User login
at 12 months, a development that indicates the promise this has a treatment for acne in the future.
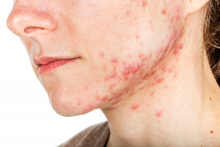
Currently, “there is no strong evidence that lasers are better than conventional treatments for acne,” Fernanda H. Sakamoto, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. Some patients struggling with acne “search for so many different options and they end up spending a lot of money,” which, she said, includes an estimated $222 million for laser treatment alone in 2019.
Unlike other existing laser and light options for acne treatment, however, Accure is the first light-based platform to selectively target and injure sebaceous glands, the main source of sebum production and the key to a durable solution for acne. The laser, which uses a 1,726-nm wavelength, is being developed by researchers at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston and was granted the European CE mark, which allows marketing of the product in Europe, in May of 2020.
In 2012, Dr. Sakamoto, a dermatologist at the center, and her Wellman colleagues were the first to describe the use of selective photothermolysis to target sebaceous glands. “We found that the peak absorption of lipids in sebaceous glands occurs between 1,700 and 1,720 nm,” she said. “Compared to water, the contrast is not high, so for us to develop a laser that is selective for acne, we needed to develop a strong cooling system and we had to create different methods to make it more selective.” She said that it took about 10 years to develop this laser.
The latest Accure prototype features a smart laser handpiece for real time thermal monitoring and precise delivery of laser emissions. “We have developed a mathematical model which permits us to predict safe and effective treatment patterns,” Dr. Sakamoto said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth?” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “It has a unique cooling system that can control and protect the skin.”
The clinical trial for Food and Drug Administration clearance, which was delayed because of the COVID-19 pandemic, is still underway, she said, and the hope is that the laser will cleared by the FDA by next year. She and her Wellman colleagues have been working with four veteran dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2021, more than 50 patients were enrolled in four IRB-approved studies and an additional 30 are enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the pilot facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment. The average lesion reduction at week 12 was 82% and the mean Visual Analog Scale score immediately after treatment was 2.10 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device with no adverse events.
“This laser is absorbed in the near-infrared spectrum, so there is no melanin absorption,” Dr. Sakamoto explained. “It’s pretty much a color-blind laser, so we can treat darker skin types safely, with no side effects.” In other findings, researchers observed a 45% reduction in acne lesions after one treatment session, which “keeps improving over time,” she said. “At 12 weeks, we have clearance of over 80% of the lesions.”
At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histological studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other follicular structures.
Dr. Sakamoto disclosed that she has received portions of patent royalties from Massachusetts General Hospital. Accure was cofounded by R. Rox Anderson, MD, the director of the Wellman Center.
at 12 months, a development that indicates the promise this has a treatment for acne in the future.
Currently, “there is no strong evidence that lasers are better than conventional treatments for acne,” Fernanda H. Sakamoto, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. Some patients struggling with acne “search for so many different options and they end up spending a lot of money,” which, she said, includes an estimated $222 million for laser treatment alone in 2019.
Unlike other existing laser and light options for acne treatment, however, Accure is the first light-based platform to selectively target and injure sebaceous glands, the main source of sebum production and the key to a durable solution for acne. The laser, which uses a 1,726-nm wavelength, is being developed by researchers at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston and was granted the European CE mark, which allows marketing of the product in Europe, in May of 2020.
In 2012, Dr. Sakamoto, a dermatologist at the center, and her Wellman colleagues were the first to describe the use of selective photothermolysis to target sebaceous glands. “We found that the peak absorption of lipids in sebaceous glands occurs between 1,700 and 1,720 nm,” she said. “Compared to water, the contrast is not high, so for us to develop a laser that is selective for acne, we needed to develop a strong cooling system and we had to create different methods to make it more selective.” She said that it took about 10 years to develop this laser.
The latest Accure prototype features a smart laser handpiece for real time thermal monitoring and precise delivery of laser emissions. “We have developed a mathematical model which permits us to predict safe and effective treatment patterns,” Dr. Sakamoto said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth?” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “It has a unique cooling system that can control and protect the skin.”
The clinical trial for Food and Drug Administration clearance, which was delayed because of the COVID-19 pandemic, is still underway, she said, and the hope is that the laser will cleared by the FDA by next year. She and her Wellman colleagues have been working with four veteran dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2021, more than 50 patients were enrolled in four IRB-approved studies and an additional 30 are enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the pilot facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment. The average lesion reduction at week 12 was 82% and the mean Visual Analog Scale score immediately after treatment was 2.10 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device with no adverse events.
“This laser is absorbed in the near-infrared spectrum, so there is no melanin absorption,” Dr. Sakamoto explained. “It’s pretty much a color-blind laser, so we can treat darker skin types safely, with no side effects.” In other findings, researchers observed a 45% reduction in acne lesions after one treatment session, which “keeps improving over time,” she said. “At 12 weeks, we have clearance of over 80% of the lesions.”
At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histological studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other follicular structures.
Dr. Sakamoto disclosed that she has received portions of patent royalties from Massachusetts General Hospital. Accure was cofounded by R. Rox Anderson, MD, the director of the Wellman Center.
at 12 months, a development that indicates the promise this has a treatment for acne in the future.
Currently, “there is no strong evidence that lasers are better than conventional treatments for acne,” Fernanda H. Sakamoto, MD, PhD, said during a virtual course on laser and aesthetic skin therapy. Some patients struggling with acne “search for so many different options and they end up spending a lot of money,” which, she said, includes an estimated $222 million for laser treatment alone in 2019.
Unlike other existing laser and light options for acne treatment, however, Accure is the first light-based platform to selectively target and injure sebaceous glands, the main source of sebum production and the key to a durable solution for acne. The laser, which uses a 1,726-nm wavelength, is being developed by researchers at the Wellman Center for Photomedicine, at Massachusetts General Hospital, Boston and was granted the European CE mark, which allows marketing of the product in Europe, in May of 2020.
In 2012, Dr. Sakamoto, a dermatologist at the center, and her Wellman colleagues were the first to describe the use of selective photothermolysis to target sebaceous glands. “We found that the peak absorption of lipids in sebaceous glands occurs between 1,700 and 1,720 nm,” she said. “Compared to water, the contrast is not high, so for us to develop a laser that is selective for acne, we needed to develop a strong cooling system and we had to create different methods to make it more selective.” She said that it took about 10 years to develop this laser.
The latest Accure prototype features a smart laser handpiece for real time thermal monitoring and precise delivery of laser emissions. “We have developed a mathematical model which permits us to predict safe and effective treatment patterns,” Dr. Sakamoto said at the meeting, which was named “Laser & Aesthetic Skin Therapy: What’s the Truth?” and was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “It has a unique cooling system that can control and protect the skin.”
The clinical trial for Food and Drug Administration clearance, which was delayed because of the COVID-19 pandemic, is still underway, she said, and the hope is that the laser will cleared by the FDA by next year. She and her Wellman colleagues have been working with four veteran dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2021, more than 50 patients were enrolled in four IRB-approved studies and an additional 30 are enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the pilot facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment. The average lesion reduction at week 12 was 82% and the mean Visual Analog Scale score immediately after treatment was 2.10 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device with no adverse events.
“This laser is absorbed in the near-infrared spectrum, so there is no melanin absorption,” Dr. Sakamoto explained. “It’s pretty much a color-blind laser, so we can treat darker skin types safely, with no side effects.” In other findings, researchers observed a 45% reduction in acne lesions after one treatment session, which “keeps improving over time,” she said. “At 12 weeks, we have clearance of over 80% of the lesions.”
At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histological studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other follicular structures.
Dr. Sakamoto disclosed that she has received portions of patent royalties from Massachusetts General Hospital. Accure was cofounded by R. Rox Anderson, MD, the director of the Wellman Center.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE