User login
A 10-mg dose of tofacitinib twice daily significantly improved remission, mucosal healing, and clinical response in adults with active ulcerative colitis (UC), based on data from a pair of identical phase III studies including nearly 900 patients. The findings were presented at the European Crohn’s and Colitis Organisation conference in Amsterdam.
“In up to one-third of patients with UC, treatment is not completely successful or complications arise,” study coauthor Dr. Geert D’Haens of the University of Amsterdam said in an interview. The goal of the studies was to evaluate the safety and efficacy of a 10-mg dose of oral tofacitinib twice daily in inducing remission in UC patients, he added. The OCTAVE (Oral Clinical Trials for Tofacitinib in Ulcerative Colitis) Induction 1 study included 476 patients taking tofacitinib and 122 patients taking placebo; the OCTAVE Induction 2 study included 429 patients on tofacitinib and 112 patients on placebo.
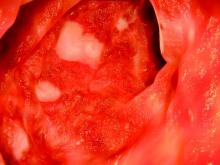
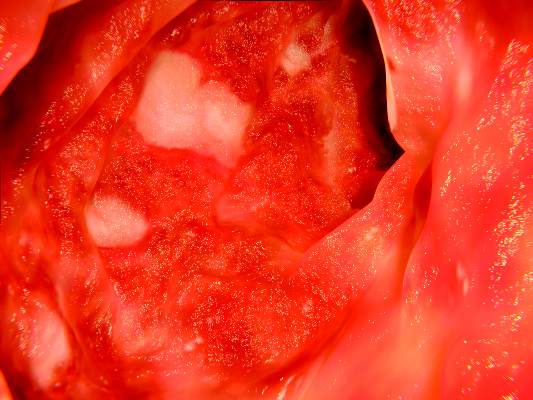
Overall, significantly more patients receiving tofacitinib 10 mg twice daily achieved remission, mucosal healing, and clinical response in both studies, compared with the placebo, at 8 weeks. In the OCTAVE Induction 1 and Induction 2 studies, remission at 8 weeks for tofacitinib compared with placebo was 19% vs. 8% and 17% vs. 4%, respectively. Mucosal healing rates in the Induction 1 and 2 studies for tofacitinib compared with placebo were 31% vs. 16% and 28% vs. 12%, respectively, and clinical response rates were 60% vs. 33% and 55% vs. 29%, respectively. Efficacy was similar for patients previously treated with tumor necrosis factor inhibitors and those who were not.
The incidence of adverse events and serious adverse events was not significantly different between treatment and placebo groups in either study. However, tofacitinib treatment was associated with increases in serum lipid (total cholesterol, low-density and high-density lipoprotein), and creatine kinase levels.
“The clinical trial data confirm our blinded observations,” Dr. D’Haens said. “Even when all other drugs have failed, tofacitinib can be effective. Since Janus kinase inhibitors reduce the production of many proinflammatory cytokines, the clinical findings are in line with what we expected. Fortunately, adverse events were limited and allowed prolonged treatment with this agent,” he noted.
Research on tofacitinib and UC is ongoing, said Dr. D’Haens. The two studies reported here, OCTAVE Induction 1 and 2, are part of the global OCTAVE program, he said. Other related studies include a third phase III study, OCTAVE Sustain, and a long-term extension trial called OCTAVE Open. “OCTAVE Sustain is a phase III placebo-controlled study evaluating oral tofacitinib 10 mg and 5 mg b.i.d. as maintenance therapy in adult patients with moderately to severely active UC. Top-line results for this study are anticipated at the end of this year,” he said. “OCTAVE Open is an ongoing open-label extension study designed to assess the safety and tolerability of tofacitinib 10 mg and 5 mg b.i.d. in patients who have completed or who have had treatment failure in OCTAVE Sustain or who were nonresponders upon completing OCTAVE Induction 1 or 2,” he added.
The study was supported in part by Pfizer. Dr. D’Haens disclosed financial relationships with multiple companies, including Pfizer.
A 10-mg dose of tofacitinib twice daily significantly improved remission, mucosal healing, and clinical response in adults with active ulcerative colitis (UC), based on data from a pair of identical phase III studies including nearly 900 patients. The findings were presented at the European Crohn’s and Colitis Organisation conference in Amsterdam.
“In up to one-third of patients with UC, treatment is not completely successful or complications arise,” study coauthor Dr. Geert D’Haens of the University of Amsterdam said in an interview. The goal of the studies was to evaluate the safety and efficacy of a 10-mg dose of oral tofacitinib twice daily in inducing remission in UC patients, he added. The OCTAVE (Oral Clinical Trials for Tofacitinib in Ulcerative Colitis) Induction 1 study included 476 patients taking tofacitinib and 122 patients taking placebo; the OCTAVE Induction 2 study included 429 patients on tofacitinib and 112 patients on placebo.
Overall, significantly more patients receiving tofacitinib 10 mg twice daily achieved remission, mucosal healing, and clinical response in both studies, compared with the placebo, at 8 weeks. In the OCTAVE Induction 1 and Induction 2 studies, remission at 8 weeks for tofacitinib compared with placebo was 19% vs. 8% and 17% vs. 4%, respectively. Mucosal healing rates in the Induction 1 and 2 studies for tofacitinib compared with placebo were 31% vs. 16% and 28% vs. 12%, respectively, and clinical response rates were 60% vs. 33% and 55% vs. 29%, respectively. Efficacy was similar for patients previously treated with tumor necrosis factor inhibitors and those who were not.
The incidence of adverse events and serious adverse events was not significantly different between treatment and placebo groups in either study. However, tofacitinib treatment was associated with increases in serum lipid (total cholesterol, low-density and high-density lipoprotein), and creatine kinase levels.
“The clinical trial data confirm our blinded observations,” Dr. D’Haens said. “Even when all other drugs have failed, tofacitinib can be effective. Since Janus kinase inhibitors reduce the production of many proinflammatory cytokines, the clinical findings are in line with what we expected. Fortunately, adverse events were limited and allowed prolonged treatment with this agent,” he noted.
Research on tofacitinib and UC is ongoing, said Dr. D’Haens. The two studies reported here, OCTAVE Induction 1 and 2, are part of the global OCTAVE program, he said. Other related studies include a third phase III study, OCTAVE Sustain, and a long-term extension trial called OCTAVE Open. “OCTAVE Sustain is a phase III placebo-controlled study evaluating oral tofacitinib 10 mg and 5 mg b.i.d. as maintenance therapy in adult patients with moderately to severely active UC. Top-line results for this study are anticipated at the end of this year,” he said. “OCTAVE Open is an ongoing open-label extension study designed to assess the safety and tolerability of tofacitinib 10 mg and 5 mg b.i.d. in patients who have completed or who have had treatment failure in OCTAVE Sustain or who were nonresponders upon completing OCTAVE Induction 1 or 2,” he added.
The study was supported in part by Pfizer. Dr. D’Haens disclosed financial relationships with multiple companies, including Pfizer.
A 10-mg dose of tofacitinib twice daily significantly improved remission, mucosal healing, and clinical response in adults with active ulcerative colitis (UC), based on data from a pair of identical phase III studies including nearly 900 patients. The findings were presented at the European Crohn’s and Colitis Organisation conference in Amsterdam.
“In up to one-third of patients with UC, treatment is not completely successful or complications arise,” study coauthor Dr. Geert D’Haens of the University of Amsterdam said in an interview. The goal of the studies was to evaluate the safety and efficacy of a 10-mg dose of oral tofacitinib twice daily in inducing remission in UC patients, he added. The OCTAVE (Oral Clinical Trials for Tofacitinib in Ulcerative Colitis) Induction 1 study included 476 patients taking tofacitinib and 122 patients taking placebo; the OCTAVE Induction 2 study included 429 patients on tofacitinib and 112 patients on placebo.
Overall, significantly more patients receiving tofacitinib 10 mg twice daily achieved remission, mucosal healing, and clinical response in both studies, compared with the placebo, at 8 weeks. In the OCTAVE Induction 1 and Induction 2 studies, remission at 8 weeks for tofacitinib compared with placebo was 19% vs. 8% and 17% vs. 4%, respectively. Mucosal healing rates in the Induction 1 and 2 studies for tofacitinib compared with placebo were 31% vs. 16% and 28% vs. 12%, respectively, and clinical response rates were 60% vs. 33% and 55% vs. 29%, respectively. Efficacy was similar for patients previously treated with tumor necrosis factor inhibitors and those who were not.
The incidence of adverse events and serious adverse events was not significantly different between treatment and placebo groups in either study. However, tofacitinib treatment was associated with increases in serum lipid (total cholesterol, low-density and high-density lipoprotein), and creatine kinase levels.
“The clinical trial data confirm our blinded observations,” Dr. D’Haens said. “Even when all other drugs have failed, tofacitinib can be effective. Since Janus kinase inhibitors reduce the production of many proinflammatory cytokines, the clinical findings are in line with what we expected. Fortunately, adverse events were limited and allowed prolonged treatment with this agent,” he noted.
Research on tofacitinib and UC is ongoing, said Dr. D’Haens. The two studies reported here, OCTAVE Induction 1 and 2, are part of the global OCTAVE program, he said. Other related studies include a third phase III study, OCTAVE Sustain, and a long-term extension trial called OCTAVE Open. “OCTAVE Sustain is a phase III placebo-controlled study evaluating oral tofacitinib 10 mg and 5 mg b.i.d. as maintenance therapy in adult patients with moderately to severely active UC. Top-line results for this study are anticipated at the end of this year,” he said. “OCTAVE Open is an ongoing open-label extension study designed to assess the safety and tolerability of tofacitinib 10 mg and 5 mg b.i.d. in patients who have completed or who have had treatment failure in OCTAVE Sustain or who were nonresponders upon completing OCTAVE Induction 1 or 2,” he added.
The study was supported in part by Pfizer. Dr. D’Haens disclosed financial relationships with multiple companies, including Pfizer.
EXPERT ANALYSIS FROM ECCO
Key clinical point: Oral tofacitinib reduced the symptoms of moderate to severe ulcerative colitis and induced remission and healing of the diseased colonic mucosa in patients previously treated with tumor necrosis factor inhibitors and those who were not.
Major finding: In the OCTAVE Induction 1 and Induction 2 studies, remission at 8 weeks for tofacitinib patients compared with placebo patients was 19% vs. 8% and 17% vs. 4%, respectively.
Data source: A pair of identical phase III studies including nearly 900 patients.
Disclosures: The study was supported in part by Pfizer. Dr. Geert D’Haens disclosed financial relationships with multiple companies, including Pfizer.