User login
Public health officials in El Paso, Texas, reported in September 2014 that more than 850 infants and 43 health care workers (HCW) at Providence Memorial Hospital may have been exposed to tuberculosis (TB) by a nurse with active infection. In collaboration with the CDC, the hospital administrators and local and state health officials advised potentially exposed individuals and their families to be screened for TB. According to press reports, five infants tested positive for TB and were to be treated.1,2 This news report highlights the importance of maintaining infection control protocols in health care facilities throughout the United States to reduce the risk for TB transmission.
SCREENING HEALTH CARE WORKERS
In the US, reported cases of TB, a treatable and curable disease, have declined since 1993. A total of 9,582 cases were reported in 2013, with 536 deaths due to TB reported in 2011 (the most recent year for which this data is available).3
Even though TB is on the decline worldwide,4 HCWs remain at increased risk for infection, confirming that TB is an occupational disease.5 It is imperative that health care facilities have effective infection control plans in place, primarily to reduce the risk for transmission of TB to HCWs.
This article reviews the available TB screening tests, CDC recommendations, parameters for evaluating test performance, and recent studies that lend support to the superiority of interferon-γ (IFN-γ) release assays (IGRAs) over tuberculin skin tests (TSTs) as part of HCW screening and infection control for TB. Primary care clinicians need to know about the status of these tests not only because we are HCWs ourselves, but because we are often responsible for the safety of other HCWs in our workplaces.
TB SCREENING TESTS
Diagnosing latent tuberculosis infection (LTBI) is key to overall control of the disease, since treatment decreases risk for conversion to active disease. Until recently, the diagnosis of LTBI relied on the TST, despite its limitations (see discussion under “Accuracy”). Now, immune-based blood tests hold promise for improving LTBI diagnosis.
Tuberculin skin test
In 1934, Florence Seibert developed what is known today as the purified protein derivative (PPD) test, which was adopted as the standard in the US in 1941.6 For 60 years, the PPD TST was the only screening method for Mycobacterium tuberculosis infection. It involves the intradermal injection of PPD; a hypersensitivity response leads to a cutaneous induration at the injection site after 48 to 72 hours.7Hence, the TST is a two-step test, requiring a follow-up visit for the result to be read.
Although strongly predictive of TB, the TST may result in false-positive test results in those previously immunized with the Bacille Calmette-Guerin (BCG) vaccine (a WHO-recommended childhood vaccination against TB, widely used outside the US),8 and in those exposed to certain nontuberculous mycobacteria such as M bovis and M africana.9 Test results may also be false negative in immunocompromised patients.9
Interferon-γ release assays
With the goal of developing a more specific and sensitive test for TB infection, IGRAs were developed in the mid-1990s as a new tool to detect active and latent TB infection.10 The first IGRA test for TB received FDA approval in 2001.11 There are currently two FDA-approved IGRAs—QuantiFERON-TB Gold In-Tube (QFT-GIT) and T-SPOT.TB—in use.
QuantiFERON-TB Gold In-Tube test. The QFT-GIT measures cell-mediated immune responses to antigens that simulate mycobacterial proteins. Requiring only one patient visit, whole blood is collected in three different tubes (negative control, TB antigen, and mitogen [positive] control), each containing a single antigen.12 After 16 to 24 hours in a temperature-controlled environment, the tubes are centrifuged to separate the plasma. IFN-γ levels are measured in each tube to calculate the test result.
T-SPOT.TB test. Using one blood sample, the T-SPOT.TB test captures IFN-γ produced by activated T-cells in response to stimulation by two M tuberculosis antigens. Addition of a substrate produces dark blue spots; the number of spots indicates the quantity of M tuberculosis-sensitive effector T-cells in the peripheral blood. A positive result is eight or more spots; a negative result is fewer than four spots, and a borderline result is five to seven spots.13 Unlike the QFT-GIT, the T-SPOT.TB has a “borderline” interpretation category.
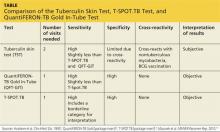
See the Table for a comparison of the TST, QFT-GIT, and T-SPOT.TB tests for TB screening.
Continue for CDC guidelines >>
CDC GUIDELINES
Subject to state and federal regulations and Occupational Safety and Health Administration (OSHA) directives, the CDC states that the goals of a TB infection control plan are
• Prompt detection of suspected or confirmed TB infection
• Airborne precautions implemented to reduce risk for TB transmission in areas in which exposure can occur
• Treatment of persons with suspected or confirmed TB.14
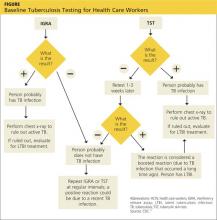
TB screening for HCWs has historically been a challenge in that, for new hires, the CDC recommends a baseline two-step process (meaning up to four visits) when TST is used.15 This is because TST-tested individuals may test false negative, even if they have LTBI, if many years have passed since their infection was acquired. As a result, guidelines for baseline testing are that, if the initial TST is negative, TST should be repeated one to three weeks later. If the person is in fact infected with TB, the first TST may stimulate the immune system’s ability to react to the TB antigens and elicit a positive or “boosted” response to the second test.
The above figure has been corrected from the print version as of December 16, 2014.
The 2005 guidelines recommended screening new-hire HCWs with either a baseline two-step TST or with one blood assay for M tuberculosis. After the introduction of the QFT-GIT and T-SPOT.TB tests, the CDC included them in its updated 2010 guidelines and indicated that either IGRAs or TSTs may be used in HCW surveillance programs for occupational exposure to M tuberculosis (see Figure).15,16 The algorithm clearly illustrates how the use of IGRAs in place of TSTs streamlines the process of HCW TB screening.17
EVALUATION OF TESTS
Given the stringent nature of the CDC’s TB infection control goals, it is essential that health care facilities use the most effective and efficient means for timely and thorough screening of HCWs for TB. When evaluating the available tests, the following factors must be considered: accuracy and reproducibility of test results; impact on results when testing is repeated frequently; interpretation of discordant test results; and specificity, sensitivity, and identification of appropriate test cutoff values so that a positive result signifies a new TB infection (ie, conversion, a change from a documented negative to positive test result within a two-year period) rather than a false positive; and costs.18,19
Accuracy
Unlike TSTs, IGRAs do not produce false-positive results in individuals vaccinated with BCG or in those infected with most nontuberculous mycobacteria.9,16 Neither TSTs nor IGRAs, however, can distinguish between active and latent TB infection.19 If either test is positive, a chest radiograph is indicated. If the x-ray reveals abnormalities in the lungs, a sputum smear to detect acid-fast-bacilli (AFB), of which M tuberculosis is one, is indicative of TB. Nuclear acid amplification testing of a respiratory specimen provides rapid laboratory confirmation, but a positive culture for M tuberculosis confirms the diagnosis.22
Specificity and sensitivity
Pai, Zwerling, and Menzies conducted a meta-analysis of 38 studies of TB testing. Most of the studies were small and had limitations, such as the lack of a gold standard test for the diagnosis of LTBI and variable TST methods and cutoff values. Nevertheless, the researchers were able to conclude that IGRAs are significantly more specific than TST and are unaffected by BCG vaccination. Although the sensitivity of IGRAs and TST is inconsistent across test populations, T-SPOT.TB appears to have greater sensitivity than QFT-GIT or TST.23
Further, TST is subject to variability in administration and interpretation, and cut points for TST positivity vary internationally.9 In contrast, the T-SPOT.TB test specifies a borderline result zone. According to the CDC, this increases test accuracy by classifying results near the cut point, making a subsequent test conversion from negative to positive more likely to represent newly acquired infection.16
On the other hand, some studies of IGRAs have found unexpectedly high rates of initial positive results and conversions among HCWs in low-risk settings that are later determined to be false positives.24 However, as noted previously, TSTs are also subject to false-positive results. In addition, the definition of an IGRA conversion is less stringent than the TST conversion definition, which may result in more IGRA conversions.16
Discordant results
Zwerling et al conducted a systematic review of all studies in which IGRAs were used for HCW screening to summarize their performance in cross-sectional and serial testing settings. The prevalence of positive IGRAs was found to be lower than that of positive TSTs. This difference was significant in low- and moderate-TB incidence settings but not in high-incidence settings. A positive association was reported between positive IGRA test results and occupational risk factors, including work in high-risk wards, TB clinics, and geriatric care, as well as length of employment.18
According to Mancuso et al, discordance of results between the TST and IGRAs in populations with low LTBI prevalence suggests that most positive test results are in fact false positives in these populations.25 Although IGRAs were designed to increase specificity, the authors found that IGRA specificity was no better than the specificity of TST. Without a gold standard for detecting M tuberculosis infection, assessing the true significance of discordance between TST and IGRAs is difficult. Further research is needed to determine the significance of test discordance, to obtain data on progression to active TB, and to better define appropriate cut points for interpreting IGRA results.25
Costs
The primary impediment to the widespread use of IGRAs has been cost, which is approximately three times that of a TST.24
Eralp et al studied the cost-effectiveness of IGRAs versus TST for screening for active LTBI in HCWs by using healthy life-years gained—defined as the number of TB cases avoided, yielding an increase in life expectancy—as the benefit metric rather than quality-adjusted life-years. Because testing is completed with a single visit, use of IGRAs increases compliance while minimizing resources needed for a second visit and eliminating loss to follow-up. Also notable is that IGRA testing takes place in a laboratory, where costs can be held in check with focused expertise and optimized staffing structures. The authors concluded that incremental IGRA costs per healthy life-year gained were justified.19
Until publication of the SWITCH (Screening health care Workers with IGRA vs. TST: impact on Costs and adHerence to testing) study in 2012, cost-effectiveness studies had shown inconsistent results. This study, conducted by The Johns Hopkins University (JHU) employee health department, was the first of its kind in the US to systematically analyze test performance and labor costs for TB screening of HCWs. The results showed that the time required to administer a TST is one of the costliest elements. For a sizeable institution such as JHU, TST screening cost more than $1.3 million annually, equivalent to approximately $73 per person; in contrast, IGRA screening amounted to less than $55 per person.26
Further research
The association between IGRA test conversion in HCWs and the risk for active TB disease has not been demonstrated.16 However, this is also true of TSTs27 and further research is needed in this area. Research is also needed to determine the significance of TSA-IGRA results discordance and to better define cut points for IGRA interpretation.25 In addition, more study of factors related to serial (periodic or ongoing) TB testing—which are very important within the context of HCW screening—is needed. As an example, serial TB testing may reveal trends in test conversions and can identify areas of concern within a health care facility. Unfortunately, current CDC recommendations do not provide specific guidelines for serial IGRA testing, such as guidance for accurate interpretation of IGRA results within a serial testing context.16 These areas should be addressed in future CDC updates.
Continue for conclusion >>
CONCLUSION
Occupational health professionals are continuously evaluating how to improve surveillance programs and reduce HCWs’ time away from work. Within this context, four advantages IGRAs offer include
• Elimination of a two-step TST for new hires (which requires up to four separate visits)
• No false-positive results caused by previous BCG vaccination or exposure to most nontuberculous mycobacteria
• Significantly improved HCW compliance because screening requires only one visit
• Cost effectiveness.
From an occupational health perspective, these are significant advantages that support the use of IGRAs to screen HCW for TB.
REFERENCES
1. Wilson J. Five babies test positive for TB in Texas. CNN. September 30, 2014. www.cnn.com/2014/09/29/health/babies-test-positive-tb/index.html. Accessed November 12, 2014.
2. Bailey J. 700 babies exposed to TB at Texas Hospital. Atlanta Journal Constitution. September 22, 2014. www.ajc.com/news/lifestyles/health/700-babies-exposed-TB-texas-hospital/nhRmC/. Accessed November 12, 2014.
3. CDC. Reported tuberculosis in the United States, 2013. www.cdc.gov/tb/statistics/reports/2013/pdf/report2013.pdf. Accessed October 23, 2014.
4. World Health Organization. Global tuberculosis report 2014. www.who.int/tb/publications/global_report/en/. Accessed November 12, 2014.
5. Baussano I, Nunn P, Williams B, et al. Tuberculosis among health care workers. Emerg Infect Dis. 2011;17(3):488-494.
6. Yang H, Kruh-Garcia NA, Dobos KM. Purified protein derivatives of tuberculin: past, present, and future. FEMS Immunol Med Microbiol. 2012;66(3):273-280.
7. Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clin Infect Dis. 1993;17(6):968-975.
8. World Health Organization. Recommendations for vaccine administration. www.who.int/immunization/policy/Immunization_routine_table1.pdf. Accessed November 12, 2014.
9. Al-Orainey IO. Diagnosis of latent tuberculosis: can we do better? Ann Thorac Med. 2009;4(1):5-9.
10. Desem N, Jones SL. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin Diagn Lab Immunol. 1998;5(4): 531-536.
11. FDA. QuantiFERON-TB - P010033. www.fda.gov/MedicalDevices/Products andMedicalProcedures/DeviceApprovalsandClearances/Recently-Approved Devices/ucm084025.htm. Accessed November 12, 2014.
12. QuantiFERON-TB Gold In-Tube Test [package insert]. www.quantiferon.com/irm/content/PI/QFT/2PK/US.pdf. Accessed November 12, 2014.
13. T-SPOT.TB [package insert]. Marlborough, MA. Oxford Immunotec, Inc. www.tspot.com/wp-content/uploads/2012/01/PI-TB-US-v4.pdf. Accessed November 12, 2014.
14. CDC. Fact sheet: infection control in health-care settings. www.cdc.gov/tb/publications/factsheets/prevention/ichcs.htm. Accessed November 12, 2014.
15. Jensen PA, Lambert LA, Iademarco MF, Ridzon R; CDC. Guidelines for preventing the transmission of Mycobacterium TB in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
16. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee, CDC. Updated guidelines for using interferon γ release assays to detect Mycobacterium TB infection: United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
17. CDC. Latent tuberculosis infection: a guide for primary health care providers. www.cdc.gov/tb/publications/ltbi/diagnosis.htm. Accessed November 3, 2014.
18. Zwerling A, van den Hof S, Scholten J, et al. Interferon-γ release assays for TB screening of healthcare workers: a systematic review. Thorax. 2012;67(1): 62-70.
19. Eralp MN, Scholtes S, Martell G, et al. Screening of healthcare workers for TB: development and validation of a new health economic model to inform practice. BMJ Open. 2012;2:e000630.
20. FDA. FDA permits marketing of first US test labeled for simultaneous detection of tuberculosis bacteria and resistance to the antibiotic rifampin [press release]. July 25, 2013.
21. CDC. Availability of an assay for detecting Mycobacterium TB, including rifampin-resistant strains, and considerations for its use: United States, 2013 [published correction appears in MMWR Morb Mortal Wkly Rep. 2013;62(45):906]. MMWR Morb Mortal Wkly Rep. 2013;62(41):821-827.
22. CDC. Fact sheet: diagnosis of tuberculosis disease. www.cdc.gov/tb/publica tions/factsheets/testing/diagnosis.htm. Accessed November 12, 2014.
23. Pai M, Zwerling A, Menzies D. Systematic review: T-cell–based assays for the diagnosis of latent TB infection: an update. Ann Intern Med. 2008;149(3): 177-184.
24. LoBue PA, Castro KG. Is it time to replace the tuberculin skin test with a blood test? JAMA. 2012;308(3):241-242.
25. Mancuso JD, Mazurek GH, Tribble D, et al. Discordance among commercially available diagnostics for latent TB infection. Am J Respir Crit Care Med. 2012;185(4):427-434.
26. Wrighton-Smith P, Sneed L, Humphrey F, et al. Screening health care workers with interferon-γ release assay versus tuberculin skin test: impact on costs and adherence to testing (the SWITCH study). J Occup Environ Med. 2012;54(7):806-815.
27. World Health Organization. Use of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle-income countries. www.who.int/tb/
features_archive/policy_statement_igra_oct2011.pdf. Accessed November 12, 2014.
Public health officials in El Paso, Texas, reported in September 2014 that more than 850 infants and 43 health care workers (HCW) at Providence Memorial Hospital may have been exposed to tuberculosis (TB) by a nurse with active infection. In collaboration with the CDC, the hospital administrators and local and state health officials advised potentially exposed individuals and their families to be screened for TB. According to press reports, five infants tested positive for TB and were to be treated.1,2 This news report highlights the importance of maintaining infection control protocols in health care facilities throughout the United States to reduce the risk for TB transmission.
SCREENING HEALTH CARE WORKERS
In the US, reported cases of TB, a treatable and curable disease, have declined since 1993. A total of 9,582 cases were reported in 2013, with 536 deaths due to TB reported in 2011 (the most recent year for which this data is available).3
Even though TB is on the decline worldwide,4 HCWs remain at increased risk for infection, confirming that TB is an occupational disease.5 It is imperative that health care facilities have effective infection control plans in place, primarily to reduce the risk for transmission of TB to HCWs.
This article reviews the available TB screening tests, CDC recommendations, parameters for evaluating test performance, and recent studies that lend support to the superiority of interferon-γ (IFN-γ) release assays (IGRAs) over tuberculin skin tests (TSTs) as part of HCW screening and infection control for TB. Primary care clinicians need to know about the status of these tests not only because we are HCWs ourselves, but because we are often responsible for the safety of other HCWs in our workplaces.
TB SCREENING TESTS
Diagnosing latent tuberculosis infection (LTBI) is key to overall control of the disease, since treatment decreases risk for conversion to active disease. Until recently, the diagnosis of LTBI relied on the TST, despite its limitations (see discussion under “Accuracy”). Now, immune-based blood tests hold promise for improving LTBI diagnosis.
Tuberculin skin test
In 1934, Florence Seibert developed what is known today as the purified protein derivative (PPD) test, which was adopted as the standard in the US in 1941.6 For 60 years, the PPD TST was the only screening method for Mycobacterium tuberculosis infection. It involves the intradermal injection of PPD; a hypersensitivity response leads to a cutaneous induration at the injection site after 48 to 72 hours.7Hence, the TST is a two-step test, requiring a follow-up visit for the result to be read.
Although strongly predictive of TB, the TST may result in false-positive test results in those previously immunized with the Bacille Calmette-Guerin (BCG) vaccine (a WHO-recommended childhood vaccination against TB, widely used outside the US),8 and in those exposed to certain nontuberculous mycobacteria such as M bovis and M africana.9 Test results may also be false negative in immunocompromised patients.9
Interferon-γ release assays
With the goal of developing a more specific and sensitive test for TB infection, IGRAs were developed in the mid-1990s as a new tool to detect active and latent TB infection.10 The first IGRA test for TB received FDA approval in 2001.11 There are currently two FDA-approved IGRAs—QuantiFERON-TB Gold In-Tube (QFT-GIT) and T-SPOT.TB—in use.
QuantiFERON-TB Gold In-Tube test. The QFT-GIT measures cell-mediated immune responses to antigens that simulate mycobacterial proteins. Requiring only one patient visit, whole blood is collected in three different tubes (negative control, TB antigen, and mitogen [positive] control), each containing a single antigen.12 After 16 to 24 hours in a temperature-controlled environment, the tubes are centrifuged to separate the plasma. IFN-γ levels are measured in each tube to calculate the test result.
T-SPOT.TB test. Using one blood sample, the T-SPOT.TB test captures IFN-γ produced by activated T-cells in response to stimulation by two M tuberculosis antigens. Addition of a substrate produces dark blue spots; the number of spots indicates the quantity of M tuberculosis-sensitive effector T-cells in the peripheral blood. A positive result is eight or more spots; a negative result is fewer than four spots, and a borderline result is five to seven spots.13 Unlike the QFT-GIT, the T-SPOT.TB has a “borderline” interpretation category.
See the Table for a comparison of the TST, QFT-GIT, and T-SPOT.TB tests for TB screening.
Continue for CDC guidelines >>
CDC GUIDELINES
Subject to state and federal regulations and Occupational Safety and Health Administration (OSHA) directives, the CDC states that the goals of a TB infection control plan are
• Prompt detection of suspected or confirmed TB infection
• Airborne precautions implemented to reduce risk for TB transmission in areas in which exposure can occur
• Treatment of persons with suspected or confirmed TB.14
TB screening for HCWs has historically been a challenge in that, for new hires, the CDC recommends a baseline two-step process (meaning up to four visits) when TST is used.15 This is because TST-tested individuals may test false negative, even if they have LTBI, if many years have passed since their infection was acquired. As a result, guidelines for baseline testing are that, if the initial TST is negative, TST should be repeated one to three weeks later. If the person is in fact infected with TB, the first TST may stimulate the immune system’s ability to react to the TB antigens and elicit a positive or “boosted” response to the second test.
The above figure has been corrected from the print version as of December 16, 2014.
The 2005 guidelines recommended screening new-hire HCWs with either a baseline two-step TST or with one blood assay for M tuberculosis. After the introduction of the QFT-GIT and T-SPOT.TB tests, the CDC included them in its updated 2010 guidelines and indicated that either IGRAs or TSTs may be used in HCW surveillance programs for occupational exposure to M tuberculosis (see Figure).15,16 The algorithm clearly illustrates how the use of IGRAs in place of TSTs streamlines the process of HCW TB screening.17
EVALUATION OF TESTS
Given the stringent nature of the CDC’s TB infection control goals, it is essential that health care facilities use the most effective and efficient means for timely and thorough screening of HCWs for TB. When evaluating the available tests, the following factors must be considered: accuracy and reproducibility of test results; impact on results when testing is repeated frequently; interpretation of discordant test results; and specificity, sensitivity, and identification of appropriate test cutoff values so that a positive result signifies a new TB infection (ie, conversion, a change from a documented negative to positive test result within a two-year period) rather than a false positive; and costs.18,19
Accuracy
Unlike TSTs, IGRAs do not produce false-positive results in individuals vaccinated with BCG or in those infected with most nontuberculous mycobacteria.9,16 Neither TSTs nor IGRAs, however, can distinguish between active and latent TB infection.19 If either test is positive, a chest radiograph is indicated. If the x-ray reveals abnormalities in the lungs, a sputum smear to detect acid-fast-bacilli (AFB), of which M tuberculosis is one, is indicative of TB. Nuclear acid amplification testing of a respiratory specimen provides rapid laboratory confirmation, but a positive culture for M tuberculosis confirms the diagnosis.22
Specificity and sensitivity
Pai, Zwerling, and Menzies conducted a meta-analysis of 38 studies of TB testing. Most of the studies were small and had limitations, such as the lack of a gold standard test for the diagnosis of LTBI and variable TST methods and cutoff values. Nevertheless, the researchers were able to conclude that IGRAs are significantly more specific than TST and are unaffected by BCG vaccination. Although the sensitivity of IGRAs and TST is inconsistent across test populations, T-SPOT.TB appears to have greater sensitivity than QFT-GIT or TST.23
Further, TST is subject to variability in administration and interpretation, and cut points for TST positivity vary internationally.9 In contrast, the T-SPOT.TB test specifies a borderline result zone. According to the CDC, this increases test accuracy by classifying results near the cut point, making a subsequent test conversion from negative to positive more likely to represent newly acquired infection.16
On the other hand, some studies of IGRAs have found unexpectedly high rates of initial positive results and conversions among HCWs in low-risk settings that are later determined to be false positives.24 However, as noted previously, TSTs are also subject to false-positive results. In addition, the definition of an IGRA conversion is less stringent than the TST conversion definition, which may result in more IGRA conversions.16
Discordant results
Zwerling et al conducted a systematic review of all studies in which IGRAs were used for HCW screening to summarize their performance in cross-sectional and serial testing settings. The prevalence of positive IGRAs was found to be lower than that of positive TSTs. This difference was significant in low- and moderate-TB incidence settings but not in high-incidence settings. A positive association was reported between positive IGRA test results and occupational risk factors, including work in high-risk wards, TB clinics, and geriatric care, as well as length of employment.18
According to Mancuso et al, discordance of results between the TST and IGRAs in populations with low LTBI prevalence suggests that most positive test results are in fact false positives in these populations.25 Although IGRAs were designed to increase specificity, the authors found that IGRA specificity was no better than the specificity of TST. Without a gold standard for detecting M tuberculosis infection, assessing the true significance of discordance between TST and IGRAs is difficult. Further research is needed to determine the significance of test discordance, to obtain data on progression to active TB, and to better define appropriate cut points for interpreting IGRA results.25
Costs
The primary impediment to the widespread use of IGRAs has been cost, which is approximately three times that of a TST.24
Eralp et al studied the cost-effectiveness of IGRAs versus TST for screening for active LTBI in HCWs by using healthy life-years gained—defined as the number of TB cases avoided, yielding an increase in life expectancy—as the benefit metric rather than quality-adjusted life-years. Because testing is completed with a single visit, use of IGRAs increases compliance while minimizing resources needed for a second visit and eliminating loss to follow-up. Also notable is that IGRA testing takes place in a laboratory, where costs can be held in check with focused expertise and optimized staffing structures. The authors concluded that incremental IGRA costs per healthy life-year gained were justified.19
Until publication of the SWITCH (Screening health care Workers with IGRA vs. TST: impact on Costs and adHerence to testing) study in 2012, cost-effectiveness studies had shown inconsistent results. This study, conducted by The Johns Hopkins University (JHU) employee health department, was the first of its kind in the US to systematically analyze test performance and labor costs for TB screening of HCWs. The results showed that the time required to administer a TST is one of the costliest elements. For a sizeable institution such as JHU, TST screening cost more than $1.3 million annually, equivalent to approximately $73 per person; in contrast, IGRA screening amounted to less than $55 per person.26
Further research
The association between IGRA test conversion in HCWs and the risk for active TB disease has not been demonstrated.16 However, this is also true of TSTs27 and further research is needed in this area. Research is also needed to determine the significance of TSA-IGRA results discordance and to better define cut points for IGRA interpretation.25 In addition, more study of factors related to serial (periodic or ongoing) TB testing—which are very important within the context of HCW screening—is needed. As an example, serial TB testing may reveal trends in test conversions and can identify areas of concern within a health care facility. Unfortunately, current CDC recommendations do not provide specific guidelines for serial IGRA testing, such as guidance for accurate interpretation of IGRA results within a serial testing context.16 These areas should be addressed in future CDC updates.
Continue for conclusion >>
CONCLUSION
Occupational health professionals are continuously evaluating how to improve surveillance programs and reduce HCWs’ time away from work. Within this context, four advantages IGRAs offer include
• Elimination of a two-step TST for new hires (which requires up to four separate visits)
• No false-positive results caused by previous BCG vaccination or exposure to most nontuberculous mycobacteria
• Significantly improved HCW compliance because screening requires only one visit
• Cost effectiveness.
From an occupational health perspective, these are significant advantages that support the use of IGRAs to screen HCW for TB.
REFERENCES
1. Wilson J. Five babies test positive for TB in Texas. CNN. September 30, 2014. www.cnn.com/2014/09/29/health/babies-test-positive-tb/index.html. Accessed November 12, 2014.
2. Bailey J. 700 babies exposed to TB at Texas Hospital. Atlanta Journal Constitution. September 22, 2014. www.ajc.com/news/lifestyles/health/700-babies-exposed-TB-texas-hospital/nhRmC/. Accessed November 12, 2014.
3. CDC. Reported tuberculosis in the United States, 2013. www.cdc.gov/tb/statistics/reports/2013/pdf/report2013.pdf. Accessed October 23, 2014.
4. World Health Organization. Global tuberculosis report 2014. www.who.int/tb/publications/global_report/en/. Accessed November 12, 2014.
5. Baussano I, Nunn P, Williams B, et al. Tuberculosis among health care workers. Emerg Infect Dis. 2011;17(3):488-494.
6. Yang H, Kruh-Garcia NA, Dobos KM. Purified protein derivatives of tuberculin: past, present, and future. FEMS Immunol Med Microbiol. 2012;66(3):273-280.
7. Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clin Infect Dis. 1993;17(6):968-975.
8. World Health Organization. Recommendations for vaccine administration. www.who.int/immunization/policy/Immunization_routine_table1.pdf. Accessed November 12, 2014.
9. Al-Orainey IO. Diagnosis of latent tuberculosis: can we do better? Ann Thorac Med. 2009;4(1):5-9.
10. Desem N, Jones SL. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin Diagn Lab Immunol. 1998;5(4): 531-536.
11. FDA. QuantiFERON-TB - P010033. www.fda.gov/MedicalDevices/Products andMedicalProcedures/DeviceApprovalsandClearances/Recently-Approved Devices/ucm084025.htm. Accessed November 12, 2014.
12. QuantiFERON-TB Gold In-Tube Test [package insert]. www.quantiferon.com/irm/content/PI/QFT/2PK/US.pdf. Accessed November 12, 2014.
13. T-SPOT.TB [package insert]. Marlborough, MA. Oxford Immunotec, Inc. www.tspot.com/wp-content/uploads/2012/01/PI-TB-US-v4.pdf. Accessed November 12, 2014.
14. CDC. Fact sheet: infection control in health-care settings. www.cdc.gov/tb/publications/factsheets/prevention/ichcs.htm. Accessed November 12, 2014.
15. Jensen PA, Lambert LA, Iademarco MF, Ridzon R; CDC. Guidelines for preventing the transmission of Mycobacterium TB in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
16. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee, CDC. Updated guidelines for using interferon γ release assays to detect Mycobacterium TB infection: United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
17. CDC. Latent tuberculosis infection: a guide for primary health care providers. www.cdc.gov/tb/publications/ltbi/diagnosis.htm. Accessed November 3, 2014.
18. Zwerling A, van den Hof S, Scholten J, et al. Interferon-γ release assays for TB screening of healthcare workers: a systematic review. Thorax. 2012;67(1): 62-70.
19. Eralp MN, Scholtes S, Martell G, et al. Screening of healthcare workers for TB: development and validation of a new health economic model to inform practice. BMJ Open. 2012;2:e000630.
20. FDA. FDA permits marketing of first US test labeled for simultaneous detection of tuberculosis bacteria and resistance to the antibiotic rifampin [press release]. July 25, 2013.
21. CDC. Availability of an assay for detecting Mycobacterium TB, including rifampin-resistant strains, and considerations for its use: United States, 2013 [published correction appears in MMWR Morb Mortal Wkly Rep. 2013;62(45):906]. MMWR Morb Mortal Wkly Rep. 2013;62(41):821-827.
22. CDC. Fact sheet: diagnosis of tuberculosis disease. www.cdc.gov/tb/publica tions/factsheets/testing/diagnosis.htm. Accessed November 12, 2014.
23. Pai M, Zwerling A, Menzies D. Systematic review: T-cell–based assays for the diagnosis of latent TB infection: an update. Ann Intern Med. 2008;149(3): 177-184.
24. LoBue PA, Castro KG. Is it time to replace the tuberculin skin test with a blood test? JAMA. 2012;308(3):241-242.
25. Mancuso JD, Mazurek GH, Tribble D, et al. Discordance among commercially available diagnostics for latent TB infection. Am J Respir Crit Care Med. 2012;185(4):427-434.
26. Wrighton-Smith P, Sneed L, Humphrey F, et al. Screening health care workers with interferon-γ release assay versus tuberculin skin test: impact on costs and adherence to testing (the SWITCH study). J Occup Environ Med. 2012;54(7):806-815.
27. World Health Organization. Use of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle-income countries. www.who.int/tb/
features_archive/policy_statement_igra_oct2011.pdf. Accessed November 12, 2014.
Public health officials in El Paso, Texas, reported in September 2014 that more than 850 infants and 43 health care workers (HCW) at Providence Memorial Hospital may have been exposed to tuberculosis (TB) by a nurse with active infection. In collaboration with the CDC, the hospital administrators and local and state health officials advised potentially exposed individuals and their families to be screened for TB. According to press reports, five infants tested positive for TB and were to be treated.1,2 This news report highlights the importance of maintaining infection control protocols in health care facilities throughout the United States to reduce the risk for TB transmission.
SCREENING HEALTH CARE WORKERS
In the US, reported cases of TB, a treatable and curable disease, have declined since 1993. A total of 9,582 cases were reported in 2013, with 536 deaths due to TB reported in 2011 (the most recent year for which this data is available).3
Even though TB is on the decline worldwide,4 HCWs remain at increased risk for infection, confirming that TB is an occupational disease.5 It is imperative that health care facilities have effective infection control plans in place, primarily to reduce the risk for transmission of TB to HCWs.
This article reviews the available TB screening tests, CDC recommendations, parameters for evaluating test performance, and recent studies that lend support to the superiority of interferon-γ (IFN-γ) release assays (IGRAs) over tuberculin skin tests (TSTs) as part of HCW screening and infection control for TB. Primary care clinicians need to know about the status of these tests not only because we are HCWs ourselves, but because we are often responsible for the safety of other HCWs in our workplaces.
TB SCREENING TESTS
Diagnosing latent tuberculosis infection (LTBI) is key to overall control of the disease, since treatment decreases risk for conversion to active disease. Until recently, the diagnosis of LTBI relied on the TST, despite its limitations (see discussion under “Accuracy”). Now, immune-based blood tests hold promise for improving LTBI diagnosis.
Tuberculin skin test
In 1934, Florence Seibert developed what is known today as the purified protein derivative (PPD) test, which was adopted as the standard in the US in 1941.6 For 60 years, the PPD TST was the only screening method for Mycobacterium tuberculosis infection. It involves the intradermal injection of PPD; a hypersensitivity response leads to a cutaneous induration at the injection site after 48 to 72 hours.7Hence, the TST is a two-step test, requiring a follow-up visit for the result to be read.
Although strongly predictive of TB, the TST may result in false-positive test results in those previously immunized with the Bacille Calmette-Guerin (BCG) vaccine (a WHO-recommended childhood vaccination against TB, widely used outside the US),8 and in those exposed to certain nontuberculous mycobacteria such as M bovis and M africana.9 Test results may also be false negative in immunocompromised patients.9
Interferon-γ release assays
With the goal of developing a more specific and sensitive test for TB infection, IGRAs were developed in the mid-1990s as a new tool to detect active and latent TB infection.10 The first IGRA test for TB received FDA approval in 2001.11 There are currently two FDA-approved IGRAs—QuantiFERON-TB Gold In-Tube (QFT-GIT) and T-SPOT.TB—in use.
QuantiFERON-TB Gold In-Tube test. The QFT-GIT measures cell-mediated immune responses to antigens that simulate mycobacterial proteins. Requiring only one patient visit, whole blood is collected in three different tubes (negative control, TB antigen, and mitogen [positive] control), each containing a single antigen.12 After 16 to 24 hours in a temperature-controlled environment, the tubes are centrifuged to separate the plasma. IFN-γ levels are measured in each tube to calculate the test result.
T-SPOT.TB test. Using one blood sample, the T-SPOT.TB test captures IFN-γ produced by activated T-cells in response to stimulation by two M tuberculosis antigens. Addition of a substrate produces dark blue spots; the number of spots indicates the quantity of M tuberculosis-sensitive effector T-cells in the peripheral blood. A positive result is eight or more spots; a negative result is fewer than four spots, and a borderline result is five to seven spots.13 Unlike the QFT-GIT, the T-SPOT.TB has a “borderline” interpretation category.
See the Table for a comparison of the TST, QFT-GIT, and T-SPOT.TB tests for TB screening.
Continue for CDC guidelines >>
CDC GUIDELINES
Subject to state and federal regulations and Occupational Safety and Health Administration (OSHA) directives, the CDC states that the goals of a TB infection control plan are
• Prompt detection of suspected or confirmed TB infection
• Airborne precautions implemented to reduce risk for TB transmission in areas in which exposure can occur
• Treatment of persons with suspected or confirmed TB.14
TB screening for HCWs has historically been a challenge in that, for new hires, the CDC recommends a baseline two-step process (meaning up to four visits) when TST is used.15 This is because TST-tested individuals may test false negative, even if they have LTBI, if many years have passed since their infection was acquired. As a result, guidelines for baseline testing are that, if the initial TST is negative, TST should be repeated one to three weeks later. If the person is in fact infected with TB, the first TST may stimulate the immune system’s ability to react to the TB antigens and elicit a positive or “boosted” response to the second test.
The above figure has been corrected from the print version as of December 16, 2014.
The 2005 guidelines recommended screening new-hire HCWs with either a baseline two-step TST or with one blood assay for M tuberculosis. After the introduction of the QFT-GIT and T-SPOT.TB tests, the CDC included them in its updated 2010 guidelines and indicated that either IGRAs or TSTs may be used in HCW surveillance programs for occupational exposure to M tuberculosis (see Figure).15,16 The algorithm clearly illustrates how the use of IGRAs in place of TSTs streamlines the process of HCW TB screening.17
EVALUATION OF TESTS
Given the stringent nature of the CDC’s TB infection control goals, it is essential that health care facilities use the most effective and efficient means for timely and thorough screening of HCWs for TB. When evaluating the available tests, the following factors must be considered: accuracy and reproducibility of test results; impact on results when testing is repeated frequently; interpretation of discordant test results; and specificity, sensitivity, and identification of appropriate test cutoff values so that a positive result signifies a new TB infection (ie, conversion, a change from a documented negative to positive test result within a two-year period) rather than a false positive; and costs.18,19
Accuracy
Unlike TSTs, IGRAs do not produce false-positive results in individuals vaccinated with BCG or in those infected with most nontuberculous mycobacteria.9,16 Neither TSTs nor IGRAs, however, can distinguish between active and latent TB infection.19 If either test is positive, a chest radiograph is indicated. If the x-ray reveals abnormalities in the lungs, a sputum smear to detect acid-fast-bacilli (AFB), of which M tuberculosis is one, is indicative of TB. Nuclear acid amplification testing of a respiratory specimen provides rapid laboratory confirmation, but a positive culture for M tuberculosis confirms the diagnosis.22
Specificity and sensitivity
Pai, Zwerling, and Menzies conducted a meta-analysis of 38 studies of TB testing. Most of the studies were small and had limitations, such as the lack of a gold standard test for the diagnosis of LTBI and variable TST methods and cutoff values. Nevertheless, the researchers were able to conclude that IGRAs are significantly more specific than TST and are unaffected by BCG vaccination. Although the sensitivity of IGRAs and TST is inconsistent across test populations, T-SPOT.TB appears to have greater sensitivity than QFT-GIT or TST.23
Further, TST is subject to variability in administration and interpretation, and cut points for TST positivity vary internationally.9 In contrast, the T-SPOT.TB test specifies a borderline result zone. According to the CDC, this increases test accuracy by classifying results near the cut point, making a subsequent test conversion from negative to positive more likely to represent newly acquired infection.16
On the other hand, some studies of IGRAs have found unexpectedly high rates of initial positive results and conversions among HCWs in low-risk settings that are later determined to be false positives.24 However, as noted previously, TSTs are also subject to false-positive results. In addition, the definition of an IGRA conversion is less stringent than the TST conversion definition, which may result in more IGRA conversions.16
Discordant results
Zwerling et al conducted a systematic review of all studies in which IGRAs were used for HCW screening to summarize their performance in cross-sectional and serial testing settings. The prevalence of positive IGRAs was found to be lower than that of positive TSTs. This difference was significant in low- and moderate-TB incidence settings but not in high-incidence settings. A positive association was reported between positive IGRA test results and occupational risk factors, including work in high-risk wards, TB clinics, and geriatric care, as well as length of employment.18
According to Mancuso et al, discordance of results between the TST and IGRAs in populations with low LTBI prevalence suggests that most positive test results are in fact false positives in these populations.25 Although IGRAs were designed to increase specificity, the authors found that IGRA specificity was no better than the specificity of TST. Without a gold standard for detecting M tuberculosis infection, assessing the true significance of discordance between TST and IGRAs is difficult. Further research is needed to determine the significance of test discordance, to obtain data on progression to active TB, and to better define appropriate cut points for interpreting IGRA results.25
Costs
The primary impediment to the widespread use of IGRAs has been cost, which is approximately three times that of a TST.24
Eralp et al studied the cost-effectiveness of IGRAs versus TST for screening for active LTBI in HCWs by using healthy life-years gained—defined as the number of TB cases avoided, yielding an increase in life expectancy—as the benefit metric rather than quality-adjusted life-years. Because testing is completed with a single visit, use of IGRAs increases compliance while minimizing resources needed for a second visit and eliminating loss to follow-up. Also notable is that IGRA testing takes place in a laboratory, where costs can be held in check with focused expertise and optimized staffing structures. The authors concluded that incremental IGRA costs per healthy life-year gained were justified.19
Until publication of the SWITCH (Screening health care Workers with IGRA vs. TST: impact on Costs and adHerence to testing) study in 2012, cost-effectiveness studies had shown inconsistent results. This study, conducted by The Johns Hopkins University (JHU) employee health department, was the first of its kind in the US to systematically analyze test performance and labor costs for TB screening of HCWs. The results showed that the time required to administer a TST is one of the costliest elements. For a sizeable institution such as JHU, TST screening cost more than $1.3 million annually, equivalent to approximately $73 per person; in contrast, IGRA screening amounted to less than $55 per person.26
Further research
The association between IGRA test conversion in HCWs and the risk for active TB disease has not been demonstrated.16 However, this is also true of TSTs27 and further research is needed in this area. Research is also needed to determine the significance of TSA-IGRA results discordance and to better define cut points for IGRA interpretation.25 In addition, more study of factors related to serial (periodic or ongoing) TB testing—which are very important within the context of HCW screening—is needed. As an example, serial TB testing may reveal trends in test conversions and can identify areas of concern within a health care facility. Unfortunately, current CDC recommendations do not provide specific guidelines for serial IGRA testing, such as guidance for accurate interpretation of IGRA results within a serial testing context.16 These areas should be addressed in future CDC updates.
Continue for conclusion >>
CONCLUSION
Occupational health professionals are continuously evaluating how to improve surveillance programs and reduce HCWs’ time away from work. Within this context, four advantages IGRAs offer include
• Elimination of a two-step TST for new hires (which requires up to four separate visits)
• No false-positive results caused by previous BCG vaccination or exposure to most nontuberculous mycobacteria
• Significantly improved HCW compliance because screening requires only one visit
• Cost effectiveness.
From an occupational health perspective, these are significant advantages that support the use of IGRAs to screen HCW for TB.
REFERENCES
1. Wilson J. Five babies test positive for TB in Texas. CNN. September 30, 2014. www.cnn.com/2014/09/29/health/babies-test-positive-tb/index.html. Accessed November 12, 2014.
2. Bailey J. 700 babies exposed to TB at Texas Hospital. Atlanta Journal Constitution. September 22, 2014. www.ajc.com/news/lifestyles/health/700-babies-exposed-TB-texas-hospital/nhRmC/. Accessed November 12, 2014.
3. CDC. Reported tuberculosis in the United States, 2013. www.cdc.gov/tb/statistics/reports/2013/pdf/report2013.pdf. Accessed October 23, 2014.
4. World Health Organization. Global tuberculosis report 2014. www.who.int/tb/publications/global_report/en/. Accessed November 12, 2014.
5. Baussano I, Nunn P, Williams B, et al. Tuberculosis among health care workers. Emerg Infect Dis. 2011;17(3):488-494.
6. Yang H, Kruh-Garcia NA, Dobos KM. Purified protein derivatives of tuberculin: past, present, and future. FEMS Immunol Med Microbiol. 2012;66(3):273-280.
7. Huebner RE, Schein MF, Bass JB Jr. The tuberculin skin test. Clin Infect Dis. 1993;17(6):968-975.
8. World Health Organization. Recommendations for vaccine administration. www.who.int/immunization/policy/Immunization_routine_table1.pdf. Accessed November 12, 2014.
9. Al-Orainey IO. Diagnosis of latent tuberculosis: can we do better? Ann Thorac Med. 2009;4(1):5-9.
10. Desem N, Jones SL. Development of a human gamma interferon enzyme immunoassay and comparison with tuberculin skin testing for detection of Mycobacterium tuberculosis infection. Clin Diagn Lab Immunol. 1998;5(4): 531-536.
11. FDA. QuantiFERON-TB - P010033. www.fda.gov/MedicalDevices/Products andMedicalProcedures/DeviceApprovalsandClearances/Recently-Approved Devices/ucm084025.htm. Accessed November 12, 2014.
12. QuantiFERON-TB Gold In-Tube Test [package insert]. www.quantiferon.com/irm/content/PI/QFT/2PK/US.pdf. Accessed November 12, 2014.
13. T-SPOT.TB [package insert]. Marlborough, MA. Oxford Immunotec, Inc. www.tspot.com/wp-content/uploads/2012/01/PI-TB-US-v4.pdf. Accessed November 12, 2014.
14. CDC. Fact sheet: infection control in health-care settings. www.cdc.gov/tb/publications/factsheets/prevention/ichcs.htm. Accessed November 12, 2014.
15. Jensen PA, Lambert LA, Iademarco MF, Ridzon R; CDC. Guidelines for preventing the transmission of Mycobacterium TB in health-care settings, 2005. MMWR Recomm Rep. 2005;54(RR-17):1-141.
16. Mazurek GH, Jereb J, Vernon A, et al; IGRA Expert Committee, CDC. Updated guidelines for using interferon γ release assays to detect Mycobacterium TB infection: United States, 2010. MMWR Recomm Rep. 2010;59(RR-5):1-25.
17. CDC. Latent tuberculosis infection: a guide for primary health care providers. www.cdc.gov/tb/publications/ltbi/diagnosis.htm. Accessed November 3, 2014.
18. Zwerling A, van den Hof S, Scholten J, et al. Interferon-γ release assays for TB screening of healthcare workers: a systematic review. Thorax. 2012;67(1): 62-70.
19. Eralp MN, Scholtes S, Martell G, et al. Screening of healthcare workers for TB: development and validation of a new health economic model to inform practice. BMJ Open. 2012;2:e000630.
20. FDA. FDA permits marketing of first US test labeled for simultaneous detection of tuberculosis bacteria and resistance to the antibiotic rifampin [press release]. July 25, 2013.
21. CDC. Availability of an assay for detecting Mycobacterium TB, including rifampin-resistant strains, and considerations for its use: United States, 2013 [published correction appears in MMWR Morb Mortal Wkly Rep. 2013;62(45):906]. MMWR Morb Mortal Wkly Rep. 2013;62(41):821-827.
22. CDC. Fact sheet: diagnosis of tuberculosis disease. www.cdc.gov/tb/publica tions/factsheets/testing/diagnosis.htm. Accessed November 12, 2014.
23. Pai M, Zwerling A, Menzies D. Systematic review: T-cell–based assays for the diagnosis of latent TB infection: an update. Ann Intern Med. 2008;149(3): 177-184.
24. LoBue PA, Castro KG. Is it time to replace the tuberculin skin test with a blood test? JAMA. 2012;308(3):241-242.
25. Mancuso JD, Mazurek GH, Tribble D, et al. Discordance among commercially available diagnostics for latent TB infection. Am J Respir Crit Care Med. 2012;185(4):427-434.
26. Wrighton-Smith P, Sneed L, Humphrey F, et al. Screening health care workers with interferon-γ release assay versus tuberculin skin test: impact on costs and adherence to testing (the SWITCH study). J Occup Environ Med. 2012;54(7):806-815.
27. World Health Organization. Use of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle-income countries. www.who.int/tb/
features_archive/policy_statement_igra_oct2011.pdf. Accessed November 12, 2014.