User login
The field of cardiology is undergoing a quiet and gradual revolution.
Emerging new technologies are bringing together subspecialties that have long been at odds with one another. Also arising is a new breed of specialists who have a new vision for cardiovascular medicine.
The procedure that lies at the heart of this movement is transcatheter valve therapy.
Although open valve replacement, a highly successful and durable treatment, remains the standard treatment for patients with severe aortic stenosis, patients too sick for surgery have few options: They are medically treated or undergo balloon aortic valvuloplasty.
But transcatheter valve replacement devices have the potential to improve the dismal landscape inoperable patients now face. Devices for transcatheter aortic valve replacement (TAVR) have been commercially available in Europe since 2007. Just this week, on Nov. 2, the Food and Drug Administration approved the first such device for use in the United States, and another is undergoing clinical trials.
"It’s a major breakthrough for [valve replacement,]" said Dr. William A. Zoghbi, the American College of Cardiology’s incoming president and the director of the Cardiovascular Imaging Institute at Methodist Hospital in Houston. "This was not present more than a decade ago, and now it’s becoming a reality. We had patients who had no other option."
What’s also significant about TAVR, experts say, is the mandated teamwork between cardiac surgeons and interventional cardiologists.
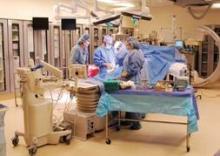
In dozens of hospitals and medical centers across the nation participating in either the Edwards Sapien valve trial or Medtronic’s CoreValve pivotal trial, cardiac surgeons, interventional cardiologists, and medical cardiologists gather almost weekly to discuss the best treatment options for patients. They perform the procedure side by side in hybrid operating rooms and learn new skills from each other.
The therapy can "change medicine forever," said Dr. Augusto D. Pichard, director of the cardiac catheterization lab at the Washington Hospital Center, which is a participant in Edwards Lifesciences’ PARTNER (Placement of Aortic Transcatheter Valve) trial of its Sapien valve (N. Engl. J. Med. 2010;363:1597-607).
"It’s proving that multidisciplinary medicine is indispensable," said Dr. Pichard, who is one of the trial’s principle investigators at the hospital.
Such multidisciplinary "Heart Teams" as required by the PARTNER trial, or Medtronic’s CoreValve trial, are not new to cardiology.
The SYNTAX trial, which compared the TAXUS drug-eluting stent with coronary artery bypass surgery, required interventional cardiologists and cardiac surgeons to review the angiography results together to decide the best treatment for patients.
Heart Teams also showed to be successful in the EVEREST II trial that compared percutaneous mitral valve repair using Abbott Vascular’s investigational MitraClip with conventional surgical repair or replacement (N. Engl. J. Med. 2011;364:1395-406).
"Such a Heart Team will be even more critical as the issue with structural heart disease become more complex, as the treatment expands to more centers, and as new technology is applied outside of the constraints of randomized clinical trials," wrote Dr. David R. Holmes Jr. of Mayo Clinic, Rochester, Minn., who is president of the ACC, and Dr. Michael J. Mack of Medical City Dallas Hospital, who is president of the Society of Thoracic Surgeons, in an expert consensus document on transcatheter valve therapy. (J. Am. Coll. Cardiol. 2011;58:445-55).
Transcatheter valve therapy is a "transformational technology," that is, "one that, when introduced, radically changes markets, creates wholly new markets, or could even eliminate existing markets for older technology," they wrote.
Having gotten a glimpse into the future through trials such as PARTNER, Dr. Mathew Williams, one of a new breed of so-called hybrid cardiovascular specialists, says it’s important to have dual training in the rapidly evolving field of invasive cardiovascular medicine.
Being a cardiac surgeon without knowing any "wire skills" is somewhat outdated. "You need to know both," said Dr. Mathew Williams.
Cardiac surgeons and interventional cardiologists "treat the same diseases and deal with the same patients. They just use different skill sets," said Dr. Williams, surgical director of Cardiovascular Transcatheter Therapies at New York–Presbyterian Hospital/Columbia University Medical Center.
He is among a handful of cardiologists who have recently trained as both cardiac surgeons and interventional cardiologists.
After completing his training in cardiac surgery, he completed a 1-year fellowship in interventional cardiology.
"I decided to do it because that’s where a lot of valve diseases are headed," said the 41-year-old in an interview. Being a cardiac surgeon without knowing any "wire skills" is somewhat outdated. "You need to know both."
In addition, "being trained in both areas will take out the financial and political battles," he said. "It will help you become the least biased decision maker."
The approval of the Edwards Sapien valve was based the results from PARTNER's Cohort B, comprising inoperable patients with severe aortic stenosis, the proportion of survival at 1 year was 50.3% for the 179 control patients and 69.3% for the 179 patients assigned to the Sapien device, a significant difference. The median survival in years was 0.97 in the control group and 2.18 in the treated group, also a significant difference. These results swayed the FDA’s Circulatory System Devices Panel to recommend the Sapien model 9000TFX, sizes 23 mm and 26 mm, for approval, despite a high neurologic event rate (stroke and transient ischemic heart attack). In anticipation of an FDA approval, the Centers for Medicare and Medicaid Services has already opened a national coverage determination analysis for TAVI and is expected to issue its decision in March 2012.
Meanwhile, inoperable aortic stenosis patients are being enrolled in the PARTNER II trial of the next-generation Sapien XT valve, which has already supplanted the first-generation Sapien in Europe.
But while Heart Teams are excited about the potential of stent-valve systems, they are aware of some apprehension, especially among some cardiovascular surgeons who have long been the gatekeepers for valve replacement surgeries.
"The rationale for having both the cardiologist and surgeon involved is that one acts as the check for the other. So that every time the cardiologist says I can stick one of those in there, it wouldn’t be easy. It takes judgment, precision, preparation, work-up and evaluation. It takes a very takes a very long time," said Dr. Alan H. Markowitz, director of the Heart Valve Center at the University Hospitals Case Medical Center, Cleveland. His center is participating in the Medtronic CoreValve U.S. Pivotal Trial. "Surgeons have to understand it, get involved in it, or it will pass them by."
Some experts outside the TAVR realm are skeptical that the interdisciplinary teams will change the trajectory of cardiology. "The collaboration that these cardiovascular specialists are enjoying is generated by the trial protocol itself. So whether this collegiality will spill over into cardiovascular science in a larger sense remains to be seen," commented Dr. Sidney Goldstein, professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. Regarding the merits of the new treatment, "TAVR represents an important step in treating high risk aortic patients. It does carry a significant risk of stroke, and its performance against standard AVR in less critically ill patients has not been determined."
The two valves in U.S. trials have more than one route of implantation. The Edwards Sapien valve can be implanted through a transfemoral or transapical route. The CoreValve routes include transfemoral, subclavian, and direct aortic access. A cardiac surgeon and interventional cardiologist collaborate and are present during all procedures.
There are still many unknowns, among them the durability of the devices or whether the patient criteria will expand beyond the "sickest of the sick," as it already has in Europe.
"This is just the very beginning," said Dr. Paul Joseph Corso, director of cardiac surgery at Washington Hospital Center and the co-principle investigator for the PARTNER trial. "We’re not going to have turf wars that will be to the detriment of the patients."
Dr. Holmes, Dr. Mack, and Dr. Zoghbi said they had no relevant disclosures. Dr. Williams, Dr. Corso, and Dr. Pichard are involved in the PARTNER trial; Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves. Dr. Markowitz is involved in the CoreValve trial.
The field of cardiology is undergoing a quiet and gradual revolution.
Emerging new technologies are bringing together subspecialties that have long been at odds with one another. Also arising is a new breed of specialists who have a new vision for cardiovascular medicine.
The procedure that lies at the heart of this movement is transcatheter valve therapy.
Although open valve replacement, a highly successful and durable treatment, remains the standard treatment for patients with severe aortic stenosis, patients too sick for surgery have few options: They are medically treated or undergo balloon aortic valvuloplasty.
But transcatheter valve replacement devices have the potential to improve the dismal landscape inoperable patients now face. Devices for transcatheter aortic valve replacement (TAVR) have been commercially available in Europe since 2007. Just this week, on Nov. 2, the Food and Drug Administration approved the first such device for use in the United States, and another is undergoing clinical trials.
"It’s a major breakthrough for [valve replacement,]" said Dr. William A. Zoghbi, the American College of Cardiology’s incoming president and the director of the Cardiovascular Imaging Institute at Methodist Hospital in Houston. "This was not present more than a decade ago, and now it’s becoming a reality. We had patients who had no other option."
What’s also significant about TAVR, experts say, is the mandated teamwork between cardiac surgeons and interventional cardiologists.
In dozens of hospitals and medical centers across the nation participating in either the Edwards Sapien valve trial or Medtronic’s CoreValve pivotal trial, cardiac surgeons, interventional cardiologists, and medical cardiologists gather almost weekly to discuss the best treatment options for patients. They perform the procedure side by side in hybrid operating rooms and learn new skills from each other.
The therapy can "change medicine forever," said Dr. Augusto D. Pichard, director of the cardiac catheterization lab at the Washington Hospital Center, which is a participant in Edwards Lifesciences’ PARTNER (Placement of Aortic Transcatheter Valve) trial of its Sapien valve (N. Engl. J. Med. 2010;363:1597-607).
"It’s proving that multidisciplinary medicine is indispensable," said Dr. Pichard, who is one of the trial’s principle investigators at the hospital.
Such multidisciplinary "Heart Teams" as required by the PARTNER trial, or Medtronic’s CoreValve trial, are not new to cardiology.
The SYNTAX trial, which compared the TAXUS drug-eluting stent with coronary artery bypass surgery, required interventional cardiologists and cardiac surgeons to review the angiography results together to decide the best treatment for patients.
Heart Teams also showed to be successful in the EVEREST II trial that compared percutaneous mitral valve repair using Abbott Vascular’s investigational MitraClip with conventional surgical repair or replacement (N. Engl. J. Med. 2011;364:1395-406).
"Such a Heart Team will be even more critical as the issue with structural heart disease become more complex, as the treatment expands to more centers, and as new technology is applied outside of the constraints of randomized clinical trials," wrote Dr. David R. Holmes Jr. of Mayo Clinic, Rochester, Minn., who is president of the ACC, and Dr. Michael J. Mack of Medical City Dallas Hospital, who is president of the Society of Thoracic Surgeons, in an expert consensus document on transcatheter valve therapy. (J. Am. Coll. Cardiol. 2011;58:445-55).
Transcatheter valve therapy is a "transformational technology," that is, "one that, when introduced, radically changes markets, creates wholly new markets, or could even eliminate existing markets for older technology," they wrote.
Having gotten a glimpse into the future through trials such as PARTNER, Dr. Mathew Williams, one of a new breed of so-called hybrid cardiovascular specialists, says it’s important to have dual training in the rapidly evolving field of invasive cardiovascular medicine.
Being a cardiac surgeon without knowing any "wire skills" is somewhat outdated. "You need to know both," said Dr. Mathew Williams.
Cardiac surgeons and interventional cardiologists "treat the same diseases and deal with the same patients. They just use different skill sets," said Dr. Williams, surgical director of Cardiovascular Transcatheter Therapies at New York–Presbyterian Hospital/Columbia University Medical Center.
He is among a handful of cardiologists who have recently trained as both cardiac surgeons and interventional cardiologists.
After completing his training in cardiac surgery, he completed a 1-year fellowship in interventional cardiology.
"I decided to do it because that’s where a lot of valve diseases are headed," said the 41-year-old in an interview. Being a cardiac surgeon without knowing any "wire skills" is somewhat outdated. "You need to know both."
In addition, "being trained in both areas will take out the financial and political battles," he said. "It will help you become the least biased decision maker."
The approval of the Edwards Sapien valve was based the results from PARTNER's Cohort B, comprising inoperable patients with severe aortic stenosis, the proportion of survival at 1 year was 50.3% for the 179 control patients and 69.3% for the 179 patients assigned to the Sapien device, a significant difference. The median survival in years was 0.97 in the control group and 2.18 in the treated group, also a significant difference. These results swayed the FDA’s Circulatory System Devices Panel to recommend the Sapien model 9000TFX, sizes 23 mm and 26 mm, for approval, despite a high neurologic event rate (stroke and transient ischemic heart attack). In anticipation of an FDA approval, the Centers for Medicare and Medicaid Services has already opened a national coverage determination analysis for TAVI and is expected to issue its decision in March 2012.
Meanwhile, inoperable aortic stenosis patients are being enrolled in the PARTNER II trial of the next-generation Sapien XT valve, which has already supplanted the first-generation Sapien in Europe.
But while Heart Teams are excited about the potential of stent-valve systems, they are aware of some apprehension, especially among some cardiovascular surgeons who have long been the gatekeepers for valve replacement surgeries.
"The rationale for having both the cardiologist and surgeon involved is that one acts as the check for the other. So that every time the cardiologist says I can stick one of those in there, it wouldn’t be easy. It takes judgment, precision, preparation, work-up and evaluation. It takes a very takes a very long time," said Dr. Alan H. Markowitz, director of the Heart Valve Center at the University Hospitals Case Medical Center, Cleveland. His center is participating in the Medtronic CoreValve U.S. Pivotal Trial. "Surgeons have to understand it, get involved in it, or it will pass them by."
Some experts outside the TAVR realm are skeptical that the interdisciplinary teams will change the trajectory of cardiology. "The collaboration that these cardiovascular specialists are enjoying is generated by the trial protocol itself. So whether this collegiality will spill over into cardiovascular science in a larger sense remains to be seen," commented Dr. Sidney Goldstein, professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. Regarding the merits of the new treatment, "TAVR represents an important step in treating high risk aortic patients. It does carry a significant risk of stroke, and its performance against standard AVR in less critically ill patients has not been determined."
The two valves in U.S. trials have more than one route of implantation. The Edwards Sapien valve can be implanted through a transfemoral or transapical route. The CoreValve routes include transfemoral, subclavian, and direct aortic access. A cardiac surgeon and interventional cardiologist collaborate and are present during all procedures.
There are still many unknowns, among them the durability of the devices or whether the patient criteria will expand beyond the "sickest of the sick," as it already has in Europe.
"This is just the very beginning," said Dr. Paul Joseph Corso, director of cardiac surgery at Washington Hospital Center and the co-principle investigator for the PARTNER trial. "We’re not going to have turf wars that will be to the detriment of the patients."
Dr. Holmes, Dr. Mack, and Dr. Zoghbi said they had no relevant disclosures. Dr. Williams, Dr. Corso, and Dr. Pichard are involved in the PARTNER trial; Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves. Dr. Markowitz is involved in the CoreValve trial.
The field of cardiology is undergoing a quiet and gradual revolution.
Emerging new technologies are bringing together subspecialties that have long been at odds with one another. Also arising is a new breed of specialists who have a new vision for cardiovascular medicine.
The procedure that lies at the heart of this movement is transcatheter valve therapy.
Although open valve replacement, a highly successful and durable treatment, remains the standard treatment for patients with severe aortic stenosis, patients too sick for surgery have few options: They are medically treated or undergo balloon aortic valvuloplasty.
But transcatheter valve replacement devices have the potential to improve the dismal landscape inoperable patients now face. Devices for transcatheter aortic valve replacement (TAVR) have been commercially available in Europe since 2007. Just this week, on Nov. 2, the Food and Drug Administration approved the first such device for use in the United States, and another is undergoing clinical trials.
"It’s a major breakthrough for [valve replacement,]" said Dr. William A. Zoghbi, the American College of Cardiology’s incoming president and the director of the Cardiovascular Imaging Institute at Methodist Hospital in Houston. "This was not present more than a decade ago, and now it’s becoming a reality. We had patients who had no other option."
What’s also significant about TAVR, experts say, is the mandated teamwork between cardiac surgeons and interventional cardiologists.
In dozens of hospitals and medical centers across the nation participating in either the Edwards Sapien valve trial or Medtronic’s CoreValve pivotal trial, cardiac surgeons, interventional cardiologists, and medical cardiologists gather almost weekly to discuss the best treatment options for patients. They perform the procedure side by side in hybrid operating rooms and learn new skills from each other.
The therapy can "change medicine forever," said Dr. Augusto D. Pichard, director of the cardiac catheterization lab at the Washington Hospital Center, which is a participant in Edwards Lifesciences’ PARTNER (Placement of Aortic Transcatheter Valve) trial of its Sapien valve (N. Engl. J. Med. 2010;363:1597-607).
"It’s proving that multidisciplinary medicine is indispensable," said Dr. Pichard, who is one of the trial’s principle investigators at the hospital.
Such multidisciplinary "Heart Teams" as required by the PARTNER trial, or Medtronic’s CoreValve trial, are not new to cardiology.
The SYNTAX trial, which compared the TAXUS drug-eluting stent with coronary artery bypass surgery, required interventional cardiologists and cardiac surgeons to review the angiography results together to decide the best treatment for patients.
Heart Teams also showed to be successful in the EVEREST II trial that compared percutaneous mitral valve repair using Abbott Vascular’s investigational MitraClip with conventional surgical repair or replacement (N. Engl. J. Med. 2011;364:1395-406).
"Such a Heart Team will be even more critical as the issue with structural heart disease become more complex, as the treatment expands to more centers, and as new technology is applied outside of the constraints of randomized clinical trials," wrote Dr. David R. Holmes Jr. of Mayo Clinic, Rochester, Minn., who is president of the ACC, and Dr. Michael J. Mack of Medical City Dallas Hospital, who is president of the Society of Thoracic Surgeons, in an expert consensus document on transcatheter valve therapy. (J. Am. Coll. Cardiol. 2011;58:445-55).
Transcatheter valve therapy is a "transformational technology," that is, "one that, when introduced, radically changes markets, creates wholly new markets, or could even eliminate existing markets for older technology," they wrote.
Having gotten a glimpse into the future through trials such as PARTNER, Dr. Mathew Williams, one of a new breed of so-called hybrid cardiovascular specialists, says it’s important to have dual training in the rapidly evolving field of invasive cardiovascular medicine.
Being a cardiac surgeon without knowing any "wire skills" is somewhat outdated. "You need to know both," said Dr. Mathew Williams.
Cardiac surgeons and interventional cardiologists "treat the same diseases and deal with the same patients. They just use different skill sets," said Dr. Williams, surgical director of Cardiovascular Transcatheter Therapies at New York–Presbyterian Hospital/Columbia University Medical Center.
He is among a handful of cardiologists who have recently trained as both cardiac surgeons and interventional cardiologists.
After completing his training in cardiac surgery, he completed a 1-year fellowship in interventional cardiology.
"I decided to do it because that’s where a lot of valve diseases are headed," said the 41-year-old in an interview. Being a cardiac surgeon without knowing any "wire skills" is somewhat outdated. "You need to know both."
In addition, "being trained in both areas will take out the financial and political battles," he said. "It will help you become the least biased decision maker."
The approval of the Edwards Sapien valve was based the results from PARTNER's Cohort B, comprising inoperable patients with severe aortic stenosis, the proportion of survival at 1 year was 50.3% for the 179 control patients and 69.3% for the 179 patients assigned to the Sapien device, a significant difference. The median survival in years was 0.97 in the control group and 2.18 in the treated group, also a significant difference. These results swayed the FDA’s Circulatory System Devices Panel to recommend the Sapien model 9000TFX, sizes 23 mm and 26 mm, for approval, despite a high neurologic event rate (stroke and transient ischemic heart attack). In anticipation of an FDA approval, the Centers for Medicare and Medicaid Services has already opened a national coverage determination analysis for TAVI and is expected to issue its decision in March 2012.
Meanwhile, inoperable aortic stenosis patients are being enrolled in the PARTNER II trial of the next-generation Sapien XT valve, which has already supplanted the first-generation Sapien in Europe.
But while Heart Teams are excited about the potential of stent-valve systems, they are aware of some apprehension, especially among some cardiovascular surgeons who have long been the gatekeepers for valve replacement surgeries.
"The rationale for having both the cardiologist and surgeon involved is that one acts as the check for the other. So that every time the cardiologist says I can stick one of those in there, it wouldn’t be easy. It takes judgment, precision, preparation, work-up and evaluation. It takes a very takes a very long time," said Dr. Alan H. Markowitz, director of the Heart Valve Center at the University Hospitals Case Medical Center, Cleveland. His center is participating in the Medtronic CoreValve U.S. Pivotal Trial. "Surgeons have to understand it, get involved in it, or it will pass them by."
Some experts outside the TAVR realm are skeptical that the interdisciplinary teams will change the trajectory of cardiology. "The collaboration that these cardiovascular specialists are enjoying is generated by the trial protocol itself. So whether this collegiality will spill over into cardiovascular science in a larger sense remains to be seen," commented Dr. Sidney Goldstein, professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. Regarding the merits of the new treatment, "TAVR represents an important step in treating high risk aortic patients. It does carry a significant risk of stroke, and its performance against standard AVR in less critically ill patients has not been determined."
The two valves in U.S. trials have more than one route of implantation. The Edwards Sapien valve can be implanted through a transfemoral or transapical route. The CoreValve routes include transfemoral, subclavian, and direct aortic access. A cardiac surgeon and interventional cardiologist collaborate and are present during all procedures.
There are still many unknowns, among them the durability of the devices or whether the patient criteria will expand beyond the "sickest of the sick," as it already has in Europe.
"This is just the very beginning," said Dr. Paul Joseph Corso, director of cardiac surgery at Washington Hospital Center and the co-principle investigator for the PARTNER trial. "We’re not going to have turf wars that will be to the detriment of the patients."
Dr. Holmes, Dr. Mack, and Dr. Zoghbi said they had no relevant disclosures. Dr. Williams, Dr. Corso, and Dr. Pichard are involved in the PARTNER trial; Dr. Pichard has received honoraria from Edwards Lifesciences as a proctor for percutaneous aortic valves. Dr. Markowitz is involved in the CoreValve trial.