User login
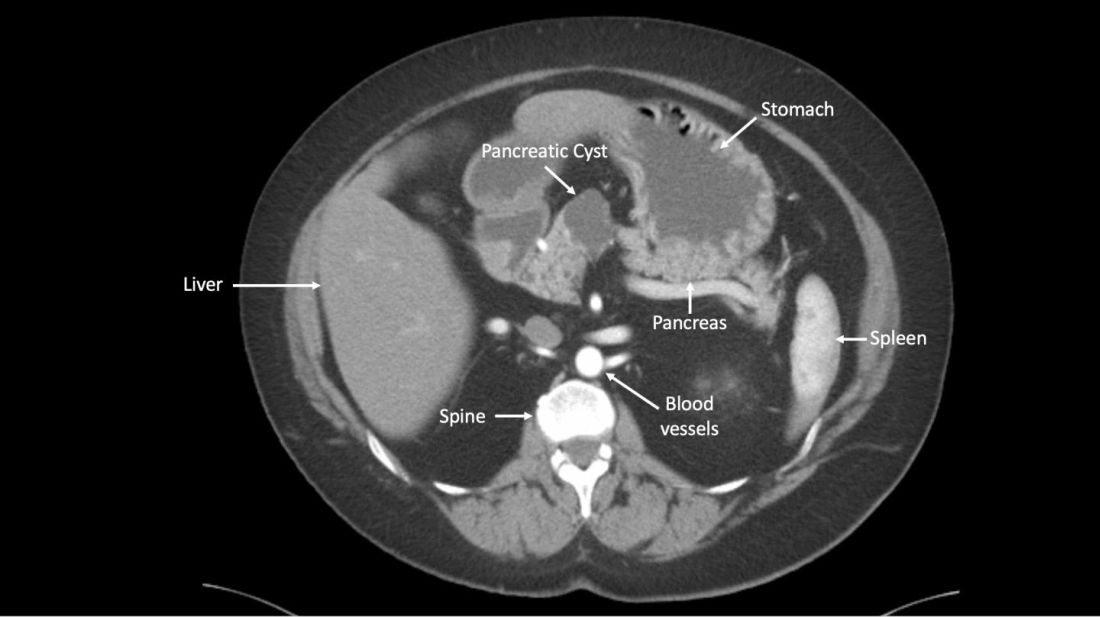
A newly developed test could help clinicians more accurately identify which patients with pancreatic cysts require surgery, according to researchers.
The test, CompCyst, incorporates clinical and imaging data as well as data on genetic and biochemical markers associated with pancreatic cancer.
CompCyst proved more effective than standard practice in estimating the risk of cancer so as to differentiate patients who should undergo surgery from patients who require monitoring and those who need no additional care.
Simeon Springer, PhD, of Ring Therapeutics in Cambridge, Mass., and colleagues described the development and testing of CompCyst in Science Translational Medicine.
The researchers collected data from 875 patients who had undergone surgical resection of pancreatic cysts. The team used clinical, imaging, and molecular data from 436 of those patients to train CompCyst to classify patients into three categories.
- Patients with benign, nonmucin-producing cysts who do not require surgery or monitoring
- Patients who require monitoring because they have mucin-producing cysts with low- or intermediate-grade dysplasia
- Patients who have invasive cancer or high-grade dysplasia and require surgery.
The researchers then tested CompCyst in the remaining 426 patients (the validation cohort), comparing CompCyst with standard practice, which involves use of clinical and imaging criteria only.
“Our aim [in developing CompCyst] was not to replace current knowledge derived from clinical data and imaging characteristics with molecular testing but, rather, to integrate all these aspects together,” study author Marco Dal Molin, MD, of Johns Hopkins University, Baltimore, said in a press conference.
“An important aspect of this paper is the comparison between the performance of our test and current clinical practice. Because the histopathology of all cysts was known from surgical specimens, we could determine, in retrospect, what the optimal treatment should have been.”
Histopathology showed that 53 patients in the validation cohort had a benign, nonmucin-producing cyst and did not require any additional intervention. Standard practice correctly identified 19% (n =10) of these patients, while CompCyst correctly identified 60% (n = 32).
There were 140 patients who had mucin-producing cysts with low- or intermediate-grade dysplasia. Standard practice correctly identified 34% (n = 48) of these patients, while CompCyst correctly identified 49% (n = 68).
“Overall, the use of CompCyst would have avoided unnecessary surgery in 60% of the patients in this study,” Dr. Dal Molin said.
Surgery was needed in 152 patients in the validation cohort. Standard practice correctly identified 89% (n = 135) of these patients, while CompCyst correctly identified 91% (n = 138). Neither method would have recommended discharge for any patient who actually required surgery, the researchers noted.
Based on these results, the researchers are hoping to make CompCyst available to patients at Johns Hopkins within the next 6-12 months.
“In the long term, we hope that a new, prospective study will be carried out, which will gain approval of this test by the FDA [Food and Drug Administration],” study author Bert Vogelstein, MD, of Johns Hopkins, said at the press conference.
“Then, at that point, we hope the technology can be commercialized and offered to the public through a company called Thrive [Earlier Detection], which has licensed the technology from Johns Hopkins.”
This research was supported by the Lustgarten Foundation for Pancreatic Cancer Research, the Virginia and D.K. Ludwig Fund for Cancer Research, the Sol Goldman Pancreatic Cancer Research Center, the Michael Rolfe Pancreatic Cancer Foundation, the Benjamin Baker Scholarship, and the National Institutes of Health. The researchers reported relationships with Thrive Earlier Detection, Personal Genome Diagnostics, Eisai-Morphotek, Sysmex Inostics, Nexus Strategy (Camden Partners), NeoPhore, and CAGE.
SOURCE: Springer S et al. Sci Transl Med. 2019 Jul 17. doi: 10.1126/scitranslmed.aav477.
A newly developed test could help clinicians more accurately identify which patients with pancreatic cysts require surgery, according to researchers.
The test, CompCyst, incorporates clinical and imaging data as well as data on genetic and biochemical markers associated with pancreatic cancer.
CompCyst proved more effective than standard practice in estimating the risk of cancer so as to differentiate patients who should undergo surgery from patients who require monitoring and those who need no additional care.
Simeon Springer, PhD, of Ring Therapeutics in Cambridge, Mass., and colleagues described the development and testing of CompCyst in Science Translational Medicine.
The researchers collected data from 875 patients who had undergone surgical resection of pancreatic cysts. The team used clinical, imaging, and molecular data from 436 of those patients to train CompCyst to classify patients into three categories.
- Patients with benign, nonmucin-producing cysts who do not require surgery or monitoring
- Patients who require monitoring because they have mucin-producing cysts with low- or intermediate-grade dysplasia
- Patients who have invasive cancer or high-grade dysplasia and require surgery.
The researchers then tested CompCyst in the remaining 426 patients (the validation cohort), comparing CompCyst with standard practice, which involves use of clinical and imaging criteria only.
“Our aim [in developing CompCyst] was not to replace current knowledge derived from clinical data and imaging characteristics with molecular testing but, rather, to integrate all these aspects together,” study author Marco Dal Molin, MD, of Johns Hopkins University, Baltimore, said in a press conference.
“An important aspect of this paper is the comparison between the performance of our test and current clinical practice. Because the histopathology of all cysts was known from surgical specimens, we could determine, in retrospect, what the optimal treatment should have been.”
Histopathology showed that 53 patients in the validation cohort had a benign, nonmucin-producing cyst and did not require any additional intervention. Standard practice correctly identified 19% (n =10) of these patients, while CompCyst correctly identified 60% (n = 32).
There were 140 patients who had mucin-producing cysts with low- or intermediate-grade dysplasia. Standard practice correctly identified 34% (n = 48) of these patients, while CompCyst correctly identified 49% (n = 68).
“Overall, the use of CompCyst would have avoided unnecessary surgery in 60% of the patients in this study,” Dr. Dal Molin said.
Surgery was needed in 152 patients in the validation cohort. Standard practice correctly identified 89% (n = 135) of these patients, while CompCyst correctly identified 91% (n = 138). Neither method would have recommended discharge for any patient who actually required surgery, the researchers noted.
Based on these results, the researchers are hoping to make CompCyst available to patients at Johns Hopkins within the next 6-12 months.
“In the long term, we hope that a new, prospective study will be carried out, which will gain approval of this test by the FDA [Food and Drug Administration],” study author Bert Vogelstein, MD, of Johns Hopkins, said at the press conference.
“Then, at that point, we hope the technology can be commercialized and offered to the public through a company called Thrive [Earlier Detection], which has licensed the technology from Johns Hopkins.”
This research was supported by the Lustgarten Foundation for Pancreatic Cancer Research, the Virginia and D.K. Ludwig Fund for Cancer Research, the Sol Goldman Pancreatic Cancer Research Center, the Michael Rolfe Pancreatic Cancer Foundation, the Benjamin Baker Scholarship, and the National Institutes of Health. The researchers reported relationships with Thrive Earlier Detection, Personal Genome Diagnostics, Eisai-Morphotek, Sysmex Inostics, Nexus Strategy (Camden Partners), NeoPhore, and CAGE.
SOURCE: Springer S et al. Sci Transl Med. 2019 Jul 17. doi: 10.1126/scitranslmed.aav477.
A newly developed test could help clinicians more accurately identify which patients with pancreatic cysts require surgery, according to researchers.
The test, CompCyst, incorporates clinical and imaging data as well as data on genetic and biochemical markers associated with pancreatic cancer.
CompCyst proved more effective than standard practice in estimating the risk of cancer so as to differentiate patients who should undergo surgery from patients who require monitoring and those who need no additional care.
Simeon Springer, PhD, of Ring Therapeutics in Cambridge, Mass., and colleagues described the development and testing of CompCyst in Science Translational Medicine.
The researchers collected data from 875 patients who had undergone surgical resection of pancreatic cysts. The team used clinical, imaging, and molecular data from 436 of those patients to train CompCyst to classify patients into three categories.
- Patients with benign, nonmucin-producing cysts who do not require surgery or monitoring
- Patients who require monitoring because they have mucin-producing cysts with low- or intermediate-grade dysplasia
- Patients who have invasive cancer or high-grade dysplasia and require surgery.
The researchers then tested CompCyst in the remaining 426 patients (the validation cohort), comparing CompCyst with standard practice, which involves use of clinical and imaging criteria only.
“Our aim [in developing CompCyst] was not to replace current knowledge derived from clinical data and imaging characteristics with molecular testing but, rather, to integrate all these aspects together,” study author Marco Dal Molin, MD, of Johns Hopkins University, Baltimore, said in a press conference.
“An important aspect of this paper is the comparison between the performance of our test and current clinical practice. Because the histopathology of all cysts was known from surgical specimens, we could determine, in retrospect, what the optimal treatment should have been.”
Histopathology showed that 53 patients in the validation cohort had a benign, nonmucin-producing cyst and did not require any additional intervention. Standard practice correctly identified 19% (n =10) of these patients, while CompCyst correctly identified 60% (n = 32).
There were 140 patients who had mucin-producing cysts with low- or intermediate-grade dysplasia. Standard practice correctly identified 34% (n = 48) of these patients, while CompCyst correctly identified 49% (n = 68).
“Overall, the use of CompCyst would have avoided unnecessary surgery in 60% of the patients in this study,” Dr. Dal Molin said.
Surgery was needed in 152 patients in the validation cohort. Standard practice correctly identified 89% (n = 135) of these patients, while CompCyst correctly identified 91% (n = 138). Neither method would have recommended discharge for any patient who actually required surgery, the researchers noted.
Based on these results, the researchers are hoping to make CompCyst available to patients at Johns Hopkins within the next 6-12 months.
“In the long term, we hope that a new, prospective study will be carried out, which will gain approval of this test by the FDA [Food and Drug Administration],” study author Bert Vogelstein, MD, of Johns Hopkins, said at the press conference.
“Then, at that point, we hope the technology can be commercialized and offered to the public through a company called Thrive [Earlier Detection], which has licensed the technology from Johns Hopkins.”
This research was supported by the Lustgarten Foundation for Pancreatic Cancer Research, the Virginia and D.K. Ludwig Fund for Cancer Research, the Sol Goldman Pancreatic Cancer Research Center, the Michael Rolfe Pancreatic Cancer Foundation, the Benjamin Baker Scholarship, and the National Institutes of Health. The researchers reported relationships with Thrive Earlier Detection, Personal Genome Diagnostics, Eisai-Morphotek, Sysmex Inostics, Nexus Strategy (Camden Partners), NeoPhore, and CAGE.
SOURCE: Springer S et al. Sci Transl Med. 2019 Jul 17. doi: 10.1126/scitranslmed.aav477.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A test called CompCyst could help clinicians more accurately identify which patients with pancreatic cysts require surgery.
Major finding: CompCyst correctly identified 91% of patients who required surgery, 49% of those who required monitoring, and 60% of patients who required no further care.
Study details: A retrospective analysis of 875 patients with pancreatic cysts
Disclosures: The research was supported by the Lustgarten Foundation for Pancreatic Cancer Research, the Virginia and D.K. Ludwig Fund for Cancer Research, the Sol Goldman Pancreatic Cancer Research Center, the Michael Rolfe Pancreatic Cancer Foundation, the Benjamin Baker Scholarship, and the National Institutes of Health. The researchers reported relationships with Thrive Earlier Detection, Personal Genome Diagnostics, Eisai-Morphotek, Sysmex Inostics, Nexus Strategy (Camden Partners), NeoPhore, and CAGE.
Source: Springer S et al. Sci Transl Med. 2019 Jul 17. doi: 10.1126/scitranslmed.aav477.