User login
ORLANDO – Patients who underwent 3 consecutive years of desensitization to grass pollen with seasonal use of a daily, sublingual pill maintained their reduced level of grass pollen allergy during the following fourth season despite stopping pill treatment, according to results from a study of 432 patients who stayed in the study for 4 years.
The findings provide the first evidence that sublingual desensitization produces allergy disease modification similar to subcutaneous desensitization, Dr. Hans-Jørgen Malling said during a poster presentation March 4 at the meeting.
"The data show sustained clinical efficacy when you stop treatment," said Dr. Malling, professor of medicine at the University of Copenhagen and chief of the allergy clinic at Gentofte Hospital in nearby Hellerup. "That is disease modifying. If there is no efficacy after you stop treatment, then this treatment will not be viable."
Although long-term efficacy is crucial, "up to now, it seems like sublingual is equivalent to subcutaneous," he said in an interview.
But Dr. Malling also cautioned that a major test will be the ability of sublingual desensitization to maintain allergy desensitization during a second year off treatment – results that will come during the fifth and final year of the study and will be available a year from now.
Prior reports from the current study documented the ability of the sublingual grass pollen pill to produce desensitization in adult patients during each of the 3 years of active treatment (J. Allergy Clin. Immunol. 2011;128:559-66).
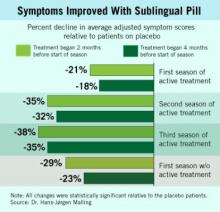
During the first 3 years, the oral desensitization regimen led to progressively higher levels of symptom reduction each year, compared with placebo, a pattern that mimicked what typically occurs with subcutaneous desensitization.
The new results showed that during patients’ first year off active treatment, symptom relief showed a trend toward a small dip in efficacy (see box), compared with placebo, although patients also self reported a maintained, positive effect on their total quality of life scores.
During the first year off treatment, the average adjusted symptom score (the study’s primary end point) was 29% below the placebo level among 136 patients who both took the pill starting 2 months before the start of their allergy season and remained in the study. The symptom score was 23% below placebo among the 142 patients who took the pill starting 4 months before the start of their allergy season and remained in the study.
The study initially randomized 633 adult patients with grass pollen allergy at several European centers. Patients began receiving either the active desensitization tablet or placebo at 2 months or 4 months before the onset of their local grass pollen season, and continued daily treatment through the end of the local pollen season. They then resumed the same treatment schedule during the subsequent 2 years. By the end of the fourth year of the study, roughly two-thirds of the initially enrolled patients remained in the study.
The most common adverse effect during treatment was mouth pruritus, followed by throat irritation. All of the adverse effects were mild or moderate, and their incidence and severity steadily declined during the 3 years of active treatment.
No patients in the study had an anaphylactic reaction, unlike what occurs with subcutaneous injections, which are know to cause anaphylaxis occasionally. The entire worldwide experience with the sublingual grass pollen tablet has so far resulted in six episodes of "doubtful" anaphylaxis, an experience that led Dr. Malling to declare that the sublingual tablet "is safer" than subcutaneous injections.
"For some patients, [sublingual] is probably optimal, while for others subcutaneous will be better," Dr. Malling said. Having both options available gives patients a choice of disease-modifying therapy. One advantage of the sublingual tablet is that it precludes the need for patients to travel to a physician’s office for regular injections, he noted, although being on a schedule of regular injections helps ensure compliance.
Stallergenes developed the sublingual tablet, and has marketed it as Oralair in Europe since 2009. The tablet contains pollen extracts from five grass types that are common throughout Europe.
A pivotal trial of the formulation is in progress in the United States, but has not yet progressed to the stage where patients have stopped active, seasonal treatment. Other companies are also conducting clinical trials to test the efficacy and safety of sublingual desensitization tablets for grass pollen and for other allergens. The new results reported by Dr. Malling and his associates are the first to report what happens once patients stop active, annual treatment.
The study was sponsored by Stallergenes, the company that markets the sublingual grass pollen tablet as Oralair. Dr. Malling said that he is a consultant to and has received research support from Stallergenes and from several other drug companies.
ORLANDO – Patients who underwent 3 consecutive years of desensitization to grass pollen with seasonal use of a daily, sublingual pill maintained their reduced level of grass pollen allergy during the following fourth season despite stopping pill treatment, according to results from a study of 432 patients who stayed in the study for 4 years.
The findings provide the first evidence that sublingual desensitization produces allergy disease modification similar to subcutaneous desensitization, Dr. Hans-Jørgen Malling said during a poster presentation March 4 at the meeting.
"The data show sustained clinical efficacy when you stop treatment," said Dr. Malling, professor of medicine at the University of Copenhagen and chief of the allergy clinic at Gentofte Hospital in nearby Hellerup. "That is disease modifying. If there is no efficacy after you stop treatment, then this treatment will not be viable."
Although long-term efficacy is crucial, "up to now, it seems like sublingual is equivalent to subcutaneous," he said in an interview.
But Dr. Malling also cautioned that a major test will be the ability of sublingual desensitization to maintain allergy desensitization during a second year off treatment – results that will come during the fifth and final year of the study and will be available a year from now.
Prior reports from the current study documented the ability of the sublingual grass pollen pill to produce desensitization in adult patients during each of the 3 years of active treatment (J. Allergy Clin. Immunol. 2011;128:559-66).
During the first 3 years, the oral desensitization regimen led to progressively higher levels of symptom reduction each year, compared with placebo, a pattern that mimicked what typically occurs with subcutaneous desensitization.
The new results showed that during patients’ first year off active treatment, symptom relief showed a trend toward a small dip in efficacy (see box), compared with placebo, although patients also self reported a maintained, positive effect on their total quality of life scores.
During the first year off treatment, the average adjusted symptom score (the study’s primary end point) was 29% below the placebo level among 136 patients who both took the pill starting 2 months before the start of their allergy season and remained in the study. The symptom score was 23% below placebo among the 142 patients who took the pill starting 4 months before the start of their allergy season and remained in the study.
The study initially randomized 633 adult patients with grass pollen allergy at several European centers. Patients began receiving either the active desensitization tablet or placebo at 2 months or 4 months before the onset of their local grass pollen season, and continued daily treatment through the end of the local pollen season. They then resumed the same treatment schedule during the subsequent 2 years. By the end of the fourth year of the study, roughly two-thirds of the initially enrolled patients remained in the study.
The most common adverse effect during treatment was mouth pruritus, followed by throat irritation. All of the adverse effects were mild or moderate, and their incidence and severity steadily declined during the 3 years of active treatment.
No patients in the study had an anaphylactic reaction, unlike what occurs with subcutaneous injections, which are know to cause anaphylaxis occasionally. The entire worldwide experience with the sublingual grass pollen tablet has so far resulted in six episodes of "doubtful" anaphylaxis, an experience that led Dr. Malling to declare that the sublingual tablet "is safer" than subcutaneous injections.
"For some patients, [sublingual] is probably optimal, while for others subcutaneous will be better," Dr. Malling said. Having both options available gives patients a choice of disease-modifying therapy. One advantage of the sublingual tablet is that it precludes the need for patients to travel to a physician’s office for regular injections, he noted, although being on a schedule of regular injections helps ensure compliance.
Stallergenes developed the sublingual tablet, and has marketed it as Oralair in Europe since 2009. The tablet contains pollen extracts from five grass types that are common throughout Europe.
A pivotal trial of the formulation is in progress in the United States, but has not yet progressed to the stage where patients have stopped active, seasonal treatment. Other companies are also conducting clinical trials to test the efficacy and safety of sublingual desensitization tablets for grass pollen and for other allergens. The new results reported by Dr. Malling and his associates are the first to report what happens once patients stop active, annual treatment.
The study was sponsored by Stallergenes, the company that markets the sublingual grass pollen tablet as Oralair. Dr. Malling said that he is a consultant to and has received research support from Stallergenes and from several other drug companies.
ORLANDO – Patients who underwent 3 consecutive years of desensitization to grass pollen with seasonal use of a daily, sublingual pill maintained their reduced level of grass pollen allergy during the following fourth season despite stopping pill treatment, according to results from a study of 432 patients who stayed in the study for 4 years.
The findings provide the first evidence that sublingual desensitization produces allergy disease modification similar to subcutaneous desensitization, Dr. Hans-Jørgen Malling said during a poster presentation March 4 at the meeting.
"The data show sustained clinical efficacy when you stop treatment," said Dr. Malling, professor of medicine at the University of Copenhagen and chief of the allergy clinic at Gentofte Hospital in nearby Hellerup. "That is disease modifying. If there is no efficacy after you stop treatment, then this treatment will not be viable."
Although long-term efficacy is crucial, "up to now, it seems like sublingual is equivalent to subcutaneous," he said in an interview.
But Dr. Malling also cautioned that a major test will be the ability of sublingual desensitization to maintain allergy desensitization during a second year off treatment – results that will come during the fifth and final year of the study and will be available a year from now.
Prior reports from the current study documented the ability of the sublingual grass pollen pill to produce desensitization in adult patients during each of the 3 years of active treatment (J. Allergy Clin. Immunol. 2011;128:559-66).
During the first 3 years, the oral desensitization regimen led to progressively higher levels of symptom reduction each year, compared with placebo, a pattern that mimicked what typically occurs with subcutaneous desensitization.
The new results showed that during patients’ first year off active treatment, symptom relief showed a trend toward a small dip in efficacy (see box), compared with placebo, although patients also self reported a maintained, positive effect on their total quality of life scores.
During the first year off treatment, the average adjusted symptom score (the study’s primary end point) was 29% below the placebo level among 136 patients who both took the pill starting 2 months before the start of their allergy season and remained in the study. The symptom score was 23% below placebo among the 142 patients who took the pill starting 4 months before the start of their allergy season and remained in the study.
The study initially randomized 633 adult patients with grass pollen allergy at several European centers. Patients began receiving either the active desensitization tablet or placebo at 2 months or 4 months before the onset of their local grass pollen season, and continued daily treatment through the end of the local pollen season. They then resumed the same treatment schedule during the subsequent 2 years. By the end of the fourth year of the study, roughly two-thirds of the initially enrolled patients remained in the study.
The most common adverse effect during treatment was mouth pruritus, followed by throat irritation. All of the adverse effects were mild or moderate, and their incidence and severity steadily declined during the 3 years of active treatment.
No patients in the study had an anaphylactic reaction, unlike what occurs with subcutaneous injections, which are know to cause anaphylaxis occasionally. The entire worldwide experience with the sublingual grass pollen tablet has so far resulted in six episodes of "doubtful" anaphylaxis, an experience that led Dr. Malling to declare that the sublingual tablet "is safer" than subcutaneous injections.
"For some patients, [sublingual] is probably optimal, while for others subcutaneous will be better," Dr. Malling said. Having both options available gives patients a choice of disease-modifying therapy. One advantage of the sublingual tablet is that it precludes the need for patients to travel to a physician’s office for regular injections, he noted, although being on a schedule of regular injections helps ensure compliance.
Stallergenes developed the sublingual tablet, and has marketed it as Oralair in Europe since 2009. The tablet contains pollen extracts from five grass types that are common throughout Europe.
A pivotal trial of the formulation is in progress in the United States, but has not yet progressed to the stage where patients have stopped active, seasonal treatment. Other companies are also conducting clinical trials to test the efficacy and safety of sublingual desensitization tablets for grass pollen and for other allergens. The new results reported by Dr. Malling and his associates are the first to report what happens once patients stop active, annual treatment.
The study was sponsored by Stallergenes, the company that markets the sublingual grass pollen tablet as Oralair. Dr. Malling said that he is a consultant to and has received research support from Stallergenes and from several other drug companies.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF ALLERGY, ASTHMA, AND IMMUNOLOGY
Major Finding: Grass-allergy patients with 3 years of desensitization with a sublingual tablet maintained desensitization in their first season without treatment.
Data Source: A multicenter, randomized, placebo-controlled study that initially enrolled 633 adult patients with grass pollen allergy.
Disclosures: The study was sponsored by Stallergenes, the company that markets the sublingual grass pollen tablet as Oralair. Dr. Malling said that he is a consultant to and has received research support from Stallergenes and from several other drug companies.