User login
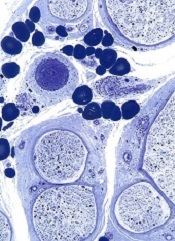
A study published in Nature Cell Biology helps explain how hemodynamic forces contribute to the formation of new vascular lumens during blood vessel morphogenesis.
Investigators found that blood flow drives lumen expansion during sprouting angiogenesis in vivo by inducing spherical deformations of the apical
membrane of endothelial cells, in a process dubbed “inverse blebbing.”
“This work, combined with previous studies, highlights the importance of balanced endothelial cell contractility in allowing the expansion and maintenance of endothelial lumens during blood vessel development,” said study author Holger Gerhardt, PhD, of the Max Delbrück Center for Molecular Medicine in Berlin, Germany.
These results challenge the previous idea that sprouting cells expand lumens independently of blood flow during angiogenesis through the generation and fusion of intracellular vacuoles.
The investigators showed that hemodynamic forces dynamically shape the apical membrane of single or groups of endothelial cells during angiogenesis to form and expand new lumenized vascular tubes.
“We find that this process relies on a tight balance between the forces applied on the membrane and the local contractile responses from the endothelial cells, as impairing this balance either way leads to lumen defects,” Dr Gerhardt said.
These findings suggest the process of blebbing does not require a specific polarity but is likely to be generally applicable to situations in which external versus internal pressure differences challenge the stability and elasticity of the actin cortex.
It more generally raises the question of the role of apical membrane contractility in the adaptation to varying hemodynamic environments, both during blood vessel morphogenesis, as connections form or remodel, and in pathological settings.
“Understanding whether and how this plasticity of the apical membrane and its underlying cortex is challenged in pathological conditions, where vessels exhibit altered perfusion and lack organized structure, has the potential to provide deeper insight into mechanisms of vascular adaptation and maladaptation,” Dr Gerhardt said. “We will definitely further investigate this.” ![]()

A study published in Nature Cell Biology helps explain how hemodynamic forces contribute to the formation of new vascular lumens during blood vessel morphogenesis.
Investigators found that blood flow drives lumen expansion during sprouting angiogenesis in vivo by inducing spherical deformations of the apical
membrane of endothelial cells, in a process dubbed “inverse blebbing.”
“This work, combined with previous studies, highlights the importance of balanced endothelial cell contractility in allowing the expansion and maintenance of endothelial lumens during blood vessel development,” said study author Holger Gerhardt, PhD, of the Max Delbrück Center for Molecular Medicine in Berlin, Germany.
These results challenge the previous idea that sprouting cells expand lumens independently of blood flow during angiogenesis through the generation and fusion of intracellular vacuoles.
The investigators showed that hemodynamic forces dynamically shape the apical membrane of single or groups of endothelial cells during angiogenesis to form and expand new lumenized vascular tubes.
“We find that this process relies on a tight balance between the forces applied on the membrane and the local contractile responses from the endothelial cells, as impairing this balance either way leads to lumen defects,” Dr Gerhardt said.
These findings suggest the process of blebbing does not require a specific polarity but is likely to be generally applicable to situations in which external versus internal pressure differences challenge the stability and elasticity of the actin cortex.
It more generally raises the question of the role of apical membrane contractility in the adaptation to varying hemodynamic environments, both during blood vessel morphogenesis, as connections form or remodel, and in pathological settings.
“Understanding whether and how this plasticity of the apical membrane and its underlying cortex is challenged in pathological conditions, where vessels exhibit altered perfusion and lack organized structure, has the potential to provide deeper insight into mechanisms of vascular adaptation and maladaptation,” Dr Gerhardt said. “We will definitely further investigate this.” ![]()

A study published in Nature Cell Biology helps explain how hemodynamic forces contribute to the formation of new vascular lumens during blood vessel morphogenesis.
Investigators found that blood flow drives lumen expansion during sprouting angiogenesis in vivo by inducing spherical deformations of the apical
membrane of endothelial cells, in a process dubbed “inverse blebbing.”
“This work, combined with previous studies, highlights the importance of balanced endothelial cell contractility in allowing the expansion and maintenance of endothelial lumens during blood vessel development,” said study author Holger Gerhardt, PhD, of the Max Delbrück Center for Molecular Medicine in Berlin, Germany.
These results challenge the previous idea that sprouting cells expand lumens independently of blood flow during angiogenesis through the generation and fusion of intracellular vacuoles.
The investigators showed that hemodynamic forces dynamically shape the apical membrane of single or groups of endothelial cells during angiogenesis to form and expand new lumenized vascular tubes.
“We find that this process relies on a tight balance between the forces applied on the membrane and the local contractile responses from the endothelial cells, as impairing this balance either way leads to lumen defects,” Dr Gerhardt said.
These findings suggest the process of blebbing does not require a specific polarity but is likely to be generally applicable to situations in which external versus internal pressure differences challenge the stability and elasticity of the actin cortex.
It more generally raises the question of the role of apical membrane contractility in the adaptation to varying hemodynamic environments, both during blood vessel morphogenesis, as connections form or remodel, and in pathological settings.
“Understanding whether and how this plasticity of the apical membrane and its underlying cortex is challenged in pathological conditions, where vessels exhibit altered perfusion and lack organized structure, has the potential to provide deeper insight into mechanisms of vascular adaptation and maladaptation,” Dr Gerhardt said. “We will definitely further investigate this.” ![]()