User login
Tourette syndrome (TS) is part of a spectrum of tic disorders. Tics are sudden, rapid, stereotyped, repetitive, nonrhythmic movements or vocalizations affecting discrete muscle groups, and are preceded by a sensory component. Patients in whom tic suppression is attempted report the experience of a sensation of inner pressure that must be released. This eventually results in the performance of motor movement or vocal sounds. TS is a disorder of childhood onset that is characterized by multiple motor and vocal tics. In some cases, there are features of obsessive compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), or other behavioral manifestations such as coprolalia, echopraxia, palilalia, and self-injury.1,2 The spectrum of tic disorders includes:
- Transient tics of childhood (tic duration less than 12 months)
- Chronic motor or vocal tics (lasting more than 12 months), and
- TS (variable motor and vocal tics lasting more than 12 months).
Many children meet the diagnostic criteria for TS between the ages of 6 and 9 years, but symptoms may improve by adulthood. The eventual loss of tics over time reflects the maturation of brain systems that control ballistic action.3
The tics that accompany TS may be defined as simple or complex, and as motor or vocal. Simple motor tics involve only a few muscles, such as eye blinking, shoulder shrugging, or facial grimacing. Complex motor tics involve multiple groups of muscles that are recruited in orchestrated bouts (eg, hand gestures, jumping, touching, or pressing), and may include copropraxia (a sudden tic-like vulgar, sexual, or obscene gesture) or echopraxia (involuntary, spontaneous imitation of someone else’s movements). Simple vocal tics are meaningless sounds such as throat clearing, grunting, sniffing, snorting, and chirping. Complex vocal tics involve speech and language such as sudden, spontaneous expression of single words or phrases, or speech blocking.4
Tics may be acquired as a consequence of other disorders, including head trauma, encephalitis, stroke, carbon monoxide poisoning, Creutzfeldt-Jakob disease, neurosyphilis, hypoglycemia, or Sydenham chorea.5 Genetic disorders such as Huntington disease may be associated with tics. Tics may also occur with certain chromosomal abnormalities or be associated with some neuropsychiatric disorders. Finally, tics may be caused by a large number of medications or illicit drugs, including cocaine, amphetamines, antipsychotics, and antidepressants.
The prevalence of all types of tics in childhood is approximately 6% to 12%, although the prevalence of chronic vocal tics is approximately 1 to 10 per 1,000 children and adolescents.6 TS is especially common among autistic children and in those with Asperger syndrome and other autistic spectrum disorders. A survey of patients at Cleveland Clinic Florida found that tics and TS accounted for 8% of all patients with movement disorders. Of patients with tics or TS who were older than 18 years, 70% were male.
PATHOPHYSIOLOGY OF TOURETTE SYNDROME: ROLE OF THE BASAL GANGLIA
Although the pathophysiology of TS is not completely understood, abnormal function of the basal ganglia is thought to be a central component of the disorder. The basal ganglia normally act to facilitate voluntary movements while suppressing competing involuntary ones. Abnormalities of basal ganglia activity are important in several disorders of motor function.7 Output neurons from the basal ganglia inhibit thalamic motor nuclei and midbrain neurons of the extrapyramidal motor system, and act to inhibit motor pattern generators in the cerebral cortex and brainstem. Hyperkinetic disorders, including tics, chorea, and dystonia, are thought to result at least in part from impaired inhibition of unwanted motor activity from the basal ganglia to downstream motor centers.7
Family heritability studies provide strong support that TS is a genetic disorder. For example, the concordance rate is 86% for monozygotic twins versus 20% for dizygotic twins.8 Chromosomes linked to TS include 2p32.2 and 13q31.1.9,10 Interactions between genetics and environment are also thought to play a significant role. The concept of pediatric autoimmune neuropsychiatric disorders associated with streptococcal (PANDAS) infections has been proposed to explain an apparent temporal association between streptococcal infections and exacerbation of tics. According to this model, molecular mimicry between streptococcal antigens and endogenous brain antigens results in an autoimmune attack.11 However, the identification of specific antibodies against basal ganglia cells remains controversial.12
MANAGEMENT OVERVIEW
Accurate diagnosis of TS is essential, and includes a complete history and neurologic examination. The tic phenomenology (complex vs simple) should be characterized, and the patient should be carefully questioned to identify the symptoms that are most bothersome (eg, motor or vocal tics, OCD, or ADHD). Pharmacotherapy should be reserved for problems that are functionally disabling and not remediable by nonpharmacologic interventions.
Treatment may also be required for other neuropsychiatric symptoms. Anxiety and depression have been reported in 19% to 80% of patients with tics, and depression is strongly correlated with the duration and severity of tics.13,14 Episodic outburst (rage), self-injurious, OCD, antisocial, and oppositional behaviors are all more common among individuals with tic disorders.15 Personality disorders may be related to OCD, ADHD, or to family or economic issues. Tic disorders are also associated with an increased incidence of somatic complaints, as well as higher rates of academic difficulties, which may be related to ADHD or medications. Sleep disturbances affect an estimated 20% to 50% of patients, and may include difficulty initiating or maintaining sleep, restlessness, movement-related arousal, or parasomnia.16
Education is an important part of treatment, and may include the patient, family members, teachers or other school staff, and work colleagues. A number of behavioral or psychosocial approaches may help to improve tics, including conditioning techniques, relaxation training, biofeedback, habit reversal, awareness training, and hypnosis.17
PHARMACOLOGIC TREATMENT: THREE TIERS
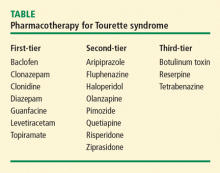
First-tier therapies
The alpha-adrenergic blockers clonidine and guanfacine are first-tier therapies. Treatment should be initiated at a low dose and escalated gradually according to response, which is determined by the severity, and not the presence, of tics. Clonidine may be administered at a dose of 0.025 mg two or three times daily or, for maintenance, 0.1 mg three times daily; another option is 0.1, 0.2, or 0.3 mg weekly by transdermal administration. Guanfacine may be administered at a dose of 1 mg once daily. Alpha-adrenergic blockers are useful for the treatment of mild tics, and are considered first-line therapy for tic suppression. Side effects may include dry mouth, somnolence, and, rarely, blood pressure fluctuations.
Agents that affect gamma-amino butyric acid (GABA) neurotransmission have been associated with improved symptoms of tic disorders.18 For example, both clonazepam and diazepam have been reported to reduce TS symptoms.18 Both of these benzodiazepines are associated with sedation, blunting of cognition, and exacerbation of depression, however.19,20
Second-tier therapies
Second-tier therapies, consisting of neuroleptics, induce a rapid treatment response. Haloperidol may be started at a dose of 0.25 mg once daily, with a maintenance dosage of 0.5 to 3.0 mg/day. Cognitive blunting or extrapyramidal side effects are rare in patients with TS, but the potential for these side effects should be thoroughly discussed with the patient or parent/guardian before treatment. Pimozide 0.5 mg (2 to 6 mg/day for maintenance) may be associated with tremor or parkinsonian symptoms (predominantly akinesia). Risperidone 0.25 mg/day (0.5 to 4 mg/day for maintenance), olanzapine 2.5 mg/day (5 to 10 mg/day for maintenance), and quetiapine 25 mg twice daily (100 to 300 mg/day for maintenance) are associated with potential adverse effects of extrapyramidal symptoms, weight gain, and diabetes.
Third-tier therapies
Dopamine agonists (reserpine and tetrabenazine) and botulinum toxin are third-tier therapies. Reserpine, although rarely used in current clinical practice, may be administered at doses of 0.1 to 0.25 mg/day, titrating upward on the basis of clinical response. Tetrabenazine may be administered at a starting dose of 12.5 mg/day, with higher doses as needed depending on the response to treatment. Adverse effects include hypotension, sedation, extrapyramidal symptoms (predominantly parkinsonism), and depression.21
The exact mechanism by which tetrabenazine produces this suppression effect is unknown, but it is believed to be related to its effect of reversibly depleting monoamines. At least three neuronal protein classes regulate the effects of dopamine on voluntary and involuntary movement.22 The two presynaptic proteins are vesicular monoamine transporter subtype 2 (VMAT2) and dopamine transporter (DAT). Postsynaptically, dopamine activity is regulated by G-protein–linked dopamine receptors (eg, the D2 receptor). Tetrabenazine reduces the uptake of monoamines (including dopamine) into synaptic vesicles by reversibly binding to VMAT2, resulting in degradation of dopamine within axon terminals by monoamine oxidases.23 By blocking dopamine transport, tetrabenazine depletes dopamine with greater selectivity than it does other monoamines.24
The dosage of tetrabenazine for the treatment of motor disorders, particularly chorea, was established in the Huntington Study Group (HSG) clinical trial.25 In the HSG trial, a starting dose of 12.5 mg on day 1 was increased to 12.5 mg twice daily on days 2 to 7, and then by 12.5 mg/day at weekly intervals until the desired clinical effect, intolerable adverse effects, or a maximum dose of 100 mg/day was reached. Daily dosages of 37.5 mg or more are administered in three divided doses. Adverse events (reported in 70% of patients who received placebo and 91% of patients who received tetrabenazine) include sedation or somnolence, insomnia, and fatigue.21 These findings may be carried over to patients with tics.
Botulinum toxin may also help to control tics—especially dystonic tics. The premonitory symptoms of TS are usually unaffected by botulinum toxin.26 The adverse effect profile for patients with TS is similar to that of patients with dystonia or facial dyskinesia, and may include soreness, transient weakness, ptosis (if injected for eye blinking), and mild transient dysphagia (if injected into the larynx).27
MANAGING COMORBID CONDITIONS
Approximately 30% of patients with TS also have OCD.28 Treatment options include selective serotonin reuptake inhibitors at standard doses, and the tricyclic antidepressant clomipramine (25 mg once or twice daily, or 75 mg/day in sustained-release form). Trazodone, a serotonin antagonist and reuptake inhibitor that is associated with a lower incidence of anticholinergic effects, may be initiated at a dose of 50 mg/day and slowly increased to 150 to 400 mg/day depending on clinical response.
As many as 60% of patients with TS may also have ADHD.28 Methylphenidate is helpful for the treatment of ADHD and does not exacerbate tics, but it is a restricted medication. The recommended dose is 20 mg once daily, titrated upward as needed based on response. Atomoxetine carries a warning regarding increased risk of suicide. It has also been associated with an increased risk of sexual dysfunction and behavioral changes, including aggressive behaviors, agitation, and irratibility.29
DEEP BRAIN STIMULATION
Deep brain stimulation (DBS) has been shown to improve TS in single-case studies and in small series, although the long-term benefit is unclear. Potential targets of stimulation include midline thalamic centromedian-parafascicular (CM-PF) nuclei, the ventralis oralis complex of the thalamus, motor and limbic globus pallidus pars interna (GPi), and the anterior limb of the internal capsule.30 In particular, stimulation of the sensorimotor GPi may ameliorate hyperkinetic states.
One report described the results of DBS implantation in a 15-year-old boy with TS who had not responded to several pharmacologic treatment options.31 Six months after implantation, the patient exhibited markedly improved tic severity as measured using the Yale Global Tic Severity Scale, including a 76% reduction in motor tic severity, 68% reduction in vocal tics, and a complete resolution of impairment.31
Published consensus criteria for the selection of suitable candidates for DBS include age greater than 25 years, chronic and severe tics with severe functional impairment for at least 12 months, tics that are frequent and noticeable in most situations most of the time, failure of conventional medical therapy, medical stability for 6 months, and willingness to participate in ongoing psychologic interventions.32 Exclusion criteria include the presence of another medical condition that could explain the tics, an unstable medical condition, being considered likely to benefit from psychologic interventions, psychosocial factors that may complicate the recovery process or make it difficult to assess outcome, and unwillingness to participate in ongoing treatment for psychosocial problems or risk factors. Other factors that should be considered include comorbidities, the variability in tic severity over time, the involvement of a multidisciplinary treatment team, results of a thorough neuropsychologic assessment, expertise of the surgical team, and access to imaging facilities for presurgical mapping and postsurgical evaluation.
SUMMARY AND CONCLUSIONS
Tourette syndrome is not uncommon among the adult population of a typical neurology practice, and should not be considered exclusively a pediatric diagnosis. Several treatment options are available, including behavioral approaches and several medications. Treatment should focus on the most disabling symptoms. Neuropsychologic assessment and psychiatric support may be necessary for some patients. The same comorbidities that are encountered in children are usually evident in adult patients as well. In medically refractory cases, DBS surgery may be helpful.
- Robertson MM. Annotation: Gilles de la Tourette syndrome—an update. J Child Psychol Psychiatry 1994; 35:597–611.
- Robertson MM, Althoff RR, Hafez A, Pauls DL. Principal components analysis of a large cohort with Tourette syndrome. Br J Psychiatry 2008; 193:31–36.
- Flaherty AW. Movement disorders. In: Stern TA, Rosenbaum JF, Fava M, Biederman J, Rauch SL, eds. Massachusetts General Hospital: Comprehensive Clinical Psychiatry E-Book. Philadelphia, PA: Mosby Elsevier; 2008.
- Müller N. Tourette’s syndrome: clinical features, pathophysiology, and therapeutic approaches. Dialogues Clin Neurosci 2007; 9:161–171.
- Bagheri MM, Kerbeshian J, Burd L. Recognition and management of Tourette’s syndrome and tic disorders. Am Fam Physician 1999; 59:2263–2272,2274.
- Lombroso PJ, Scahill L. Tourette syndrome and obsessive–compulsive disorder [published online ahead of print October 15, 2007]. Brain Dev 2008; 30:231–237. 10.1016/j.braindev.2007.09.001
- Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol 2003; 60:1365–1368.
- Singer HS, Smith-Hicks C, Lieberman D. Tourette syndrome. In: LeDoux M, ed. Animal Models of Movement Disorders. Academic Press; 2005.
- Tourette Syndrome Association International Consortium for Genetics. Genome scan for Tourette disorder in affected-sibling-pair and multigenerational families. Am J Hum Genet 2007; 80:265–272.
- Abelson JF, Kwan KY, O’Roak BJ, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science 2005; 310:317–320.
- Kurlan R. Tourette’s syndrome and ‘PANDAS’: will the relation bear out? Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Neurology 1998; 50:1530–1534.
- Morris CM, Pardo-Villamizar C, Gause CD, Singer HS. Serum autoantibodies measured by immunofluorescence confirm a failure to differentiate PANDAS and Tourette syndrome from controls [published online ahead of print September 27, 2008]. J Neurol Sci 2009; 276:45–48. 10.1016/j.jns.2008.08.032
- Comings BG, Comings DE. A controlled study of Tourette syndrome. V. Depression and mania. Am J Hum Genet 1987; 41:804–821.
- Robertson MM, Williamson F, Eapen V. Depressive symptomatology in young people with Gilles de la Tourette syndrome—a comparison of self-report scales [published online ahead of print February 7, 2006]. J Affect Disord 2006; 91:265–268. 10.1016/j.jad.2005.12.046
- Budman CL, Bruun RD, Park KS, Lesser M, Olson M. Explosive outbursts in children with Tourette’s disorder. J Am Acad Child Adolesc Psychiatry 2000; 39:1270–1276.
- Kostanecka-Endress T, Banaschewski T, Kinkelbur J, et al. Disturbed sleep in children with Tourette syndrome: a polysomnographic study. J Psychosom Res 2003; 55:23–29.
- Peterson AL. Psychosocial management of tics and intentional repetitive behaviors associated with Tourette syndrome. In:Woods DW, Piacentini J, Walkup JT, eds. Treating Tourette Syndrome and Tic Disorders: A Guide for Practitioners. Guilford Press; 2007.
- Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain 2000; 123:425–462.
- Klonopin [package insert]. South San Francisco, CA: Genentech USA, Inc.; 2010.
- Valium [package insert]. Nutley, NJ: Roche Laboratories, Inc.; 2008.
- Xenazine [package insert]. Deerfield, IL: Lundbeck Inc.; 2011.
- Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem 2003; 87:273–289.
- Morrow T. Gene therapy offers HD patients relief from some symptoms. Tetrabenazine inhibits the transport of a molecule called vesicular monoamine transporter type 2 or VMAT2. Manag Care 2008; 17:46–47.
- Pearson SJ, Reynolds GP. Depletion of monoamine transmitters by tetrabenazine in brain tissue in Huntington’s disease. Neuropharmacology 1988; 27:717–719.
- Huntington Study Group. Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology 2006; 66:366–372.
- Jankovic J, Kurlan R. Tourette syndrome: evolving concepts [published online ahead of print April 11, 2011]. Mov Disord 2011; 26:1149–1156. 10.1002/mds.23618
- Swain JE, Leckman JF. Tourette syndrome and tic disorders: overview and practical guide to diagnosis and treatment. Psychiatry (Edgmont) 2005; 2:26–36.
- Gorman DA, Thompson N, Plessen KJ, Robertson MM, Leckman JF, Peterson BS. Psychosocial outcome and psychiatric comorbidity in older adolescents with Tourette syndrome: controlled study. Br J Psychiatry 2010; 197:36–44.
- Strattera [package insert]. Indianapolis, IN: Eli Lilly and Company; 2011.
- Ackermans L, Temel Y, Visser-Vandewalle V. Deep brain stimulation in Tourette’s syndrome. Neurotherapeutics 2008; 5:339–344.
- Shahed J, Poysky J, Kenney C, Simpson R, Jankovic J. GPi deep brain stimulation for Tourette syndrome improves tics and psychiatric comorbidities. Neurology 2007; 68:159–160.
- Mink JW, Walkup J, Frey KA, et al .Patient selection and assessment recommendations for deep brain stimulation in Tourette syndrome. Mov Disord 2006; 21:1831–1838.
Tourette syndrome (TS) is part of a spectrum of tic disorders. Tics are sudden, rapid, stereotyped, repetitive, nonrhythmic movements or vocalizations affecting discrete muscle groups, and are preceded by a sensory component. Patients in whom tic suppression is attempted report the experience of a sensation of inner pressure that must be released. This eventually results in the performance of motor movement or vocal sounds. TS is a disorder of childhood onset that is characterized by multiple motor and vocal tics. In some cases, there are features of obsessive compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), or other behavioral manifestations such as coprolalia, echopraxia, palilalia, and self-injury.1,2 The spectrum of tic disorders includes:
- Transient tics of childhood (tic duration less than 12 months)
- Chronic motor or vocal tics (lasting more than 12 months), and
- TS (variable motor and vocal tics lasting more than 12 months).
Many children meet the diagnostic criteria for TS between the ages of 6 and 9 years, but symptoms may improve by adulthood. The eventual loss of tics over time reflects the maturation of brain systems that control ballistic action.3
The tics that accompany TS may be defined as simple or complex, and as motor or vocal. Simple motor tics involve only a few muscles, such as eye blinking, shoulder shrugging, or facial grimacing. Complex motor tics involve multiple groups of muscles that are recruited in orchestrated bouts (eg, hand gestures, jumping, touching, or pressing), and may include copropraxia (a sudden tic-like vulgar, sexual, or obscene gesture) or echopraxia (involuntary, spontaneous imitation of someone else’s movements). Simple vocal tics are meaningless sounds such as throat clearing, grunting, sniffing, snorting, and chirping. Complex vocal tics involve speech and language such as sudden, spontaneous expression of single words or phrases, or speech blocking.4
Tics may be acquired as a consequence of other disorders, including head trauma, encephalitis, stroke, carbon monoxide poisoning, Creutzfeldt-Jakob disease, neurosyphilis, hypoglycemia, or Sydenham chorea.5 Genetic disorders such as Huntington disease may be associated with tics. Tics may also occur with certain chromosomal abnormalities or be associated with some neuropsychiatric disorders. Finally, tics may be caused by a large number of medications or illicit drugs, including cocaine, amphetamines, antipsychotics, and antidepressants.
The prevalence of all types of tics in childhood is approximately 6% to 12%, although the prevalence of chronic vocal tics is approximately 1 to 10 per 1,000 children and adolescents.6 TS is especially common among autistic children and in those with Asperger syndrome and other autistic spectrum disorders. A survey of patients at Cleveland Clinic Florida found that tics and TS accounted for 8% of all patients with movement disorders. Of patients with tics or TS who were older than 18 years, 70% were male.
PATHOPHYSIOLOGY OF TOURETTE SYNDROME: ROLE OF THE BASAL GANGLIA
Although the pathophysiology of TS is not completely understood, abnormal function of the basal ganglia is thought to be a central component of the disorder. The basal ganglia normally act to facilitate voluntary movements while suppressing competing involuntary ones. Abnormalities of basal ganglia activity are important in several disorders of motor function.7 Output neurons from the basal ganglia inhibit thalamic motor nuclei and midbrain neurons of the extrapyramidal motor system, and act to inhibit motor pattern generators in the cerebral cortex and brainstem. Hyperkinetic disorders, including tics, chorea, and dystonia, are thought to result at least in part from impaired inhibition of unwanted motor activity from the basal ganglia to downstream motor centers.7
Family heritability studies provide strong support that TS is a genetic disorder. For example, the concordance rate is 86% for monozygotic twins versus 20% for dizygotic twins.8 Chromosomes linked to TS include 2p32.2 and 13q31.1.9,10 Interactions between genetics and environment are also thought to play a significant role. The concept of pediatric autoimmune neuropsychiatric disorders associated with streptococcal (PANDAS) infections has been proposed to explain an apparent temporal association between streptococcal infections and exacerbation of tics. According to this model, molecular mimicry between streptococcal antigens and endogenous brain antigens results in an autoimmune attack.11 However, the identification of specific antibodies against basal ganglia cells remains controversial.12
MANAGEMENT OVERVIEW
Accurate diagnosis of TS is essential, and includes a complete history and neurologic examination. The tic phenomenology (complex vs simple) should be characterized, and the patient should be carefully questioned to identify the symptoms that are most bothersome (eg, motor or vocal tics, OCD, or ADHD). Pharmacotherapy should be reserved for problems that are functionally disabling and not remediable by nonpharmacologic interventions.
Treatment may also be required for other neuropsychiatric symptoms. Anxiety and depression have been reported in 19% to 80% of patients with tics, and depression is strongly correlated with the duration and severity of tics.13,14 Episodic outburst (rage), self-injurious, OCD, antisocial, and oppositional behaviors are all more common among individuals with tic disorders.15 Personality disorders may be related to OCD, ADHD, or to family or economic issues. Tic disorders are also associated with an increased incidence of somatic complaints, as well as higher rates of academic difficulties, which may be related to ADHD or medications. Sleep disturbances affect an estimated 20% to 50% of patients, and may include difficulty initiating or maintaining sleep, restlessness, movement-related arousal, or parasomnia.16
Education is an important part of treatment, and may include the patient, family members, teachers or other school staff, and work colleagues. A number of behavioral or psychosocial approaches may help to improve tics, including conditioning techniques, relaxation training, biofeedback, habit reversal, awareness training, and hypnosis.17
PHARMACOLOGIC TREATMENT: THREE TIERS
First-tier therapies
The alpha-adrenergic blockers clonidine and guanfacine are first-tier therapies. Treatment should be initiated at a low dose and escalated gradually according to response, which is determined by the severity, and not the presence, of tics. Clonidine may be administered at a dose of 0.025 mg two or three times daily or, for maintenance, 0.1 mg three times daily; another option is 0.1, 0.2, or 0.3 mg weekly by transdermal administration. Guanfacine may be administered at a dose of 1 mg once daily. Alpha-adrenergic blockers are useful for the treatment of mild tics, and are considered first-line therapy for tic suppression. Side effects may include dry mouth, somnolence, and, rarely, blood pressure fluctuations.
Agents that affect gamma-amino butyric acid (GABA) neurotransmission have been associated with improved symptoms of tic disorders.18 For example, both clonazepam and diazepam have been reported to reduce TS symptoms.18 Both of these benzodiazepines are associated with sedation, blunting of cognition, and exacerbation of depression, however.19,20
Second-tier therapies
Second-tier therapies, consisting of neuroleptics, induce a rapid treatment response. Haloperidol may be started at a dose of 0.25 mg once daily, with a maintenance dosage of 0.5 to 3.0 mg/day. Cognitive blunting or extrapyramidal side effects are rare in patients with TS, but the potential for these side effects should be thoroughly discussed with the patient or parent/guardian before treatment. Pimozide 0.5 mg (2 to 6 mg/day for maintenance) may be associated with tremor or parkinsonian symptoms (predominantly akinesia). Risperidone 0.25 mg/day (0.5 to 4 mg/day for maintenance), olanzapine 2.5 mg/day (5 to 10 mg/day for maintenance), and quetiapine 25 mg twice daily (100 to 300 mg/day for maintenance) are associated with potential adverse effects of extrapyramidal symptoms, weight gain, and diabetes.
Third-tier therapies
Dopamine agonists (reserpine and tetrabenazine) and botulinum toxin are third-tier therapies. Reserpine, although rarely used in current clinical practice, may be administered at doses of 0.1 to 0.25 mg/day, titrating upward on the basis of clinical response. Tetrabenazine may be administered at a starting dose of 12.5 mg/day, with higher doses as needed depending on the response to treatment. Adverse effects include hypotension, sedation, extrapyramidal symptoms (predominantly parkinsonism), and depression.21
The exact mechanism by which tetrabenazine produces this suppression effect is unknown, but it is believed to be related to its effect of reversibly depleting monoamines. At least three neuronal protein classes regulate the effects of dopamine on voluntary and involuntary movement.22 The two presynaptic proteins are vesicular monoamine transporter subtype 2 (VMAT2) and dopamine transporter (DAT). Postsynaptically, dopamine activity is regulated by G-protein–linked dopamine receptors (eg, the D2 receptor). Tetrabenazine reduces the uptake of monoamines (including dopamine) into synaptic vesicles by reversibly binding to VMAT2, resulting in degradation of dopamine within axon terminals by monoamine oxidases.23 By blocking dopamine transport, tetrabenazine depletes dopamine with greater selectivity than it does other monoamines.24
The dosage of tetrabenazine for the treatment of motor disorders, particularly chorea, was established in the Huntington Study Group (HSG) clinical trial.25 In the HSG trial, a starting dose of 12.5 mg on day 1 was increased to 12.5 mg twice daily on days 2 to 7, and then by 12.5 mg/day at weekly intervals until the desired clinical effect, intolerable adverse effects, or a maximum dose of 100 mg/day was reached. Daily dosages of 37.5 mg or more are administered in three divided doses. Adverse events (reported in 70% of patients who received placebo and 91% of patients who received tetrabenazine) include sedation or somnolence, insomnia, and fatigue.21 These findings may be carried over to patients with tics.
Botulinum toxin may also help to control tics—especially dystonic tics. The premonitory symptoms of TS are usually unaffected by botulinum toxin.26 The adverse effect profile for patients with TS is similar to that of patients with dystonia or facial dyskinesia, and may include soreness, transient weakness, ptosis (if injected for eye blinking), and mild transient dysphagia (if injected into the larynx).27
MANAGING COMORBID CONDITIONS
Approximately 30% of patients with TS also have OCD.28 Treatment options include selective serotonin reuptake inhibitors at standard doses, and the tricyclic antidepressant clomipramine (25 mg once or twice daily, or 75 mg/day in sustained-release form). Trazodone, a serotonin antagonist and reuptake inhibitor that is associated with a lower incidence of anticholinergic effects, may be initiated at a dose of 50 mg/day and slowly increased to 150 to 400 mg/day depending on clinical response.
As many as 60% of patients with TS may also have ADHD.28 Methylphenidate is helpful for the treatment of ADHD and does not exacerbate tics, but it is a restricted medication. The recommended dose is 20 mg once daily, titrated upward as needed based on response. Atomoxetine carries a warning regarding increased risk of suicide. It has also been associated with an increased risk of sexual dysfunction and behavioral changes, including aggressive behaviors, agitation, and irratibility.29
DEEP BRAIN STIMULATION
Deep brain stimulation (DBS) has been shown to improve TS in single-case studies and in small series, although the long-term benefit is unclear. Potential targets of stimulation include midline thalamic centromedian-parafascicular (CM-PF) nuclei, the ventralis oralis complex of the thalamus, motor and limbic globus pallidus pars interna (GPi), and the anterior limb of the internal capsule.30 In particular, stimulation of the sensorimotor GPi may ameliorate hyperkinetic states.
One report described the results of DBS implantation in a 15-year-old boy with TS who had not responded to several pharmacologic treatment options.31 Six months after implantation, the patient exhibited markedly improved tic severity as measured using the Yale Global Tic Severity Scale, including a 76% reduction in motor tic severity, 68% reduction in vocal tics, and a complete resolution of impairment.31
Published consensus criteria for the selection of suitable candidates for DBS include age greater than 25 years, chronic and severe tics with severe functional impairment for at least 12 months, tics that are frequent and noticeable in most situations most of the time, failure of conventional medical therapy, medical stability for 6 months, and willingness to participate in ongoing psychologic interventions.32 Exclusion criteria include the presence of another medical condition that could explain the tics, an unstable medical condition, being considered likely to benefit from psychologic interventions, psychosocial factors that may complicate the recovery process or make it difficult to assess outcome, and unwillingness to participate in ongoing treatment for psychosocial problems or risk factors. Other factors that should be considered include comorbidities, the variability in tic severity over time, the involvement of a multidisciplinary treatment team, results of a thorough neuropsychologic assessment, expertise of the surgical team, and access to imaging facilities for presurgical mapping and postsurgical evaluation.
SUMMARY AND CONCLUSIONS
Tourette syndrome is not uncommon among the adult population of a typical neurology practice, and should not be considered exclusively a pediatric diagnosis. Several treatment options are available, including behavioral approaches and several medications. Treatment should focus on the most disabling symptoms. Neuropsychologic assessment and psychiatric support may be necessary for some patients. The same comorbidities that are encountered in children are usually evident in adult patients as well. In medically refractory cases, DBS surgery may be helpful.
Tourette syndrome (TS) is part of a spectrum of tic disorders. Tics are sudden, rapid, stereotyped, repetitive, nonrhythmic movements or vocalizations affecting discrete muscle groups, and are preceded by a sensory component. Patients in whom tic suppression is attempted report the experience of a sensation of inner pressure that must be released. This eventually results in the performance of motor movement or vocal sounds. TS is a disorder of childhood onset that is characterized by multiple motor and vocal tics. In some cases, there are features of obsessive compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), or other behavioral manifestations such as coprolalia, echopraxia, palilalia, and self-injury.1,2 The spectrum of tic disorders includes:
- Transient tics of childhood (tic duration less than 12 months)
- Chronic motor or vocal tics (lasting more than 12 months), and
- TS (variable motor and vocal tics lasting more than 12 months).
Many children meet the diagnostic criteria for TS between the ages of 6 and 9 years, but symptoms may improve by adulthood. The eventual loss of tics over time reflects the maturation of brain systems that control ballistic action.3
The tics that accompany TS may be defined as simple or complex, and as motor or vocal. Simple motor tics involve only a few muscles, such as eye blinking, shoulder shrugging, or facial grimacing. Complex motor tics involve multiple groups of muscles that are recruited in orchestrated bouts (eg, hand gestures, jumping, touching, or pressing), and may include copropraxia (a sudden tic-like vulgar, sexual, or obscene gesture) or echopraxia (involuntary, spontaneous imitation of someone else’s movements). Simple vocal tics are meaningless sounds such as throat clearing, grunting, sniffing, snorting, and chirping. Complex vocal tics involve speech and language such as sudden, spontaneous expression of single words or phrases, or speech blocking.4
Tics may be acquired as a consequence of other disorders, including head trauma, encephalitis, stroke, carbon monoxide poisoning, Creutzfeldt-Jakob disease, neurosyphilis, hypoglycemia, or Sydenham chorea.5 Genetic disorders such as Huntington disease may be associated with tics. Tics may also occur with certain chromosomal abnormalities or be associated with some neuropsychiatric disorders. Finally, tics may be caused by a large number of medications or illicit drugs, including cocaine, amphetamines, antipsychotics, and antidepressants.
The prevalence of all types of tics in childhood is approximately 6% to 12%, although the prevalence of chronic vocal tics is approximately 1 to 10 per 1,000 children and adolescents.6 TS is especially common among autistic children and in those with Asperger syndrome and other autistic spectrum disorders. A survey of patients at Cleveland Clinic Florida found that tics and TS accounted for 8% of all patients with movement disorders. Of patients with tics or TS who were older than 18 years, 70% were male.
PATHOPHYSIOLOGY OF TOURETTE SYNDROME: ROLE OF THE BASAL GANGLIA
Although the pathophysiology of TS is not completely understood, abnormal function of the basal ganglia is thought to be a central component of the disorder. The basal ganglia normally act to facilitate voluntary movements while suppressing competing involuntary ones. Abnormalities of basal ganglia activity are important in several disorders of motor function.7 Output neurons from the basal ganglia inhibit thalamic motor nuclei and midbrain neurons of the extrapyramidal motor system, and act to inhibit motor pattern generators in the cerebral cortex and brainstem. Hyperkinetic disorders, including tics, chorea, and dystonia, are thought to result at least in part from impaired inhibition of unwanted motor activity from the basal ganglia to downstream motor centers.7
Family heritability studies provide strong support that TS is a genetic disorder. For example, the concordance rate is 86% for monozygotic twins versus 20% for dizygotic twins.8 Chromosomes linked to TS include 2p32.2 and 13q31.1.9,10 Interactions between genetics and environment are also thought to play a significant role. The concept of pediatric autoimmune neuropsychiatric disorders associated with streptococcal (PANDAS) infections has been proposed to explain an apparent temporal association between streptococcal infections and exacerbation of tics. According to this model, molecular mimicry between streptococcal antigens and endogenous brain antigens results in an autoimmune attack.11 However, the identification of specific antibodies against basal ganglia cells remains controversial.12
MANAGEMENT OVERVIEW
Accurate diagnosis of TS is essential, and includes a complete history and neurologic examination. The tic phenomenology (complex vs simple) should be characterized, and the patient should be carefully questioned to identify the symptoms that are most bothersome (eg, motor or vocal tics, OCD, or ADHD). Pharmacotherapy should be reserved for problems that are functionally disabling and not remediable by nonpharmacologic interventions.
Treatment may also be required for other neuropsychiatric symptoms. Anxiety and depression have been reported in 19% to 80% of patients with tics, and depression is strongly correlated with the duration and severity of tics.13,14 Episodic outburst (rage), self-injurious, OCD, antisocial, and oppositional behaviors are all more common among individuals with tic disorders.15 Personality disorders may be related to OCD, ADHD, or to family or economic issues. Tic disorders are also associated with an increased incidence of somatic complaints, as well as higher rates of academic difficulties, which may be related to ADHD or medications. Sleep disturbances affect an estimated 20% to 50% of patients, and may include difficulty initiating or maintaining sleep, restlessness, movement-related arousal, or parasomnia.16
Education is an important part of treatment, and may include the patient, family members, teachers or other school staff, and work colleagues. A number of behavioral or psychosocial approaches may help to improve tics, including conditioning techniques, relaxation training, biofeedback, habit reversal, awareness training, and hypnosis.17
PHARMACOLOGIC TREATMENT: THREE TIERS
First-tier therapies
The alpha-adrenergic blockers clonidine and guanfacine are first-tier therapies. Treatment should be initiated at a low dose and escalated gradually according to response, which is determined by the severity, and not the presence, of tics. Clonidine may be administered at a dose of 0.025 mg two or three times daily or, for maintenance, 0.1 mg three times daily; another option is 0.1, 0.2, or 0.3 mg weekly by transdermal administration. Guanfacine may be administered at a dose of 1 mg once daily. Alpha-adrenergic blockers are useful for the treatment of mild tics, and are considered first-line therapy for tic suppression. Side effects may include dry mouth, somnolence, and, rarely, blood pressure fluctuations.
Agents that affect gamma-amino butyric acid (GABA) neurotransmission have been associated with improved symptoms of tic disorders.18 For example, both clonazepam and diazepam have been reported to reduce TS symptoms.18 Both of these benzodiazepines are associated with sedation, blunting of cognition, and exacerbation of depression, however.19,20
Second-tier therapies
Second-tier therapies, consisting of neuroleptics, induce a rapid treatment response. Haloperidol may be started at a dose of 0.25 mg once daily, with a maintenance dosage of 0.5 to 3.0 mg/day. Cognitive blunting or extrapyramidal side effects are rare in patients with TS, but the potential for these side effects should be thoroughly discussed with the patient or parent/guardian before treatment. Pimozide 0.5 mg (2 to 6 mg/day for maintenance) may be associated with tremor or parkinsonian symptoms (predominantly akinesia). Risperidone 0.25 mg/day (0.5 to 4 mg/day for maintenance), olanzapine 2.5 mg/day (5 to 10 mg/day for maintenance), and quetiapine 25 mg twice daily (100 to 300 mg/day for maintenance) are associated with potential adverse effects of extrapyramidal symptoms, weight gain, and diabetes.
Third-tier therapies
Dopamine agonists (reserpine and tetrabenazine) and botulinum toxin are third-tier therapies. Reserpine, although rarely used in current clinical practice, may be administered at doses of 0.1 to 0.25 mg/day, titrating upward on the basis of clinical response. Tetrabenazine may be administered at a starting dose of 12.5 mg/day, with higher doses as needed depending on the response to treatment. Adverse effects include hypotension, sedation, extrapyramidal symptoms (predominantly parkinsonism), and depression.21
The exact mechanism by which tetrabenazine produces this suppression effect is unknown, but it is believed to be related to its effect of reversibly depleting monoamines. At least three neuronal protein classes regulate the effects of dopamine on voluntary and involuntary movement.22 The two presynaptic proteins are vesicular monoamine transporter subtype 2 (VMAT2) and dopamine transporter (DAT). Postsynaptically, dopamine activity is regulated by G-protein–linked dopamine receptors (eg, the D2 receptor). Tetrabenazine reduces the uptake of monoamines (including dopamine) into synaptic vesicles by reversibly binding to VMAT2, resulting in degradation of dopamine within axon terminals by monoamine oxidases.23 By blocking dopamine transport, tetrabenazine depletes dopamine with greater selectivity than it does other monoamines.24
The dosage of tetrabenazine for the treatment of motor disorders, particularly chorea, was established in the Huntington Study Group (HSG) clinical trial.25 In the HSG trial, a starting dose of 12.5 mg on day 1 was increased to 12.5 mg twice daily on days 2 to 7, and then by 12.5 mg/day at weekly intervals until the desired clinical effect, intolerable adverse effects, or a maximum dose of 100 mg/day was reached. Daily dosages of 37.5 mg or more are administered in three divided doses. Adverse events (reported in 70% of patients who received placebo and 91% of patients who received tetrabenazine) include sedation or somnolence, insomnia, and fatigue.21 These findings may be carried over to patients with tics.
Botulinum toxin may also help to control tics—especially dystonic tics. The premonitory symptoms of TS are usually unaffected by botulinum toxin.26 The adverse effect profile for patients with TS is similar to that of patients with dystonia or facial dyskinesia, and may include soreness, transient weakness, ptosis (if injected for eye blinking), and mild transient dysphagia (if injected into the larynx).27
MANAGING COMORBID CONDITIONS
Approximately 30% of patients with TS also have OCD.28 Treatment options include selective serotonin reuptake inhibitors at standard doses, and the tricyclic antidepressant clomipramine (25 mg once or twice daily, or 75 mg/day in sustained-release form). Trazodone, a serotonin antagonist and reuptake inhibitor that is associated with a lower incidence of anticholinergic effects, may be initiated at a dose of 50 mg/day and slowly increased to 150 to 400 mg/day depending on clinical response.
As many as 60% of patients with TS may also have ADHD.28 Methylphenidate is helpful for the treatment of ADHD and does not exacerbate tics, but it is a restricted medication. The recommended dose is 20 mg once daily, titrated upward as needed based on response. Atomoxetine carries a warning regarding increased risk of suicide. It has also been associated with an increased risk of sexual dysfunction and behavioral changes, including aggressive behaviors, agitation, and irratibility.29
DEEP BRAIN STIMULATION
Deep brain stimulation (DBS) has been shown to improve TS in single-case studies and in small series, although the long-term benefit is unclear. Potential targets of stimulation include midline thalamic centromedian-parafascicular (CM-PF) nuclei, the ventralis oralis complex of the thalamus, motor and limbic globus pallidus pars interna (GPi), and the anterior limb of the internal capsule.30 In particular, stimulation of the sensorimotor GPi may ameliorate hyperkinetic states.
One report described the results of DBS implantation in a 15-year-old boy with TS who had not responded to several pharmacologic treatment options.31 Six months after implantation, the patient exhibited markedly improved tic severity as measured using the Yale Global Tic Severity Scale, including a 76% reduction in motor tic severity, 68% reduction in vocal tics, and a complete resolution of impairment.31
Published consensus criteria for the selection of suitable candidates for DBS include age greater than 25 years, chronic and severe tics with severe functional impairment for at least 12 months, tics that are frequent and noticeable in most situations most of the time, failure of conventional medical therapy, medical stability for 6 months, and willingness to participate in ongoing psychologic interventions.32 Exclusion criteria include the presence of another medical condition that could explain the tics, an unstable medical condition, being considered likely to benefit from psychologic interventions, psychosocial factors that may complicate the recovery process or make it difficult to assess outcome, and unwillingness to participate in ongoing treatment for psychosocial problems or risk factors. Other factors that should be considered include comorbidities, the variability in tic severity over time, the involvement of a multidisciplinary treatment team, results of a thorough neuropsychologic assessment, expertise of the surgical team, and access to imaging facilities for presurgical mapping and postsurgical evaluation.
SUMMARY AND CONCLUSIONS
Tourette syndrome is not uncommon among the adult population of a typical neurology practice, and should not be considered exclusively a pediatric diagnosis. Several treatment options are available, including behavioral approaches and several medications. Treatment should focus on the most disabling symptoms. Neuropsychologic assessment and psychiatric support may be necessary for some patients. The same comorbidities that are encountered in children are usually evident in adult patients as well. In medically refractory cases, DBS surgery may be helpful.
- Robertson MM. Annotation: Gilles de la Tourette syndrome—an update. J Child Psychol Psychiatry 1994; 35:597–611.
- Robertson MM, Althoff RR, Hafez A, Pauls DL. Principal components analysis of a large cohort with Tourette syndrome. Br J Psychiatry 2008; 193:31–36.
- Flaherty AW. Movement disorders. In: Stern TA, Rosenbaum JF, Fava M, Biederman J, Rauch SL, eds. Massachusetts General Hospital: Comprehensive Clinical Psychiatry E-Book. Philadelphia, PA: Mosby Elsevier; 2008.
- Müller N. Tourette’s syndrome: clinical features, pathophysiology, and therapeutic approaches. Dialogues Clin Neurosci 2007; 9:161–171.
- Bagheri MM, Kerbeshian J, Burd L. Recognition and management of Tourette’s syndrome and tic disorders. Am Fam Physician 1999; 59:2263–2272,2274.
- Lombroso PJ, Scahill L. Tourette syndrome and obsessive–compulsive disorder [published online ahead of print October 15, 2007]. Brain Dev 2008; 30:231–237. 10.1016/j.braindev.2007.09.001
- Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol 2003; 60:1365–1368.
- Singer HS, Smith-Hicks C, Lieberman D. Tourette syndrome. In: LeDoux M, ed. Animal Models of Movement Disorders. Academic Press; 2005.
- Tourette Syndrome Association International Consortium for Genetics. Genome scan for Tourette disorder in affected-sibling-pair and multigenerational families. Am J Hum Genet 2007; 80:265–272.
- Abelson JF, Kwan KY, O’Roak BJ, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science 2005; 310:317–320.
- Kurlan R. Tourette’s syndrome and ‘PANDAS’: will the relation bear out? Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Neurology 1998; 50:1530–1534.
- Morris CM, Pardo-Villamizar C, Gause CD, Singer HS. Serum autoantibodies measured by immunofluorescence confirm a failure to differentiate PANDAS and Tourette syndrome from controls [published online ahead of print September 27, 2008]. J Neurol Sci 2009; 276:45–48. 10.1016/j.jns.2008.08.032
- Comings BG, Comings DE. A controlled study of Tourette syndrome. V. Depression and mania. Am J Hum Genet 1987; 41:804–821.
- Robertson MM, Williamson F, Eapen V. Depressive symptomatology in young people with Gilles de la Tourette syndrome—a comparison of self-report scales [published online ahead of print February 7, 2006]. J Affect Disord 2006; 91:265–268. 10.1016/j.jad.2005.12.046
- Budman CL, Bruun RD, Park KS, Lesser M, Olson M. Explosive outbursts in children with Tourette’s disorder. J Am Acad Child Adolesc Psychiatry 2000; 39:1270–1276.
- Kostanecka-Endress T, Banaschewski T, Kinkelbur J, et al. Disturbed sleep in children with Tourette syndrome: a polysomnographic study. J Psychosom Res 2003; 55:23–29.
- Peterson AL. Psychosocial management of tics and intentional repetitive behaviors associated with Tourette syndrome. In:Woods DW, Piacentini J, Walkup JT, eds. Treating Tourette Syndrome and Tic Disorders: A Guide for Practitioners. Guilford Press; 2007.
- Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain 2000; 123:425–462.
- Klonopin [package insert]. South San Francisco, CA: Genentech USA, Inc.; 2010.
- Valium [package insert]. Nutley, NJ: Roche Laboratories, Inc.; 2008.
- Xenazine [package insert]. Deerfield, IL: Lundbeck Inc.; 2011.
- Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem 2003; 87:273–289.
- Morrow T. Gene therapy offers HD patients relief from some symptoms. Tetrabenazine inhibits the transport of a molecule called vesicular monoamine transporter type 2 or VMAT2. Manag Care 2008; 17:46–47.
- Pearson SJ, Reynolds GP. Depletion of monoamine transmitters by tetrabenazine in brain tissue in Huntington’s disease. Neuropharmacology 1988; 27:717–719.
- Huntington Study Group. Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology 2006; 66:366–372.
- Jankovic J, Kurlan R. Tourette syndrome: evolving concepts [published online ahead of print April 11, 2011]. Mov Disord 2011; 26:1149–1156. 10.1002/mds.23618
- Swain JE, Leckman JF. Tourette syndrome and tic disorders: overview and practical guide to diagnosis and treatment. Psychiatry (Edgmont) 2005; 2:26–36.
- Gorman DA, Thompson N, Plessen KJ, Robertson MM, Leckman JF, Peterson BS. Psychosocial outcome and psychiatric comorbidity in older adolescents with Tourette syndrome: controlled study. Br J Psychiatry 2010; 197:36–44.
- Strattera [package insert]. Indianapolis, IN: Eli Lilly and Company; 2011.
- Ackermans L, Temel Y, Visser-Vandewalle V. Deep brain stimulation in Tourette’s syndrome. Neurotherapeutics 2008; 5:339–344.
- Shahed J, Poysky J, Kenney C, Simpson R, Jankovic J. GPi deep brain stimulation for Tourette syndrome improves tics and psychiatric comorbidities. Neurology 2007; 68:159–160.
- Mink JW, Walkup J, Frey KA, et al .Patient selection and assessment recommendations for deep brain stimulation in Tourette syndrome. Mov Disord 2006; 21:1831–1838.
- Robertson MM. Annotation: Gilles de la Tourette syndrome—an update. J Child Psychol Psychiatry 1994; 35:597–611.
- Robertson MM, Althoff RR, Hafez A, Pauls DL. Principal components analysis of a large cohort with Tourette syndrome. Br J Psychiatry 2008; 193:31–36.
- Flaherty AW. Movement disorders. In: Stern TA, Rosenbaum JF, Fava M, Biederman J, Rauch SL, eds. Massachusetts General Hospital: Comprehensive Clinical Psychiatry E-Book. Philadelphia, PA: Mosby Elsevier; 2008.
- Müller N. Tourette’s syndrome: clinical features, pathophysiology, and therapeutic approaches. Dialogues Clin Neurosci 2007; 9:161–171.
- Bagheri MM, Kerbeshian J, Burd L. Recognition and management of Tourette’s syndrome and tic disorders. Am Fam Physician 1999; 59:2263–2272,2274.
- Lombroso PJ, Scahill L. Tourette syndrome and obsessive–compulsive disorder [published online ahead of print October 15, 2007]. Brain Dev 2008; 30:231–237. 10.1016/j.braindev.2007.09.001
- Mink JW. The basal ganglia and involuntary movements: impaired inhibition of competing motor patterns. Arch Neurol 2003; 60:1365–1368.
- Singer HS, Smith-Hicks C, Lieberman D. Tourette syndrome. In: LeDoux M, ed. Animal Models of Movement Disorders. Academic Press; 2005.
- Tourette Syndrome Association International Consortium for Genetics. Genome scan for Tourette disorder in affected-sibling-pair and multigenerational families. Am J Hum Genet 2007; 80:265–272.
- Abelson JF, Kwan KY, O’Roak BJ, et al. Sequence variants in SLITRK1 are associated with Tourette’s syndrome. Science 2005; 310:317–320.
- Kurlan R. Tourette’s syndrome and ‘PANDAS’: will the relation bear out? Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Neurology 1998; 50:1530–1534.
- Morris CM, Pardo-Villamizar C, Gause CD, Singer HS. Serum autoantibodies measured by immunofluorescence confirm a failure to differentiate PANDAS and Tourette syndrome from controls [published online ahead of print September 27, 2008]. J Neurol Sci 2009; 276:45–48. 10.1016/j.jns.2008.08.032
- Comings BG, Comings DE. A controlled study of Tourette syndrome. V. Depression and mania. Am J Hum Genet 1987; 41:804–821.
- Robertson MM, Williamson F, Eapen V. Depressive symptomatology in young people with Gilles de la Tourette syndrome—a comparison of self-report scales [published online ahead of print February 7, 2006]. J Affect Disord 2006; 91:265–268. 10.1016/j.jad.2005.12.046
- Budman CL, Bruun RD, Park KS, Lesser M, Olson M. Explosive outbursts in children with Tourette’s disorder. J Am Acad Child Adolesc Psychiatry 2000; 39:1270–1276.
- Kostanecka-Endress T, Banaschewski T, Kinkelbur J, et al. Disturbed sleep in children with Tourette syndrome: a polysomnographic study. J Psychosom Res 2003; 55:23–29.
- Peterson AL. Psychosocial management of tics and intentional repetitive behaviors associated with Tourette syndrome. In:Woods DW, Piacentini J, Walkup JT, eds. Treating Tourette Syndrome and Tic Disorders: A Guide for Practitioners. Guilford Press; 2007.
- Robertson MM. Tourette syndrome, associated conditions and the complexities of treatment. Brain 2000; 123:425–462.
- Klonopin [package insert]. South San Francisco, CA: Genentech USA, Inc.; 2010.
- Valium [package insert]. Nutley, NJ: Roche Laboratories, Inc.; 2008.
- Xenazine [package insert]. Deerfield, IL: Lundbeck Inc.; 2011.
- Schmitz Y, Benoit-Marand M, Gonon F, Sulzer D. Presynaptic regulation of dopaminergic neurotransmission. J Neurochem 2003; 87:273–289.
- Morrow T. Gene therapy offers HD patients relief from some symptoms. Tetrabenazine inhibits the transport of a molecule called vesicular monoamine transporter type 2 or VMAT2. Manag Care 2008; 17:46–47.
- Pearson SJ, Reynolds GP. Depletion of monoamine transmitters by tetrabenazine in brain tissue in Huntington’s disease. Neuropharmacology 1988; 27:717–719.
- Huntington Study Group. Tetrabenazine as antichorea therapy in Huntington disease: a randomized controlled trial. Neurology 2006; 66:366–372.
- Jankovic J, Kurlan R. Tourette syndrome: evolving concepts [published online ahead of print April 11, 2011]. Mov Disord 2011; 26:1149–1156. 10.1002/mds.23618
- Swain JE, Leckman JF. Tourette syndrome and tic disorders: overview and practical guide to diagnosis and treatment. Psychiatry (Edgmont) 2005; 2:26–36.
- Gorman DA, Thompson N, Plessen KJ, Robertson MM, Leckman JF, Peterson BS. Psychosocial outcome and psychiatric comorbidity in older adolescents with Tourette syndrome: controlled study. Br J Psychiatry 2010; 197:36–44.
- Strattera [package insert]. Indianapolis, IN: Eli Lilly and Company; 2011.
- Ackermans L, Temel Y, Visser-Vandewalle V. Deep brain stimulation in Tourette’s syndrome. Neurotherapeutics 2008; 5:339–344.
- Shahed J, Poysky J, Kenney C, Simpson R, Jankovic J. GPi deep brain stimulation for Tourette syndrome improves tics and psychiatric comorbidities. Neurology 2007; 68:159–160.
- Mink JW, Walkup J, Frey KA, et al .Patient selection and assessment recommendations for deep brain stimulation in Tourette syndrome. Mov Disord 2006; 21:1831–1838.