User login
A 19-year-old man with Duchenne Muscular Dystrophy (DMD) presented to the Emergency Department (ED) for left knee pain after ejection from his motorized wheelchair at a low velocity. In the ED, he developed increasing respiratory distress.
When addressing a new problem in a patient with a chronic condition, it is crucial to first understand the chronic condition and then consider whether the presenting symptoms relate to that condition or stem from an unrelated inciting event.
Patients with DMD are at risk of pulmonary complications relating to their underlying disease. For instance, dysphagia and ineffective cough can predispose them to recurrent aspiration pneumonitis and/or pneumonia, whereas decreased lung compliance (from scoliosis, atelectasis, and/or pulmonary fibrosis) and respiratory muscle weakness can progress to ventilatory failure. In addition, patients with DMD are at risk for pulmonary thromboembolism in the setting of immobility. Patients with DMD may also develop congestive heart failure resulting from myocardial fibrosis and nonischemic cardiomyopathy.
The ejection from his wheelchair signals potential trauma-associated conditions that could explain his respiratory distress. Respiratory complications of blunt thoracic trauma include pulmonary contusion, pneumothorax, flail chest (resulting from fractured ribs), and acute respiratory distress syndrome (ARDS). Lower extremity injury can result in venous thrombosis and pulmonary thromboembolism. While classically associated with long bone fractures, fat embolism syndrome (FES) may rarely occur with rib fractures and soft-tissue trauma. Respiratory compromise may also result from cervical spinal cord injury or severe anemia from trauma-associated hemorrhage.
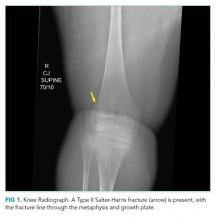
Additional past medical history included growth hormone deficiency, migraine headaches, osteoporosis secondary to chronic steroid use, cardiac fibrosis of the inferolateral wall and septum with a baseline left ventricular ejection fraction of 65%, and atrial fibrillation. His medications included calcium carbonate, vitamin D, omeprazole, lisinopril, metoprolol, prednisone, escitalopram, and testosterone. Physical examination revealed an ill-appearing obese man in respiratory distress. Temperature was 37.3°C, heart rate was 102 beats per minute (bpm), blood pressure was 110/74 mm Hg. His oxygen saturation was 93% with a respiratory rate of 25 breaths per minute while breathing ambient air. His lung sounds were clear, and his heart was without murmur. The left knee was diffusely tender to palpation without specific point tenderness. Strength was 2/5 with flexion and extension at the bilateral knees and hips and 3/5 flexion and extension at the bilateral elbows. He reported this level of weakness was his baseline. Radiographs revealed a minimally displaced Salter Harris II fracture (fracture line through the metaphysis and growth plate) of the left distal femur. His fracture was splinted early in his ED course. During his ED evaluation, the patient had acute worsening of tachycardia to 130 bpm, increased respiratory rate of 34 breaths per minute, and hypoxemia with an oxygen saturation of 83% on ambient air. He was placed on 3 L/min of oxygen via nasal cannula with improvement in his oxygen saturation to 90%. A chest radiograph was unremarkable, without evidence of pneumothorax, effusion, or pneumonia. The patient was admitted to the hospital.
The acute onset of tachypnea, tachycardia, and hypoxia, accompanied by a clear lung exam and normal chest radiograph, increases the likelihood of a pulmonary embolism. Obesity, testosterone therapy, and trauma increase his susceptibility to venous thromboembolism, while a distal femur fracture increases his risk for FES. Acute pulmonary aspiration often presents with initially absent or subtle radiographic findings. An arterial blood gas analysis would determine the presence and extent of an alveolar-arterial (A-a) gradient; a normal A-a gradient is seen in hypoventilation, while an elevated A-a gradient is seen in conditions affecting gas exchange, including pulmonary emboli and alveolar filling processes. His hypoxemia only partially corrects with supplemental oxygen, raising the possibility of capillary or anatomic shunting. Capillary shunting may occur with atelectasis, aspiration/pneumonia and pulmonary edema, whereas anatomic shunting can be intra-cardiac (eg, patent foramen ovale or septic defect) or intrapulmonary (eg, arteriovenous malformations). Patients with pulmonary emboli may also develop right-to-left shunting because of increased pulmonary vascular resistance, although hypoxemia with pulmonary emboli largely relates to ventilation/perfusion mismatch and decreased level of mixed venous blood oxygen (PvO2).
This patient’s complex medical history warrants a broadened differential with consideration of his cardiac history, including myocardial fibrosis and arrhythmia, and the impact of exposure to steroids on his immune and musculoskeletal systems. He has a history of atrial fibrillation, and an electrocardiogram is warranted to determine the underlying rhythm. Prolonged periods of rapid ventricular response may lead to tachycardia-induced cardiomyopathy. Myocardial fibrosis may progress despite use of angiotensin-converting enzyme inhibitors and is associated with systolic and/or diastolic dysfunction, although neither the examination findings provided nor the chest radiograph are suggestive of decompensated heart failure. Chronic exposure to corticosteroids (used in DMD to improve muscle strength and function) may predispose to numerous infectious and metabolic complications. Up to 10%-15% of patients with Pneumocystis jirovecii pneumonia may present with a normal chest radiograph. Acute adrenal insufficiency can present with tachycardia, weakness, and respiratory distress, so recent prednisone dose changes or interruptions should be assessed.
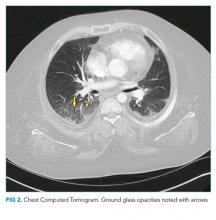
The patient’s respiratory status worsened. In light of his complex medical history, he was transferred to a children’s hospital for a higher level of care with a presumptive diagnosis of aspiration pneumonia. Upon reassessment at the new facility, the patient reported an ongoing and severe headache since his initial injury. NSAIDs had been given prior to transfer. His exam continued to be significant for tachycardia, tachypnea, and hypoxemia. His cardiac and lung examinations were otherwise normal. A comprehensive metabolic panel, procalcitonin, complete blood count with differential, and lactate were normal; his C-reactive protein (CRP) was 46.8 mg/dL (Normal <8 mg/dL). A computed tomography (CT) angiogram of the chest revealed small multifocal nodular ground-glass opacities, especially in the lower lobes, concerning for microatelectasis, multifocal pneumonia, or aspiration pneumonia. After consultation with pediatric pulmonology consultants, antimicrobials were held during the initial phase of work-up.
His headache may reflect a migraine, although further characterization and assessment for the presence and extent of head or neck trauma is warranted. Headache following trauma warrants consideration of cerebral contusion, diffuse axonal injury, intracranial hemorrhage, and carotid or vertebral artery dissection. Screening for concussion should also be performed. Hypoxemia may increase cerebral blood flow and raise intracranial pressure, resulting in headache.
CRP elevation is nonspecific and signals the presence of focal or systemic inflammation and is often elevated to a milder extent in obese patients with DMD. While normal procalcitonin argues against bacterial pneumonia, the precise level can be informative, and serial procalcitonin values may be more helpful than a single value. Although antecedent respiratory symptoms were not mentioned, viral or fungal pneumonia can present insidiously. An occult malignancy may be incidentally discovered when patients present for unrelated issues, although this and other sources of elevated CRP (eg, exacerbation of an autoimmune disease or drug reaction) remain less likely given the acuity of his presentation. Acute pulmonary embolism may be associated with a systemic inflammatory response and elevation in CRP.
In addition to the radiographic differential diagnosis already presented, the appearance of multifocal opacifications with hypoxemia raises the possibility of pulmonary infarcts or noncardiogenic pulmonary edema.
On hospital day 2, the patient continued to complain of “the worst headache of his life” as well as blurry vision and seeing “dark spots.” His headache did not improve with NSAIDs. A noncontrast CT scan of the head was normal. Neurology was consulted. Given his symptoms, history of migraines, stable neurological examination, and normal head CT, he was diagnosed with migraines and administered fluids, prochlorperazine, diphenhydramine, ondansetron, and NSAIDs. His headache continued and he continued to require supplemental oxygen.
The combination of hypoxemia, severe headache, and vision changes remains consistent with systemic emboli caused by thromboembolism or fat embolism. Headache assessment must also involve screening for “red flags,” which include sudden onset, antecedent head trauma, systemic illness (eg, fever or meningismus), focal neurologic findings, papilledema, changes with position or Valsalva, and immunosuppression. Although primary headache syndromes (eg, migraines or tension and cluster headache) may be triggered in the setting of trauma and systemic illness, “the worst headache of my life” is a concerning symptom that warrants urgent attention. While this invokes the possibility of a subarachnoid hemorrhage (SAH), headache severity is nonspecific, and rapid onset (ie, thunderclap headache) would be more suggestive. After 6 hours of symptoms, the sensitivity of head CT for detecting SAH declines, and lumbar puncture would be warranted to evaluate for xanthochromia.
His blurry vision and dark spots require testing of visual acuity and visual fields, as well as fundoscopic examination to assess for embolic phenomena or papilledema. Migraine is classically associated with “positive” or scintillating scotomata, although dark spots may occur. The presence of horizontal diplopia would indicate a cranial nerve VI palsy, which can occur with increased intracranial pressure. Visual-field cuts may also present as blurry vision, and monocular vs binocular deficits signal whether the issue is anterior or involving/posterior to the optic chiasm, respectively. Magnetic resonance imaging (MRI) may reveal the presence or sequelae of cerebral emboli (eg, fat emboli), including vasogenic edema.
Dilated fundus examination revealed Purtscher retinopathy: bilateral cotton-wool spots and larger areas of retinal whitening (Purtscher flecken).
Typical findings of Purtscher retinopathy include Purtscher flecken, cotton-wool spots, retinal hemorrhage, and optic disc edema. Purtscher retinopathy is classically associated with severe head trauma. Without associated head trauma, the term “Purtscher-like retinopathy” is used. Conditions that can cause Purtscher-like retinopathy include pancreatitis, vasculitis, microangiopathy, chronic renal failure, and systemic embolization. The most likely source of systemic embolization remains fat emboli stemming from his femur fracture. Treatment of FES is largely supportive.
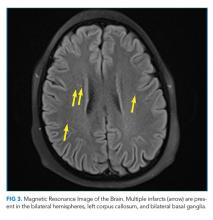
The possibility of fat emboli had been repeatedly raised by the patient’s mother since admission. While providers had considered this a possibility, it was discounted early on because of the minor nature of the patient’s orthopedic trauma, the lack of clear radiographic evidence for pulmonary emboli on chest CT, and the normal head CT. The findings on the ophthalmologist’s fundoscopic examination led the primary team to reconsider FES, along with thromboemboli and pancreatitis. Lipase was normal. MRI of the brain with contrast revealed >20 microinfarcts in the bilateral hemispheres, left corpus callosum, and bilateral basal ganglia. The CT angiogram of the chest was rereviewed; the pediatric radiologists suggested that microinfarcts could explain the patchy small ground glass opacities seen in the lungs. A transthoracic echocardiogram and electrocardiogram were normal. The diagnosis of FES was made, and the patient was started on aspirin and enoxaparin prophylaxis. His headache and respiratory status improved, and he was discharged home with close follow-up.
DISCUSSION
FES is a rare complication associated with long bone fractures and orthopedic manipulation.1,2 The exact mechanism of fat emboli production is unknown, but two theories prevail. The mechanical theory states that an outside mechanical source causes bone marrow contents or adipose tissue contents to be dislodged into the circulation where they travel through the venous circulation to become embedded in the lungs.1,2 These fragments may also migrate to the arterial circulation, through a patent foramen ovale or intrapulmonary shunts, leading to end organ damage.1,2 The biochemical theory suggests that fat emboli in the venous circulation precipitate an inflammatory and prothrombotic cascade that triggers fibrin production, platelet aggregation, and release of free fatty acids into the circulation, predisposing patients to develop multifocal systemic emboli.1
Although the classic triad in FES includes respiratory symptoms, rash, and CNS symptoms, all three findings are only present in 1%-29% of cases.1,2 Respiratory abnormalities, ranging from tachypnea and dyspnea to ARDS and hypoxic respiratory failure, occur in up to 75% of patients with FES.1 Central nervous system (CNS) complications, including headache, confusion, coma, seizures, and death caused by cerebral ischemia, occur in up to 86% of patients.1,2 Petechiae may occur in 20%-60% of patients and are usually located on nondependent regions of the body such as the head, neck, and chest.
Diagnosis of FES is largely clinical and requires a high index of suspicion and elimination of other conditions, including pulmonary thromboembolism, diffuse intravascular coagulation, and sepsis. The CNS complications must be differentiated from CNS infection, stroke, migraine, benign intracranial hypertension, and intracranial hemorrhage. There is no gold standard test for diagnosis. The Gurd and Wilson criteria, modified Gurd criteria, and Schonfeld’s criteria (Table) are commonly used but have not been clinically validated.1,3-5 These use a combination of clinical signs of respiratory distress, neurological symptoms, petechial rash, and various other diagnostic factors. When patients have risk factors, such as trauma, surgery, or predisposing conditions (eg, mobility-limiting neuromuscular disorders) and signs and symptoms potentially consistent with FES, the diagnostic evaluation should include arterial blood gas analysis, complete blood count, chest radiographs, and coagulation studies. Ophthalmological exam to evaluate for cotton-wool spots, brain MRI to detect cerebral emboli, and CT pulmonary angiogram to assess for pulmonary infarcts may help to eliminate other diagnoses and/or confirm FES.
Diagnosis of uncommon conditions that present with nonspecific symptoms, like FES, can be challenging because the symptoms may overlap with many other possible diagnoses. This challenge is further exacerbated in patients with significant medical complexity, as with the patient discussed here. Specifically, this patient had multiple plausible explanations for CNS symptoms and respiratory symptoms. It was ultimately the visual symptoms that began to link his symptoms together into a unifying syndrome and the ophthalmologic examination that prompted confirmatory imaging. It is important to continually revisit and revise the differential diagnosis in patients with medical complexity and avoid the competing temptations to abandon the search for a unifying diagnosis and attribute all symptoms to a patient’s underlying condition.
Treatment of FES is largely supportive with close monitoring of neurological status and providing supplemental oxygen as needed. Corticosteroids have been suggested to help prevent FES in patients with long bone fractures, but there is no evidence to suggest they are helpful once FES is diagnosed.2 There is conflicting evidence for the efficacy of heparin or low-molecular-weight heparin as treatment in FES.2,6 After discussions with consulting physician teams, the patient, and his family, enoxaparin and aspirin were started for this patient in light of his tenuous condition in order to decrease the risk of further embolic complications.
Patients with DMD and other neuromuscular disorders likely have a greater propensity to develop FES even after minor trauma.1,6 This is believed to be caused by patients becoming nonambulatory early in life and receiving substantial corticosteroid therapy, which can lead to osteopenia and fatty replacement of the bone marrow.1,6 This population is also often obese by the second or third decade of life, which contributes to their already increased propensity to fall.1,6
To our knowledge, this patient is 1 of 18 reported cases of FES after trauma in DMD patients. Two-thirds of these cases occurred when an unrestrained patient fell from their wheelchair. The other cases occurred while walking, during physical therapy, and during assisted transfers.6-12 In these cases, FES had a guarded prognosis, with 7 of the 18 patients dying and 1 of the patients remaining in a persistent vegetative state.8,9 While caution is warranted in generalizing these findings, given the small number of reported cases and likely publication bias,education of caregivers and patients on use of restraints and safe transfers is paramount to limit the risk of trauma.
TEACHING POINTS
- FES is a rare condition that most commonly manifests with respiratory, neurological, and cutaneous findings.
- Patients with Duchenne’s Muscular Dystrophy are likely at increased risk for FES even with minor trauma; this makes wheelchair restraints and safe transfers fundamental.
- Patients with medical complexity and their caregivers are key members of the diagnostic team.
1. Fukumoto LE, Fukumoto KD. Fat embolism syndrome. Nurs Clin North Am. 2018;53(3):335-347. https://doi.org/10.1016/j.cnur.2018.04.003.
2. Scarpino M, Lanzo G, Lolli F, Grippo A. From the diagnosis to the therapeutic management: Cerebral fat embolism, a clinical challenge. Int J Gen Med. 2019;2019(12):39-48. https://doi.org/10.2147/IJGM.S177407.
A 19-year-old man with Duchenne Muscular Dystrophy (DMD) presented to the Emergency Department (ED) for left knee pain after ejection from his motorized wheelchair at a low velocity. In the ED, he developed increasing respiratory distress.
When addressing a new problem in a patient with a chronic condition, it is crucial to first understand the chronic condition and then consider whether the presenting symptoms relate to that condition or stem from an unrelated inciting event.
Patients with DMD are at risk of pulmonary complications relating to their underlying disease. For instance, dysphagia and ineffective cough can predispose them to recurrent aspiration pneumonitis and/or pneumonia, whereas decreased lung compliance (from scoliosis, atelectasis, and/or pulmonary fibrosis) and respiratory muscle weakness can progress to ventilatory failure. In addition, patients with DMD are at risk for pulmonary thromboembolism in the setting of immobility. Patients with DMD may also develop congestive heart failure resulting from myocardial fibrosis and nonischemic cardiomyopathy.
The ejection from his wheelchair signals potential trauma-associated conditions that could explain his respiratory distress. Respiratory complications of blunt thoracic trauma include pulmonary contusion, pneumothorax, flail chest (resulting from fractured ribs), and acute respiratory distress syndrome (ARDS). Lower extremity injury can result in venous thrombosis and pulmonary thromboembolism. While classically associated with long bone fractures, fat embolism syndrome (FES) may rarely occur with rib fractures and soft-tissue trauma. Respiratory compromise may also result from cervical spinal cord injury or severe anemia from trauma-associated hemorrhage.
Additional past medical history included growth hormone deficiency, migraine headaches, osteoporosis secondary to chronic steroid use, cardiac fibrosis of the inferolateral wall and septum with a baseline left ventricular ejection fraction of 65%, and atrial fibrillation. His medications included calcium carbonate, vitamin D, omeprazole, lisinopril, metoprolol, prednisone, escitalopram, and testosterone. Physical examination revealed an ill-appearing obese man in respiratory distress. Temperature was 37.3°C, heart rate was 102 beats per minute (bpm), blood pressure was 110/74 mm Hg. His oxygen saturation was 93% with a respiratory rate of 25 breaths per minute while breathing ambient air. His lung sounds were clear, and his heart was without murmur. The left knee was diffusely tender to palpation without specific point tenderness. Strength was 2/5 with flexion and extension at the bilateral knees and hips and 3/5 flexion and extension at the bilateral elbows. He reported this level of weakness was his baseline. Radiographs revealed a minimally displaced Salter Harris II fracture (fracture line through the metaphysis and growth plate) of the left distal femur. His fracture was splinted early in his ED course. During his ED evaluation, the patient had acute worsening of tachycardia to 130 bpm, increased respiratory rate of 34 breaths per minute, and hypoxemia with an oxygen saturation of 83% on ambient air. He was placed on 3 L/min of oxygen via nasal cannula with improvement in his oxygen saturation to 90%. A chest radiograph was unremarkable, without evidence of pneumothorax, effusion, or pneumonia. The patient was admitted to the hospital.
The acute onset of tachypnea, tachycardia, and hypoxia, accompanied by a clear lung exam and normal chest radiograph, increases the likelihood of a pulmonary embolism. Obesity, testosterone therapy, and trauma increase his susceptibility to venous thromboembolism, while a distal femur fracture increases his risk for FES. Acute pulmonary aspiration often presents with initially absent or subtle radiographic findings. An arterial blood gas analysis would determine the presence and extent of an alveolar-arterial (A-a) gradient; a normal A-a gradient is seen in hypoventilation, while an elevated A-a gradient is seen in conditions affecting gas exchange, including pulmonary emboli and alveolar filling processes. His hypoxemia only partially corrects with supplemental oxygen, raising the possibility of capillary or anatomic shunting. Capillary shunting may occur with atelectasis, aspiration/pneumonia and pulmonary edema, whereas anatomic shunting can be intra-cardiac (eg, patent foramen ovale or septic defect) or intrapulmonary (eg, arteriovenous malformations). Patients with pulmonary emboli may also develop right-to-left shunting because of increased pulmonary vascular resistance, although hypoxemia with pulmonary emboli largely relates to ventilation/perfusion mismatch and decreased level of mixed venous blood oxygen (PvO2).
This patient’s complex medical history warrants a broadened differential with consideration of his cardiac history, including myocardial fibrosis and arrhythmia, and the impact of exposure to steroids on his immune and musculoskeletal systems. He has a history of atrial fibrillation, and an electrocardiogram is warranted to determine the underlying rhythm. Prolonged periods of rapid ventricular response may lead to tachycardia-induced cardiomyopathy. Myocardial fibrosis may progress despite use of angiotensin-converting enzyme inhibitors and is associated with systolic and/or diastolic dysfunction, although neither the examination findings provided nor the chest radiograph are suggestive of decompensated heart failure. Chronic exposure to corticosteroids (used in DMD to improve muscle strength and function) may predispose to numerous infectious and metabolic complications. Up to 10%-15% of patients with Pneumocystis jirovecii pneumonia may present with a normal chest radiograph. Acute adrenal insufficiency can present with tachycardia, weakness, and respiratory distress, so recent prednisone dose changes or interruptions should be assessed.
The patient’s respiratory status worsened. In light of his complex medical history, he was transferred to a children’s hospital for a higher level of care with a presumptive diagnosis of aspiration pneumonia. Upon reassessment at the new facility, the patient reported an ongoing and severe headache since his initial injury. NSAIDs had been given prior to transfer. His exam continued to be significant for tachycardia, tachypnea, and hypoxemia. His cardiac and lung examinations were otherwise normal. A comprehensive metabolic panel, procalcitonin, complete blood count with differential, and lactate were normal; his C-reactive protein (CRP) was 46.8 mg/dL (Normal <8 mg/dL). A computed tomography (CT) angiogram of the chest revealed small multifocal nodular ground-glass opacities, especially in the lower lobes, concerning for microatelectasis, multifocal pneumonia, or aspiration pneumonia. After consultation with pediatric pulmonology consultants, antimicrobials were held during the initial phase of work-up.
His headache may reflect a migraine, although further characterization and assessment for the presence and extent of head or neck trauma is warranted. Headache following trauma warrants consideration of cerebral contusion, diffuse axonal injury, intracranial hemorrhage, and carotid or vertebral artery dissection. Screening for concussion should also be performed. Hypoxemia may increase cerebral blood flow and raise intracranial pressure, resulting in headache.
CRP elevation is nonspecific and signals the presence of focal or systemic inflammation and is often elevated to a milder extent in obese patients with DMD. While normal procalcitonin argues against bacterial pneumonia, the precise level can be informative, and serial procalcitonin values may be more helpful than a single value. Although antecedent respiratory symptoms were not mentioned, viral or fungal pneumonia can present insidiously. An occult malignancy may be incidentally discovered when patients present for unrelated issues, although this and other sources of elevated CRP (eg, exacerbation of an autoimmune disease or drug reaction) remain less likely given the acuity of his presentation. Acute pulmonary embolism may be associated with a systemic inflammatory response and elevation in CRP.
In addition to the radiographic differential diagnosis already presented, the appearance of multifocal opacifications with hypoxemia raises the possibility of pulmonary infarcts or noncardiogenic pulmonary edema.
On hospital day 2, the patient continued to complain of “the worst headache of his life” as well as blurry vision and seeing “dark spots.” His headache did not improve with NSAIDs. A noncontrast CT scan of the head was normal. Neurology was consulted. Given his symptoms, history of migraines, stable neurological examination, and normal head CT, he was diagnosed with migraines and administered fluids, prochlorperazine, diphenhydramine, ondansetron, and NSAIDs. His headache continued and he continued to require supplemental oxygen.
The combination of hypoxemia, severe headache, and vision changes remains consistent with systemic emboli caused by thromboembolism or fat embolism. Headache assessment must also involve screening for “red flags,” which include sudden onset, antecedent head trauma, systemic illness (eg, fever or meningismus), focal neurologic findings, papilledema, changes with position or Valsalva, and immunosuppression. Although primary headache syndromes (eg, migraines or tension and cluster headache) may be triggered in the setting of trauma and systemic illness, “the worst headache of my life” is a concerning symptom that warrants urgent attention. While this invokes the possibility of a subarachnoid hemorrhage (SAH), headache severity is nonspecific, and rapid onset (ie, thunderclap headache) would be more suggestive. After 6 hours of symptoms, the sensitivity of head CT for detecting SAH declines, and lumbar puncture would be warranted to evaluate for xanthochromia.
His blurry vision and dark spots require testing of visual acuity and visual fields, as well as fundoscopic examination to assess for embolic phenomena or papilledema. Migraine is classically associated with “positive” or scintillating scotomata, although dark spots may occur. The presence of horizontal diplopia would indicate a cranial nerve VI palsy, which can occur with increased intracranial pressure. Visual-field cuts may also present as blurry vision, and monocular vs binocular deficits signal whether the issue is anterior or involving/posterior to the optic chiasm, respectively. Magnetic resonance imaging (MRI) may reveal the presence or sequelae of cerebral emboli (eg, fat emboli), including vasogenic edema.
Dilated fundus examination revealed Purtscher retinopathy: bilateral cotton-wool spots and larger areas of retinal whitening (Purtscher flecken).
Typical findings of Purtscher retinopathy include Purtscher flecken, cotton-wool spots, retinal hemorrhage, and optic disc edema. Purtscher retinopathy is classically associated with severe head trauma. Without associated head trauma, the term “Purtscher-like retinopathy” is used. Conditions that can cause Purtscher-like retinopathy include pancreatitis, vasculitis, microangiopathy, chronic renal failure, and systemic embolization. The most likely source of systemic embolization remains fat emboli stemming from his femur fracture. Treatment of FES is largely supportive.
The possibility of fat emboli had been repeatedly raised by the patient’s mother since admission. While providers had considered this a possibility, it was discounted early on because of the minor nature of the patient’s orthopedic trauma, the lack of clear radiographic evidence for pulmonary emboli on chest CT, and the normal head CT. The findings on the ophthalmologist’s fundoscopic examination led the primary team to reconsider FES, along with thromboemboli and pancreatitis. Lipase was normal. MRI of the brain with contrast revealed >20 microinfarcts in the bilateral hemispheres, left corpus callosum, and bilateral basal ganglia. The CT angiogram of the chest was rereviewed; the pediatric radiologists suggested that microinfarcts could explain the patchy small ground glass opacities seen in the lungs. A transthoracic echocardiogram and electrocardiogram were normal. The diagnosis of FES was made, and the patient was started on aspirin and enoxaparin prophylaxis. His headache and respiratory status improved, and he was discharged home with close follow-up.
DISCUSSION
FES is a rare complication associated with long bone fractures and orthopedic manipulation.1,2 The exact mechanism of fat emboli production is unknown, but two theories prevail. The mechanical theory states that an outside mechanical source causes bone marrow contents or adipose tissue contents to be dislodged into the circulation where they travel through the venous circulation to become embedded in the lungs.1,2 These fragments may also migrate to the arterial circulation, through a patent foramen ovale or intrapulmonary shunts, leading to end organ damage.1,2 The biochemical theory suggests that fat emboli in the venous circulation precipitate an inflammatory and prothrombotic cascade that triggers fibrin production, platelet aggregation, and release of free fatty acids into the circulation, predisposing patients to develop multifocal systemic emboli.1
Although the classic triad in FES includes respiratory symptoms, rash, and CNS symptoms, all three findings are only present in 1%-29% of cases.1,2 Respiratory abnormalities, ranging from tachypnea and dyspnea to ARDS and hypoxic respiratory failure, occur in up to 75% of patients with FES.1 Central nervous system (CNS) complications, including headache, confusion, coma, seizures, and death caused by cerebral ischemia, occur in up to 86% of patients.1,2 Petechiae may occur in 20%-60% of patients and are usually located on nondependent regions of the body such as the head, neck, and chest.
Diagnosis of FES is largely clinical and requires a high index of suspicion and elimination of other conditions, including pulmonary thromboembolism, diffuse intravascular coagulation, and sepsis. The CNS complications must be differentiated from CNS infection, stroke, migraine, benign intracranial hypertension, and intracranial hemorrhage. There is no gold standard test for diagnosis. The Gurd and Wilson criteria, modified Gurd criteria, and Schonfeld’s criteria (Table) are commonly used but have not been clinically validated.1,3-5 These use a combination of clinical signs of respiratory distress, neurological symptoms, petechial rash, and various other diagnostic factors. When patients have risk factors, such as trauma, surgery, or predisposing conditions (eg, mobility-limiting neuromuscular disorders) and signs and symptoms potentially consistent with FES, the diagnostic evaluation should include arterial blood gas analysis, complete blood count, chest radiographs, and coagulation studies. Ophthalmological exam to evaluate for cotton-wool spots, brain MRI to detect cerebral emboli, and CT pulmonary angiogram to assess for pulmonary infarcts may help to eliminate other diagnoses and/or confirm FES.
Diagnosis of uncommon conditions that present with nonspecific symptoms, like FES, can be challenging because the symptoms may overlap with many other possible diagnoses. This challenge is further exacerbated in patients with significant medical complexity, as with the patient discussed here. Specifically, this patient had multiple plausible explanations for CNS symptoms and respiratory symptoms. It was ultimately the visual symptoms that began to link his symptoms together into a unifying syndrome and the ophthalmologic examination that prompted confirmatory imaging. It is important to continually revisit and revise the differential diagnosis in patients with medical complexity and avoid the competing temptations to abandon the search for a unifying diagnosis and attribute all symptoms to a patient’s underlying condition.
Treatment of FES is largely supportive with close monitoring of neurological status and providing supplemental oxygen as needed. Corticosteroids have been suggested to help prevent FES in patients with long bone fractures, but there is no evidence to suggest they are helpful once FES is diagnosed.2 There is conflicting evidence for the efficacy of heparin or low-molecular-weight heparin as treatment in FES.2,6 After discussions with consulting physician teams, the patient, and his family, enoxaparin and aspirin were started for this patient in light of his tenuous condition in order to decrease the risk of further embolic complications.
Patients with DMD and other neuromuscular disorders likely have a greater propensity to develop FES even after minor trauma.1,6 This is believed to be caused by patients becoming nonambulatory early in life and receiving substantial corticosteroid therapy, which can lead to osteopenia and fatty replacement of the bone marrow.1,6 This population is also often obese by the second or third decade of life, which contributes to their already increased propensity to fall.1,6
To our knowledge, this patient is 1 of 18 reported cases of FES after trauma in DMD patients. Two-thirds of these cases occurred when an unrestrained patient fell from their wheelchair. The other cases occurred while walking, during physical therapy, and during assisted transfers.6-12 In these cases, FES had a guarded prognosis, with 7 of the 18 patients dying and 1 of the patients remaining in a persistent vegetative state.8,9 While caution is warranted in generalizing these findings, given the small number of reported cases and likely publication bias,education of caregivers and patients on use of restraints and safe transfers is paramount to limit the risk of trauma.
TEACHING POINTS
- FES is a rare condition that most commonly manifests with respiratory, neurological, and cutaneous findings.
- Patients with Duchenne’s Muscular Dystrophy are likely at increased risk for FES even with minor trauma; this makes wheelchair restraints and safe transfers fundamental.
- Patients with medical complexity and their caregivers are key members of the diagnostic team.
A 19-year-old man with Duchenne Muscular Dystrophy (DMD) presented to the Emergency Department (ED) for left knee pain after ejection from his motorized wheelchair at a low velocity. In the ED, he developed increasing respiratory distress.
When addressing a new problem in a patient with a chronic condition, it is crucial to first understand the chronic condition and then consider whether the presenting symptoms relate to that condition or stem from an unrelated inciting event.
Patients with DMD are at risk of pulmonary complications relating to their underlying disease. For instance, dysphagia and ineffective cough can predispose them to recurrent aspiration pneumonitis and/or pneumonia, whereas decreased lung compliance (from scoliosis, atelectasis, and/or pulmonary fibrosis) and respiratory muscle weakness can progress to ventilatory failure. In addition, patients with DMD are at risk for pulmonary thromboembolism in the setting of immobility. Patients with DMD may also develop congestive heart failure resulting from myocardial fibrosis and nonischemic cardiomyopathy.
The ejection from his wheelchair signals potential trauma-associated conditions that could explain his respiratory distress. Respiratory complications of blunt thoracic trauma include pulmonary contusion, pneumothorax, flail chest (resulting from fractured ribs), and acute respiratory distress syndrome (ARDS). Lower extremity injury can result in venous thrombosis and pulmonary thromboembolism. While classically associated with long bone fractures, fat embolism syndrome (FES) may rarely occur with rib fractures and soft-tissue trauma. Respiratory compromise may also result from cervical spinal cord injury or severe anemia from trauma-associated hemorrhage.
Additional past medical history included growth hormone deficiency, migraine headaches, osteoporosis secondary to chronic steroid use, cardiac fibrosis of the inferolateral wall and septum with a baseline left ventricular ejection fraction of 65%, and atrial fibrillation. His medications included calcium carbonate, vitamin D, omeprazole, lisinopril, metoprolol, prednisone, escitalopram, and testosterone. Physical examination revealed an ill-appearing obese man in respiratory distress. Temperature was 37.3°C, heart rate was 102 beats per minute (bpm), blood pressure was 110/74 mm Hg. His oxygen saturation was 93% with a respiratory rate of 25 breaths per minute while breathing ambient air. His lung sounds were clear, and his heart was without murmur. The left knee was diffusely tender to palpation without specific point tenderness. Strength was 2/5 with flexion and extension at the bilateral knees and hips and 3/5 flexion and extension at the bilateral elbows. He reported this level of weakness was his baseline. Radiographs revealed a minimally displaced Salter Harris II fracture (fracture line through the metaphysis and growth plate) of the left distal femur. His fracture was splinted early in his ED course. During his ED evaluation, the patient had acute worsening of tachycardia to 130 bpm, increased respiratory rate of 34 breaths per minute, and hypoxemia with an oxygen saturation of 83% on ambient air. He was placed on 3 L/min of oxygen via nasal cannula with improvement in his oxygen saturation to 90%. A chest radiograph was unremarkable, without evidence of pneumothorax, effusion, or pneumonia. The patient was admitted to the hospital.
The acute onset of tachypnea, tachycardia, and hypoxia, accompanied by a clear lung exam and normal chest radiograph, increases the likelihood of a pulmonary embolism. Obesity, testosterone therapy, and trauma increase his susceptibility to venous thromboembolism, while a distal femur fracture increases his risk for FES. Acute pulmonary aspiration often presents with initially absent or subtle radiographic findings. An arterial blood gas analysis would determine the presence and extent of an alveolar-arterial (A-a) gradient; a normal A-a gradient is seen in hypoventilation, while an elevated A-a gradient is seen in conditions affecting gas exchange, including pulmonary emboli and alveolar filling processes. His hypoxemia only partially corrects with supplemental oxygen, raising the possibility of capillary or anatomic shunting. Capillary shunting may occur with atelectasis, aspiration/pneumonia and pulmonary edema, whereas anatomic shunting can be intra-cardiac (eg, patent foramen ovale or septic defect) or intrapulmonary (eg, arteriovenous malformations). Patients with pulmonary emboli may also develop right-to-left shunting because of increased pulmonary vascular resistance, although hypoxemia with pulmonary emboli largely relates to ventilation/perfusion mismatch and decreased level of mixed venous blood oxygen (PvO2).
This patient’s complex medical history warrants a broadened differential with consideration of his cardiac history, including myocardial fibrosis and arrhythmia, and the impact of exposure to steroids on his immune and musculoskeletal systems. He has a history of atrial fibrillation, and an electrocardiogram is warranted to determine the underlying rhythm. Prolonged periods of rapid ventricular response may lead to tachycardia-induced cardiomyopathy. Myocardial fibrosis may progress despite use of angiotensin-converting enzyme inhibitors and is associated with systolic and/or diastolic dysfunction, although neither the examination findings provided nor the chest radiograph are suggestive of decompensated heart failure. Chronic exposure to corticosteroids (used in DMD to improve muscle strength and function) may predispose to numerous infectious and metabolic complications. Up to 10%-15% of patients with Pneumocystis jirovecii pneumonia may present with a normal chest radiograph. Acute adrenal insufficiency can present with tachycardia, weakness, and respiratory distress, so recent prednisone dose changes or interruptions should be assessed.
The patient’s respiratory status worsened. In light of his complex medical history, he was transferred to a children’s hospital for a higher level of care with a presumptive diagnosis of aspiration pneumonia. Upon reassessment at the new facility, the patient reported an ongoing and severe headache since his initial injury. NSAIDs had been given prior to transfer. His exam continued to be significant for tachycardia, tachypnea, and hypoxemia. His cardiac and lung examinations were otherwise normal. A comprehensive metabolic panel, procalcitonin, complete blood count with differential, and lactate were normal; his C-reactive protein (CRP) was 46.8 mg/dL (Normal <8 mg/dL). A computed tomography (CT) angiogram of the chest revealed small multifocal nodular ground-glass opacities, especially in the lower lobes, concerning for microatelectasis, multifocal pneumonia, or aspiration pneumonia. After consultation with pediatric pulmonology consultants, antimicrobials were held during the initial phase of work-up.
His headache may reflect a migraine, although further characterization and assessment for the presence and extent of head or neck trauma is warranted. Headache following trauma warrants consideration of cerebral contusion, diffuse axonal injury, intracranial hemorrhage, and carotid or vertebral artery dissection. Screening for concussion should also be performed. Hypoxemia may increase cerebral blood flow and raise intracranial pressure, resulting in headache.
CRP elevation is nonspecific and signals the presence of focal or systemic inflammation and is often elevated to a milder extent in obese patients with DMD. While normal procalcitonin argues against bacterial pneumonia, the precise level can be informative, and serial procalcitonin values may be more helpful than a single value. Although antecedent respiratory symptoms were not mentioned, viral or fungal pneumonia can present insidiously. An occult malignancy may be incidentally discovered when patients present for unrelated issues, although this and other sources of elevated CRP (eg, exacerbation of an autoimmune disease or drug reaction) remain less likely given the acuity of his presentation. Acute pulmonary embolism may be associated with a systemic inflammatory response and elevation in CRP.
In addition to the radiographic differential diagnosis already presented, the appearance of multifocal opacifications with hypoxemia raises the possibility of pulmonary infarcts or noncardiogenic pulmonary edema.
On hospital day 2, the patient continued to complain of “the worst headache of his life” as well as blurry vision and seeing “dark spots.” His headache did not improve with NSAIDs. A noncontrast CT scan of the head was normal. Neurology was consulted. Given his symptoms, history of migraines, stable neurological examination, and normal head CT, he was diagnosed with migraines and administered fluids, prochlorperazine, diphenhydramine, ondansetron, and NSAIDs. His headache continued and he continued to require supplemental oxygen.
The combination of hypoxemia, severe headache, and vision changes remains consistent with systemic emboli caused by thromboembolism or fat embolism. Headache assessment must also involve screening for “red flags,” which include sudden onset, antecedent head trauma, systemic illness (eg, fever or meningismus), focal neurologic findings, papilledema, changes with position or Valsalva, and immunosuppression. Although primary headache syndromes (eg, migraines or tension and cluster headache) may be triggered in the setting of trauma and systemic illness, “the worst headache of my life” is a concerning symptom that warrants urgent attention. While this invokes the possibility of a subarachnoid hemorrhage (SAH), headache severity is nonspecific, and rapid onset (ie, thunderclap headache) would be more suggestive. After 6 hours of symptoms, the sensitivity of head CT for detecting SAH declines, and lumbar puncture would be warranted to evaluate for xanthochromia.
His blurry vision and dark spots require testing of visual acuity and visual fields, as well as fundoscopic examination to assess for embolic phenomena or papilledema. Migraine is classically associated with “positive” or scintillating scotomata, although dark spots may occur. The presence of horizontal diplopia would indicate a cranial nerve VI palsy, which can occur with increased intracranial pressure. Visual-field cuts may also present as blurry vision, and monocular vs binocular deficits signal whether the issue is anterior or involving/posterior to the optic chiasm, respectively. Magnetic resonance imaging (MRI) may reveal the presence or sequelae of cerebral emboli (eg, fat emboli), including vasogenic edema.
Dilated fundus examination revealed Purtscher retinopathy: bilateral cotton-wool spots and larger areas of retinal whitening (Purtscher flecken).
Typical findings of Purtscher retinopathy include Purtscher flecken, cotton-wool spots, retinal hemorrhage, and optic disc edema. Purtscher retinopathy is classically associated with severe head trauma. Without associated head trauma, the term “Purtscher-like retinopathy” is used. Conditions that can cause Purtscher-like retinopathy include pancreatitis, vasculitis, microangiopathy, chronic renal failure, and systemic embolization. The most likely source of systemic embolization remains fat emboli stemming from his femur fracture. Treatment of FES is largely supportive.
The possibility of fat emboli had been repeatedly raised by the patient’s mother since admission. While providers had considered this a possibility, it was discounted early on because of the minor nature of the patient’s orthopedic trauma, the lack of clear radiographic evidence for pulmonary emboli on chest CT, and the normal head CT. The findings on the ophthalmologist’s fundoscopic examination led the primary team to reconsider FES, along with thromboemboli and pancreatitis. Lipase was normal. MRI of the brain with contrast revealed >20 microinfarcts in the bilateral hemispheres, left corpus callosum, and bilateral basal ganglia. The CT angiogram of the chest was rereviewed; the pediatric radiologists suggested that microinfarcts could explain the patchy small ground glass opacities seen in the lungs. A transthoracic echocardiogram and electrocardiogram were normal. The diagnosis of FES was made, and the patient was started on aspirin and enoxaparin prophylaxis. His headache and respiratory status improved, and he was discharged home with close follow-up.
DISCUSSION
FES is a rare complication associated with long bone fractures and orthopedic manipulation.1,2 The exact mechanism of fat emboli production is unknown, but two theories prevail. The mechanical theory states that an outside mechanical source causes bone marrow contents or adipose tissue contents to be dislodged into the circulation where they travel through the venous circulation to become embedded in the lungs.1,2 These fragments may also migrate to the arterial circulation, through a patent foramen ovale or intrapulmonary shunts, leading to end organ damage.1,2 The biochemical theory suggests that fat emboli in the venous circulation precipitate an inflammatory and prothrombotic cascade that triggers fibrin production, platelet aggregation, and release of free fatty acids into the circulation, predisposing patients to develop multifocal systemic emboli.1
Although the classic triad in FES includes respiratory symptoms, rash, and CNS symptoms, all three findings are only present in 1%-29% of cases.1,2 Respiratory abnormalities, ranging from tachypnea and dyspnea to ARDS and hypoxic respiratory failure, occur in up to 75% of patients with FES.1 Central nervous system (CNS) complications, including headache, confusion, coma, seizures, and death caused by cerebral ischemia, occur in up to 86% of patients.1,2 Petechiae may occur in 20%-60% of patients and are usually located on nondependent regions of the body such as the head, neck, and chest.
Diagnosis of FES is largely clinical and requires a high index of suspicion and elimination of other conditions, including pulmonary thromboembolism, diffuse intravascular coagulation, and sepsis. The CNS complications must be differentiated from CNS infection, stroke, migraine, benign intracranial hypertension, and intracranial hemorrhage. There is no gold standard test for diagnosis. The Gurd and Wilson criteria, modified Gurd criteria, and Schonfeld’s criteria (Table) are commonly used but have not been clinically validated.1,3-5 These use a combination of clinical signs of respiratory distress, neurological symptoms, petechial rash, and various other diagnostic factors. When patients have risk factors, such as trauma, surgery, or predisposing conditions (eg, mobility-limiting neuromuscular disorders) and signs and symptoms potentially consistent with FES, the diagnostic evaluation should include arterial blood gas analysis, complete blood count, chest radiographs, and coagulation studies. Ophthalmological exam to evaluate for cotton-wool spots, brain MRI to detect cerebral emboli, and CT pulmonary angiogram to assess for pulmonary infarcts may help to eliminate other diagnoses and/or confirm FES.
Diagnosis of uncommon conditions that present with nonspecific symptoms, like FES, can be challenging because the symptoms may overlap with many other possible diagnoses. This challenge is further exacerbated in patients with significant medical complexity, as with the patient discussed here. Specifically, this patient had multiple plausible explanations for CNS symptoms and respiratory symptoms. It was ultimately the visual symptoms that began to link his symptoms together into a unifying syndrome and the ophthalmologic examination that prompted confirmatory imaging. It is important to continually revisit and revise the differential diagnosis in patients with medical complexity and avoid the competing temptations to abandon the search for a unifying diagnosis and attribute all symptoms to a patient’s underlying condition.
Treatment of FES is largely supportive with close monitoring of neurological status and providing supplemental oxygen as needed. Corticosteroids have been suggested to help prevent FES in patients with long bone fractures, but there is no evidence to suggest they are helpful once FES is diagnosed.2 There is conflicting evidence for the efficacy of heparin or low-molecular-weight heparin as treatment in FES.2,6 After discussions with consulting physician teams, the patient, and his family, enoxaparin and aspirin were started for this patient in light of his tenuous condition in order to decrease the risk of further embolic complications.
Patients with DMD and other neuromuscular disorders likely have a greater propensity to develop FES even after minor trauma.1,6 This is believed to be caused by patients becoming nonambulatory early in life and receiving substantial corticosteroid therapy, which can lead to osteopenia and fatty replacement of the bone marrow.1,6 This population is also often obese by the second or third decade of life, which contributes to their already increased propensity to fall.1,6
To our knowledge, this patient is 1 of 18 reported cases of FES after trauma in DMD patients. Two-thirds of these cases occurred when an unrestrained patient fell from their wheelchair. The other cases occurred while walking, during physical therapy, and during assisted transfers.6-12 In these cases, FES had a guarded prognosis, with 7 of the 18 patients dying and 1 of the patients remaining in a persistent vegetative state.8,9 While caution is warranted in generalizing these findings, given the small number of reported cases and likely publication bias,education of caregivers and patients on use of restraints and safe transfers is paramount to limit the risk of trauma.
TEACHING POINTS
- FES is a rare condition that most commonly manifests with respiratory, neurological, and cutaneous findings.
- Patients with Duchenne’s Muscular Dystrophy are likely at increased risk for FES even with minor trauma; this makes wheelchair restraints and safe transfers fundamental.
- Patients with medical complexity and their caregivers are key members of the diagnostic team.
1. Fukumoto LE, Fukumoto KD. Fat embolism syndrome. Nurs Clin North Am. 2018;53(3):335-347. https://doi.org/10.1016/j.cnur.2018.04.003.
2. Scarpino M, Lanzo G, Lolli F, Grippo A. From the diagnosis to the therapeutic management: Cerebral fat embolism, a clinical challenge. Int J Gen Med. 2019;2019(12):39-48. https://doi.org/10.2147/IJGM.S177407.
1. Fukumoto LE, Fukumoto KD. Fat embolism syndrome. Nurs Clin North Am. 2018;53(3):335-347. https://doi.org/10.1016/j.cnur.2018.04.003.
2. Scarpino M, Lanzo G, Lolli F, Grippo A. From the diagnosis to the therapeutic management: Cerebral fat embolism, a clinical challenge. Int J Gen Med. 2019;2019(12):39-48. https://doi.org/10.2147/IJGM.S177407.
© 2020 Society of Hospital Medicine