User login
Antibiotic Stewardship in Acne: Practical Tips From Dr. Lorraine L. Rosamilia
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
What clinical signs suggest antimicrobial resistance is affecting acne treatment response, and how can dermatologists identify them early?
DR. ROSAMILIA: Antibiotic resistance is a difficult phenomenon to define clinically for acne due to many pathogenic contributors, namely the increase in sebum production stoked by hormonal changes, which further provokes Cutibacterium acnes biofilms, follicular plugs, and various inflammatory cascades. The sequence and primacy of these steps are enigmatic in each patient, therefore the role and extent of true antimicrobial therapy are debatable. Acne is more complex than other conditions that utilize antimicrobials, such as tinea corporis. In acne, lack of treatment response may be due to various factors, including long-term adherence challenges (such as inconsistent home dosing and trending complex over-the-counter [OTC] regimens), hormonal fluctuation, and confounders such as gram-negative or pityrosporum folliculitis. Therefore, determining if resistant bacteria are “causal” in acne recalcitrance or exacerbation is vague. In older patients (or younger patients with chronic conditions), proof of bacterial resistance from wound, pulmonary, or gastrointestinal studies might be available, but a typical acne patient would not present with these data.
Do you routinely rotate patients off oral antibiotics after a fixed treatment period, or is it symptom based? How do you balance the risk for disease recurrence with resistance concerns?
DR. ROSAMILIA: For my patients, the typical “triple threat” for moderate acne—oral antibiotics, topical benzoyl peroxide, and topical retinoids—still is tried and true. I typically prescribe 6 weeks of low-dose antibiotic therapy (doxycycline 50 mg daily) and arrange a telemedicine visit at 4 to 6 weeks to assess progress and adherence. Subsequently, I might substitute topical for oral antibiotics, with long-term plans to discontinue all antibiotics. In females, I might add spironolactone and/or oral contraceptive pills, and for recalcitrant or progressive acne, I would discuss isotretinoin. If the patient’s acne is under good control without antibiotics but they still experience intermittent deeper papules, I consider adding burst therapy of low-dose doxycycline for 1 week as needed, or for instance, during sports seasons. I try to maintain the lowest possible dosage of doxycycline while toeing the line between short-term antibacterial and longer-term anti-inflammatory control. In fact, I typically recommend that patients take it with their morning meal to absorb slightly less than the full 50-mg dosage, mitigate adverse effects, and increase adherence. All of these regimens include a benzoyl peroxide wash for its many anti-acne properties and in the context of this discussion to mitigate C acnes on acne-prone skin without creating antibiotic resistance.
Do you see a future for point-of-care microbiome or resistance testing in acne management?
DR. ROSAMILIA: I think we should be receptive to the evolution of these tests, and depending on the patient’s insurance coverage, efficient collection methods, and applicability to all patients, we someday may approach antimicrobial pharmacotherapy in a more personalized way. The microbiome is a broad topic with protean approaches to testing and prebiotic/probiotic supplementation, so openminded but cautious and well-studied utilization is key.
What language do you find effective when setting expectations for acne treatment that avoids overreliance on antibiotics?
DR. ROSAMILIA: I find it important to first determine the patient’s prior therapies. Many patients with acne present to dermatology after taking a full dosage of various antibiotics for broad amounts of time, and they may have experienced acne exacerbation (or at least perception of such) when the refills ran out. Also, I ask them to outline their past and current OTC regimens, which provides context for where and how the patient gets their information and advice. I like providing the patient’s next steps in written form, even telling them to tape the instructions to their bathroom mirror. It is just as vital to take time at the first office visit to explain the expected time to improvement and why acne is a multifactorial condition for which antibiotics are only part of the approach with benzoyl peroxide and retinoids.
What are your top practical tips for incoming dermatologists to practice antibiotic stewardship in acne management?
DR. ROSAMILIA: The American Academy of Dermatology (AAD) guidelines recommend 3 to 4 months as the maximum threshold for systemic antibiotics for moderate to severe acne, with tetracyclines having the best evidence for efficacy and safety. The AAD recommends never utilizing these as monotherapy and always including concomitant benzoyl peroxide to avoid bacterial resistance and topicals such as retinoids to provide a bridge to a maintenance phase without antibiotics. Starting there gives trainees structure within which to build their own acne management approach and style for their patient population. Some dermatologists might prescribe middle to high antibiotic dosages at first followed by a taper or initiate low antibiotic dosages with a standard 3- to 4-month follow-up, or a bit of a hybrid of these, as outlined in my approach. As mentioned, standardized testing for resistance to guide our dosing is not mainstream. There are countless ways to apply these guardrails, consider a place for hormonal or future isotretinoin therapy, and include the many varieties of OTC and prescription acne topicals to round out a personalized regimen for each patient based on their schedule, medication intolerances, skin type, fertility plans, and lifestyle.
What’s the single most impactful change a busy dermatology clinic could make right now to reduce antibiotic overuse in acne care?
DR. ROSAMILIA: I think telemedicine or in-person check-ins at the 1- or 2-month mark are vital to the assessment of the patient’s and/or family’s understanding of the treatment schedule, their ability to procure the prescription and OTC products successfully, and their consistency in using them. This is a good opportunity to remind them that our goal is to see true acne improvement; take fewer medications, not more; and create a reality where their acne regimen is intuitive and safe.
Path of Least Resistance: Guidance for Antibiotic Stewardship in Acne
Path of Least Resistance: Guidance for Antibiotic Stewardship in Acne
Dermatologists have long relied on oral antibiotics to manage moderate to severe acne1-4; however, it is critical to reassess how these medications are used in clinical practice as concerns about antibiotic resistance grow.5 The question is not whether antibiotics are effective for acne treatment—we know they are—but how to optimize their use to balance clinical benefit with responsible prescribing. Resistance in Cutibacterium acnes has been well documented in laboratory settings, but clinical treatment failure due to resistance remains rare and difficult to quantify.6,7 Still, minimizing unnecessary exposure is good clinical practice. Whether antibiotic resistance ultimately proves to drive clinical failure or remains largely theoretical, stewardship safeguards future treatment options.
In this article, we present a practical, expert-based framework aligned with American Academy of Dermatology (AAD) guidelines to support responsible antibiotic use in acne management. Seven prescribing principles are outlined to help clinicians maintain efficacy while minimizing resistance risk. Mechanisms of resistance in C acnes and broader microbiome impacts also are discussed.
MECHANISMS OF RESISTANCE IN ACNE THERAPY
Antibiotic resistance in acne primarily involves C acnes and arises through selective pressure from prolonged or subtherapeutic antibiotic exposure. Resistance mechanisms include point mutations in ribosomal binding sites, leading to decreased binding affinity for tetracyclines and macrolides as well as efflux pump activation and biofilm formation.8,9 Over time, resistant strains may proliferate and outcompete susceptible populations, potentially contributing to reduced clinical efficacy. Importantly, the use of broad-spectrum antibiotics may disrupt the skin and gut microbiota, promoting resistance among nontarget organisms.5 These concerns underscore the importance of limiting antibiotic use to appropriate indications, combining antibiotics with adjunctive nonantibiotic therapies, and avoiding monotherapy.
PRESCRIBING PRINCIPLES FOR RESPONSIBLE ORAL ANTIBIOTIC USE IN ACNE
The following principles are derived from our clinical experience and are aligned with AAD guidelines on acne treatment.10 This practical framework supports safe, effective, and streamlined prescribing.
Reserve Oral Antibiotics for Appropriate Cases
Oral antibiotics should be considered for patients with moderate to severe inflammatory acne when rapid anti-inflammatory control is needed. They are not indicated for comedonal or mild papulopustular acne. Before initiating treatment, clinicians should weigh the potential benefits against the risks associated with antibiotic exposure, including resistance and microbiome disruption.
Combine Oral Antibiotics With Topical Retinoids
Oral antibiotics should not be used as monotherapy. Topical retinoids should be initiated concurrently with oral antibiotics to maximize anti-inflammatory benefit, support transition to maintenance therapy, and reduce risk for resistance.
Consider Adding an Adjunctive Topical Antimicrobial Agent
Adjunctive topical antimicrobials can help reduce bacterial load. Benzoyl peroxide remains a first-line option due to its bactericidal activity and lack of resistance induction; however, recent product recalls involving benzene contamination may have raised safety concerns among some clinicians and patients.11,12 While no definitive harm has been established, alternative topical agents approved by the US Food and Drug Administration (eg, azelaic acid) may be used based on shared decision-making, tolerability, cost, access, and patient preference. Use of topical antibiotics (eg, clindamycin, erythromycin) as monotherapy is discouraged due to their higher resistance potential, which is consistent with AAD guidance.
Limit Treatment Duration to 12 Weeks or Less
Antibiotic use should be time limited, with discontinuation ideally within 8 to 12 weeks as clinical improvement is demonstrated. Repeated or prolonged courses should be avoided to minimize risk for resistance.
Simplify Treatment Regimens to Enhance Adherence
Regimen simplicity improves adherence, especially in adolescents. A two-agent regimen of an oral antibiotic and a topical retinoid typically is sufficient during the induction phase.13,14
Select Narrower-Spectrum Antibiotics When Feasible
Using a narrower-spectrum antibiotic may help minimize disruption to nontarget microbiota.15,16 Sarecycline has shown narrower in vitro activity within the tetracycline class,17,18 though clinical decisions should be informed by access, availability, and cost. Regardless of the agent used (eg, doxycycline, minocycline, or sarecycline), all antibiotics should be used judiciously and for the shortest effective duration.
Use Systemic Nonantibiotic Therapies When Appropriate
If there is inadequate response to oral antibiotic therapy, consider switching to systemic nonantibiotic options. Hormonal therapy may be appropriate for select female patients. Oral isotretinoin should be considered for patients with severe, recalcitrant, or scarring acne. Cycling between antibiotic classes without clear benefit is discouraged.
FINAL THOUGHTS
Oral antibiotics remain a foundational component in the management of moderate to severe acne; however, their use must be intentional, time limited, and guided by best practices to minimize the emergence of antimicrobial resistance. By adhering to the prescribing principles we have outlined here, which are rooted in clinical expertise and consistent with AAD guidelines, dermatologists can preserve antibiotic efficacy, optimize patient outcomes, and reduce long-term microbiologic risks. Stewardship is not about withholding treatment; it is about optimizing care today to protect treatment options for tomorrow.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
- Grada A, Armstrong A, Bunick C, et al. Trends in oral antibiotic use for acne treatment: a retrospective, population-based study in the United States, 2014 to 2016. J Drugs Dermatol. 2023;22:265-270.
- Perche PO, Peck GM, Robinson L, et al. Prescribing trends for acne vulgaris visits in the United States. Antibiotics. 2023;12:269.
- Karadag A, Aslan Kayıran M, Wu CY, et al. Antibiotic resistance in acne: changes, consequences and concerns. J Eur Acad Dermatol Venereol. 2021;35:73-78.
- Eady AE, Cove JH, Layton AM. Is antibiotic resistance in cutaneous propionibacteria clinically relevant? implications of resistance for acne patients and prescribers. Am J Clin Dermatol. 2003;4:813-831.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J Dermatol. 1989;121:51-57.
- Grossman TH. Tetracycline antibiotics and resistance. Cold Spring Harb Perspect Med. 2016;6:a025387.
- Kayiran M AS, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006-1035.
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:037702.
- Kucera K, Zenzola N, Hudspeth A, et al. Evaluation of benzene presence and formation in benzoyl peroxide drug products. J Invest Dermatol. 2025;145:1147-1154.E11.
- Grada A, Perche P, Feldman S. Adherence and persistence to acne medications: a population-based claims database analysis. J Drugs Dermatol. 2022;21:758-764.<.li>
- Anderson KL, Dothard EH, Huang KE, et al. Frequency of primary nonadherence to acne treatment. JAMA Dermatol. 2015;151:623-626.
- Grada A, Bunick CG. Spectrum of antibiotic activity and its relevance to the microbiome. JAMA Netw Open. 2021;4:E215357-E215357.
- Francino M. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:164577.
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911.
- Zhanel G, Critchley I, Lin L-Y, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2019;63:1297-1318.
Dermatologists have long relied on oral antibiotics to manage moderate to severe acne1-4; however, it is critical to reassess how these medications are used in clinical practice as concerns about antibiotic resistance grow.5 The question is not whether antibiotics are effective for acne treatment—we know they are—but how to optimize their use to balance clinical benefit with responsible prescribing. Resistance in Cutibacterium acnes has been well documented in laboratory settings, but clinical treatment failure due to resistance remains rare and difficult to quantify.6,7 Still, minimizing unnecessary exposure is good clinical practice. Whether antibiotic resistance ultimately proves to drive clinical failure or remains largely theoretical, stewardship safeguards future treatment options.
In this article, we present a practical, expert-based framework aligned with American Academy of Dermatology (AAD) guidelines to support responsible antibiotic use in acne management. Seven prescribing principles are outlined to help clinicians maintain efficacy while minimizing resistance risk. Mechanisms of resistance in C acnes and broader microbiome impacts also are discussed.
MECHANISMS OF RESISTANCE IN ACNE THERAPY
Antibiotic resistance in acne primarily involves C acnes and arises through selective pressure from prolonged or subtherapeutic antibiotic exposure. Resistance mechanisms include point mutations in ribosomal binding sites, leading to decreased binding affinity for tetracyclines and macrolides as well as efflux pump activation and biofilm formation.8,9 Over time, resistant strains may proliferate and outcompete susceptible populations, potentially contributing to reduced clinical efficacy. Importantly, the use of broad-spectrum antibiotics may disrupt the skin and gut microbiota, promoting resistance among nontarget organisms.5 These concerns underscore the importance of limiting antibiotic use to appropriate indications, combining antibiotics with adjunctive nonantibiotic therapies, and avoiding monotherapy.
PRESCRIBING PRINCIPLES FOR RESPONSIBLE ORAL ANTIBIOTIC USE IN ACNE
The following principles are derived from our clinical experience and are aligned with AAD guidelines on acne treatment.10 This practical framework supports safe, effective, and streamlined prescribing.
Reserve Oral Antibiotics for Appropriate Cases
Oral antibiotics should be considered for patients with moderate to severe inflammatory acne when rapid anti-inflammatory control is needed. They are not indicated for comedonal or mild papulopustular acne. Before initiating treatment, clinicians should weigh the potential benefits against the risks associated with antibiotic exposure, including resistance and microbiome disruption.
Combine Oral Antibiotics With Topical Retinoids
Oral antibiotics should not be used as monotherapy. Topical retinoids should be initiated concurrently with oral antibiotics to maximize anti-inflammatory benefit, support transition to maintenance therapy, and reduce risk for resistance.
Consider Adding an Adjunctive Topical Antimicrobial Agent
Adjunctive topical antimicrobials can help reduce bacterial load. Benzoyl peroxide remains a first-line option due to its bactericidal activity and lack of resistance induction; however, recent product recalls involving benzene contamination may have raised safety concerns among some clinicians and patients.11,12 While no definitive harm has been established, alternative topical agents approved by the US Food and Drug Administration (eg, azelaic acid) may be used based on shared decision-making, tolerability, cost, access, and patient preference. Use of topical antibiotics (eg, clindamycin, erythromycin) as monotherapy is discouraged due to their higher resistance potential, which is consistent with AAD guidance.
Limit Treatment Duration to 12 Weeks or Less
Antibiotic use should be time limited, with discontinuation ideally within 8 to 12 weeks as clinical improvement is demonstrated. Repeated or prolonged courses should be avoided to minimize risk for resistance.
Simplify Treatment Regimens to Enhance Adherence
Regimen simplicity improves adherence, especially in adolescents. A two-agent regimen of an oral antibiotic and a topical retinoid typically is sufficient during the induction phase.13,14
Select Narrower-Spectrum Antibiotics When Feasible
Using a narrower-spectrum antibiotic may help minimize disruption to nontarget microbiota.15,16 Sarecycline has shown narrower in vitro activity within the tetracycline class,17,18 though clinical decisions should be informed by access, availability, and cost. Regardless of the agent used (eg, doxycycline, minocycline, or sarecycline), all antibiotics should be used judiciously and for the shortest effective duration.
Use Systemic Nonantibiotic Therapies When Appropriate
If there is inadequate response to oral antibiotic therapy, consider switching to systemic nonantibiotic options. Hormonal therapy may be appropriate for select female patients. Oral isotretinoin should be considered for patients with severe, recalcitrant, or scarring acne. Cycling between antibiotic classes without clear benefit is discouraged.
FINAL THOUGHTS
Oral antibiotics remain a foundational component in the management of moderate to severe acne; however, their use must be intentional, time limited, and guided by best practices to minimize the emergence of antimicrobial resistance. By adhering to the prescribing principles we have outlined here, which are rooted in clinical expertise and consistent with AAD guidelines, dermatologists can preserve antibiotic efficacy, optimize patient outcomes, and reduce long-term microbiologic risks. Stewardship is not about withholding treatment; it is about optimizing care today to protect treatment options for tomorrow.
Dermatologists have long relied on oral antibiotics to manage moderate to severe acne1-4; however, it is critical to reassess how these medications are used in clinical practice as concerns about antibiotic resistance grow.5 The question is not whether antibiotics are effective for acne treatment—we know they are—but how to optimize their use to balance clinical benefit with responsible prescribing. Resistance in Cutibacterium acnes has been well documented in laboratory settings, but clinical treatment failure due to resistance remains rare and difficult to quantify.6,7 Still, minimizing unnecessary exposure is good clinical practice. Whether antibiotic resistance ultimately proves to drive clinical failure or remains largely theoretical, stewardship safeguards future treatment options.
In this article, we present a practical, expert-based framework aligned with American Academy of Dermatology (AAD) guidelines to support responsible antibiotic use in acne management. Seven prescribing principles are outlined to help clinicians maintain efficacy while minimizing resistance risk. Mechanisms of resistance in C acnes and broader microbiome impacts also are discussed.
MECHANISMS OF RESISTANCE IN ACNE THERAPY
Antibiotic resistance in acne primarily involves C acnes and arises through selective pressure from prolonged or subtherapeutic antibiotic exposure. Resistance mechanisms include point mutations in ribosomal binding sites, leading to decreased binding affinity for tetracyclines and macrolides as well as efflux pump activation and biofilm formation.8,9 Over time, resistant strains may proliferate and outcompete susceptible populations, potentially contributing to reduced clinical efficacy. Importantly, the use of broad-spectrum antibiotics may disrupt the skin and gut microbiota, promoting resistance among nontarget organisms.5 These concerns underscore the importance of limiting antibiotic use to appropriate indications, combining antibiotics with adjunctive nonantibiotic therapies, and avoiding monotherapy.
PRESCRIBING PRINCIPLES FOR RESPONSIBLE ORAL ANTIBIOTIC USE IN ACNE
The following principles are derived from our clinical experience and are aligned with AAD guidelines on acne treatment.10 This practical framework supports safe, effective, and streamlined prescribing.
Reserve Oral Antibiotics for Appropriate Cases
Oral antibiotics should be considered for patients with moderate to severe inflammatory acne when rapid anti-inflammatory control is needed. They are not indicated for comedonal or mild papulopustular acne. Before initiating treatment, clinicians should weigh the potential benefits against the risks associated with antibiotic exposure, including resistance and microbiome disruption.
Combine Oral Antibiotics With Topical Retinoids
Oral antibiotics should not be used as monotherapy. Topical retinoids should be initiated concurrently with oral antibiotics to maximize anti-inflammatory benefit, support transition to maintenance therapy, and reduce risk for resistance.
Consider Adding an Adjunctive Topical Antimicrobial Agent
Adjunctive topical antimicrobials can help reduce bacterial load. Benzoyl peroxide remains a first-line option due to its bactericidal activity and lack of resistance induction; however, recent product recalls involving benzene contamination may have raised safety concerns among some clinicians and patients.11,12 While no definitive harm has been established, alternative topical agents approved by the US Food and Drug Administration (eg, azelaic acid) may be used based on shared decision-making, tolerability, cost, access, and patient preference. Use of topical antibiotics (eg, clindamycin, erythromycin) as monotherapy is discouraged due to their higher resistance potential, which is consistent with AAD guidance.
Limit Treatment Duration to 12 Weeks or Less
Antibiotic use should be time limited, with discontinuation ideally within 8 to 12 weeks as clinical improvement is demonstrated. Repeated or prolonged courses should be avoided to minimize risk for resistance.
Simplify Treatment Regimens to Enhance Adherence
Regimen simplicity improves adherence, especially in adolescents. A two-agent regimen of an oral antibiotic and a topical retinoid typically is sufficient during the induction phase.13,14
Select Narrower-Spectrum Antibiotics When Feasible
Using a narrower-spectrum antibiotic may help minimize disruption to nontarget microbiota.15,16 Sarecycline has shown narrower in vitro activity within the tetracycline class,17,18 though clinical decisions should be informed by access, availability, and cost. Regardless of the agent used (eg, doxycycline, minocycline, or sarecycline), all antibiotics should be used judiciously and for the shortest effective duration.
Use Systemic Nonantibiotic Therapies When Appropriate
If there is inadequate response to oral antibiotic therapy, consider switching to systemic nonantibiotic options. Hormonal therapy may be appropriate for select female patients. Oral isotretinoin should be considered for patients with severe, recalcitrant, or scarring acne. Cycling between antibiotic classes without clear benefit is discouraged.
FINAL THOUGHTS
Oral antibiotics remain a foundational component in the management of moderate to severe acne; however, their use must be intentional, time limited, and guided by best practices to minimize the emergence of antimicrobial resistance. By adhering to the prescribing principles we have outlined here, which are rooted in clinical expertise and consistent with AAD guidelines, dermatologists can preserve antibiotic efficacy, optimize patient outcomes, and reduce long-term microbiologic risks. Stewardship is not about withholding treatment; it is about optimizing care today to protect treatment options for tomorrow.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
- Grada A, Armstrong A, Bunick C, et al. Trends in oral antibiotic use for acne treatment: a retrospective, population-based study in the United States, 2014 to 2016. J Drugs Dermatol. 2023;22:265-270.
- Perche PO, Peck GM, Robinson L, et al. Prescribing trends for acne vulgaris visits in the United States. Antibiotics. 2023;12:269.
- Karadag A, Aslan Kayıran M, Wu CY, et al. Antibiotic resistance in acne: changes, consequences and concerns. J Eur Acad Dermatol Venereol. 2021;35:73-78.
- Eady AE, Cove JH, Layton AM. Is antibiotic resistance in cutaneous propionibacteria clinically relevant? implications of resistance for acne patients and prescribers. Am J Clin Dermatol. 2003;4:813-831.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J Dermatol. 1989;121:51-57.
- Grossman TH. Tetracycline antibiotics and resistance. Cold Spring Harb Perspect Med. 2016;6:a025387.
- Kayiran M AS, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006-1035.
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:037702.
- Kucera K, Zenzola N, Hudspeth A, et al. Evaluation of benzene presence and formation in benzoyl peroxide drug products. J Invest Dermatol. 2025;145:1147-1154.E11.
- Grada A, Perche P, Feldman S. Adherence and persistence to acne medications: a population-based claims database analysis. J Drugs Dermatol. 2022;21:758-764.<.li>
- Anderson KL, Dothard EH, Huang KE, et al. Frequency of primary nonadherence to acne treatment. JAMA Dermatol. 2015;151:623-626.
- Grada A, Bunick CG. Spectrum of antibiotic activity and its relevance to the microbiome. JAMA Netw Open. 2021;4:E215357-E215357.
- Francino M. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:164577.
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911.
- Zhanel G, Critchley I, Lin L-Y, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2019;63:1297-1318.
- Bhate K, Williams H. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168:474-485.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol. 2019;155:290-297.
- Grada A, Armstrong A, Bunick C, et al. Trends in oral antibiotic use for acne treatment: a retrospective, population-based study in the United States, 2014 to 2016. J Drugs Dermatol. 2023;22:265-270.
- Perche PO, Peck GM, Robinson L, et al. Prescribing trends for acne vulgaris visits in the United States. Antibiotics. 2023;12:269.
- Karadag A, Aslan Kayıran M, Wu CY, et al. Antibiotic resistance in acne: changes, consequences and concerns. J Eur Acad Dermatol Venereol. 2021;35:73-78.
- Eady AE, Cove JH, Layton AM. Is antibiotic resistance in cutaneous propionibacteria clinically relevant? implications of resistance for acne patients and prescribers. Am J Clin Dermatol. 2003;4:813-831.
- Eady EA, Cove J, Holland K, et al. Erythromycin resistant propionibacteria in antibiotic treated acne patients: association with therapeutic failure. Br J Dermatol. 1989;121:51-57.
- Grossman TH. Tetracycline antibiotics and resistance. Cold Spring Harb Perspect Med. 2016;6:a025387.
- Kayiran M AS, Karadag AS, Al-Khuzaei S, et al. Antibiotic resistance in acne: mechanisms, complications and management. Am J Clin Dermatol. 2020;21:813-819.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006-1035.
- Kucera K, Zenzola N, Hudspeth A, et al. Benzoyl peroxide drug products form benzene. Environ Health Perspect. 2024;132:037702.
- Kucera K, Zenzola N, Hudspeth A, et al. Evaluation of benzene presence and formation in benzoyl peroxide drug products. J Invest Dermatol. 2025;145:1147-1154.E11.
- Grada A, Perche P, Feldman S. Adherence and persistence to acne medications: a population-based claims database analysis. J Drugs Dermatol. 2022;21:758-764.<.li>
- Anderson KL, Dothard EH, Huang KE, et al. Frequency of primary nonadherence to acne treatment. JAMA Dermatol. 2015;151:623-626.
- Grada A, Bunick CG. Spectrum of antibiotic activity and its relevance to the microbiome. JAMA Netw Open. 2021;4:E215357-E215357.
- Francino M. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:164577.
- Moura IB, Grada A, Spittal W, et al. Profiling the effects of systemic antibiotics for acne, including the narrow-spectrum antibiotic sarecycline, on the human gut microbiota. Front Microbiol. 2022;13:901911.
- Zhanel G, Critchley I, Lin L-Y, et al. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob Agents Chemother. 2019;63:1297-1318.
Path of Least Resistance: Guidance for Antibiotic Stewardship in Acne
Path of Least Resistance: Guidance for Antibiotic Stewardship in Acne
Practice Point
- Oral antibiotics remain a cornerstone in the treatment of moderate to severe acne, but growing concerns about antibiotic resistance necessitate more intentional prescribing.
Irritable Bowel Syndrome Risk in Acne Patients: Implications for Dermatologic Care
To the Editor:
Acne vulgaris and irritable bowel syndrome (IBS) are both associated with microbial dysbiosis and chronic inflammation.1-3 While the prevalence of IBS among patients with acne has been examined previously,4,5 there has been limited focus on the risk for new-onset IBS following acne diagnosis. Current evidence suggests isotretinoin may be associated with a lower risk for IBS compared to oral antibiotics6; however, evidence supporting this association is limited outside these cohorts, highlighting the need for further investigation. In this large-scale study, we sought to investigate the incidence of new-onset IBS among patients with acne compared with healthy controls as well as to evaluate whether oral acne treatments (ie, oral antibiotics or isotretinoin) are associated with new-onset IBS in this population.
A retrospective cohort study was conducted using data from the US Collaborative Network in TriNetX from October 2014 to October 2024. Patients were identified using International Classification of Diseases, Tenth Revision, Clinical Modification codes, Current Procedural Terminology codes, Anatomical Therapeutic Chemical Classification System codes, and RxNorm codes (Table 1). These codes were selected based on prior literature review, clinical relevance, and their ability to capture diagnoses of acne and IBS as well as relevant exclusion criteria. Patients were considered eligible if they were between the ages of 18 and 90 years. Individuals with a history of IBS, inflammatory bowel disease, infectious gastroenteritis, or celiac disease were excluded from our analysis.
To examine potential associations between acne and IBS, 2 primary cohorts were established: patients with acne who were managed without systemic medications and healthy controls (ie, patients with no history of acne) who had no exposure to systemic acne treatments (Figure). Further, to assess the relationship between oral acne treatments (macrolides, tetracyclines, isotretinoin) and IBS, additional cohorts were created for each therapy and were compared to a cohort of patients with acne who were managed without systemic medications. To control for potential concomitant treatments, patients who had received any systemic treatment other than the specific therapy for their treatment cohort were excluded from our analysis.
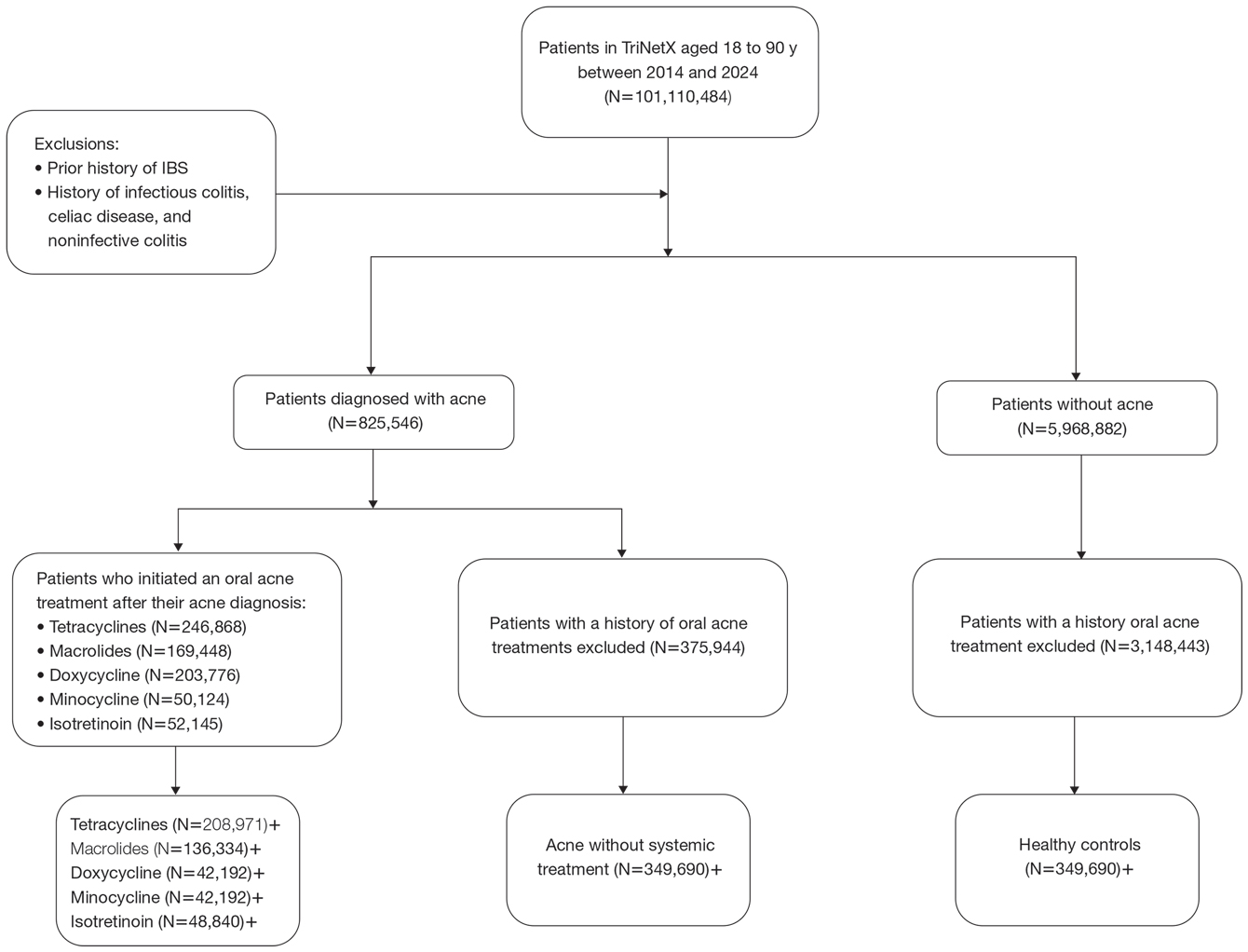
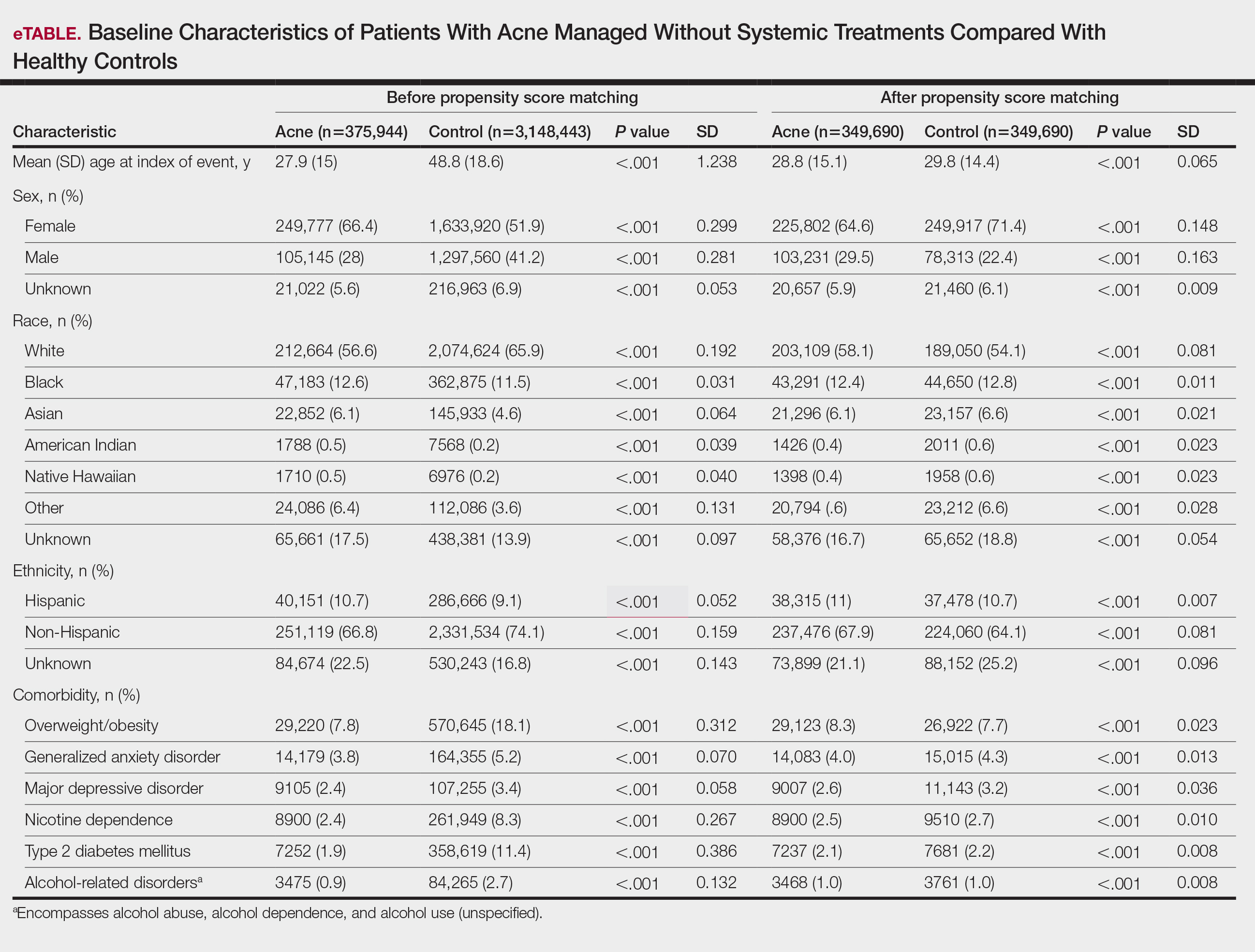
To account for potential confounders, all cohorts were 1:1 propensity score matched by demographics, tobacco and alcohol use, type 2 diabetes, obesity, anxiety, and depression (eTable). Each cohort was followed for 2 years after their index of event: the date of acne diagnosis for the acne cohort, the date of systemic treatment initiation for the treatment cohorts, and the date of the general adult encounter without abnormal findings for the control cohort. The primary outcome was the incidence of IBS, assessed by odds ratio (OR) and 95% CIs.
We identified 375,944 patients with acne managed without systemic treatment and 3,148,443 healthy controls who met study criteria. After the 1:1 propensity score match, each cohort included 49,690 patients (eTable). In the 2-year period after acne diagnosis, patients were more likely to develop IBS compared with controls (1421 vs 1285 [OR, 1.10; 95% CI, 1.02-1.19])(Table 2). Patients with acne who were treated with tetracyclines (n=208,971) were 30% more likely to develop IBS than those managed without systemic medications (1114 vs 856 [OR, 1.30; 95% CI, 1.19-1.42]). Within the tetracycline cohort, doxycycline-treated patients were 25% more likely to develop IBS compared with those treated with minocycline (213 vs 170 [OR, 1.25; 95% CI, 1.02-1.53]). Similarly, the use of macrolides (n=136,334) for acne treatment was significantly associated with an increased risk for IBS (1023 vs 595 [OR, 1.73; 95% CI, 1.57-1.92; P<.0001]) compared with controls. No statistically significant association was observed between isotretinoin and the incidence of IBS (Table 2).
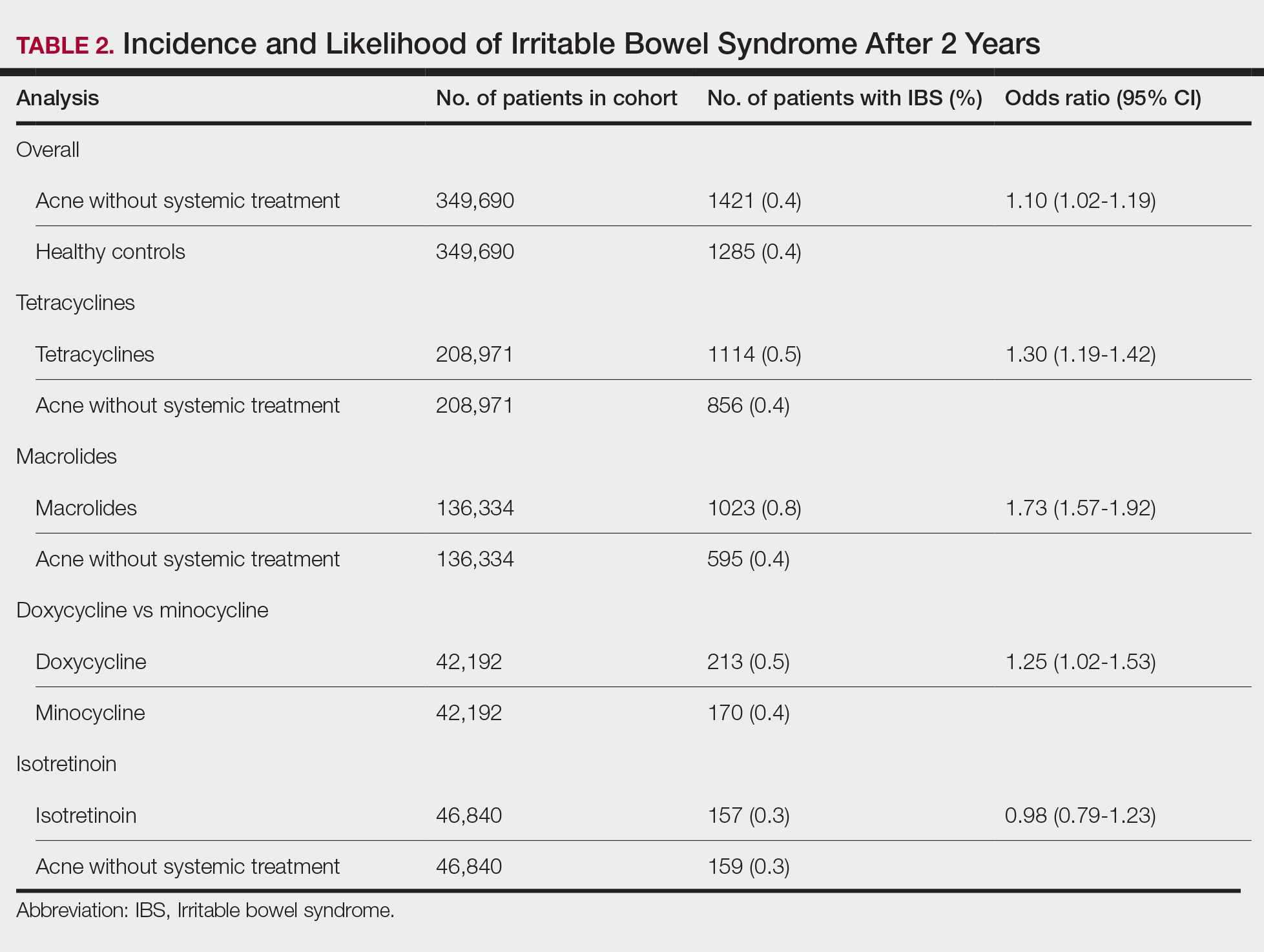
In this large-scale cohort study, acne was associated with an increased likelihood of developing IBS within 2 years of an acne diagnosis compared with healthy controls. While a prior study also identified this association, it had a broader follow-up window ranging from 8 to 10 years.2 In contrast, our analysis specifically quantified the risk within the first 2 years of diagnosis. This distinction suggested potential for earlier IBS onset in patients with acne than has previously been recognized and may serve as an early clinical indicator for IBS risk in this population.
Our findings further suggested an association between oral tetracyclines and macrolides and an increased risk for IBS. This aligns with existing literature suggesting that oral antibiotic use can disrupt the gut microbiota and lead to potential gastrointestinal complications7 and reinforces the importance of careful antibiotic stewardship in dermatologic practice.
Although isotretinoin initially was surrounded by substantial controversy regarding its potential impact on gut health—particularly in inflammatory bowel disease8—our results do not support an increased risk for IBS among patients with acne who use isotretinoin. These findings challenge previous concerns and align with research suggesting that isotretinoin could be a safer alternative to antibiotic use for eligible patients who have a history of gastrointestinal disorders.6
This study highlights an important but underrecognized link between acne and IBS risk, emphasizing the need for early monitoring of gastrointestinal symptoms and careful antibiotic stewardship in dermatologic practice. Gastroenterology consultation may be advisable for patients with acne who have persistent gastrointestinal symptoms to facilitate a more integrated, patient-centered approach to care.
Limitations of this study include potential misclassification of International Classification of Diseases, Tenth Revision, Clinical Modification codes, selection bias, and residual confounding from unmeasured factors such as diet, lifestyle, disease severity, and treatment adherence due to the reliance on electronic health record data.
Our findings build upon prior evidence linking acne and IBS and offer important insights into the timing of this association following acne diagnosis. Future research should explore biological mechanisms underlying the gut-skin axis and evaluate targeted interventions to mitigate IBS risk in patients with acne.
Menees S, Chey W. The gut microbiome and irritable bowel syndrome. F1000Res. 2018;7:F1000 Faculty Rev-1029. doi:10.12688/f1000research.14592.1
Yu-Wen C, Chun-Ying W, Yi-Ju C. Gastrointestinal comorbidities in patients with acne vulgaris: a population-based retrospective study. JAAD Int. 2025;18:62-68. doi:10.1016/j.jdin.2024.08.022
Deng Y, Wang H, Zhou J, et al. Patients with acne vulgaris have a distinct gut microbiota in comparison with healthy controls. Acta Derm Venereol. 2018;98:783-790. doi:10.2340/00015555-2968
Demirbas¸ A, Elmas ÖF. The relationship between acne vulgaris and irritable bowel syndrome: a preliminary study. J Cosmet Dermatol. 2021;20:316-320. doi:10.1111/jocd.13481
Daye M, Cihan FG, Is¸ık B, et al. Evaluation of bowel habits in patients with acne vulgaris. Int J Clin Pract. 2021;75:e14903. doi:10.1111/ijcp.14903
Kridin K, Ludwig RJ. Isotretinoin and the risk of inflammatory bowel disease and irritable bowel syndrome: a large-scale global study. J Am Acad Dermatol. 2023;88:824-830. doi:10.1016/j.jaad.2022.12.015
Villarreal AA, Aberger FJ, Benrud R, et al. Use of broad-spectrum antibiotics and the development of irritable bowel syndrome. WMJ. 2012;111:17-20.
Yu C-L, Chou P-Y, Liang C-S, et al. Isotretinoin exposure and risk of inflammatory bowel disease: a systematic review with meta-analysis and trial sequential analysis. Am J Clin Dermatol. 2023;24:721-730. doi:10.1007/s40257-023-00765-9
To the Editor:
Acne vulgaris and irritable bowel syndrome (IBS) are both associated with microbial dysbiosis and chronic inflammation.1-3 While the prevalence of IBS among patients with acne has been examined previously,4,5 there has been limited focus on the risk for new-onset IBS following acne diagnosis. Current evidence suggests isotretinoin may be associated with a lower risk for IBS compared to oral antibiotics6; however, evidence supporting this association is limited outside these cohorts, highlighting the need for further investigation. In this large-scale study, we sought to investigate the incidence of new-onset IBS among patients with acne compared with healthy controls as well as to evaluate whether oral acne treatments (ie, oral antibiotics or isotretinoin) are associated with new-onset IBS in this population.
A retrospective cohort study was conducted using data from the US Collaborative Network in TriNetX from October 2014 to October 2024. Patients were identified using International Classification of Diseases, Tenth Revision, Clinical Modification codes, Current Procedural Terminology codes, Anatomical Therapeutic Chemical Classification System codes, and RxNorm codes (Table 1). These codes were selected based on prior literature review, clinical relevance, and their ability to capture diagnoses of acne and IBS as well as relevant exclusion criteria. Patients were considered eligible if they were between the ages of 18 and 90 years. Individuals with a history of IBS, inflammatory bowel disease, infectious gastroenteritis, or celiac disease were excluded from our analysis.

To examine potential associations between acne and IBS, 2 primary cohorts were established: patients with acne who were managed without systemic medications and healthy controls (ie, patients with no history of acne) who had no exposure to systemic acne treatments (Figure). Further, to assess the relationship between oral acne treatments (macrolides, tetracyclines, isotretinoin) and IBS, additional cohorts were created for each therapy and were compared to a cohort of patients with acne who were managed without systemic medications. To control for potential concomitant treatments, patients who had received any systemic treatment other than the specific therapy for their treatment cohort were excluded from our analysis.

To account for potential confounders, all cohorts were 1:1 propensity score matched by demographics, tobacco and alcohol use, type 2 diabetes, obesity, anxiety, and depression (eTable). Each cohort was followed for 2 years after their index of event: the date of acne diagnosis for the acne cohort, the date of systemic treatment initiation for the treatment cohorts, and the date of the general adult encounter without abnormal findings for the control cohort. The primary outcome was the incidence of IBS, assessed by odds ratio (OR) and 95% CIs.
We identified 375,944 patients with acne managed without systemic treatment and 3,148,443 healthy controls who met study criteria. After the 1:1 propensity score match, each cohort included 49,690 patients (eTable). In the 2-year period after acne diagnosis, patients were more likely to develop IBS compared with controls (1421 vs 1285 [OR, 1.10; 95% CI, 1.02-1.19])(Table 2). Patients with acne who were treated with tetracyclines (n=208,971) were 30% more likely to develop IBS than those managed without systemic medications (1114 vs 856 [OR, 1.30; 95% CI, 1.19-1.42]). Within the tetracycline cohort, doxycycline-treated patients were 25% more likely to develop IBS compared with those treated with minocycline (213 vs 170 [OR, 1.25; 95% CI, 1.02-1.53]). Similarly, the use of macrolides (n=136,334) for acne treatment was significantly associated with an increased risk for IBS (1023 vs 595 [OR, 1.73; 95% CI, 1.57-1.92; P<.0001]) compared with controls. No statistically significant association was observed between isotretinoin and the incidence of IBS (Table 2).


In this large-scale cohort study, acne was associated with an increased likelihood of developing IBS within 2 years of an acne diagnosis compared with healthy controls. While a prior study also identified this association, it had a broader follow-up window ranging from 8 to 10 years.2 In contrast, our analysis specifically quantified the risk within the first 2 years of diagnosis. This distinction suggested potential for earlier IBS onset in patients with acne than has previously been recognized and may serve as an early clinical indicator for IBS risk in this population.
Our findings further suggested an association between oral tetracyclines and macrolides and an increased risk for IBS. This aligns with existing literature suggesting that oral antibiotic use can disrupt the gut microbiota and lead to potential gastrointestinal complications7 and reinforces the importance of careful antibiotic stewardship in dermatologic practice.
Although isotretinoin initially was surrounded by substantial controversy regarding its potential impact on gut health—particularly in inflammatory bowel disease8—our results do not support an increased risk for IBS among patients with acne who use isotretinoin. These findings challenge previous concerns and align with research suggesting that isotretinoin could be a safer alternative to antibiotic use for eligible patients who have a history of gastrointestinal disorders.6
This study highlights an important but underrecognized link between acne and IBS risk, emphasizing the need for early monitoring of gastrointestinal symptoms and careful antibiotic stewardship in dermatologic practice. Gastroenterology consultation may be advisable for patients with acne who have persistent gastrointestinal symptoms to facilitate a more integrated, patient-centered approach to care.
Limitations of this study include potential misclassification of International Classification of Diseases, Tenth Revision, Clinical Modification codes, selection bias, and residual confounding from unmeasured factors such as diet, lifestyle, disease severity, and treatment adherence due to the reliance on electronic health record data.
Our findings build upon prior evidence linking acne and IBS and offer important insights into the timing of this association following acne diagnosis. Future research should explore biological mechanisms underlying the gut-skin axis and evaluate targeted interventions to mitigate IBS risk in patients with acne.
To the Editor:
Acne vulgaris and irritable bowel syndrome (IBS) are both associated with microbial dysbiosis and chronic inflammation.1-3 While the prevalence of IBS among patients with acne has been examined previously,4,5 there has been limited focus on the risk for new-onset IBS following acne diagnosis. Current evidence suggests isotretinoin may be associated with a lower risk for IBS compared to oral antibiotics6; however, evidence supporting this association is limited outside these cohorts, highlighting the need for further investigation. In this large-scale study, we sought to investigate the incidence of new-onset IBS among patients with acne compared with healthy controls as well as to evaluate whether oral acne treatments (ie, oral antibiotics or isotretinoin) are associated with new-onset IBS in this population.
A retrospective cohort study was conducted using data from the US Collaborative Network in TriNetX from October 2014 to October 2024. Patients were identified using International Classification of Diseases, Tenth Revision, Clinical Modification codes, Current Procedural Terminology codes, Anatomical Therapeutic Chemical Classification System codes, and RxNorm codes (Table 1). These codes were selected based on prior literature review, clinical relevance, and their ability to capture diagnoses of acne and IBS as well as relevant exclusion criteria. Patients were considered eligible if they were between the ages of 18 and 90 years. Individuals with a history of IBS, inflammatory bowel disease, infectious gastroenteritis, or celiac disease were excluded from our analysis.

To examine potential associations between acne and IBS, 2 primary cohorts were established: patients with acne who were managed without systemic medications and healthy controls (ie, patients with no history of acne) who had no exposure to systemic acne treatments (Figure). Further, to assess the relationship between oral acne treatments (macrolides, tetracyclines, isotretinoin) and IBS, additional cohorts were created for each therapy and were compared to a cohort of patients with acne who were managed without systemic medications. To control for potential concomitant treatments, patients who had received any systemic treatment other than the specific therapy for their treatment cohort were excluded from our analysis.

To account for potential confounders, all cohorts were 1:1 propensity score matched by demographics, tobacco and alcohol use, type 2 diabetes, obesity, anxiety, and depression (eTable). Each cohort was followed for 2 years after their index of event: the date of acne diagnosis for the acne cohort, the date of systemic treatment initiation for the treatment cohorts, and the date of the general adult encounter without abnormal findings for the control cohort. The primary outcome was the incidence of IBS, assessed by odds ratio (OR) and 95% CIs.
We identified 375,944 patients with acne managed without systemic treatment and 3,148,443 healthy controls who met study criteria. After the 1:1 propensity score match, each cohort included 49,690 patients (eTable). In the 2-year period after acne diagnosis, patients were more likely to develop IBS compared with controls (1421 vs 1285 [OR, 1.10; 95% CI, 1.02-1.19])(Table 2). Patients with acne who were treated with tetracyclines (n=208,971) were 30% more likely to develop IBS than those managed without systemic medications (1114 vs 856 [OR, 1.30; 95% CI, 1.19-1.42]). Within the tetracycline cohort, doxycycline-treated patients were 25% more likely to develop IBS compared with those treated with minocycline (213 vs 170 [OR, 1.25; 95% CI, 1.02-1.53]). Similarly, the use of macrolides (n=136,334) for acne treatment was significantly associated with an increased risk for IBS (1023 vs 595 [OR, 1.73; 95% CI, 1.57-1.92; P<.0001]) compared with controls. No statistically significant association was observed between isotretinoin and the incidence of IBS (Table 2).


In this large-scale cohort study, acne was associated with an increased likelihood of developing IBS within 2 years of an acne diagnosis compared with healthy controls. While a prior study also identified this association, it had a broader follow-up window ranging from 8 to 10 years.2 In contrast, our analysis specifically quantified the risk within the first 2 years of diagnosis. This distinction suggested potential for earlier IBS onset in patients with acne than has previously been recognized and may serve as an early clinical indicator for IBS risk in this population.
Our findings further suggested an association between oral tetracyclines and macrolides and an increased risk for IBS. This aligns with existing literature suggesting that oral antibiotic use can disrupt the gut microbiota and lead to potential gastrointestinal complications7 and reinforces the importance of careful antibiotic stewardship in dermatologic practice.
Although isotretinoin initially was surrounded by substantial controversy regarding its potential impact on gut health—particularly in inflammatory bowel disease8—our results do not support an increased risk for IBS among patients with acne who use isotretinoin. These findings challenge previous concerns and align with research suggesting that isotretinoin could be a safer alternative to antibiotic use for eligible patients who have a history of gastrointestinal disorders.6
This study highlights an important but underrecognized link between acne and IBS risk, emphasizing the need for early monitoring of gastrointestinal symptoms and careful antibiotic stewardship in dermatologic practice. Gastroenterology consultation may be advisable for patients with acne who have persistent gastrointestinal symptoms to facilitate a more integrated, patient-centered approach to care.
Limitations of this study include potential misclassification of International Classification of Diseases, Tenth Revision, Clinical Modification codes, selection bias, and residual confounding from unmeasured factors such as diet, lifestyle, disease severity, and treatment adherence due to the reliance on electronic health record data.
Our findings build upon prior evidence linking acne and IBS and offer important insights into the timing of this association following acne diagnosis. Future research should explore biological mechanisms underlying the gut-skin axis and evaluate targeted interventions to mitigate IBS risk in patients with acne.
Menees S, Chey W. The gut microbiome and irritable bowel syndrome. F1000Res. 2018;7:F1000 Faculty Rev-1029. doi:10.12688/f1000research.14592.1
Yu-Wen C, Chun-Ying W, Yi-Ju C. Gastrointestinal comorbidities in patients with acne vulgaris: a population-based retrospective study. JAAD Int. 2025;18:62-68. doi:10.1016/j.jdin.2024.08.022
Deng Y, Wang H, Zhou J, et al. Patients with acne vulgaris have a distinct gut microbiota in comparison with healthy controls. Acta Derm Venereol. 2018;98:783-790. doi:10.2340/00015555-2968
Demirbas¸ A, Elmas ÖF. The relationship between acne vulgaris and irritable bowel syndrome: a preliminary study. J Cosmet Dermatol. 2021;20:316-320. doi:10.1111/jocd.13481
Daye M, Cihan FG, Is¸ık B, et al. Evaluation of bowel habits in patients with acne vulgaris. Int J Clin Pract. 2021;75:e14903. doi:10.1111/ijcp.14903
Kridin K, Ludwig RJ. Isotretinoin and the risk of inflammatory bowel disease and irritable bowel syndrome: a large-scale global study. J Am Acad Dermatol. 2023;88:824-830. doi:10.1016/j.jaad.2022.12.015
Villarreal AA, Aberger FJ, Benrud R, et al. Use of broad-spectrum antibiotics and the development of irritable bowel syndrome. WMJ. 2012;111:17-20.
Yu C-L, Chou P-Y, Liang C-S, et al. Isotretinoin exposure and risk of inflammatory bowel disease: a systematic review with meta-analysis and trial sequential analysis. Am J Clin Dermatol. 2023;24:721-730. doi:10.1007/s40257-023-00765-9
Menees S, Chey W. The gut microbiome and irritable bowel syndrome. F1000Res. 2018;7:F1000 Faculty Rev-1029. doi:10.12688/f1000research.14592.1
Yu-Wen C, Chun-Ying W, Yi-Ju C. Gastrointestinal comorbidities in patients with acne vulgaris: a population-based retrospective study. JAAD Int. 2025;18:62-68. doi:10.1016/j.jdin.2024.08.022
Deng Y, Wang H, Zhou J, et al. Patients with acne vulgaris have a distinct gut microbiota in comparison with healthy controls. Acta Derm Venereol. 2018;98:783-790. doi:10.2340/00015555-2968
Demirbas¸ A, Elmas ÖF. The relationship between acne vulgaris and irritable bowel syndrome: a preliminary study. J Cosmet Dermatol. 2021;20:316-320. doi:10.1111/jocd.13481
Daye M, Cihan FG, Is¸ık B, et al. Evaluation of bowel habits in patients with acne vulgaris. Int J Clin Pract. 2021;75:e14903. doi:10.1111/ijcp.14903
Kridin K, Ludwig RJ. Isotretinoin and the risk of inflammatory bowel disease and irritable bowel syndrome: a large-scale global study. J Am Acad Dermatol. 2023;88:824-830. doi:10.1016/j.jaad.2022.12.015
Villarreal AA, Aberger FJ, Benrud R, et al. Use of broad-spectrum antibiotics and the development of irritable bowel syndrome. WMJ. 2012;111:17-20.
Yu C-L, Chou P-Y, Liang C-S, et al. Isotretinoin exposure and risk of inflammatory bowel disease: a systematic review with meta-analysis and trial sequential analysis. Am J Clin Dermatol. 2023;24:721-730. doi:10.1007/s40257-023-00765-9
Spironolactone for Acne: Practical Strategies for Optimal Clinical Outcomes
Spironolactone for Acne: Practical Strategies for Optimal Clinical Outcomes
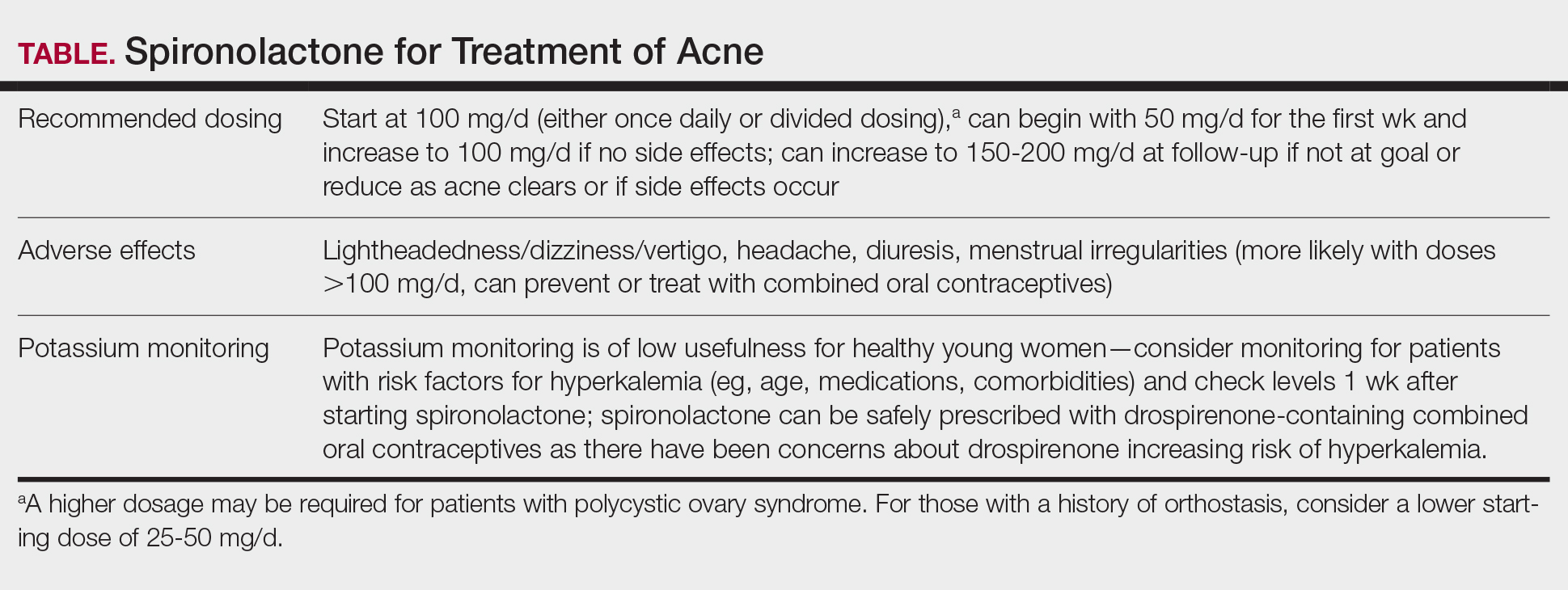
Spironolactone is increasingly used off label for acne treatment and is now being prescribed for women with acne at a frequency similar to oral antibiotics.1,2 In this article, we provide an overview of spironolactone use for acne treatment and discuss recent clinical trials and practical strategies for patient selection, dosing, adverse effect management, and monitoring (Table).
History and Mechanism of Action
Because sebaceous gland activity is an important component of acne pathogenesis and is regulated by androgens,3 there has long been interest in identifying treatment strategies that can target the role of hormones in activating the sebaceous gland. In the 1980s, it became apparent that spironolactone, originally developed as a potassium-sparing diuretic, also might possess antiandrogenic properties that could be useful in the treatment of acne.4 Spironolactone has been found to decrease testosterone production, inhibit testosterone and dihydrotestosterone binding to androgen receptors,5-8 and block 5α-reductase receptors of the sebaceous glands of skin.9
In 1984, Goodfellow et al10 conducted a trial in which 36 male and female patients with severe acne were randomized to placebo or spironolactone doses ranging from 50 to 200 mg/d. They found that spironolactone resulted in dose-dependent reductions of sebum production as well as improvement in patient- and clinician-reported assessments of acne. In 1986, another placebo-controlled crossover trial by Muhlemann et al11 provided further support for the effectiveness of spironolactone for acne. This trial randomized 21 women to placebo or spironolactone 200 mg/d and found that spironolactone was associated with statistically significant (P<.001) improvements in acne lesion counts.
Recent Observational Studies and Trials
Following these early trials, several large case series have been published describing the successful use of spironolactone for acne, including a 2020 retrospective case series from the Mayo Clinic describing 395 patients.12 The investigators found that almost 66% of patients had a complete response and almost 85% had a complete response or a partial response greater than 50%. They also found that the median time to initial response and maximal response were 3 and 5 months, respectively, and that efficacy was observed across acne subtypes, including for nodulocystic acne.12 In addition, a 2021 case series describing 403 patients treated with spironolactone found that approximately 80% had reduction or complete clearance of acne, with improvements observed for both facial and truncal acne. In this cohort, doses of 100 to 150 mg/d typically were the most successful.13 A case series of 80 adolescent females also highlighted the efficacy of spironolactone in younger populations.14
Adding to these observational data, the multicenter, phase 3, double-blind Spironolactone for Adult Female Acne (SAFA) trial included 410 women (mean age, 29.2 years) who were randomized to receive either placebo or intervention (spironolactone 50 mg/d until week 6 and 100 mg/d until week 24).15 At 24 weeks, greater improvement in quality of life and participant self-assessed improvement were observed in the spironolactone group. In addition, at 12 weeks, rates of success were higher in the spironolactone group using the Investigator Global Assessment score (adjusted odds ratio 5.18 [95% CI, 2.18- 12.28]). Those randomized to receive spironolactone also had lower rates of oral antibiotic use at 52 weeks than the placebo group did (5.8% vs 13.5%, respectively).
In the SAFA trial, spironolactone was well tolerated; the most common adverse effects relative to placebo were lightheadedness (19% for spironolactone vs 12% for placebo) and headache (20% for spironolactone vs 12% for placebo). Notably, more than 95% of patients were able to increase from 50 mg/d to 100 mg/d at week 6, with greater than 90% tolerating 100 mg/d. As observational data suggest that spironolactone takes 3 to 5 months to reach peak efficacy, these findings provide further support that starting at a dose of at least 100 mg/d is likely optimal for most patients.16
A Potential Alternative to Oral Antibiotics
Oral antibiotics such as tetracyclines have long played a central role in the treatment of acne and remain a first-line treatment option.17 In addition, many of these antibiotic courses exceed 6 months in duration.1 In fact, dermatologists prescribe more antibiotics per capita than any other specialty1,18-20; however, this can be associated with the development of antibiotic resistance,21,22 as well as other antibiotic-associated complications, including inflammatory bowel disease,23 pharyngitis,24 Clostridium difficile infections, and cancer.25-29
In addition to these concerns, many patients may prefer nonantibiotic alternatives to oral antibiotics, with more than 75% preferring a nonantibiotic option if available. For female patients with acne, antiandrogens such as spironolactone have been suggested as a potential alternative.30 A 10-year retrospective study of female patients with acne found that those who had ever received hormonal therapy (ie, spironolactone or a combined oral contraceptive) received fewer cumulative days of oral antibiotics than those who did not (226 days vs 302 days, respectively).31 In addition, while oral antibiotics were the most common initial therapy prescribed for patients, as they progressed through their treatment course, more patients ended up on hormonal therapy than oral antibiotics. This study suggests that hormonal therapy such as spironolactone could represent an alternative to the use of systemic antibiotics.31
Further supporting the role of spironolactone as an alternative to oral antibiotics, a 2018 analysis of claims data found that spironolactone may have similar effectiveness to oral antibiotics for the treatment of acne.32 After adjusting for age and topical retinoid and oral contraceptive use, this study found that there was no significant difference in the odds of being prescribed a different systemic treatment within 1 year (ie, treatment failure) among those starting spironolactone vs those starting oral tetracycline-class antibiotics as their initial therapy for acne.
A multicenter, randomized, double-blind trial (Female Acne Spironolactone vs doxyCycline Efficacy [FASCE]) also evaluated the comparative effectiveness of doxycycline 100 mg/d for 3 months followed by an oral placebo for 3 months vs spironolactone 150 mg/d for 6 months among 133 adult women with acne. This study found that spironolactone had statistically significantly greater rates of Investigator Global Assessment treatment success after 6 months (odds ratio 2.87 [95% CI, 1.38-5.99; P=.007]).33 Since spironolactone historically has been prescribed less often than oral antibiotics for women with acne, these findings support spironolactone as an underutilized treatment alternative. The ongoing Spironolactone versus Doxycycline for Acne: A Comparative Effectiveness, Noninferiority Evaluation trial—a 16-week, blinded trial comparing 100 mg/d doses of both drugs—should provide additional evidence regarding the relative role of spironolactone and oral antibiotics in the management of acne.34
Ultimately, the decision to use spironolactone or other treatments such as oral antibiotics should be based on shared decision making between clinician and patient. Spironolactone has a relatively slow onset of efficacy, and other options such as oral antibiotics might be preferred by those looking for more immediate results; however, as women with acne often have activity that persists into adulthood, spironolactone might be preferable as a long-term maintenance therapy to avoid complications of prolonged antibiotic use.35 Comorbidities also will influence the optimal choice of therapy (eg, spironolactone might be preferred in someone with inflammatory bowel disease, and oral antibiotics might be preferred in someone with orthostatic hypotension).
Patient Selection
Acne occurring along the lower face or jawline in adult women sometimes is referred to as hormonal acne, but this dogma is not particularly evidence based. An observational study of 374 patients found that almost 90% of adult women had acne involving multiple facial zones with a spectrum of facial acne severity similar to that in adolescents.36 Only a small subset of these patients (11.2%) had acne localized solely to the mandibular area. In addition, acne along the lower face is not predictive of hyperandrogenism (eg, polycystic ovary syndrome).37 Antiandrogen therapies such as spironolactone and clascoterone are effective in both men and women with acne10,38 and in adolescents and adults, suggesting that hormones play a fundamental role in all acne and that addressing this mechanism can be useful broadly. Therefore, hormonal therapies such as spironolactone should not be restricted to only adult women with acne along the lower face.
While spironolactone can be effective for acne treatment in any age group, it may be most effective for adult women with acne. In the SAFA trial, prespecified subgroup analyses showed a statistically significant (P=.005) interaction term for age (categorized as <25 years and ≥25 years), which suggested that spironolactone might be a more effective treatment for women 25 years and older.15 In addition, subgroup analyses in the aforementioned 2018 analysis of claims data found that spironolactone was more effective relative to oral antibiotics in adults vs adolescents.32 Despite these limitations, several case series have highlighted that spironolactone is effective among adolescent populations with acne. A case series of spironolactone use in 73 patients aged 19 years or younger found that 68% of patients demonstrated resolution or improvement in their acne after spironolactone treatment.39 Another case series among 80 adolescent females reported 80% of patients experiencing improvement of their acne.14
For those with more severe acne, spironolactone can be combined with other complementary treatment approaches such as topicals, oral antibiotics, or procedural modalities.40
Dosing
We recommend starting spironolactone at a dose of 100 mg/d (the patient can take 50 mg/d for 1 week, then increase to 100 mg/d if there are no adverse effects at the lower dose). In the 1984 trial by Goodfellow et al,10 participants were randomized to doses of 50 mg/d, 100 mg/d, 150 mg/d, and 200 mg/d. In this trial, efficacy assessed by objective and subjective outcomes did not plateau until doses of 100 mg/d to 150 mg/d. In addition, a case series of 403 patients found that the most successful dosage of spironolactone generally was 100 mg/d or higher.13 Most of the patients who were started at this dosage either stayed at this level or escalated, whereas patients who started at lower dosages (25-75 mg/d) frequently increased their dosage over time. The SAFA trial also highlighted that most patients can tolerate a spironolactone dose of 100 mg/d.15 For specific populations, such as patients with polycystic ovary syndrome, a higher dose (mean dosage of 143 mg/d) may be required for efficacy.41 Given the slow onset of efficacy, typically taking 3 to 5 months, and the low rate of adverse effects, we believe the optimal starting dose is 100 mg/s to 150 mg/d. If adverse effects occur or lesions clear, then the dosage may be reduced.
Adverse Effects
Spironolactone generally is well tolerated; in the SAFA and FASCE trials, fewer than 1% of participants discontinued due to adverse effects.15,33 Rates of discontinuation due to adverse effects typically have been less than 5% in case series of patients treated in routine clinical practice.12-14
Because spironolactone is a diuretic and antihypertensive, the most common adverse effects are related to these characteristics. In the SAFA trial, dizziness, lightheadedness, and vertigo were reported more commonly in the spironolactone group than in the placebo group (19% vs 12%, respectively). Similarly, headaches also were reported more frequently in the spironolactone group than in the placebo group (20% vs 12%, respectively).15 One case series found that, among the 267 patients on spironolactone whose blood pressure was monitored, the mean reduction in systolic blood pressure was 3.5 mm Hg and the mean reduction in diastolic blood pressure was 0.9 mm Hg.13 For those with baseline orthostasis or in those who experience adverse effects related to hypotension, reducing the dose often can be helpful. Of note, while doses of 100 mg/d to 150 mg/d often are the most effective, randomized trials have found that spironolactone still can be effective for acne at doses as low as 25 mg/d to 50 mg/d.10,38
Menstrual irregularities are another commonly cited adverse effect of spironolactone. While a systematic review found that 15% to 30% of patients treated with spironolactone experience menstrual irregularities, it has been difficult to evaluate whether this is due to the medication or other comorbidities, such as polycystic ovary syndrome.42 Notably, in the SAFA trial, rates of menstrual irregularities were equivalent between the spironolactone and placebo groups at a dose of 100 mg/d (32% vs 35%, respectively).15 In contrast, in the FASCE trial, menstrual irregularities were more commonly reported at a dose of 150 mg/d.33 These findings are consistent with observational data suggesting that menstrual irregularities are much more common at spironolactone doses greater than 100 mg/d.42 Additionally, some evidence supports that for some patients these menstrual irregularities may resolve within 2 to 3 months of continued treatment.43 It has been noted in several studies that menstrual irregularities are less likely to occur in patients who are using combined oral contraceptives; therefore, for patients who are amenable and have no contraindications, combined oral contraceptives can be considered to prevent or address menstrual irregularities.13,42,44
More generally, combined oral contraceptives can be an excellent combination with spironolactone, as they have complementary characteristics. Spironolactone primarily blocks the effects of androgens, while combined oral contraceptives predominantly block the production of androgens. Whereas spironolactone typically causes hypotension and menstrual irregularities, combined oral contraceptives cause hypertension and help to regulate the menstrual cycle.
Spironolactone carries an official US Food and Drug Administration warning regarding possible tumorigenicity that is based on animal studies that used up to 150 times the normal dose of spironolactone used in humans45; however, observational studies in humans have not identified such an association when spironolactone is used in normal clinical settings. A systematic review and metanalysis in 2022 reviewed data from a total population of more than 4 million individuals and found that there was no statistically significant association between spironolactone use and the risk for breast, ovarian, bladder, kidney, gastric, or esophageal cancers.46 Additional studies also found no association between spironolactone use and cancers.48 A more recent cohort study specifically among patients treated with spironolactone for acne also found no significant increased risk for breast cancer.49
Combined oral contraceptives are associated with an increased risk for venous thromboembolisms, and there have been concerns that this risk may be greater in combined oral contraceptives that contain drospirenone.50 Drospirenone is molecularly related to spironolactone, which has prompted the consideration of whether spironolactone use also conveys a risk for venous thromboembolism. Reassuringly, a retrospective study of claims data found that individuals on spironolactone were not more likely to develop a pulmonary embolism or a deep venous thrombosis than matched controls treated with tetracycline antibiotics, with a point estimate favoring decreased risk.51
Monitoring
Given that one of spironolactone’s mechanisms of action is aldosterone antagonism and thus the inhibition of potassium excretion, there have been concerns regarding risk for hyperkalemia. A retrospective study analyzing data from 2000 to 2014 found that, among 974 young women receiving spironolactone therapy, the rate of hyperkalemia was 0.72%, which is equivalent to the 0.76% baseline rate of hyperkalemia in the same population.52 Subsequent studies also have found that spironolactone does not appear to be associated with a meaningful risk for hyperkalemia among young healthy patients treated for acne.38,53 These studies suggest that routine potassium monitoring is of low usefulness for healthy young women taking spironolactone for acne. The 2024 American Academy of Dermatology guidelines on the management of acne also state that potassium monitoring is not needed in healthy patients but that potassium testing should be considered for those with risk factors for hyperkalemia (eg, older age, medical comorbidities, medications).40 Clinicians should still engage in shared decision making with patients to determine whether to check potassium. If potassium is to be monitored, it should be checked 1 to 2 weeks after spironolactone is started.45,54
Since drospirenone also has aldosterone antagonistic properties,55 there have been concerns about whether concomitant use of spironolactone and drospirenone-containing combined oral contraceptives might increase the risk for hyperkalemia.56 However, a retrospective cohort study analyzing data from more than 1 million women found that drospirenone is not any more likely than levonorgestrel to cause hyperkalemia and that there is no interaction between drospirenone and spironolactone for hyperkalemia.57 A subsequent prospective study of 27 women treated with combined oral contraceptives containing ethinyl estradiol/drospirenone and spironolactone also did not find any significant elevations in potassium.58 Data from these studies suggest that spironolactone can safely be co-administered with drospirenone-containing combined oral contraceptives.
Reproductive Risks
Despite its utility in treating acne, spironolactone should not be used during pregnancy, and appropriate pregnancy prevention is recommended. Spironolactone crosses the placenta, and some animal studies have shown feminization of male fetuses.59 While human data are limited to a few case reports that did not demonstrate an association of major malformations,60 it generally is recommended to avoid spironolactone during pregnancy. Small studies have found that spironolactone has minimal transfer to breastmilk and is not associated with adverse effects in breastfed infants.61-63 Accordingly, the World Health Organization considers spironolactone to be compatible with breastfeeding.64 Notably, spironolactone may be associated with lactation suppression65,66; therefore, it may be best if lactating patients ensure that their milk production is established prior to starting spironolactone and to increase their water intake to offset the diuretic effects.
Spironolactone also can result in gynecomastia in men and therefore typically is not prescribed for the treatment of acne in this population in oral form10; however, topical antiandrogens such as clascoterone can be used in both women and men with acne.67
Conclusion
Spironolactone is a well-tolerated and effective treatment for women with acne, both in adult and adolescent populations. It is a potentially underutilized alternative to oral antibiotics. Spironolactone also is affordable, fully covered without any requirements in almost 90% of states under Medicaid and with a monthly cost of only $4.00 when obtained through major retailers in the United States, making it an optimal long-term treatment option for many patients.52,68 We recommend a starting dose of 100 mg/d, which can be increased to 150 mg/d to 200 mg/d if needed for better acne control or decreased if adverse effects occur or acne clears. Potassium monitoring is of low usefulness in young healthy women, and studies have not identified an association between spironolactone use and increased risk for cancer.
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS. Temporal trends in the use of systemic medications for acne from 2017 to 2020. JAMA Dermatol. 2023;159:1135-1136. doi:10.1001 /jamadermatol.2023.2363
- Strauss JS, Pochi PE, Downing DT. Acne: perspectives. J Invest Dermatol. 1974;62:321-325. doi:10.1111/1523-1747.ep12724280
- Luderschmidt C, Bidlingmaier F, Plewig G. Inhibition of sebaceous gland activity by spironolactone in Syrian hamster. J Invest Dermatol. 1982;78:253-255. doi:10.1111/1523-1747.ep12506612
- Boisselle A, Dionne FT, Tremblay RR. Interaction of spironolactone with rat skin androgen receptor. Can J Biochem. 1979;57:1042-1046. doi:10.1139/o79-131
- Menard RH, Stripp B, Gillette JR. Spironolactone and testicular cytochrome P-450: decreased testosterone formation in several species and changes in hepatic drug metabolism. Endocrinology. 1974;94:1628-1636. doi:10.1210/endo-94-6-1628
- Rifka SM, Pita JC, Vigersky RA, et al. Interaction of digitalis and spironolactone with human sex steroid receptors. J Clin Endocrinol Metab. 1978;46:338-344. doi:10.1210/jcem-46-2-338
- Corvol P, Michaud A, Menard J, et al. Antiandrogenic effect of spirolactones: mechanism of action. Endocrinology. 1975;97:52-58. doi:10.1210/endo-97-1-52
- Akamatsu H, Zouboulis CC, Orfanos CE. Spironolactone directly inhibits proliferation of cultured human facial sebocytes and acts antagonistically to testosterone and 5 alpha-dihydrotestosterone in vitro. J Invest Dermatol. 1993;100:660-662. doi:10.1111/1523-1747 .ep12472325
- Goodfellow A, Alaghband-Zadeh J, Carter G, et al. Oral spironolactone improves acne vulgaris and reduces sebum excretion. Br J Dermatol. 1984;111:209-214. doi:10.1111/j.1365-2133.1984.tb04045.x
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232. doi:10.1111/j.1365-2133.1986.tb05722.x
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Santer M, Lawrence M, Renz S, et al. Effectiveness of spironolactone for women with acne vulgaris (SAFA) in England and Wales: pragmatic, multicentre, phase 3, double blind, randomised controlled trial. BMJ. 2023;381:E074349. doi:10.1136/bmj-2022-074349
- Shields A, Barbieri JS. Effectiveness of spironolactone for women with acne vulgaris (SAFA) trial: a critically appraised topic. Br J Dermatol. 2023;189:509-510. doi:10.1093/bjd/ljad270
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344. doi:10.1007/s40257-018-00417-3
- Knutsen-Larson S, Dawson AL, Dunnick CA, et al. Acne vulgaris: pathogenesis, treatment, and needs assessment. Dermatol Clin. 2012;30:99-106, viii-ix. doi:10.1016/j.det.2011.09.001
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States, 2021. Accessed May 21, 2025. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/antibiotic-use/data/report-2021.html
- Adler BL, Kornmehl H, Armstrong AW. Antibiotic resistance in acne treatment. JAMA Dermatol. 2017;153:810-811. doi:10.1001 /jamadermatol.2017.1297
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33. doi:10.1016/S1473-3099(15)00527-7
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616. doi:10.1038/ajg.2010.303?
- Margolis DJ, Fanelli M, Kupperman E, et al. Association of pharyngitis with oral antibiotic use for the treatment of acne: a cross-sectional and prospective cohort study. Arch Dermatol. 2012;148:326-332. doi:10.1001 /archdermatol.2011.355
- Bartlett JG, Chang TW, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531-534. doi:10.1056/NEJM197803092981003
- Carroll KC, Bartlett JG. Biology of Clostridium difficile: implications for epidemiology and diagnosis. Annu Rev Microbiol. 2011;65:501-521. doi:10.1146/annurev-micro-090110-102824
- Velicer CM, Heckbert SR, Lampe JW, et al. Antibiotic use in relation to the risk of breast cancer. JAMA. 2004;291:827-835. doi:10.1001/jama.291.7.827
- Song M, Nguyen LH, Emilsson L, et al. Antibiotic use associated with risk of colorectal polyps in a nationwide study. Clin Gastroenterol Hepatol. 2021;19:1426-1435.e6. doi:10.1016/j.cgh.2020.05.036
- Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67:672-678. doi:10.1136 /gutjnl-2016-313413
- Del Rosso JQ, Rosen T, Palceski D, et al. Patient awareness of antimicrobial resistance and antibiotic use in acne vulgaris. J Clin Aesthetic Dermatol. 2019;12:30-41.
- Park JH, Bienenfeld A, Orlow SJ, et al. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol. 2018;19:449-455. doi:10.1007/s40257-018-0349-6
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) Study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Barbieri JS, Ellenberg S, Grice E, et al. Challenges in designing a randomized, double-blind noninferiority trial for treatment of acne: The SDACNE trial. Clin Trials. 2025;22:66-76. doi:10.1177/17407745241265094
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Dréno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106. doi:10.1111/jdv.12757
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2016;152:391-398. doi:10.1001/jamadermatol.2015.4498
- Plante J, Robinson I, Elston D. The need for potassium monitoring in women on spironolactone for dermatologic conditions. J Am Acad Dermatol. 2022;87:1097-1099. doi:10.1016/j.jaad.2022.01.010
- Berman HS, Cheng CE, Hogeling M. Spironolactone in the treatment of adolescent acne: a retrospective review. J Am Acad Dermatol. 2021;85:269-271. doi:10.1016/j.jaad.2020.11.044
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006.e1-1006 .e30. doi:10.1016/j.jaad.2023.12.017
- Basu P. High-dose spironolactone for acne in patients with polycystic ovarian syndrome: a single-institution retrospective study. J Am Acad Dermatol. 2021;85:740-741.
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Yemisci A, Gorgulu A, Piskin S. Effects and side-effects of spironolactone therapy in women with acne. J Eur Acad Dermatol Venereol. 2005;19:163-166. doi:10.1111/j.1468-3083.2005.01072.x
- Patiyasikunt M, Chancheewa B, Asawanonda P, et al. Efficacy and tolerability of low-dose spironolactone and topical benzoyl peroxide in adult female acne: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2020;47:1411-1416. doi:10.1111/1346-8138.15559
- Aldactone (spironolactone) tablets. Prescribing information. Pfizer; 2008. Accessed May 21, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/012151s062lbl.pdf
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001/jamadermatol.2021.5866
- Mackenzie IS, Morant SV, Wei L, et al. Spironolactone use and risk of incident cancers: a retrospective, matched cohort study. Br J Clin Pharmacol. 2017;83:653-663. doi:10.1111/bcp.13152
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875. doi:10.1016/j.canep.2013.10.004
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007/s00403-024-02936-y
- Jick SS, Hernandez RK. Risk of nonfatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: casecontrol study using United States claims data. BMJ. 2011;342:d2151. doi:10.1136/bmj.d2151
- Shields A, Flood K, Barbieri JS. Spironolactone use for acne is not associated with an increased risk of venous thromboembolism: a matched, retrospective cohort study. J Am Acad Dermatol. 2023;88:1396-1397. doi:10.1016/j.jaad.2023.02.028
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Muhn P, Fuhrmann U, Fritzemeier KH, et al. Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity. Ann N Y Acad Sci. 1995;761:311-335. doi:10.1111/j.1749-6632.1995.tb31386.x
- Yaz (drospirenone/ethinyl estradiol) tablets. Prescribing information. Bayer HealthCare Pharmaceuticals; 2012. Accessed May 21, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021676s012lbl.pdf
- Bird ST, Pepe SR, Etminan M, et al. The association between drospirenone and hyperkalemia: a comparative-safety study. BMC Clin Pharmacol. 2011;11:23. doi:10.1186/1472-6904-11-23
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Liszewski W, Boull C. Lack of evidence for feminization of males exposed to spironolactone in utero: a systematic review. J Am Acad Dermatol. 2019;80:1147-1148. doi:10.1016/j.jaad.2018.10.023
- de Jong MFC, Riphagen IJ, Kootstra-Ros JE, et al. Potassium and magnesium in breast milk of a woman with gitelman syndrome. Kidney Int Rep. 2022;7:1720-1721. doi:10.1016/j.ekir.2022.05.006
- Reisman T, Goldstein Z. Case report: induced lactation in a transgender woman. Transgender Health. 2018;3:24-26. doi:10.1089 /trgh.2017.0044
- Phelps DL, Karim A. Spironolactone: relationship between concentrations of dethioacetylated metabolite in human serum and milk. J Pharm Sci. 1977;66:1203. doi:10.1002/jps.2600660841
- World Health Organization. Breastfeeding and maternal medication: recommendations for drugs in the eleventh WHO model list of essential drugs. February 25, 2002. Accessed May 21, 2025. https://www.who.int/publications/i/item/55732
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. Lactation. J Am Acad Dermatol. 2014;70:417.e1-10; quiz 427. doi:10.1016/j.jaad.2013.09.009
- Cominos DC, van der Walt A, van Rooyen AJ. Suppression of postpartum lactation with furosemide. S Afr Med J. 1976;50:251-252.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Ershadi S, Choe J, Barbieri JS. Medicaid formularies for acne treatments are difficult to access and reflect inconsistent coverage policies. J Am Acad Dermatol. 2024;90:1074-1076. doi:10.1016/j.jaad.2024.01.033
Spironolactone is increasingly used off label for acne treatment and is now being prescribed for women with acne at a frequency similar to oral antibiotics.1,2 In this article, we provide an overview of spironolactone use for acne treatment and discuss recent clinical trials and practical strategies for patient selection, dosing, adverse effect management, and monitoring (Table).

History and Mechanism of Action
Because sebaceous gland activity is an important component of acne pathogenesis and is regulated by androgens,3 there has long been interest in identifying treatment strategies that can target the role of hormones in activating the sebaceous gland. In the 1980s, it became apparent that spironolactone, originally developed as a potassium-sparing diuretic, also might possess antiandrogenic properties that could be useful in the treatment of acne.4 Spironolactone has been found to decrease testosterone production, inhibit testosterone and dihydrotestosterone binding to androgen receptors,5-8 and block 5α-reductase receptors of the sebaceous glands of skin.9
In 1984, Goodfellow et al10 conducted a trial in which 36 male and female patients with severe acne were randomized to placebo or spironolactone doses ranging from 50 to 200 mg/d. They found that spironolactone resulted in dose-dependent reductions of sebum production as well as improvement in patient- and clinician-reported assessments of acne. In 1986, another placebo-controlled crossover trial by Muhlemann et al11 provided further support for the effectiveness of spironolactone for acne. This trial randomized 21 women to placebo or spironolactone 200 mg/d and found that spironolactone was associated with statistically significant (P<.001) improvements in acne lesion counts.
Recent Observational Studies and Trials
Following these early trials, several large case series have been published describing the successful use of spironolactone for acne, including a 2020 retrospective case series from the Mayo Clinic describing 395 patients.12 The investigators found that almost 66% of patients had a complete response and almost 85% had a complete response or a partial response greater than 50%. They also found that the median time to initial response and maximal response were 3 and 5 months, respectively, and that efficacy was observed across acne subtypes, including for nodulocystic acne.12 In addition, a 2021 case series describing 403 patients treated with spironolactone found that approximately 80% had reduction or complete clearance of acne, with improvements observed for both facial and truncal acne. In this cohort, doses of 100 to 150 mg/d typically were the most successful.13 A case series of 80 adolescent females also highlighted the efficacy of spironolactone in younger populations.14
Adding to these observational data, the multicenter, phase 3, double-blind Spironolactone for Adult Female Acne (SAFA) trial included 410 women (mean age, 29.2 years) who were randomized to receive either placebo or intervention (spironolactone 50 mg/d until week 6 and 100 mg/d until week 24).15 At 24 weeks, greater improvement in quality of life and participant self-assessed improvement were observed in the spironolactone group. In addition, at 12 weeks, rates of success were higher in the spironolactone group using the Investigator Global Assessment score (adjusted odds ratio 5.18 [95% CI, 2.18- 12.28]). Those randomized to receive spironolactone also had lower rates of oral antibiotic use at 52 weeks than the placebo group did (5.8% vs 13.5%, respectively).
In the SAFA trial, spironolactone was well tolerated; the most common adverse effects relative to placebo were lightheadedness (19% for spironolactone vs 12% for placebo) and headache (20% for spironolactone vs 12% for placebo). Notably, more than 95% of patients were able to increase from 50 mg/d to 100 mg/d at week 6, with greater than 90% tolerating 100 mg/d. As observational data suggest that spironolactone takes 3 to 5 months to reach peak efficacy, these findings provide further support that starting at a dose of at least 100 mg/d is likely optimal for most patients.16
A Potential Alternative to Oral Antibiotics
Oral antibiotics such as tetracyclines have long played a central role in the treatment of acne and remain a first-line treatment option.17 In addition, many of these antibiotic courses exceed 6 months in duration.1 In fact, dermatologists prescribe more antibiotics per capita than any other specialty1,18-20; however, this can be associated with the development of antibiotic resistance,21,22 as well as other antibiotic-associated complications, including inflammatory bowel disease,23 pharyngitis,24 Clostridium difficile infections, and cancer.25-29
In addition to these concerns, many patients may prefer nonantibiotic alternatives to oral antibiotics, with more than 75% preferring a nonantibiotic option if available. For female patients with acne, antiandrogens such as spironolactone have been suggested as a potential alternative.30 A 10-year retrospective study of female patients with acne found that those who had ever received hormonal therapy (ie, spironolactone or a combined oral contraceptive) received fewer cumulative days of oral antibiotics than those who did not (226 days vs 302 days, respectively).31 In addition, while oral antibiotics were the most common initial therapy prescribed for patients, as they progressed through their treatment course, more patients ended up on hormonal therapy than oral antibiotics. This study suggests that hormonal therapy such as spironolactone could represent an alternative to the use of systemic antibiotics.31
Further supporting the role of spironolactone as an alternative to oral antibiotics, a 2018 analysis of claims data found that spironolactone may have similar effectiveness to oral antibiotics for the treatment of acne.32 After adjusting for age and topical retinoid and oral contraceptive use, this study found that there was no significant difference in the odds of being prescribed a different systemic treatment within 1 year (ie, treatment failure) among those starting spironolactone vs those starting oral tetracycline-class antibiotics as their initial therapy for acne.
A multicenter, randomized, double-blind trial (Female Acne Spironolactone vs doxyCycline Efficacy [FASCE]) also evaluated the comparative effectiveness of doxycycline 100 mg/d for 3 months followed by an oral placebo for 3 months vs spironolactone 150 mg/d for 6 months among 133 adult women with acne. This study found that spironolactone had statistically significantly greater rates of Investigator Global Assessment treatment success after 6 months (odds ratio 2.87 [95% CI, 1.38-5.99; P=.007]).33 Since spironolactone historically has been prescribed less often than oral antibiotics for women with acne, these findings support spironolactone as an underutilized treatment alternative. The ongoing Spironolactone versus Doxycycline for Acne: A Comparative Effectiveness, Noninferiority Evaluation trial—a 16-week, blinded trial comparing 100 mg/d doses of both drugs—should provide additional evidence regarding the relative role of spironolactone and oral antibiotics in the management of acne.34
Ultimately, the decision to use spironolactone or other treatments such as oral antibiotics should be based on shared decision making between clinician and patient. Spironolactone has a relatively slow onset of efficacy, and other options such as oral antibiotics might be preferred by those looking for more immediate results; however, as women with acne often have activity that persists into adulthood, spironolactone might be preferable as a long-term maintenance therapy to avoid complications of prolonged antibiotic use.35 Comorbidities also will influence the optimal choice of therapy (eg, spironolactone might be preferred in someone with inflammatory bowel disease, and oral antibiotics might be preferred in someone with orthostatic hypotension).
Patient Selection
Acne occurring along the lower face or jawline in adult women sometimes is referred to as hormonal acne, but this dogma is not particularly evidence based. An observational study of 374 patients found that almost 90% of adult women had acne involving multiple facial zones with a spectrum of facial acne severity similar to that in adolescents.36 Only a small subset of these patients (11.2%) had acne localized solely to the mandibular area. In addition, acne along the lower face is not predictive of hyperandrogenism (eg, polycystic ovary syndrome).37 Antiandrogen therapies such as spironolactone and clascoterone are effective in both men and women with acne10,38 and in adolescents and adults, suggesting that hormones play a fundamental role in all acne and that addressing this mechanism can be useful broadly. Therefore, hormonal therapies such as spironolactone should not be restricted to only adult women with acne along the lower face.
While spironolactone can be effective for acne treatment in any age group, it may be most effective for adult women with acne. In the SAFA trial, prespecified subgroup analyses showed a statistically significant (P=.005) interaction term for age (categorized as <25 years and ≥25 years), which suggested that spironolactone might be a more effective treatment for women 25 years and older.15 In addition, subgroup analyses in the aforementioned 2018 analysis of claims data found that spironolactone was more effective relative to oral antibiotics in adults vs adolescents.32 Despite these limitations, several case series have highlighted that spironolactone is effective among adolescent populations with acne. A case series of spironolactone use in 73 patients aged 19 years or younger found that 68% of patients demonstrated resolution or improvement in their acne after spironolactone treatment.39 Another case series among 80 adolescent females reported 80% of patients experiencing improvement of their acne.14
For those with more severe acne, spironolactone can be combined with other complementary treatment approaches such as topicals, oral antibiotics, or procedural modalities.40
Dosing
We recommend starting spironolactone at a dose of 100 mg/d (the patient can take 50 mg/d for 1 week, then increase to 100 mg/d if there are no adverse effects at the lower dose). In the 1984 trial by Goodfellow et al,10 participants were randomized to doses of 50 mg/d, 100 mg/d, 150 mg/d, and 200 mg/d. In this trial, efficacy assessed by objective and subjective outcomes did not plateau until doses of 100 mg/d to 150 mg/d. In addition, a case series of 403 patients found that the most successful dosage of spironolactone generally was 100 mg/d or higher.13 Most of the patients who were started at this dosage either stayed at this level or escalated, whereas patients who started at lower dosages (25-75 mg/d) frequently increased their dosage over time. The SAFA trial also highlighted that most patients can tolerate a spironolactone dose of 100 mg/d.15 For specific populations, such as patients with polycystic ovary syndrome, a higher dose (mean dosage of 143 mg/d) may be required for efficacy.41 Given the slow onset of efficacy, typically taking 3 to 5 months, and the low rate of adverse effects, we believe the optimal starting dose is 100 mg/s to 150 mg/d. If adverse effects occur or lesions clear, then the dosage may be reduced.
Adverse Effects
Spironolactone generally is well tolerated; in the SAFA and FASCE trials, fewer than 1% of participants discontinued due to adverse effects.15,33 Rates of discontinuation due to adverse effects typically have been less than 5% in case series of patients treated in routine clinical practice.12-14
Because spironolactone is a diuretic and antihypertensive, the most common adverse effects are related to these characteristics. In the SAFA trial, dizziness, lightheadedness, and vertigo were reported more commonly in the spironolactone group than in the placebo group (19% vs 12%, respectively). Similarly, headaches also were reported more frequently in the spironolactone group than in the placebo group (20% vs 12%, respectively).15 One case series found that, among the 267 patients on spironolactone whose blood pressure was monitored, the mean reduction in systolic blood pressure was 3.5 mm Hg and the mean reduction in diastolic blood pressure was 0.9 mm Hg.13 For those with baseline orthostasis or in those who experience adverse effects related to hypotension, reducing the dose often can be helpful. Of note, while doses of 100 mg/d to 150 mg/d often are the most effective, randomized trials have found that spironolactone still can be effective for acne at doses as low as 25 mg/d to 50 mg/d.10,38
Menstrual irregularities are another commonly cited adverse effect of spironolactone. While a systematic review found that 15% to 30% of patients treated with spironolactone experience menstrual irregularities, it has been difficult to evaluate whether this is due to the medication or other comorbidities, such as polycystic ovary syndrome.42 Notably, in the SAFA trial, rates of menstrual irregularities were equivalent between the spironolactone and placebo groups at a dose of 100 mg/d (32% vs 35%, respectively).15 In contrast, in the FASCE trial, menstrual irregularities were more commonly reported at a dose of 150 mg/d.33 These findings are consistent with observational data suggesting that menstrual irregularities are much more common at spironolactone doses greater than 100 mg/d.42 Additionally, some evidence supports that for some patients these menstrual irregularities may resolve within 2 to 3 months of continued treatment.43 It has been noted in several studies that menstrual irregularities are less likely to occur in patients who are using combined oral contraceptives; therefore, for patients who are amenable and have no contraindications, combined oral contraceptives can be considered to prevent or address menstrual irregularities.13,42,44
More generally, combined oral contraceptives can be an excellent combination with spironolactone, as they have complementary characteristics. Spironolactone primarily blocks the effects of androgens, while combined oral contraceptives predominantly block the production of androgens. Whereas spironolactone typically causes hypotension and menstrual irregularities, combined oral contraceptives cause hypertension and help to regulate the menstrual cycle.
Spironolactone carries an official US Food and Drug Administration warning regarding possible tumorigenicity that is based on animal studies that used up to 150 times the normal dose of spironolactone used in humans45; however, observational studies in humans have not identified such an association when spironolactone is used in normal clinical settings. A systematic review and metanalysis in 2022 reviewed data from a total population of more than 4 million individuals and found that there was no statistically significant association between spironolactone use and the risk for breast, ovarian, bladder, kidney, gastric, or esophageal cancers.46 Additional studies also found no association between spironolactone use and cancers.48 A more recent cohort study specifically among patients treated with spironolactone for acne also found no significant increased risk for breast cancer.49
Combined oral contraceptives are associated with an increased risk for venous thromboembolisms, and there have been concerns that this risk may be greater in combined oral contraceptives that contain drospirenone.50 Drospirenone is molecularly related to spironolactone, which has prompted the consideration of whether spironolactone use also conveys a risk for venous thromboembolism. Reassuringly, a retrospective study of claims data found that individuals on spironolactone were not more likely to develop a pulmonary embolism or a deep venous thrombosis than matched controls treated with tetracycline antibiotics, with a point estimate favoring decreased risk.51
Monitoring
Given that one of spironolactone’s mechanisms of action is aldosterone antagonism and thus the inhibition of potassium excretion, there have been concerns regarding risk for hyperkalemia. A retrospective study analyzing data from 2000 to 2014 found that, among 974 young women receiving spironolactone therapy, the rate of hyperkalemia was 0.72%, which is equivalent to the 0.76% baseline rate of hyperkalemia in the same population.52 Subsequent studies also have found that spironolactone does not appear to be associated with a meaningful risk for hyperkalemia among young healthy patients treated for acne.38,53 These studies suggest that routine potassium monitoring is of low usefulness for healthy young women taking spironolactone for acne. The 2024 American Academy of Dermatology guidelines on the management of acne also state that potassium monitoring is not needed in healthy patients but that potassium testing should be considered for those with risk factors for hyperkalemia (eg, older age, medical comorbidities, medications).40 Clinicians should still engage in shared decision making with patients to determine whether to check potassium. If potassium is to be monitored, it should be checked 1 to 2 weeks after spironolactone is started.45,54
Since drospirenone also has aldosterone antagonistic properties,55 there have been concerns about whether concomitant use of spironolactone and drospirenone-containing combined oral contraceptives might increase the risk for hyperkalemia.56 However, a retrospective cohort study analyzing data from more than 1 million women found that drospirenone is not any more likely than levonorgestrel to cause hyperkalemia and that there is no interaction between drospirenone and spironolactone for hyperkalemia.57 A subsequent prospective study of 27 women treated with combined oral contraceptives containing ethinyl estradiol/drospirenone and spironolactone also did not find any significant elevations in potassium.58 Data from these studies suggest that spironolactone can safely be co-administered with drospirenone-containing combined oral contraceptives.
Reproductive Risks
Despite its utility in treating acne, spironolactone should not be used during pregnancy, and appropriate pregnancy prevention is recommended. Spironolactone crosses the placenta, and some animal studies have shown feminization of male fetuses.59 While human data are limited to a few case reports that did not demonstrate an association of major malformations,60 it generally is recommended to avoid spironolactone during pregnancy. Small studies have found that spironolactone has minimal transfer to breastmilk and is not associated with adverse effects in breastfed infants.61-63 Accordingly, the World Health Organization considers spironolactone to be compatible with breastfeeding.64 Notably, spironolactone may be associated with lactation suppression65,66; therefore, it may be best if lactating patients ensure that their milk production is established prior to starting spironolactone and to increase their water intake to offset the diuretic effects.
Spironolactone also can result in gynecomastia in men and therefore typically is not prescribed for the treatment of acne in this population in oral form10; however, topical antiandrogens such as clascoterone can be used in both women and men with acne.67
Conclusion
Spironolactone is a well-tolerated and effective treatment for women with acne, both in adult and adolescent populations. It is a potentially underutilized alternative to oral antibiotics. Spironolactone also is affordable, fully covered without any requirements in almost 90% of states under Medicaid and with a monthly cost of only $4.00 when obtained through major retailers in the United States, making it an optimal long-term treatment option for many patients.52,68 We recommend a starting dose of 100 mg/d, which can be increased to 150 mg/d to 200 mg/d if needed for better acne control or decreased if adverse effects occur or acne clears. Potassium monitoring is of low usefulness in young healthy women, and studies have not identified an association between spironolactone use and increased risk for cancer.
Spironolactone is increasingly used off label for acne treatment and is now being prescribed for women with acne at a frequency similar to oral antibiotics.1,2 In this article, we provide an overview of spironolactone use for acne treatment and discuss recent clinical trials and practical strategies for patient selection, dosing, adverse effect management, and monitoring (Table).

History and Mechanism of Action
Because sebaceous gland activity is an important component of acne pathogenesis and is regulated by androgens,3 there has long been interest in identifying treatment strategies that can target the role of hormones in activating the sebaceous gland. In the 1980s, it became apparent that spironolactone, originally developed as a potassium-sparing diuretic, also might possess antiandrogenic properties that could be useful in the treatment of acne.4 Spironolactone has been found to decrease testosterone production, inhibit testosterone and dihydrotestosterone binding to androgen receptors,5-8 and block 5α-reductase receptors of the sebaceous glands of skin.9
In 1984, Goodfellow et al10 conducted a trial in which 36 male and female patients with severe acne were randomized to placebo or spironolactone doses ranging from 50 to 200 mg/d. They found that spironolactone resulted in dose-dependent reductions of sebum production as well as improvement in patient- and clinician-reported assessments of acne. In 1986, another placebo-controlled crossover trial by Muhlemann et al11 provided further support for the effectiveness of spironolactone for acne. This trial randomized 21 women to placebo or spironolactone 200 mg/d and found that spironolactone was associated with statistically significant (P<.001) improvements in acne lesion counts.
Recent Observational Studies and Trials
Following these early trials, several large case series have been published describing the successful use of spironolactone for acne, including a 2020 retrospective case series from the Mayo Clinic describing 395 patients.12 The investigators found that almost 66% of patients had a complete response and almost 85% had a complete response or a partial response greater than 50%. They also found that the median time to initial response and maximal response were 3 and 5 months, respectively, and that efficacy was observed across acne subtypes, including for nodulocystic acne.12 In addition, a 2021 case series describing 403 patients treated with spironolactone found that approximately 80% had reduction or complete clearance of acne, with improvements observed for both facial and truncal acne. In this cohort, doses of 100 to 150 mg/d typically were the most successful.13 A case series of 80 adolescent females also highlighted the efficacy of spironolactone in younger populations.14
Adding to these observational data, the multicenter, phase 3, double-blind Spironolactone for Adult Female Acne (SAFA) trial included 410 women (mean age, 29.2 years) who were randomized to receive either placebo or intervention (spironolactone 50 mg/d until week 6 and 100 mg/d until week 24).15 At 24 weeks, greater improvement in quality of life and participant self-assessed improvement were observed in the spironolactone group. In addition, at 12 weeks, rates of success were higher in the spironolactone group using the Investigator Global Assessment score (adjusted odds ratio 5.18 [95% CI, 2.18- 12.28]). Those randomized to receive spironolactone also had lower rates of oral antibiotic use at 52 weeks than the placebo group did (5.8% vs 13.5%, respectively).
In the SAFA trial, spironolactone was well tolerated; the most common adverse effects relative to placebo were lightheadedness (19% for spironolactone vs 12% for placebo) and headache (20% for spironolactone vs 12% for placebo). Notably, more than 95% of patients were able to increase from 50 mg/d to 100 mg/d at week 6, with greater than 90% tolerating 100 mg/d. As observational data suggest that spironolactone takes 3 to 5 months to reach peak efficacy, these findings provide further support that starting at a dose of at least 100 mg/d is likely optimal for most patients.16
A Potential Alternative to Oral Antibiotics
Oral antibiotics such as tetracyclines have long played a central role in the treatment of acne and remain a first-line treatment option.17 In addition, many of these antibiotic courses exceed 6 months in duration.1 In fact, dermatologists prescribe more antibiotics per capita than any other specialty1,18-20; however, this can be associated with the development of antibiotic resistance,21,22 as well as other antibiotic-associated complications, including inflammatory bowel disease,23 pharyngitis,24 Clostridium difficile infections, and cancer.25-29
In addition to these concerns, many patients may prefer nonantibiotic alternatives to oral antibiotics, with more than 75% preferring a nonantibiotic option if available. For female patients with acne, antiandrogens such as spironolactone have been suggested as a potential alternative.30 A 10-year retrospective study of female patients with acne found that those who had ever received hormonal therapy (ie, spironolactone or a combined oral contraceptive) received fewer cumulative days of oral antibiotics than those who did not (226 days vs 302 days, respectively).31 In addition, while oral antibiotics were the most common initial therapy prescribed for patients, as they progressed through their treatment course, more patients ended up on hormonal therapy than oral antibiotics. This study suggests that hormonal therapy such as spironolactone could represent an alternative to the use of systemic antibiotics.31
Further supporting the role of spironolactone as an alternative to oral antibiotics, a 2018 analysis of claims data found that spironolactone may have similar effectiveness to oral antibiotics for the treatment of acne.32 After adjusting for age and topical retinoid and oral contraceptive use, this study found that there was no significant difference in the odds of being prescribed a different systemic treatment within 1 year (ie, treatment failure) among those starting spironolactone vs those starting oral tetracycline-class antibiotics as their initial therapy for acne.
A multicenter, randomized, double-blind trial (Female Acne Spironolactone vs doxyCycline Efficacy [FASCE]) also evaluated the comparative effectiveness of doxycycline 100 mg/d for 3 months followed by an oral placebo for 3 months vs spironolactone 150 mg/d for 6 months among 133 adult women with acne. This study found that spironolactone had statistically significantly greater rates of Investigator Global Assessment treatment success after 6 months (odds ratio 2.87 [95% CI, 1.38-5.99; P=.007]).33 Since spironolactone historically has been prescribed less often than oral antibiotics for women with acne, these findings support spironolactone as an underutilized treatment alternative. The ongoing Spironolactone versus Doxycycline for Acne: A Comparative Effectiveness, Noninferiority Evaluation trial—a 16-week, blinded trial comparing 100 mg/d doses of both drugs—should provide additional evidence regarding the relative role of spironolactone and oral antibiotics in the management of acne.34
Ultimately, the decision to use spironolactone or other treatments such as oral antibiotics should be based on shared decision making between clinician and patient. Spironolactone has a relatively slow onset of efficacy, and other options such as oral antibiotics might be preferred by those looking for more immediate results; however, as women with acne often have activity that persists into adulthood, spironolactone might be preferable as a long-term maintenance therapy to avoid complications of prolonged antibiotic use.35 Comorbidities also will influence the optimal choice of therapy (eg, spironolactone might be preferred in someone with inflammatory bowel disease, and oral antibiotics might be preferred in someone with orthostatic hypotension).
Patient Selection
Acne occurring along the lower face or jawline in adult women sometimes is referred to as hormonal acne, but this dogma is not particularly evidence based. An observational study of 374 patients found that almost 90% of adult women had acne involving multiple facial zones with a spectrum of facial acne severity similar to that in adolescents.36 Only a small subset of these patients (11.2%) had acne localized solely to the mandibular area. In addition, acne along the lower face is not predictive of hyperandrogenism (eg, polycystic ovary syndrome).37 Antiandrogen therapies such as spironolactone and clascoterone are effective in both men and women with acne10,38 and in adolescents and adults, suggesting that hormones play a fundamental role in all acne and that addressing this mechanism can be useful broadly. Therefore, hormonal therapies such as spironolactone should not be restricted to only adult women with acne along the lower face.
While spironolactone can be effective for acne treatment in any age group, it may be most effective for adult women with acne. In the SAFA trial, prespecified subgroup analyses showed a statistically significant (P=.005) interaction term for age (categorized as <25 years and ≥25 years), which suggested that spironolactone might be a more effective treatment for women 25 years and older.15 In addition, subgroup analyses in the aforementioned 2018 analysis of claims data found that spironolactone was more effective relative to oral antibiotics in adults vs adolescents.32 Despite these limitations, several case series have highlighted that spironolactone is effective among adolescent populations with acne. A case series of spironolactone use in 73 patients aged 19 years or younger found that 68% of patients demonstrated resolution or improvement in their acne after spironolactone treatment.39 Another case series among 80 adolescent females reported 80% of patients experiencing improvement of their acne.14
For those with more severe acne, spironolactone can be combined with other complementary treatment approaches such as topicals, oral antibiotics, or procedural modalities.40
Dosing
We recommend starting spironolactone at a dose of 100 mg/d (the patient can take 50 mg/d for 1 week, then increase to 100 mg/d if there are no adverse effects at the lower dose). In the 1984 trial by Goodfellow et al,10 participants were randomized to doses of 50 mg/d, 100 mg/d, 150 mg/d, and 200 mg/d. In this trial, efficacy assessed by objective and subjective outcomes did not plateau until doses of 100 mg/d to 150 mg/d. In addition, a case series of 403 patients found that the most successful dosage of spironolactone generally was 100 mg/d or higher.13 Most of the patients who were started at this dosage either stayed at this level or escalated, whereas patients who started at lower dosages (25-75 mg/d) frequently increased their dosage over time. The SAFA trial also highlighted that most patients can tolerate a spironolactone dose of 100 mg/d.15 For specific populations, such as patients with polycystic ovary syndrome, a higher dose (mean dosage of 143 mg/d) may be required for efficacy.41 Given the slow onset of efficacy, typically taking 3 to 5 months, and the low rate of adverse effects, we believe the optimal starting dose is 100 mg/s to 150 mg/d. If adverse effects occur or lesions clear, then the dosage may be reduced.
Adverse Effects
Spironolactone generally is well tolerated; in the SAFA and FASCE trials, fewer than 1% of participants discontinued due to adverse effects.15,33 Rates of discontinuation due to adverse effects typically have been less than 5% in case series of patients treated in routine clinical practice.12-14
Because spironolactone is a diuretic and antihypertensive, the most common adverse effects are related to these characteristics. In the SAFA trial, dizziness, lightheadedness, and vertigo were reported more commonly in the spironolactone group than in the placebo group (19% vs 12%, respectively). Similarly, headaches also were reported more frequently in the spironolactone group than in the placebo group (20% vs 12%, respectively).15 One case series found that, among the 267 patients on spironolactone whose blood pressure was monitored, the mean reduction in systolic blood pressure was 3.5 mm Hg and the mean reduction in diastolic blood pressure was 0.9 mm Hg.13 For those with baseline orthostasis or in those who experience adverse effects related to hypotension, reducing the dose often can be helpful. Of note, while doses of 100 mg/d to 150 mg/d often are the most effective, randomized trials have found that spironolactone still can be effective for acne at doses as low as 25 mg/d to 50 mg/d.10,38
Menstrual irregularities are another commonly cited adverse effect of spironolactone. While a systematic review found that 15% to 30% of patients treated with spironolactone experience menstrual irregularities, it has been difficult to evaluate whether this is due to the medication or other comorbidities, such as polycystic ovary syndrome.42 Notably, in the SAFA trial, rates of menstrual irregularities were equivalent between the spironolactone and placebo groups at a dose of 100 mg/d (32% vs 35%, respectively).15 In contrast, in the FASCE trial, menstrual irregularities were more commonly reported at a dose of 150 mg/d.33 These findings are consistent with observational data suggesting that menstrual irregularities are much more common at spironolactone doses greater than 100 mg/d.42 Additionally, some evidence supports that for some patients these menstrual irregularities may resolve within 2 to 3 months of continued treatment.43 It has been noted in several studies that menstrual irregularities are less likely to occur in patients who are using combined oral contraceptives; therefore, for patients who are amenable and have no contraindications, combined oral contraceptives can be considered to prevent or address menstrual irregularities.13,42,44
More generally, combined oral contraceptives can be an excellent combination with spironolactone, as they have complementary characteristics. Spironolactone primarily blocks the effects of androgens, while combined oral contraceptives predominantly block the production of androgens. Whereas spironolactone typically causes hypotension and menstrual irregularities, combined oral contraceptives cause hypertension and help to regulate the menstrual cycle.
Spironolactone carries an official US Food and Drug Administration warning regarding possible tumorigenicity that is based on animal studies that used up to 150 times the normal dose of spironolactone used in humans45; however, observational studies in humans have not identified such an association when spironolactone is used in normal clinical settings. A systematic review and metanalysis in 2022 reviewed data from a total population of more than 4 million individuals and found that there was no statistically significant association between spironolactone use and the risk for breast, ovarian, bladder, kidney, gastric, or esophageal cancers.46 Additional studies also found no association between spironolactone use and cancers.48 A more recent cohort study specifically among patients treated with spironolactone for acne also found no significant increased risk for breast cancer.49
Combined oral contraceptives are associated with an increased risk for venous thromboembolisms, and there have been concerns that this risk may be greater in combined oral contraceptives that contain drospirenone.50 Drospirenone is molecularly related to spironolactone, which has prompted the consideration of whether spironolactone use also conveys a risk for venous thromboembolism. Reassuringly, a retrospective study of claims data found that individuals on spironolactone were not more likely to develop a pulmonary embolism or a deep venous thrombosis than matched controls treated with tetracycline antibiotics, with a point estimate favoring decreased risk.51
Monitoring
Given that one of spironolactone’s mechanisms of action is aldosterone antagonism and thus the inhibition of potassium excretion, there have been concerns regarding risk for hyperkalemia. A retrospective study analyzing data from 2000 to 2014 found that, among 974 young women receiving spironolactone therapy, the rate of hyperkalemia was 0.72%, which is equivalent to the 0.76% baseline rate of hyperkalemia in the same population.52 Subsequent studies also have found that spironolactone does not appear to be associated with a meaningful risk for hyperkalemia among young healthy patients treated for acne.38,53 These studies suggest that routine potassium monitoring is of low usefulness for healthy young women taking spironolactone for acne. The 2024 American Academy of Dermatology guidelines on the management of acne also state that potassium monitoring is not needed in healthy patients but that potassium testing should be considered for those with risk factors for hyperkalemia (eg, older age, medical comorbidities, medications).40 Clinicians should still engage in shared decision making with patients to determine whether to check potassium. If potassium is to be monitored, it should be checked 1 to 2 weeks after spironolactone is started.45,54
Since drospirenone also has aldosterone antagonistic properties,55 there have been concerns about whether concomitant use of spironolactone and drospirenone-containing combined oral contraceptives might increase the risk for hyperkalemia.56 However, a retrospective cohort study analyzing data from more than 1 million women found that drospirenone is not any more likely than levonorgestrel to cause hyperkalemia and that there is no interaction between drospirenone and spironolactone for hyperkalemia.57 A subsequent prospective study of 27 women treated with combined oral contraceptives containing ethinyl estradiol/drospirenone and spironolactone also did not find any significant elevations in potassium.58 Data from these studies suggest that spironolactone can safely be co-administered with drospirenone-containing combined oral contraceptives.
Reproductive Risks
Despite its utility in treating acne, spironolactone should not be used during pregnancy, and appropriate pregnancy prevention is recommended. Spironolactone crosses the placenta, and some animal studies have shown feminization of male fetuses.59 While human data are limited to a few case reports that did not demonstrate an association of major malformations,60 it generally is recommended to avoid spironolactone during pregnancy. Small studies have found that spironolactone has minimal transfer to breastmilk and is not associated with adverse effects in breastfed infants.61-63 Accordingly, the World Health Organization considers spironolactone to be compatible with breastfeeding.64 Notably, spironolactone may be associated with lactation suppression65,66; therefore, it may be best if lactating patients ensure that their milk production is established prior to starting spironolactone and to increase their water intake to offset the diuretic effects.
Spironolactone also can result in gynecomastia in men and therefore typically is not prescribed for the treatment of acne in this population in oral form10; however, topical antiandrogens such as clascoterone can be used in both women and men with acne.67
Conclusion
Spironolactone is a well-tolerated and effective treatment for women with acne, both in adult and adolescent populations. It is a potentially underutilized alternative to oral antibiotics. Spironolactone also is affordable, fully covered without any requirements in almost 90% of states under Medicaid and with a monthly cost of only $4.00 when obtained through major retailers in the United States, making it an optimal long-term treatment option for many patients.52,68 We recommend a starting dose of 100 mg/d, which can be increased to 150 mg/d to 200 mg/d if needed for better acne control or decreased if adverse effects occur or acne clears. Potassium monitoring is of low usefulness in young healthy women, and studies have not identified an association between spironolactone use and increased risk for cancer.
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS. Temporal trends in the use of systemic medications for acne from 2017 to 2020. JAMA Dermatol. 2023;159:1135-1136. doi:10.1001 /jamadermatol.2023.2363
- Strauss JS, Pochi PE, Downing DT. Acne: perspectives. J Invest Dermatol. 1974;62:321-325. doi:10.1111/1523-1747.ep12724280
- Luderschmidt C, Bidlingmaier F, Plewig G. Inhibition of sebaceous gland activity by spironolactone in Syrian hamster. J Invest Dermatol. 1982;78:253-255. doi:10.1111/1523-1747.ep12506612
- Boisselle A, Dionne FT, Tremblay RR. Interaction of spironolactone with rat skin androgen receptor. Can J Biochem. 1979;57:1042-1046. doi:10.1139/o79-131
- Menard RH, Stripp B, Gillette JR. Spironolactone and testicular cytochrome P-450: decreased testosterone formation in several species and changes in hepatic drug metabolism. Endocrinology. 1974;94:1628-1636. doi:10.1210/endo-94-6-1628
- Rifka SM, Pita JC, Vigersky RA, et al. Interaction of digitalis and spironolactone with human sex steroid receptors. J Clin Endocrinol Metab. 1978;46:338-344. doi:10.1210/jcem-46-2-338
- Corvol P, Michaud A, Menard J, et al. Antiandrogenic effect of spirolactones: mechanism of action. Endocrinology. 1975;97:52-58. doi:10.1210/endo-97-1-52
- Akamatsu H, Zouboulis CC, Orfanos CE. Spironolactone directly inhibits proliferation of cultured human facial sebocytes and acts antagonistically to testosterone and 5 alpha-dihydrotestosterone in vitro. J Invest Dermatol. 1993;100:660-662. doi:10.1111/1523-1747 .ep12472325
- Goodfellow A, Alaghband-Zadeh J, Carter G, et al. Oral spironolactone improves acne vulgaris and reduces sebum excretion. Br J Dermatol. 1984;111:209-214. doi:10.1111/j.1365-2133.1984.tb04045.x
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232. doi:10.1111/j.1365-2133.1986.tb05722.x
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Santer M, Lawrence M, Renz S, et al. Effectiveness of spironolactone for women with acne vulgaris (SAFA) in England and Wales: pragmatic, multicentre, phase 3, double blind, randomised controlled trial. BMJ. 2023;381:E074349. doi:10.1136/bmj-2022-074349
- Shields A, Barbieri JS. Effectiveness of spironolactone for women with acne vulgaris (SAFA) trial: a critically appraised topic. Br J Dermatol. 2023;189:509-510. doi:10.1093/bjd/ljad270
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344. doi:10.1007/s40257-018-00417-3
- Knutsen-Larson S, Dawson AL, Dunnick CA, et al. Acne vulgaris: pathogenesis, treatment, and needs assessment. Dermatol Clin. 2012;30:99-106, viii-ix. doi:10.1016/j.det.2011.09.001
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States, 2021. Accessed May 21, 2025. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/antibiotic-use/data/report-2021.html
- Adler BL, Kornmehl H, Armstrong AW. Antibiotic resistance in acne treatment. JAMA Dermatol. 2017;153:810-811. doi:10.1001 /jamadermatol.2017.1297
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33. doi:10.1016/S1473-3099(15)00527-7
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616. doi:10.1038/ajg.2010.303?
- Margolis DJ, Fanelli M, Kupperman E, et al. Association of pharyngitis with oral antibiotic use for the treatment of acne: a cross-sectional and prospective cohort study. Arch Dermatol. 2012;148:326-332. doi:10.1001 /archdermatol.2011.355
- Bartlett JG, Chang TW, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531-534. doi:10.1056/NEJM197803092981003
- Carroll KC, Bartlett JG. Biology of Clostridium difficile: implications for epidemiology and diagnosis. Annu Rev Microbiol. 2011;65:501-521. doi:10.1146/annurev-micro-090110-102824
- Velicer CM, Heckbert SR, Lampe JW, et al. Antibiotic use in relation to the risk of breast cancer. JAMA. 2004;291:827-835. doi:10.1001/jama.291.7.827
- Song M, Nguyen LH, Emilsson L, et al. Antibiotic use associated with risk of colorectal polyps in a nationwide study. Clin Gastroenterol Hepatol. 2021;19:1426-1435.e6. doi:10.1016/j.cgh.2020.05.036
- Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67:672-678. doi:10.1136 /gutjnl-2016-313413
- Del Rosso JQ, Rosen T, Palceski D, et al. Patient awareness of antimicrobial resistance and antibiotic use in acne vulgaris. J Clin Aesthetic Dermatol. 2019;12:30-41.
- Park JH, Bienenfeld A, Orlow SJ, et al. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol. 2018;19:449-455. doi:10.1007/s40257-018-0349-6
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) Study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Barbieri JS, Ellenberg S, Grice E, et al. Challenges in designing a randomized, double-blind noninferiority trial for treatment of acne: The SDACNE trial. Clin Trials. 2025;22:66-76. doi:10.1177/17407745241265094
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Dréno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106. doi:10.1111/jdv.12757
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2016;152:391-398. doi:10.1001/jamadermatol.2015.4498
- Plante J, Robinson I, Elston D. The need for potassium monitoring in women on spironolactone for dermatologic conditions. J Am Acad Dermatol. 2022;87:1097-1099. doi:10.1016/j.jaad.2022.01.010
- Berman HS, Cheng CE, Hogeling M. Spironolactone in the treatment of adolescent acne: a retrospective review. J Am Acad Dermatol. 2021;85:269-271. doi:10.1016/j.jaad.2020.11.044
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006.e1-1006 .e30. doi:10.1016/j.jaad.2023.12.017
- Basu P. High-dose spironolactone for acne in patients with polycystic ovarian syndrome: a single-institution retrospective study. J Am Acad Dermatol. 2021;85:740-741.
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Yemisci A, Gorgulu A, Piskin S. Effects and side-effects of spironolactone therapy in women with acne. J Eur Acad Dermatol Venereol. 2005;19:163-166. doi:10.1111/j.1468-3083.2005.01072.x
- Patiyasikunt M, Chancheewa B, Asawanonda P, et al. Efficacy and tolerability of low-dose spironolactone and topical benzoyl peroxide in adult female acne: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2020;47:1411-1416. doi:10.1111/1346-8138.15559
- Aldactone (spironolactone) tablets. Prescribing information. Pfizer; 2008. Accessed May 21, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/012151s062lbl.pdf
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001/jamadermatol.2021.5866
- Mackenzie IS, Morant SV, Wei L, et al. Spironolactone use and risk of incident cancers: a retrospective, matched cohort study. Br J Clin Pharmacol. 2017;83:653-663. doi:10.1111/bcp.13152
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875. doi:10.1016/j.canep.2013.10.004
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007/s00403-024-02936-y
- Jick SS, Hernandez RK. Risk of nonfatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: casecontrol study using United States claims data. BMJ. 2011;342:d2151. doi:10.1136/bmj.d2151
- Shields A, Flood K, Barbieri JS. Spironolactone use for acne is not associated with an increased risk of venous thromboembolism: a matched, retrospective cohort study. J Am Acad Dermatol. 2023;88:1396-1397. doi:10.1016/j.jaad.2023.02.028
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Muhn P, Fuhrmann U, Fritzemeier KH, et al. Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity. Ann N Y Acad Sci. 1995;761:311-335. doi:10.1111/j.1749-6632.1995.tb31386.x
- Yaz (drospirenone/ethinyl estradiol) tablets. Prescribing information. Bayer HealthCare Pharmaceuticals; 2012. Accessed May 21, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021676s012lbl.pdf
- Bird ST, Pepe SR, Etminan M, et al. The association between drospirenone and hyperkalemia: a comparative-safety study. BMC Clin Pharmacol. 2011;11:23. doi:10.1186/1472-6904-11-23
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Liszewski W, Boull C. Lack of evidence for feminization of males exposed to spironolactone in utero: a systematic review. J Am Acad Dermatol. 2019;80:1147-1148. doi:10.1016/j.jaad.2018.10.023
- de Jong MFC, Riphagen IJ, Kootstra-Ros JE, et al. Potassium and magnesium in breast milk of a woman with gitelman syndrome. Kidney Int Rep. 2022;7:1720-1721. doi:10.1016/j.ekir.2022.05.006
- Reisman T, Goldstein Z. Case report: induced lactation in a transgender woman. Transgender Health. 2018;3:24-26. doi:10.1089 /trgh.2017.0044
- Phelps DL, Karim A. Spironolactone: relationship between concentrations of dethioacetylated metabolite in human serum and milk. J Pharm Sci. 1977;66:1203. doi:10.1002/jps.2600660841
- World Health Organization. Breastfeeding and maternal medication: recommendations for drugs in the eleventh WHO model list of essential drugs. February 25, 2002. Accessed May 21, 2025. https://www.who.int/publications/i/item/55732
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. Lactation. J Am Acad Dermatol. 2014;70:417.e1-10; quiz 427. doi:10.1016/j.jaad.2013.09.009
- Cominos DC, van der Walt A, van Rooyen AJ. Suppression of postpartum lactation with furosemide. S Afr Med J. 1976;50:251-252.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Ershadi S, Choe J, Barbieri JS. Medicaid formularies for acne treatments are difficult to access and reflect inconsistent coverage policies. J Am Acad Dermatol. 2024;90:1074-1076. doi:10.1016/j.jaad.2024.01.033
- Barbieri JS, James WD, Margolis DJ. Trends in prescribing behavior of systemic agents used in the treatment of acne among dermatologists and nondermatologists: a retrospective analysis, 2004-2013. J Am Acad Dermatol. 2017;77:456-463.e4. doi:10.1016/j.jaad.2017.04.016
- Barbieri JS. Temporal trends in the use of systemic medications for acne from 2017 to 2020. JAMA Dermatol. 2023;159:1135-1136. doi:10.1001 /jamadermatol.2023.2363
- Strauss JS, Pochi PE, Downing DT. Acne: perspectives. J Invest Dermatol. 1974;62:321-325. doi:10.1111/1523-1747.ep12724280
- Luderschmidt C, Bidlingmaier F, Plewig G. Inhibition of sebaceous gland activity by spironolactone in Syrian hamster. J Invest Dermatol. 1982;78:253-255. doi:10.1111/1523-1747.ep12506612
- Boisselle A, Dionne FT, Tremblay RR. Interaction of spironolactone with rat skin androgen receptor. Can J Biochem. 1979;57:1042-1046. doi:10.1139/o79-131
- Menard RH, Stripp B, Gillette JR. Spironolactone and testicular cytochrome P-450: decreased testosterone formation in several species and changes in hepatic drug metabolism. Endocrinology. 1974;94:1628-1636. doi:10.1210/endo-94-6-1628
- Rifka SM, Pita JC, Vigersky RA, et al. Interaction of digitalis and spironolactone with human sex steroid receptors. J Clin Endocrinol Metab. 1978;46:338-344. doi:10.1210/jcem-46-2-338
- Corvol P, Michaud A, Menard J, et al. Antiandrogenic effect of spirolactones: mechanism of action. Endocrinology. 1975;97:52-58. doi:10.1210/endo-97-1-52
- Akamatsu H, Zouboulis CC, Orfanos CE. Spironolactone directly inhibits proliferation of cultured human facial sebocytes and acts antagonistically to testosterone and 5 alpha-dihydrotestosterone in vitro. J Invest Dermatol. 1993;100:660-662. doi:10.1111/1523-1747 .ep12472325
- Goodfellow A, Alaghband-Zadeh J, Carter G, et al. Oral spironolactone improves acne vulgaris and reduces sebum excretion. Br J Dermatol. 1984;111:209-214. doi:10.1111/j.1365-2133.1984.tb04045.x
- Muhlemann MF, Carter GD, Cream JJ, et al. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115:227-232. doi:10.1111/j.1365-2133.1986.tb05722.x
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Santer M, Lawrence M, Renz S, et al. Effectiveness of spironolactone for women with acne vulgaris (SAFA) in England and Wales: pragmatic, multicentre, phase 3, double blind, randomised controlled trial. BMJ. 2023;381:E074349. doi:10.1136/bmj-2022-074349
- Shields A, Barbieri JS. Effectiveness of spironolactone for women with acne vulgaris (SAFA) trial: a critically appraised topic. Br J Dermatol. 2023;189:509-510. doi:10.1093/bjd/ljad270
- Xu H, Li H. Acne, the skin microbiome, and antibiotic treatment. Am J Clin Dermatol. 2019;20:335-344. doi:10.1007/s40257-018-00417-3
- Knutsen-Larson S, Dawson AL, Dunnick CA, et al. Acne vulgaris: pathogenesis, treatment, and needs assessment. Dermatol Clin. 2012;30:99-106, viii-ix. doi:10.1016/j.det.2011.09.001
- Han JJ, Faletsky A, Barbieri JS, et al. New acne therapies and updates on use of spironolactone and isotretinoin: a narrative review. Dermatol Ther (Heidelb). 2021;11:79-91.
- Centers for Disease Control and Prevention. Outpatient antibiotic prescriptions—United States, 2021. Accessed May 21, 2025. https://archive.cdc.gov/#/details?url=https://www.cdc.gov/antibiotic-use/data/report-2021.html
- Adler BL, Kornmehl H, Armstrong AW. Antibiotic resistance in acne treatment. JAMA Dermatol. 2017;153:810-811. doi:10.1001 /jamadermatol.2017.1297
- Walsh TR, Efthimiou J, Dréno B. Systematic review of antibiotic resistance in acne: an increasing topical and oral threat. Lancet Infect Dis. 2016;16:E23-E33. doi:10.1016/S1473-3099(15)00527-7
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616. doi:10.1038/ajg.2010.303?
- Margolis DJ, Fanelli M, Kupperman E, et al. Association of pharyngitis with oral antibiotic use for the treatment of acne: a cross-sectional and prospective cohort study. Arch Dermatol. 2012;148:326-332. doi:10.1001 /archdermatol.2011.355
- Bartlett JG, Chang TW, Gurwith M, et al. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978;298:531-534. doi:10.1056/NEJM197803092981003
- Carroll KC, Bartlett JG. Biology of Clostridium difficile: implications for epidemiology and diagnosis. Annu Rev Microbiol. 2011;65:501-521. doi:10.1146/annurev-micro-090110-102824
- Velicer CM, Heckbert SR, Lampe JW, et al. Antibiotic use in relation to the risk of breast cancer. JAMA. 2004;291:827-835. doi:10.1001/jama.291.7.827
- Song M, Nguyen LH, Emilsson L, et al. Antibiotic use associated with risk of colorectal polyps in a nationwide study. Clin Gastroenterol Hepatol. 2021;19:1426-1435.e6. doi:10.1016/j.cgh.2020.05.036
- Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67:672-678. doi:10.1136 /gutjnl-2016-313413
- Del Rosso JQ, Rosen T, Palceski D, et al. Patient awareness of antimicrobial resistance and antibiotic use in acne vulgaris. J Clin Aesthetic Dermatol. 2019;12:30-41.
- Park JH, Bienenfeld A, Orlow SJ, et al. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol. 2018;19:449-455. doi:10.1007/s40257-018-0349-6
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) Study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Barbieri JS, Ellenberg S, Grice E, et al. Challenges in designing a randomized, double-blind noninferiority trial for treatment of acne: The SDACNE trial. Clin Trials. 2025;22:66-76. doi:10.1177/17407745241265094
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59. doi:10.1016/j.jaad.2007.06.045
- Dréno B, Thiboutot D, Layton AM, et al. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106. doi:10.1111/jdv.12757
- Schmidt TH, Khanijow K, Cedars MI, et al. Cutaneous findings and systemic associations in women with polycystic ovary syndrome. JAMA Dermatol. 2016;152:391-398. doi:10.1001/jamadermatol.2015.4498
- Plante J, Robinson I, Elston D. The need for potassium monitoring in women on spironolactone for dermatologic conditions. J Am Acad Dermatol. 2022;87:1097-1099. doi:10.1016/j.jaad.2022.01.010
- Berman HS, Cheng CE, Hogeling M. Spironolactone in the treatment of adolescent acne: a retrospective review. J Am Acad Dermatol. 2021;85:269-271. doi:10.1016/j.jaad.2020.11.044
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006.e1-1006 .e30. doi:10.1016/j.jaad.2023.12.017
- Basu P. High-dose spironolactone for acne in patients with polycystic ovarian syndrome: a single-institution retrospective study. J Am Acad Dermatol. 2021;85:740-741.
- Layton AM, Eady EA, Whitehouse H, et al. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18:169-191. doi:10.1007/s40257-016-0245-x
- Yemisci A, Gorgulu A, Piskin S. Effects and side-effects of spironolactone therapy in women with acne. J Eur Acad Dermatol Venereol. 2005;19:163-166. doi:10.1111/j.1468-3083.2005.01072.x
- Patiyasikunt M, Chancheewa B, Asawanonda P, et al. Efficacy and tolerability of low-dose spironolactone and topical benzoyl peroxide in adult female acne: a randomized, double-blind, placebo-controlled trial. J Dermatol. 2020;47:1411-1416. doi:10.1111/1346-8138.15559
- Aldactone (spironolactone) tablets. Prescribing information. Pfizer; 2008. Accessed May 21, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/012151s062lbl.pdf
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001/jamadermatol.2021.5866
- Mackenzie IS, Morant SV, Wei L, et al. Spironolactone use and risk of incident cancers: a retrospective, matched cohort study. Br J Clin Pharmacol. 2017;83:653-663. doi:10.1111/bcp.13152
- Biggar RJ, Andersen EW, Wohlfahrt J, et al. Spironolactone use and the risk of breast and gynecologic cancers. Cancer Epidemiol. 2013;37:870-875. doi:10.1016/j.canep.2013.10.004
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007/s00403-024-02936-y
- Jick SS, Hernandez RK. Risk of nonfatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with women using oral contraceptives containing levonorgestrel: casecontrol study using United States claims data. BMJ. 2011;342:d2151. doi:10.1136/bmj.d2151
- Shields A, Flood K, Barbieri JS. Spironolactone use for acne is not associated with an increased risk of venous thromboembolism: a matched, retrospective cohort study. J Am Acad Dermatol. 2023;88:1396-1397. doi:10.1016/j.jaad.2023.02.028
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Muhn P, Fuhrmann U, Fritzemeier KH, et al. Drospirenone: a novel progestogen with antimineralocorticoid and antiandrogenic activity. Ann N Y Acad Sci. 1995;761:311-335. doi:10.1111/j.1749-6632.1995.tb31386.x
- Yaz (drospirenone/ethinyl estradiol) tablets. Prescribing information. Bayer HealthCare Pharmaceuticals; 2012. Accessed May 21, 2025. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/021676s012lbl.pdf
- Bird ST, Pepe SR, Etminan M, et al. The association between drospirenone and hyperkalemia: a comparative-safety study. BMC Clin Pharmacol. 2011;11:23. doi:10.1186/1472-6904-11-23
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Liszewski W, Boull C. Lack of evidence for feminization of males exposed to spironolactone in utero: a systematic review. J Am Acad Dermatol. 2019;80:1147-1148. doi:10.1016/j.jaad.2018.10.023
- de Jong MFC, Riphagen IJ, Kootstra-Ros JE, et al. Potassium and magnesium in breast milk of a woman with gitelman syndrome. Kidney Int Rep. 2022;7:1720-1721. doi:10.1016/j.ekir.2022.05.006
- Reisman T, Goldstein Z. Case report: induced lactation in a transgender woman. Transgender Health. 2018;3:24-26. doi:10.1089 /trgh.2017.0044
- Phelps DL, Karim A. Spironolactone: relationship between concentrations of dethioacetylated metabolite in human serum and milk. J Pharm Sci. 1977;66:1203. doi:10.1002/jps.2600660841
- World Health Organization. Breastfeeding and maternal medication: recommendations for drugs in the eleventh WHO model list of essential drugs. February 25, 2002. Accessed May 21, 2025. https://www.who.int/publications/i/item/55732
- Butler DC, Heller MM, Murase JE. Safety of dermatologic medications in pregnancy and lactation: part II. Lactation. J Am Acad Dermatol. 2014;70:417.e1-10; quiz 427. doi:10.1016/j.jaad.2013.09.009
- Cominos DC, van der Walt A, van Rooyen AJ. Suppression of postpartum lactation with furosemide. S Afr Med J. 1976;50:251-252.
- Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA Dermatol. 2020;156:621-630. doi:10.1001/jamadermatol.2020.0465
- Ershadi S, Choe J, Barbieri JS. Medicaid formularies for acne treatments are difficult to access and reflect inconsistent coverage policies. J Am Acad Dermatol. 2024;90:1074-1076. doi:10.1016/j.jaad.2024.01.033
Spironolactone for Acne: Practical Strategies for Optimal Clinical Outcomes
Spironolactone for Acne: Practical Strategies for Optimal Clinical Outcomes
PRACTICE POINTS
- Spironolactone is an effective systemic treatment for women with acne and likely is an underutilized alternative to oral antibiotics.
- We recommend a starting dose of 100 mg/d, which is well tolerated by most patients and has superior effectiveness to lower doses.
- Potassium monitoring is of low usefulness in young healthy women, and an association between spironolactone use and increased risk for cancer has not been identified.
Can We Successfully Adapt to Changes in Direction and Support for Acne?
Can We Successfully Adapt to Changes in Direction and Support for Acne?
How did I develop a strong interest in acne and rosacea? Interest on a personal level was with me throughout my adolescence and post-teen years as I suffered with very severe facial acne from ages 13 through 23 (1967-1977). I was sometimes called “pizza face” in high school, and biweekly trips to a dermatology office that always had a packed waiting room were of little help that I could appreciate visibly. Six straight years of extractions, intralesional injections, draining of fluctuant cysts, UVC light treatments, oral tetracycline, irritating topical formulations of benzoyl peroxide and tretinoin, and topical sulfacetamide-sulfur products resulted in minimal improvement. However, maybe all of this did something to what was happening underneath the skin surface, as I have no residual acne scars. I do recall vividly that I walked the halls in high school and college consistently affected by a very red face from the topical agents and smelling like rotten eggs from the topical sulfur application. I fortunately handled it well emotionally and socially, for which I am very thankful. Many people affected with acne do not.
In dermatology, I have always had a strong interest in pathophysiology and therapeutics, rooted I am sure in my background as a pharmacist. Although I was always interested in acne therapy, I was fully captivated by a presentation given by Dr. Jim Leyden many years ago at a small meeting in Myrtle Beach, South Carolina. He brought the subject of acne to life in a way that more than grabbed my complete attention and ignited an interest in learning everything I could about it. Over time, I was fortunate enough to work alongside Dr. Leyden and many other household names in acne at meetings and publications to further education on one of the most common disease states seen in ambulatory dermatology practices worldwide. The rest is history, leading to almost 4 decades of work in acne on many levels in dermatology, all being efforts that I am grateful for.
What I have observed to date is that we have had few revolutionary advances in acne therapy, the major one being oral isotretinoin, which was first brought to market in 1982. We are still utilizing many of the same therapeutic agents that I used back when I was treated for acne. A few new topical compounds have emerged, such as dapsone and clascoterone, and a narrow-spectrum tetracycline agent, sarecycline, also was developed. These agents do represent important advances with some specific benefits. There have been many major improvements in drug delivery formulations, including several vehicle technologies that allow augmented skin tolerability, increased efficacy, and improved stability, allowing for combination therapy products containing 2 or 3 active ingredients. A recent example is the first triple-combination topical acne therapy with excellent supporting data on speed of onset, efficacy, and safety.1
Technological advances also have aided in the development of modified- or extended-release formulations of oral antibiotics, such as doxycycline and minocycline, which allow for reduced adverse effects and lower daily dosages. Lidose formulations of isotretinoin have circumvented the need for concurrent ingestion of a high-fat meal to facilitate its absorption in the gastrointestinal tract (as required with conventional formulations). Many hours also have been spent on delivery devices and vehicles such as pumps, foams, and aqueous-based gels. Let us not forget the efforts and myriad products directed at skin care, cosmeceuticals, and physical devices (lasers and lights) for acne. Regardless of the above, we have not seen the monumental therapeutic and research revolution for acne that we have experienced more recently with biologic agents, Janus kinase inhibitors, and other modes of action for many common disease states such as atopic dermatitis, psoriasis, alopecia areata, vitiligo, hidradenitis suppurativa, prurigo nodularis, and chronic spontaneous urticaria.
Unfortunately, the slow development of advances in treatments for acne has been compounded further by the widespread availability of generic equivalents of most topical and oral therapies along with several over-the- counter topical medications. The expanded skin care and cosmeceutical product world has further diluted the perceived value of topical prescription therapies for acne. The marked difficulty in achieving and sustaining total clearance of acne, with the exception of many individuals treated with oral isotretinoin, results in many patients searching for other options, often through sources beyond dermatology practices (eg, the internet). While some of these sources may provide valid suggestions, they often are not truly substantiated by valid clinical research and are not formally regulated by the US Food and Drug Administration.
All of the above, in addition to the barriers to medication coverage put in place by third-party organizations such as pharmacy benefit managers, have contributed to the extreme slowdown in the development of new prescription therapies for acne. What this leads me to believe is that until there is a true meeting of the minds of all stakeholders on policies that facilitate access to both established and newly available acne therapies, there will be an enduring diminished incentive to support the development of newer acne treatments that will continue to spiral progressively downward. Some research on acne will always continue, such as the search for an acne vaccine and cutaneous microbiome alterations that are in progress.2,3 However, I do not see much happening in the foreseeable future. I am not inherently a pessimist or a “prophet of doom,” so I sincerely hope I am wrong.
- Stein Gold L, Baldwin H, Kircik LH, et al. Efficacy and safety of a fixed-dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: a randomized phase II study of the first triple-combination drug. Am J Clin Dermatol. 2022;23:93-104. doi:10.1007/s40257-021-00650-3
- Keshari S, Kumar M, Balasubramaniam A, et al. Prospects of acne vaccines targeting secreted virulence factors of Cutibacterium acnes. Expert Rev Vaccines. 2019;18:433-437. doi:10.1080/14760584
- Dreno B, Dekio I, Baldwin H, et al. Acne microbiome: from phyla to phylotypes. J Eur Acad Dermatol Venereol. 2024;38:657- 664. doi:10.1111/jdv.19540 .2019.1593830
How did I develop a strong interest in acne and rosacea? Interest on a personal level was with me throughout my adolescence and post-teen years as I suffered with very severe facial acne from ages 13 through 23 (1967-1977). I was sometimes called “pizza face” in high school, and biweekly trips to a dermatology office that always had a packed waiting room were of little help that I could appreciate visibly. Six straight years of extractions, intralesional injections, draining of fluctuant cysts, UVC light treatments, oral tetracycline, irritating topical formulations of benzoyl peroxide and tretinoin, and topical sulfacetamide-sulfur products resulted in minimal improvement. However, maybe all of this did something to what was happening underneath the skin surface, as I have no residual acne scars. I do recall vividly that I walked the halls in high school and college consistently affected by a very red face from the topical agents and smelling like rotten eggs from the topical sulfur application. I fortunately handled it well emotionally and socially, for which I am very thankful. Many people affected with acne do not.
In dermatology, I have always had a strong interest in pathophysiology and therapeutics, rooted I am sure in my background as a pharmacist. Although I was always interested in acne therapy, I was fully captivated by a presentation given by Dr. Jim Leyden many years ago at a small meeting in Myrtle Beach, South Carolina. He brought the subject of acne to life in a way that more than grabbed my complete attention and ignited an interest in learning everything I could about it. Over time, I was fortunate enough to work alongside Dr. Leyden and many other household names in acne at meetings and publications to further education on one of the most common disease states seen in ambulatory dermatology practices worldwide. The rest is history, leading to almost 4 decades of work in acne on many levels in dermatology, all being efforts that I am grateful for.
What I have observed to date is that we have had few revolutionary advances in acne therapy, the major one being oral isotretinoin, which was first brought to market in 1982. We are still utilizing many of the same therapeutic agents that I used back when I was treated for acne. A few new topical compounds have emerged, such as dapsone and clascoterone, and a narrow-spectrum tetracycline agent, sarecycline, also was developed. These agents do represent important advances with some specific benefits. There have been many major improvements in drug delivery formulations, including several vehicle technologies that allow augmented skin tolerability, increased efficacy, and improved stability, allowing for combination therapy products containing 2 or 3 active ingredients. A recent example is the first triple-combination topical acne therapy with excellent supporting data on speed of onset, efficacy, and safety.1
Technological advances also have aided in the development of modified- or extended-release formulations of oral antibiotics, such as doxycycline and minocycline, which allow for reduced adverse effects and lower daily dosages. Lidose formulations of isotretinoin have circumvented the need for concurrent ingestion of a high-fat meal to facilitate its absorption in the gastrointestinal tract (as required with conventional formulations). Many hours also have been spent on delivery devices and vehicles such as pumps, foams, and aqueous-based gels. Let us not forget the efforts and myriad products directed at skin care, cosmeceuticals, and physical devices (lasers and lights) for acne. Regardless of the above, we have not seen the monumental therapeutic and research revolution for acne that we have experienced more recently with biologic agents, Janus kinase inhibitors, and other modes of action for many common disease states such as atopic dermatitis, psoriasis, alopecia areata, vitiligo, hidradenitis suppurativa, prurigo nodularis, and chronic spontaneous urticaria.
Unfortunately, the slow development of advances in treatments for acne has been compounded further by the widespread availability of generic equivalents of most topical and oral therapies along with several over-the- counter topical medications. The expanded skin care and cosmeceutical product world has further diluted the perceived value of topical prescription therapies for acne. The marked difficulty in achieving and sustaining total clearance of acne, with the exception of many individuals treated with oral isotretinoin, results in many patients searching for other options, often through sources beyond dermatology practices (eg, the internet). While some of these sources may provide valid suggestions, they often are not truly substantiated by valid clinical research and are not formally regulated by the US Food and Drug Administration.
All of the above, in addition to the barriers to medication coverage put in place by third-party organizations such as pharmacy benefit managers, have contributed to the extreme slowdown in the development of new prescription therapies for acne. What this leads me to believe is that until there is a true meeting of the minds of all stakeholders on policies that facilitate access to both established and newly available acne therapies, there will be an enduring diminished incentive to support the development of newer acne treatments that will continue to spiral progressively downward. Some research on acne will always continue, such as the search for an acne vaccine and cutaneous microbiome alterations that are in progress.2,3 However, I do not see much happening in the foreseeable future. I am not inherently a pessimist or a “prophet of doom,” so I sincerely hope I am wrong.
How did I develop a strong interest in acne and rosacea? Interest on a personal level was with me throughout my adolescence and post-teen years as I suffered with very severe facial acne from ages 13 through 23 (1967-1977). I was sometimes called “pizza face” in high school, and biweekly trips to a dermatology office that always had a packed waiting room were of little help that I could appreciate visibly. Six straight years of extractions, intralesional injections, draining of fluctuant cysts, UVC light treatments, oral tetracycline, irritating topical formulations of benzoyl peroxide and tretinoin, and topical sulfacetamide-sulfur products resulted in minimal improvement. However, maybe all of this did something to what was happening underneath the skin surface, as I have no residual acne scars. I do recall vividly that I walked the halls in high school and college consistently affected by a very red face from the topical agents and smelling like rotten eggs from the topical sulfur application. I fortunately handled it well emotionally and socially, for which I am very thankful. Many people affected with acne do not.
In dermatology, I have always had a strong interest in pathophysiology and therapeutics, rooted I am sure in my background as a pharmacist. Although I was always interested in acne therapy, I was fully captivated by a presentation given by Dr. Jim Leyden many years ago at a small meeting in Myrtle Beach, South Carolina. He brought the subject of acne to life in a way that more than grabbed my complete attention and ignited an interest in learning everything I could about it. Over time, I was fortunate enough to work alongside Dr. Leyden and many other household names in acne at meetings and publications to further education on one of the most common disease states seen in ambulatory dermatology practices worldwide. The rest is history, leading to almost 4 decades of work in acne on many levels in dermatology, all being efforts that I am grateful for.
What I have observed to date is that we have had few revolutionary advances in acne therapy, the major one being oral isotretinoin, which was first brought to market in 1982. We are still utilizing many of the same therapeutic agents that I used back when I was treated for acne. A few new topical compounds have emerged, such as dapsone and clascoterone, and a narrow-spectrum tetracycline agent, sarecycline, also was developed. These agents do represent important advances with some specific benefits. There have been many major improvements in drug delivery formulations, including several vehicle technologies that allow augmented skin tolerability, increased efficacy, and improved stability, allowing for combination therapy products containing 2 or 3 active ingredients. A recent example is the first triple-combination topical acne therapy with excellent supporting data on speed of onset, efficacy, and safety.1
Technological advances also have aided in the development of modified- or extended-release formulations of oral antibiotics, such as doxycycline and minocycline, which allow for reduced adverse effects and lower daily dosages. Lidose formulations of isotretinoin have circumvented the need for concurrent ingestion of a high-fat meal to facilitate its absorption in the gastrointestinal tract (as required with conventional formulations). Many hours also have been spent on delivery devices and vehicles such as pumps, foams, and aqueous-based gels. Let us not forget the efforts and myriad products directed at skin care, cosmeceuticals, and physical devices (lasers and lights) for acne. Regardless of the above, we have not seen the monumental therapeutic and research revolution for acne that we have experienced more recently with biologic agents, Janus kinase inhibitors, and other modes of action for many common disease states such as atopic dermatitis, psoriasis, alopecia areata, vitiligo, hidradenitis suppurativa, prurigo nodularis, and chronic spontaneous urticaria.
Unfortunately, the slow development of advances in treatments for acne has been compounded further by the widespread availability of generic equivalents of most topical and oral therapies along with several over-the- counter topical medications. The expanded skin care and cosmeceutical product world has further diluted the perceived value of topical prescription therapies for acne. The marked difficulty in achieving and sustaining total clearance of acne, with the exception of many individuals treated with oral isotretinoin, results in many patients searching for other options, often through sources beyond dermatology practices (eg, the internet). While some of these sources may provide valid suggestions, they often are not truly substantiated by valid clinical research and are not formally regulated by the US Food and Drug Administration.
All of the above, in addition to the barriers to medication coverage put in place by third-party organizations such as pharmacy benefit managers, have contributed to the extreme slowdown in the development of new prescription therapies for acne. What this leads me to believe is that until there is a true meeting of the minds of all stakeholders on policies that facilitate access to both established and newly available acne therapies, there will be an enduring diminished incentive to support the development of newer acne treatments that will continue to spiral progressively downward. Some research on acne will always continue, such as the search for an acne vaccine and cutaneous microbiome alterations that are in progress.2,3 However, I do not see much happening in the foreseeable future. I am not inherently a pessimist or a “prophet of doom,” so I sincerely hope I am wrong.
- Stein Gold L, Baldwin H, Kircik LH, et al. Efficacy and safety of a fixed-dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: a randomized phase II study of the first triple-combination drug. Am J Clin Dermatol. 2022;23:93-104. doi:10.1007/s40257-021-00650-3
- Keshari S, Kumar M, Balasubramaniam A, et al. Prospects of acne vaccines targeting secreted virulence factors of Cutibacterium acnes. Expert Rev Vaccines. 2019;18:433-437. doi:10.1080/14760584
- Dreno B, Dekio I, Baldwin H, et al. Acne microbiome: from phyla to phylotypes. J Eur Acad Dermatol Venereol. 2024;38:657- 664. doi:10.1111/jdv.19540 .2019.1593830
- Stein Gold L, Baldwin H, Kircik LH, et al. Efficacy and safety of a fixed-dose clindamycin phosphate 1.2%, benzoyl peroxide 3.1%, and adapalene 0.15% gel for moderate-to-severe acne: a randomized phase II study of the first triple-combination drug. Am J Clin Dermatol. 2022;23:93-104. doi:10.1007/s40257-021-00650-3
- Keshari S, Kumar M, Balasubramaniam A, et al. Prospects of acne vaccines targeting secreted virulence factors of Cutibacterium acnes. Expert Rev Vaccines. 2019;18:433-437. doi:10.1080/14760584
- Dreno B, Dekio I, Baldwin H, et al. Acne microbiome: from phyla to phylotypes. J Eur Acad Dermatol Venereol. 2024;38:657- 664. doi:10.1111/jdv.19540 .2019.1593830
Can We Successfully Adapt to Changes in Direction and Support for Acne?
Can We Successfully Adapt to Changes in Direction and Support for Acne?
Efficacy and Safety of Spironolactone in Acne Management
Efficacy and Safety of Spironolactone in Acne Management
Spironolactone is an aldosterone antagonist that first was used as a potassium-sparing diuretic to treat heart failure and hypertension. It also possesses antiandrogenic mechanisms including competitively inhibiting androgen receptors, increasing steroid hormone–binding globulin production, and decreasing 5α-reductase activity.1 These properties have been leveraged in off-label use for dermatologic conditions including acne, hidradenitis suppurativa, androgenic alopecia, and hirsutism.1,2 Despite being used off-label to treat acne for more than 40 years, spironolactone has not received US Food and Drug Administration approval for this indication.3 Herein, we review the current evidence for use of spironolactone in acne management.
Spironolactone Efficacy
Spironolactone is efficacious for facial and truncal acne in adult females; it cannot be used in males given its anti-androgenic effects.4,5 In 2 large studies, spironolactone completely or partially cleared facial acne in 75.5% to 85.1% of patients.4,5 In the first study, which included 395 patients on a median dose of 100 mg/d (range, 25-200 mg/d), clearance of comedonal, papulopustular, and nodulocystic acne was observed.4 The second study included 403 patients, most of whom started on spironolactone at 100 mg/d (range, 25-200 mg/d). In addition to facial clearance, patients in this study demonstrated similar rates of partial or complete clearance of acne on the chest (84.0%) and back (80.2%) assessed via a comprehensive acne severity scale.5 In both studies, doses of 100 mg/d or higher were most effective, and the median time to initial acne improvement was 3 months, with peak effects occurring after 4 to 6 months of treatment.4,5 Most patients were using spironolactone monotherapy or spironolactone in combination with topical therapies; however, a minority used it concurrently with oral antibiotics and/or combined oral contraceptives.
Spironolactone has demonstrated comparable efficacy to tetracycline antibiotics. A study comparing the rate of switching to another systemic therapy within 1 year of treatment initiation identified similar rates in patients started on spironolactone (n=962) and those started on tetracyclines (n=4236)(14.4% vs 13.4%, respectively). As switching may indicate treatment failure due to insufficient efficacy, adverse effects, or other causes, these findings may suggest similar effectiveness for spironolactone and tetracyclines.6 These treatments also were compared in a randomized controlled trial of 133 patients receiving topical benzoyl peroxide 5% for 6 months and either spironolactone 150 mg/d for 6 months or doxycycline 100 mg/d for 3 months followed by oral placebo for 3 months. At 4 months, spironolactone performed better than doxycycline as assessed using the Adult Female Acne Scoring Tool.3 Although doxycycline was stopped after 3 months and only topical therapy was continued, this finding is notable because guidelines from the American Academy of Dermatology recommend limiting tetracycline use to 3 to 4 months, whereas spironolactone may be continued for prolonged durations.1,4
While most studies have evaluated the efficacy of spironolactone in adult females, it is increasingly being prescribed in adolescents.7 In a study that included 80 females aged 14 to 20 years, 80% (64/80) experienced acne improvement on a median dose of 100 mg/d.8 Additionally, in the study evaluating treatment switching rates, more than 80% of 1139 adolescents who were started on spironolactone were not switched to a different systemic therapy within the first year of treatment, demonstrating the efficacy of spironolactone in this demographic.6 However, treatment switching was more common among adolescents started on spironolactone compared with those who started on tetracyclines. As noted for adults, the treatment switching rates were the same for spironolactone and tetracycline users; the difference in adolescents may be due to lower influence of hormonal factors or higher therapeutic expectations in this population.6
Spironolactone Safety
Spironolactone is well tolerated at doses of 25 to 200 mg/d for acne management. Common adverse effects include diuresis (29% [26/90]), menstrual irregularities (22% [20/90]), fatigue (17% [15/90]), headache (14% [13/90]), and dizziness (12% [11/90]), but they infrequently lead to treatment discontinuation.4,9 Rates of adverse effects are lower in adolescents compared to adults, although the effects of spironolactone on early endocrine development in adolescents are unknown.7 Spironolactone should not be used during pregnancy, and concurrent contraception use is advised because spironolactone has caused feminization of male fetuses in animal studies.1,10-11
While concerns about potentially severe adverse effects including hypotension, hyperkalemia, and tumorigenicity have been raised, their occurrence in the literature is rare.5,12-18 In a study evaluating hypotension in 2084 patients taking spironolactone 50 to 200 mg/day for acne, hair loss, and/or hirsutism, 3.1% experienced absolute hypotension, and only 0.26% required dose reduction or discontinuation.12 Another study of 403 patients taking spironolactone for acne reported a statistically significant but clinically insignificant mean reduction in systolic blood pressure of 3.5 mm Hg.5 While clinically relevant hypotension is unlikely to occur, some authors still recommend measuring baseline blood pressure before spironolactone initiation.12
Many large studies have demonstrated that hyperkalemia with spironolactone use is rare in young healthy women.13-15 In one study of patients aged 18 to 45 years treated with spironolactone for acne, only 0.72% of 1802 serum potassium measurements fell within the range of mild hyperkalemia.13 Another study found a significantly greater incidence of hyperkalemia in healthy women aged 46 to 65 years compared with women younger than 45 years (16.7% vs <1%; P=.0245).14 Additionally, among 27 patients taking spironolactone and oral contraceptives containing drospirenone (a spironolactone analog), none had elevated potassium levels.15 Given these findings, American Academy of Dermatology guidelines suggest that monitoring potassium in young healthy women has low utility but should be considered in those with risk factors including older age; renal and cardiovascular disease; and concurrent medications that interfere with renal, adrenal, and hepatic function.1 If performed, monitoring should be done within the first few weeks of initiating spironolactone for early detection of hyperkalemia.16
Spironolactone has a US Food and Drug Administration warning for tumorigenicity based on studies in rats that were given up to 150 times the amount for human therapeutic doses and subsequently developed thyroid, hepatic, testicular, and breast adenomas.1 However, several large studies in humans have not found an association between spironolactone and breast cancer (BC) development.1,17,18 Furthermore, a large retrospective study found no increased risk for recurrence in BC survivors treated with spironolactone.2 Most carcinogenicity studies include older women, which may limit generalizability of the findings to younger women, who comprise the majority of patients being treated for acne. Recently, however, a retrospective study evaluating healthy females aged 9 to 40 years with acne identified no significant increased risk for BC in patients treated with spironolactone.17 When compared to tetracyclines, there was a slightly decreased BC risk with spironolactone, providing further support for the latter’s safety. Finally, a large systematic review identified no association between spironolactone and ovarian, bladder, kidney, gastric, or esophageal cancers.18
Final Thoughts
Over the past several years, an ever-expanding body of literature supporting the efficacy and safety of spironolactone has emerged. While spironolactone has been used off label for decades to treat acne in healthy adult females, there are now strong data to support its efficacy in adolescent females. Notably, spironolactone consistently demonstrates similar effectiveness to first-line tetracycline antibiotics. Additionally, data suggest that spironolactone is safe in patients with a history of BC. Overall, spironolactone is a safe, comparable, and promising alternative to antibiotics for acne management in adult and adolescent females.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006. e1-1006.e30. doi:10.1016/j.jaad.2023.12.017
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Horissian M, Maczuga S, Barbieri JS, et al. Trends in the prescribing pattern of spironolactone for acne and hidradenitis suppurativa in adolescents. J Am Acad Dermatol. 2022;87:684-686. doi:10.1016/j.jaad.2021.12.005
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545. doi:10.1007/s10227-001-0152-4
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Jaussan V, Lemarchand-Béraud T, Gómez F. Modifications of the gonadal function in the adult rat after fetal exposure to spironolactone. Biol Reprod. 1985;32:1051-1061. doi:10.1095 /biolreprod32.5.1051
- Hill RC, Wang Y, Shaikh B, et al. Spironolactone treatment for dermatologic indications is not associated with hypotension in a single-center retrospective study. J Am Acad Dermatol. 2024;90: 1245-1247. doi:10.1016/j.jaad.2024.01.057
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. ,em>JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007 /s00403-024-02936-y
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001 /jamadermatol.2021.5866
Spironolactone is an aldosterone antagonist that first was used as a potassium-sparing diuretic to treat heart failure and hypertension. It also possesses antiandrogenic mechanisms including competitively inhibiting androgen receptors, increasing steroid hormone–binding globulin production, and decreasing 5α-reductase activity.1 These properties have been leveraged in off-label use for dermatologic conditions including acne, hidradenitis suppurativa, androgenic alopecia, and hirsutism.1,2 Despite being used off-label to treat acne for more than 40 years, spironolactone has not received US Food and Drug Administration approval for this indication.3 Herein, we review the current evidence for use of spironolactone in acne management.
Spironolactone Efficacy
Spironolactone is efficacious for facial and truncal acne in adult females; it cannot be used in males given its anti-androgenic effects.4,5 In 2 large studies, spironolactone completely or partially cleared facial acne in 75.5% to 85.1% of patients.4,5 In the first study, which included 395 patients on a median dose of 100 mg/d (range, 25-200 mg/d), clearance of comedonal, papulopustular, and nodulocystic acne was observed.4 The second study included 403 patients, most of whom started on spironolactone at 100 mg/d (range, 25-200 mg/d). In addition to facial clearance, patients in this study demonstrated similar rates of partial or complete clearance of acne on the chest (84.0%) and back (80.2%) assessed via a comprehensive acne severity scale.5 In both studies, doses of 100 mg/d or higher were most effective, and the median time to initial acne improvement was 3 months, with peak effects occurring after 4 to 6 months of treatment.4,5 Most patients were using spironolactone monotherapy or spironolactone in combination with topical therapies; however, a minority used it concurrently with oral antibiotics and/or combined oral contraceptives.
Spironolactone has demonstrated comparable efficacy to tetracycline antibiotics. A study comparing the rate of switching to another systemic therapy within 1 year of treatment initiation identified similar rates in patients started on spironolactone (n=962) and those started on tetracyclines (n=4236)(14.4% vs 13.4%, respectively). As switching may indicate treatment failure due to insufficient efficacy, adverse effects, or other causes, these findings may suggest similar effectiveness for spironolactone and tetracyclines.6 These treatments also were compared in a randomized controlled trial of 133 patients receiving topical benzoyl peroxide 5% for 6 months and either spironolactone 150 mg/d for 6 months or doxycycline 100 mg/d for 3 months followed by oral placebo for 3 months. At 4 months, spironolactone performed better than doxycycline as assessed using the Adult Female Acne Scoring Tool.3 Although doxycycline was stopped after 3 months and only topical therapy was continued, this finding is notable because guidelines from the American Academy of Dermatology recommend limiting tetracycline use to 3 to 4 months, whereas spironolactone may be continued for prolonged durations.1,4
While most studies have evaluated the efficacy of spironolactone in adult females, it is increasingly being prescribed in adolescents.7 In a study that included 80 females aged 14 to 20 years, 80% (64/80) experienced acne improvement on a median dose of 100 mg/d.8 Additionally, in the study evaluating treatment switching rates, more than 80% of 1139 adolescents who were started on spironolactone were not switched to a different systemic therapy within the first year of treatment, demonstrating the efficacy of spironolactone in this demographic.6 However, treatment switching was more common among adolescents started on spironolactone compared with those who started on tetracyclines. As noted for adults, the treatment switching rates were the same for spironolactone and tetracycline users; the difference in adolescents may be due to lower influence of hormonal factors or higher therapeutic expectations in this population.6
Spironolactone Safety
Spironolactone is well tolerated at doses of 25 to 200 mg/d for acne management. Common adverse effects include diuresis (29% [26/90]), menstrual irregularities (22% [20/90]), fatigue (17% [15/90]), headache (14% [13/90]), and dizziness (12% [11/90]), but they infrequently lead to treatment discontinuation.4,9 Rates of adverse effects are lower in adolescents compared to adults, although the effects of spironolactone on early endocrine development in adolescents are unknown.7 Spironolactone should not be used during pregnancy, and concurrent contraception use is advised because spironolactone has caused feminization of male fetuses in animal studies.1,10-11
While concerns about potentially severe adverse effects including hypotension, hyperkalemia, and tumorigenicity have been raised, their occurrence in the literature is rare.5,12-18 In a study evaluating hypotension in 2084 patients taking spironolactone 50 to 200 mg/day for acne, hair loss, and/or hirsutism, 3.1% experienced absolute hypotension, and only 0.26% required dose reduction or discontinuation.12 Another study of 403 patients taking spironolactone for acne reported a statistically significant but clinically insignificant mean reduction in systolic blood pressure of 3.5 mm Hg.5 While clinically relevant hypotension is unlikely to occur, some authors still recommend measuring baseline blood pressure before spironolactone initiation.12
Many large studies have demonstrated that hyperkalemia with spironolactone use is rare in young healthy women.13-15 In one study of patients aged 18 to 45 years treated with spironolactone for acne, only 0.72% of 1802 serum potassium measurements fell within the range of mild hyperkalemia.13 Another study found a significantly greater incidence of hyperkalemia in healthy women aged 46 to 65 years compared with women younger than 45 years (16.7% vs <1%; P=.0245).14 Additionally, among 27 patients taking spironolactone and oral contraceptives containing drospirenone (a spironolactone analog), none had elevated potassium levels.15 Given these findings, American Academy of Dermatology guidelines suggest that monitoring potassium in young healthy women has low utility but should be considered in those with risk factors including older age; renal and cardiovascular disease; and concurrent medications that interfere with renal, adrenal, and hepatic function.1 If performed, monitoring should be done within the first few weeks of initiating spironolactone for early detection of hyperkalemia.16
Spironolactone has a US Food and Drug Administration warning for tumorigenicity based on studies in rats that were given up to 150 times the amount for human therapeutic doses and subsequently developed thyroid, hepatic, testicular, and breast adenomas.1 However, several large studies in humans have not found an association between spironolactone and breast cancer (BC) development.1,17,18 Furthermore, a large retrospective study found no increased risk for recurrence in BC survivors treated with spironolactone.2 Most carcinogenicity studies include older women, which may limit generalizability of the findings to younger women, who comprise the majority of patients being treated for acne. Recently, however, a retrospective study evaluating healthy females aged 9 to 40 years with acne identified no significant increased risk for BC in patients treated with spironolactone.17 When compared to tetracyclines, there was a slightly decreased BC risk with spironolactone, providing further support for the latter’s safety. Finally, a large systematic review identified no association between spironolactone and ovarian, bladder, kidney, gastric, or esophageal cancers.18
Final Thoughts
Over the past several years, an ever-expanding body of literature supporting the efficacy and safety of spironolactone has emerged. While spironolactone has been used off label for decades to treat acne in healthy adult females, there are now strong data to support its efficacy in adolescent females. Notably, spironolactone consistently demonstrates similar effectiveness to first-line tetracycline antibiotics. Additionally, data suggest that spironolactone is safe in patients with a history of BC. Overall, spironolactone is a safe, comparable, and promising alternative to antibiotics for acne management in adult and adolescent females.
Spironolactone is an aldosterone antagonist that first was used as a potassium-sparing diuretic to treat heart failure and hypertension. It also possesses antiandrogenic mechanisms including competitively inhibiting androgen receptors, increasing steroid hormone–binding globulin production, and decreasing 5α-reductase activity.1 These properties have been leveraged in off-label use for dermatologic conditions including acne, hidradenitis suppurativa, androgenic alopecia, and hirsutism.1,2 Despite being used off-label to treat acne for more than 40 years, spironolactone has not received US Food and Drug Administration approval for this indication.3 Herein, we review the current evidence for use of spironolactone in acne management.
Spironolactone Efficacy
Spironolactone is efficacious for facial and truncal acne in adult females; it cannot be used in males given its anti-androgenic effects.4,5 In 2 large studies, spironolactone completely or partially cleared facial acne in 75.5% to 85.1% of patients.4,5 In the first study, which included 395 patients on a median dose of 100 mg/d (range, 25-200 mg/d), clearance of comedonal, papulopustular, and nodulocystic acne was observed.4 The second study included 403 patients, most of whom started on spironolactone at 100 mg/d (range, 25-200 mg/d). In addition to facial clearance, patients in this study demonstrated similar rates of partial or complete clearance of acne on the chest (84.0%) and back (80.2%) assessed via a comprehensive acne severity scale.5 In both studies, doses of 100 mg/d or higher were most effective, and the median time to initial acne improvement was 3 months, with peak effects occurring after 4 to 6 months of treatment.4,5 Most patients were using spironolactone monotherapy or spironolactone in combination with topical therapies; however, a minority used it concurrently with oral antibiotics and/or combined oral contraceptives.
Spironolactone has demonstrated comparable efficacy to tetracycline antibiotics. A study comparing the rate of switching to another systemic therapy within 1 year of treatment initiation identified similar rates in patients started on spironolactone (n=962) and those started on tetracyclines (n=4236)(14.4% vs 13.4%, respectively). As switching may indicate treatment failure due to insufficient efficacy, adverse effects, or other causes, these findings may suggest similar effectiveness for spironolactone and tetracyclines.6 These treatments also were compared in a randomized controlled trial of 133 patients receiving topical benzoyl peroxide 5% for 6 months and either spironolactone 150 mg/d for 6 months or doxycycline 100 mg/d for 3 months followed by oral placebo for 3 months. At 4 months, spironolactone performed better than doxycycline as assessed using the Adult Female Acne Scoring Tool.3 Although doxycycline was stopped after 3 months and only topical therapy was continued, this finding is notable because guidelines from the American Academy of Dermatology recommend limiting tetracycline use to 3 to 4 months, whereas spironolactone may be continued for prolonged durations.1,4
While most studies have evaluated the efficacy of spironolactone in adult females, it is increasingly being prescribed in adolescents.7 In a study that included 80 females aged 14 to 20 years, 80% (64/80) experienced acne improvement on a median dose of 100 mg/d.8 Additionally, in the study evaluating treatment switching rates, more than 80% of 1139 adolescents who were started on spironolactone were not switched to a different systemic therapy within the first year of treatment, demonstrating the efficacy of spironolactone in this demographic.6 However, treatment switching was more common among adolescents started on spironolactone compared with those who started on tetracyclines. As noted for adults, the treatment switching rates were the same for spironolactone and tetracycline users; the difference in adolescents may be due to lower influence of hormonal factors or higher therapeutic expectations in this population.6
Spironolactone Safety
Spironolactone is well tolerated at doses of 25 to 200 mg/d for acne management. Common adverse effects include diuresis (29% [26/90]), menstrual irregularities (22% [20/90]), fatigue (17% [15/90]), headache (14% [13/90]), and dizziness (12% [11/90]), but they infrequently lead to treatment discontinuation.4,9 Rates of adverse effects are lower in adolescents compared to adults, although the effects of spironolactone on early endocrine development in adolescents are unknown.7 Spironolactone should not be used during pregnancy, and concurrent contraception use is advised because spironolactone has caused feminization of male fetuses in animal studies.1,10-11
While concerns about potentially severe adverse effects including hypotension, hyperkalemia, and tumorigenicity have been raised, their occurrence in the literature is rare.5,12-18 In a study evaluating hypotension in 2084 patients taking spironolactone 50 to 200 mg/day for acne, hair loss, and/or hirsutism, 3.1% experienced absolute hypotension, and only 0.26% required dose reduction or discontinuation.12 Another study of 403 patients taking spironolactone for acne reported a statistically significant but clinically insignificant mean reduction in systolic blood pressure of 3.5 mm Hg.5 While clinically relevant hypotension is unlikely to occur, some authors still recommend measuring baseline blood pressure before spironolactone initiation.12
Many large studies have demonstrated that hyperkalemia with spironolactone use is rare in young healthy women.13-15 In one study of patients aged 18 to 45 years treated with spironolactone for acne, only 0.72% of 1802 serum potassium measurements fell within the range of mild hyperkalemia.13 Another study found a significantly greater incidence of hyperkalemia in healthy women aged 46 to 65 years compared with women younger than 45 years (16.7% vs <1%; P=.0245).14 Additionally, among 27 patients taking spironolactone and oral contraceptives containing drospirenone (a spironolactone analog), none had elevated potassium levels.15 Given these findings, American Academy of Dermatology guidelines suggest that monitoring potassium in young healthy women has low utility but should be considered in those with risk factors including older age; renal and cardiovascular disease; and concurrent medications that interfere with renal, adrenal, and hepatic function.1 If performed, monitoring should be done within the first few weeks of initiating spironolactone for early detection of hyperkalemia.16
Spironolactone has a US Food and Drug Administration warning for tumorigenicity based on studies in rats that were given up to 150 times the amount for human therapeutic doses and subsequently developed thyroid, hepatic, testicular, and breast adenomas.1 However, several large studies in humans have not found an association between spironolactone and breast cancer (BC) development.1,17,18 Furthermore, a large retrospective study found no increased risk for recurrence in BC survivors treated with spironolactone.2 Most carcinogenicity studies include older women, which may limit generalizability of the findings to younger women, who comprise the majority of patients being treated for acne. Recently, however, a retrospective study evaluating healthy females aged 9 to 40 years with acne identified no significant increased risk for BC in patients treated with spironolactone.17 When compared to tetracyclines, there was a slightly decreased BC risk with spironolactone, providing further support for the latter’s safety. Finally, a large systematic review identified no association between spironolactone and ovarian, bladder, kidney, gastric, or esophageal cancers.18
Final Thoughts
Over the past several years, an ever-expanding body of literature supporting the efficacy and safety of spironolactone has emerged. While spironolactone has been used off label for decades to treat acne in healthy adult females, there are now strong data to support its efficacy in adolescent females. Notably, spironolactone consistently demonstrates similar effectiveness to first-line tetracycline antibiotics. Additionally, data suggest that spironolactone is safe in patients with a history of BC. Overall, spironolactone is a safe, comparable, and promising alternative to antibiotics for acne management in adult and adolescent females.
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006. e1-1006.e30. doi:10.1016/j.jaad.2023.12.017
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Horissian M, Maczuga S, Barbieri JS, et al. Trends in the prescribing pattern of spironolactone for acne and hidradenitis suppurativa in adolescents. J Am Acad Dermatol. 2022;87:684-686. doi:10.1016/j.jaad.2021.12.005
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545. doi:10.1007/s10227-001-0152-4
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Jaussan V, Lemarchand-Béraud T, Gómez F. Modifications of the gonadal function in the adult rat after fetal exposure to spironolactone. Biol Reprod. 1985;32:1051-1061. doi:10.1095 /biolreprod32.5.1051
- Hill RC, Wang Y, Shaikh B, et al. Spironolactone treatment for dermatologic indications is not associated with hypotension in a single-center retrospective study. J Am Acad Dermatol. 2024;90: 1245-1247. doi:10.1016/j.jaad.2024.01.057
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. ,em>JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007 /s00403-024-02936-y
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001 /jamadermatol.2021.5866
- Reynolds RV, Yeung H, Cheng CE, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2024;90:1006. e1-1006.e30. doi:10.1016/j.jaad.2023.12.017
- Wei C, Bovonratwet P, Gu A, et al. Spironolactone use does not increase the risk of female breast cancer recurrence: a retrospective analysis. J Am Acad Dermatol. 2020;83:1021-1027. doi:10.1016/j.jaad.2020.05.081
- Dréno B, Nguyen JM, Hainaut E, et al. Efficacy of spironolactone compared with doxycycline in moderate acne in adult females: results of the multicentre, controlled, randomized, double-blind prospective and parallel Female Acne Spironolactone vs doxyCycline Efficacy (FASCE) study. Acta Derm Venereol. 2024;104:adv26002. doi:10.2340/actadv.v104.26002
- Roberts EE, Nowsheen S, Davis MDP, et al. Treatment of acne with spironolactone: a retrospective review of 395 adult patients at Mayo Clinic, 2007-2017. J Eur Acad Dermatol Venereol. 2020;34:2106-2110. doi:10.1111/jdv.16302
- Garg V, Choi JK, James WD, et al. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. 2021;84:1348-1355. doi:10.1016/j.jaad.2020.12.071
- Barbieri JS, Choi JK, Mitra N, et al. Frequency of treatment switching for spironolactone compared to oral tetracycline-class antibiotics for women with acne: a retrospective cohort study 2010-2016. J Drugs Dermatol. 2018;17:632-638.
- Horissian M, Maczuga S, Barbieri JS, et al. Trends in the prescribing pattern of spironolactone for acne and hidradenitis suppurativa in adolescents. J Am Acad Dermatol. 2022;87:684-686. doi:10.1016/j.jaad.2021.12.005
- Roberts EE, Nowsheen S, Davis DMR, et al. Use of spironolactone to treat acne in adolescent females. Pediatr Dermatol. 2021;38:72-76. doi:10.1111/pde.14391
- Shaw JC, White LE. Long-term safety of spironolactone in acne: results of an 8-year follow-up study. J Cutan Med Surg. 2002;6:541-545. doi:10.1007/s10227-001-0152-4
- Hecker A, Hasan SH, Neumann F. Disturbances in sexual differentiation of rat foetuses following spironolactone treatment. Acta Endocrinol (Copenh). 1980;95:540-545. doi:10.1530/acta.0.0950540
- Jaussan V, Lemarchand-Béraud T, Gómez F. Modifications of the gonadal function in the adult rat after fetal exposure to spironolactone. Biol Reprod. 1985;32:1051-1061. doi:10.1095 /biolreprod32.5.1051
- Hill RC, Wang Y, Shaikh B, et al. Spironolactone treatment for dermatologic indications is not associated with hypotension in a single-center retrospective study. J Am Acad Dermatol. 2024;90: 1245-1247. doi:10.1016/j.jaad.2024.01.057
- Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. ,em>JAMA Dermatol. 2015;151:941-944. doi:10.1001 /jamadermatol.2015.34
- Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157. doi:10.1016/j.ijwd.2019.04.024
- Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60-62. doi:10.1016/j.jaad.2007.09.024
- Lai J, Zaenglein AL, Barbieri JS. Timing of potassium monitoring in females treated for acne with spironolactone is not optimal: a retrospective cohort study. J Am Acad Dermatol. 2024;91:982-984. doi:10.1016/j.jaad.2024.07.1446
- Garate D, Thang CJ, Golovko G, et al. A matched cohort study evaluating whether spironolactone or tetracycline-class antibiotic use among female acne patients is associated with breast cancer development risk. Arch Dermatol Res. 2024;316:196. doi:10.1007 /s00403-024-02936-y
- Bommareddy K, Hamade H, Lopez-Olivo MA, et al. Association of spironolactone use with risk of cancer: a systematic review and meta-analysis. JAMA Dermatol. 2022;158:275-282. doi:10.1001 /jamadermatol.2021.5866
Efficacy and Safety of Spironolactone in Acne Management
Efficacy and Safety of Spironolactone in Acne Management
Probiotics, Prebiotics, and Provocative Claims About Bacillus Lysate
Outrageous assertions with little evidence are not new. Even the famous statement “There’s a sucker born every minute,” long attributed to 1800s showman P.T. Barnum, lacks evidence that the circus founder uttered the remark. The message itself and the snippet of a story about the message may be pertinent, though, when we consider the touted benefits of Bacillus lysate for the skin. The focus of this column will be the foundation for the use of probiotics and prebiotics in skin care and then claims made about this skin care ingredient derived from a particular strain of Bacillus bacteria.
. Typically, this topic is broached in the context of the gut-skin axis and the skin and gut microbiomes.1-3 In 2014, Miyazaki et al. found that phenols produced by gut bacteria spurred skin disorders and that decreasing phenols with probiotics and/or prebiotics can restore or maintain cutaneous health.4
Probiotics have been associated with antioxidant activity, primarily because of the presence of antioxidant enzymes (eg, superoxide dismutase), the delivery of antioxidant substances (eg, glutathione), and extracellular polysaccharide synthesis.5-8 Further, probiotics are known to synthesize a cascade of substances with anti-inflammatory, antibacterial, immunomodulatory, and angiogenetic functions that can contribute to wound healing.9 The use of probiotics in skin health largely relies on applying inactivated beneficial bacteria.10 Prebiotics, which are non-digestible plant-based carbohydrates that aid digestion, inhibit pathogens, and support beneficial bacteria, are known to rebalance the skin microflora.10 In addition, prebiotics are considered a robust option to replace live bacteria in skin formulations.11 Bacterial cell lysates, which include bacterial metabolites, cell walls, and dead bacteria, are incorporated into skin care products as well.12
Probiotics and Wound Healing
In 2020, Ashoori et al. reported on their study of three formulations composed of probiotic supernatant (Lactobacillus reuteri, L. fermentum, and Bacillus subtilis sp. natto)-loaded chitosan nanogels prepared from cultures. They evaluated the effectiveness and dressing activity of the formulations by gauging wound closure and histological results in Sprague-Dawley rats. The researchers found that all probiotic lysate preparations conferred healing properties, with the Bacillus subtilis natto yielding the best wound healing quality. They concluded that probiotic lysate nanogels impart a range of benefits, such as favorable wound closure rates, improved appearance, and suitable histological results upon in vivo examination, supporting the potential use of such formulations to treat wounds.9
Probiotics and Treating Skin Disorders
A 2015 review by Roudsari et al. suggests that probiotics display the potential for preventing and treating various skin disorders, including acne, atopic dermatitis, allergic inflammation or hypersensitivity, eczema, photodamage, and wounds.8 They reported that in a US patent, Gueniche revealed ways to employ at least one probiotic microorganism (from Lactobacillus and/or Bifidobacterium) as an active agent to prevent or treat skin irritation.8,13 In addition, they noted that L. brevis was used successfully by DeSimone in 2003 to promote apoptosis and/or diminish inflammation, particularly in creams and ointments to alleviate inflammation.8
At around the same time, Miyazaki et al. reported that Bifidobacterium-fermented soy milk extract stimulated the production of hyaluronic acid (HA) in organotypic cultures of human keratinocytes, cultures of human skin fibroblasts, and hairless mouse skin after 2 weeks of topical application and has the potential to promote HA synthesis in the epidermis and dermis and thus act as an anti-aging agent.14 In another study, Miyazaki et al. investigated the impact of Bifidobacterium-fermented soy milk extract containing genistein and daidzein on the HA content of hairless mouse as well as human skin. After 6 weeks of topical application in mice, skin elasticity, viscoelasticity, hydration, and thickness improved, and HA content increased. In addition, after 3 months of topical application of a 10% Bifidobacterium-fermented soy milk extract gel to the human forearm, decreases in skin elasticity were significantly mitigated.15More recently, in 2023, Xie et al. reviewed clinical and experimental data on the use of various species of Lactobacillus for the treatment and prevention of atopic dermatitis (AD). They found evidence that multiple species (L. rhamnosus in animal and clinical experiments) appeared to be effective in preventing and treating AD, with L. acidophilus lessening symptoms and reported to be safe, L. plantarum improving symptoms through immunomodulatory activity, and L. sakei demonstrating anti-inflammatory and skin barrier protective activity. The authors also noted that L. paracasei exhibited anti-inflammatory effects on AD-like skin lesions, and L. reuteri supplementation prevented AD development. Overall, they called for more in vivo studies and randomized controlled clinical trials to fully elucidate the wide-ranging potential of Lactobacillus species in treating and preventing AD.16
The Darker Side of Using Prebiotic Species in Skin Care?
According to manufacturer Delavie Sciences, its Aeonia product line was based on research conducted on the International Space Station, which allowed for its patented microorganism to be exposed to the conditions of outer space. This cornerstone ingredient, Bacillus lysate, once returned to Earth, reportedly exhibited anti-aging and UV-protective characteristics. The product line has been described as a prebiotic that contributes to a healthy skin barrier.17
In a September 2023 interview in CosmeticsDesign, the president of Delavie Sciences clarified that its Bacillus lysate contains no live bacteria and that it is not a probiotic, but rather, the certified prebiotic lysate is a Bacillus extract that has been used to strengthen the SPF potency of skin care formulations.18 Because of the research performed on the International Space Station, the manufacturers are claiming these ingredients could be “out-of-this-world” as a way to promote results that have, as yet, not been verified by peer review.
Conclusion
Probiotics and prebiotics continue to be the focus of multiple lines of research for their applications and further potential in skin care. In the case of the Bacillus lysate prebiotic compound, there is a kernel of an interesting idea here, at the very least. But proprietary research limits our ability to render a comprehensive evaluation at this time. Such bold and outrageous claims spur more skepticism than optimism. However, lysates are the latest thing in skin care — so we need to keep watch on the developments to stay current. But that’s what you have me for, I’ll help keep you current on new ingredient findings. If you are on LinkedIn, come connect with me. I post breaking ingredient news and skin care trends there to help you answer patient questions. When you are asked if these lysates work, the answer is: All the data we have on bacillus extract are from computer analysis of the ingredient properties and not on the actual formulations or products. Stay tuned.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. Mahmud MR et al. Gut Microbes. 2022 Jan-Dec;14(1):2096995. doi: 10.1080/19490976.2022.2096995.
2. Sinha S et al. Clin Dermatol. 2021 Sep-Oct;39(5):829-839. doi: 10.1016/j.clindermatol.2021.08.021.
3. Gao T et al. Nutrients. 2023 Jul 13;15(14):3123. doi: 10.3390/nu15143123.
4. Miyazaki K et al. Benef Microbes. 2014 Jun 1;5(2):121-128. doi: 10.3920/BM2012.0066.
5. Shen Q et al. Anaerobe. 2010 Aug;16(4):380-386. doi: 10.1016/j.anaerobe.2010.06.006.
6. Peran L et al. Int J Colorectal Dis. 2006 Dec;21(8):737-746. doi: 10.1007/s00384-005-0773-y.
7. Kodali VP, Sen R. Biotechnol J. 2008 Feb;3(2):245-251. doi: 10.1002/biot.200700208.
8. Roudsari MR et al. Health effects of probiotics on the skin. Crit Rev Food Sci Nutr. 2015;55(9):1219-40. doi: 10.1080/10408398.2012.680078.
9. Ashoori Y et al. Biomed Res Int. 2020 Dec 28;2020:8868618. doi: 10.1155/2020/8868618.
10. Simmering R, Breves R. Hautarzt. 2009 Oct;60(10):809-814. doi: 10.1007/s00105-009-1759-4.
11. Bockmuhl D. IFSSC Mag. 2006 Sep 30;9[3]:1-5.
12. Lew LC, Liong MT. J Appl Microbiol. 2013 May;114(5):1241-1253. doi: 10.1111/jam.12137.
13. Gueniche A. US Patent, US 20100226892. 2010.
14. Miyazaki K et al. Skin Pharmacol Appl Skin Physiol. 2003 Mar-Apr;16(2):108-116. doi: 10.1159/000069031.
15. Miyazaki et al. J Cosmet Sci. 2004 Sep-Oct;55(5):473-479.16. Xie A et al. Front Cell Infect Microbiol. 2023 Feb 16;13:1137275. doi: 10.3389/fcimb.2023.1137275.
17. Delavie Sciences. Skincare Science: Aeonia. Skincare from the Stars.
. Accessed December 12, 2024.
18. Stern C. CosmeticsDesign USA. September 7, 2023.
Outrageous assertions with little evidence are not new. Even the famous statement “There’s a sucker born every minute,” long attributed to 1800s showman P.T. Barnum, lacks evidence that the circus founder uttered the remark. The message itself and the snippet of a story about the message may be pertinent, though, when we consider the touted benefits of Bacillus lysate for the skin. The focus of this column will be the foundation for the use of probiotics and prebiotics in skin care and then claims made about this skin care ingredient derived from a particular strain of Bacillus bacteria.
. Typically, this topic is broached in the context of the gut-skin axis and the skin and gut microbiomes.1-3 In 2014, Miyazaki et al. found that phenols produced by gut bacteria spurred skin disorders and that decreasing phenols with probiotics and/or prebiotics can restore or maintain cutaneous health.4
Probiotics have been associated with antioxidant activity, primarily because of the presence of antioxidant enzymes (eg, superoxide dismutase), the delivery of antioxidant substances (eg, glutathione), and extracellular polysaccharide synthesis.5-8 Further, probiotics are known to synthesize a cascade of substances with anti-inflammatory, antibacterial, immunomodulatory, and angiogenetic functions that can contribute to wound healing.9 The use of probiotics in skin health largely relies on applying inactivated beneficial bacteria.10 Prebiotics, which are non-digestible plant-based carbohydrates that aid digestion, inhibit pathogens, and support beneficial bacteria, are known to rebalance the skin microflora.10 In addition, prebiotics are considered a robust option to replace live bacteria in skin formulations.11 Bacterial cell lysates, which include bacterial metabolites, cell walls, and dead bacteria, are incorporated into skin care products as well.12
Probiotics and Wound Healing
In 2020, Ashoori et al. reported on their study of three formulations composed of probiotic supernatant (Lactobacillus reuteri, L. fermentum, and Bacillus subtilis sp. natto)-loaded chitosan nanogels prepared from cultures. They evaluated the effectiveness and dressing activity of the formulations by gauging wound closure and histological results in Sprague-Dawley rats. The researchers found that all probiotic lysate preparations conferred healing properties, with the Bacillus subtilis natto yielding the best wound healing quality. They concluded that probiotic lysate nanogels impart a range of benefits, such as favorable wound closure rates, improved appearance, and suitable histological results upon in vivo examination, supporting the potential use of such formulations to treat wounds.9
Probiotics and Treating Skin Disorders
A 2015 review by Roudsari et al. suggests that probiotics display the potential for preventing and treating various skin disorders, including acne, atopic dermatitis, allergic inflammation or hypersensitivity, eczema, photodamage, and wounds.8 They reported that in a US patent, Gueniche revealed ways to employ at least one probiotic microorganism (from Lactobacillus and/or Bifidobacterium) as an active agent to prevent or treat skin irritation.8,13 In addition, they noted that L. brevis was used successfully by DeSimone in 2003 to promote apoptosis and/or diminish inflammation, particularly in creams and ointments to alleviate inflammation.8
At around the same time, Miyazaki et al. reported that Bifidobacterium-fermented soy milk extract stimulated the production of hyaluronic acid (HA) in organotypic cultures of human keratinocytes, cultures of human skin fibroblasts, and hairless mouse skin after 2 weeks of topical application and has the potential to promote HA synthesis in the epidermis and dermis and thus act as an anti-aging agent.14 In another study, Miyazaki et al. investigated the impact of Bifidobacterium-fermented soy milk extract containing genistein and daidzein on the HA content of hairless mouse as well as human skin. After 6 weeks of topical application in mice, skin elasticity, viscoelasticity, hydration, and thickness improved, and HA content increased. In addition, after 3 months of topical application of a 10% Bifidobacterium-fermented soy milk extract gel to the human forearm, decreases in skin elasticity were significantly mitigated.15More recently, in 2023, Xie et al. reviewed clinical and experimental data on the use of various species of Lactobacillus for the treatment and prevention of atopic dermatitis (AD). They found evidence that multiple species (L. rhamnosus in animal and clinical experiments) appeared to be effective in preventing and treating AD, with L. acidophilus lessening symptoms and reported to be safe, L. plantarum improving symptoms through immunomodulatory activity, and L. sakei demonstrating anti-inflammatory and skin barrier protective activity. The authors also noted that L. paracasei exhibited anti-inflammatory effects on AD-like skin lesions, and L. reuteri supplementation prevented AD development. Overall, they called for more in vivo studies and randomized controlled clinical trials to fully elucidate the wide-ranging potential of Lactobacillus species in treating and preventing AD.16
The Darker Side of Using Prebiotic Species in Skin Care?
According to manufacturer Delavie Sciences, its Aeonia product line was based on research conducted on the International Space Station, which allowed for its patented microorganism to be exposed to the conditions of outer space. This cornerstone ingredient, Bacillus lysate, once returned to Earth, reportedly exhibited anti-aging and UV-protective characteristics. The product line has been described as a prebiotic that contributes to a healthy skin barrier.17
In a September 2023 interview in CosmeticsDesign, the president of Delavie Sciences clarified that its Bacillus lysate contains no live bacteria and that it is not a probiotic, but rather, the certified prebiotic lysate is a Bacillus extract that has been used to strengthen the SPF potency of skin care formulations.18 Because of the research performed on the International Space Station, the manufacturers are claiming these ingredients could be “out-of-this-world” as a way to promote results that have, as yet, not been verified by peer review.
Conclusion
Probiotics and prebiotics continue to be the focus of multiple lines of research for their applications and further potential in skin care. In the case of the Bacillus lysate prebiotic compound, there is a kernel of an interesting idea here, at the very least. But proprietary research limits our ability to render a comprehensive evaluation at this time. Such bold and outrageous claims spur more skepticism than optimism. However, lysates are the latest thing in skin care — so we need to keep watch on the developments to stay current. But that’s what you have me for, I’ll help keep you current on new ingredient findings. If you are on LinkedIn, come connect with me. I post breaking ingredient news and skin care trends there to help you answer patient questions. When you are asked if these lysates work, the answer is: All the data we have on bacillus extract are from computer analysis of the ingredient properties and not on the actual formulations or products. Stay tuned.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. Mahmud MR et al. Gut Microbes. 2022 Jan-Dec;14(1):2096995. doi: 10.1080/19490976.2022.2096995.
2. Sinha S et al. Clin Dermatol. 2021 Sep-Oct;39(5):829-839. doi: 10.1016/j.clindermatol.2021.08.021.
3. Gao T et al. Nutrients. 2023 Jul 13;15(14):3123. doi: 10.3390/nu15143123.
4. Miyazaki K et al. Benef Microbes. 2014 Jun 1;5(2):121-128. doi: 10.3920/BM2012.0066.
5. Shen Q et al. Anaerobe. 2010 Aug;16(4):380-386. doi: 10.1016/j.anaerobe.2010.06.006.
6. Peran L et al. Int J Colorectal Dis. 2006 Dec;21(8):737-746. doi: 10.1007/s00384-005-0773-y.
7. Kodali VP, Sen R. Biotechnol J. 2008 Feb;3(2):245-251. doi: 10.1002/biot.200700208.
8. Roudsari MR et al. Health effects of probiotics on the skin. Crit Rev Food Sci Nutr. 2015;55(9):1219-40. doi: 10.1080/10408398.2012.680078.
9. Ashoori Y et al. Biomed Res Int. 2020 Dec 28;2020:8868618. doi: 10.1155/2020/8868618.
10. Simmering R, Breves R. Hautarzt. 2009 Oct;60(10):809-814. doi: 10.1007/s00105-009-1759-4.
11. Bockmuhl D. IFSSC Mag. 2006 Sep 30;9[3]:1-5.
12. Lew LC, Liong MT. J Appl Microbiol. 2013 May;114(5):1241-1253. doi: 10.1111/jam.12137.
13. Gueniche A. US Patent, US 20100226892. 2010.
14. Miyazaki K et al. Skin Pharmacol Appl Skin Physiol. 2003 Mar-Apr;16(2):108-116. doi: 10.1159/000069031.
15. Miyazaki et al. J Cosmet Sci. 2004 Sep-Oct;55(5):473-479.16. Xie A et al. Front Cell Infect Microbiol. 2023 Feb 16;13:1137275. doi: 10.3389/fcimb.2023.1137275.
17. Delavie Sciences. Skincare Science: Aeonia. Skincare from the Stars.
. Accessed December 12, 2024.
18. Stern C. CosmeticsDesign USA. September 7, 2023.
Outrageous assertions with little evidence are not new. Even the famous statement “There’s a sucker born every minute,” long attributed to 1800s showman P.T. Barnum, lacks evidence that the circus founder uttered the remark. The message itself and the snippet of a story about the message may be pertinent, though, when we consider the touted benefits of Bacillus lysate for the skin. The focus of this column will be the foundation for the use of probiotics and prebiotics in skin care and then claims made about this skin care ingredient derived from a particular strain of Bacillus bacteria.
. Typically, this topic is broached in the context of the gut-skin axis and the skin and gut microbiomes.1-3 In 2014, Miyazaki et al. found that phenols produced by gut bacteria spurred skin disorders and that decreasing phenols with probiotics and/or prebiotics can restore or maintain cutaneous health.4
Probiotics have been associated with antioxidant activity, primarily because of the presence of antioxidant enzymes (eg, superoxide dismutase), the delivery of antioxidant substances (eg, glutathione), and extracellular polysaccharide synthesis.5-8 Further, probiotics are known to synthesize a cascade of substances with anti-inflammatory, antibacterial, immunomodulatory, and angiogenetic functions that can contribute to wound healing.9 The use of probiotics in skin health largely relies on applying inactivated beneficial bacteria.10 Prebiotics, which are non-digestible plant-based carbohydrates that aid digestion, inhibit pathogens, and support beneficial bacteria, are known to rebalance the skin microflora.10 In addition, prebiotics are considered a robust option to replace live bacteria in skin formulations.11 Bacterial cell lysates, which include bacterial metabolites, cell walls, and dead bacteria, are incorporated into skin care products as well.12
Probiotics and Wound Healing
In 2020, Ashoori et al. reported on their study of three formulations composed of probiotic supernatant (Lactobacillus reuteri, L. fermentum, and Bacillus subtilis sp. natto)-loaded chitosan nanogels prepared from cultures. They evaluated the effectiveness and dressing activity of the formulations by gauging wound closure and histological results in Sprague-Dawley rats. The researchers found that all probiotic lysate preparations conferred healing properties, with the Bacillus subtilis natto yielding the best wound healing quality. They concluded that probiotic lysate nanogels impart a range of benefits, such as favorable wound closure rates, improved appearance, and suitable histological results upon in vivo examination, supporting the potential use of such formulations to treat wounds.9
Probiotics and Treating Skin Disorders
A 2015 review by Roudsari et al. suggests that probiotics display the potential for preventing and treating various skin disorders, including acne, atopic dermatitis, allergic inflammation or hypersensitivity, eczema, photodamage, and wounds.8 They reported that in a US patent, Gueniche revealed ways to employ at least one probiotic microorganism (from Lactobacillus and/or Bifidobacterium) as an active agent to prevent or treat skin irritation.8,13 In addition, they noted that L. brevis was used successfully by DeSimone in 2003 to promote apoptosis and/or diminish inflammation, particularly in creams and ointments to alleviate inflammation.8
At around the same time, Miyazaki et al. reported that Bifidobacterium-fermented soy milk extract stimulated the production of hyaluronic acid (HA) in organotypic cultures of human keratinocytes, cultures of human skin fibroblasts, and hairless mouse skin after 2 weeks of topical application and has the potential to promote HA synthesis in the epidermis and dermis and thus act as an anti-aging agent.14 In another study, Miyazaki et al. investigated the impact of Bifidobacterium-fermented soy milk extract containing genistein and daidzein on the HA content of hairless mouse as well as human skin. After 6 weeks of topical application in mice, skin elasticity, viscoelasticity, hydration, and thickness improved, and HA content increased. In addition, after 3 months of topical application of a 10% Bifidobacterium-fermented soy milk extract gel to the human forearm, decreases in skin elasticity were significantly mitigated.15More recently, in 2023, Xie et al. reviewed clinical and experimental data on the use of various species of Lactobacillus for the treatment and prevention of atopic dermatitis (AD). They found evidence that multiple species (L. rhamnosus in animal and clinical experiments) appeared to be effective in preventing and treating AD, with L. acidophilus lessening symptoms and reported to be safe, L. plantarum improving symptoms through immunomodulatory activity, and L. sakei demonstrating anti-inflammatory and skin barrier protective activity. The authors also noted that L. paracasei exhibited anti-inflammatory effects on AD-like skin lesions, and L. reuteri supplementation prevented AD development. Overall, they called for more in vivo studies and randomized controlled clinical trials to fully elucidate the wide-ranging potential of Lactobacillus species in treating and preventing AD.16
The Darker Side of Using Prebiotic Species in Skin Care?
According to manufacturer Delavie Sciences, its Aeonia product line was based on research conducted on the International Space Station, which allowed for its patented microorganism to be exposed to the conditions of outer space. This cornerstone ingredient, Bacillus lysate, once returned to Earth, reportedly exhibited anti-aging and UV-protective characteristics. The product line has been described as a prebiotic that contributes to a healthy skin barrier.17
In a September 2023 interview in CosmeticsDesign, the president of Delavie Sciences clarified that its Bacillus lysate contains no live bacteria and that it is not a probiotic, but rather, the certified prebiotic lysate is a Bacillus extract that has been used to strengthen the SPF potency of skin care formulations.18 Because of the research performed on the International Space Station, the manufacturers are claiming these ingredients could be “out-of-this-world” as a way to promote results that have, as yet, not been verified by peer review.
Conclusion
Probiotics and prebiotics continue to be the focus of multiple lines of research for their applications and further potential in skin care. In the case of the Bacillus lysate prebiotic compound, there is a kernel of an interesting idea here, at the very least. But proprietary research limits our ability to render a comprehensive evaluation at this time. Such bold and outrageous claims spur more skepticism than optimism. However, lysates are the latest thing in skin care — so we need to keep watch on the developments to stay current. But that’s what you have me for, I’ll help keep you current on new ingredient findings. If you are on LinkedIn, come connect with me. I post breaking ingredient news and skin care trends there to help you answer patient questions. When you are asked if these lysates work, the answer is: All the data we have on bacillus extract are from computer analysis of the ingredient properties and not on the actual formulations or products. Stay tuned.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a SaaS company used to generate skin care routines in office and as a ecommerce solution. Write to her at [email protected].
References
1. Mahmud MR et al. Gut Microbes. 2022 Jan-Dec;14(1):2096995. doi: 10.1080/19490976.2022.2096995.
2. Sinha S et al. Clin Dermatol. 2021 Sep-Oct;39(5):829-839. doi: 10.1016/j.clindermatol.2021.08.021.
3. Gao T et al. Nutrients. 2023 Jul 13;15(14):3123. doi: 10.3390/nu15143123.
4. Miyazaki K et al. Benef Microbes. 2014 Jun 1;5(2):121-128. doi: 10.3920/BM2012.0066.
5. Shen Q et al. Anaerobe. 2010 Aug;16(4):380-386. doi: 10.1016/j.anaerobe.2010.06.006.
6. Peran L et al. Int J Colorectal Dis. 2006 Dec;21(8):737-746. doi: 10.1007/s00384-005-0773-y.
7. Kodali VP, Sen R. Biotechnol J. 2008 Feb;3(2):245-251. doi: 10.1002/biot.200700208.
8. Roudsari MR et al. Health effects of probiotics on the skin. Crit Rev Food Sci Nutr. 2015;55(9):1219-40. doi: 10.1080/10408398.2012.680078.
9. Ashoori Y et al. Biomed Res Int. 2020 Dec 28;2020:8868618. doi: 10.1155/2020/8868618.
10. Simmering R, Breves R. Hautarzt. 2009 Oct;60(10):809-814. doi: 10.1007/s00105-009-1759-4.
11. Bockmuhl D. IFSSC Mag. 2006 Sep 30;9[3]:1-5.
12. Lew LC, Liong MT. J Appl Microbiol. 2013 May;114(5):1241-1253. doi: 10.1111/jam.12137.
13. Gueniche A. US Patent, US 20100226892. 2010.
14. Miyazaki K et al. Skin Pharmacol Appl Skin Physiol. 2003 Mar-Apr;16(2):108-116. doi: 10.1159/000069031.
15. Miyazaki et al. J Cosmet Sci. 2004 Sep-Oct;55(5):473-479.16. Xie A et al. Front Cell Infect Microbiol. 2023 Feb 16;13:1137275. doi: 10.3389/fcimb.2023.1137275.
17. Delavie Sciences. Skincare Science: Aeonia. Skincare from the Stars.
. Accessed December 12, 2024.
18. Stern C. CosmeticsDesign USA. September 7, 2023.
Acne Outcome Measures: Do they Incorporate LGBTQ+ Inclusive Language?
TOPLINE:
with heteronormative terms used in three of six measures addressing intimate relationships.
METHODOLOGY:
- Researchers conducted an inductive thematic analysis of 22 PROMs for acne, identified through a PubMed search.
- LGBTQ+-inclusive language was defined per the National Institutes of Health style guide.
- The analysis included 16 PROMs: Nine were acne-specific with 56 relevant items, 4 were dermatology-specific with 28 items, and 4 were health-related with 43 items.
TAKEAWAY:
- LGBTQ+-noninclusive language was identified in four of nine acne-specific PROMs — the Acne Disability Index (ADI), Acne Quality of Life Scale (AQOL), Acne-Quality of Life (Acne-QoL), and Cardiff Acne Disability Index (CADI) — but not in health-related or dermatology-specific PROMs.
- Among PROMs addressing intimate relationships, three of six acne-specific measures (CADI, ADI, and Acne-QoL) used heteronormative language, while three acne-specific PROMs, three dermatology-specific PROMs, and one health-related PROM used nonheteronormative terminology (such as “partner”).
- All PROMs contained items with nongendered pronouns (such as “I” or “you” instead of “he” or “she”). However, the AQOL included gendered language (“brothers” and “sisters,” rather than “siblings”).
- Two acne-specific PROMs demonstrated partial LGBTQ+ inclusivity, incorporating some but not all LGBTQ+ identities.
IN PRACTICE:
“Using LGBTQ+-inclusive language may promote the acquisition of accurate and relevant data for patient care and clinical trials and even enhance patient-clinician relationships,” the authors of the study wrote. “While demographics such as sex, age, race, and ethnicity are commonly considered during patient-reported outcome development and validation,” wrote the authors of an accompanying editorial, the study highlights that “sexual orientation and gender identity should also be considered to ensure these measures have similar performance across diverse populations.”
SOURCE:
The study was led by Twan Sia, BA, Department of Dermatology, Stanford University School of Medicine in California. The authors of the editorial were John S. Barbieri, MD, MBA, Department of Dermatology, Brigham and Women’s Hospital, Boston, Massachusetts, and Mya L. Roberson, MSPH, PhD, University of North Carolina at Chapel Hill.
LIMITATIONS:
The study was limited to the analysis of only English-language PROMs.
DISCLOSURES:
Two study authors disclosed receiving grants or personal fees from various sources, including pharmaceutical companies outside the submitted work. Barbieri disclosed receiving consulting fees from Dexcel Pharma and Honeydew Care; Roberson disclosed receiving consulting fees from the National Committee for Quality Assurance.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with heteronormative terms used in three of six measures addressing intimate relationships.
METHODOLOGY:
- Researchers conducted an inductive thematic analysis of 22 PROMs for acne, identified through a PubMed search.
- LGBTQ+-inclusive language was defined per the National Institutes of Health style guide.
- The analysis included 16 PROMs: Nine were acne-specific with 56 relevant items, 4 were dermatology-specific with 28 items, and 4 were health-related with 43 items.
TAKEAWAY:
- LGBTQ+-noninclusive language was identified in four of nine acne-specific PROMs — the Acne Disability Index (ADI), Acne Quality of Life Scale (AQOL), Acne-Quality of Life (Acne-QoL), and Cardiff Acne Disability Index (CADI) — but not in health-related or dermatology-specific PROMs.
- Among PROMs addressing intimate relationships, three of six acne-specific measures (CADI, ADI, and Acne-QoL) used heteronormative language, while three acne-specific PROMs, three dermatology-specific PROMs, and one health-related PROM used nonheteronormative terminology (such as “partner”).
- All PROMs contained items with nongendered pronouns (such as “I” or “you” instead of “he” or “she”). However, the AQOL included gendered language (“brothers” and “sisters,” rather than “siblings”).
- Two acne-specific PROMs demonstrated partial LGBTQ+ inclusivity, incorporating some but not all LGBTQ+ identities.
IN PRACTICE:
“Using LGBTQ+-inclusive language may promote the acquisition of accurate and relevant data for patient care and clinical trials and even enhance patient-clinician relationships,” the authors of the study wrote. “While demographics such as sex, age, race, and ethnicity are commonly considered during patient-reported outcome development and validation,” wrote the authors of an accompanying editorial, the study highlights that “sexual orientation and gender identity should also be considered to ensure these measures have similar performance across diverse populations.”
SOURCE:
The study was led by Twan Sia, BA, Department of Dermatology, Stanford University School of Medicine in California. The authors of the editorial were John S. Barbieri, MD, MBA, Department of Dermatology, Brigham and Women’s Hospital, Boston, Massachusetts, and Mya L. Roberson, MSPH, PhD, University of North Carolina at Chapel Hill.
LIMITATIONS:
The study was limited to the analysis of only English-language PROMs.
DISCLOSURES:
Two study authors disclosed receiving grants or personal fees from various sources, including pharmaceutical companies outside the submitted work. Barbieri disclosed receiving consulting fees from Dexcel Pharma and Honeydew Care; Roberson disclosed receiving consulting fees from the National Committee for Quality Assurance.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with heteronormative terms used in three of six measures addressing intimate relationships.
METHODOLOGY:
- Researchers conducted an inductive thematic analysis of 22 PROMs for acne, identified through a PubMed search.
- LGBTQ+-inclusive language was defined per the National Institutes of Health style guide.
- The analysis included 16 PROMs: Nine were acne-specific with 56 relevant items, 4 were dermatology-specific with 28 items, and 4 were health-related with 43 items.
TAKEAWAY:
- LGBTQ+-noninclusive language was identified in four of nine acne-specific PROMs — the Acne Disability Index (ADI), Acne Quality of Life Scale (AQOL), Acne-Quality of Life (Acne-QoL), and Cardiff Acne Disability Index (CADI) — but not in health-related or dermatology-specific PROMs.
- Among PROMs addressing intimate relationships, three of six acne-specific measures (CADI, ADI, and Acne-QoL) used heteronormative language, while three acne-specific PROMs, three dermatology-specific PROMs, and one health-related PROM used nonheteronormative terminology (such as “partner”).
- All PROMs contained items with nongendered pronouns (such as “I” or “you” instead of “he” or “she”). However, the AQOL included gendered language (“brothers” and “sisters,” rather than “siblings”).
- Two acne-specific PROMs demonstrated partial LGBTQ+ inclusivity, incorporating some but not all LGBTQ+ identities.
IN PRACTICE:
“Using LGBTQ+-inclusive language may promote the acquisition of accurate and relevant data for patient care and clinical trials and even enhance patient-clinician relationships,” the authors of the study wrote. “While demographics such as sex, age, race, and ethnicity are commonly considered during patient-reported outcome development and validation,” wrote the authors of an accompanying editorial, the study highlights that “sexual orientation and gender identity should also be considered to ensure these measures have similar performance across diverse populations.”
SOURCE:
The study was led by Twan Sia, BA, Department of Dermatology, Stanford University School of Medicine in California. The authors of the editorial were John S. Barbieri, MD, MBA, Department of Dermatology, Brigham and Women’s Hospital, Boston, Massachusetts, and Mya L. Roberson, MSPH, PhD, University of North Carolina at Chapel Hill.
LIMITATIONS:
The study was limited to the analysis of only English-language PROMs.
DISCLOSURES:
Two study authors disclosed receiving grants or personal fees from various sources, including pharmaceutical companies outside the submitted work. Barbieri disclosed receiving consulting fees from Dexcel Pharma and Honeydew Care; Roberson disclosed receiving consulting fees from the National Committee for Quality Assurance.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
There Are ‘Four Pillars of Acne Pathogenesis’: Make Sure Treatment Hits as Many as Possible
LAS VEGAS — For clinicians who rely on generic tretinoin 0.5% as their go-to treatment for patients with acne, Shanna Miranti, MPAS, PA-C, offers some straightforward advice: You can do better.
“Friends don’t let friends write generic tretinoin only because there are so many better options out there,” Miranti, who practices dermatology in Naples, Florida, said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference. “Don’t get lazy; your patients deserve better.”
In her wide-ranging presentation, Miranti described the four pillars of acne pathogenesis as increased sebum production caused by androgens, follicular hyperkeratinization in the pilosebaceous unit, colonization by Cutibacterium acnes (formerly Proprionibacterium acnes), and inflammation. Acne “starts with androgens, but this is a cascade, so you have to find treatment options that hit as many of these four pillars as possible,” Miranti explained. “If you’re only using generic tretinoin, you’re only hitting maybe two of the four pillars at best.”
She then discussed the best treatment options for each pillar:
Follicular plugging and hyperkeratinization. Topical retinoids, including tretinoin, adapalene, tazarotene, and trifarotene, are highly effective for this issue. Systemic isotretinoin is also a strong option. For patients who are pregnant or trying to conceive, azelaic acid is a helpful alternative.
Excessive sebum production and androgens. “This may be the genesis of when acne begins — during puberty,” Miranti said. “With rising androgens comes rising amounts of sebum.” The only topical treatment that specifically targets this is clascoterone (Winlevi), which should be applied twice daily. For systemic management of excessive sebum, isotretinoin is highly effective. In women, spironolactone (50 mg daily, or split into two doses) and oral contraceptives are also options.
Inflammation. Topical options include retinoids, antibiotics, benzoyl peroxide (BPO), topical dapsone, azelaic acid, and clascoterone. Systemic options include isotretinoin; the antibiotics doxycycline, minocycline, and sarecycline; spironolactone; and oral contraceptives. “So, when you see patients with intense inflammation, and they’re starting to get post-inflammatory erythema or post-inflammatory hyperpigmentation, you need something to address this inflammatory problem,” she noted.
C acnes. Topical treatment options include BPO and antibiotics. However, topical antibiotics should never be used alone, Miranti said; they must always be combined with BPO to prevent bacterial resistance. Oral options include sarecycline, “which has a low propensity for antibiotic resistance and spares the gut microbiome to some degree,” and the “old-school” antibiotics doxycycline, minocycline, and tetracycline. “But all oral antibiotics should be used concomitantly with BPO,” she added.
Regardless of which treatment is chosen for any pillar, Miranti emphasized that monotherapy with a single agent is often insufficient. “Historically, we have combined therapies to treat the multiple causes of acne,” she said. “The average number of acne products used per patient is 2.53, but that’s also the average number of copays. We have to be conscious of that. If you are a mom with four kids who are on acne medication, you want to minimize your copay burden. So, if you can find a topical medication that hits three out of the four pillars of acne pathogenesis, that would be fantastic.” The only topical that targets excess sebum is clascoterone, she noted, and the only medication that hits all four pillars is isotretinoin.
In October 2023, the Food and Drug Administration approved a once-daily topical gel for patients aged 12 years or older that contains clindamycin 1.2%, adapalene 0.15%, and BPO 3.1%. The first-ever triple combination therapy, known as Cabtreo, was released to pharmacies in March 2024. In a phase 2 trial, researchers randomized 394 patients aged 9 years or older with moderate to severe acne to once-daily IDP-126, one of three dyad combination gels, or vehicle gel for 12 weeks. Patients in the Cabtreo arm achieved significantly greater lesion reductions than those in the vehicle arm (inflammatory: 78.3% vs 45.1%; noninflammatory: 70.0% vs 37.6%; P < .001 for both). They also experienced lesion reductions that were 9.2%-16.6% greater than those observed with any of the dyad combination gels. Miranti characterized the study results as “pretty phenomenal,” noting that the ease of use makes Cabtreo stand out as a treatment option. “Simplicity drives compliance, and compliance drives results,” she said. “This is one product to apply once a day. Any of you who have a teenage son like me, you know it is hard to get them to brush their teeth twice a day, let alone take medicine before they leave the house in the morning. This can be a home run for a lot of patients, and not just our teenagers. Adult females have done very well with this medication.”
In a network meta-analysis, researchers reviewed 221 randomized controlled trials to compare the efficacy of pharmacologic treatment for acne. The most effective treatment in reducing inflammatory and noninflammatory lesions was oral isotretinoin, followed by Cabtreo.
Miranti disclosed being a speaker, consultant, and/or an advisory board member for Arcutis Biotherapeutics, Bausch Health, Dermavant Sciences, Galderma, Incyte, LEO Pharma, Eli Lilly, Sun Pharma, Swift USA, and Verrica Pharmaceuticals.
A version of this article first appeared on Medscape.com.
LAS VEGAS — For clinicians who rely on generic tretinoin 0.5% as their go-to treatment for patients with acne, Shanna Miranti, MPAS, PA-C, offers some straightforward advice: You can do better.
“Friends don’t let friends write generic tretinoin only because there are so many better options out there,” Miranti, who practices dermatology in Naples, Florida, said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference. “Don’t get lazy; your patients deserve better.”
In her wide-ranging presentation, Miranti described the four pillars of acne pathogenesis as increased sebum production caused by androgens, follicular hyperkeratinization in the pilosebaceous unit, colonization by Cutibacterium acnes (formerly Proprionibacterium acnes), and inflammation. Acne “starts with androgens, but this is a cascade, so you have to find treatment options that hit as many of these four pillars as possible,” Miranti explained. “If you’re only using generic tretinoin, you’re only hitting maybe two of the four pillars at best.”
She then discussed the best treatment options for each pillar:
Follicular plugging and hyperkeratinization. Topical retinoids, including tretinoin, adapalene, tazarotene, and trifarotene, are highly effective for this issue. Systemic isotretinoin is also a strong option. For patients who are pregnant or trying to conceive, azelaic acid is a helpful alternative.
Excessive sebum production and androgens. “This may be the genesis of when acne begins — during puberty,” Miranti said. “With rising androgens comes rising amounts of sebum.” The only topical treatment that specifically targets this is clascoterone (Winlevi), which should be applied twice daily. For systemic management of excessive sebum, isotretinoin is highly effective. In women, spironolactone (50 mg daily, or split into two doses) and oral contraceptives are also options.
Inflammation. Topical options include retinoids, antibiotics, benzoyl peroxide (BPO), topical dapsone, azelaic acid, and clascoterone. Systemic options include isotretinoin; the antibiotics doxycycline, minocycline, and sarecycline; spironolactone; and oral contraceptives. “So, when you see patients with intense inflammation, and they’re starting to get post-inflammatory erythema or post-inflammatory hyperpigmentation, you need something to address this inflammatory problem,” she noted.
C acnes. Topical treatment options include BPO and antibiotics. However, topical antibiotics should never be used alone, Miranti said; they must always be combined with BPO to prevent bacterial resistance. Oral options include sarecycline, “which has a low propensity for antibiotic resistance and spares the gut microbiome to some degree,” and the “old-school” antibiotics doxycycline, minocycline, and tetracycline. “But all oral antibiotics should be used concomitantly with BPO,” she added.
Regardless of which treatment is chosen for any pillar, Miranti emphasized that monotherapy with a single agent is often insufficient. “Historically, we have combined therapies to treat the multiple causes of acne,” she said. “The average number of acne products used per patient is 2.53, but that’s also the average number of copays. We have to be conscious of that. If you are a mom with four kids who are on acne medication, you want to minimize your copay burden. So, if you can find a topical medication that hits three out of the four pillars of acne pathogenesis, that would be fantastic.” The only topical that targets excess sebum is clascoterone, she noted, and the only medication that hits all four pillars is isotretinoin.
In October 2023, the Food and Drug Administration approved a once-daily topical gel for patients aged 12 years or older that contains clindamycin 1.2%, adapalene 0.15%, and BPO 3.1%. The first-ever triple combination therapy, known as Cabtreo, was released to pharmacies in March 2024. In a phase 2 trial, researchers randomized 394 patients aged 9 years or older with moderate to severe acne to once-daily IDP-126, one of three dyad combination gels, or vehicle gel for 12 weeks. Patients in the Cabtreo arm achieved significantly greater lesion reductions than those in the vehicle arm (inflammatory: 78.3% vs 45.1%; noninflammatory: 70.0% vs 37.6%; P < .001 for both). They also experienced lesion reductions that were 9.2%-16.6% greater than those observed with any of the dyad combination gels. Miranti characterized the study results as “pretty phenomenal,” noting that the ease of use makes Cabtreo stand out as a treatment option. “Simplicity drives compliance, and compliance drives results,” she said. “This is one product to apply once a day. Any of you who have a teenage son like me, you know it is hard to get them to brush their teeth twice a day, let alone take medicine before they leave the house in the morning. This can be a home run for a lot of patients, and not just our teenagers. Adult females have done very well with this medication.”
In a network meta-analysis, researchers reviewed 221 randomized controlled trials to compare the efficacy of pharmacologic treatment for acne. The most effective treatment in reducing inflammatory and noninflammatory lesions was oral isotretinoin, followed by Cabtreo.
Miranti disclosed being a speaker, consultant, and/or an advisory board member for Arcutis Biotherapeutics, Bausch Health, Dermavant Sciences, Galderma, Incyte, LEO Pharma, Eli Lilly, Sun Pharma, Swift USA, and Verrica Pharmaceuticals.
A version of this article first appeared on Medscape.com.
LAS VEGAS — For clinicians who rely on generic tretinoin 0.5% as their go-to treatment for patients with acne, Shanna Miranti, MPAS, PA-C, offers some straightforward advice: You can do better.
“Friends don’t let friends write generic tretinoin only because there are so many better options out there,” Miranti, who practices dermatology in Naples, Florida, said at the Society of Dermatology Physician Associates (SDPA) 22nd Annual Fall Dermatology Conference. “Don’t get lazy; your patients deserve better.”
In her wide-ranging presentation, Miranti described the four pillars of acne pathogenesis as increased sebum production caused by androgens, follicular hyperkeratinization in the pilosebaceous unit, colonization by Cutibacterium acnes (formerly Proprionibacterium acnes), and inflammation. Acne “starts with androgens, but this is a cascade, so you have to find treatment options that hit as many of these four pillars as possible,” Miranti explained. “If you’re only using generic tretinoin, you’re only hitting maybe two of the four pillars at best.”
She then discussed the best treatment options for each pillar:
Follicular plugging and hyperkeratinization. Topical retinoids, including tretinoin, adapalene, tazarotene, and trifarotene, are highly effective for this issue. Systemic isotretinoin is also a strong option. For patients who are pregnant or trying to conceive, azelaic acid is a helpful alternative.
Excessive sebum production and androgens. “This may be the genesis of when acne begins — during puberty,” Miranti said. “With rising androgens comes rising amounts of sebum.” The only topical treatment that specifically targets this is clascoterone (Winlevi), which should be applied twice daily. For systemic management of excessive sebum, isotretinoin is highly effective. In women, spironolactone (50 mg daily, or split into two doses) and oral contraceptives are also options.
Inflammation. Topical options include retinoids, antibiotics, benzoyl peroxide (BPO), topical dapsone, azelaic acid, and clascoterone. Systemic options include isotretinoin; the antibiotics doxycycline, minocycline, and sarecycline; spironolactone; and oral contraceptives. “So, when you see patients with intense inflammation, and they’re starting to get post-inflammatory erythema or post-inflammatory hyperpigmentation, you need something to address this inflammatory problem,” she noted.
C acnes. Topical treatment options include BPO and antibiotics. However, topical antibiotics should never be used alone, Miranti said; they must always be combined with BPO to prevent bacterial resistance. Oral options include sarecycline, “which has a low propensity for antibiotic resistance and spares the gut microbiome to some degree,” and the “old-school” antibiotics doxycycline, minocycline, and tetracycline. “But all oral antibiotics should be used concomitantly with BPO,” she added.
Regardless of which treatment is chosen for any pillar, Miranti emphasized that monotherapy with a single agent is often insufficient. “Historically, we have combined therapies to treat the multiple causes of acne,” she said. “The average number of acne products used per patient is 2.53, but that’s also the average number of copays. We have to be conscious of that. If you are a mom with four kids who are on acne medication, you want to minimize your copay burden. So, if you can find a topical medication that hits three out of the four pillars of acne pathogenesis, that would be fantastic.” The only topical that targets excess sebum is clascoterone, she noted, and the only medication that hits all four pillars is isotretinoin.
In October 2023, the Food and Drug Administration approved a once-daily topical gel for patients aged 12 years or older that contains clindamycin 1.2%, adapalene 0.15%, and BPO 3.1%. The first-ever triple combination therapy, known as Cabtreo, was released to pharmacies in March 2024. In a phase 2 trial, researchers randomized 394 patients aged 9 years or older with moderate to severe acne to once-daily IDP-126, one of three dyad combination gels, or vehicle gel for 12 weeks. Patients in the Cabtreo arm achieved significantly greater lesion reductions than those in the vehicle arm (inflammatory: 78.3% vs 45.1%; noninflammatory: 70.0% vs 37.6%; P < .001 for both). They also experienced lesion reductions that were 9.2%-16.6% greater than those observed with any of the dyad combination gels. Miranti characterized the study results as “pretty phenomenal,” noting that the ease of use makes Cabtreo stand out as a treatment option. “Simplicity drives compliance, and compliance drives results,” she said. “This is one product to apply once a day. Any of you who have a teenage son like me, you know it is hard to get them to brush their teeth twice a day, let alone take medicine before they leave the house in the morning. This can be a home run for a lot of patients, and not just our teenagers. Adult females have done very well with this medication.”
In a network meta-analysis, researchers reviewed 221 randomized controlled trials to compare the efficacy of pharmacologic treatment for acne. The most effective treatment in reducing inflammatory and noninflammatory lesions was oral isotretinoin, followed by Cabtreo.
Miranti disclosed being a speaker, consultant, and/or an advisory board member for Arcutis Biotherapeutics, Bausch Health, Dermavant Sciences, Galderma, Incyte, LEO Pharma, Eli Lilly, Sun Pharma, Swift USA, and Verrica Pharmaceuticals.
A version of this article first appeared on Medscape.com.
FROM SDPA 2024
Levonorgestrel IUDs Linked to Higher Skin Side Effects
TOPLINE:
, with some differences between the available levonorgestrel IUDs.
METHODOLOGY:
- Researchers reviewed the US Food and Drug Administration (FDA) Adverse Events Reporting System (FAERS) through December 2023 for adverse events associated with levonorgestrel IUDs where IUDs were the only suspected cause, focusing on acne, alopecia, and hirsutism.
- They included 139,348 reports for the levonorgestrel IUDs (Mirena, Liletta, Kyleena, Skyla) and 50,450 reports for the copper IUD (Paragard).
TAKEAWAY:
- Levonorgestrel IUD users showed higher odds of reporting acne (odds ratio [OR], 3.21), alopecia (OR, 5.96), and hirsutism (OR, 15.48; all P < .0001) than copper IUD users.
- The Kyleena 19.5 mg levonorgestrel IUD was associated with the highest odds of acne reports (OR, 3.42), followed by the Mirena 52 mg (OR, 3.40) and Skyla 13.5 mg (OR, 2.30) levonorgestrel IUDs (all P < .0001).
- The Mirena IUD was associated with the highest odds of alopecia and hirsutism reports (OR, 6.62 and 17.43, respectively), followed by the Kyleena (ORs, 2.90 and 8.17, respectively) and Skyla (ORs, 2.69 and 1.48, respectively) IUDs (all P < .0001).
- Reports of acne, alopecia, and hirsutism were not significantly different between the Liletta 52 mg levonorgestrel IUD and the copper IUD.
IN PRACTICE:
“Overall, we identified significant associations between levonorgestrel IUDs and androgenic cutaneous adverse events,” the authors wrote. “Counseling prior to initiation of levonorgestrel IUDs should include information on possible cutaneous AEs including acne, alopecia, and hirsutism to guide contraceptive shared decision making,” they added.
SOURCE:
The study was led by Lydia Cassard, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio, and was published online November 3 in Journal of the American Academy of Dermatology.
LIMITATIONS:
FAERS database reports could not be verified, and differences in FDA approval dates for IUDs could have influenced reporting rates. Moreover, a lack of data on prior medication use limits the ability to determine if these AEs are a result of changes in androgenic or antiandrogenic medication use. Cutaneous adverse events associated with copper IUDs may have been underreported because of assumptions that a nonhormonal device would not cause these adverse events.
DISCLOSURES:
The authors did not report any funding source or conflict of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, with some differences between the available levonorgestrel IUDs.
METHODOLOGY:
- Researchers reviewed the US Food and Drug Administration (FDA) Adverse Events Reporting System (FAERS) through December 2023 for adverse events associated with levonorgestrel IUDs where IUDs were the only suspected cause, focusing on acne, alopecia, and hirsutism.
- They included 139,348 reports for the levonorgestrel IUDs (Mirena, Liletta, Kyleena, Skyla) and 50,450 reports for the copper IUD (Paragard).
TAKEAWAY:
- Levonorgestrel IUD users showed higher odds of reporting acne (odds ratio [OR], 3.21), alopecia (OR, 5.96), and hirsutism (OR, 15.48; all P < .0001) than copper IUD users.
- The Kyleena 19.5 mg levonorgestrel IUD was associated with the highest odds of acne reports (OR, 3.42), followed by the Mirena 52 mg (OR, 3.40) and Skyla 13.5 mg (OR, 2.30) levonorgestrel IUDs (all P < .0001).
- The Mirena IUD was associated with the highest odds of alopecia and hirsutism reports (OR, 6.62 and 17.43, respectively), followed by the Kyleena (ORs, 2.90 and 8.17, respectively) and Skyla (ORs, 2.69 and 1.48, respectively) IUDs (all P < .0001).
- Reports of acne, alopecia, and hirsutism were not significantly different between the Liletta 52 mg levonorgestrel IUD and the copper IUD.
IN PRACTICE:
“Overall, we identified significant associations between levonorgestrel IUDs and androgenic cutaneous adverse events,” the authors wrote. “Counseling prior to initiation of levonorgestrel IUDs should include information on possible cutaneous AEs including acne, alopecia, and hirsutism to guide contraceptive shared decision making,” they added.
SOURCE:
The study was led by Lydia Cassard, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio, and was published online November 3 in Journal of the American Academy of Dermatology.
LIMITATIONS:
FAERS database reports could not be verified, and differences in FDA approval dates for IUDs could have influenced reporting rates. Moreover, a lack of data on prior medication use limits the ability to determine if these AEs are a result of changes in androgenic or antiandrogenic medication use. Cutaneous adverse events associated with copper IUDs may have been underreported because of assumptions that a nonhormonal device would not cause these adverse events.
DISCLOSURES:
The authors did not report any funding source or conflict of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, with some differences between the available levonorgestrel IUDs.
METHODOLOGY:
- Researchers reviewed the US Food and Drug Administration (FDA) Adverse Events Reporting System (FAERS) through December 2023 for adverse events associated with levonorgestrel IUDs where IUDs were the only suspected cause, focusing on acne, alopecia, and hirsutism.
- They included 139,348 reports for the levonorgestrel IUDs (Mirena, Liletta, Kyleena, Skyla) and 50,450 reports for the copper IUD (Paragard).
TAKEAWAY:
- Levonorgestrel IUD users showed higher odds of reporting acne (odds ratio [OR], 3.21), alopecia (OR, 5.96), and hirsutism (OR, 15.48; all P < .0001) than copper IUD users.
- The Kyleena 19.5 mg levonorgestrel IUD was associated with the highest odds of acne reports (OR, 3.42), followed by the Mirena 52 mg (OR, 3.40) and Skyla 13.5 mg (OR, 2.30) levonorgestrel IUDs (all P < .0001).
- The Mirena IUD was associated with the highest odds of alopecia and hirsutism reports (OR, 6.62 and 17.43, respectively), followed by the Kyleena (ORs, 2.90 and 8.17, respectively) and Skyla (ORs, 2.69 and 1.48, respectively) IUDs (all P < .0001).
- Reports of acne, alopecia, and hirsutism were not significantly different between the Liletta 52 mg levonorgestrel IUD and the copper IUD.
IN PRACTICE:
“Overall, we identified significant associations between levonorgestrel IUDs and androgenic cutaneous adverse events,” the authors wrote. “Counseling prior to initiation of levonorgestrel IUDs should include information on possible cutaneous AEs including acne, alopecia, and hirsutism to guide contraceptive shared decision making,” they added.
SOURCE:
The study was led by Lydia Cassard, Cleveland Clinic Lerner College of Medicine, Cleveland, Ohio, and was published online November 3 in Journal of the American Academy of Dermatology.
LIMITATIONS:
FAERS database reports could not be verified, and differences in FDA approval dates for IUDs could have influenced reporting rates. Moreover, a lack of data on prior medication use limits the ability to determine if these AEs are a result of changes in androgenic or antiandrogenic medication use. Cutaneous adverse events associated with copper IUDs may have been underreported because of assumptions that a nonhormonal device would not cause these adverse events.
DISCLOSURES:
The authors did not report any funding source or conflict of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.