User login
Tumor necrosis factor (TNF)–alpha inhibitors achieve better endoscopic healing than the newer biologic drugs vedolizumab (Entyvio) and ustekinumab (Stelara) in moderate to severe Crohn’s disease, a new meta-analysis suggests.
The advantage for the TNF blockers infliximab (Remicade) and adalimumab (Humira) came in treating larger ileal ulcers and colonic disease.
This finding could help physicians choose among the four biologic drugs approved in recent years in the United States, Canada, and Western Europe to treat this disease. None of these drugs has emerged as clearly superior to all the others.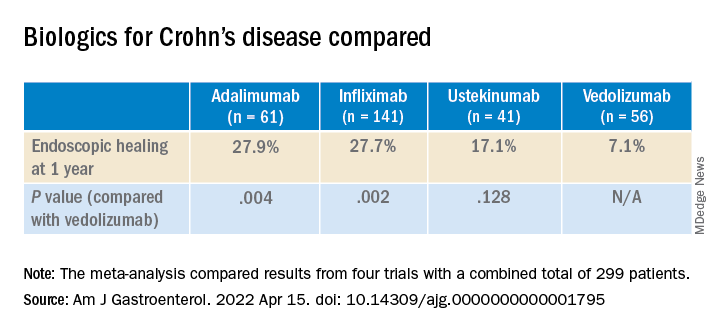
“For patients with high-risk or difficult-to-treat disease, such as those with larger ileal ulcers, the use of anti-TNF may be preferable as a first-line option,” said lead author Neeraj Narula, MD, MPH, of the department of medicine at McMaster University in Toronto, in an email to this news organization.
The study was published online in the American Journal of Gastroenterology.
Few head-to-head trials
In contrast to the TNF blockers infliximab and adalimumab, ustekinumab blocks interleukin-12 and interleukin-23, and vedolizumab blocks integrin–alpha4-beta7.
Only one trial, SEAVUE, has compared any of these drugs head to head for the treatment of Crohn’s disease. This trial found no difference between ustekinumab and adalimumab in rates of clinical remission or endoscopic healing. However, the patients in the trial had a relatively low baseline Simple Endoscopic Score for Crohn’s disease (SES-CD).
In the VARSITY trial, vedolizumab showed better results than adalimumab in clinical remission and endoscopic improvement, but that trial involved patients with ulcerative colitis.
“None of these medications are clearly head and shoulders above the rest; they all work in similar ways,” said Simon Hong, MD, of the Inflammatory Bowel Disease Center at New York University Langone Health, who was not involved in the study. “It’s not clear, at least from a rigorous scientific standpoint, which is better.”
Four biologic drugs compared
In their meta-analysis, Dr. Narula and colleagues compared results from four previous trials, which combined had a total of 299 patients. The investigators assessed the difference in results for specific ileocolonic segments. They focused on endoscopic healing because it is believed to be a more reliable indicator of long-term health than symptoms, which are more susceptible to the placebo effect.
Although the rates of endoscopic healing were low overall, they were significantly better for the TNF blockers than with the newer drugs. The difference between ustekinumab and vedolizumab was not statistically significant.
Among patients with a baseline ileal SES-CD of 3 or greater, the researchers found no significant differences between biologics for 1-year ileal endoscopic healing.
But in patients with ileal ulcers larger than 0.5 cm, the ulcers disappeared after a year in 40.8% of patients who took infliximab vs. 30% of those who took adalimumab, 17.7% of those who took ustekinumab, and 8.7% of those who took vedolizumab. Compared to vedolizumab, the difference was statistically significant for infliximab (P = .045) but not for adalimumab (P = .077) or ustekinumab (P = .259).
Among those patients who had at least one colonic segment with an SES-CD of 3 or greater, the patients taking adalimumab did the best, with 62.5% achieving endoscopic healing of the colon. The rate with infliximab was 52.4%. For vedolizumab, the rate was 31.3%, and for ustekinumab, it was 29.0%. Only the differences between the TNF blockers and the newer biologics were statistically significant for this comparison.
In general, the ileum does not heal as well as the colon, Dr. Narula and colleagues note.
“This confirms, or at least supports, our experience,” Dr. Hong told this news organization. The explanation for the greater efficacy of the TNF blockers could be their more systemic mechanism of action, he said.
The study authors acknowledge that their meta-analysis cannot take the place of true head-to-head trials.
“Safety, convenience, and cost of therapy all are relevant factors that impact decision-making, and the availability of biosimilar TNF-alpha antagonist therapies in routine practice adds additional consideration for cost-effectiveness in population health decisions,” Dr. Narula said.
The study was self-funded. Dr. Narula has received honoraria from Janssen, AbbVie, Takeda, Pfizer, Merck, Sandoz, Novartis, and Ferring. Dr. Hong reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tumor necrosis factor (TNF)–alpha inhibitors achieve better endoscopic healing than the newer biologic drugs vedolizumab (Entyvio) and ustekinumab (Stelara) in moderate to severe Crohn’s disease, a new meta-analysis suggests.
The advantage for the TNF blockers infliximab (Remicade) and adalimumab (Humira) came in treating larger ileal ulcers and colonic disease.
This finding could help physicians choose among the four biologic drugs approved in recent years in the United States, Canada, and Western Europe to treat this disease. None of these drugs has emerged as clearly superior to all the others.
“For patients with high-risk or difficult-to-treat disease, such as those with larger ileal ulcers, the use of anti-TNF may be preferable as a first-line option,” said lead author Neeraj Narula, MD, MPH, of the department of medicine at McMaster University in Toronto, in an email to this news organization.
The study was published online in the American Journal of Gastroenterology.
Few head-to-head trials
In contrast to the TNF blockers infliximab and adalimumab, ustekinumab blocks interleukin-12 and interleukin-23, and vedolizumab blocks integrin–alpha4-beta7.
Only one trial, SEAVUE, has compared any of these drugs head to head for the treatment of Crohn’s disease. This trial found no difference between ustekinumab and adalimumab in rates of clinical remission or endoscopic healing. However, the patients in the trial had a relatively low baseline Simple Endoscopic Score for Crohn’s disease (SES-CD).
In the VARSITY trial, vedolizumab showed better results than adalimumab in clinical remission and endoscopic improvement, but that trial involved patients with ulcerative colitis.
“None of these medications are clearly head and shoulders above the rest; they all work in similar ways,” said Simon Hong, MD, of the Inflammatory Bowel Disease Center at New York University Langone Health, who was not involved in the study. “It’s not clear, at least from a rigorous scientific standpoint, which is better.”
Four biologic drugs compared
In their meta-analysis, Dr. Narula and colleagues compared results from four previous trials, which combined had a total of 299 patients. The investigators assessed the difference in results for specific ileocolonic segments. They focused on endoscopic healing because it is believed to be a more reliable indicator of long-term health than symptoms, which are more susceptible to the placebo effect.
Although the rates of endoscopic healing were low overall, they were significantly better for the TNF blockers than with the newer drugs. The difference between ustekinumab and vedolizumab was not statistically significant.
Among patients with a baseline ileal SES-CD of 3 or greater, the researchers found no significant differences between biologics for 1-year ileal endoscopic healing.
But in patients with ileal ulcers larger than 0.5 cm, the ulcers disappeared after a year in 40.8% of patients who took infliximab vs. 30% of those who took adalimumab, 17.7% of those who took ustekinumab, and 8.7% of those who took vedolizumab. Compared to vedolizumab, the difference was statistically significant for infliximab (P = .045) but not for adalimumab (P = .077) or ustekinumab (P = .259).
Among those patients who had at least one colonic segment with an SES-CD of 3 or greater, the patients taking adalimumab did the best, with 62.5% achieving endoscopic healing of the colon. The rate with infliximab was 52.4%. For vedolizumab, the rate was 31.3%, and for ustekinumab, it was 29.0%. Only the differences between the TNF blockers and the newer biologics were statistically significant for this comparison.
In general, the ileum does not heal as well as the colon, Dr. Narula and colleagues note.
“This confirms, or at least supports, our experience,” Dr. Hong told this news organization. The explanation for the greater efficacy of the TNF blockers could be their more systemic mechanism of action, he said.
The study authors acknowledge that their meta-analysis cannot take the place of true head-to-head trials.
“Safety, convenience, and cost of therapy all are relevant factors that impact decision-making, and the availability of biosimilar TNF-alpha antagonist therapies in routine practice adds additional consideration for cost-effectiveness in population health decisions,” Dr. Narula said.
The study was self-funded. Dr. Narula has received honoraria from Janssen, AbbVie, Takeda, Pfizer, Merck, Sandoz, Novartis, and Ferring. Dr. Hong reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Tumor necrosis factor (TNF)–alpha inhibitors achieve better endoscopic healing than the newer biologic drugs vedolizumab (Entyvio) and ustekinumab (Stelara) in moderate to severe Crohn’s disease, a new meta-analysis suggests.
The advantage for the TNF blockers infliximab (Remicade) and adalimumab (Humira) came in treating larger ileal ulcers and colonic disease.
This finding could help physicians choose among the four biologic drugs approved in recent years in the United States, Canada, and Western Europe to treat this disease. None of these drugs has emerged as clearly superior to all the others.
“For patients with high-risk or difficult-to-treat disease, such as those with larger ileal ulcers, the use of anti-TNF may be preferable as a first-line option,” said lead author Neeraj Narula, MD, MPH, of the department of medicine at McMaster University in Toronto, in an email to this news organization.
The study was published online in the American Journal of Gastroenterology.
Few head-to-head trials
In contrast to the TNF blockers infliximab and adalimumab, ustekinumab blocks interleukin-12 and interleukin-23, and vedolizumab blocks integrin–alpha4-beta7.
Only one trial, SEAVUE, has compared any of these drugs head to head for the treatment of Crohn’s disease. This trial found no difference between ustekinumab and adalimumab in rates of clinical remission or endoscopic healing. However, the patients in the trial had a relatively low baseline Simple Endoscopic Score for Crohn’s disease (SES-CD).
In the VARSITY trial, vedolizumab showed better results than adalimumab in clinical remission and endoscopic improvement, but that trial involved patients with ulcerative colitis.
“None of these medications are clearly head and shoulders above the rest; they all work in similar ways,” said Simon Hong, MD, of the Inflammatory Bowel Disease Center at New York University Langone Health, who was not involved in the study. “It’s not clear, at least from a rigorous scientific standpoint, which is better.”
Four biologic drugs compared
In their meta-analysis, Dr. Narula and colleagues compared results from four previous trials, which combined had a total of 299 patients. The investigators assessed the difference in results for specific ileocolonic segments. They focused on endoscopic healing because it is believed to be a more reliable indicator of long-term health than symptoms, which are more susceptible to the placebo effect.
Although the rates of endoscopic healing were low overall, they were significantly better for the TNF blockers than with the newer drugs. The difference between ustekinumab and vedolizumab was not statistically significant.
Among patients with a baseline ileal SES-CD of 3 or greater, the researchers found no significant differences between biologics for 1-year ileal endoscopic healing.
But in patients with ileal ulcers larger than 0.5 cm, the ulcers disappeared after a year in 40.8% of patients who took infliximab vs. 30% of those who took adalimumab, 17.7% of those who took ustekinumab, and 8.7% of those who took vedolizumab. Compared to vedolizumab, the difference was statistically significant for infliximab (P = .045) but not for adalimumab (P = .077) or ustekinumab (P = .259).
Among those patients who had at least one colonic segment with an SES-CD of 3 or greater, the patients taking adalimumab did the best, with 62.5% achieving endoscopic healing of the colon. The rate with infliximab was 52.4%. For vedolizumab, the rate was 31.3%, and for ustekinumab, it was 29.0%. Only the differences between the TNF blockers and the newer biologics were statistically significant for this comparison.
In general, the ileum does not heal as well as the colon, Dr. Narula and colleagues note.
“This confirms, or at least supports, our experience,” Dr. Hong told this news organization. The explanation for the greater efficacy of the TNF blockers could be their more systemic mechanism of action, he said.
The study authors acknowledge that their meta-analysis cannot take the place of true head-to-head trials.
“Safety, convenience, and cost of therapy all are relevant factors that impact decision-making, and the availability of biosimilar TNF-alpha antagonist therapies in routine practice adds additional consideration for cost-effectiveness in population health decisions,” Dr. Narula said.
The study was self-funded. Dr. Narula has received honoraria from Janssen, AbbVie, Takeda, Pfizer, Merck, Sandoz, Novartis, and Ferring. Dr. Hong reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF GASTROENTEROLOGY