User login
Malignant mesothelioma poses a significant challenge for clinicians because of its ability to resist chemotherapy, but the use of three-dimensional tumor spheroid models has shown that local administration of paclitaxel in a nanoparticle platform achieved better tumor penetration than conventional paclitaxel therapy, investigators reported. The study is in the May issue of the Journal of Thoracic and Cardiovascular Surgery.
Dr. Hongyi Lei of Brigham and Women’s Hospital, Boston, and his colleagues used the in vitro mesothelioma spheroid model because two-dimensional in vitro monolayer cell culture experiments do not replicate the superior efficacy of paclitaxel-loaded expansile nanoparticles (Pax-eNPs), suggesting that Pax-eNPs utilize a unique drug delivery mechanism.
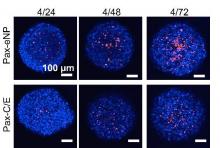
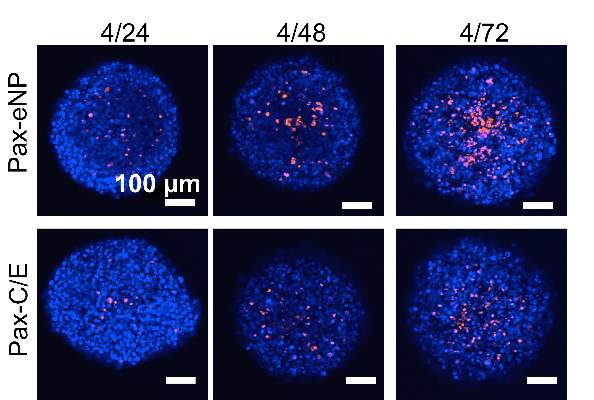
The study observed that spheroids treated with Pax-eNP showed increased drug penetration and a 38-fold higher intraspheroidal drug concentration at 24 hours than that of paclitaxel dissolved in Cremophor EL/ethanol (J. Thorac. Cardiovasc. Surg. 2014 [doi:10.1016/j.jtcvs.2015.02.020]).
The researchers said their findings showed that three-dimensional spheroid models “are valuable tools for investigating cytotoxic mechanisms and nanoparticle-tumor interactions, particularly given the costs and limitations of in vivo animal studies.” Their findings were first presented at the 94th Annual Meeting of the American Association of Thoracic Surgery last year in Toronto.
Despite advances of nanoparticle-based drug delivery systems, difficulties in evaluating the effectiveness of these drugs in local chemotherapy have hindered their adoption in the clinic. Studies of the same agent utilizing in vitro vs. in vivo methods have shown conflicting results.
The observation that Pax-eNP treatment of intraperitoneal mesothelioma significantly improved survival in lab animals in vivo compared to conventional paclitaxel led to the use of the three-dimensional spheroid model. Dr. Lei and colleagues called this revelation “striking” because Pax-eNP exposure of the identical mesothelioma tumor cells plated as a two-dimensional monolayer in vitro demonstrated equal or worse results. “This suggested that eNP may be more effective at penetration and/or persistence within multicellular tumors and led to the use of a 3-D tumor spheroid mode,” they said.
“Given the high cost and limitations of in vivo animal studies, spheroid models may present a clinically relevant platform for screening novel pharmaceuticals and unique drug-delivery systems during the preclinical phase,” the researchers indicated.
They also investigated spheroid cytotoxicity in a clinic-like setting following a 4-hour, high-dose (1,000 ng/mL) paclitaxel exposure via conventional and eNP vehicles. They found that Pax-eNP exposure led to greater tumor cytotoxicity at 72 hours, and that cytotoxicity continued seven days later because Pax-eNPs rapidly enter the tumor spheroid and remain intracellular, slowly releasing the drug.
“The prolonged drug release mechanism that pH-triggered Pax-eNP uses appears to be unique, leading to markedly higher intraspheroidal drug delivery, prolonged intratumoral drug release and superior antitumor efficacy,” the investigators concluded. The authors had no disclosures.
“While this study contributes greatly to the body of knowledge available regarding potential treatment strategies for malignant mesothelioma, one might argue that the more important impact of this paper relates to the successful implementation of an unconventional tumor model,” Dr. Mara B. Antonoff wrote in her invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi: 10.1016/j.jtcvs.2015.02.015].
The investigators’ earlier studies noted the limitations of the two-dimensional cell monolayer for in vitro experiments, but Dr. Antonoff acknowledged their quest for a solution that was more cost-effective than animal models and better approximated in vivo biological actions of the drug.

|
Dr. Mara B. Antonoff |
However, she noted the three-dimensional models “are not without limits, either.” Widespread adoption is limited because of the time and expertise involved in spheroid formation. “Nonetheless, it is clear that such models are a huge improvement over our current in vitro models,” she wrote.
Among the limits of the three-dimensional spheroid model she pointed out are that it lacks the multiple cell types typical in an actual tumor and quantitative assessment of the results can be difficult.
But the spheroid model does enhance the ability to screen novel drugs and drug-delivery systems, she wrote. “This timely and well-constructed study provides a foundation upon which we may build our knowledge of chemotherapeutic delivery mechanisms, while setting an outstanding example, and perhaps a new standard, for in vitro methodology,” she wrote.
Dr. Antonoff is a clinical instructor at University of Texas M.D. Anderson Cancer Center in Houston.
“While this study contributes greatly to the body of knowledge available regarding potential treatment strategies for malignant mesothelioma, one might argue that the more important impact of this paper relates to the successful implementation of an unconventional tumor model,” Dr. Mara B. Antonoff wrote in her invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi: 10.1016/j.jtcvs.2015.02.015].
The investigators’ earlier studies noted the limitations of the two-dimensional cell monolayer for in vitro experiments, but Dr. Antonoff acknowledged their quest for a solution that was more cost-effective than animal models and better approximated in vivo biological actions of the drug.

|
Dr. Mara B. Antonoff |
However, she noted the three-dimensional models “are not without limits, either.” Widespread adoption is limited because of the time and expertise involved in spheroid formation. “Nonetheless, it is clear that such models are a huge improvement over our current in vitro models,” she wrote.
Among the limits of the three-dimensional spheroid model she pointed out are that it lacks the multiple cell types typical in an actual tumor and quantitative assessment of the results can be difficult.
But the spheroid model does enhance the ability to screen novel drugs and drug-delivery systems, she wrote. “This timely and well-constructed study provides a foundation upon which we may build our knowledge of chemotherapeutic delivery mechanisms, while setting an outstanding example, and perhaps a new standard, for in vitro methodology,” she wrote.
Dr. Antonoff is a clinical instructor at University of Texas M.D. Anderson Cancer Center in Houston.
“While this study contributes greatly to the body of knowledge available regarding potential treatment strategies for malignant mesothelioma, one might argue that the more important impact of this paper relates to the successful implementation of an unconventional tumor model,” Dr. Mara B. Antonoff wrote in her invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi: 10.1016/j.jtcvs.2015.02.015].
The investigators’ earlier studies noted the limitations of the two-dimensional cell monolayer for in vitro experiments, but Dr. Antonoff acknowledged their quest for a solution that was more cost-effective than animal models and better approximated in vivo biological actions of the drug.

|
Dr. Mara B. Antonoff |
However, she noted the three-dimensional models “are not without limits, either.” Widespread adoption is limited because of the time and expertise involved in spheroid formation. “Nonetheless, it is clear that such models are a huge improvement over our current in vitro models,” she wrote.
Among the limits of the three-dimensional spheroid model she pointed out are that it lacks the multiple cell types typical in an actual tumor and quantitative assessment of the results can be difficult.
But the spheroid model does enhance the ability to screen novel drugs and drug-delivery systems, she wrote. “This timely and well-constructed study provides a foundation upon which we may build our knowledge of chemotherapeutic delivery mechanisms, while setting an outstanding example, and perhaps a new standard, for in vitro methodology,” she wrote.
Dr. Antonoff is a clinical instructor at University of Texas M.D. Anderson Cancer Center in Houston.
Malignant mesothelioma poses a significant challenge for clinicians because of its ability to resist chemotherapy, but the use of three-dimensional tumor spheroid models has shown that local administration of paclitaxel in a nanoparticle platform achieved better tumor penetration than conventional paclitaxel therapy, investigators reported. The study is in the May issue of the Journal of Thoracic and Cardiovascular Surgery.
Dr. Hongyi Lei of Brigham and Women’s Hospital, Boston, and his colleagues used the in vitro mesothelioma spheroid model because two-dimensional in vitro monolayer cell culture experiments do not replicate the superior efficacy of paclitaxel-loaded expansile nanoparticles (Pax-eNPs), suggesting that Pax-eNPs utilize a unique drug delivery mechanism.
The study observed that spheroids treated with Pax-eNP showed increased drug penetration and a 38-fold higher intraspheroidal drug concentration at 24 hours than that of paclitaxel dissolved in Cremophor EL/ethanol (J. Thorac. Cardiovasc. Surg. 2014 [doi:10.1016/j.jtcvs.2015.02.020]).
The researchers said their findings showed that three-dimensional spheroid models “are valuable tools for investigating cytotoxic mechanisms and nanoparticle-tumor interactions, particularly given the costs and limitations of in vivo animal studies.” Their findings were first presented at the 94th Annual Meeting of the American Association of Thoracic Surgery last year in Toronto.
Despite advances of nanoparticle-based drug delivery systems, difficulties in evaluating the effectiveness of these drugs in local chemotherapy have hindered their adoption in the clinic. Studies of the same agent utilizing in vitro vs. in vivo methods have shown conflicting results.
The observation that Pax-eNP treatment of intraperitoneal mesothelioma significantly improved survival in lab animals in vivo compared to conventional paclitaxel led to the use of the three-dimensional spheroid model. Dr. Lei and colleagues called this revelation “striking” because Pax-eNP exposure of the identical mesothelioma tumor cells plated as a two-dimensional monolayer in vitro demonstrated equal or worse results. “This suggested that eNP may be more effective at penetration and/or persistence within multicellular tumors and led to the use of a 3-D tumor spheroid mode,” they said.
“Given the high cost and limitations of in vivo animal studies, spheroid models may present a clinically relevant platform for screening novel pharmaceuticals and unique drug-delivery systems during the preclinical phase,” the researchers indicated.
They also investigated spheroid cytotoxicity in a clinic-like setting following a 4-hour, high-dose (1,000 ng/mL) paclitaxel exposure via conventional and eNP vehicles. They found that Pax-eNP exposure led to greater tumor cytotoxicity at 72 hours, and that cytotoxicity continued seven days later because Pax-eNPs rapidly enter the tumor spheroid and remain intracellular, slowly releasing the drug.
“The prolonged drug release mechanism that pH-triggered Pax-eNP uses appears to be unique, leading to markedly higher intraspheroidal drug delivery, prolonged intratumoral drug release and superior antitumor efficacy,” the investigators concluded. The authors had no disclosures.
Malignant mesothelioma poses a significant challenge for clinicians because of its ability to resist chemotherapy, but the use of three-dimensional tumor spheroid models has shown that local administration of paclitaxel in a nanoparticle platform achieved better tumor penetration than conventional paclitaxel therapy, investigators reported. The study is in the May issue of the Journal of Thoracic and Cardiovascular Surgery.
Dr. Hongyi Lei of Brigham and Women’s Hospital, Boston, and his colleagues used the in vitro mesothelioma spheroid model because two-dimensional in vitro monolayer cell culture experiments do not replicate the superior efficacy of paclitaxel-loaded expansile nanoparticles (Pax-eNPs), suggesting that Pax-eNPs utilize a unique drug delivery mechanism.
The study observed that spheroids treated with Pax-eNP showed increased drug penetration and a 38-fold higher intraspheroidal drug concentration at 24 hours than that of paclitaxel dissolved in Cremophor EL/ethanol (J. Thorac. Cardiovasc. Surg. 2014 [doi:10.1016/j.jtcvs.2015.02.020]).
The researchers said their findings showed that three-dimensional spheroid models “are valuable tools for investigating cytotoxic mechanisms and nanoparticle-tumor interactions, particularly given the costs and limitations of in vivo animal studies.” Their findings were first presented at the 94th Annual Meeting of the American Association of Thoracic Surgery last year in Toronto.
Despite advances of nanoparticle-based drug delivery systems, difficulties in evaluating the effectiveness of these drugs in local chemotherapy have hindered their adoption in the clinic. Studies of the same agent utilizing in vitro vs. in vivo methods have shown conflicting results.
The observation that Pax-eNP treatment of intraperitoneal mesothelioma significantly improved survival in lab animals in vivo compared to conventional paclitaxel led to the use of the three-dimensional spheroid model. Dr. Lei and colleagues called this revelation “striking” because Pax-eNP exposure of the identical mesothelioma tumor cells plated as a two-dimensional monolayer in vitro demonstrated equal or worse results. “This suggested that eNP may be more effective at penetration and/or persistence within multicellular tumors and led to the use of a 3-D tumor spheroid mode,” they said.
“Given the high cost and limitations of in vivo animal studies, spheroid models may present a clinically relevant platform for screening novel pharmaceuticals and unique drug-delivery systems during the preclinical phase,” the researchers indicated.
They also investigated spheroid cytotoxicity in a clinic-like setting following a 4-hour, high-dose (1,000 ng/mL) paclitaxel exposure via conventional and eNP vehicles. They found that Pax-eNP exposure led to greater tumor cytotoxicity at 72 hours, and that cytotoxicity continued seven days later because Pax-eNPs rapidly enter the tumor spheroid and remain intracellular, slowly releasing the drug.
“The prolonged drug release mechanism that pH-triggered Pax-eNP uses appears to be unique, leading to markedly higher intraspheroidal drug delivery, prolonged intratumoral drug release and superior antitumor efficacy,” the investigators concluded. The authors had no disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Three-dimensional spheroid models, as opposed to monolayer cell cultures, are valuable tools for predicting the efficacy of nanoparticle-tumor interactions in malignant mesothelioma.
Major finding: There was increased drug penetration and a 38-fold higher drug concentration 24 hours after human malignant mesothelioma spheroids were treated with paclitaxel-loaded expansile nanoparticles, compared to conventional drug delivery.
Data source: A study of a mesothelioma spheroid model comparing treatment with Pax-eNP and paclitaxel dissolved in Cremophor EL/ethanol.
Disclosures: This work was supported by the Brigham and Women’s Hospital International Mesothelioma Program, National Science Foundation and Boston University’s Nanomedicine Program and Cross-Disciplinary Training in Nanotechnology for Cancer, and the Zhujiang Hospital (Guangzhou, China) Scholarship Program. The authors had no relevant disclosures.