User login
• Initiate antiviral treatment as soon as possible; rapid resolution of acute pain and reduction in the development of postherpetic neuralgia (PHN) are most likely when therapy is started within 72 hours of the outbreak. A
• Discuss herpes zoster (HZ) vaccination with healthy patients 60 years of age and older during their first office visit; the vaccine markedly reduces the incidence of HZ and PHN. A
• Do not prescribe tricyclic antidepressants or corticosteroids in the acute phase of HZ. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
We don’t do enough to protect our patients from the pain of herpes zoster (HZ). Consider:
- Each year in the United States, about 1 million new cases of herpes zoster (HZ) occur.1 The incidence is estimated at 3 to 4 per 1,000 in the general population,2 but climbs to more than 10 per 1000 among people 60 years of age and older.3,4
- Overall, between 13% and 26% of patients with HZ develop postherpetic neuralgia (PHN), defined as pain that continues for more than 1 month after the rash has healed. Among patients who are 70 or older, however, the likelihood that HZ will progress to PHN is approximately 50%.5 (In a study of 7595 patients being treated for HZ or PHN by general practitioners or dermatologists in France, 45% reported pain that was severe or very severe, and 42% reported permanent pain.6 )
- Between 10% and 25% of HZ patients develop ocular complications, which have the potential to result in vision loss, facial scarring, or prolonged or permanent pain.7 Encephalitis, myelitis, and peripheral nerve palsies are potential complications, as well.
Yet HZ and its complications are largely preventable.
A live attenuated vaccine (Zostavax) received US Food and Drug Administration approval in 2006.8 But many patients have not yet heard of it, and many physicians fail to recommend it. (See “Herpes zoster vaccine: Why aren’t more people receiving it?”.)
As a family physician, you can play a key role in reducing the burden of shingles by rapidly identifying and treating HZ, minimizing the risk of prolonged pain, and, notably, by talking to older patients about the benefits of vaccination.
Zostavax, a live attenuated herpes zoster (HZ) vaccine, was licensed by the US Food and Drug Administration in 2006 for use in people 60 years of age and older—the first new vaccine targeting this age group in years. In 2007, researchers at the Centers for Disease Control and Prevention (CDC) conducted a national survey,20 in part to gauge the knowledge of, and interest in, the HZ vaccine among the intended recipients.
Their findings: Of more than 3500 respondents, only 1.9% had received the HZ vaccine. Most (72%) were unaware of the vaccine’s existence, but the majority said they would agree to vaccination if their physician were to recommend it.
Among those who were aware of it, the key reasons for rejecting the vaccine were that it was not needed (cited by 35%), they were not at risk (13%), and a lack of trust in doctors or the US health care system (10%). Both the limited awareness of the vaccine and the lack of physician recommendations are barriers to HZ vaccination, the researchers concluded.20
On its Web site, the CDC broaches another potential barrier to greater use of the HZ vaccine: cost. The vaccine is not covered by Medicare Part B, nor by some private insurers. While it is covered by all Medicare Part D plans, the extent of coverage depends on the particular plan. The CDC recommends that physicians encourage patients to contact their insurers to determine the extent of their coverage.21
Start antiviral therapy without delay
Several meta-analyses and many (though not all) randomized controlled trials (RCTs) of HZ treatment have demonstrated that prompt antiviral therapy—with oral acyclovir (ACV), valacyclovir (VCV), or famciclovir (FCV)—reduces the duration of acute pain and the likelihood that PHN will develop.9,10 Without antiviral therapy, up to 45% of patients over the age of 60 experience pain that persists for 6 months to a year. Even with therapy, studies have found that about 20% of patients older than 50 years continued to have pain for 6 months after their rash appeared.10 Risk factors for PHN include age (>50 years), sex (female), a disseminated rash, a severe pain presentation, and polymerase chain reaction-detectable varicella zoster virus viremia.11
Which agent? What the research reveals
For most people with HZ, any of the 3 antiviral agents can be used, based on physician and patient preference. (See TABLE for treatment guidelines.) Here’s a look at the evidence for each.
Oral ACV has long been the mainstay of treatment for HZ, but its poor bioavailability and the need for 5 daily doses has led to the development of newer antiviral agents.12 When initiated within 48 to 72 hours of the onset of the rash, ACV has demonstrated clinical benefit. (The value of starting ACV therapy beyond the 72-hour mark has not been established, though treatment should be considered if new lesions are still appearing.)
In 1 meta-analysis of 4 placebo-controlled trials, ACV accelerated resolution of acute pain, with the greatest effect in those older than 50 years.13 In a second meta-analysis, treatment with ACV reduced the incidence of PHN at 3 months by 46% (number needed to treat [NNT]=3.2-8).12
VCV is well absorbed in the gastrointestinal tract, providing 3- to 5-fold greater bioavailability compared with ACV.13 VCV’s efficacy was demonstrated in an RCT in which the researchers conducted an intent-to-treat analysis: Compared with ACV therapy for 7 to 14 days, VCV significantly accelerated the resolution of acute pain, reduced the duration of PHN, and decreased the proportion of patients with pain persisting for more than 6 months (19% vs 26%). The incidence of adverse events was similar in both groups.14
FCV has broad activity against varicella-zoster virus.15 In an RCT that evaluated oral FCV in 419 immunocompetent adults (mean age 50 years) with uncomplicated HZ, FCV was well tolerated and accelerated lesion healing compared with placebo. Among those who developed PHN, the pain resolved twice as fast for patients in the FCV group compared with the controls, and the median duration of PHN was reduced by 2 months.15
TABLE
Antiviral therapy dosing guidelines*
| Dosage adjusted for creatinine clearance† (mL/min) | ||||
|---|---|---|---|---|
| Drug | Standard dose | <10 | 10-25 | Duration |
| ACV | 800 mg 5x/d | 1600 mg/d | 2400 mg/d | 7-10 days |
| VCV | 1000 mg tid | 1000 mg/d | 2000 mg/d | 7 days |
| FCV | 750 mg/d or 250 mg tid | 250 mg/d | 500 mg/d | 7 days |
| *All 3 drugs reduce acute pain and development of postherpetic neuralgia, and are most effective when started within 72 hours of onset of rash. | ||||
| †Patients with creatinine clearance >25 mL/min receive the standard dose. | ||||
| ACV, acyclovir; FCV, famciclovir; VCV, valacyclovir. | ||||
Analgesics and other drugs: What to consider
While antiviral therapy helps to relieve the pain of HZ, several trials have shown that none of the available agents completely alleviates it or routinely prevents the development of PHN. As a result, adjunctive therapy, including pain medication, is often required. But prescribing analgesics to frail elderly patients and those who have comorbidities and take multiple medications is not without risk.
The ability of a tricyclic antidepressant to alleviate pain and prevent PHN when therapy is initiated within 48 hours of the eruption of lesions was tested in a double-blind trial in which 72 patients 60 years of age or older were randomized to amitriptyline 25 mg daily for 90 days or placebo.16 Antiviral agents were administered according to the preference of the primary physician. At 6 weeks, the pain prevalence—the primary outcome measure—was reduced by about 50% in the amitriptyline group.16 There is no other evidence to support the use of tricyclics in the acute phase of HZ, however, and concerns about orthostatic hypotension and anticholinergic side effects limit their use, particularly in older patients.
Corticosteroids are sometimes used, too, often in conjunction with antiviral therapy, but there are problems with this approach, as well. One RCT comparing an ACV-prednisone combination with ACV alone in HZ patients over the age of 50 found that patients who received both drugs had faster resolution of acute pain and earlier discontinuation of analgesics.17 But several serious adverse effects of prednisone were reported in patients in the combination therapy group, despite the fact that individuals with contraindications to corticosteroids were excluded from the study. Overall, there is little evidence to suggest that steroids can be safely used to reduce the incidence or severity of PHN. There is no specific recommendation regarding analgesic therapy for PHN, but physicians often adopt a stepwise approach.
Recommend the shingles vaccine
In view of the toll that shingles often takes, vaccination is the best way to prevent HZ and its complications. In a randomized, double-blind, placebo-controlled study involving 38,546 adults who were 60 years of age or older, researchers demonstrated that cell-mediated immunity to the varicella-zoster virus was boosted by the live attenuated HZ vaccine.18 The enhanced immunity was associated with a 51% reduction in the incidence of HZ (NNT=58 to prevent 1 case over 3 years), a 66% reduction in the incidence of PHN (NNT=364 to prevent 1 case of PHN over 3 years), and a 61% reduction in disease burden. The vaccine was well tolerated, and injection site reactions were generally mild.18 Accurate cost-effectiveness analyses of immunization are not available because the duration of vaccine protection is unknown.19
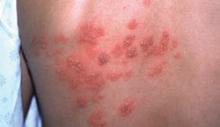
FIGURE
A unilateral vesicular rash
A shingles outbreak, like the rash shown on this patient’s back, usually appears as a patch or band of blisters on 1 side of the body.
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination to prevent both HZ and PHN in healthy adults who are 60 years of age and older (individuals with primary or acquired immunodeficiencies or patients on immunosuppressive therapies should not be vaccinated), and suggests that practitioners offer the HZ vaccine to appropriate patients at their first visit.7 It is not necessary, however, to ask about the patient’s history of varicella or to conduct serologic testing to determine varicella immunity before administering the HZ vaccine.7 A history of shingles is not a contraindication, so advise patients who develop HZ to come in for vaccination soon after the rash and pain resolve.
CORRESPONDENCE
Pierre-Olivier Lang, MD, MPH, PhD, University Hospitals of Geneva, Department of Rehabilitation and Geriatrics, Chemin du Pont-Bochet, 3, CH-1226, Thonex-Geneva, Switzerland; [email protected]
1. Centers for Disease Control and Prevention. Shingles disease – questions and answers (herpes zoster). Available at http://www.cdc.gov/vaccines/vpd-vac/shingles/dis-faqs.htm. Accessed September 1, 2009.
2. Wareham DW, Breuer J. Herpes zoster. BMJ. 2007;334:1211-1215.
3. Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571-575.
4. Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26-33.
5. Scott FT, Johnson RW, Leedham-Green M, et al. The burden of herpes zoster: a prospective population based study. Vaccine. 2006;24:1308-1314.
6. Chidiac C, Buxelles J, Daures JP, et al. Characteristics of patients with herpes zoster on presentation to practitioners in France. Clin Infect Dis. 2001;33:62-69.
7. Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2008;57:1-30.
8. US Food and Drug Administration. FDA licenses new vaccine to reduce older Americans’ risk of shingles. May 26, 2006. Available at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108659.htm. Accessed September 1, 2009.
9. Johnson RW, Dworkin RH. Treatment of herpes zoster and postherpetic neuralgia. BMJ. 2003;326:748-50.
10. Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
11. Jung BF, Johnson RW, Griffin DR, et al. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62:1545-1551.
12. Jackson JL, Gibbons R, Meyer G, et al. The effect of treating herpes zoster with oral acyclovir in preventing postherpetic neuralgia. A meta-analysis. Arch Intern Med. 1997;157:909-912.
13. Wood MJ, Kay R, Dworkin RH, et al. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis. 1996;22:341-347.
14. Beutner KR, Friedman DJ, Forszpaniak C, et al. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546-1553.
15. Tyring S, Barbarash RA, Nahlik JE, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89-96.
16. Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327-331.
17. Whitley RJ, Weiss H, Gnann JW, Jr, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376-383.
18. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271-2284.
19. Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med. 2006;145:317-325.
20. Lu P, Euler GL, Jumaan AO, et al. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine. 2009;27:882-887.
21. Centers for Disease Control and Prevention. Herpes zoster – vaccine Q&As for providers. Available at: http://www.cdc.gov/vaccines/vpd-vac/shingles/vac-faqs-hcp.htm. Accessed September 1, 2009.
• Initiate antiviral treatment as soon as possible; rapid resolution of acute pain and reduction in the development of postherpetic neuralgia (PHN) are most likely when therapy is started within 72 hours of the outbreak. A
• Discuss herpes zoster (HZ) vaccination with healthy patients 60 years of age and older during their first office visit; the vaccine markedly reduces the incidence of HZ and PHN. A
• Do not prescribe tricyclic antidepressants or corticosteroids in the acute phase of HZ. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
We don’t do enough to protect our patients from the pain of herpes zoster (HZ). Consider:
- Each year in the United States, about 1 million new cases of herpes zoster (HZ) occur.1 The incidence is estimated at 3 to 4 per 1,000 in the general population,2 but climbs to more than 10 per 1000 among people 60 years of age and older.3,4
- Overall, between 13% and 26% of patients with HZ develop postherpetic neuralgia (PHN), defined as pain that continues for more than 1 month after the rash has healed. Among patients who are 70 or older, however, the likelihood that HZ will progress to PHN is approximately 50%.5 (In a study of 7595 patients being treated for HZ or PHN by general practitioners or dermatologists in France, 45% reported pain that was severe or very severe, and 42% reported permanent pain.6 )
- Between 10% and 25% of HZ patients develop ocular complications, which have the potential to result in vision loss, facial scarring, or prolonged or permanent pain.7 Encephalitis, myelitis, and peripheral nerve palsies are potential complications, as well.
Yet HZ and its complications are largely preventable.
A live attenuated vaccine (Zostavax) received US Food and Drug Administration approval in 2006.8 But many patients have not yet heard of it, and many physicians fail to recommend it. (See “Herpes zoster vaccine: Why aren’t more people receiving it?”.)
As a family physician, you can play a key role in reducing the burden of shingles by rapidly identifying and treating HZ, minimizing the risk of prolonged pain, and, notably, by talking to older patients about the benefits of vaccination.
Zostavax, a live attenuated herpes zoster (HZ) vaccine, was licensed by the US Food and Drug Administration in 2006 for use in people 60 years of age and older—the first new vaccine targeting this age group in years. In 2007, researchers at the Centers for Disease Control and Prevention (CDC) conducted a national survey,20 in part to gauge the knowledge of, and interest in, the HZ vaccine among the intended recipients.
Their findings: Of more than 3500 respondents, only 1.9% had received the HZ vaccine. Most (72%) were unaware of the vaccine’s existence, but the majority said they would agree to vaccination if their physician were to recommend it.
Among those who were aware of it, the key reasons for rejecting the vaccine were that it was not needed (cited by 35%), they were not at risk (13%), and a lack of trust in doctors or the US health care system (10%). Both the limited awareness of the vaccine and the lack of physician recommendations are barriers to HZ vaccination, the researchers concluded.20
On its Web site, the CDC broaches another potential barrier to greater use of the HZ vaccine: cost. The vaccine is not covered by Medicare Part B, nor by some private insurers. While it is covered by all Medicare Part D plans, the extent of coverage depends on the particular plan. The CDC recommends that physicians encourage patients to contact their insurers to determine the extent of their coverage.21
Start antiviral therapy without delay
Several meta-analyses and many (though not all) randomized controlled trials (RCTs) of HZ treatment have demonstrated that prompt antiviral therapy—with oral acyclovir (ACV), valacyclovir (VCV), or famciclovir (FCV)—reduces the duration of acute pain and the likelihood that PHN will develop.9,10 Without antiviral therapy, up to 45% of patients over the age of 60 experience pain that persists for 6 months to a year. Even with therapy, studies have found that about 20% of patients older than 50 years continued to have pain for 6 months after their rash appeared.10 Risk factors for PHN include age (>50 years), sex (female), a disseminated rash, a severe pain presentation, and polymerase chain reaction-detectable varicella zoster virus viremia.11
Which agent? What the research reveals
For most people with HZ, any of the 3 antiviral agents can be used, based on physician and patient preference. (See TABLE for treatment guidelines.) Here’s a look at the evidence for each.
Oral ACV has long been the mainstay of treatment for HZ, but its poor bioavailability and the need for 5 daily doses has led to the development of newer antiviral agents.12 When initiated within 48 to 72 hours of the onset of the rash, ACV has demonstrated clinical benefit. (The value of starting ACV therapy beyond the 72-hour mark has not been established, though treatment should be considered if new lesions are still appearing.)
In 1 meta-analysis of 4 placebo-controlled trials, ACV accelerated resolution of acute pain, with the greatest effect in those older than 50 years.13 In a second meta-analysis, treatment with ACV reduced the incidence of PHN at 3 months by 46% (number needed to treat [NNT]=3.2-8).12
VCV is well absorbed in the gastrointestinal tract, providing 3- to 5-fold greater bioavailability compared with ACV.13 VCV’s efficacy was demonstrated in an RCT in which the researchers conducted an intent-to-treat analysis: Compared with ACV therapy for 7 to 14 days, VCV significantly accelerated the resolution of acute pain, reduced the duration of PHN, and decreased the proportion of patients with pain persisting for more than 6 months (19% vs 26%). The incidence of adverse events was similar in both groups.14
FCV has broad activity against varicella-zoster virus.15 In an RCT that evaluated oral FCV in 419 immunocompetent adults (mean age 50 years) with uncomplicated HZ, FCV was well tolerated and accelerated lesion healing compared with placebo. Among those who developed PHN, the pain resolved twice as fast for patients in the FCV group compared with the controls, and the median duration of PHN was reduced by 2 months.15
TABLE
Antiviral therapy dosing guidelines*
| Dosage adjusted for creatinine clearance† (mL/min) | ||||
|---|---|---|---|---|
| Drug | Standard dose | <10 | 10-25 | Duration |
| ACV | 800 mg 5x/d | 1600 mg/d | 2400 mg/d | 7-10 days |
| VCV | 1000 mg tid | 1000 mg/d | 2000 mg/d | 7 days |
| FCV | 750 mg/d or 250 mg tid | 250 mg/d | 500 mg/d | 7 days |
| *All 3 drugs reduce acute pain and development of postherpetic neuralgia, and are most effective when started within 72 hours of onset of rash. | ||||
| †Patients with creatinine clearance >25 mL/min receive the standard dose. | ||||
| ACV, acyclovir; FCV, famciclovir; VCV, valacyclovir. | ||||
Analgesics and other drugs: What to consider
While antiviral therapy helps to relieve the pain of HZ, several trials have shown that none of the available agents completely alleviates it or routinely prevents the development of PHN. As a result, adjunctive therapy, including pain medication, is often required. But prescribing analgesics to frail elderly patients and those who have comorbidities and take multiple medications is not without risk.
The ability of a tricyclic antidepressant to alleviate pain and prevent PHN when therapy is initiated within 48 hours of the eruption of lesions was tested in a double-blind trial in which 72 patients 60 years of age or older were randomized to amitriptyline 25 mg daily for 90 days or placebo.16 Antiviral agents were administered according to the preference of the primary physician. At 6 weeks, the pain prevalence—the primary outcome measure—was reduced by about 50% in the amitriptyline group.16 There is no other evidence to support the use of tricyclics in the acute phase of HZ, however, and concerns about orthostatic hypotension and anticholinergic side effects limit their use, particularly in older patients.
Corticosteroids are sometimes used, too, often in conjunction with antiviral therapy, but there are problems with this approach, as well. One RCT comparing an ACV-prednisone combination with ACV alone in HZ patients over the age of 50 found that patients who received both drugs had faster resolution of acute pain and earlier discontinuation of analgesics.17 But several serious adverse effects of prednisone were reported in patients in the combination therapy group, despite the fact that individuals with contraindications to corticosteroids were excluded from the study. Overall, there is little evidence to suggest that steroids can be safely used to reduce the incidence or severity of PHN. There is no specific recommendation regarding analgesic therapy for PHN, but physicians often adopt a stepwise approach.
Recommend the shingles vaccine
In view of the toll that shingles often takes, vaccination is the best way to prevent HZ and its complications. In a randomized, double-blind, placebo-controlled study involving 38,546 adults who were 60 years of age or older, researchers demonstrated that cell-mediated immunity to the varicella-zoster virus was boosted by the live attenuated HZ vaccine.18 The enhanced immunity was associated with a 51% reduction in the incidence of HZ (NNT=58 to prevent 1 case over 3 years), a 66% reduction in the incidence of PHN (NNT=364 to prevent 1 case of PHN over 3 years), and a 61% reduction in disease burden. The vaccine was well tolerated, and injection site reactions were generally mild.18 Accurate cost-effectiveness analyses of immunization are not available because the duration of vaccine protection is unknown.19
FIGURE
A unilateral vesicular rash
A shingles outbreak, like the rash shown on this patient’s back, usually appears as a patch or band of blisters on 1 side of the body.
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination to prevent both HZ and PHN in healthy adults who are 60 years of age and older (individuals with primary or acquired immunodeficiencies or patients on immunosuppressive therapies should not be vaccinated), and suggests that practitioners offer the HZ vaccine to appropriate patients at their first visit.7 It is not necessary, however, to ask about the patient’s history of varicella or to conduct serologic testing to determine varicella immunity before administering the HZ vaccine.7 A history of shingles is not a contraindication, so advise patients who develop HZ to come in for vaccination soon after the rash and pain resolve.
CORRESPONDENCE
Pierre-Olivier Lang, MD, MPH, PhD, University Hospitals of Geneva, Department of Rehabilitation and Geriatrics, Chemin du Pont-Bochet, 3, CH-1226, Thonex-Geneva, Switzerland; [email protected]
• Initiate antiviral treatment as soon as possible; rapid resolution of acute pain and reduction in the development of postherpetic neuralgia (PHN) are most likely when therapy is started within 72 hours of the outbreak. A
• Discuss herpes zoster (HZ) vaccination with healthy patients 60 years of age and older during their first office visit; the vaccine markedly reduces the incidence of HZ and PHN. A
• Do not prescribe tricyclic antidepressants or corticosteroids in the acute phase of HZ. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
We don’t do enough to protect our patients from the pain of herpes zoster (HZ). Consider:
- Each year in the United States, about 1 million new cases of herpes zoster (HZ) occur.1 The incidence is estimated at 3 to 4 per 1,000 in the general population,2 but climbs to more than 10 per 1000 among people 60 years of age and older.3,4
- Overall, between 13% and 26% of patients with HZ develop postherpetic neuralgia (PHN), defined as pain that continues for more than 1 month after the rash has healed. Among patients who are 70 or older, however, the likelihood that HZ will progress to PHN is approximately 50%.5 (In a study of 7595 patients being treated for HZ or PHN by general practitioners or dermatologists in France, 45% reported pain that was severe or very severe, and 42% reported permanent pain.6 )
- Between 10% and 25% of HZ patients develop ocular complications, which have the potential to result in vision loss, facial scarring, or prolonged or permanent pain.7 Encephalitis, myelitis, and peripheral nerve palsies are potential complications, as well.
Yet HZ and its complications are largely preventable.
A live attenuated vaccine (Zostavax) received US Food and Drug Administration approval in 2006.8 But many patients have not yet heard of it, and many physicians fail to recommend it. (See “Herpes zoster vaccine: Why aren’t more people receiving it?”.)
As a family physician, you can play a key role in reducing the burden of shingles by rapidly identifying and treating HZ, minimizing the risk of prolonged pain, and, notably, by talking to older patients about the benefits of vaccination.
Zostavax, a live attenuated herpes zoster (HZ) vaccine, was licensed by the US Food and Drug Administration in 2006 for use in people 60 years of age and older—the first new vaccine targeting this age group in years. In 2007, researchers at the Centers for Disease Control and Prevention (CDC) conducted a national survey,20 in part to gauge the knowledge of, and interest in, the HZ vaccine among the intended recipients.
Their findings: Of more than 3500 respondents, only 1.9% had received the HZ vaccine. Most (72%) were unaware of the vaccine’s existence, but the majority said they would agree to vaccination if their physician were to recommend it.
Among those who were aware of it, the key reasons for rejecting the vaccine were that it was not needed (cited by 35%), they were not at risk (13%), and a lack of trust in doctors or the US health care system (10%). Both the limited awareness of the vaccine and the lack of physician recommendations are barriers to HZ vaccination, the researchers concluded.20
On its Web site, the CDC broaches another potential barrier to greater use of the HZ vaccine: cost. The vaccine is not covered by Medicare Part B, nor by some private insurers. While it is covered by all Medicare Part D plans, the extent of coverage depends on the particular plan. The CDC recommends that physicians encourage patients to contact their insurers to determine the extent of their coverage.21
Start antiviral therapy without delay
Several meta-analyses and many (though not all) randomized controlled trials (RCTs) of HZ treatment have demonstrated that prompt antiviral therapy—with oral acyclovir (ACV), valacyclovir (VCV), or famciclovir (FCV)—reduces the duration of acute pain and the likelihood that PHN will develop.9,10 Without antiviral therapy, up to 45% of patients over the age of 60 experience pain that persists for 6 months to a year. Even with therapy, studies have found that about 20% of patients older than 50 years continued to have pain for 6 months after their rash appeared.10 Risk factors for PHN include age (>50 years), sex (female), a disseminated rash, a severe pain presentation, and polymerase chain reaction-detectable varicella zoster virus viremia.11
Which agent? What the research reveals
For most people with HZ, any of the 3 antiviral agents can be used, based on physician and patient preference. (See TABLE for treatment guidelines.) Here’s a look at the evidence for each.
Oral ACV has long been the mainstay of treatment for HZ, but its poor bioavailability and the need for 5 daily doses has led to the development of newer antiviral agents.12 When initiated within 48 to 72 hours of the onset of the rash, ACV has demonstrated clinical benefit. (The value of starting ACV therapy beyond the 72-hour mark has not been established, though treatment should be considered if new lesions are still appearing.)
In 1 meta-analysis of 4 placebo-controlled trials, ACV accelerated resolution of acute pain, with the greatest effect in those older than 50 years.13 In a second meta-analysis, treatment with ACV reduced the incidence of PHN at 3 months by 46% (number needed to treat [NNT]=3.2-8).12
VCV is well absorbed in the gastrointestinal tract, providing 3- to 5-fold greater bioavailability compared with ACV.13 VCV’s efficacy was demonstrated in an RCT in which the researchers conducted an intent-to-treat analysis: Compared with ACV therapy for 7 to 14 days, VCV significantly accelerated the resolution of acute pain, reduced the duration of PHN, and decreased the proportion of patients with pain persisting for more than 6 months (19% vs 26%). The incidence of adverse events was similar in both groups.14
FCV has broad activity against varicella-zoster virus.15 In an RCT that evaluated oral FCV in 419 immunocompetent adults (mean age 50 years) with uncomplicated HZ, FCV was well tolerated and accelerated lesion healing compared with placebo. Among those who developed PHN, the pain resolved twice as fast for patients in the FCV group compared with the controls, and the median duration of PHN was reduced by 2 months.15
TABLE
Antiviral therapy dosing guidelines*
| Dosage adjusted for creatinine clearance† (mL/min) | ||||
|---|---|---|---|---|
| Drug | Standard dose | <10 | 10-25 | Duration |
| ACV | 800 mg 5x/d | 1600 mg/d | 2400 mg/d | 7-10 days |
| VCV | 1000 mg tid | 1000 mg/d | 2000 mg/d | 7 days |
| FCV | 750 mg/d or 250 mg tid | 250 mg/d | 500 mg/d | 7 days |
| *All 3 drugs reduce acute pain and development of postherpetic neuralgia, and are most effective when started within 72 hours of onset of rash. | ||||
| †Patients with creatinine clearance >25 mL/min receive the standard dose. | ||||
| ACV, acyclovir; FCV, famciclovir; VCV, valacyclovir. | ||||
Analgesics and other drugs: What to consider
While antiviral therapy helps to relieve the pain of HZ, several trials have shown that none of the available agents completely alleviates it or routinely prevents the development of PHN. As a result, adjunctive therapy, including pain medication, is often required. But prescribing analgesics to frail elderly patients and those who have comorbidities and take multiple medications is not without risk.
The ability of a tricyclic antidepressant to alleviate pain and prevent PHN when therapy is initiated within 48 hours of the eruption of lesions was tested in a double-blind trial in which 72 patients 60 years of age or older were randomized to amitriptyline 25 mg daily for 90 days or placebo.16 Antiviral agents were administered according to the preference of the primary physician. At 6 weeks, the pain prevalence—the primary outcome measure—was reduced by about 50% in the amitriptyline group.16 There is no other evidence to support the use of tricyclics in the acute phase of HZ, however, and concerns about orthostatic hypotension and anticholinergic side effects limit their use, particularly in older patients.
Corticosteroids are sometimes used, too, often in conjunction with antiviral therapy, but there are problems with this approach, as well. One RCT comparing an ACV-prednisone combination with ACV alone in HZ patients over the age of 50 found that patients who received both drugs had faster resolution of acute pain and earlier discontinuation of analgesics.17 But several serious adverse effects of prednisone were reported in patients in the combination therapy group, despite the fact that individuals with contraindications to corticosteroids were excluded from the study. Overall, there is little evidence to suggest that steroids can be safely used to reduce the incidence or severity of PHN. There is no specific recommendation regarding analgesic therapy for PHN, but physicians often adopt a stepwise approach.
Recommend the shingles vaccine
In view of the toll that shingles often takes, vaccination is the best way to prevent HZ and its complications. In a randomized, double-blind, placebo-controlled study involving 38,546 adults who were 60 years of age or older, researchers demonstrated that cell-mediated immunity to the varicella-zoster virus was boosted by the live attenuated HZ vaccine.18 The enhanced immunity was associated with a 51% reduction in the incidence of HZ (NNT=58 to prevent 1 case over 3 years), a 66% reduction in the incidence of PHN (NNT=364 to prevent 1 case of PHN over 3 years), and a 61% reduction in disease burden. The vaccine was well tolerated, and injection site reactions were generally mild.18 Accurate cost-effectiveness analyses of immunization are not available because the duration of vaccine protection is unknown.19
FIGURE
A unilateral vesicular rash
A shingles outbreak, like the rash shown on this patient’s back, usually appears as a patch or band of blisters on 1 side of the body.
The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination to prevent both HZ and PHN in healthy adults who are 60 years of age and older (individuals with primary or acquired immunodeficiencies or patients on immunosuppressive therapies should not be vaccinated), and suggests that practitioners offer the HZ vaccine to appropriate patients at their first visit.7 It is not necessary, however, to ask about the patient’s history of varicella or to conduct serologic testing to determine varicella immunity before administering the HZ vaccine.7 A history of shingles is not a contraindication, so advise patients who develop HZ to come in for vaccination soon after the rash and pain resolve.
CORRESPONDENCE
Pierre-Olivier Lang, MD, MPH, PhD, University Hospitals of Geneva, Department of Rehabilitation and Geriatrics, Chemin du Pont-Bochet, 3, CH-1226, Thonex-Geneva, Switzerland; [email protected]
1. Centers for Disease Control and Prevention. Shingles disease – questions and answers (herpes zoster). Available at http://www.cdc.gov/vaccines/vpd-vac/shingles/dis-faqs.htm. Accessed September 1, 2009.
2. Wareham DW, Breuer J. Herpes zoster. BMJ. 2007;334:1211-1215.
3. Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571-575.
4. Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26-33.
5. Scott FT, Johnson RW, Leedham-Green M, et al. The burden of herpes zoster: a prospective population based study. Vaccine. 2006;24:1308-1314.
6. Chidiac C, Buxelles J, Daures JP, et al. Characteristics of patients with herpes zoster on presentation to practitioners in France. Clin Infect Dis. 2001;33:62-69.
7. Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2008;57:1-30.
8. US Food and Drug Administration. FDA licenses new vaccine to reduce older Americans’ risk of shingles. May 26, 2006. Available at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108659.htm. Accessed September 1, 2009.
9. Johnson RW, Dworkin RH. Treatment of herpes zoster and postherpetic neuralgia. BMJ. 2003;326:748-50.
10. Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
11. Jung BF, Johnson RW, Griffin DR, et al. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62:1545-1551.
12. Jackson JL, Gibbons R, Meyer G, et al. The effect of treating herpes zoster with oral acyclovir in preventing postherpetic neuralgia. A meta-analysis. Arch Intern Med. 1997;157:909-912.
13. Wood MJ, Kay R, Dworkin RH, et al. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis. 1996;22:341-347.
14. Beutner KR, Friedman DJ, Forszpaniak C, et al. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546-1553.
15. Tyring S, Barbarash RA, Nahlik JE, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89-96.
16. Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327-331.
17. Whitley RJ, Weiss H, Gnann JW, Jr, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376-383.
18. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271-2284.
19. Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med. 2006;145:317-325.
20. Lu P, Euler GL, Jumaan AO, et al. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine. 2009;27:882-887.
21. Centers for Disease Control and Prevention. Herpes zoster – vaccine Q&As for providers. Available at: http://www.cdc.gov/vaccines/vpd-vac/shingles/vac-faqs-hcp.htm. Accessed September 1, 2009.
1. Centers for Disease Control and Prevention. Shingles disease – questions and answers (herpes zoster). Available at http://www.cdc.gov/vaccines/vpd-vac/shingles/dis-faqs.htm. Accessed September 1, 2009.
2. Wareham DW, Breuer J. Herpes zoster. BMJ. 2007;334:1211-1215.
3. Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571-575.
4. Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26-33.
5. Scott FT, Johnson RW, Leedham-Green M, et al. The burden of herpes zoster: a prospective population based study. Vaccine. 2006;24:1308-1314.
6. Chidiac C, Buxelles J, Daures JP, et al. Characteristics of patients with herpes zoster on presentation to practitioners in France. Clin Infect Dis. 2001;33:62-69.
7. Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2008;57:1-30.
8. US Food and Drug Administration. FDA licenses new vaccine to reduce older Americans’ risk of shingles. May 26, 2006. Available at www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108659.htm. Accessed September 1, 2009.
9. Johnson RW, Dworkin RH. Treatment of herpes zoster and postherpetic neuralgia. BMJ. 2003;326:748-50.
10. Dworkin RH, Johnson RW, Breuer J, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007;44(suppl 1):S1-S26.
11. Jung BF, Johnson RW, Griffin DR, et al. Risk factors for postherpetic neuralgia in patients with herpes zoster. Neurology. 2004;62:1545-1551.
12. Jackson JL, Gibbons R, Meyer G, et al. The effect of treating herpes zoster with oral acyclovir in preventing postherpetic neuralgia. A meta-analysis. Arch Intern Med. 1997;157:909-912.
13. Wood MJ, Kay R, Dworkin RH, et al. Oral acyclovir therapy accelerates pain resolution in patients with herpes zoster: a meta-analysis of placebo-controlled trials. Clin Infect Dis. 1996;22:341-347.
14. Beutner KR, Friedman DJ, Forszpaniak C, et al. Valaciclovir compared with acyclovir for improved therapy for herpes zoster in immunocompetent adults. Antimicrob Agents Chemother. 1995;39:1546-1553.
15. Tyring S, Barbarash RA, Nahlik JE, et al. Famciclovir for the treatment of acute herpes zoster: effects on acute disease and postherpetic neuralgia. A randomized, double-blind, placebo-controlled trial. Collaborative Famciclovir Herpes Zoster Study Group. Ann Intern Med. 1995;123:89-96.
16. Bowsher D. The effects of pre-emptive treatment of postherpetic neuralgia with amitriptyline: a randomized, double-blind, placebo-controlled trial. J Pain Symptom Manage. 1997;13:327-331.
17. Whitley RJ, Weiss H, Gnann JW, Jr, et al. Acyclovir with and without prednisone for the treatment of herpes zoster. A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Ann Intern Med. 1996;125:376-383.
18. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271-2284.
19. Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med. 2006;145:317-325.
20. Lu P, Euler GL, Jumaan AO, et al. Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: uptake of the first new vaccine to target seniors. Vaccine. 2009;27:882-887.
21. Centers for Disease Control and Prevention. Herpes zoster – vaccine Q&As for providers. Available at: http://www.cdc.gov/vaccines/vpd-vac/shingles/vac-faqs-hcp.htm. Accessed September 1, 2009.