefore, if exercise programming is limited, or arthritis-specific programs and interventions are not available, nonspecific exercise programs will also be beneficial to individuals with arthritis. Thus, individuals with arthritis should be encouraged to participate in any available exercise programming to achieve improvements in physical function. In addition, many older adults have multiple comorbidities, most of which improve with participation in exercise. 37 Disease-specific exercise programs can offer tailored exercises and coaching related to common barriers in participation, such as joint pain for arthritis.31 It is unclear whether these additional programmatic components are associated with greater improvements in outcomes, such as physical function. More research is needed to explore the benefits of disease-specific tailored exercise programs compared with general exercise programs.
Strengths and Limitations
This study demonstrated the effect of participation in a clinical, supervised exercise program in a real-world setting. It suggests that even exercise programs not specifically targeted for arthritis populations can improve physical function among those with arthritis.
As a VHA clinical supervised exercise program, Gerofit may not be generalizable to all older adults or other exercise programs. In addition, this analysis only included a veteran population that was > 95% male and may not be generalizable to other populations. Arthritis status was defined by self-report and not verified in the health record. However, this approach has been shown to be acceptable in this setting and the most common type of arthritis in this population (OA) is a painful musculoskeletal condition associated with functional limitations.4,22,38,39 Self-reported arthritis or rheumatism is associated with functional limitations.1 Therefore, it is unlikely that the results would differ for physician-diagnosed or radiographically defined OA. Additionally, the study did not have data on the total number of joints with arthritis or arthritis severity but rather used upper body, lower body, and both upper and lower body arthritis as a proxy for arthritis status. While our models were adjusted for age and BMI, 2 known confounding factors for the association between arthritis and physical function, there are other potential confounding factors that were not included in the models. 40,41 Finally, this study only included individuals with completed baseline and 3-month follow-up assessments, and the individuals who participated for longer or shorter periods may have had different physical function outcomes than individuals included in this study.
Conclusions
Participation in 3 months VHA Gerofit outpatient supervised exercise programs can improve physical function for all older adults, regardless of arthritis status. These programs may increase access to exercise programming that is beneficial for common conditions affecting older adults, such as arthritis.
- Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults- -United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421-426.
- Theis KA, Murphy LB, Guglielmo D, et al. Prevalence of arthritis and arthritis-attributable activity limitation—United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2021;70:1401-1407. doi:10.15585/mmwr.mm7040a2
- Murphy LB, Helmick CG, Allen KD, et al. Arthritis among veterans—United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2014;63:999-1003.
- Park J, Mendy A, Vieira ER. Various types of arthritis in the United States: prevalence and age-related trends from 1999 to 2014. Am J Public Health. 2018;108:256-258.
- Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis Rheum. 2011;63:2974-2982. doi:10.1002/art.30498
- Patzkowski JC, Rivera JC, Ficke JR, Wenke JC. The changing face of disability in the US Army: the Operation Enduring Freedom and Operation Iraqi Freedom effect. J Am Acad Orthop Surg. 2012;20(suppl 1):S23-S30. doi:10.5435/JAAOS-20-08-S23
- Rivera JC, Amuan ME, Morris RM, Johnson AE, Pugh MJ. Arthritis, comorbidities, and care utilization in veterans of Operations Enduring and Iraqi Freedom. J Orthop Res. 2017;35:682-687. doi:10.1002/jor.23323
- Singh JA, Nelson DB, Fink HA, Nichol KL. Health-related quality of life predicts future health care utilization and mortality in veterans with self-reported physician-diagnosed arthritis: the Veterans Arthritis Quality of Life Study. Semin Arthritis Rheum. 2005;34:755- 765. doi:10.1016/j.semarthrit.2004.08.001
- Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712. doi:10.1016/j.semarthrit.2013.11.012
- Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit Program: a VA innovation. South Med J. 1994;87:S83-S87.
- Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20:873-899. doi:10.1080/026404102320761787
- Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44:1226-1231. doi:10.1111/j.1532-5415.1996.tb01374.x
- Allen KD, Gol ight ly YM. State of the evidence. Curr Opin Rheumatol. 2015;27:276-283. doi:10.1097/BOR.0000000000000161
- den Ouden MEM, Schuurmans MJ, Arts IEMA, van der Schouw YT. Association between physical performance characteristics and independence in activities of daily living in middle-aged and elderly men. Geriatr Gerontol Int. 2013;13:274-280. doi:10.1111/j.1447-0594.2012.00890.x
- Daly M, Vidt ME, Eggebeen JD, et al. Upper extremity muscle volumes and functional strength after resistance training in older adults. J Aging Phys Act. 2013;21:186-207. doi:10.1123/japa.21.2.186
- Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit Program. J Am Geriatr Soc. 2018;66:1009-1016. doi:10.1111/jgs.15276
- Jennings SC, Manning KM, Bettger JP, et al. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol Geriatr Med. 2020;6:2333721420980313. doi:10.1177/ 2333721420980313
- Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314-322. doi:10.1046/j.1532-5415.2003.51104.x
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community residing older adults. Res Q Exerc Sport. 1999;70:113- 119. doi:10.1080/02701367.1999.10608028
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129-161. doi:10.1123/japa.7.2.129
- Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837-841. doi:10.1016/s0003-9993(99)90236-8
- Peeters GGME, Alshurafa M, Schaap L, de Vet HCW. Diagnostic accuracy of self-reported arthritis in the general adult population is acceptable. J Clin Epidemiol. 2015;68:452-459. doi:10.1016/j.jclinepi.2014.09.019
- Cuperus N, Vliet Vlieland TPM, Mahler EAM, Kersten CC, Hoogeboom TJ, van den Ende CHM. The clinical burden of generalized osteoarthritis represented by self-reported health-related quality of life and activity limitations: a cross-sectional study. Rheumatol Int. 2015;35:871-877. doi:10.1007/s00296-014-3149-1
- Coleman G, Dobson F, Hinman RS, Bennell K, White DK. Measures of physical performance. Arthritis Care Res (Hoboken). 2020;72(suppl 10):452-485. doi:10.1002/acr.24373
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743-749. doi:10.1111/j.1532-5415.2006.00701.x
- Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther. 2011;41:319-327. doi:10.2519/jospt.2011.3515
- Dobson F, Hinman R, Roos EM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1042- 1052. doi:10.1016/j.joca.2013.05.002
- Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217-228. doi:10.1093/geront/44.2.217
- Callahan LF, Shreffler JH, Altpeter M, et al. Evaluation of group and self-directed formats of the Arthritis Foundation's Walk With Ease Program. Arthritis Care Res (Hoboken). 2011;63:1098-1107. doi:10.1002/acr.20490
- Boutaugh ML. Arthritis Foundation community-based physical activity programs: effectiveness and implementation issues. Arthritis Rheum. 2003;49:463-470. doi:10.1002/art.11050
- Callahan LF, Mielenz T, Freburger J, et al. A randomized controlled trial of the People with Arthritis Can Exercise Program: symptoms, function, physical activity, and psychosocial outcomes. Arthritis Rheum. 2008;59:92-101. doi:10.1002/art.23239
- Hall KS, Jennings SC, Pearson MP. Outpatient care models: the Gerofit model of care for exercise promotion in older adults. In: Malone ML, Boltz M, Macias Tejada J, White H, eds. Geriatrics Models of Care. Springer; 2024:205-213. doi:10.1007/978-3-031-56204-4_21
- Pepin MJ, Valencia WM, Bettger JP, et al. Impact of supervised exercise on one-year medication use in older veterans with multiple morbidities. Gerontol Geriatr Med. 2020;6:2333721420956751. doi:10.1177/ 2333721420956751
- Abbate L, Li J, Veazie P, et al. Does Gerofit exercise reduce veterans’ use of emergency department and inpatient care? Innov Aging. 2020;4(suppl 1):771. doi:10.1093/geroni/igaa057.2786
- Morey MC, Pieper CF, Crowley GM, Sullivan RJ Jr, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50:1929-1933. doi:10.1046/j.1532-5415.2002.50602.x
- Manning KM, Hall KS, Sloane R, et al. Longitudinal analysis of physical function in older adults: the effects of physical inactivity and exercise training. Aging Cell. 2024;23:e13987. doi:10.1111/acel.13987
- Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85(7 suppl 3):S31-S42; quiz S3-S4. doi:10.1016/j.apmr.2004.03.010
- Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009; 57:1556-1561. doi:10.1111/j.1532-5415.2009.02388.x
- Katz JN, Wright EA, Baron JA, Losina E. Development and validation of an index of musculoskeletal functional limitations. BMC Musculoskelet Disord. 2009;10:62. doi:10.1186/1471-2474-10-62
- Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30:184-195. doi:10.1016/j.joca.2021.04.020
- Riebe D, Blissmer BJ, Greaney ML, Ewing Garber C, Lees FD, Clark PG. The relationship between obesity, physical activity, and physical function in older adults. J Aging Health. 2009;21:1159-1178. doi:10.1177/0898264309350076
- Centers for Disease Control and Prevention. Prevalence and most common causes of disability among adults- -United States, 2005. MMWR Morb Mortal Wkly Rep. 2009;58:421-426.
- Theis KA, Murphy LB, Guglielmo D, et al. Prevalence of arthritis and arthritis-attributable activity limitation—United States, 2016–2018. MMWR Morb Mortal Wkly Rep. 2021;70:1401-1407. doi:10.15585/mmwr.mm7040a2
- Murphy LB, Helmick CG, Allen KD, et al. Arthritis among veterans—United States, 2011–2013. MMWR Morb Mortal Wkly Rep. 2014;63:999-1003.
- Park J, Mendy A, Vieira ER. Various types of arthritis in the United States: prevalence and age-related trends from 1999 to 2014. Am J Public Health. 2018;108:256-258.
- Cameron KL, Hsiao MS, Owens BD, Burks R, Svoboda SJ. Incidence of physician-diagnosed osteoarthritis among active duty United States military service members. Arthritis Rheum. 2011;63:2974-2982. doi:10.1002/art.30498
- Patzkowski JC, Rivera JC, Ficke JR, Wenke JC. The changing face of disability in the US Army: the Operation Enduring Freedom and Operation Iraqi Freedom effect. J Am Acad Orthop Surg. 2012;20(suppl 1):S23-S30. doi:10.5435/JAAOS-20-08-S23
- Rivera JC, Amuan ME, Morris RM, Johnson AE, Pugh MJ. Arthritis, comorbidities, and care utilization in veterans of Operations Enduring and Iraqi Freedom. J Orthop Res. 2017;35:682-687. doi:10.1002/jor.23323
- Singh JA, Nelson DB, Fink HA, Nichol KL. Health-related quality of life predicts future health care utilization and mortality in veterans with self-reported physician-diagnosed arthritis: the Veterans Arthritis Quality of Life Study. Semin Arthritis Rheum. 2005;34:755- 765. doi:10.1016/j.semarthrit.2004.08.001
- Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin Arthritis Rheum. 2014;43:701-712. doi:10.1016/j.semarthrit.2013.11.012
- Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit Program: a VA innovation. South Med J. 1994;87:S83-S87.
- Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20:873-899. doi:10.1080/026404102320761787
- Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44:1226-1231. doi:10.1111/j.1532-5415.1996.tb01374.x
- Allen KD, Gol ight ly YM. State of the evidence. Curr Opin Rheumatol. 2015;27:276-283. doi:10.1097/BOR.0000000000000161
- den Ouden MEM, Schuurmans MJ, Arts IEMA, van der Schouw YT. Association between physical performance characteristics and independence in activities of daily living in middle-aged and elderly men. Geriatr Gerontol Int. 2013;13:274-280. doi:10.1111/j.1447-0594.2012.00890.x
- Daly M, Vidt ME, Eggebeen JD, et al. Upper extremity muscle volumes and functional strength after resistance training in older adults. J Aging Phys Act. 2013;21:186-207. doi:10.1123/japa.21.2.186
- Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit Program. J Am Geriatr Soc. 2018;66:1009-1016. doi:10.1111/jgs.15276
- Jennings SC, Manning KM, Bettger JP, et al. Rapid transition to telehealth group exercise and functional assessments in response to COVID-19. Gerontol Geriatr Med. 2020;6:2333721420980313. doi:10.1177/ 2333721420980313
- Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314-322. doi:10.1046/j.1532-5415.2003.51104.x
- Jones CJ, Rikli RE, Beam WC. A 30-s chair-stand test as a measure of lower body strength in community residing older adults. Res Q Exerc Sport. 1999;70:113- 119. doi:10.1080/02701367.1999.10608028
- Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129-161. doi:10.1123/japa.7.2.129
- Harada ND, Chiu V, Stewart AL. Mobility-related function in older adults: assessment with a 6-minute walk test. Arch Phys Med Rehabil. 1999;80:837-841. doi:10.1016/s0003-9993(99)90236-8
- Peeters GGME, Alshurafa M, Schaap L, de Vet HCW. Diagnostic accuracy of self-reported arthritis in the general adult population is acceptable. J Clin Epidemiol. 2015;68:452-459. doi:10.1016/j.jclinepi.2014.09.019
- Cuperus N, Vliet Vlieland TPM, Mahler EAM, Kersten CC, Hoogeboom TJ, van den Ende CHM. The clinical burden of generalized osteoarthritis represented by self-reported health-related quality of life and activity limitations: a cross-sectional study. Rheumatol Int. 2015;35:871-877. doi:10.1007/s00296-014-3149-1
- Coleman G, Dobson F, Hinman RS, Bennell K, White DK. Measures of physical performance. Arthritis Care Res (Hoboken). 2020;72(suppl 10):452-485. doi:10.1002/acr.24373
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743-749. doi:10.1111/j.1532-5415.2006.00701.x
- Wright AA, Cook CE, Baxter GD, Dockerty JD, Abbott JH. A comparison of 3 methodological approaches to defining major clinically important improvement of 4 performance measures in patients with hip osteoarthritis. J Orthop Sports Phys Ther. 2011;41:319-327. doi:10.2519/jospt.2011.3515
- Dobson F, Hinman R, Roos EM, et al. OARSI recommended performance-based tests to assess physical function in people diagnosed with hip or knee osteoarthritis. Osteoarthritis Cartilage. 2013;21:1042- 1052. doi:10.1016/j.joca.2013.05.002
- Hughes SL, Seymour RB, Campbell R, Pollak N, Huber G, Sharma L. Impact of the fit and strong intervention on older adults with osteoarthritis. Gerontologist. 2004;44:217-228. doi:10.1093/geront/44.2.217
- Callahan LF, Shreffler JH, Altpeter M, et al. Evaluation of group and self-directed formats of the Arthritis Foundation's Walk With Ease Program. Arthritis Care Res (Hoboken). 2011;63:1098-1107. doi:10.1002/acr.20490
- Boutaugh ML. Arthritis Foundation community-based physical activity programs: effectiveness and implementation issues. Arthritis Rheum. 2003;49:463-470. doi:10.1002/art.11050
- Callahan LF, Mielenz T, Freburger J, et al. A randomized controlled trial of the People with Arthritis Can Exercise Program: symptoms, function, physical activity, and psychosocial outcomes. Arthritis Rheum. 2008;59:92-101. doi:10.1002/art.23239
- Hall KS, Jennings SC, Pearson MP. Outpatient care models: the Gerofit model of care for exercise promotion in older adults. In: Malone ML, Boltz M, Macias Tejada J, White H, eds. Geriatrics Models of Care. Springer; 2024:205-213. doi:10.1007/978-3-031-56204-4_21
- Pepin MJ, Valencia WM, Bettger JP, et al. Impact of supervised exercise on one-year medication use in older veterans with multiple morbidities. Gerontol Geriatr Med. 2020;6:2333721420956751. doi:10.1177/ 2333721420956751
- Abbate L, Li J, Veazie P, et al. Does Gerofit exercise reduce veterans’ use of emergency department and inpatient care? Innov Aging. 2020;4(suppl 1):771. doi:10.1093/geroni/igaa057.2786
- Morey MC, Pieper CF, Crowley GM, Sullivan RJ Jr, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50:1929-1933. doi:10.1046/j.1532-5415.2002.50602.x
- Manning KM, Hall KS, Sloane R, et al. Longitudinal analysis of physical function in older adults: the effects of physical inactivity and exercise training. Aging Cell. 2024;23:e13987. doi:10.1111/acel.13987
- Bean JF, Vora A, Frontera WR. Benefits of exercise for community-dwelling older adults. Arch Phys Med Rehabil. 2004;85(7 suppl 3):S31-S42; quiz S3-S4. doi:10.1016/j.apmr.2004.03.010
- Covinsky KE, Lindquist K, Dunlop DD, Yelin E. Pain, functional limitations, and aging. J Am Geriatr Soc. 2009; 57:1556-1561. doi:10.1111/j.1532-5415.2009.02388.x
- Katz JN, Wright EA, Baron JA, Losina E. Development and validation of an index of musculoskeletal functional limitations. BMC Musculoskelet Disord. 2009;10:62. doi:10.1186/1471-2474-10-62
- Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30:184-195. doi:10.1016/j.joca.2021.04.020
- Riebe D, Blissmer BJ, Greaney ML, Ewing Garber C, Lees FD, Clark PG. The relationship between obesity, physical activity, and physical function in older adults. J Aging Health. 2009;21:1159-1178. doi:10.1177/0898264309350076
Impact of 3 Months of Supervised Exercise on Function by Arthritis Status
Impact of 3 Months of Supervised Exercise on Function by Arthritis Status
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Osteoporotic fragility fractures constitute a significant public health concern, with 1 in 2 women and 1 in 5 men aged > 50 years sustaining an osteoporotic fracture.1 Osteoporotic fractures are costly and associated with reduced quality of life and impaired survival.2-6 Many interventions including fall mitigation, calcium, vitamin D supplementation, and osteoporosis—specific medications reduce fracture risk.7 New medications for treating osteoporosis, including anabolic therapies, are costly and require clinical oversight to ensure safe delivery. This includes laboratory monitoring, timing of in-clinic dosing and provision of sequence therapy.8,9 COVID-19 introduced numerous barriers to osteoporosis care, raising concerns for medication interruption and patients lost to follow-up, which made monitoring these high risk and costly medications even more important.
The US Department of Veterans Affairs (VA) was an early adopter of using the electronic health record to analyze and implement system-wide processes for population management and quality improvement.10 This enabled the creation of clinical dashboards to display key performance indicator data that support quality improvement and patient care initiatives.11-15 The VA Puget Sound Health Care System (VAPSHCS) has a dedicated osteoporosis clinic focused on preventing and treating veterans at high risk for fracture. Considering the growing utilization of osteoporosis medications, particularly those requiring timed sequential therapy to prevent bone mineral density loss and rebound osteoporotic fractures, close monitoring and follow-up is required. The COVID-19 pandemic made clear the need for proactive osteoporosis management. This article describes the creation and use of an automated clinic dashboard to identify and contact veterans with osteoporosis-related care needs, such as prescription refills, laboratory tests, and clinical visits.
Methods
An automated dashboard was created in partnership with VA pharmacy clinical informatics to display the osteoporosis medication prescription (including last refill), monitoring laboratory test values and most recent osteoporosis clinic visit for each clinic patient. Data from the VA Corporate Data Warehouse were extracted. The resulting tables were used to create a patient cohort with ≥ 1 active medication for alendronate, zoledronic acid, the parathyroid hormone analogues (PTH) teriparatide or abaloparatide, denosumab, or romosozumab. Notably, alendronate was the only oral bisphosphonate prescribed in the clinic. These data were formatted and displayed using Microsoft SQL Server Reporting Services. The secure and encrypted dashboard alerts the clinic staff when prescriptions, appointments, or laboratory tests, such as estimated glomerular filtration rate, 25-hydroxy vitamin D, calcium, and PTH are overdue or out of reference range. The dashboard tracked the most recent clinic visit or dual-energy X-ray absorptiometry (DXA) scan if performed within the VA. Overdue laboratory test alerts for bisphosphonates were flagged if delayed 12 months and 6 months for all other medications.
On March 20, 2021, the VAPSHCS osteoporosis clinic was staffed by 1 endocrinologist, 1 geriatrician, 1 rheumatologist, and 1 registered nurse (RN) coordinator. Overdue or out-of-range alerts were reviewed weekly by the RN coordinator, who addressed alerts. For any overdue laboratory work or prescription refills, the RN coordinator alerted the primary osteoporosis physician via the electronic health record for updated orders. Patients were contacted by phone to schedule a clinic visit, complete ordered laboratory work, or discuss osteoporosis medication refills based on the need identified by the dashboard. A letter was mailed to the patient requesting they contact the osteoporosis clinic for patients who could not be reached by phone after 2 attempts. If 3 attempts (2 phone calls and a letter) were unsuccessful, the osteoporosis physician was alerted so they could either call the patient, alert the primary referring clinician, or discontinue the osteoporosis medication.
Results
As of March 20, 2021, 139 patients were included on the dashboard. Ninety-two patients (66%) had unmet care needs and 29% were female. Ages ranged from 40 to 100 years (Table). The dashboard alerted the team to 3 patients lost to follow-up, all of whom had transferred to care outside the clinic. Twenty-three patients (17%) had overdue medications, including 2 (9%) who had not refilled oral bisphosphonate and 18 (78%) who were overdue for intravenous bisphosphonate treatment. One veteran flagged as overdue for their denosumab injection was unable to receive it due to a significant change in health status. Two veterans were overdue for a PTH analogue refill, 1 of whom had completed their course and transitioned to bisphosphonate.
The most common alert was 40 patients (29%) with overdue laboratory tests, 37 of which were receiving bisphosphonates. One patient included on the dashboard was taking romosozumab and all their monitoring parameters were up to date, thus their data were not included in the Table to prevent possible identification.
Discussion
A dashboard alerted the osteoporosis clinic team to veterans who were overdue for visits, laboratory work, and prescription renewals. Overall, 92 patients (66%) had unmet care needs identified by the dashboard, all of which were addressed with phone calls and/or letters. Most of the overdue medication refills and laboratory tests were for patients taking bisphosphonates avoiding VAPSHCS during the COVID-19 pandemic. The dashboard enabled the RN coordinator to promptly contact the patient, facilitate coordination of care requirements, and guarantee the safe and efficient delivery of osteoporosis care.
The VA has historically been a leader in the creation of clinical dashboards to support health campaigns.11,12 These dashboards have successfully improved quality metrics towards the treatment of hepatitis C virus, heart failure, and highrisk opioid prescribing.13-15 Data have shown that successful clinical dashboard implementation must be done in conjunction with protected time or staff to support care improvements.16 Additionally, the time required for clinical dashboards can limit their sustainability and feasibility.17 A study aimed at improving osteoporosis care for patients with Parkinson disease found that weekly multidisciplinary review of at-risk patients resulted in all new patients and 91% of follow-up patients receiving evidence- based osteoporosis treatments.17 However, despite the benefits, the intervention required significant time and resources. In contrast, the osteoporosis dashboard implemented at VAPSHCS was not time or resource intensive, requiring about 1 hour per week for the RN coordinator to review the dashboard and coordinate patient care needs.
Limitations
This study setting is unique from other health care organizations or VA health care systems. Implementation of a similar dashboard in other clinical settings where patients receive medical care in multiple health care systems may differ. The VA dedicates resources to support veteran population health management, which may not be available in other health care systems.11,12 These issues may pose a barrier to implementing a similar osteoporosis dashboard in non-VA facilities. In addition, it is significant that while the dashboard can be reconfigured and adapted to track veterans across different VA facilities, certain complexities arise if essential data, such as laboratory tests and DXA imaging, are conducted outside of VA facilities. In such cases, manual entry of this information into the dashboard would be necessary. Because the dashboard was quickly developed during the COVID-19 pandemic, this study lacked preimplementation data on laboratory testing, medication refills, and DXA imaging, which would have enabled a comparison of adherence before and after dashboard implementation. Finally, we acknowledge the delay in publishing these findings; however, we believe sharing innovative approaches to providing care for high-risk populations is essential, as demonstrated during the COVID-19 pandemic.
Conclusions
An osteoporosis clinic dashboard served as a valuable clinical support tool to ensure safe and effective osteoporosis medication delivery at VAPSHCS. Considering the growing utilization of osteoporosis medications, this dashboard plays a vital role in facilitating care coordination for patients receiving these high-risk treatments.18 Use of the dashboard supported the effective use of high-cost osteoporosis medications and is likely to improve clinical osteoporosis outcomes.
Despite the known fracture risk reduction, osteoporosis medication adherence is low.19,20 Maintaining consistent pharmacotherapy for osteoporosis is essential not only for fracture prevention but also reducing health care costs related to osteoporosis and preserving patient independence and functionality.21-24 While initially developed in response to the COVID-19 pandemic, the dashboard remains useful. The VAPSHCS osteoporosis clinic is now staffed by 2 physicians (endocrine and rheumatology) and the dashboard is still in use. The RN coordinator spends about 15 minutes per week using the dashboard and managing the 67 veterans on osteoporosis therapy. This dashboard represents a sustainable clinical tool with the capacity to minimize osteoporosis care gaps and improve outcomes.
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(suppl 2):S3-S7. doi:10.1007/s00198-004-1702-6
- van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517-522. doi:10.1016/s8756-3282(01)00614-7
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(suppl 1):58-63. doi:10.1159/000063449
- Cooper C. Epidemiology and public health impact of osteoporosis. Baillieres Clin Rheumatol. 1993;7:459-477. doi:10.1016/s0950-3579(05)80073-1
- Dolan P, Torgerson DJ. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int. 1998;8:611-617. doi:10.1007/s001980050107
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. doi:10.1359/jbmr.061113
- Palacios S. Medical treatment of osteoporosis. Climacteric. 2022;25:43-49. doi:10.1080/13697137.2021.1951697
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. doi:10.1210/jc.2019-00221
- Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822. doi:10.1210/jc.2011-3045
- Lau MK, Bounthavong M, Kay CL, Harvey MA, Christopher MLD. Clinical dashboard development and use for academic detailing in the U.S. Department of Veterans Affairs. J Am Pharm Assoc (2003). 2019;59(2S):S96-S103.e3. doi:10.1016/j.japh.2018.12.006
- Mould DR, D’Haens G, Upton RN. Clinical decision support tools: the evolution of a revolution. Clin Pharmacol Ther. 2016;99:405-418. doi:10.1002/cpt.334
- Kizer KW, Fonseca ML, Long LM. The veterans healthcare system: preparing for the twenty-first century. Hosp Health Serv Adm. 1997;42:283-298.
- Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis c in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35:24-29.
- Brownell N, Kay C, Parra D, et al. Development and optimization of the Veterans Affairs’ national heart failure dashboard for population health management. J Card Fail. 2024;30:452-459. doi:10.1016/j.cardfail.2023.08.024
- Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the opioid safety initiative on opioidrelated prescribing in veterans. Pain. 2017;158:833-839. doi:10.1097/j.pain.0000000000000837
- Twohig PA, Rivington JR, Gunzler D, Daprano J, Margolius D. Clinician dashboard views and improvement in preventative health outcome measures: a retrospective analysis. BMC Health Serv Res. 2019;19:475. doi:10.1186/s12913-019-4327-3
- Singh I, Fletcher R, Scanlon L, Tyler M, Aithal S. A quality improvement initiative on the management of osteoporosis in older people with Parkinsonism. BMJ Qual Improv Rep. 2016;5:u210921.w5756. doi:10.1136/bmjquality.u210921.w5756
- Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med. 2021;10:152. doi:10.3390/jcm10010152
- Sharman Moser S, Yu J, Goldshtein I, et al. Cost and consequences of nonadherence with oral bisphosphonate therapy: findings from a real-world data analysis. Ann Pharmacother. 2016;50:262-269. doi:10.1177/1060028015626935
- Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs--an analysis using national health databases. Osteoporos Int. 2013;24:2639-2647. doi:10.1007/s00198-013-2365-y
- Blouin J, Dragomir A, Fredette M, Ste-Marie LG, Fernandes JC, Perreault S. Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int. 2009;20:1571-1581. doi:10.1007/s00198-008-0818-5
- Cotté F-E, De Pouvourville G. Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC Health Serv Res. 2011;11:151. doi:10.1186/1472-6963-11-151
- Cho H, Byun J-H, Song I, et al. Effect of improved medication adherence on health care costs in osteoporosis patients. Medicine (Baltimore). 2018;97:e11470. doi:10.1097/MD.0000000000011470
- Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181-209. doi:10.1007/s40273-020-00965-9
Osteoporotic fragility fractures constitute a significant public health concern, with 1 in 2 women and 1 in 5 men aged > 50 years sustaining an osteoporotic fracture.1 Osteoporotic fractures are costly and associated with reduced quality of life and impaired survival.2-6 Many interventions including fall mitigation, calcium, vitamin D supplementation, and osteoporosis—specific medications reduce fracture risk.7 New medications for treating osteoporosis, including anabolic therapies, are costly and require clinical oversight to ensure safe delivery. This includes laboratory monitoring, timing of in-clinic dosing and provision of sequence therapy.8,9 COVID-19 introduced numerous barriers to osteoporosis care, raising concerns for medication interruption and patients lost to follow-up, which made monitoring these high risk and costly medications even more important.
The US Department of Veterans Affairs (VA) was an early adopter of using the electronic health record to analyze and implement system-wide processes for population management and quality improvement.10 This enabled the creation of clinical dashboards to display key performance indicator data that support quality improvement and patient care initiatives.11-15 The VA Puget Sound Health Care System (VAPSHCS) has a dedicated osteoporosis clinic focused on preventing and treating veterans at high risk for fracture. Considering the growing utilization of osteoporosis medications, particularly those requiring timed sequential therapy to prevent bone mineral density loss and rebound osteoporotic fractures, close monitoring and follow-up is required. The COVID-19 pandemic made clear the need for proactive osteoporosis management. This article describes the creation and use of an automated clinic dashboard to identify and contact veterans with osteoporosis-related care needs, such as prescription refills, laboratory tests, and clinical visits.
Methods
An automated dashboard was created in partnership with VA pharmacy clinical informatics to display the osteoporosis medication prescription (including last refill), monitoring laboratory test values and most recent osteoporosis clinic visit for each clinic patient. Data from the VA Corporate Data Warehouse were extracted. The resulting tables were used to create a patient cohort with ≥ 1 active medication for alendronate, zoledronic acid, the parathyroid hormone analogues (PTH) teriparatide or abaloparatide, denosumab, or romosozumab. Notably, alendronate was the only oral bisphosphonate prescribed in the clinic. These data were formatted and displayed using Microsoft SQL Server Reporting Services. The secure and encrypted dashboard alerts the clinic staff when prescriptions, appointments, or laboratory tests, such as estimated glomerular filtration rate, 25-hydroxy vitamin D, calcium, and PTH are overdue or out of reference range. The dashboard tracked the most recent clinic visit or dual-energy X-ray absorptiometry (DXA) scan if performed within the VA. Overdue laboratory test alerts for bisphosphonates were flagged if delayed 12 months and 6 months for all other medications.
On March 20, 2021, the VAPSHCS osteoporosis clinic was staffed by 1 endocrinologist, 1 geriatrician, 1 rheumatologist, and 1 registered nurse (RN) coordinator. Overdue or out-of-range alerts were reviewed weekly by the RN coordinator, who addressed alerts. For any overdue laboratory work or prescription refills, the RN coordinator alerted the primary osteoporosis physician via the electronic health record for updated orders. Patients were contacted by phone to schedule a clinic visit, complete ordered laboratory work, or discuss osteoporosis medication refills based on the need identified by the dashboard. A letter was mailed to the patient requesting they contact the osteoporosis clinic for patients who could not be reached by phone after 2 attempts. If 3 attempts (2 phone calls and a letter) were unsuccessful, the osteoporosis physician was alerted so they could either call the patient, alert the primary referring clinician, or discontinue the osteoporosis medication.
Results
As of March 20, 2021, 139 patients were included on the dashboard. Ninety-two patients (66%) had unmet care needs and 29% were female. Ages ranged from 40 to 100 years (Table). The dashboard alerted the team to 3 patients lost to follow-up, all of whom had transferred to care outside the clinic. Twenty-three patients (17%) had overdue medications, including 2 (9%) who had not refilled oral bisphosphonate and 18 (78%) who were overdue for intravenous bisphosphonate treatment. One veteran flagged as overdue for their denosumab injection was unable to receive it due to a significant change in health status. Two veterans were overdue for a PTH analogue refill, 1 of whom had completed their course and transitioned to bisphosphonate.

The most common alert was 40 patients (29%) with overdue laboratory tests, 37 of which were receiving bisphosphonates. One patient included on the dashboard was taking romosozumab and all their monitoring parameters were up to date, thus their data were not included in the Table to prevent possible identification.
Discussion
A dashboard alerted the osteoporosis clinic team to veterans who were overdue for visits, laboratory work, and prescription renewals. Overall, 92 patients (66%) had unmet care needs identified by the dashboard, all of which were addressed with phone calls and/or letters. Most of the overdue medication refills and laboratory tests were for patients taking bisphosphonates avoiding VAPSHCS during the COVID-19 pandemic. The dashboard enabled the RN coordinator to promptly contact the patient, facilitate coordination of care requirements, and guarantee the safe and efficient delivery of osteoporosis care.
The VA has historically been a leader in the creation of clinical dashboards to support health campaigns.11,12 These dashboards have successfully improved quality metrics towards the treatment of hepatitis C virus, heart failure, and highrisk opioid prescribing.13-15 Data have shown that successful clinical dashboard implementation must be done in conjunction with protected time or staff to support care improvements.16 Additionally, the time required for clinical dashboards can limit their sustainability and feasibility.17 A study aimed at improving osteoporosis care for patients with Parkinson disease found that weekly multidisciplinary review of at-risk patients resulted in all new patients and 91% of follow-up patients receiving evidence- based osteoporosis treatments.17 However, despite the benefits, the intervention required significant time and resources. In contrast, the osteoporosis dashboard implemented at VAPSHCS was not time or resource intensive, requiring about 1 hour per week for the RN coordinator to review the dashboard and coordinate patient care needs.
Limitations
This study setting is unique from other health care organizations or VA health care systems. Implementation of a similar dashboard in other clinical settings where patients receive medical care in multiple health care systems may differ. The VA dedicates resources to support veteran population health management, which may not be available in other health care systems.11,12 These issues may pose a barrier to implementing a similar osteoporosis dashboard in non-VA facilities. In addition, it is significant that while the dashboard can be reconfigured and adapted to track veterans across different VA facilities, certain complexities arise if essential data, such as laboratory tests and DXA imaging, are conducted outside of VA facilities. In such cases, manual entry of this information into the dashboard would be necessary. Because the dashboard was quickly developed during the COVID-19 pandemic, this study lacked preimplementation data on laboratory testing, medication refills, and DXA imaging, which would have enabled a comparison of adherence before and after dashboard implementation. Finally, we acknowledge the delay in publishing these findings; however, we believe sharing innovative approaches to providing care for high-risk populations is essential, as demonstrated during the COVID-19 pandemic.
Conclusions
An osteoporosis clinic dashboard served as a valuable clinical support tool to ensure safe and effective osteoporosis medication delivery at VAPSHCS. Considering the growing utilization of osteoporosis medications, this dashboard plays a vital role in facilitating care coordination for patients receiving these high-risk treatments.18 Use of the dashboard supported the effective use of high-cost osteoporosis medications and is likely to improve clinical osteoporosis outcomes.
Despite the known fracture risk reduction, osteoporosis medication adherence is low.19,20 Maintaining consistent pharmacotherapy for osteoporosis is essential not only for fracture prevention but also reducing health care costs related to osteoporosis and preserving patient independence and functionality.21-24 While initially developed in response to the COVID-19 pandemic, the dashboard remains useful. The VAPSHCS osteoporosis clinic is now staffed by 2 physicians (endocrine and rheumatology) and the dashboard is still in use. The RN coordinator spends about 15 minutes per week using the dashboard and managing the 67 veterans on osteoporosis therapy. This dashboard represents a sustainable clinical tool with the capacity to minimize osteoporosis care gaps and improve outcomes.
Osteoporotic fragility fractures constitute a significant public health concern, with 1 in 2 women and 1 in 5 men aged > 50 years sustaining an osteoporotic fracture.1 Osteoporotic fractures are costly and associated with reduced quality of life and impaired survival.2-6 Many interventions including fall mitigation, calcium, vitamin D supplementation, and osteoporosis—specific medications reduce fracture risk.7 New medications for treating osteoporosis, including anabolic therapies, are costly and require clinical oversight to ensure safe delivery. This includes laboratory monitoring, timing of in-clinic dosing and provision of sequence therapy.8,9 COVID-19 introduced numerous barriers to osteoporosis care, raising concerns for medication interruption and patients lost to follow-up, which made monitoring these high risk and costly medications even more important.
The US Department of Veterans Affairs (VA) was an early adopter of using the electronic health record to analyze and implement system-wide processes for population management and quality improvement.10 This enabled the creation of clinical dashboards to display key performance indicator data that support quality improvement and patient care initiatives.11-15 The VA Puget Sound Health Care System (VAPSHCS) has a dedicated osteoporosis clinic focused on preventing and treating veterans at high risk for fracture. Considering the growing utilization of osteoporosis medications, particularly those requiring timed sequential therapy to prevent bone mineral density loss and rebound osteoporotic fractures, close monitoring and follow-up is required. The COVID-19 pandemic made clear the need for proactive osteoporosis management. This article describes the creation and use of an automated clinic dashboard to identify and contact veterans with osteoporosis-related care needs, such as prescription refills, laboratory tests, and clinical visits.
Methods
An automated dashboard was created in partnership with VA pharmacy clinical informatics to display the osteoporosis medication prescription (including last refill), monitoring laboratory test values and most recent osteoporosis clinic visit for each clinic patient. Data from the VA Corporate Data Warehouse were extracted. The resulting tables were used to create a patient cohort with ≥ 1 active medication for alendronate, zoledronic acid, the parathyroid hormone analogues (PTH) teriparatide or abaloparatide, denosumab, or romosozumab. Notably, alendronate was the only oral bisphosphonate prescribed in the clinic. These data were formatted and displayed using Microsoft SQL Server Reporting Services. The secure and encrypted dashboard alerts the clinic staff when prescriptions, appointments, or laboratory tests, such as estimated glomerular filtration rate, 25-hydroxy vitamin D, calcium, and PTH are overdue or out of reference range. The dashboard tracked the most recent clinic visit or dual-energy X-ray absorptiometry (DXA) scan if performed within the VA. Overdue laboratory test alerts for bisphosphonates were flagged if delayed 12 months and 6 months for all other medications.
On March 20, 2021, the VAPSHCS osteoporosis clinic was staffed by 1 endocrinologist, 1 geriatrician, 1 rheumatologist, and 1 registered nurse (RN) coordinator. Overdue or out-of-range alerts were reviewed weekly by the RN coordinator, who addressed alerts. For any overdue laboratory work or prescription refills, the RN coordinator alerted the primary osteoporosis physician via the electronic health record for updated orders. Patients were contacted by phone to schedule a clinic visit, complete ordered laboratory work, or discuss osteoporosis medication refills based on the need identified by the dashboard. A letter was mailed to the patient requesting they contact the osteoporosis clinic for patients who could not be reached by phone after 2 attempts. If 3 attempts (2 phone calls and a letter) were unsuccessful, the osteoporosis physician was alerted so they could either call the patient, alert the primary referring clinician, or discontinue the osteoporosis medication.
Results
As of March 20, 2021, 139 patients were included on the dashboard. Ninety-two patients (66%) had unmet care needs and 29% were female. Ages ranged from 40 to 100 years (Table). The dashboard alerted the team to 3 patients lost to follow-up, all of whom had transferred to care outside the clinic. Twenty-three patients (17%) had overdue medications, including 2 (9%) who had not refilled oral bisphosphonate and 18 (78%) who were overdue for intravenous bisphosphonate treatment. One veteran flagged as overdue for their denosumab injection was unable to receive it due to a significant change in health status. Two veterans were overdue for a PTH analogue refill, 1 of whom had completed their course and transitioned to bisphosphonate.

The most common alert was 40 patients (29%) with overdue laboratory tests, 37 of which were receiving bisphosphonates. One patient included on the dashboard was taking romosozumab and all their monitoring parameters were up to date, thus their data were not included in the Table to prevent possible identification.
Discussion
A dashboard alerted the osteoporosis clinic team to veterans who were overdue for visits, laboratory work, and prescription renewals. Overall, 92 patients (66%) had unmet care needs identified by the dashboard, all of which were addressed with phone calls and/or letters. Most of the overdue medication refills and laboratory tests were for patients taking bisphosphonates avoiding VAPSHCS during the COVID-19 pandemic. The dashboard enabled the RN coordinator to promptly contact the patient, facilitate coordination of care requirements, and guarantee the safe and efficient delivery of osteoporosis care.
The VA has historically been a leader in the creation of clinical dashboards to support health campaigns.11,12 These dashboards have successfully improved quality metrics towards the treatment of hepatitis C virus, heart failure, and highrisk opioid prescribing.13-15 Data have shown that successful clinical dashboard implementation must be done in conjunction with protected time or staff to support care improvements.16 Additionally, the time required for clinical dashboards can limit their sustainability and feasibility.17 A study aimed at improving osteoporosis care for patients with Parkinson disease found that weekly multidisciplinary review of at-risk patients resulted in all new patients and 91% of follow-up patients receiving evidence- based osteoporosis treatments.17 However, despite the benefits, the intervention required significant time and resources. In contrast, the osteoporosis dashboard implemented at VAPSHCS was not time or resource intensive, requiring about 1 hour per week for the RN coordinator to review the dashboard and coordinate patient care needs.
Limitations
This study setting is unique from other health care organizations or VA health care systems. Implementation of a similar dashboard in other clinical settings where patients receive medical care in multiple health care systems may differ. The VA dedicates resources to support veteran population health management, which may not be available in other health care systems.11,12 These issues may pose a barrier to implementing a similar osteoporosis dashboard in non-VA facilities. In addition, it is significant that while the dashboard can be reconfigured and adapted to track veterans across different VA facilities, certain complexities arise if essential data, such as laboratory tests and DXA imaging, are conducted outside of VA facilities. In such cases, manual entry of this information into the dashboard would be necessary. Because the dashboard was quickly developed during the COVID-19 pandemic, this study lacked preimplementation data on laboratory testing, medication refills, and DXA imaging, which would have enabled a comparison of adherence before and after dashboard implementation. Finally, we acknowledge the delay in publishing these findings; however, we believe sharing innovative approaches to providing care for high-risk populations is essential, as demonstrated during the COVID-19 pandemic.
Conclusions
An osteoporosis clinic dashboard served as a valuable clinical support tool to ensure safe and effective osteoporosis medication delivery at VAPSHCS. Considering the growing utilization of osteoporosis medications, this dashboard plays a vital role in facilitating care coordination for patients receiving these high-risk treatments.18 Use of the dashboard supported the effective use of high-cost osteoporosis medications and is likely to improve clinical osteoporosis outcomes.
Despite the known fracture risk reduction, osteoporosis medication adherence is low.19,20 Maintaining consistent pharmacotherapy for osteoporosis is essential not only for fracture prevention but also reducing health care costs related to osteoporosis and preserving patient independence and functionality.21-24 While initially developed in response to the COVID-19 pandemic, the dashboard remains useful. The VAPSHCS osteoporosis clinic is now staffed by 2 physicians (endocrine and rheumatology) and the dashboard is still in use. The RN coordinator spends about 15 minutes per week using the dashboard and managing the 67 veterans on osteoporosis therapy. This dashboard represents a sustainable clinical tool with the capacity to minimize osteoporosis care gaps and improve outcomes.
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(suppl 2):S3-S7. doi:10.1007/s00198-004-1702-6
- van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517-522. doi:10.1016/s8756-3282(01)00614-7
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(suppl 1):58-63. doi:10.1159/000063449
- Cooper C. Epidemiology and public health impact of osteoporosis. Baillieres Clin Rheumatol. 1993;7:459-477. doi:10.1016/s0950-3579(05)80073-1
- Dolan P, Torgerson DJ. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int. 1998;8:611-617. doi:10.1007/s001980050107
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. doi:10.1359/jbmr.061113
- Palacios S. Medical treatment of osteoporosis. Climacteric. 2022;25:43-49. doi:10.1080/13697137.2021.1951697
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. doi:10.1210/jc.2019-00221
- Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822. doi:10.1210/jc.2011-3045
- Lau MK, Bounthavong M, Kay CL, Harvey MA, Christopher MLD. Clinical dashboard development and use for academic detailing in the U.S. Department of Veterans Affairs. J Am Pharm Assoc (2003). 2019;59(2S):S96-S103.e3. doi:10.1016/j.japh.2018.12.006
- Mould DR, D’Haens G, Upton RN. Clinical decision support tools: the evolution of a revolution. Clin Pharmacol Ther. 2016;99:405-418. doi:10.1002/cpt.334
- Kizer KW, Fonseca ML, Long LM. The veterans healthcare system: preparing for the twenty-first century. Hosp Health Serv Adm. 1997;42:283-298.
- Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis c in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35:24-29.
- Brownell N, Kay C, Parra D, et al. Development and optimization of the Veterans Affairs’ national heart failure dashboard for population health management. J Card Fail. 2024;30:452-459. doi:10.1016/j.cardfail.2023.08.024
- Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the opioid safety initiative on opioidrelated prescribing in veterans. Pain. 2017;158:833-839. doi:10.1097/j.pain.0000000000000837
- Twohig PA, Rivington JR, Gunzler D, Daprano J, Margolius D. Clinician dashboard views and improvement in preventative health outcome measures: a retrospective analysis. BMC Health Serv Res. 2019;19:475. doi:10.1186/s12913-019-4327-3
- Singh I, Fletcher R, Scanlon L, Tyler M, Aithal S. A quality improvement initiative on the management of osteoporosis in older people with Parkinsonism. BMJ Qual Improv Rep. 2016;5:u210921.w5756. doi:10.1136/bmjquality.u210921.w5756
- Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med. 2021;10:152. doi:10.3390/jcm10010152
- Sharman Moser S, Yu J, Goldshtein I, et al. Cost and consequences of nonadherence with oral bisphosphonate therapy: findings from a real-world data analysis. Ann Pharmacother. 2016;50:262-269. doi:10.1177/1060028015626935
- Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs--an analysis using national health databases. Osteoporos Int. 2013;24:2639-2647. doi:10.1007/s00198-013-2365-y
- Blouin J, Dragomir A, Fredette M, Ste-Marie LG, Fernandes JC, Perreault S. Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int. 2009;20:1571-1581. doi:10.1007/s00198-008-0818-5
- Cotté F-E, De Pouvourville G. Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC Health Serv Res. 2011;11:151. doi:10.1186/1472-6963-11-151
- Cho H, Byun J-H, Song I, et al. Effect of improved medication adherence on health care costs in osteoporosis patients. Medicine (Baltimore). 2018;97:e11470. doi:10.1097/MD.0000000000011470
- Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181-209. doi:10.1007/s40273-020-00965-9
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16(suppl 2):S3-S7. doi:10.1007/s00198-004-1702-6
- van Staa TP, Dennison EM, Leufkens HG, Cooper C. Epidemiology of fractures in England and Wales. Bone. 2001;29:517-522. doi:10.1016/s8756-3282(01)00614-7
- Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm Res. 2000;54(suppl 1):58-63. doi:10.1159/000063449
- Cooper C. Epidemiology and public health impact of osteoporosis. Baillieres Clin Rheumatol. 1993;7:459-477. doi:10.1016/s0950-3579(05)80073-1
- Dolan P, Torgerson DJ. The cost of treating osteoporotic fractures in the United Kingdom female population. Osteoporos Int. 1998;8:611-617. doi:10.1007/s001980050107
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22:465-475. doi:10.1359/jbmr.061113
- Palacios S. Medical treatment of osteoporosis. Climacteric. 2022;25:43-49. doi:10.1080/13697137.2021.1951697
- Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104:1595-1622. doi:10.1210/jc.2019-00221
- Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:1802-1822. doi:10.1210/jc.2011-3045
- Lau MK, Bounthavong M, Kay CL, Harvey MA, Christopher MLD. Clinical dashboard development and use for academic detailing in the U.S. Department of Veterans Affairs. J Am Pharm Assoc (2003). 2019;59(2S):S96-S103.e3. doi:10.1016/j.japh.2018.12.006
- Mould DR, D’Haens G, Upton RN. Clinical decision support tools: the evolution of a revolution. Clin Pharmacol Ther. 2016;99:405-418. doi:10.1002/cpt.334
- Kizer KW, Fonseca ML, Long LM. The veterans healthcare system: preparing for the twenty-first century. Hosp Health Serv Adm. 1997;42:283-298.
- Park A, Gonzalez R, Chartier M, et al. Screening and treating hepatitis c in the VA: achieving excellence using lean and system redesign. Fed Pract. 2018;35:24-29.
- Brownell N, Kay C, Parra D, et al. Development and optimization of the Veterans Affairs’ national heart failure dashboard for population health management. J Card Fail. 2024;30:452-459. doi:10.1016/j.cardfail.2023.08.024
- Lin LA, Bohnert ASB, Kerns RD, Clay MA, Ganoczy D, Ilgen MA. Impact of the opioid safety initiative on opioidrelated prescribing in veterans. Pain. 2017;158:833-839. doi:10.1097/j.pain.0000000000000837
- Twohig PA, Rivington JR, Gunzler D, Daprano J, Margolius D. Clinician dashboard views and improvement in preventative health outcome measures: a retrospective analysis. BMC Health Serv Res. 2019;19:475. doi:10.1186/s12913-019-4327-3
- Singh I, Fletcher R, Scanlon L, Tyler M, Aithal S. A quality improvement initiative on the management of osteoporosis in older people with Parkinsonism. BMJ Qual Improv Rep. 2016;5:u210921.w5756. doi:10.1136/bmjquality.u210921.w5756
- Anastasilakis AD, Makras P, Yavropoulou MP, Tabacco G, Naciu AM, Palermo A. Denosumab discontinuation and the rebound phenomenon: a narrative review. J Clin Med. 2021;10:152. doi:10.3390/jcm10010152
- Sharman Moser S, Yu J, Goldshtein I, et al. Cost and consequences of nonadherence with oral bisphosphonate therapy: findings from a real-world data analysis. Ann Pharmacother. 2016;50:262-269. doi:10.1177/1060028015626935
- Olsen KR, Hansen C, Abrahamsen B. Association between refill compliance to oral bisphosphonate treatment, incident fractures, and health care costs--an analysis using national health databases. Osteoporos Int. 2013;24:2639-2647. doi:10.1007/s00198-013-2365-y
- Blouin J, Dragomir A, Fredette M, Ste-Marie LG, Fernandes JC, Perreault S. Comparison of direct health care costs related to the pharmacological treatment of osteoporosis and to the management of osteoporotic fractures among compliant and noncompliant users of alendronate and risedronate: a population-based study. Osteoporos Int. 2009;20:1571-1581. doi:10.1007/s00198-008-0818-5
- Cotté F-E, De Pouvourville G. Cost of non-persistence with oral bisphosphonates in post-menopausal osteoporosis treatment in France. BMC Health Serv Res. 2011;11:151. doi:10.1186/1472-6963-11-151
- Cho H, Byun J-H, Song I, et al. Effect of improved medication adherence on health care costs in osteoporosis patients. Medicine (Baltimore). 2018;97:e11470. doi:10.1097/MD.0000000000011470
- Li N, Cornelissen D, Silverman S, et al. An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics. 2021;39:181-209. doi:10.1007/s40273-020-00965-9
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Improving High-Risk Osteoporosis Medication Adherence and Safety With an Automated Dashboard
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
The impact of obesity in the United States is significant. Between August 2021 and August 2023, the prevalence of obesity (body mass index ≥ 30) in US adults was 40.3%.1 The prevalence of obesity in adults aged 40 to 59 years was 46.4%, higher than the prevalence in adults aged 20 to 39 years (35.5%) and those aged ≥ 60 years (38.9%).1 The excess annual medical costs associated with obesity in the US are estimated at nearly $173 billion.2
The first-line treatment for obesity is lifestyle modifications, including a healthy diet and exercise. When lifestyle modifications are not enough to achieve weight-loss goals, bariatric surgery and anti-obesity medications (AOMs) are often considered. Five medications were approved for the long-term tretament of obesity by the US Food and Drug Administration (FDA) between 2021 and 2023, when this study was conducted: semaglutide (Wegovy), liraglutide (Saxenda), phentermine and topiramate, naltrexone and bupropion, and orlistat. The clinically meaningful (and commonly accepted) weight-loss target for these medications is ≥ 5% from baseline by week 12 of the maximally tolerated dose of therapy. A 5% weight loss has been shown to be clinically significant in improving cardiometabolic risk factors.3,4 These medications are intended to be used as an adjunct to healthy diet and exercise. Of note, semaglutide and liraglutide carry brand names, which are associated with different dosing for the treatment of type 2 diabetes mellitus (T2DM).
All 5 FDA-approved AOMs were available at the Veterans Affairs Sioux Falls Health Care System (VASFHCS) for the treatment of obesity at the time of the study. To qualify for an AOM, a veteran at VASFHCS must first work with a dietitian or be enrolled in the MOVE! clinic to participate in the weight management program, which focuses on dietary, exercise, and behavioral changes. At VASFHCS, AOMs are prescribed by primary care practitioners, clinical pharmacy providers, and advanced practitioners within the MOVE! program.
Ample data exist for the efficacy of AOMs. However, no published research has reported on AOM efficacy by age group (Appendix).5-11 While most of the AOM clinical trials included older adults, the average age of participants was typically between 40 and 50 years. It is well-known that pharmacokinetic and pharmacodynamic changes occur as age increases. Renal and hepatic clearance is reduced while the volume distribution and sensitivities to some medications may increase. 12 Although this study did not focus on specific pharmacokinetic and pharmacodynamic changes with respect to AOM, it is important to recognize that this may play a role in the efficacy and safety of AOMs in older adults.
Methods
This retrospective single-center chart review was performed using the VASFHCS Computerized Patient Record System to compare the efficacy of AOMs in older adults (aged ≥ 65 years) vs adults (aged < 65 years). The primary endpoint was the percent change in body weight from baseline to 6 and 12 months after initiation of AOM therapy in the older adult vs adult population. Secondary endpoints included changes in low-density lipoprotein (LDL), hemoglobin A1c (HbA1c), and blood pressure (BP) from baseline compared to 12 months on AOM therapy. HbA1c was assessed in patients with T2DM or prediabetes at the time of AOM initiation. Two safety endpoints were also explored to determine the incidence of medication adverse events (AEs) and subsequent discontinuation of AOM. A subset analysis was performed to determine whether there was a difference in percent change in body weight between patients in 3 age groups: 18 to 40 years, 41 to 64 years, and ≥ 65 years.
The study population included patients who were prescribed an AOM between January 1, 2021, and June 30, 2023. Patients were excluded if they did not continue AOM therapy for ≥ 6 months after initiation or if they underwent gastric bypass surgery while undergoing AOM therapy. Patients taking semaglutide (Ozempic) or liraglutide (Victoza) for both T2DM and weight loss who were eventually switched to the weight loss formulations (Wegovy or Saxenda) were included. Patients who switched between semaglutide and liraglutide for weight loss were also included. Those taking semaglutide or liraglutide solely for T2DM treatment were excluded because they are dosed differently.
Collected data included age, gender, race, weight (baseline, 6 and 12 months after initiation of AOM), metabolic laboratory values/vital signs (HbA1c, LDL, and BP at baseline and 12 months after initiation of AOM), diagnosis of T2DM or prediabetes, reported AEs associated with AOM therapy, and date of AOM initiation and discontinuation (if applicable). Baseline values were defined at the time of medication initiation or values documented within 6 months prior to medication initiation if true baseline data were not reported. If values were not recorded at months 6 and 12 after AOM initiation, values documented closest to those targets were used. Weights were used for baseline, 6-, and 12-month data unless they were unavailable due to use of virtual care modalities. In these cases, patient-reported weights were used. Patients were included in the 6-month data, but not the 12-month data, if they were taking AOMs for > 6 months but not for 12 months. If patients had been on multiple AOMs, baseline data were recorded at the start of the first medication that was used for 6 months or longer. Twelve-month data were recorded after subsequent medication change. Twelve-month metabolic laboratory values/vital signs were recorded for patients included in the study even if they did not complete ≥ 12 months of AOM therapy.
Statistical Analysis
Data from patients who were prescribed an AOM from January 2021 to June 2023 and who remained on the medication for ≥ 6 months were analyzed. Baseline characteristics were analyzed using descriptive statistics. The primary and secondary endpoints were evaluated using the t test. The safety endpoints were analyzed using descriptive statistics. An analysis of variance test was used for the subset analysis. Results with P < .05 were statistically significant.
Results
A total of 144 participants were included in this study, 116 in the adult group (aged < 65 years) and 28 in the older adult group (aged ≥ 65 years). Sixty-seven patients were excluded due to prespecified inclusion and exclusion criteria.
Other than the predetermined mean age differences (48 years vs 71 years), there were multiple differences in patient baseline characteristics. When comparing older adults and adults, average weight (283 lb vs 269 lb) and White race (89% vs 87%) were slightly higher in the older adult group. Also, a higher prevalence of T2DM (54% and 18%) and a lower prevalence of prediabetes (21% and 33%) was noted in the older adult group. HbA1c and BP were similar between both groups at baseline, while LDL was slightly lower in the older adult group (Table 1).
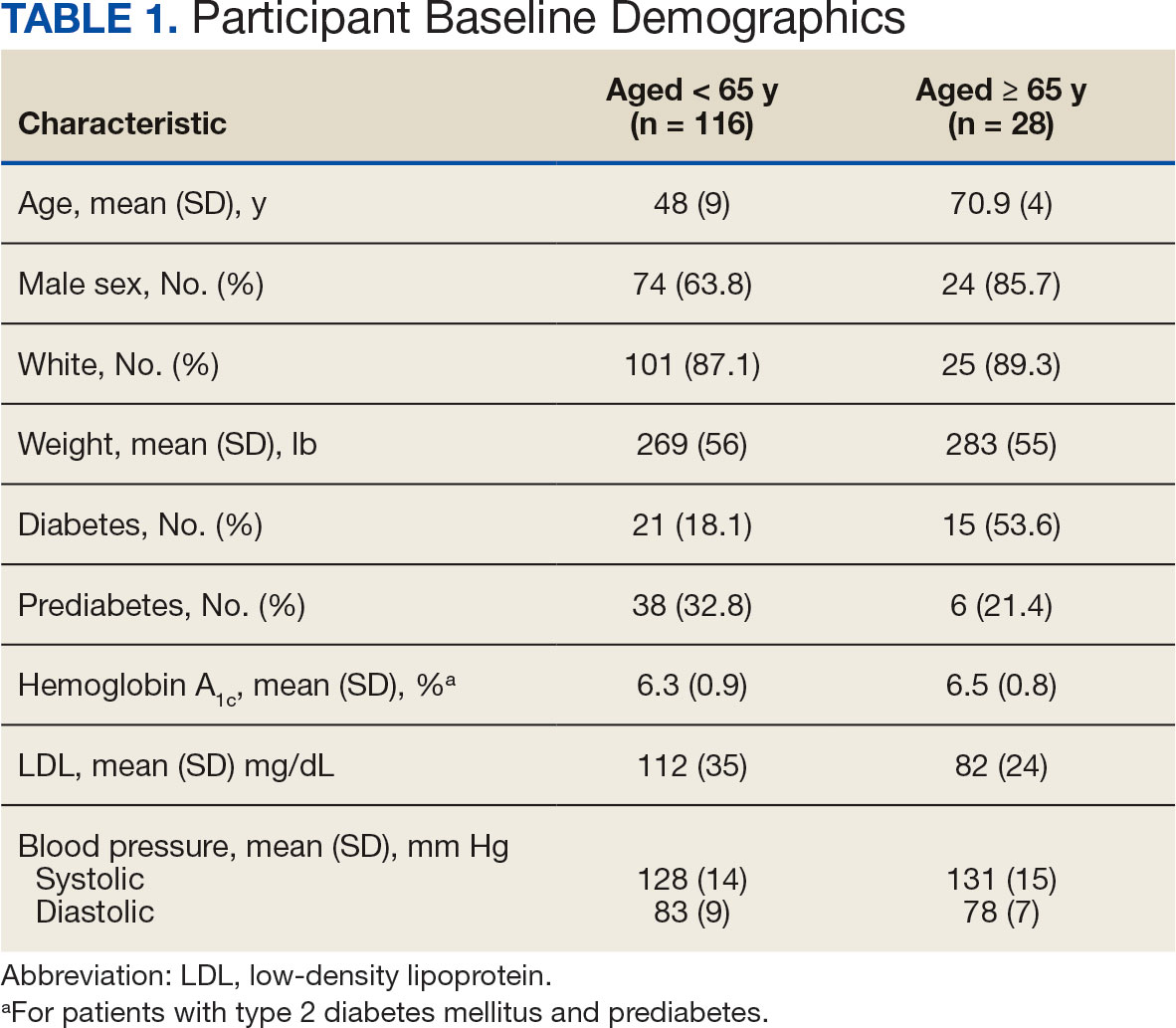
Patients in the adult group lost a mean 7.0% and 8.7% of body weight at 6 and 12 months, respectively, while the older adult group lost 5.0% and 6.6% body weight at 6 and 12 months, respectively. The difference in percent change in body weight was not statistically different at 6 (P = .08) or 12 (P = .26) months between patients in the adult group vs the older adult group or in the specific age groups (18-40 years, 41-64 years, ≥ 65 years) at 6 months (P = .24) or 12 months (P = .53) (Figure).
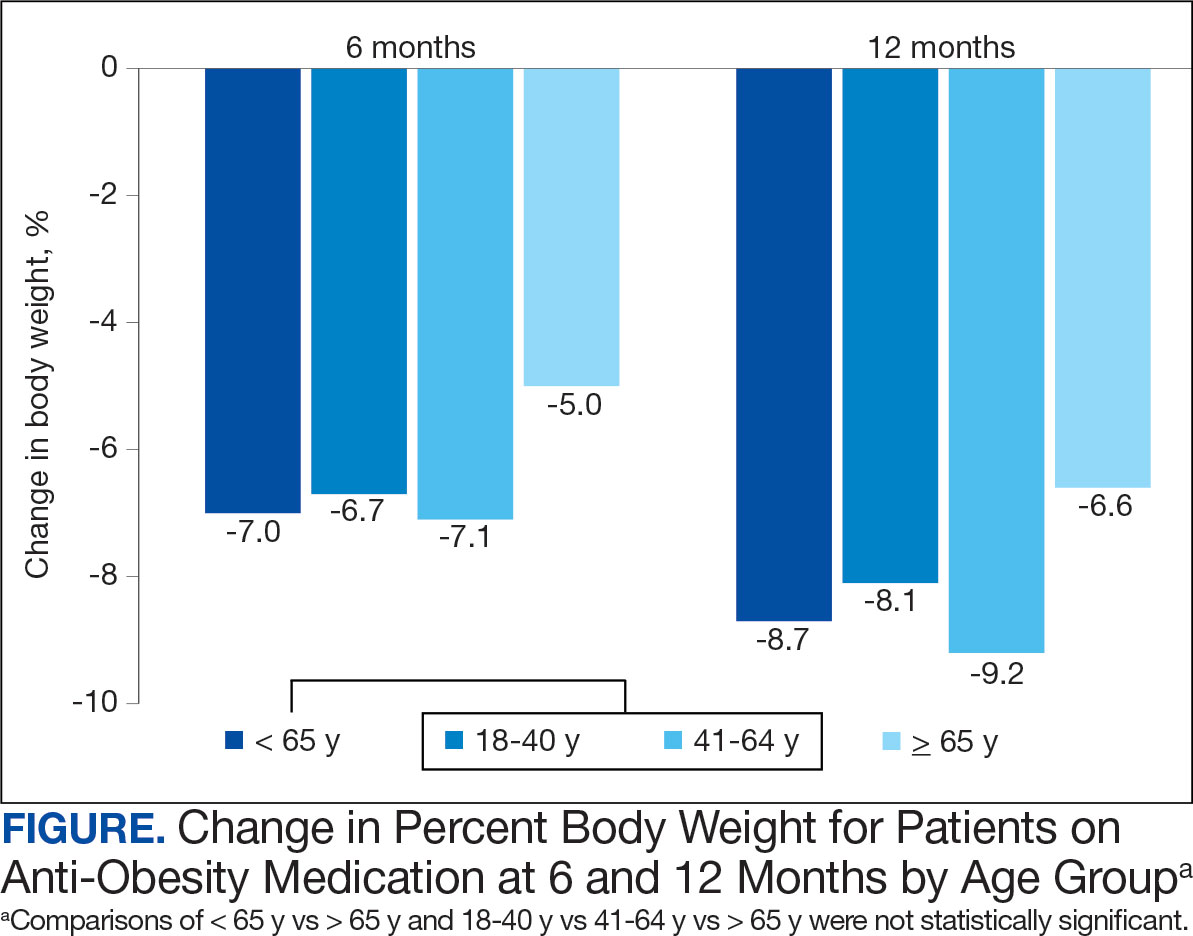
At 12 months, the difference between the adult group vs the older adult group was not statistically significant for HbA1c in patients with T2DM or prediabetes (P = .73), LDL (P = .95), systolic BP (P = .58), or diastolic BP (P = .51) (Table 2).
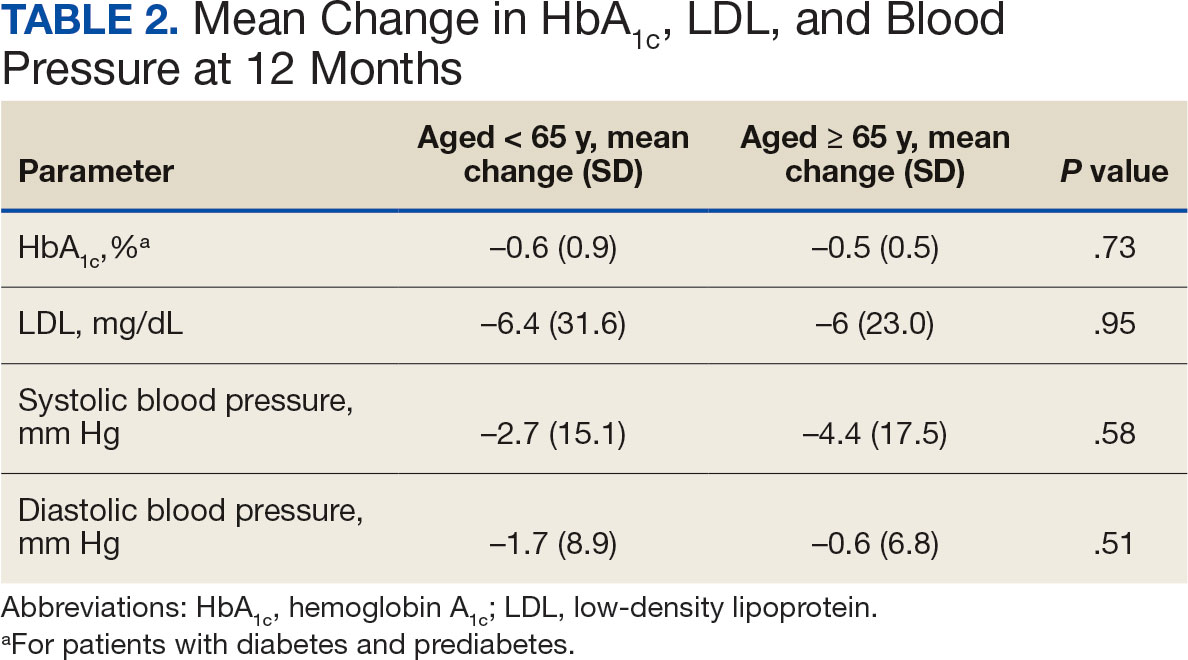
For the safety endpoint, the incidence of AEs was found to be different between groups. There were more reported AEs (61.2% vs 39.3%) and a greater increase in therapy discontinuation due to AEs (6.0% vs 0%) in the adult group compared to the older adult group (Table 3).
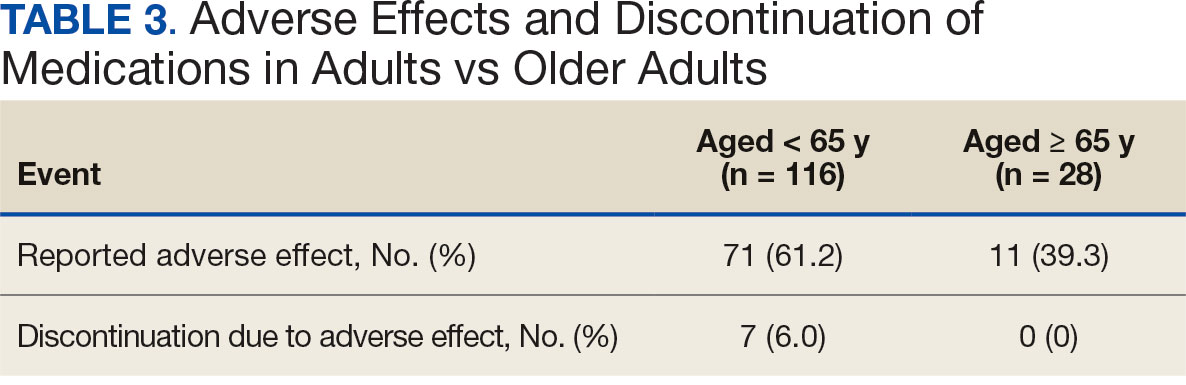
Discussion
Patients taking AOMs revealed no statistically significant difference in percent change in body weight at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. The subset analysis also showed no statistically significant difference in change in percent body weight between more narrowly defined age groups of 18 to 40 years, 41 to 64 years, and ≥ 65 years. This suggests that AOM may have similar efficacy for weight loss in all ages of adults.
Secondary endpoint findings showed no statistically significant difference in HbA1c (in patients with T2DM or prediabetes), LDL, or BP at 12 months between the 2 groups. Although this study did not differentiate secondary outcomes based on the individual AOM, the change in HbA1c in both groups was expected, given that 70% of the patients included in this study were taking a glucagon-like peptide-1 agonist (liraglutide and semaglutide) at some point during the study. It’s also worth noting that secondary endpoints were collected for patients who discontinued the AOM between 6 and 12 months. Therefore, the patients’ HbA1c, LDL, and BP may not have accurately reflected the change that could have been expected if they had continued AOM therapy beyond the 12-month period.
Due to the different mechanisms and range in efficacy that AOMs have in regard to weight loss, changes in all outcomes, including weight, HbA1c, LDL, and BP were expected to vary as patients were included even after switching AOM (collection of data started after ≥ 6 months on a single AOM). Switching of AOM after the first 6 months of therapy was recorded in 25% of the patients in the ≥ 65 years group and 330% of the patients in the < 65 years group.
The incidence of AEs and subsequent discontinuation of AOMs in this study was higher in the adult group. This study excluded patients who did not continue taking an AOM for at least 6 months. As a result, the incidence of AEs between the 2 groups within the first 6 months of AOM therapy remains unknown. It is possible that during the first 6 months of therapy, patients aged < 65 years were more willing to tolerate or had fewer severe AEs compared with the older adult group. It’s also possible that the smaller number of patients in the older adult group was due to increased AEs that led them to discontinue early (before completion of 6 months of therapy) and/or prescriber discomfort in using AOMs in the older adult population. In addition, because the specific medication(s) taken by patients in each group were not detailed, it is unknown whether the adult group was taking AOMs associated with a greater number of AEs.
Limitations
This was a retrospective study with a relatively small sample size. A larger sample size may have shown more precise differences between age groups and may be more representative of the general population. Additionally, data were reliant on appropriate documentation, and adherence to AOM therapy was not assessed due to the retrospective nature of this study. At times, the study relied on patient reported data points, such as weight, if a clinic weight was not available. Also, this study did not account for many potential confounding factors such as other medications taken by the patient, which can affect outcomes including weight, HbA1c, LDL, blood pressure, and AEs.
Conclusions
This retrospective study of patients taking AOMs showed no statistically significant difference in weight loss at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. A subset analysis found no statistically significant difference in change in body weight between specific age groups (18-40 years, 41-64 years, and ≥ 65 years). There was also no statistically significant difference in secondary outcomes, including change in HbA1c (in patients with T2DM or prediabetes), LDL or BP between age groups. The safety endpoints showed a higher incidence of medication AEs in the adult group, with more of these adults discontinuing therapy due to AEs. This study indicates that AOM may have similar outcomes for weight loss and metabolic laboratory values/vital sign changes between adults and older adults. Also, our findings suggest that patients aged < 65 years may experience more AEs than patients aged ≥ 65 years after ≥ 6 months of AOM therapy. Larger studies are needed to further evaluate these age-specific findings.
- Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and severe obesity prevalence in adults: United States, August 2021-August 2023. NCHS Data Brief No. 508. National Center for Health Statistics; 2024. Accessed December 11, 2024. https://www.cdc.gov/nchs/products/databriefs/db508.htm
- Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi:10.1371/journal.pone.0247307
- Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134(4):359-375. doi:10.1080/00325481.2022.2051366
- American Diabetes Association (ADA). Standards of care in diabetes–2023. Diabetes Care. 2023;46(suppl 1):S128- S2139. doi:10.2337/dc23-S008
- Wilding JPH, Batterham RL, Calanna S, et al. Onceweekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342. doi:10.1038/oby.2011.330
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308. doi:10.3945/ajcn.111.024927
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
- Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016s0140-6736(97)11509-4
- Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14. doi:10.1046/j.1365-2125.2003.02007.x
The impact of obesity in the United States is significant. Between August 2021 and August 2023, the prevalence of obesity (body mass index ≥ 30) in US adults was 40.3%.1 The prevalence of obesity in adults aged 40 to 59 years was 46.4%, higher than the prevalence in adults aged 20 to 39 years (35.5%) and those aged ≥ 60 years (38.9%).1 The excess annual medical costs associated with obesity in the US are estimated at nearly $173 billion.2
The first-line treatment for obesity is lifestyle modifications, including a healthy diet and exercise. When lifestyle modifications are not enough to achieve weight-loss goals, bariatric surgery and anti-obesity medications (AOMs) are often considered. Five medications were approved for the long-term tretament of obesity by the US Food and Drug Administration (FDA) between 2021 and 2023, when this study was conducted: semaglutide (Wegovy), liraglutide (Saxenda), phentermine and topiramate, naltrexone and bupropion, and orlistat. The clinically meaningful (and commonly accepted) weight-loss target for these medications is ≥ 5% from baseline by week 12 of the maximally tolerated dose of therapy. A 5% weight loss has been shown to be clinically significant in improving cardiometabolic risk factors.3,4 These medications are intended to be used as an adjunct to healthy diet and exercise. Of note, semaglutide and liraglutide carry brand names, which are associated with different dosing for the treatment of type 2 diabetes mellitus (T2DM).
All 5 FDA-approved AOMs were available at the Veterans Affairs Sioux Falls Health Care System (VASFHCS) for the treatment of obesity at the time of the study. To qualify for an AOM, a veteran at VASFHCS must first work with a dietitian or be enrolled in the MOVE! clinic to participate in the weight management program, which focuses on dietary, exercise, and behavioral changes. At VASFHCS, AOMs are prescribed by primary care practitioners, clinical pharmacy providers, and advanced practitioners within the MOVE! program.
Ample data exist for the efficacy of AOMs. However, no published research has reported on AOM efficacy by age group (Appendix).5-11 While most of the AOM clinical trials included older adults, the average age of participants was typically between 40 and 50 years. It is well-known that pharmacokinetic and pharmacodynamic changes occur as age increases. Renal and hepatic clearance is reduced while the volume distribution and sensitivities to some medications may increase. 12 Although this study did not focus on specific pharmacokinetic and pharmacodynamic changes with respect to AOM, it is important to recognize that this may play a role in the efficacy and safety of AOMs in older adults.

Methods
This retrospective single-center chart review was performed using the VASFHCS Computerized Patient Record System to compare the efficacy of AOMs in older adults (aged ≥ 65 years) vs adults (aged < 65 years). The primary endpoint was the percent change in body weight from baseline to 6 and 12 months after initiation of AOM therapy in the older adult vs adult population. Secondary endpoints included changes in low-density lipoprotein (LDL), hemoglobin A1c (HbA1c), and blood pressure (BP) from baseline compared to 12 months on AOM therapy. HbA1c was assessed in patients with T2DM or prediabetes at the time of AOM initiation. Two safety endpoints were also explored to determine the incidence of medication adverse events (AEs) and subsequent discontinuation of AOM. A subset analysis was performed to determine whether there was a difference in percent change in body weight between patients in 3 age groups: 18 to 40 years, 41 to 64 years, and ≥ 65 years.
The study population included patients who were prescribed an AOM between January 1, 2021, and June 30, 2023. Patients were excluded if they did not continue AOM therapy for ≥ 6 months after initiation or if they underwent gastric bypass surgery while undergoing AOM therapy. Patients taking semaglutide (Ozempic) or liraglutide (Victoza) for both T2DM and weight loss who were eventually switched to the weight loss formulations (Wegovy or Saxenda) were included. Patients who switched between semaglutide and liraglutide for weight loss were also included. Those taking semaglutide or liraglutide solely for T2DM treatment were excluded because they are dosed differently.
Collected data included age, gender, race, weight (baseline, 6 and 12 months after initiation of AOM), metabolic laboratory values/vital signs (HbA1c, LDL, and BP at baseline and 12 months after initiation of AOM), diagnosis of T2DM or prediabetes, reported AEs associated with AOM therapy, and date of AOM initiation and discontinuation (if applicable). Baseline values were defined at the time of medication initiation or values documented within 6 months prior to medication initiation if true baseline data were not reported. If values were not recorded at months 6 and 12 after AOM initiation, values documented closest to those targets were used. Weights were used for baseline, 6-, and 12-month data unless they were unavailable due to use of virtual care modalities. In these cases, patient-reported weights were used. Patients were included in the 6-month data, but not the 12-month data, if they were taking AOMs for > 6 months but not for 12 months. If patients had been on multiple AOMs, baseline data were recorded at the start of the first medication that was used for 6 months or longer. Twelve-month data were recorded after subsequent medication change. Twelve-month metabolic laboratory values/vital signs were recorded for patients included in the study even if they did not complete ≥ 12 months of AOM therapy.
Statistical Analysis
Data from patients who were prescribed an AOM from January 2021 to June 2023 and who remained on the medication for ≥ 6 months were analyzed. Baseline characteristics were analyzed using descriptive statistics. The primary and secondary endpoints were evaluated using the t test. The safety endpoints were analyzed using descriptive statistics. An analysis of variance test was used for the subset analysis. Results with P < .05 were statistically significant.
Results
A total of 144 participants were included in this study, 116 in the adult group (aged < 65 years) and 28 in the older adult group (aged ≥ 65 years). Sixty-seven patients were excluded due to prespecified inclusion and exclusion criteria.
Other than the predetermined mean age differences (48 years vs 71 years), there were multiple differences in patient baseline characteristics. When comparing older adults and adults, average weight (283 lb vs 269 lb) and White race (89% vs 87%) were slightly higher in the older adult group. Also, a higher prevalence of T2DM (54% and 18%) and a lower prevalence of prediabetes (21% and 33%) was noted in the older adult group. HbA1c and BP were similar between both groups at baseline, while LDL was slightly lower in the older adult group (Table 1).

Patients in the adult group lost a mean 7.0% and 8.7% of body weight at 6 and 12 months, respectively, while the older adult group lost 5.0% and 6.6% body weight at 6 and 12 months, respectively. The difference in percent change in body weight was not statistically different at 6 (P = .08) or 12 (P = .26) months between patients in the adult group vs the older adult group or in the specific age groups (18-40 years, 41-64 years, ≥ 65 years) at 6 months (P = .24) or 12 months (P = .53) (Figure).

At 12 months, the difference between the adult group vs the older adult group was not statistically significant for HbA1c in patients with T2DM or prediabetes (P = .73), LDL (P = .95), systolic BP (P = .58), or diastolic BP (P = .51) (Table 2).

For the safety endpoint, the incidence of AEs was found to be different between groups. There were more reported AEs (61.2% vs 39.3%) and a greater increase in therapy discontinuation due to AEs (6.0% vs 0%) in the adult group compared to the older adult group (Table 3).

Discussion
Patients taking AOMs revealed no statistically significant difference in percent change in body weight at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. The subset analysis also showed no statistically significant difference in change in percent body weight between more narrowly defined age groups of 18 to 40 years, 41 to 64 years, and ≥ 65 years. This suggests that AOM may have similar efficacy for weight loss in all ages of adults.
Secondary endpoint findings showed no statistically significant difference in HbA1c (in patients with T2DM or prediabetes), LDL, or BP at 12 months between the 2 groups. Although this study did not differentiate secondary outcomes based on the individual AOM, the change in HbA1c in both groups was expected, given that 70% of the patients included in this study were taking a glucagon-like peptide-1 agonist (liraglutide and semaglutide) at some point during the study. It’s also worth noting that secondary endpoints were collected for patients who discontinued the AOM between 6 and 12 months. Therefore, the patients’ HbA1c, LDL, and BP may not have accurately reflected the change that could have been expected if they had continued AOM therapy beyond the 12-month period.
Due to the different mechanisms and range in efficacy that AOMs have in regard to weight loss, changes in all outcomes, including weight, HbA1c, LDL, and BP were expected to vary as patients were included even after switching AOM (collection of data started after ≥ 6 months on a single AOM). Switching of AOM after the first 6 months of therapy was recorded in 25% of the patients in the ≥ 65 years group and 330% of the patients in the < 65 years group.
The incidence of AEs and subsequent discontinuation of AOMs in this study was higher in the adult group. This study excluded patients who did not continue taking an AOM for at least 6 months. As a result, the incidence of AEs between the 2 groups within the first 6 months of AOM therapy remains unknown. It is possible that during the first 6 months of therapy, patients aged < 65 years were more willing to tolerate or had fewer severe AEs compared with the older adult group. It’s also possible that the smaller number of patients in the older adult group was due to increased AEs that led them to discontinue early (before completion of 6 months of therapy) and/or prescriber discomfort in using AOMs in the older adult population. In addition, because the specific medication(s) taken by patients in each group were not detailed, it is unknown whether the adult group was taking AOMs associated with a greater number of AEs.
Limitations
This was a retrospective study with a relatively small sample size. A larger sample size may have shown more precise differences between age groups and may be more representative of the general population. Additionally, data were reliant on appropriate documentation, and adherence to AOM therapy was not assessed due to the retrospective nature of this study. At times, the study relied on patient reported data points, such as weight, if a clinic weight was not available. Also, this study did not account for many potential confounding factors such as other medications taken by the patient, which can affect outcomes including weight, HbA1c, LDL, blood pressure, and AEs.
Conclusions
This retrospective study of patients taking AOMs showed no statistically significant difference in weight loss at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. A subset analysis found no statistically significant difference in change in body weight between specific age groups (18-40 years, 41-64 years, and ≥ 65 years). There was also no statistically significant difference in secondary outcomes, including change in HbA1c (in patients with T2DM or prediabetes), LDL or BP between age groups. The safety endpoints showed a higher incidence of medication AEs in the adult group, with more of these adults discontinuing therapy due to AEs. This study indicates that AOM may have similar outcomes for weight loss and metabolic laboratory values/vital sign changes between adults and older adults. Also, our findings suggest that patients aged < 65 years may experience more AEs than patients aged ≥ 65 years after ≥ 6 months of AOM therapy. Larger studies are needed to further evaluate these age-specific findings.
The impact of obesity in the United States is significant. Between August 2021 and August 2023, the prevalence of obesity (body mass index ≥ 30) in US adults was 40.3%.1 The prevalence of obesity in adults aged 40 to 59 years was 46.4%, higher than the prevalence in adults aged 20 to 39 years (35.5%) and those aged ≥ 60 years (38.9%).1 The excess annual medical costs associated with obesity in the US are estimated at nearly $173 billion.2
The first-line treatment for obesity is lifestyle modifications, including a healthy diet and exercise. When lifestyle modifications are not enough to achieve weight-loss goals, bariatric surgery and anti-obesity medications (AOMs) are often considered. Five medications were approved for the long-term tretament of obesity by the US Food and Drug Administration (FDA) between 2021 and 2023, when this study was conducted: semaglutide (Wegovy), liraglutide (Saxenda), phentermine and topiramate, naltrexone and bupropion, and orlistat. The clinically meaningful (and commonly accepted) weight-loss target for these medications is ≥ 5% from baseline by week 12 of the maximally tolerated dose of therapy. A 5% weight loss has been shown to be clinically significant in improving cardiometabolic risk factors.3,4 These medications are intended to be used as an adjunct to healthy diet and exercise. Of note, semaglutide and liraglutide carry brand names, which are associated with different dosing for the treatment of type 2 diabetes mellitus (T2DM).
All 5 FDA-approved AOMs were available at the Veterans Affairs Sioux Falls Health Care System (VASFHCS) for the treatment of obesity at the time of the study. To qualify for an AOM, a veteran at VASFHCS must first work with a dietitian or be enrolled in the MOVE! clinic to participate in the weight management program, which focuses on dietary, exercise, and behavioral changes. At VASFHCS, AOMs are prescribed by primary care practitioners, clinical pharmacy providers, and advanced practitioners within the MOVE! program.
Ample data exist for the efficacy of AOMs. However, no published research has reported on AOM efficacy by age group (Appendix).5-11 While most of the AOM clinical trials included older adults, the average age of participants was typically between 40 and 50 years. It is well-known that pharmacokinetic and pharmacodynamic changes occur as age increases. Renal and hepatic clearance is reduced while the volume distribution and sensitivities to some medications may increase. 12 Although this study did not focus on specific pharmacokinetic and pharmacodynamic changes with respect to AOM, it is important to recognize that this may play a role in the efficacy and safety of AOMs in older adults.

Methods
This retrospective single-center chart review was performed using the VASFHCS Computerized Patient Record System to compare the efficacy of AOMs in older adults (aged ≥ 65 years) vs adults (aged < 65 years). The primary endpoint was the percent change in body weight from baseline to 6 and 12 months after initiation of AOM therapy in the older adult vs adult population. Secondary endpoints included changes in low-density lipoprotein (LDL), hemoglobin A1c (HbA1c), and blood pressure (BP) from baseline compared to 12 months on AOM therapy. HbA1c was assessed in patients with T2DM or prediabetes at the time of AOM initiation. Two safety endpoints were also explored to determine the incidence of medication adverse events (AEs) and subsequent discontinuation of AOM. A subset analysis was performed to determine whether there was a difference in percent change in body weight between patients in 3 age groups: 18 to 40 years, 41 to 64 years, and ≥ 65 years.
The study population included patients who were prescribed an AOM between January 1, 2021, and June 30, 2023. Patients were excluded if they did not continue AOM therapy for ≥ 6 months after initiation or if they underwent gastric bypass surgery while undergoing AOM therapy. Patients taking semaglutide (Ozempic) or liraglutide (Victoza) for both T2DM and weight loss who were eventually switched to the weight loss formulations (Wegovy or Saxenda) were included. Patients who switched between semaglutide and liraglutide for weight loss were also included. Those taking semaglutide or liraglutide solely for T2DM treatment were excluded because they are dosed differently.
Collected data included age, gender, race, weight (baseline, 6 and 12 months after initiation of AOM), metabolic laboratory values/vital signs (HbA1c, LDL, and BP at baseline and 12 months after initiation of AOM), diagnosis of T2DM or prediabetes, reported AEs associated with AOM therapy, and date of AOM initiation and discontinuation (if applicable). Baseline values were defined at the time of medication initiation or values documented within 6 months prior to medication initiation if true baseline data were not reported. If values were not recorded at months 6 and 12 after AOM initiation, values documented closest to those targets were used. Weights were used for baseline, 6-, and 12-month data unless they were unavailable due to use of virtual care modalities. In these cases, patient-reported weights were used. Patients were included in the 6-month data, but not the 12-month data, if they were taking AOMs for > 6 months but not for 12 months. If patients had been on multiple AOMs, baseline data were recorded at the start of the first medication that was used for 6 months or longer. Twelve-month data were recorded after subsequent medication change. Twelve-month metabolic laboratory values/vital signs were recorded for patients included in the study even if they did not complete ≥ 12 months of AOM therapy.
Statistical Analysis
Data from patients who were prescribed an AOM from January 2021 to June 2023 and who remained on the medication for ≥ 6 months were analyzed. Baseline characteristics were analyzed using descriptive statistics. The primary and secondary endpoints were evaluated using the t test. The safety endpoints were analyzed using descriptive statistics. An analysis of variance test was used for the subset analysis. Results with P < .05 were statistically significant.
Results
A total of 144 participants were included in this study, 116 in the adult group (aged < 65 years) and 28 in the older adult group (aged ≥ 65 years). Sixty-seven patients were excluded due to prespecified inclusion and exclusion criteria.
Other than the predetermined mean age differences (48 years vs 71 years), there were multiple differences in patient baseline characteristics. When comparing older adults and adults, average weight (283 lb vs 269 lb) and White race (89% vs 87%) were slightly higher in the older adult group. Also, a higher prevalence of T2DM (54% and 18%) and a lower prevalence of prediabetes (21% and 33%) was noted in the older adult group. HbA1c and BP were similar between both groups at baseline, while LDL was slightly lower in the older adult group (Table 1).

Patients in the adult group lost a mean 7.0% and 8.7% of body weight at 6 and 12 months, respectively, while the older adult group lost 5.0% and 6.6% body weight at 6 and 12 months, respectively. The difference in percent change in body weight was not statistically different at 6 (P = .08) or 12 (P = .26) months between patients in the adult group vs the older adult group or in the specific age groups (18-40 years, 41-64 years, ≥ 65 years) at 6 months (P = .24) or 12 months (P = .53) (Figure).

At 12 months, the difference between the adult group vs the older adult group was not statistically significant for HbA1c in patients with T2DM or prediabetes (P = .73), LDL (P = .95), systolic BP (P = .58), or diastolic BP (P = .51) (Table 2).

For the safety endpoint, the incidence of AEs was found to be different between groups. There were more reported AEs (61.2% vs 39.3%) and a greater increase in therapy discontinuation due to AEs (6.0% vs 0%) in the adult group compared to the older adult group (Table 3).

Discussion
Patients taking AOMs revealed no statistically significant difference in percent change in body weight at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. The subset analysis also showed no statistically significant difference in change in percent body weight between more narrowly defined age groups of 18 to 40 years, 41 to 64 years, and ≥ 65 years. This suggests that AOM may have similar efficacy for weight loss in all ages of adults.
Secondary endpoint findings showed no statistically significant difference in HbA1c (in patients with T2DM or prediabetes), LDL, or BP at 12 months between the 2 groups. Although this study did not differentiate secondary outcomes based on the individual AOM, the change in HbA1c in both groups was expected, given that 70% of the patients included in this study were taking a glucagon-like peptide-1 agonist (liraglutide and semaglutide) at some point during the study. It’s also worth noting that secondary endpoints were collected for patients who discontinued the AOM between 6 and 12 months. Therefore, the patients’ HbA1c, LDL, and BP may not have accurately reflected the change that could have been expected if they had continued AOM therapy beyond the 12-month period.
Due to the different mechanisms and range in efficacy that AOMs have in regard to weight loss, changes in all outcomes, including weight, HbA1c, LDL, and BP were expected to vary as patients were included even after switching AOM (collection of data started after ≥ 6 months on a single AOM). Switching of AOM after the first 6 months of therapy was recorded in 25% of the patients in the ≥ 65 years group and 330% of the patients in the < 65 years group.
The incidence of AEs and subsequent discontinuation of AOMs in this study was higher in the adult group. This study excluded patients who did not continue taking an AOM for at least 6 months. As a result, the incidence of AEs between the 2 groups within the first 6 months of AOM therapy remains unknown. It is possible that during the first 6 months of therapy, patients aged < 65 years were more willing to tolerate or had fewer severe AEs compared with the older adult group. It’s also possible that the smaller number of patients in the older adult group was due to increased AEs that led them to discontinue early (before completion of 6 months of therapy) and/or prescriber discomfort in using AOMs in the older adult population. In addition, because the specific medication(s) taken by patients in each group were not detailed, it is unknown whether the adult group was taking AOMs associated with a greater number of AEs.
Limitations
This was a retrospective study with a relatively small sample size. A larger sample size may have shown more precise differences between age groups and may be more representative of the general population. Additionally, data were reliant on appropriate documentation, and adherence to AOM therapy was not assessed due to the retrospective nature of this study. At times, the study relied on patient reported data points, such as weight, if a clinic weight was not available. Also, this study did not account for many potential confounding factors such as other medications taken by the patient, which can affect outcomes including weight, HbA1c, LDL, blood pressure, and AEs.
Conclusions
This retrospective study of patients taking AOMs showed no statistically significant difference in weight loss at 6 or 12 months between adults aged < 65 years and older adults aged ≥ 65 years. A subset analysis found no statistically significant difference in change in body weight between specific age groups (18-40 years, 41-64 years, and ≥ 65 years). There was also no statistically significant difference in secondary outcomes, including change in HbA1c (in patients with T2DM or prediabetes), LDL or BP between age groups. The safety endpoints showed a higher incidence of medication AEs in the adult group, with more of these adults discontinuing therapy due to AEs. This study indicates that AOM may have similar outcomes for weight loss and metabolic laboratory values/vital sign changes between adults and older adults. Also, our findings suggest that patients aged < 65 years may experience more AEs than patients aged ≥ 65 years after ≥ 6 months of AOM therapy. Larger studies are needed to further evaluate these age-specific findings.
- Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and severe obesity prevalence in adults: United States, August 2021-August 2023. NCHS Data Brief No. 508. National Center for Health Statistics; 2024. Accessed December 11, 2024. https://www.cdc.gov/nchs/products/databriefs/db508.htm
- Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi:10.1371/journal.pone.0247307
- Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134(4):359-375. doi:10.1080/00325481.2022.2051366
- American Diabetes Association (ADA). Standards of care in diabetes–2023. Diabetes Care. 2023;46(suppl 1):S128- S2139. doi:10.2337/dc23-S008
- Wilding JPH, Batterham RL, Calanna S, et al. Onceweekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342. doi:10.1038/oby.2011.330
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308. doi:10.3945/ajcn.111.024927
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
- Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016s0140-6736(97)11509-4
- Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14. doi:10.1046/j.1365-2125.2003.02007.x
- Emmerich SD, Fryar CD, Stierman B, Ogden CL. Obesity and severe obesity prevalence in adults: United States, August 2021-August 2023. NCHS Data Brief No. 508. National Center for Health Statistics; 2024. Accessed December 11, 2024. https://www.cdc.gov/nchs/products/databriefs/db508.htm
- Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. doi:10.1371/journal.pone.0247307
- Horn DB, Almandoz JP, Look M. What is clinically relevant weight loss for your patients and how can it be achieved? A narrative review. Postgrad Med. 2022;134(4):359-375. doi:10.1080/00325481.2022.2051366
- American Diabetes Association (ADA). Standards of care in diabetes–2023. Diabetes Care. 2023;46(suppl 1):S128- S2139. doi:10.2337/dc23-S008
- Wilding JPH, Batterham RL, Calanna S, et al. Onceweekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11-22. doi:10.1056/NEJMoa1411892
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20(2):330-342. doi:10.1038/oby.2011.330
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9774):1341-1352. doi:10.1016/S0140-6736(11)60205-5
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95(2):297-308. doi:10.3945/ajcn.111.024927
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595-605. doi:10.1016/S0140-6736(10)60888-4
- Sjöström L, Rissanen A, Andersen T, et al. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. European Multicentre Orlistat Study Group. Lancet. 1998;352(9123):167-172. doi:10.1016s0140-6736(97)11509-4
- Mangoni AA, Jackson SHD. Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol. 2004;57(1):6-14. doi:10.1046/j.1365-2125.2003.02007.x
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
Efficacy of Anti-Obesity Medications in Adult and Older Adult Veteran Populations
Pruritus: Diagnosing and Treating Older Adults
Chronic pruritus is a common problem among older individuals. During a session at the Dermatology Days of Paris 2024 conference dedicated to general practitioners, Juliette Delaunay, MD, a dermatologist and venereologist at Angers University Hospital Center in Angers, France, and Gabrielle Lisembard, MD, a general practitioner in the French town Grand-Fort-Philippe, discussed diagnostic approaches and key principles for the therapeutic management of pruritus.
Identifying Causes
“Pruritus in older people is most often linked to physiological changes in the skin caused by aging, leading to significant xerosis. However, before attributing it to aging, we need to rule out several causes,” Delaunay noted.
Beyond simple aging, one must consider autoimmune bullous dermatoses (bullous pemphigoid), drug-related causes, metabolic disorders (can occur at any age), cutaneous T-cell lymphomas, scabies, lice, and HIV infection.
Senile Pruritus
Aging-related xerosis can cause senile pruritus, often presenting as itching with scratch marks and dry skin. “This is a diagnosis of exclusion,” Delaunay insisted.
In older individuals with pruritus, initial examinations should include complete blood cell count (CBC), liver function tests, and thyroid-stimulating hormone levels. Syphilis serology, HIV testing, and beta-2 microglobulin levels are secondary evaluations. Renal function analysis may also be performed, and imaging may be required to investigate neoplasia.
“Annual etiological reassessment is essential if the initial evaluation is negative, as patients may later develop or report a neoplasia or hematological disorder,” Delaunay emphasized.
Paraneoplastic pruritus can occur, particularly those linked to hematological disorders (lymphomas, polycythemia, or myeloma).
Bullous Pemphigoid
Bullous pemphigoid often begins with pruritus, which can be severe and lead to insomnia. General practitioners should consider bullous pemphigoids when there is a bullous rash (tense blisters with citrine content) or an urticarial or chronic eczematous rash that does not heal spontaneously within a few days. The first-line biologic test to confirm the diagnosis is the CBC, which may reveal significant hypereosinophilia.
The diagnosis is confirmed by a skin biopsy showing a subepidermal blister with a preserved roof, unlike intraepidermal dermatoses, where the roof ruptures.
Direct immunofluorescence revealed deposits of immunoglobulin G antibodies along the dermoepidermal junction.
Approximately 40% of cases of bullous pemphigoid are associated with neurodegenerative diseases, such as stroke, parkinsonism, or dementia syndromes — occurring at a rate two to three times higher than in the general population.
It’s important to identify drugs that induce bullous pemphigoid, such as gliptins, anti-programmed cell death protein 1-programmed death-ligand 1 agents, loop diuretics (furosemide and bumetanide), anti-aldosterones (spironolactone), antiarrhythmics (amiodarone), and neuroleptics (phenothiazines).
“Stopping the medication is not mandatory if the bullous pemphigoid is well controlled by local or systemic treatments and the medication is essential. The decision to stop should be made on a case-by-case basis in consultation with the treating specialist,” Delaunay emphasized.
Treatment consists of very strong local corticosteroid therapy as the first-line treatment. If ineffective, systemic treatments based on methotrexate, oral corticosteroids, or immunomodulatory agents may be considered. Hospitalization is sometimes required.
Drug-Induced Pruritus
Drug-induced pruritus is common because older individuals often take multiple medications (antihypertensives, statins, oral hypoglycemics, psychotropic drugs, antiarrhythmics, etc.). “Sometimes, drug-induced pruritus can occur even if the medication was started several months or years ago,” Delaunay emphasized.
The lesions are generally nonspecific and scratching.
“This is a diagnosis of exclusion for other causes of pruritus. In the absence of specific lesions pointing to a dermatosis, eviction/reintroduction tests with treatments should be conducted one by one, which can be quite lengthy,” she explained.
Awareness for Scabies
Delaunay reminded attendees to consider scabies in older individuals when classic signs of pruritus flare up at night, with a rash affecting the face, scabs, or vesicles in the interdigital spaces of the hands, wrists, scrotal area, or the peri-mammary region.
“The incidence is increasing, particularly in nursing homes, where outbreaks pose a significant risk of rapid spread. Treatment involves three courses of topical and oral treatments administered on days 0, 7, and 14. All contact cases must also be treated. Sometimes, these thick lesions are stripped with 10% salicylated petroleum jelly. Environmental treatment with acaricides is essential, along with strict isolation measures,” Delaunay emphasized.
Adherent nits on the scalp or other hairy areas should raise suspicion of pediculosis.
Neurogenic and Psychogenic Origins
Neurogenic pruritus can occur during a stroke, presenting as contralateral pruritus, or in the presence of a brain tumor or following neurosurgery. Opioid-containing medications may also induce neurogenic pruritus.
The presence of unilateral painful or itchy sensations should prompt the investigation of shingles in older individuals.
Psychogenic pruritus is also common and can arise in the context of psychosis with parasitophobia or as part of anxiety-depression syndromes.
Supportive Measures
For managing pruritus, it is essential to:
- Keep nails trimmed short
- Wash with cold or lukewarm water
- Use lipid-rice soaps and syndets
- Avoid irritants, including antiseptics, cologne, no-rinse cleansers, and steroidal or nonsteroidal anti-inflammatory drugs
- Limit bathing frequency
- Avoid wearing nylon, wool, or tight clothing
- Minimize exposure to heat and excessive heating
“Alternatives to scratching, such as applying a moisturizing emollient, can be beneficial and may have a placebo effect,” explained the dermatologist. She further emphasized that local corticosteroids are effective only in the presence of inflammatory dermatosis and should not be applied to healthy skin. Similarly, antihistamines should only be prescribed if the pruritus is histamine-mediated.
Capsaicin may be useful in the treatment of localized neuropathic pruritus.
In cases of neurogenic pruritus, gabapentin and pregabalin may be prescribed, but tolerance can be problematic at this age. Other measures include acupuncture, cryotherapy, relaxation, hypnosis, psychotherapy, and music therapy. In cases of repeated therapeutic failure, patients may be treated with biotherapy (dupilumab) by a dermatologist.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Chronic pruritus is a common problem among older individuals. During a session at the Dermatology Days of Paris 2024 conference dedicated to general practitioners, Juliette Delaunay, MD, a dermatologist and venereologist at Angers University Hospital Center in Angers, France, and Gabrielle Lisembard, MD, a general practitioner in the French town Grand-Fort-Philippe, discussed diagnostic approaches and key principles for the therapeutic management of pruritus.
Identifying Causes
“Pruritus in older people is most often linked to physiological changes in the skin caused by aging, leading to significant xerosis. However, before attributing it to aging, we need to rule out several causes,” Delaunay noted.
Beyond simple aging, one must consider autoimmune bullous dermatoses (bullous pemphigoid), drug-related causes, metabolic disorders (can occur at any age), cutaneous T-cell lymphomas, scabies, lice, and HIV infection.
Senile Pruritus
Aging-related xerosis can cause senile pruritus, often presenting as itching with scratch marks and dry skin. “This is a diagnosis of exclusion,” Delaunay insisted.
In older individuals with pruritus, initial examinations should include complete blood cell count (CBC), liver function tests, and thyroid-stimulating hormone levels. Syphilis serology, HIV testing, and beta-2 microglobulin levels are secondary evaluations. Renal function analysis may also be performed, and imaging may be required to investigate neoplasia.
“Annual etiological reassessment is essential if the initial evaluation is negative, as patients may later develop or report a neoplasia or hematological disorder,” Delaunay emphasized.
Paraneoplastic pruritus can occur, particularly those linked to hematological disorders (lymphomas, polycythemia, or myeloma).
Bullous Pemphigoid
Bullous pemphigoid often begins with pruritus, which can be severe and lead to insomnia. General practitioners should consider bullous pemphigoids when there is a bullous rash (tense blisters with citrine content) or an urticarial or chronic eczematous rash that does not heal spontaneously within a few days. The first-line biologic test to confirm the diagnosis is the CBC, which may reveal significant hypereosinophilia.
The diagnosis is confirmed by a skin biopsy showing a subepidermal blister with a preserved roof, unlike intraepidermal dermatoses, where the roof ruptures.
Direct immunofluorescence revealed deposits of immunoglobulin G antibodies along the dermoepidermal junction.
Approximately 40% of cases of bullous pemphigoid are associated with neurodegenerative diseases, such as stroke, parkinsonism, or dementia syndromes — occurring at a rate two to three times higher than in the general population.
It’s important to identify drugs that induce bullous pemphigoid, such as gliptins, anti-programmed cell death protein 1-programmed death-ligand 1 agents, loop diuretics (furosemide and bumetanide), anti-aldosterones (spironolactone), antiarrhythmics (amiodarone), and neuroleptics (phenothiazines).
“Stopping the medication is not mandatory if the bullous pemphigoid is well controlled by local or systemic treatments and the medication is essential. The decision to stop should be made on a case-by-case basis in consultation with the treating specialist,” Delaunay emphasized.
Treatment consists of very strong local corticosteroid therapy as the first-line treatment. If ineffective, systemic treatments based on methotrexate, oral corticosteroids, or immunomodulatory agents may be considered. Hospitalization is sometimes required.
Drug-Induced Pruritus
Drug-induced pruritus is common because older individuals often take multiple medications (antihypertensives, statins, oral hypoglycemics, psychotropic drugs, antiarrhythmics, etc.). “Sometimes, drug-induced pruritus can occur even if the medication was started several months or years ago,” Delaunay emphasized.
The lesions are generally nonspecific and scratching.
“This is a diagnosis of exclusion for other causes of pruritus. In the absence of specific lesions pointing to a dermatosis, eviction/reintroduction tests with treatments should be conducted one by one, which can be quite lengthy,” she explained.
Awareness for Scabies
Delaunay reminded attendees to consider scabies in older individuals when classic signs of pruritus flare up at night, with a rash affecting the face, scabs, or vesicles in the interdigital spaces of the hands, wrists, scrotal area, or the peri-mammary region.
“The incidence is increasing, particularly in nursing homes, where outbreaks pose a significant risk of rapid spread. Treatment involves three courses of topical and oral treatments administered on days 0, 7, and 14. All contact cases must also be treated. Sometimes, these thick lesions are stripped with 10% salicylated petroleum jelly. Environmental treatment with acaricides is essential, along with strict isolation measures,” Delaunay emphasized.
Adherent nits on the scalp or other hairy areas should raise suspicion of pediculosis.
Neurogenic and Psychogenic Origins
Neurogenic pruritus can occur during a stroke, presenting as contralateral pruritus, or in the presence of a brain tumor or following neurosurgery. Opioid-containing medications may also induce neurogenic pruritus.
The presence of unilateral painful or itchy sensations should prompt the investigation of shingles in older individuals.
Psychogenic pruritus is also common and can arise in the context of psychosis with parasitophobia or as part of anxiety-depression syndromes.
Supportive Measures
For managing pruritus, it is essential to:
- Keep nails trimmed short
- Wash with cold or lukewarm water
- Use lipid-rice soaps and syndets
- Avoid irritants, including antiseptics, cologne, no-rinse cleansers, and steroidal or nonsteroidal anti-inflammatory drugs
- Limit bathing frequency
- Avoid wearing nylon, wool, or tight clothing
- Minimize exposure to heat and excessive heating
“Alternatives to scratching, such as applying a moisturizing emollient, can be beneficial and may have a placebo effect,” explained the dermatologist. She further emphasized that local corticosteroids are effective only in the presence of inflammatory dermatosis and should not be applied to healthy skin. Similarly, antihistamines should only be prescribed if the pruritus is histamine-mediated.
Capsaicin may be useful in the treatment of localized neuropathic pruritus.
In cases of neurogenic pruritus, gabapentin and pregabalin may be prescribed, but tolerance can be problematic at this age. Other measures include acupuncture, cryotherapy, relaxation, hypnosis, psychotherapy, and music therapy. In cases of repeated therapeutic failure, patients may be treated with biotherapy (dupilumab) by a dermatologist.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Chronic pruritus is a common problem among older individuals. During a session at the Dermatology Days of Paris 2024 conference dedicated to general practitioners, Juliette Delaunay, MD, a dermatologist and venereologist at Angers University Hospital Center in Angers, France, and Gabrielle Lisembard, MD, a general practitioner in the French town Grand-Fort-Philippe, discussed diagnostic approaches and key principles for the therapeutic management of pruritus.
Identifying Causes
“Pruritus in older people is most often linked to physiological changes in the skin caused by aging, leading to significant xerosis. However, before attributing it to aging, we need to rule out several causes,” Delaunay noted.
Beyond simple aging, one must consider autoimmune bullous dermatoses (bullous pemphigoid), drug-related causes, metabolic disorders (can occur at any age), cutaneous T-cell lymphomas, scabies, lice, and HIV infection.
Senile Pruritus
Aging-related xerosis can cause senile pruritus, often presenting as itching with scratch marks and dry skin. “This is a diagnosis of exclusion,” Delaunay insisted.
In older individuals with pruritus, initial examinations should include complete blood cell count (CBC), liver function tests, and thyroid-stimulating hormone levels. Syphilis serology, HIV testing, and beta-2 microglobulin levels are secondary evaluations. Renal function analysis may also be performed, and imaging may be required to investigate neoplasia.
“Annual etiological reassessment is essential if the initial evaluation is negative, as patients may later develop or report a neoplasia or hematological disorder,” Delaunay emphasized.
Paraneoplastic pruritus can occur, particularly those linked to hematological disorders (lymphomas, polycythemia, or myeloma).
Bullous Pemphigoid
Bullous pemphigoid often begins with pruritus, which can be severe and lead to insomnia. General practitioners should consider bullous pemphigoids when there is a bullous rash (tense blisters with citrine content) or an urticarial or chronic eczematous rash that does not heal spontaneously within a few days. The first-line biologic test to confirm the diagnosis is the CBC, which may reveal significant hypereosinophilia.
The diagnosis is confirmed by a skin biopsy showing a subepidermal blister with a preserved roof, unlike intraepidermal dermatoses, where the roof ruptures.
Direct immunofluorescence revealed deposits of immunoglobulin G antibodies along the dermoepidermal junction.
Approximately 40% of cases of bullous pemphigoid are associated with neurodegenerative diseases, such as stroke, parkinsonism, or dementia syndromes — occurring at a rate two to three times higher than in the general population.
It’s important to identify drugs that induce bullous pemphigoid, such as gliptins, anti-programmed cell death protein 1-programmed death-ligand 1 agents, loop diuretics (furosemide and bumetanide), anti-aldosterones (spironolactone), antiarrhythmics (amiodarone), and neuroleptics (phenothiazines).
“Stopping the medication is not mandatory if the bullous pemphigoid is well controlled by local or systemic treatments and the medication is essential. The decision to stop should be made on a case-by-case basis in consultation with the treating specialist,” Delaunay emphasized.
Treatment consists of very strong local corticosteroid therapy as the first-line treatment. If ineffective, systemic treatments based on methotrexate, oral corticosteroids, or immunomodulatory agents may be considered. Hospitalization is sometimes required.
Drug-Induced Pruritus
Drug-induced pruritus is common because older individuals often take multiple medications (antihypertensives, statins, oral hypoglycemics, psychotropic drugs, antiarrhythmics, etc.). “Sometimes, drug-induced pruritus can occur even if the medication was started several months or years ago,” Delaunay emphasized.
The lesions are generally nonspecific and scratching.
“This is a diagnosis of exclusion for other causes of pruritus. In the absence of specific lesions pointing to a dermatosis, eviction/reintroduction tests with treatments should be conducted one by one, which can be quite lengthy,” she explained.
Awareness for Scabies
Delaunay reminded attendees to consider scabies in older individuals when classic signs of pruritus flare up at night, with a rash affecting the face, scabs, or vesicles in the interdigital spaces of the hands, wrists, scrotal area, or the peri-mammary region.
“The incidence is increasing, particularly in nursing homes, where outbreaks pose a significant risk of rapid spread. Treatment involves three courses of topical and oral treatments administered on days 0, 7, and 14. All contact cases must also be treated. Sometimes, these thick lesions are stripped with 10% salicylated petroleum jelly. Environmental treatment with acaricides is essential, along with strict isolation measures,” Delaunay emphasized.
Adherent nits on the scalp or other hairy areas should raise suspicion of pediculosis.
Neurogenic and Psychogenic Origins
Neurogenic pruritus can occur during a stroke, presenting as contralateral pruritus, or in the presence of a brain tumor or following neurosurgery. Opioid-containing medications may also induce neurogenic pruritus.
The presence of unilateral painful or itchy sensations should prompt the investigation of shingles in older individuals.
Psychogenic pruritus is also common and can arise in the context of psychosis with parasitophobia or as part of anxiety-depression syndromes.
Supportive Measures
For managing pruritus, it is essential to:
- Keep nails trimmed short
- Wash with cold or lukewarm water
- Use lipid-rice soaps and syndets
- Avoid irritants, including antiseptics, cologne, no-rinse cleansers, and steroidal or nonsteroidal anti-inflammatory drugs
- Limit bathing frequency
- Avoid wearing nylon, wool, or tight clothing
- Minimize exposure to heat and excessive heating
“Alternatives to scratching, such as applying a moisturizing emollient, can be beneficial and may have a placebo effect,” explained the dermatologist. She further emphasized that local corticosteroids are effective only in the presence of inflammatory dermatosis and should not be applied to healthy skin. Similarly, antihistamines should only be prescribed if the pruritus is histamine-mediated.
Capsaicin may be useful in the treatment of localized neuropathic pruritus.
In cases of neurogenic pruritus, gabapentin and pregabalin may be prescribed, but tolerance can be problematic at this age. Other measures include acupuncture, cryotherapy, relaxation, hypnosis, psychotherapy, and music therapy. In cases of repeated therapeutic failure, patients may be treated with biotherapy (dupilumab) by a dermatologist.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.