User login
Current uncertainty over the best approach for preventing fatal perioperative myocardial infarction (MI) lies in our inability, despite sophisticated testing methods, to detect unstable coronary plaque prior to surgery. Unstable plaque can be present in patients with coronary lumina that appear normal on coronary angiography. Therefore, reliance on medical therapy to blunt inflammation is currently the best practice for minimizing the risk that unstable plaque poses.
Perioperative use of statins is a cornerstone of such therapy. This article briefly reviews the rationale for perioperative statin use in the setting of noncardiac surgery, presents the latest evidence on the clinical effects of perioperative statin use, and considers the potential role for statins in promoting recovery from acute kidney injury after vascular surgery.
FATAL MI: ORIGINS AND APPROACHES TO RISK REDUCTION
Fatal perioperative MI has two potential origins.1,2 One is a culprit coronary plaque that fissures and ruptures, causing a cascade of thrombogenic events (hemorrhage and thrombosis) inside the vessel wall, culminating in an MI. Less often, fatal perioperative MI results from long-lasting myocardial ischemia (a demand/supply mismatch of oxygen), typically as a consequence of a fixed coronary stenosis.
In nearly half of patients with fatal MI, coronary inflammation is a key contributor. In the perioperative setting, surgical stress induces the release of inflammatory cytokines that disrupt smooth muscle cells in the endothelium and contribute to disruption of a nonobstructing coronary plaque, predisposing to acute thrombus formation.
Risk reduction depends on pathophysiology
Strategies for minimizing the risk of perioperative MI depend on the pathophysiology involved. In the case of oxygen demand/supply mismatch as a result of flow-limiting stenosis, a beta-blocker and coronary revascularization, if possible, may be useful.
In the more common case of unstable plaque, a multifactorial strategy appears optimal, involving the following:
- Statin therapy to reduce coronary inflammation
- Aspirin to blunt the prothrombotic milieu postoperatively
- Chronic low-dose beta-blockade to decrease myocardial oxygen demand or inhibit plaque rupture.
A particular role for statins
Ridker et al found that patients with an acute coronary syndrome who experience a decline in high-sensitivity C-reactive protein (hsCRP) level after treatment with a statin have improved clinical outcomes compared with those whose hsCRP level remains high, regardless of their resultant low-density lipoprotein (LDL) cholesterol level.3
Among surgical patients, those most at risk for poor cardiovascular outcomes are those who undergo vascular surgery. In Europe, the cardiovascular death rate in such patients is approximately 2%.4
Retrospective cohort data and data from randomized clinical trials have demonstrated reductions in perioperative cardiac complications with statin use in patients undergoing various types of noncardiac vascular surgery.5–9 In light of these data, my colleagues and I recently undertook a prospective study to examine the effect of perioperative statin use on cardiovascular complications in patients undergoing vascular surgery.10 Key details and findings are surveyed in the following section.
DECREASE III: PROSPECTIVE EVIDENCE FOR ISCHEMIC BENEFIT FROM PERIOPERATIVE STATINS
The Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echo III (DECREASE III) was conducted at a single center (Erasmus Medical Center, Rotterdam, the Netherlands) in a randomized, double-blind, placebo-controlled manner.10
Patients and study design
The study population included 497 statin-naïve patients who were scheduled for one of four noncardiac vascular surgical procedures (repair or revascularization for abdominal aortic aneurysm, abdominal aortic stenosis, lower limb arterial stenosis, or carotid artery stenosis). Patients with unstable coronary artery disease or left main disease were excluded.
Patients were randomized to placebo or extended-release fluvastatin (80 mg/day) starting on the day of randomization, which was a median of 37 days before surgery. Treatment was continued until 30 days after surgery.
Extended-release fluvastatin was chosen because its long half-life permits a bridge to the early postoperative period, during which oral medications are not permitted in patients undergoing high-risk vascular surgery.
The primary end point was the occurrence of myocardial ischemia as assessed by three methods:
- Holter monitoring during the first 72 postoperative hours
- Measurement of troponin T on days 1, 3, 7, and 30
- Additional electrocardiographic recordings on days 7 and 30.
The secondary end point was a composite of cardiovascular death and nonfatal MI during the first 30 postoperative days.
Baseline characteristics were similar between the two randomized groups, with a median age approaching 66 years. About three-fourths of the patients were male, one-fourth had a history of MI, one-fourth had angina pectoris, one-fifth had diabetes mellitus, and nearly 30% had a history of cerebrovascular accident or transient ischemic attack.
All patients were being treated with a beta-blocker, about 60% with antiplatelet therapy, more than one-fourth with anticoagulant therapy, nearly half with either an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and more than one-fourth with diuretics. There were no significant differences between the groups in the proportion of patients on each of these therapies.
Results: Reductions in inflammatory markers
Baseline levels of hsCRP and interleukin-6 (IL-6) were comparable between the groups. In patients randomized to placebo, the hsCRP level increased by 3%, from a median of 5.80 mg/L at randomization to 6.00 mg/L immediately prior to surgery. In contrast, the hsCRP level in patients randomized to extended-release fluvastatin decreased by 21%, from a median of 5.93 mg/L to 4.66 mg/L. The between-group difference in the change in hsCRP level was statistically significant (P < .001). There was also a significantly greater reduction from baseline in median level of IL-6 among fluvastatin recipients compared with placebo recipients (–33% vs –4%; P < .001).
The specificity of hsCRP for cardiac inflammation is not yet known, but measures of hsCRP and IL-6 can provide a fingerprint of inflammatory activity prior to surgery. Other inflammatory and noninflammatory markers are being investigated to better identify (prior to surgery) those high-risk patients most likely to benefit from perioperative statin use.
Results: Favorable effect on clinical end points
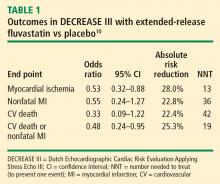
The incidence of the primary end point—myocardial ischemia 30 days after surgery—was significantly lower in the patients randomized to extended-release fluvastatin compared with placebo (10.9% vs 18.9%; P = .016), as was the incidence of the secondary end point of cardiovascular death or nonfatal MI (4.8% vs 10.1%; P = .039).
Safety: No effects on liver function or evidence of increased myopathy
We concluded that initiation of therapy with a long-acting statin should be considered in statin-naïve patients undergoing vascular surgery.
PERIOPERATIVE STATIN USE: PRACTICAL CONSIDERATIONS
Inflammation, not cholesterol, should be the target
The optimal statin choice and the target level of LDL cholesterol immediately prior to surgery remain controversial. It may be that the more potent statins induce more side effects during surgery, but any such claim is speculative since no comparative studies exist. Regardless, the purpose of perioperative statin use should be reduction of the inflammatory stress response to surgery, with the long-term goal being achievement of recommended target lipid levels.
In particular, patients with peripheral arterial disease should have a statin initiated prior to high-risk vascular surgery (if they are not already receiving one), to increase the odds of recovering renal function after surgery (see section below) and to improve long-term outcomes.
Who are the best candidates?
Patients with multiple cardiac risk factors represent an especially high-risk group that benefits the most from statin therapy prior to vascular surgery, as they are likely to have more extensive disease and more extensive inflammation in the coronary artery tree.
Given the low incidence of side effects associated with statins, initiating a statin in patients with multiple cardiac risk factors who are undergoing intermediate-risk surgery may seem appropriate, but no data from large randomized trials are available to support this practice. Caution is in order when extrapolating data from studies conducted in the high-risk vascular surgery context to other surgical settings, since statins may pose hidden side effects such as liver dysfunction and myopathy, which may be missed in patients under anesthesia.
My personal practice is to initiate a statin prior to high-risk surgery or in patients with multiple cardiac risk factors if the risk-factor profile presents a clear indication for long-term statin use. If no risk factors are present, I am more reluctant to initiate a statin because of a lack of supportive data.
Beware the rebound effect with statin withdrawal
Statin withdrawal for several days following surgery is a common practice, since statins are given orally and their pleiotropic effects are underappreciated.
A withdrawal effect leading to abrogation of clinical benefit has been observed with perioperative use of short-acting statins, whose anti-inflammatory properties do not effectively extend to the postoperative period. Acute withdrawal has been associated with an increase in markers of inflammation and oxidative stress, and an increase in cardiac events has been observed with acute withdrawal of statins during periods of instability when compared with continuation of statin therapy.11
For these reasons, a long-acting statin is preferred preoperatively in patients whose oral intake will be compromised for several days after surgery (eg, in gastric surgery). The optimal statin for preventing the withdrawal effect is unknown. We chose extended-release fluvastatin in DECREASE III because its biological effect appears to last at least 4 days12 even though analysis of serum levels of the drug indicates a shorter half-life.
ANOTHER POTENTIAL BENEFIT: ENHANCED RECOVERY OF KIDNEY FUNCTION
Postoperative renal dysfunction is an ominous sign
Renal ischemic reperfusion injury is inevitable after vascular surgery that requires aortic cross-clamping. This is significant, as renal dysfunction after surgery is an ominous long-term sign that indicates abundant atherosclerosis. Complete recovery after acute kidney injury portends an improved long-term outcome, whereas patients with persistent renal dysfunction after vascular surgery have poor long-term outcomes.
A benefit from statins?
Statins may offer an effective means of preventing or shortening the course of acute kidney injury after surgery. Statins have been reported to lengthen survival of chronic kidney disease patients with sepsis or infectious complications and to improve the course of acute kidney injury in aging rats.13–15 These findings prompted my colleagues and I to conduct a retrospective study to evaluate whether statins may ameliorate reperfusion injury in the kidney after aortic cross-clamping.16
Promising findings from an observational review
We reviewed the records of all patients who had undergone vascular surgery at Erasmus Medical Center from January 1995 to June 2006 to examine the relation between preoperative statin use and renal function after suprarenal aortic cross-clamping.16 Of the 1,944 patients who met inclusion criteria, 515 (26.5%) were statin users. Postoperative kidney injury was defined as more than a 10% reduction in creatinine clearance on postoperative day 1 or 2 compared with baseline. Recovery of kidney function was defined as a creatinine clearance of greater than 90% of the baseline value by postoperative day 3.
The clinical characteristics of the populations with and without kidney injury after aortic cross-clamping were similar, including baseline creatinine clearance and serum creatinine.
Acute kidney injury within 2 days of surgery occurred in 664 patients (34%), of which 313 (47%) had complete recovery of kidney function at postoperative day 3. Although the incidence of postoperative kidney injury was similar among statin users and nonusers, statin use was associated with an increased chance of complete recovery of kidney function at day 3 (odds ratio = 2.0; 95% CI, 1.0–3.8).
All-cause mortality was assessed during a mean follow-up of 6.24 years. Statin use was associated with improved long-term survival, regardless of any change in kidney function (hazard ratio for death = 0.60; 95% CI, 0.48–0.75). Among the four broad patient groups, survival was highest among statin users with no postoperative kidney injury, followed by statin users who had kidney injury, then by nonusers of statins with no kidney injury, and finally by nonusers of statins who had kidney injury.
We concluded that perioperative statin use was associated with clinically significant recovery from acute kidney injury after high-risk vascular surgery and, more importantly, with improved long-term survival regardless of the presence of kidney injury. These promising findings require confirmation in prospective trials.
SUMMARY
Vascular surgery carries a high risk of perioperative mortality. Perioperative use of extended-release fluvastatin is associated with a reduced incidence of myocardial ischemia and the composite of MI and cardiovascular death at 30 days following surgery. These beneficial clinical outcomes are achieved without an increase in the incidence of side effects, including liver dysfunction and myopathy. Preoperative initiation of a long-acting statin is a reasonable strategy for reducing the risks associated with vascular surgery, and offers a bridge to postoperative statin continuation to blunt the inflammatory stress of surgery. Ischemic reperfusion injury is a major cause of renal dysfunction following vascular surgery. Statin therapy appears to help restore kidney function after aortic cross-clamping in patients undergoing high-risk vascular surgery.
DISCUSSION
Question from the audience: The majority of patients randomized in DECREASE III had relatively normal cholesterol levels. Do you believe those patients are biologically different from patients with physiologic vascular disease and elevated cholesterol levels?
Dr. Poldermans: We enrolled patients with various baseline cholesterol levels, and we found that these levels were not related to postoperative outcome. It would be a good idea to examine inflammation status just prior to surgery in patients with lower cholesterol levels to see if they have different outcomes from those with high cholesterol.
Question from the audience: If a patient is already on a short-acting statin and we know that he or she won’t be able to take a statin postoperatively, should we change to a long-acting statin just prior to surgery?
Dr. Poldermans: To be honest, this is a financial issue. If you have the opportunity, the best course would be to prescribe a statin with a prolonged half-life or an extended-release formulation. Of course, it’s not always possible to prescribe one particular statin. You have to negotiate what is feasible and hope to initiate the statin as early as possible to reduce risk.
Question from the audience: In studies conducted outside the perioperative setting, such as PROVE IT (Pravastatin or Atorvastatin Evaluation and Infection Therapy) and a substudy of REVERSAL (Reversing Atherosclerosis with Aggressive Lipid Lowering), it took about 30 days after statin initiation for hsCRP levels to minimize, and at least that long for halting of plaque progression to be detected by intravascular ultrasonography. Given that, does it make sense to delay nonurgent surgery in a patient in whom you’re worried about a postoperative MI?
Dr. Poldermans: Rat studies show improved blood flow and reduced thrombosis within hours of statin initiation. In the perioperative setting, therefore, initiating a statin within 30 days may be appropriate, but nobody knows the exact timing for optimal effect. Since there are no data to answer this question, I would not postpone surgery for this reason.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- Dawood MM, Gutpa DK, Southern J, et al. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996; 57:37–44.
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–28.
- Poldermans D, Bax J, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. European Society of Cardiology Web site. http://www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-perioperative-cardiac-care-FT.pdf. Posted September 1, 2009. Accessed September 3, 2009.
- Durazzo AE, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–976.
- Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- Kertai MD, Boersma E, Westerhout CM, et al. A combination of statins and beta-blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 2004; 28:343–352.
- O’Neil-Callahan K, Katsimaglis G, Tepper MR, et al. Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS). J Am Coll Cardiol 2005; 45:336–342.
- Poldermans D, Bax JJ, Kertai MD, et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation 2003; 107:1848–1851.
- Schouten O, Boersma E, Hoeks SE, et al, for the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med 2009; 361:980–989.
- Heeschen C, Hamm CW, Laufs U, Snapinn S, Bohm M, White HD. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation 2002; 105:1446–1452.
- Schouten O, Hoeks SE, Welten GM, et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol 2007; 100:316–320.
- Gupta R, Plantinga LC, Fink NE, et al. Statin use and hospitalization for sepsis in patients with chronic kidney disease. JAMA 2007; 297:1455–1464.
- Yasuda H, Yuen PS, Hu X, et al. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int 2006; 69:1535–1542.
- Sabbatini M, Pisani A, Uccello F, et al. Atorvastatin improves the course of ischemic acute renal failure in aging rats. J Am Soc Nephrol 2004; 15:901–909.
- Welten GMJM, Chonchol M, Schouten O, et al. Statin use is associated with early recovery of kidney injury after vascular surgery and improved long-term outcome. Nephrol Dial Transplant 2008; 23:3867–3873.
Current uncertainty over the best approach for preventing fatal perioperative myocardial infarction (MI) lies in our inability, despite sophisticated testing methods, to detect unstable coronary plaque prior to surgery. Unstable plaque can be present in patients with coronary lumina that appear normal on coronary angiography. Therefore, reliance on medical therapy to blunt inflammation is currently the best practice for minimizing the risk that unstable plaque poses.
Perioperative use of statins is a cornerstone of such therapy. This article briefly reviews the rationale for perioperative statin use in the setting of noncardiac surgery, presents the latest evidence on the clinical effects of perioperative statin use, and considers the potential role for statins in promoting recovery from acute kidney injury after vascular surgery.
FATAL MI: ORIGINS AND APPROACHES TO RISK REDUCTION
Fatal perioperative MI has two potential origins.1,2 One is a culprit coronary plaque that fissures and ruptures, causing a cascade of thrombogenic events (hemorrhage and thrombosis) inside the vessel wall, culminating in an MI. Less often, fatal perioperative MI results from long-lasting myocardial ischemia (a demand/supply mismatch of oxygen), typically as a consequence of a fixed coronary stenosis.
In nearly half of patients with fatal MI, coronary inflammation is a key contributor. In the perioperative setting, surgical stress induces the release of inflammatory cytokines that disrupt smooth muscle cells in the endothelium and contribute to disruption of a nonobstructing coronary plaque, predisposing to acute thrombus formation.
Risk reduction depends on pathophysiology
Strategies for minimizing the risk of perioperative MI depend on the pathophysiology involved. In the case of oxygen demand/supply mismatch as a result of flow-limiting stenosis, a beta-blocker and coronary revascularization, if possible, may be useful.
In the more common case of unstable plaque, a multifactorial strategy appears optimal, involving the following:
- Statin therapy to reduce coronary inflammation
- Aspirin to blunt the prothrombotic milieu postoperatively
- Chronic low-dose beta-blockade to decrease myocardial oxygen demand or inhibit plaque rupture.
A particular role for statins
Ridker et al found that patients with an acute coronary syndrome who experience a decline in high-sensitivity C-reactive protein (hsCRP) level after treatment with a statin have improved clinical outcomes compared with those whose hsCRP level remains high, regardless of their resultant low-density lipoprotein (LDL) cholesterol level.3
Among surgical patients, those most at risk for poor cardiovascular outcomes are those who undergo vascular surgery. In Europe, the cardiovascular death rate in such patients is approximately 2%.4
Retrospective cohort data and data from randomized clinical trials have demonstrated reductions in perioperative cardiac complications with statin use in patients undergoing various types of noncardiac vascular surgery.5–9 In light of these data, my colleagues and I recently undertook a prospective study to examine the effect of perioperative statin use on cardiovascular complications in patients undergoing vascular surgery.10 Key details and findings are surveyed in the following section.
DECREASE III: PROSPECTIVE EVIDENCE FOR ISCHEMIC BENEFIT FROM PERIOPERATIVE STATINS
The Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echo III (DECREASE III) was conducted at a single center (Erasmus Medical Center, Rotterdam, the Netherlands) in a randomized, double-blind, placebo-controlled manner.10
Patients and study design
The study population included 497 statin-naïve patients who were scheduled for one of four noncardiac vascular surgical procedures (repair or revascularization for abdominal aortic aneurysm, abdominal aortic stenosis, lower limb arterial stenosis, or carotid artery stenosis). Patients with unstable coronary artery disease or left main disease were excluded.
Patients were randomized to placebo or extended-release fluvastatin (80 mg/day) starting on the day of randomization, which was a median of 37 days before surgery. Treatment was continued until 30 days after surgery.
Extended-release fluvastatin was chosen because its long half-life permits a bridge to the early postoperative period, during which oral medications are not permitted in patients undergoing high-risk vascular surgery.
The primary end point was the occurrence of myocardial ischemia as assessed by three methods:
- Holter monitoring during the first 72 postoperative hours
- Measurement of troponin T on days 1, 3, 7, and 30
- Additional electrocardiographic recordings on days 7 and 30.
The secondary end point was a composite of cardiovascular death and nonfatal MI during the first 30 postoperative days.
Baseline characteristics were similar between the two randomized groups, with a median age approaching 66 years. About three-fourths of the patients were male, one-fourth had a history of MI, one-fourth had angina pectoris, one-fifth had diabetes mellitus, and nearly 30% had a history of cerebrovascular accident or transient ischemic attack.
All patients were being treated with a beta-blocker, about 60% with antiplatelet therapy, more than one-fourth with anticoagulant therapy, nearly half with either an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and more than one-fourth with diuretics. There were no significant differences between the groups in the proportion of patients on each of these therapies.
Results: Reductions in inflammatory markers
Baseline levels of hsCRP and interleukin-6 (IL-6) were comparable between the groups. In patients randomized to placebo, the hsCRP level increased by 3%, from a median of 5.80 mg/L at randomization to 6.00 mg/L immediately prior to surgery. In contrast, the hsCRP level in patients randomized to extended-release fluvastatin decreased by 21%, from a median of 5.93 mg/L to 4.66 mg/L. The between-group difference in the change in hsCRP level was statistically significant (P < .001). There was also a significantly greater reduction from baseline in median level of IL-6 among fluvastatin recipients compared with placebo recipients (–33% vs –4%; P < .001).
The specificity of hsCRP for cardiac inflammation is not yet known, but measures of hsCRP and IL-6 can provide a fingerprint of inflammatory activity prior to surgery. Other inflammatory and noninflammatory markers are being investigated to better identify (prior to surgery) those high-risk patients most likely to benefit from perioperative statin use.
Results: Favorable effect on clinical end points
The incidence of the primary end point—myocardial ischemia 30 days after surgery—was significantly lower in the patients randomized to extended-release fluvastatin compared with placebo (10.9% vs 18.9%; P = .016), as was the incidence of the secondary end point of cardiovascular death or nonfatal MI (4.8% vs 10.1%; P = .039).
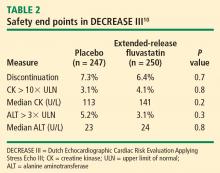
Safety: No effects on liver function or evidence of increased myopathy
We concluded that initiation of therapy with a long-acting statin should be considered in statin-naïve patients undergoing vascular surgery.
PERIOPERATIVE STATIN USE: PRACTICAL CONSIDERATIONS
Inflammation, not cholesterol, should be the target
The optimal statin choice and the target level of LDL cholesterol immediately prior to surgery remain controversial. It may be that the more potent statins induce more side effects during surgery, but any such claim is speculative since no comparative studies exist. Regardless, the purpose of perioperative statin use should be reduction of the inflammatory stress response to surgery, with the long-term goal being achievement of recommended target lipid levels.
In particular, patients with peripheral arterial disease should have a statin initiated prior to high-risk vascular surgery (if they are not already receiving one), to increase the odds of recovering renal function after surgery (see section below) and to improve long-term outcomes.
Who are the best candidates?
Patients with multiple cardiac risk factors represent an especially high-risk group that benefits the most from statin therapy prior to vascular surgery, as they are likely to have more extensive disease and more extensive inflammation in the coronary artery tree.
Given the low incidence of side effects associated with statins, initiating a statin in patients with multiple cardiac risk factors who are undergoing intermediate-risk surgery may seem appropriate, but no data from large randomized trials are available to support this practice. Caution is in order when extrapolating data from studies conducted in the high-risk vascular surgery context to other surgical settings, since statins may pose hidden side effects such as liver dysfunction and myopathy, which may be missed in patients under anesthesia.
My personal practice is to initiate a statin prior to high-risk surgery or in patients with multiple cardiac risk factors if the risk-factor profile presents a clear indication for long-term statin use. If no risk factors are present, I am more reluctant to initiate a statin because of a lack of supportive data.
Beware the rebound effect with statin withdrawal
Statin withdrawal for several days following surgery is a common practice, since statins are given orally and their pleiotropic effects are underappreciated.
A withdrawal effect leading to abrogation of clinical benefit has been observed with perioperative use of short-acting statins, whose anti-inflammatory properties do not effectively extend to the postoperative period. Acute withdrawal has been associated with an increase in markers of inflammation and oxidative stress, and an increase in cardiac events has been observed with acute withdrawal of statins during periods of instability when compared with continuation of statin therapy.11
For these reasons, a long-acting statin is preferred preoperatively in patients whose oral intake will be compromised for several days after surgery (eg, in gastric surgery). The optimal statin for preventing the withdrawal effect is unknown. We chose extended-release fluvastatin in DECREASE III because its biological effect appears to last at least 4 days12 even though analysis of serum levels of the drug indicates a shorter half-life.
ANOTHER POTENTIAL BENEFIT: ENHANCED RECOVERY OF KIDNEY FUNCTION
Postoperative renal dysfunction is an ominous sign
Renal ischemic reperfusion injury is inevitable after vascular surgery that requires aortic cross-clamping. This is significant, as renal dysfunction after surgery is an ominous long-term sign that indicates abundant atherosclerosis. Complete recovery after acute kidney injury portends an improved long-term outcome, whereas patients with persistent renal dysfunction after vascular surgery have poor long-term outcomes.
A benefit from statins?
Statins may offer an effective means of preventing or shortening the course of acute kidney injury after surgery. Statins have been reported to lengthen survival of chronic kidney disease patients with sepsis or infectious complications and to improve the course of acute kidney injury in aging rats.13–15 These findings prompted my colleagues and I to conduct a retrospective study to evaluate whether statins may ameliorate reperfusion injury in the kidney after aortic cross-clamping.16
Promising findings from an observational review
We reviewed the records of all patients who had undergone vascular surgery at Erasmus Medical Center from January 1995 to June 2006 to examine the relation between preoperative statin use and renal function after suprarenal aortic cross-clamping.16 Of the 1,944 patients who met inclusion criteria, 515 (26.5%) were statin users. Postoperative kidney injury was defined as more than a 10% reduction in creatinine clearance on postoperative day 1 or 2 compared with baseline. Recovery of kidney function was defined as a creatinine clearance of greater than 90% of the baseline value by postoperative day 3.
The clinical characteristics of the populations with and without kidney injury after aortic cross-clamping were similar, including baseline creatinine clearance and serum creatinine.
Acute kidney injury within 2 days of surgery occurred in 664 patients (34%), of which 313 (47%) had complete recovery of kidney function at postoperative day 3. Although the incidence of postoperative kidney injury was similar among statin users and nonusers, statin use was associated with an increased chance of complete recovery of kidney function at day 3 (odds ratio = 2.0; 95% CI, 1.0–3.8).
All-cause mortality was assessed during a mean follow-up of 6.24 years. Statin use was associated with improved long-term survival, regardless of any change in kidney function (hazard ratio for death = 0.60; 95% CI, 0.48–0.75). Among the four broad patient groups, survival was highest among statin users with no postoperative kidney injury, followed by statin users who had kidney injury, then by nonusers of statins with no kidney injury, and finally by nonusers of statins who had kidney injury.
We concluded that perioperative statin use was associated with clinically significant recovery from acute kidney injury after high-risk vascular surgery and, more importantly, with improved long-term survival regardless of the presence of kidney injury. These promising findings require confirmation in prospective trials.
SUMMARY
Vascular surgery carries a high risk of perioperative mortality. Perioperative use of extended-release fluvastatin is associated with a reduced incidence of myocardial ischemia and the composite of MI and cardiovascular death at 30 days following surgery. These beneficial clinical outcomes are achieved without an increase in the incidence of side effects, including liver dysfunction and myopathy. Preoperative initiation of a long-acting statin is a reasonable strategy for reducing the risks associated with vascular surgery, and offers a bridge to postoperative statin continuation to blunt the inflammatory stress of surgery. Ischemic reperfusion injury is a major cause of renal dysfunction following vascular surgery. Statin therapy appears to help restore kidney function after aortic cross-clamping in patients undergoing high-risk vascular surgery.
DISCUSSION
Question from the audience: The majority of patients randomized in DECREASE III had relatively normal cholesterol levels. Do you believe those patients are biologically different from patients with physiologic vascular disease and elevated cholesterol levels?
Dr. Poldermans: We enrolled patients with various baseline cholesterol levels, and we found that these levels were not related to postoperative outcome. It would be a good idea to examine inflammation status just prior to surgery in patients with lower cholesterol levels to see if they have different outcomes from those with high cholesterol.
Question from the audience: If a patient is already on a short-acting statin and we know that he or she won’t be able to take a statin postoperatively, should we change to a long-acting statin just prior to surgery?
Dr. Poldermans: To be honest, this is a financial issue. If you have the opportunity, the best course would be to prescribe a statin with a prolonged half-life or an extended-release formulation. Of course, it’s not always possible to prescribe one particular statin. You have to negotiate what is feasible and hope to initiate the statin as early as possible to reduce risk.
Question from the audience: In studies conducted outside the perioperative setting, such as PROVE IT (Pravastatin or Atorvastatin Evaluation and Infection Therapy) and a substudy of REVERSAL (Reversing Atherosclerosis with Aggressive Lipid Lowering), it took about 30 days after statin initiation for hsCRP levels to minimize, and at least that long for halting of plaque progression to be detected by intravascular ultrasonography. Given that, does it make sense to delay nonurgent surgery in a patient in whom you’re worried about a postoperative MI?
Dr. Poldermans: Rat studies show improved blood flow and reduced thrombosis within hours of statin initiation. In the perioperative setting, therefore, initiating a statin within 30 days may be appropriate, but nobody knows the exact timing for optimal effect. Since there are no data to answer this question, I would not postpone surgery for this reason.
Current uncertainty over the best approach for preventing fatal perioperative myocardial infarction (MI) lies in our inability, despite sophisticated testing methods, to detect unstable coronary plaque prior to surgery. Unstable plaque can be present in patients with coronary lumina that appear normal on coronary angiography. Therefore, reliance on medical therapy to blunt inflammation is currently the best practice for minimizing the risk that unstable plaque poses.
Perioperative use of statins is a cornerstone of such therapy. This article briefly reviews the rationale for perioperative statin use in the setting of noncardiac surgery, presents the latest evidence on the clinical effects of perioperative statin use, and considers the potential role for statins in promoting recovery from acute kidney injury after vascular surgery.
FATAL MI: ORIGINS AND APPROACHES TO RISK REDUCTION
Fatal perioperative MI has two potential origins.1,2 One is a culprit coronary plaque that fissures and ruptures, causing a cascade of thrombogenic events (hemorrhage and thrombosis) inside the vessel wall, culminating in an MI. Less often, fatal perioperative MI results from long-lasting myocardial ischemia (a demand/supply mismatch of oxygen), typically as a consequence of a fixed coronary stenosis.
In nearly half of patients with fatal MI, coronary inflammation is a key contributor. In the perioperative setting, surgical stress induces the release of inflammatory cytokines that disrupt smooth muscle cells in the endothelium and contribute to disruption of a nonobstructing coronary plaque, predisposing to acute thrombus formation.
Risk reduction depends on pathophysiology
Strategies for minimizing the risk of perioperative MI depend on the pathophysiology involved. In the case of oxygen demand/supply mismatch as a result of flow-limiting stenosis, a beta-blocker and coronary revascularization, if possible, may be useful.
In the more common case of unstable plaque, a multifactorial strategy appears optimal, involving the following:
- Statin therapy to reduce coronary inflammation
- Aspirin to blunt the prothrombotic milieu postoperatively
- Chronic low-dose beta-blockade to decrease myocardial oxygen demand or inhibit plaque rupture.
A particular role for statins
Ridker et al found that patients with an acute coronary syndrome who experience a decline in high-sensitivity C-reactive protein (hsCRP) level after treatment with a statin have improved clinical outcomes compared with those whose hsCRP level remains high, regardless of their resultant low-density lipoprotein (LDL) cholesterol level.3
Among surgical patients, those most at risk for poor cardiovascular outcomes are those who undergo vascular surgery. In Europe, the cardiovascular death rate in such patients is approximately 2%.4
Retrospective cohort data and data from randomized clinical trials have demonstrated reductions in perioperative cardiac complications with statin use in patients undergoing various types of noncardiac vascular surgery.5–9 In light of these data, my colleagues and I recently undertook a prospective study to examine the effect of perioperative statin use on cardiovascular complications in patients undergoing vascular surgery.10 Key details and findings are surveyed in the following section.
DECREASE III: PROSPECTIVE EVIDENCE FOR ISCHEMIC BENEFIT FROM PERIOPERATIVE STATINS
The Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echo III (DECREASE III) was conducted at a single center (Erasmus Medical Center, Rotterdam, the Netherlands) in a randomized, double-blind, placebo-controlled manner.10
Patients and study design
The study population included 497 statin-naïve patients who were scheduled for one of four noncardiac vascular surgical procedures (repair or revascularization for abdominal aortic aneurysm, abdominal aortic stenosis, lower limb arterial stenosis, or carotid artery stenosis). Patients with unstable coronary artery disease or left main disease were excluded.
Patients were randomized to placebo or extended-release fluvastatin (80 mg/day) starting on the day of randomization, which was a median of 37 days before surgery. Treatment was continued until 30 days after surgery.
Extended-release fluvastatin was chosen because its long half-life permits a bridge to the early postoperative period, during which oral medications are not permitted in patients undergoing high-risk vascular surgery.
The primary end point was the occurrence of myocardial ischemia as assessed by three methods:
- Holter monitoring during the first 72 postoperative hours
- Measurement of troponin T on days 1, 3, 7, and 30
- Additional electrocardiographic recordings on days 7 and 30.
The secondary end point was a composite of cardiovascular death and nonfatal MI during the first 30 postoperative days.
Baseline characteristics were similar between the two randomized groups, with a median age approaching 66 years. About three-fourths of the patients were male, one-fourth had a history of MI, one-fourth had angina pectoris, one-fifth had diabetes mellitus, and nearly 30% had a history of cerebrovascular accident or transient ischemic attack.
All patients were being treated with a beta-blocker, about 60% with antiplatelet therapy, more than one-fourth with anticoagulant therapy, nearly half with either an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and more than one-fourth with diuretics. There were no significant differences between the groups in the proportion of patients on each of these therapies.
Results: Reductions in inflammatory markers
Baseline levels of hsCRP and interleukin-6 (IL-6) were comparable between the groups. In patients randomized to placebo, the hsCRP level increased by 3%, from a median of 5.80 mg/L at randomization to 6.00 mg/L immediately prior to surgery. In contrast, the hsCRP level in patients randomized to extended-release fluvastatin decreased by 21%, from a median of 5.93 mg/L to 4.66 mg/L. The between-group difference in the change in hsCRP level was statistically significant (P < .001). There was also a significantly greater reduction from baseline in median level of IL-6 among fluvastatin recipients compared with placebo recipients (–33% vs –4%; P < .001).
The specificity of hsCRP for cardiac inflammation is not yet known, but measures of hsCRP and IL-6 can provide a fingerprint of inflammatory activity prior to surgery. Other inflammatory and noninflammatory markers are being investigated to better identify (prior to surgery) those high-risk patients most likely to benefit from perioperative statin use.
Results: Favorable effect on clinical end points
The incidence of the primary end point—myocardial ischemia 30 days after surgery—was significantly lower in the patients randomized to extended-release fluvastatin compared with placebo (10.9% vs 18.9%; P = .016), as was the incidence of the secondary end point of cardiovascular death or nonfatal MI (4.8% vs 10.1%; P = .039).
Safety: No effects on liver function or evidence of increased myopathy
We concluded that initiation of therapy with a long-acting statin should be considered in statin-naïve patients undergoing vascular surgery.
PERIOPERATIVE STATIN USE: PRACTICAL CONSIDERATIONS
Inflammation, not cholesterol, should be the target
The optimal statin choice and the target level of LDL cholesterol immediately prior to surgery remain controversial. It may be that the more potent statins induce more side effects during surgery, but any such claim is speculative since no comparative studies exist. Regardless, the purpose of perioperative statin use should be reduction of the inflammatory stress response to surgery, with the long-term goal being achievement of recommended target lipid levels.
In particular, patients with peripheral arterial disease should have a statin initiated prior to high-risk vascular surgery (if they are not already receiving one), to increase the odds of recovering renal function after surgery (see section below) and to improve long-term outcomes.
Who are the best candidates?
Patients with multiple cardiac risk factors represent an especially high-risk group that benefits the most from statin therapy prior to vascular surgery, as they are likely to have more extensive disease and more extensive inflammation in the coronary artery tree.
Given the low incidence of side effects associated with statins, initiating a statin in patients with multiple cardiac risk factors who are undergoing intermediate-risk surgery may seem appropriate, but no data from large randomized trials are available to support this practice. Caution is in order when extrapolating data from studies conducted in the high-risk vascular surgery context to other surgical settings, since statins may pose hidden side effects such as liver dysfunction and myopathy, which may be missed in patients under anesthesia.
My personal practice is to initiate a statin prior to high-risk surgery or in patients with multiple cardiac risk factors if the risk-factor profile presents a clear indication for long-term statin use. If no risk factors are present, I am more reluctant to initiate a statin because of a lack of supportive data.
Beware the rebound effect with statin withdrawal
Statin withdrawal for several days following surgery is a common practice, since statins are given orally and their pleiotropic effects are underappreciated.
A withdrawal effect leading to abrogation of clinical benefit has been observed with perioperative use of short-acting statins, whose anti-inflammatory properties do not effectively extend to the postoperative period. Acute withdrawal has been associated with an increase in markers of inflammation and oxidative stress, and an increase in cardiac events has been observed with acute withdrawal of statins during periods of instability when compared with continuation of statin therapy.11
For these reasons, a long-acting statin is preferred preoperatively in patients whose oral intake will be compromised for several days after surgery (eg, in gastric surgery). The optimal statin for preventing the withdrawal effect is unknown. We chose extended-release fluvastatin in DECREASE III because its biological effect appears to last at least 4 days12 even though analysis of serum levels of the drug indicates a shorter half-life.
ANOTHER POTENTIAL BENEFIT: ENHANCED RECOVERY OF KIDNEY FUNCTION
Postoperative renal dysfunction is an ominous sign
Renal ischemic reperfusion injury is inevitable after vascular surgery that requires aortic cross-clamping. This is significant, as renal dysfunction after surgery is an ominous long-term sign that indicates abundant atherosclerosis. Complete recovery after acute kidney injury portends an improved long-term outcome, whereas patients with persistent renal dysfunction after vascular surgery have poor long-term outcomes.
A benefit from statins?
Statins may offer an effective means of preventing or shortening the course of acute kidney injury after surgery. Statins have been reported to lengthen survival of chronic kidney disease patients with sepsis or infectious complications and to improve the course of acute kidney injury in aging rats.13–15 These findings prompted my colleagues and I to conduct a retrospective study to evaluate whether statins may ameliorate reperfusion injury in the kidney after aortic cross-clamping.16
Promising findings from an observational review
We reviewed the records of all patients who had undergone vascular surgery at Erasmus Medical Center from January 1995 to June 2006 to examine the relation between preoperative statin use and renal function after suprarenal aortic cross-clamping.16 Of the 1,944 patients who met inclusion criteria, 515 (26.5%) were statin users. Postoperative kidney injury was defined as more than a 10% reduction in creatinine clearance on postoperative day 1 or 2 compared with baseline. Recovery of kidney function was defined as a creatinine clearance of greater than 90% of the baseline value by postoperative day 3.
The clinical characteristics of the populations with and without kidney injury after aortic cross-clamping were similar, including baseline creatinine clearance and serum creatinine.
Acute kidney injury within 2 days of surgery occurred in 664 patients (34%), of which 313 (47%) had complete recovery of kidney function at postoperative day 3. Although the incidence of postoperative kidney injury was similar among statin users and nonusers, statin use was associated with an increased chance of complete recovery of kidney function at day 3 (odds ratio = 2.0; 95% CI, 1.0–3.8).
All-cause mortality was assessed during a mean follow-up of 6.24 years. Statin use was associated with improved long-term survival, regardless of any change in kidney function (hazard ratio for death = 0.60; 95% CI, 0.48–0.75). Among the four broad patient groups, survival was highest among statin users with no postoperative kidney injury, followed by statin users who had kidney injury, then by nonusers of statins with no kidney injury, and finally by nonusers of statins who had kidney injury.
We concluded that perioperative statin use was associated with clinically significant recovery from acute kidney injury after high-risk vascular surgery and, more importantly, with improved long-term survival regardless of the presence of kidney injury. These promising findings require confirmation in prospective trials.
SUMMARY
Vascular surgery carries a high risk of perioperative mortality. Perioperative use of extended-release fluvastatin is associated with a reduced incidence of myocardial ischemia and the composite of MI and cardiovascular death at 30 days following surgery. These beneficial clinical outcomes are achieved without an increase in the incidence of side effects, including liver dysfunction and myopathy. Preoperative initiation of a long-acting statin is a reasonable strategy for reducing the risks associated with vascular surgery, and offers a bridge to postoperative statin continuation to blunt the inflammatory stress of surgery. Ischemic reperfusion injury is a major cause of renal dysfunction following vascular surgery. Statin therapy appears to help restore kidney function after aortic cross-clamping in patients undergoing high-risk vascular surgery.
DISCUSSION
Question from the audience: The majority of patients randomized in DECREASE III had relatively normal cholesterol levels. Do you believe those patients are biologically different from patients with physiologic vascular disease and elevated cholesterol levels?
Dr. Poldermans: We enrolled patients with various baseline cholesterol levels, and we found that these levels were not related to postoperative outcome. It would be a good idea to examine inflammation status just prior to surgery in patients with lower cholesterol levels to see if they have different outcomes from those with high cholesterol.
Question from the audience: If a patient is already on a short-acting statin and we know that he or she won’t be able to take a statin postoperatively, should we change to a long-acting statin just prior to surgery?
Dr. Poldermans: To be honest, this is a financial issue. If you have the opportunity, the best course would be to prescribe a statin with a prolonged half-life or an extended-release formulation. Of course, it’s not always possible to prescribe one particular statin. You have to negotiate what is feasible and hope to initiate the statin as early as possible to reduce risk.
Question from the audience: In studies conducted outside the perioperative setting, such as PROVE IT (Pravastatin or Atorvastatin Evaluation and Infection Therapy) and a substudy of REVERSAL (Reversing Atherosclerosis with Aggressive Lipid Lowering), it took about 30 days after statin initiation for hsCRP levels to minimize, and at least that long for halting of plaque progression to be detected by intravascular ultrasonography. Given that, does it make sense to delay nonurgent surgery in a patient in whom you’re worried about a postoperative MI?
Dr. Poldermans: Rat studies show improved blood flow and reduced thrombosis within hours of statin initiation. In the perioperative setting, therefore, initiating a statin within 30 days may be appropriate, but nobody knows the exact timing for optimal effect. Since there are no data to answer this question, I would not postpone surgery for this reason.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- Dawood MM, Gutpa DK, Southern J, et al. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996; 57:37–44.
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–28.
- Poldermans D, Bax J, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. European Society of Cardiology Web site. http://www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-perioperative-cardiac-care-FT.pdf. Posted September 1, 2009. Accessed September 3, 2009.
- Durazzo AE, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–976.
- Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- Kertai MD, Boersma E, Westerhout CM, et al. A combination of statins and beta-blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 2004; 28:343–352.
- O’Neil-Callahan K, Katsimaglis G, Tepper MR, et al. Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS). J Am Coll Cardiol 2005; 45:336–342.
- Poldermans D, Bax JJ, Kertai MD, et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation 2003; 107:1848–1851.
- Schouten O, Boersma E, Hoeks SE, et al, for the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med 2009; 361:980–989.
- Heeschen C, Hamm CW, Laufs U, Snapinn S, Bohm M, White HD. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation 2002; 105:1446–1452.
- Schouten O, Hoeks SE, Welten GM, et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol 2007; 100:316–320.
- Gupta R, Plantinga LC, Fink NE, et al. Statin use and hospitalization for sepsis in patients with chronic kidney disease. JAMA 2007; 297:1455–1464.
- Yasuda H, Yuen PS, Hu X, et al. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int 2006; 69:1535–1542.
- Sabbatini M, Pisani A, Uccello F, et al. Atorvastatin improves the course of ischemic acute renal failure in aging rats. J Am Soc Nephrol 2004; 15:901–909.
- Welten GMJM, Chonchol M, Schouten O, et al. Statin use is associated with early recovery of kidney injury after vascular surgery and improved long-term outcome. Nephrol Dial Transplant 2008; 23:3867–3873.
- Cohen MC, Aretz TH. Histological analysis of coronary artery lesions in fatal postoperative myocardial infarction. Cardiovasc Pathol 1999; 8:133–139.
- Dawood MM, Gutpa DK, Southern J, et al. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996; 57:37–44.
- Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–28.
- Poldermans D, Bax J, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. European Society of Cardiology Web site. http://www.escardio.org/guidelines-surveys/esc-guidelines/GuidelinesDocuments/guidelines-perioperative-cardiac-care-FT.pdf. Posted September 1, 2009. Accessed September 3, 2009.
- Durazzo AE, Machado FS, Ikeoka DT, et al. Reduction in cardiovascular events after vascular surgery with atorvastatin: a randomized trial. J Vasc Surg 2004; 39:967–976.
- Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA 2004; 291:2092–2099.
- Kertai MD, Boersma E, Westerhout CM, et al. A combination of statins and beta-blockers is independently associated with a reduction in the incidence of perioperative mortality and nonfatal myocardial infarction in patients undergoing abdominal aortic aneurysm surgery. Eur J Vasc Endovasc Surg 2004; 28:343–352.
- O’Neil-Callahan K, Katsimaglis G, Tepper MR, et al. Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: the Statins for Risk Reduction in Surgery (StaRRS). J Am Coll Cardiol 2005; 45:336–342.
- Poldermans D, Bax JJ, Kertai MD, et al. Statins are associated with a reduced incidence of perioperative mortality in patients undergoing major noncardiac vascular surgery. Circulation 2003; 107:1848–1851.
- Schouten O, Boersma E, Hoeks SE, et al, for the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med 2009; 361:980–989.
- Heeschen C, Hamm CW, Laufs U, Snapinn S, Bohm M, White HD. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation 2002; 105:1446–1452.
- Schouten O, Hoeks SE, Welten GM, et al. Effect of statin withdrawal on frequency of cardiac events after vascular surgery. Am J Cardiol 2007; 100:316–320.
- Gupta R, Plantinga LC, Fink NE, et al. Statin use and hospitalization for sepsis in patients with chronic kidney disease. JAMA 2007; 297:1455–1464.
- Yasuda H, Yuen PS, Hu X, et al. Simvastatin improves sepsis-induced mortality and acute kidney injury via renal vascular effects. Kidney Int 2006; 69:1535–1542.
- Sabbatini M, Pisani A, Uccello F, et al. Atorvastatin improves the course of ischemic acute renal failure in aging rats. J Am Soc Nephrol 2004; 15:901–909.
- Welten GMJM, Chonchol M, Schouten O, et al. Statin use is associated with early recovery of kidney injury after vascular surgery and improved long-term outcome. Nephrol Dial Transplant 2008; 23:3867–3873.
KEY POINTS
- The inflammatory and oxidative stress induced by vascular surgery can be blunted by statin therapy.
- Statin therapy started preoperatively can reduce the incidence of myocardial ischemia and the level of inflammatory markers in patients undergoing high-risk vascular surgery.
- The purpose of perioperative statin use should be reduction of the inflammatory stress response to surgery, with the long-term goal being achievement of target lipid levels.
- A long-acting statin is preferred preoperatively to best extend the anti-inflammatory effects into the postoperative period. Statin therapy should be continued postoperatively, if possible, to avoid deleterious acute withdrawal effects.