User login
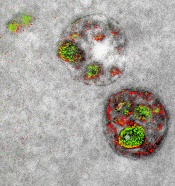
of endosomal uptake
of peptide proteins
Image by Adams et al/
Cell Chemical Biology 2016
Researchers say they have found a way to image specific cellular components using multicolor electron microscopy.
With their method, which the team worked on for nearly 15 years, up to 3 colors at a time (green, red, or yellow) can be used in an image.
A detector on the microscope captures electrons lost from metal ions painted over the specimen and records the metal’s energy loss signature as a color.
A technician must add the ionized metals one at a time and then lay the full color map over the still microscopy image.
The researchers described this method in detail in Cell Chemical Biology.
“This method has many potential applications in biology,” said study author Stephen Adams, PhD, of the University of California, San Diego in La Jolla.
“In the paper, we demonstrate how it can distinguish cellular compartments or track proteins and tag cells.”
For the multicolor effect to work, the researchers needed metal complexes that are stable enough to withstand application (meaning they don’t quickly deteriorate and blur the image) and have a distinct electron energy loss signature.
The researchers used ionized lanthanum (La), cerium (Ce), and praseodymium (Pr)—all metals in the lanthanide family. Each metal complex was laid down sequentially as a precipitate onto the specimen as it sat in the microscope.
“One challenge that kept us from publishing this much earlier, because we had the chemistry and we had an instrument that worked about 4 years ago, was we needed a way to deposit the metal compounds sequentially,” said study author Mark Ellisman, PhD, of the University of California, San Diego.
“We spent an awful lot of time trying to figure out how to deposit one of the lanthanides and then clear it so that it didn’t react when we deposited a second signal on the first site.”
Once the application process had been established, the researchers illustrated the power of multicolor electron microscopy by visualizing 2 brain cells sharing a single synapse. They also showed peptides entering through a cell membrane.
The researchers said there is more chemistry to be done to perfect the metal ion application process as well as produce images with more colors. There may also be ways to increase the amount of metal ions that can be deposited, which could help with resolution.
Many in the biochemical community should be able to begin using this technique right away, as it takes advantage of tools that are already found in laboratories.
This study is one of the last that Roger Tsien, PhD, who won a 2008 Nobel Prize in Chemistry for the discovery and application of green fluorescent protein to biochemical imaging, saw accepted by a journal before his death last August.
He did the first experiments to develop the chemical compounds needed for the multicolor imaging method nearly 15 years ago.
“One theme that has gone through all of Roger’s work is the desire to peer more closely into the workings of the cell,” Dr Adams said.
“With all of the fluorescence techniques that he’s introduced, he was able to do that in live cells and make action movies of them in vivid colors. But he always wanted to look closer, and now he’s left the beginnings for a method where we can add colors to electron microscopy.”
“This is clearly an example of Roger’s brilliance at chemistry and how he saw that, if we could do this, we would be able to enjoy the advantages of electron microscopy,” Dr Ellisman added.
“The biggest advantage of electron microscopy that we saw is that you have weak contrasts by the nature of the way that staining works, so color-specific labels give context to all of the rich information in the scene of which molecules are operating.” ![]()

of endosomal uptake
of peptide proteins
Image by Adams et al/
Cell Chemical Biology 2016
Researchers say they have found a way to image specific cellular components using multicolor electron microscopy.
With their method, which the team worked on for nearly 15 years, up to 3 colors at a time (green, red, or yellow) can be used in an image.
A detector on the microscope captures electrons lost from metal ions painted over the specimen and records the metal’s energy loss signature as a color.
A technician must add the ionized metals one at a time and then lay the full color map over the still microscopy image.
The researchers described this method in detail in Cell Chemical Biology.
“This method has many potential applications in biology,” said study author Stephen Adams, PhD, of the University of California, San Diego in La Jolla.
“In the paper, we demonstrate how it can distinguish cellular compartments or track proteins and tag cells.”
For the multicolor effect to work, the researchers needed metal complexes that are stable enough to withstand application (meaning they don’t quickly deteriorate and blur the image) and have a distinct electron energy loss signature.
The researchers used ionized lanthanum (La), cerium (Ce), and praseodymium (Pr)—all metals in the lanthanide family. Each metal complex was laid down sequentially as a precipitate onto the specimen as it sat in the microscope.
“One challenge that kept us from publishing this much earlier, because we had the chemistry and we had an instrument that worked about 4 years ago, was we needed a way to deposit the metal compounds sequentially,” said study author Mark Ellisman, PhD, of the University of California, San Diego.
“We spent an awful lot of time trying to figure out how to deposit one of the lanthanides and then clear it so that it didn’t react when we deposited a second signal on the first site.”
Once the application process had been established, the researchers illustrated the power of multicolor electron microscopy by visualizing 2 brain cells sharing a single synapse. They also showed peptides entering through a cell membrane.
The researchers said there is more chemistry to be done to perfect the metal ion application process as well as produce images with more colors. There may also be ways to increase the amount of metal ions that can be deposited, which could help with resolution.
Many in the biochemical community should be able to begin using this technique right away, as it takes advantage of tools that are already found in laboratories.
This study is one of the last that Roger Tsien, PhD, who won a 2008 Nobel Prize in Chemistry for the discovery and application of green fluorescent protein to biochemical imaging, saw accepted by a journal before his death last August.
He did the first experiments to develop the chemical compounds needed for the multicolor imaging method nearly 15 years ago.
“One theme that has gone through all of Roger’s work is the desire to peer more closely into the workings of the cell,” Dr Adams said.
“With all of the fluorescence techniques that he’s introduced, he was able to do that in live cells and make action movies of them in vivid colors. But he always wanted to look closer, and now he’s left the beginnings for a method where we can add colors to electron microscopy.”
“This is clearly an example of Roger’s brilliance at chemistry and how he saw that, if we could do this, we would be able to enjoy the advantages of electron microscopy,” Dr Ellisman added.
“The biggest advantage of electron microscopy that we saw is that you have weak contrasts by the nature of the way that staining works, so color-specific labels give context to all of the rich information in the scene of which molecules are operating.” ![]()

of endosomal uptake
of peptide proteins
Image by Adams et al/
Cell Chemical Biology 2016
Researchers say they have found a way to image specific cellular components using multicolor electron microscopy.
With their method, which the team worked on for nearly 15 years, up to 3 colors at a time (green, red, or yellow) can be used in an image.
A detector on the microscope captures electrons lost from metal ions painted over the specimen and records the metal’s energy loss signature as a color.
A technician must add the ionized metals one at a time and then lay the full color map over the still microscopy image.
The researchers described this method in detail in Cell Chemical Biology.
“This method has many potential applications in biology,” said study author Stephen Adams, PhD, of the University of California, San Diego in La Jolla.
“In the paper, we demonstrate how it can distinguish cellular compartments or track proteins and tag cells.”
For the multicolor effect to work, the researchers needed metal complexes that are stable enough to withstand application (meaning they don’t quickly deteriorate and blur the image) and have a distinct electron energy loss signature.
The researchers used ionized lanthanum (La), cerium (Ce), and praseodymium (Pr)—all metals in the lanthanide family. Each metal complex was laid down sequentially as a precipitate onto the specimen as it sat in the microscope.
“One challenge that kept us from publishing this much earlier, because we had the chemistry and we had an instrument that worked about 4 years ago, was we needed a way to deposit the metal compounds sequentially,” said study author Mark Ellisman, PhD, of the University of California, San Diego.
“We spent an awful lot of time trying to figure out how to deposit one of the lanthanides and then clear it so that it didn’t react when we deposited a second signal on the first site.”
Once the application process had been established, the researchers illustrated the power of multicolor electron microscopy by visualizing 2 brain cells sharing a single synapse. They also showed peptides entering through a cell membrane.
The researchers said there is more chemistry to be done to perfect the metal ion application process as well as produce images with more colors. There may also be ways to increase the amount of metal ions that can be deposited, which could help with resolution.
Many in the biochemical community should be able to begin using this technique right away, as it takes advantage of tools that are already found in laboratories.
This study is one of the last that Roger Tsien, PhD, who won a 2008 Nobel Prize in Chemistry for the discovery and application of green fluorescent protein to biochemical imaging, saw accepted by a journal before his death last August.
He did the first experiments to develop the chemical compounds needed for the multicolor imaging method nearly 15 years ago.
“One theme that has gone through all of Roger’s work is the desire to peer more closely into the workings of the cell,” Dr Adams said.
“With all of the fluorescence techniques that he’s introduced, he was able to do that in live cells and make action movies of them in vivid colors. But he always wanted to look closer, and now he’s left the beginnings for a method where we can add colors to electron microscopy.”
“This is clearly an example of Roger’s brilliance at chemistry and how he saw that, if we could do this, we would be able to enjoy the advantages of electron microscopy,” Dr Ellisman added.
“The biggest advantage of electron microscopy that we saw is that you have weak contrasts by the nature of the way that staining works, so color-specific labels give context to all of the rich information in the scene of which molecules are operating.” ![]()