User login
Obstructive sleep apnea (OSA) is a common cause of daytime sleepiness, and severe OSA is a risk factor for hypertension, cardiovascular events, atrial fibrillation (AF), insulin resistance, cognitive impairment, motor vehicle crashes, adverse pregnancy outcomes, and overall mortality.1-8 The hazard ratio for mortality for patients with severe OSA may be as high as 3.8.5
OSA is diagnosed by the apnea-hypopnea index (AHI), defined as the number of apnea or hypopnea events per hour as determined by polysomnography. An AHI score ≤ 5 is considered normal; > 5 to ≤ 15 is mild; > 15 to < 30 is moderate; and ≥ 30 is severe. Most studies of OSA treatment use reduction of AHI as the measure of treatment effectiveness, and several types of treatment improve AHI.
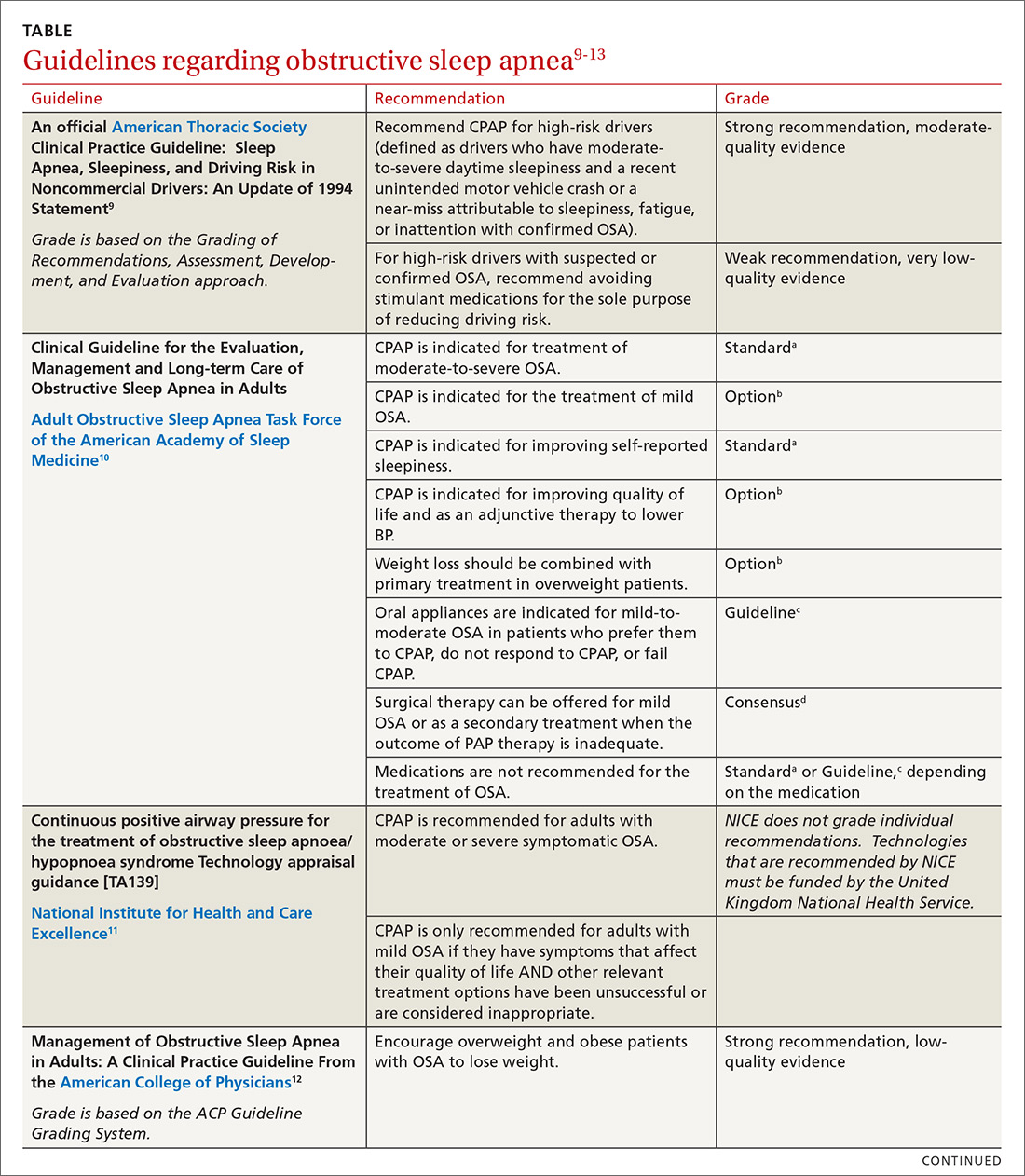
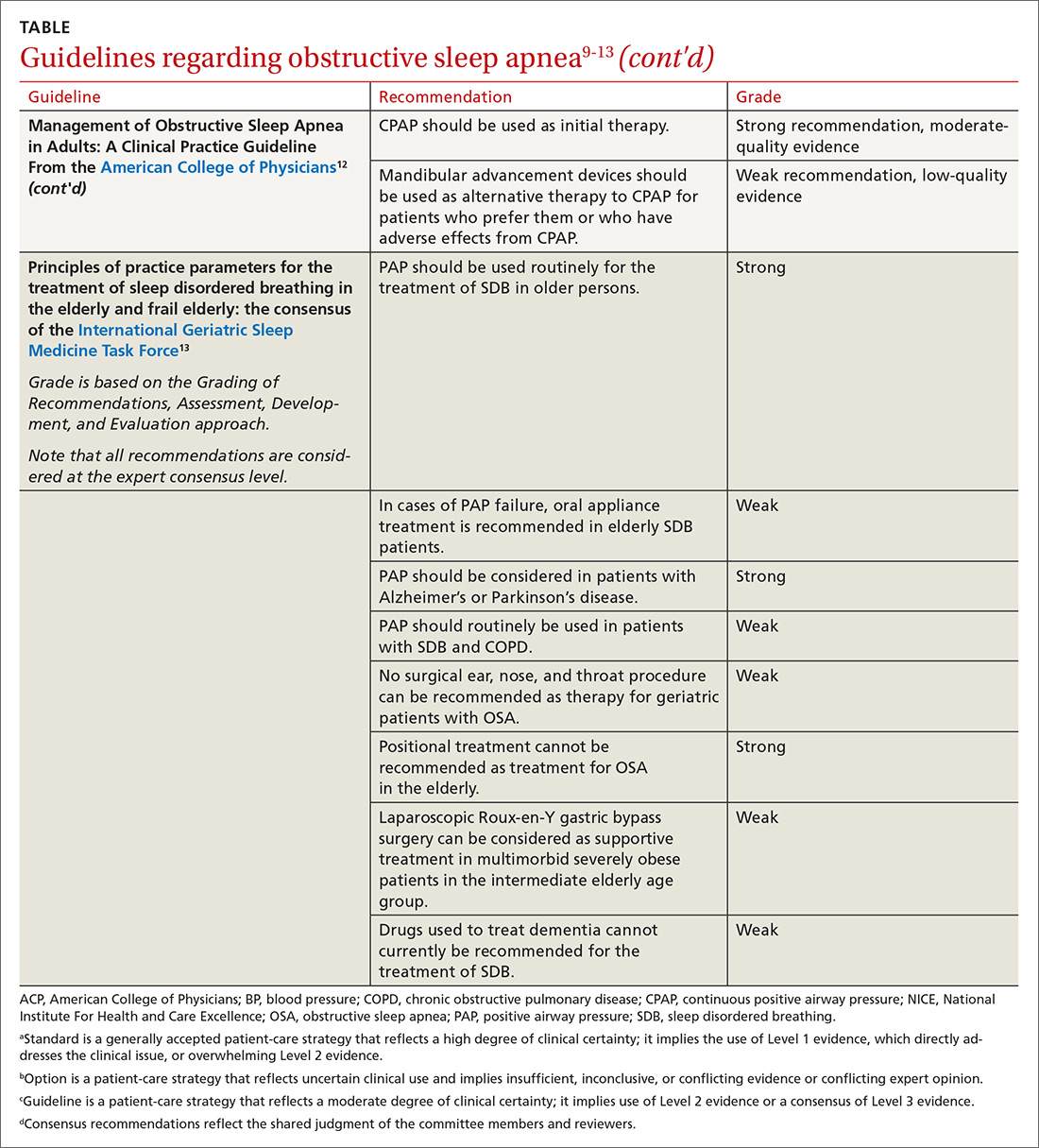
In family medicine, we generally want to know whether treatment of OSA will improve outcomes of significance to patients. A recent systematic review of evidence for the US Preventive Services Task Force found that it was unclear whether OSA treatment improved most health outcomes, including mortality, cardiovascular events, or motor vehicle crashes.6 Several other organizations have published guidelines regarding OSA treatment; these guidelines are reviewed in the TABLE.9-13
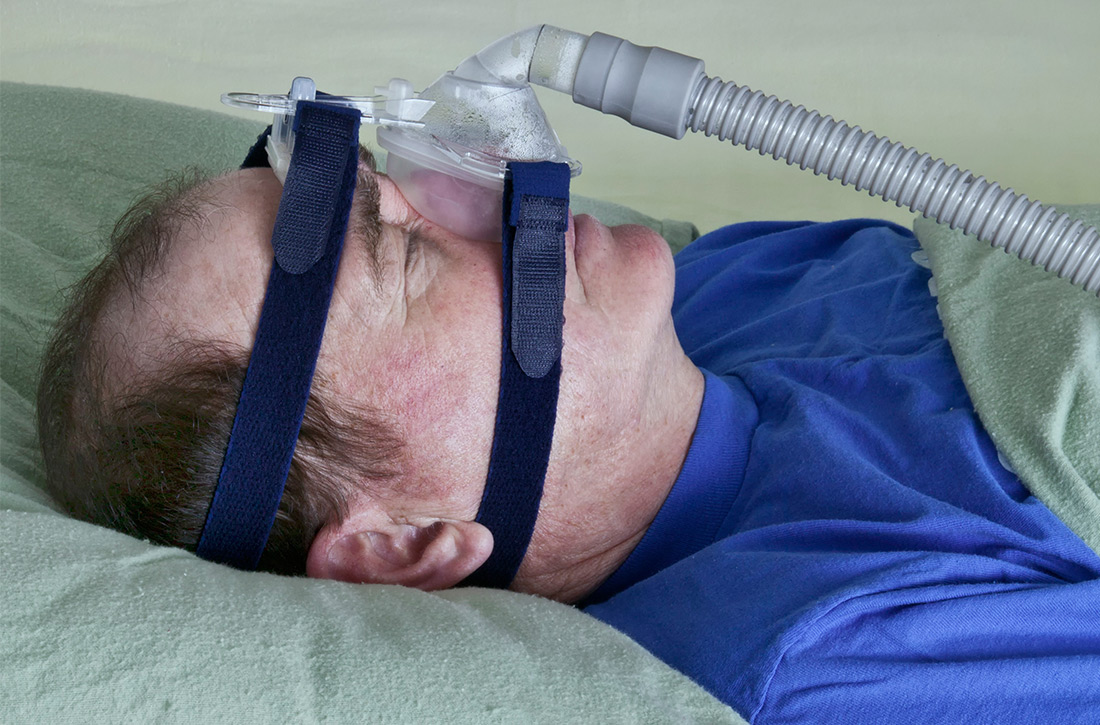
This article summarizes the current evidence surrounding the effect of treatment of OSA on outcomes of significance to patients. While multiple treatments have been advocated for patients with OSA, positive airway pressure (PAP) is the most widely used and studied and is recommended as standard treatment by most guidelines.9-13 Most available evidence about patient-oriented outcomes involves treatment with PAP; where there is evidence about the effect of other OSA treatments on a particular outcome, that evidence is also summarized.
Benefits of OSA treatment
Patients with OSA who have excessive daytime sleepiness can gain substantial symptomatic benefit from treatment of their OSA with PAP or oral appliances (OAs), and might benefit from hypoglossal nerve stimulation or other surgical treatment. PAP is probably more effective than OAs in patients who use it ≥ 4 hours/night, but it is more difficult to comply with PAP.14
Evidence that treatment of asymptomatic OSA benefits other medical conditions is often conflicting. Given the low risk of treatment, it is reasonable to consider offering a trial of treatment, preferably with PAP, to asymptomatic patients with moderate-to-severe OSA and certain comorbidities, including obesity, resistant hypertension, high cardiovascular risk, congestive heart failure (CHF), AF, diabetes that is difficult to control, and pregnancy. Such patients should be strongly encouraged to use PAP ≥ 4 hours/night, and should be advised that benefits may not be immediately apparent.
Treatment of OSA improves daytime sleepiness
Daytime sleepiness is typically measured with the Epworth Sleepiness Scale (ESS), a self-administered questionnaire assessing a person’s level of drowsiness and propensity to fall asleep in 8 different daytime situations. Each situation is scored between 0 (would never doze) and 3 (high chance of dozing), with the scores then totaled to provide an overall score between 0 and 24. A score > 10 is considered abnormal.
Continue to: Treament of OSA...
Treatment of OSA with either PAP or OAs significantly improves ESS scores, with PAP being more effective.13 The difference appears to widen in patients with greater daytime sleepiness; in other words, patients with greater daytime sleepiness will gain even greater benefit from PAP, both overall and when compared with OAs.15
One randomized trial of an intensive lifestyle modification program for patients with OSA failed to show improvement in the ESS in the intention-to-treat analysis, but did demonstrate a 2.4-point greater reduction in ESS scores in those patients who successfully followed the program (achieving weight loss).16 Surgical treatments for OSA, such as uvulopalatopharyngoplasty or maxillary advancement, have been shown in some (but not all) studies to improve ESS scores; the different types of surgical treatment and the heterogeneity of studies prevents estimation of effect size.17 A meta-analysis of case series studies of hypoglossal nerve stimulation reported a mean improvement of 4.5 points on the ESS;18 comparison with other interventions is lacking.
Improved quality of life
Both PAP and OAs have been shown to improve sleep-related quality of life in patients with OSA. However, while the improvement is statistically significant, the effect size is small.14
That could be said of a study by Lewis et al.19 These researchers randomized patients with moderate-to-severe OSA and known coronary artery disease (CAD) or at least 3 risk factors for CAD to receive PAP, nocturnal oxygen, or lifestyle education.19 The patients randomized to receive PAP improved vitality scores by only 3.6 points on a 100-point scale; this was significantly better statistically than the improvement achieved by those randomized to lifestyle education. Smaller improvements were noted in depression, social function, and general health. Patients who had more daytime sleepiness at baseline had greater improvements in function.19
Cognitive function findings are mixed
In a systematic review published in 2004, Aloia et al4 found measurable impairments on neuropsychological tests of global cognitive functioning, attention/vigilance, executive functioning, memory, psychomotor function, and constructional abilities in patients with OSA. The results of treatment studies (all but 1 using PAP) were mixed. No studies showed improvement in psychomotor speed or language, and studies disagreed on whether treatment produced benefits in global cognition, attention, or executive functions.4
Continue to: Findings of more recent studies...
Findings of more recent studies remain mixed. A 3-month Spanish trial of PAP in older adults with severe OSA showed improvement in 2 of 4 neuropsychological tests of cognitive function; this was a secondary outcome measure.20 The PREDICT trial in the United Kingdom demonstrated a reduction in daytime sleepiness but no improvement in cognitive function in PAP-treated older adults with OSA but without dementia over a 1-year period.21
In contrast, a French long-term study of adults ages ≥ 65 years with severe (but not necessarily symptomatic) OSA showed better maintenance of memory performance; these results must be interpreted with caution, however, because the study was not randomized, controlled, or blinded, and the results were not adjusted for potential confounders.22 The severity of OSA may influence the impact of PAP treatment on cognitive function.
The prevalence of OSA in patients with dementia is high, and more severe dementia is associated with more severe OSA.23 Although it is intuitive that disrupted sleep may worsen cognitive function, and that treatment could improve it, minimal benefit on cognitive function was shown by neuropsychological testing in patients with Alzheimer’s disease and OSA treated with continuous positive airway pressure (CPAP) vs sham CPAP in 1 small short-term randomized trial.23
In another study of patients with Alzheimer’s disease, this time an observational (nonrandomized, non-controlled, single-blind) study of patients who also had severe symptomatic OSA, researchers followed the patients for 3 years and found a significant delay in median annual cognitive decline of 1.5 points per year on the Mini-Mental Status Examination in patients treated with PAP compared with those who did not receive PAP treatment.24
Hypertension: Small but positive results
A meta-analysis of PAP use in patients with OSA and resistant hypertension (defined as inadequate control while taking at least 3 antihypertensive agents or control requiring at least 4 agents) documented significant blood pressure (BP) lowering, with a pooled estimate of -7.21 mm Hg systolic and -4.99 mm Hg diastolic.25 The decrease in BP was demonstrated in both sleepy and non-sleepy subjects.
Continue to: Multiple studies have...
Multiple studies have shown a small reduction in BP readings (generally about 2 mm Hg) with PAP treatment in nonresistant hypertensive patients with OSA who are sleepy.26 Conversely, the literature is mixed on whether treatment of non-sleepy patients with OSA reduces BP. One long-term study demonstrated a small (1.89 mm Hg systolic, 2.19 mm Hg diastolic) BP reduction effect of PAP in non-sleepy subjects with OSA.27 Similarly, research has shown mandibular advancement devices to lower BP in patients with OSA, in a range similar to that achieved with PAP.28 Whether very small reductions in BP improve important clinical outcomes such as stroke or heart disease is unknown.
CV risk: Again, findings are mixed
The SAVE study is the largest randomized investigation of the effect of treatment of OSA with PAP for secondary prevention of cardiovascular events.29 The trial involved 2717 adults with cardiovascular disease, moderate-to-severe OSA, and minimal sleepiness, and had as its primary composite endpoint death from cardiovascular causes, myocardial infarction (MI), stroke, hospitalization for unstable angina, heart failure, or transient ischemic attack. Patients with severe daytime sleepiness or severe hypoxemia were excluded. The study found no difference between PAP and usual care in the primary outcome, despite a significant reduction in the AHI from a mean of 29 at baseline to 3.7 with PAP treatment.
Similarly, a randomized controlled trial (RCT) of 725 patients with non-sleepy OSA failed to show a reduction in cardiovascular events or in the development of hypertension.30 Peker et al31 randomized 244 adults with recently revascularized coronary artery disease and OSA without daytime sleepiness to auto-titrating CPAP or usual care and did not find a statistically significant difference in revascularization, MI, stroke, or cardiovascular mortality; however, those patients who were compliant with CPAP for ≥ 4 hours/night did have a statistically significant reduction in the combined endpoint.
In contrast, a trial of patients with first-ever stroke and moderate-to-severe OSA who were randomized to early nasal CPAP or usual care demonstrated better 5-year cardiovascular survival for the patients in the CPAP group, and a trend toward better cardiovascular event-free survival.32 Degree of daytime sleepiness was not stated in this study.
A recent meta-analysis of RCTs failed to find a reduction in major adverse cardiovascular events (MACE) in patients with moderate-to-severe OSA treated with PAP.33 In this study, subgroup analysis documented benefit in patients who were adherent with PAP for ≥ 4 hours/night. A larger meta-analysis, however, did not find a reduction in MACE even in the adherent subgroup.34
Continue to: AF and OSA
AF and OSA: An interesting relationship
OSA is an independent risk factor for AF, approximately doubling the risk.35 A review of 10,132 patients with AF (1841 with OSA) in a large observational study demonstrated no difference in outcomes of all-cause mortality, first hospitalization, major bleeding, or major cardiovascular events in OSA patients who were or were not treated with PAP. The PAP-treated patients did have a slightly lower (16% vs 18%) risk of worsening of AF over 2 years.36 Overall, AF patients with OSA had more symptoms and higher admission rates, but no difference in overall mortality or MACE. Observational studies have suggested that PAP treatment of OSA facilitates maintenance of normal sinus rhythm after cardioversion and after ablation.37
CHF: Results look promising
In one small study, 24 patients with heart failure with reduced ejection fraction who were optimally medically treated were randomized to receive PAP or sham PAP for 1 month.38 The treatment group demonstrated reduced systolic BP, reduced end systolic dimension, and significant improvement in ejection fraction from 25 ± 2.8% to 33.8 ± 2.4%.
OSA Tx improves insulin sensitivity
OSA is associated with impaired glucose tolerance, and PAP treatment of OSA has been documented to improve insulin sensitivity.39,40 An efficacy study utilizing PAP in a laboratory setting for 8 hours/night demonstrated significant reduction in fasting blood sugar and a reduction in the dawn phenomenon (an increase in early morning fasting glucose as a result of rebound from hypoglycemia during sleep).39 A 2015 meta-analysis of short-term studies also showed improvement in insulin sensitivity in OSA patients treated with PAP, but failed to find any reduction in A1C or in body mass index.40
All-cause mortality: Difference in findings between short- and long-term studies
Yu et al’s34 meta-analysis of 10 RCTs involving 7266 participants found no difference in mortality in treated (vs no treatment or sham treatment) OSA patients. This was true even in the more adherent subgroup. These studies were relatively short-term, with the longest mean follow-up being 68 months.
However, several longer-term population-based studies have suggested that OSA treatment improves all-cause mortality. An 18-year follow-up of a Wisconsin cohort documented dramatically increased mortality in patients with severe sleep apnea; mortality was even higher when patients treated with PAP were removed from the analysis, suggesting that PAP treatment was protective, mainly for cardiovascular death.5
Continue to: A Danish registry...
A Danish registry documented that patients treated with CPAP had higher rates of comorbidities before and during treatment; when these comorbidities were controlled, men ages ≥ 60 years had improved survival when treated with CPAP. There was no survival benefit in women.41
A recent analysis—the Sleep Heart Health Study—followed patients with obesity and severe OSA for a mean of 11.1 years and calculated a hazard ratio for all-cause mortality associated with prescribed PAP therapy of 0.58 (95% confidence interval [CI], 0.35-0.96) after propensity matching.42 The difference in mortality appeared 6 to 7 years after PAP therapy was prescribed. This delay may explain the failure of shorter-term studies to demonstrate evidence of benefit.
OSA Tx reduces motor vehicle crashes
Drowsy driving is widely accepted as a risk for motor vehicle crashes. Successful treatment of OSA with PAP has been shown to improve driving performance on a driving simulator.43 An analysis of 15 studies similarly demonstrated a significant reduction in driving accidents (incident rate ratio [IRR] = 0.45) and in near-miss accidents (IRR = 0.23) in patients with OSA treated with CPAP.44
Pulmonary hypertension: OSA Tx lowers pulmonary arterial pressure
Patients with OSA have higher than expected rates of pulmonary arterial hypertension—as high as 22%—documented by pulmonary artery catheterization findings.45 A meta-analysis of studies that examined the effect of PAP in patients with OSA and coexisting pulmonary hypertension but without other overt pulmonary or cardiac disease found significant reductions in pulmonary artery pressure.46 Whether this finding translates into improved patient-oriented outcomes is unknown.
OSA and pregnancy outcomes
A national cohort study demonstrated that OSA is an independent risk factor for multiple adverse pregnancy outcomes, including gestational diabetes, hypertensive disorders in pregnancy, intrauterine growth retardation, and stillbirth.7 OSA was also associated with the rare serious adverse outcomes of congestive heart failure, cardiomyopathy, and pulmonary embolism.7 There is little evidence to date with which to determine whether treatment of OSA improves outcomes, but PAP treatment is documented to be safe in pregnant women.8
CORRESPONDENCE
Stephen C. Sorsby, MD, MHA, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 530, Little Rock, AR 72205; [email protected].
1. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384.
2. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
3. Iftikhar IH, Hoyos CM, Phillips CL, et al. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med. 2015;11:475-485.
4. Aloia MS, Arnedt JT, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772-785.
5. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071-1078.
6. Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:415-433.
7. Bourjeily G, Danilack VA, Bublitz MA, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;35:50-57.
8. Booth JM, Tonidandel AM. Peripartum management of obstructive sleep apnea. Clin Obstet Gyn. 2017;60:405-417.
9. Strohl KP, Brown DB, Collop N, et al. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med. 2013;187:1259-1266.
10. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
11. National Institute for Health and Care Excellence. Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. Technology appraisal guidance [TA139]. https://www.nice.org.uk/guidance/ta139. Revised February 2012. Accessed October 28, 2019.
12. Qaseem A, Holty JE, Owens DK, et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471-483.
13. Netzer NC, Ancoli-Israel S, Bliwise DL, et al. Principles of practice parameters for the treatment of sleep disordered breathing in the elderly and frail elderly: the consensus of the International Geriatric Sleep Medicine Task Force. Eur Respir J. 2016;48:992-1018.
14. Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879-887.
15. Bratton DJ, Gaisl T, Schlatzer C, et al. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med. 2015;3:869-878.
16. Ng SSS, Chan RSM, Woo J, et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest. 2015;148:1193-1203.
17. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;4:CD001004.
18. Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015; 125:1254-1264.
19. Lewis EF, Rui W, Punjabi N, et al. Impact of continuous positive airway pressure and oxygen on health status in patients with coronary heart disease, cardiovascular risk factors, and obstructive sleep apnea: A Heart Biomarker Evaluation in Apnea Treatment (HEARTBEAT) analysis. Am Heart J. 2017;189:59-67.
20. Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. 2015;46:142-151.
21. McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804-812.
22. Crawford-Achour E, Dauphinot V, Martin MS, et al. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe OSA: the PROOF study. J Clin Sleep Med. 2015;11:519-524.
23. Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076-2081.
24. Troussière AC, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1405-1408.
25. Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757-764.
26. Montesi SB, Edwards BA, Malhotra A, et al. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587-596.
27. Barbé F, Durán-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718-726.
28. Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280-2293.
29. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919-931.
30. Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161-2168.
31. Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613-620.
32. Parra O, Sánchez-Armengol Á, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24:47-53.
33. Abuzaid AS, Al Ashray HS, Elbadaway A, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure in patients with obstructive sleep apnea. Am J Card. 2017;120:693-699.
34. Yu J, Zhou Z, McEvoy D, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318:156-166.
35. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the incident risk of atrial fibrillation. J Amer Coll of Card. 2007;49:565-571.
36. Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169:647-654.e2.
37. Nalliah CJ, Sanders P, Kalman JM. Obstructive sleep apnea treatment and atrial fibrillation: a need for definitive evidence. J Cardiovasc Electrophysiol. 2016;27:1001-1010.
38. Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233-1241
39. Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes: a randomized controlled trial. Am J Respir Crit Care Med. 2015;192:96-105.
40. Feng Y, Zhang Z, Dong ZZ. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15005.
41. Jennum P, Tonnesen P, Ibsen R, et al. Obstructive sleep apnea: effect of comorbidities and positive airway pressure on all-cause mortality. Sleep Med. 2017;36:62-66.
42. Lisan Q, Van Sloten T, Marques Vidal P, et al. Association of positive airway pressure prescription with mortality in patients with obesity and severe obstructive sleep apnea: the sleep heart health study. JAMA Otolaryngol Head Neck Surg. 2019;145:509-515.
43. Mazza S, Pépin JL, Naëgelé B, et al. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. Eur Respir J. 2006;28:1020-1028.
44. Antonopoulos CN, Sergentanis TN, Daskalopoulou SS, et al. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev. 2011;15:301-310.
45. Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol. 2009;104:1300-1306.
46. Imran TF, Ghazipura M, Liu S, et al. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: a meta-analysis. Heart Fail Rev. 2016;21:591-598.
Obstructive sleep apnea (OSA) is a common cause of daytime sleepiness, and severe OSA is a risk factor for hypertension, cardiovascular events, atrial fibrillation (AF), insulin resistance, cognitive impairment, motor vehicle crashes, adverse pregnancy outcomes, and overall mortality.1-8 The hazard ratio for mortality for patients with severe OSA may be as high as 3.8.5
OSA is diagnosed by the apnea-hypopnea index (AHI), defined as the number of apnea or hypopnea events per hour as determined by polysomnography. An AHI score ≤ 5 is considered normal; > 5 to ≤ 15 is mild; > 15 to < 30 is moderate; and ≥ 30 is severe. Most studies of OSA treatment use reduction of AHI as the measure of treatment effectiveness, and several types of treatment improve AHI.
In family medicine, we generally want to know whether treatment of OSA will improve outcomes of significance to patients. A recent systematic review of evidence for the US Preventive Services Task Force found that it was unclear whether OSA treatment improved most health outcomes, including mortality, cardiovascular events, or motor vehicle crashes.6 Several other organizations have published guidelines regarding OSA treatment; these guidelines are reviewed in the TABLE.9-13

This article summarizes the current evidence surrounding the effect of treatment of OSA on outcomes of significance to patients. While multiple treatments have been advocated for patients with OSA, positive airway pressure (PAP) is the most widely used and studied and is recommended as standard treatment by most guidelines.9-13 Most available evidence about patient-oriented outcomes involves treatment with PAP; where there is evidence about the effect of other OSA treatments on a particular outcome, that evidence is also summarized.

Benefits of OSA treatment
Patients with OSA who have excessive daytime sleepiness can gain substantial symptomatic benefit from treatment of their OSA with PAP or oral appliances (OAs), and might benefit from hypoglossal nerve stimulation or other surgical treatment. PAP is probably more effective than OAs in patients who use it ≥ 4 hours/night, but it is more difficult to comply with PAP.14
Evidence that treatment of asymptomatic OSA benefits other medical conditions is often conflicting. Given the low risk of treatment, it is reasonable to consider offering a trial of treatment, preferably with PAP, to asymptomatic patients with moderate-to-severe OSA and certain comorbidities, including obesity, resistant hypertension, high cardiovascular risk, congestive heart failure (CHF), AF, diabetes that is difficult to control, and pregnancy. Such patients should be strongly encouraged to use PAP ≥ 4 hours/night, and should be advised that benefits may not be immediately apparent.
Treatment of OSA improves daytime sleepiness
Daytime sleepiness is typically measured with the Epworth Sleepiness Scale (ESS), a self-administered questionnaire assessing a person’s level of drowsiness and propensity to fall asleep in 8 different daytime situations. Each situation is scored between 0 (would never doze) and 3 (high chance of dozing), with the scores then totaled to provide an overall score between 0 and 24. A score > 10 is considered abnormal.
Continue to: Treament of OSA...
Treatment of OSA with either PAP or OAs significantly improves ESS scores, with PAP being more effective.13 The difference appears to widen in patients with greater daytime sleepiness; in other words, patients with greater daytime sleepiness will gain even greater benefit from PAP, both overall and when compared with OAs.15
One randomized trial of an intensive lifestyle modification program for patients with OSA failed to show improvement in the ESS in the intention-to-treat analysis, but did demonstrate a 2.4-point greater reduction in ESS scores in those patients who successfully followed the program (achieving weight loss).16 Surgical treatments for OSA, such as uvulopalatopharyngoplasty or maxillary advancement, have been shown in some (but not all) studies to improve ESS scores; the different types of surgical treatment and the heterogeneity of studies prevents estimation of effect size.17 A meta-analysis of case series studies of hypoglossal nerve stimulation reported a mean improvement of 4.5 points on the ESS;18 comparison with other interventions is lacking.
Improved quality of life
Both PAP and OAs have been shown to improve sleep-related quality of life in patients with OSA. However, while the improvement is statistically significant, the effect size is small.14
That could be said of a study by Lewis et al.19 These researchers randomized patients with moderate-to-severe OSA and known coronary artery disease (CAD) or at least 3 risk factors for CAD to receive PAP, nocturnal oxygen, or lifestyle education.19 The patients randomized to receive PAP improved vitality scores by only 3.6 points on a 100-point scale; this was significantly better statistically than the improvement achieved by those randomized to lifestyle education. Smaller improvements were noted in depression, social function, and general health. Patients who had more daytime sleepiness at baseline had greater improvements in function.19
Cognitive function findings are mixed
In a systematic review published in 2004, Aloia et al4 found measurable impairments on neuropsychological tests of global cognitive functioning, attention/vigilance, executive functioning, memory, psychomotor function, and constructional abilities in patients with OSA. The results of treatment studies (all but 1 using PAP) were mixed. No studies showed improvement in psychomotor speed or language, and studies disagreed on whether treatment produced benefits in global cognition, attention, or executive functions.4
Continue to: Findings of more recent studies...
Findings of more recent studies remain mixed. A 3-month Spanish trial of PAP in older adults with severe OSA showed improvement in 2 of 4 neuropsychological tests of cognitive function; this was a secondary outcome measure.20 The PREDICT trial in the United Kingdom demonstrated a reduction in daytime sleepiness but no improvement in cognitive function in PAP-treated older adults with OSA but without dementia over a 1-year period.21
In contrast, a French long-term study of adults ages ≥ 65 years with severe (but not necessarily symptomatic) OSA showed better maintenance of memory performance; these results must be interpreted with caution, however, because the study was not randomized, controlled, or blinded, and the results were not adjusted for potential confounders.22 The severity of OSA may influence the impact of PAP treatment on cognitive function.
The prevalence of OSA in patients with dementia is high, and more severe dementia is associated with more severe OSA.23 Although it is intuitive that disrupted sleep may worsen cognitive function, and that treatment could improve it, minimal benefit on cognitive function was shown by neuropsychological testing in patients with Alzheimer’s disease and OSA treated with continuous positive airway pressure (CPAP) vs sham CPAP in 1 small short-term randomized trial.23
In another study of patients with Alzheimer’s disease, this time an observational (nonrandomized, non-controlled, single-blind) study of patients who also had severe symptomatic OSA, researchers followed the patients for 3 years and found a significant delay in median annual cognitive decline of 1.5 points per year on the Mini-Mental Status Examination in patients treated with PAP compared with those who did not receive PAP treatment.24
Hypertension: Small but positive results
A meta-analysis of PAP use in patients with OSA and resistant hypertension (defined as inadequate control while taking at least 3 antihypertensive agents or control requiring at least 4 agents) documented significant blood pressure (BP) lowering, with a pooled estimate of -7.21 mm Hg systolic and -4.99 mm Hg diastolic.25 The decrease in BP was demonstrated in both sleepy and non-sleepy subjects.
Continue to: Multiple studies have...
Multiple studies have shown a small reduction in BP readings (generally about 2 mm Hg) with PAP treatment in nonresistant hypertensive patients with OSA who are sleepy.26 Conversely, the literature is mixed on whether treatment of non-sleepy patients with OSA reduces BP. One long-term study demonstrated a small (1.89 mm Hg systolic, 2.19 mm Hg diastolic) BP reduction effect of PAP in non-sleepy subjects with OSA.27 Similarly, research has shown mandibular advancement devices to lower BP in patients with OSA, in a range similar to that achieved with PAP.28 Whether very small reductions in BP improve important clinical outcomes such as stroke or heart disease is unknown.
CV risk: Again, findings are mixed
The SAVE study is the largest randomized investigation of the effect of treatment of OSA with PAP for secondary prevention of cardiovascular events.29 The trial involved 2717 adults with cardiovascular disease, moderate-to-severe OSA, and minimal sleepiness, and had as its primary composite endpoint death from cardiovascular causes, myocardial infarction (MI), stroke, hospitalization for unstable angina, heart failure, or transient ischemic attack. Patients with severe daytime sleepiness or severe hypoxemia were excluded. The study found no difference between PAP and usual care in the primary outcome, despite a significant reduction in the AHI from a mean of 29 at baseline to 3.7 with PAP treatment.
Similarly, a randomized controlled trial (RCT) of 725 patients with non-sleepy OSA failed to show a reduction in cardiovascular events or in the development of hypertension.30 Peker et al31 randomized 244 adults with recently revascularized coronary artery disease and OSA without daytime sleepiness to auto-titrating CPAP or usual care and did not find a statistically significant difference in revascularization, MI, stroke, or cardiovascular mortality; however, those patients who were compliant with CPAP for ≥ 4 hours/night did have a statistically significant reduction in the combined endpoint.
In contrast, a trial of patients with first-ever stroke and moderate-to-severe OSA who were randomized to early nasal CPAP or usual care demonstrated better 5-year cardiovascular survival for the patients in the CPAP group, and a trend toward better cardiovascular event-free survival.32 Degree of daytime sleepiness was not stated in this study.
A recent meta-analysis of RCTs failed to find a reduction in major adverse cardiovascular events (MACE) in patients with moderate-to-severe OSA treated with PAP.33 In this study, subgroup analysis documented benefit in patients who were adherent with PAP for ≥ 4 hours/night. A larger meta-analysis, however, did not find a reduction in MACE even in the adherent subgroup.34
Continue to: AF and OSA
AF and OSA: An interesting relationship
OSA is an independent risk factor for AF, approximately doubling the risk.35 A review of 10,132 patients with AF (1841 with OSA) in a large observational study demonstrated no difference in outcomes of all-cause mortality, first hospitalization, major bleeding, or major cardiovascular events in OSA patients who were or were not treated with PAP. The PAP-treated patients did have a slightly lower (16% vs 18%) risk of worsening of AF over 2 years.36 Overall, AF patients with OSA had more symptoms and higher admission rates, but no difference in overall mortality or MACE. Observational studies have suggested that PAP treatment of OSA facilitates maintenance of normal sinus rhythm after cardioversion and after ablation.37
CHF: Results look promising
In one small study, 24 patients with heart failure with reduced ejection fraction who were optimally medically treated were randomized to receive PAP or sham PAP for 1 month.38 The treatment group demonstrated reduced systolic BP, reduced end systolic dimension, and significant improvement in ejection fraction from 25 ± 2.8% to 33.8 ± 2.4%.
OSA Tx improves insulin sensitivity
OSA is associated with impaired glucose tolerance, and PAP treatment of OSA has been documented to improve insulin sensitivity.39,40 An efficacy study utilizing PAP in a laboratory setting for 8 hours/night demonstrated significant reduction in fasting blood sugar and a reduction in the dawn phenomenon (an increase in early morning fasting glucose as a result of rebound from hypoglycemia during sleep).39 A 2015 meta-analysis of short-term studies also showed improvement in insulin sensitivity in OSA patients treated with PAP, but failed to find any reduction in A1C or in body mass index.40
All-cause mortality: Difference in findings between short- and long-term studies
Yu et al’s34 meta-analysis of 10 RCTs involving 7266 participants found no difference in mortality in treated (vs no treatment or sham treatment) OSA patients. This was true even in the more adherent subgroup. These studies were relatively short-term, with the longest mean follow-up being 68 months.
However, several longer-term population-based studies have suggested that OSA treatment improves all-cause mortality. An 18-year follow-up of a Wisconsin cohort documented dramatically increased mortality in patients with severe sleep apnea; mortality was even higher when patients treated with PAP were removed from the analysis, suggesting that PAP treatment was protective, mainly for cardiovascular death.5
Continue to: A Danish registry...
A Danish registry documented that patients treated with CPAP had higher rates of comorbidities before and during treatment; when these comorbidities were controlled, men ages ≥ 60 years had improved survival when treated with CPAP. There was no survival benefit in women.41
A recent analysis—the Sleep Heart Health Study—followed patients with obesity and severe OSA for a mean of 11.1 years and calculated a hazard ratio for all-cause mortality associated with prescribed PAP therapy of 0.58 (95% confidence interval [CI], 0.35-0.96) after propensity matching.42 The difference in mortality appeared 6 to 7 years after PAP therapy was prescribed. This delay may explain the failure of shorter-term studies to demonstrate evidence of benefit.
OSA Tx reduces motor vehicle crashes
Drowsy driving is widely accepted as a risk for motor vehicle crashes. Successful treatment of OSA with PAP has been shown to improve driving performance on a driving simulator.43 An analysis of 15 studies similarly demonstrated a significant reduction in driving accidents (incident rate ratio [IRR] = 0.45) and in near-miss accidents (IRR = 0.23) in patients with OSA treated with CPAP.44
Pulmonary hypertension: OSA Tx lowers pulmonary arterial pressure
Patients with OSA have higher than expected rates of pulmonary arterial hypertension—as high as 22%—documented by pulmonary artery catheterization findings.45 A meta-analysis of studies that examined the effect of PAP in patients with OSA and coexisting pulmonary hypertension but without other overt pulmonary or cardiac disease found significant reductions in pulmonary artery pressure.46 Whether this finding translates into improved patient-oriented outcomes is unknown.
OSA and pregnancy outcomes
A national cohort study demonstrated that OSA is an independent risk factor for multiple adverse pregnancy outcomes, including gestational diabetes, hypertensive disorders in pregnancy, intrauterine growth retardation, and stillbirth.7 OSA was also associated with the rare serious adverse outcomes of congestive heart failure, cardiomyopathy, and pulmonary embolism.7 There is little evidence to date with which to determine whether treatment of OSA improves outcomes, but PAP treatment is documented to be safe in pregnant women.8
CORRESPONDENCE
Stephen C. Sorsby, MD, MHA, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 530, Little Rock, AR 72205; [email protected].
Obstructive sleep apnea (OSA) is a common cause of daytime sleepiness, and severe OSA is a risk factor for hypertension, cardiovascular events, atrial fibrillation (AF), insulin resistance, cognitive impairment, motor vehicle crashes, adverse pregnancy outcomes, and overall mortality.1-8 The hazard ratio for mortality for patients with severe OSA may be as high as 3.8.5
OSA is diagnosed by the apnea-hypopnea index (AHI), defined as the number of apnea or hypopnea events per hour as determined by polysomnography. An AHI score ≤ 5 is considered normal; > 5 to ≤ 15 is mild; > 15 to < 30 is moderate; and ≥ 30 is severe. Most studies of OSA treatment use reduction of AHI as the measure of treatment effectiveness, and several types of treatment improve AHI.
In family medicine, we generally want to know whether treatment of OSA will improve outcomes of significance to patients. A recent systematic review of evidence for the US Preventive Services Task Force found that it was unclear whether OSA treatment improved most health outcomes, including mortality, cardiovascular events, or motor vehicle crashes.6 Several other organizations have published guidelines regarding OSA treatment; these guidelines are reviewed in the TABLE.9-13

This article summarizes the current evidence surrounding the effect of treatment of OSA on outcomes of significance to patients. While multiple treatments have been advocated for patients with OSA, positive airway pressure (PAP) is the most widely used and studied and is recommended as standard treatment by most guidelines.9-13 Most available evidence about patient-oriented outcomes involves treatment with PAP; where there is evidence about the effect of other OSA treatments on a particular outcome, that evidence is also summarized.

Benefits of OSA treatment
Patients with OSA who have excessive daytime sleepiness can gain substantial symptomatic benefit from treatment of their OSA with PAP or oral appliances (OAs), and might benefit from hypoglossal nerve stimulation or other surgical treatment. PAP is probably more effective than OAs in patients who use it ≥ 4 hours/night, but it is more difficult to comply with PAP.14
Evidence that treatment of asymptomatic OSA benefits other medical conditions is often conflicting. Given the low risk of treatment, it is reasonable to consider offering a trial of treatment, preferably with PAP, to asymptomatic patients with moderate-to-severe OSA and certain comorbidities, including obesity, resistant hypertension, high cardiovascular risk, congestive heart failure (CHF), AF, diabetes that is difficult to control, and pregnancy. Such patients should be strongly encouraged to use PAP ≥ 4 hours/night, and should be advised that benefits may not be immediately apparent.
Treatment of OSA improves daytime sleepiness
Daytime sleepiness is typically measured with the Epworth Sleepiness Scale (ESS), a self-administered questionnaire assessing a person’s level of drowsiness and propensity to fall asleep in 8 different daytime situations. Each situation is scored between 0 (would never doze) and 3 (high chance of dozing), with the scores then totaled to provide an overall score between 0 and 24. A score > 10 is considered abnormal.
Continue to: Treament of OSA...
Treatment of OSA with either PAP or OAs significantly improves ESS scores, with PAP being more effective.13 The difference appears to widen in patients with greater daytime sleepiness; in other words, patients with greater daytime sleepiness will gain even greater benefit from PAP, both overall and when compared with OAs.15
One randomized trial of an intensive lifestyle modification program for patients with OSA failed to show improvement in the ESS in the intention-to-treat analysis, but did demonstrate a 2.4-point greater reduction in ESS scores in those patients who successfully followed the program (achieving weight loss).16 Surgical treatments for OSA, such as uvulopalatopharyngoplasty or maxillary advancement, have been shown in some (but not all) studies to improve ESS scores; the different types of surgical treatment and the heterogeneity of studies prevents estimation of effect size.17 A meta-analysis of case series studies of hypoglossal nerve stimulation reported a mean improvement of 4.5 points on the ESS;18 comparison with other interventions is lacking.
Improved quality of life
Both PAP and OAs have been shown to improve sleep-related quality of life in patients with OSA. However, while the improvement is statistically significant, the effect size is small.14
That could be said of a study by Lewis et al.19 These researchers randomized patients with moderate-to-severe OSA and known coronary artery disease (CAD) or at least 3 risk factors for CAD to receive PAP, nocturnal oxygen, or lifestyle education.19 The patients randomized to receive PAP improved vitality scores by only 3.6 points on a 100-point scale; this was significantly better statistically than the improvement achieved by those randomized to lifestyle education. Smaller improvements were noted in depression, social function, and general health. Patients who had more daytime sleepiness at baseline had greater improvements in function.19
Cognitive function findings are mixed
In a systematic review published in 2004, Aloia et al4 found measurable impairments on neuropsychological tests of global cognitive functioning, attention/vigilance, executive functioning, memory, psychomotor function, and constructional abilities in patients with OSA. The results of treatment studies (all but 1 using PAP) were mixed. No studies showed improvement in psychomotor speed or language, and studies disagreed on whether treatment produced benefits in global cognition, attention, or executive functions.4
Continue to: Findings of more recent studies...
Findings of more recent studies remain mixed. A 3-month Spanish trial of PAP in older adults with severe OSA showed improvement in 2 of 4 neuropsychological tests of cognitive function; this was a secondary outcome measure.20 The PREDICT trial in the United Kingdom demonstrated a reduction in daytime sleepiness but no improvement in cognitive function in PAP-treated older adults with OSA but without dementia over a 1-year period.21
In contrast, a French long-term study of adults ages ≥ 65 years with severe (but not necessarily symptomatic) OSA showed better maintenance of memory performance; these results must be interpreted with caution, however, because the study was not randomized, controlled, or blinded, and the results were not adjusted for potential confounders.22 The severity of OSA may influence the impact of PAP treatment on cognitive function.
The prevalence of OSA in patients with dementia is high, and more severe dementia is associated with more severe OSA.23 Although it is intuitive that disrupted sleep may worsen cognitive function, and that treatment could improve it, minimal benefit on cognitive function was shown by neuropsychological testing in patients with Alzheimer’s disease and OSA treated with continuous positive airway pressure (CPAP) vs sham CPAP in 1 small short-term randomized trial.23
In another study of patients with Alzheimer’s disease, this time an observational (nonrandomized, non-controlled, single-blind) study of patients who also had severe symptomatic OSA, researchers followed the patients for 3 years and found a significant delay in median annual cognitive decline of 1.5 points per year on the Mini-Mental Status Examination in patients treated with PAP compared with those who did not receive PAP treatment.24
Hypertension: Small but positive results
A meta-analysis of PAP use in patients with OSA and resistant hypertension (defined as inadequate control while taking at least 3 antihypertensive agents or control requiring at least 4 agents) documented significant blood pressure (BP) lowering, with a pooled estimate of -7.21 mm Hg systolic and -4.99 mm Hg diastolic.25 The decrease in BP was demonstrated in both sleepy and non-sleepy subjects.
Continue to: Multiple studies have...
Multiple studies have shown a small reduction in BP readings (generally about 2 mm Hg) with PAP treatment in nonresistant hypertensive patients with OSA who are sleepy.26 Conversely, the literature is mixed on whether treatment of non-sleepy patients with OSA reduces BP. One long-term study demonstrated a small (1.89 mm Hg systolic, 2.19 mm Hg diastolic) BP reduction effect of PAP in non-sleepy subjects with OSA.27 Similarly, research has shown mandibular advancement devices to lower BP in patients with OSA, in a range similar to that achieved with PAP.28 Whether very small reductions in BP improve important clinical outcomes such as stroke or heart disease is unknown.
CV risk: Again, findings are mixed
The SAVE study is the largest randomized investigation of the effect of treatment of OSA with PAP for secondary prevention of cardiovascular events.29 The trial involved 2717 adults with cardiovascular disease, moderate-to-severe OSA, and minimal sleepiness, and had as its primary composite endpoint death from cardiovascular causes, myocardial infarction (MI), stroke, hospitalization for unstable angina, heart failure, or transient ischemic attack. Patients with severe daytime sleepiness or severe hypoxemia were excluded. The study found no difference between PAP and usual care in the primary outcome, despite a significant reduction in the AHI from a mean of 29 at baseline to 3.7 with PAP treatment.
Similarly, a randomized controlled trial (RCT) of 725 patients with non-sleepy OSA failed to show a reduction in cardiovascular events or in the development of hypertension.30 Peker et al31 randomized 244 adults with recently revascularized coronary artery disease and OSA without daytime sleepiness to auto-titrating CPAP or usual care and did not find a statistically significant difference in revascularization, MI, stroke, or cardiovascular mortality; however, those patients who were compliant with CPAP for ≥ 4 hours/night did have a statistically significant reduction in the combined endpoint.
In contrast, a trial of patients with first-ever stroke and moderate-to-severe OSA who were randomized to early nasal CPAP or usual care demonstrated better 5-year cardiovascular survival for the patients in the CPAP group, and a trend toward better cardiovascular event-free survival.32 Degree of daytime sleepiness was not stated in this study.
A recent meta-analysis of RCTs failed to find a reduction in major adverse cardiovascular events (MACE) in patients with moderate-to-severe OSA treated with PAP.33 In this study, subgroup analysis documented benefit in patients who were adherent with PAP for ≥ 4 hours/night. A larger meta-analysis, however, did not find a reduction in MACE even in the adherent subgroup.34
Continue to: AF and OSA
AF and OSA: An interesting relationship
OSA is an independent risk factor for AF, approximately doubling the risk.35 A review of 10,132 patients with AF (1841 with OSA) in a large observational study demonstrated no difference in outcomes of all-cause mortality, first hospitalization, major bleeding, or major cardiovascular events in OSA patients who were or were not treated with PAP. The PAP-treated patients did have a slightly lower (16% vs 18%) risk of worsening of AF over 2 years.36 Overall, AF patients with OSA had more symptoms and higher admission rates, but no difference in overall mortality or MACE. Observational studies have suggested that PAP treatment of OSA facilitates maintenance of normal sinus rhythm after cardioversion and after ablation.37
CHF: Results look promising
In one small study, 24 patients with heart failure with reduced ejection fraction who were optimally medically treated were randomized to receive PAP or sham PAP for 1 month.38 The treatment group demonstrated reduced systolic BP, reduced end systolic dimension, and significant improvement in ejection fraction from 25 ± 2.8% to 33.8 ± 2.4%.
OSA Tx improves insulin sensitivity
OSA is associated with impaired glucose tolerance, and PAP treatment of OSA has been documented to improve insulin sensitivity.39,40 An efficacy study utilizing PAP in a laboratory setting for 8 hours/night demonstrated significant reduction in fasting blood sugar and a reduction in the dawn phenomenon (an increase in early morning fasting glucose as a result of rebound from hypoglycemia during sleep).39 A 2015 meta-analysis of short-term studies also showed improvement in insulin sensitivity in OSA patients treated with PAP, but failed to find any reduction in A1C or in body mass index.40
All-cause mortality: Difference in findings between short- and long-term studies
Yu et al’s34 meta-analysis of 10 RCTs involving 7266 participants found no difference in mortality in treated (vs no treatment or sham treatment) OSA patients. This was true even in the more adherent subgroup. These studies were relatively short-term, with the longest mean follow-up being 68 months.
However, several longer-term population-based studies have suggested that OSA treatment improves all-cause mortality. An 18-year follow-up of a Wisconsin cohort documented dramatically increased mortality in patients with severe sleep apnea; mortality was even higher when patients treated with PAP were removed from the analysis, suggesting that PAP treatment was protective, mainly for cardiovascular death.5
Continue to: A Danish registry...
A Danish registry documented that patients treated with CPAP had higher rates of comorbidities before and during treatment; when these comorbidities were controlled, men ages ≥ 60 years had improved survival when treated with CPAP. There was no survival benefit in women.41
A recent analysis—the Sleep Heart Health Study—followed patients with obesity and severe OSA for a mean of 11.1 years and calculated a hazard ratio for all-cause mortality associated with prescribed PAP therapy of 0.58 (95% confidence interval [CI], 0.35-0.96) after propensity matching.42 The difference in mortality appeared 6 to 7 years after PAP therapy was prescribed. This delay may explain the failure of shorter-term studies to demonstrate evidence of benefit.
OSA Tx reduces motor vehicle crashes
Drowsy driving is widely accepted as a risk for motor vehicle crashes. Successful treatment of OSA with PAP has been shown to improve driving performance on a driving simulator.43 An analysis of 15 studies similarly demonstrated a significant reduction in driving accidents (incident rate ratio [IRR] = 0.45) and in near-miss accidents (IRR = 0.23) in patients with OSA treated with CPAP.44
Pulmonary hypertension: OSA Tx lowers pulmonary arterial pressure
Patients with OSA have higher than expected rates of pulmonary arterial hypertension—as high as 22%—documented by pulmonary artery catheterization findings.45 A meta-analysis of studies that examined the effect of PAP in patients with OSA and coexisting pulmonary hypertension but without other overt pulmonary or cardiac disease found significant reductions in pulmonary artery pressure.46 Whether this finding translates into improved patient-oriented outcomes is unknown.
OSA and pregnancy outcomes
A national cohort study demonstrated that OSA is an independent risk factor for multiple adverse pregnancy outcomes, including gestational diabetes, hypertensive disorders in pregnancy, intrauterine growth retardation, and stillbirth.7 OSA was also associated with the rare serious adverse outcomes of congestive heart failure, cardiomyopathy, and pulmonary embolism.7 There is little evidence to date with which to determine whether treatment of OSA improves outcomes, but PAP treatment is documented to be safe in pregnant women.8
CORRESPONDENCE
Stephen C. Sorsby, MD, MHA, University of Arkansas for Medical Sciences, 4301 West Markham, Slot 530, Little Rock, AR 72205; [email protected].
1. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384.
2. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
3. Iftikhar IH, Hoyos CM, Phillips CL, et al. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med. 2015;11:475-485.
4. Aloia MS, Arnedt JT, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772-785.
5. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071-1078.
6. Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:415-433.
7. Bourjeily G, Danilack VA, Bublitz MA, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;35:50-57.
8. Booth JM, Tonidandel AM. Peripartum management of obstructive sleep apnea. Clin Obstet Gyn. 2017;60:405-417.
9. Strohl KP, Brown DB, Collop N, et al. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med. 2013;187:1259-1266.
10. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
11. National Institute for Health and Care Excellence. Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. Technology appraisal guidance [TA139]. https://www.nice.org.uk/guidance/ta139. Revised February 2012. Accessed October 28, 2019.
12. Qaseem A, Holty JE, Owens DK, et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471-483.
13. Netzer NC, Ancoli-Israel S, Bliwise DL, et al. Principles of practice parameters for the treatment of sleep disordered breathing in the elderly and frail elderly: the consensus of the International Geriatric Sleep Medicine Task Force. Eur Respir J. 2016;48:992-1018.
14. Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879-887.
15. Bratton DJ, Gaisl T, Schlatzer C, et al. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med. 2015;3:869-878.
16. Ng SSS, Chan RSM, Woo J, et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest. 2015;148:1193-1203.
17. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;4:CD001004.
18. Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015; 125:1254-1264.
19. Lewis EF, Rui W, Punjabi N, et al. Impact of continuous positive airway pressure and oxygen on health status in patients with coronary heart disease, cardiovascular risk factors, and obstructive sleep apnea: A Heart Biomarker Evaluation in Apnea Treatment (HEARTBEAT) analysis. Am Heart J. 2017;189:59-67.
20. Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. 2015;46:142-151.
21. McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804-812.
22. Crawford-Achour E, Dauphinot V, Martin MS, et al. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe OSA: the PROOF study. J Clin Sleep Med. 2015;11:519-524.
23. Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076-2081.
24. Troussière AC, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1405-1408.
25. Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757-764.
26. Montesi SB, Edwards BA, Malhotra A, et al. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587-596.
27. Barbé F, Durán-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718-726.
28. Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280-2293.
29. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919-931.
30. Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161-2168.
31. Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613-620.
32. Parra O, Sánchez-Armengol Á, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24:47-53.
33. Abuzaid AS, Al Ashray HS, Elbadaway A, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure in patients with obstructive sleep apnea. Am J Card. 2017;120:693-699.
34. Yu J, Zhou Z, McEvoy D, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318:156-166.
35. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the incident risk of atrial fibrillation. J Amer Coll of Card. 2007;49:565-571.
36. Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169:647-654.e2.
37. Nalliah CJ, Sanders P, Kalman JM. Obstructive sleep apnea treatment and atrial fibrillation: a need for definitive evidence. J Cardiovasc Electrophysiol. 2016;27:1001-1010.
38. Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233-1241
39. Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes: a randomized controlled trial. Am J Respir Crit Care Med. 2015;192:96-105.
40. Feng Y, Zhang Z, Dong ZZ. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15005.
41. Jennum P, Tonnesen P, Ibsen R, et al. Obstructive sleep apnea: effect of comorbidities and positive airway pressure on all-cause mortality. Sleep Med. 2017;36:62-66.
42. Lisan Q, Van Sloten T, Marques Vidal P, et al. Association of positive airway pressure prescription with mortality in patients with obesity and severe obstructive sleep apnea: the sleep heart health study. JAMA Otolaryngol Head Neck Surg. 2019;145:509-515.
43. Mazza S, Pépin JL, Naëgelé B, et al. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. Eur Respir J. 2006;28:1020-1028.
44. Antonopoulos CN, Sergentanis TN, Daskalopoulou SS, et al. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev. 2011;15:301-310.
45. Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol. 2009;104:1300-1306.
46. Imran TF, Ghazipura M, Liu S, et al. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: a meta-analysis. Heart Fail Rev. 2016;21:591-598.
1. Peppard PE, Young T, Palta M, et al. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378-1384.
2. Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046-1053.
3. Iftikhar IH, Hoyos CM, Phillips CL, et al. Meta-analyses of the association of sleep apnea with insulin resistance, and the effects of CPAP on HOMA-IR, adiponectin, and visceral adipose fat. J Clin Sleep Med. 2015;11:475-485.
4. Aloia MS, Arnedt JT, Davis JD, et al. Neuropsychological sequelae of obstructive sleep apnea-hypopnea syndrome: a critical review. J Int Neuropsychol Soc. 2004;10:772-785.
5. Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071-1078.
6. Jonas DE, Amick HR, Feltner C, et al. Screening for obstructive sleep apnea in adults: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317:415-433.
7. Bourjeily G, Danilack VA, Bublitz MA, et al. Obstructive sleep apnea in pregnancy is associated with adverse maternal outcomes: a national cohort. Sleep Med. 2017;35:50-57.
8. Booth JM, Tonidandel AM. Peripartum management of obstructive sleep apnea. Clin Obstet Gyn. 2017;60:405-417.
9. Strohl KP, Brown DB, Collop N, et al. An official American Thoracic Society Clinical Practice Guideline: sleep apnea, sleepiness, and driving risk in noncommercial drivers. An update of a 1994 Statement. Am J Respir Crit Care Med. 2013;187:1259-1266.
10. Epstein LJ, Kristo D, Strollo PJ Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
11. National Institute for Health and Care Excellence. Continuous positive airway pressure for the treatment of obstructive sleep apnoea/hypopnoea syndrome. Technology appraisal guidance [TA139]. https://www.nice.org.uk/guidance/ta139. Revised February 2012. Accessed October 28, 2019.
12. Qaseem A, Holty JE, Owens DK, et al. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2013;159:471-483.
13. Netzer NC, Ancoli-Israel S, Bliwise DL, et al. Principles of practice parameters for the treatment of sleep disordered breathing in the elderly and frail elderly: the consensus of the International Geriatric Sleep Medicine Task Force. Eur Respir J. 2016;48:992-1018.
14. Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879-887.
15. Bratton DJ, Gaisl T, Schlatzer C, et al. Comparison of the effects of continuous positive airway pressure and mandibular advancement devices on sleepiness in patients with obstructive sleep apnoea: a network meta-analysis. Lancet Respir Med. 2015;3:869-878.
16. Ng SSS, Chan RSM, Woo J, et al. A randomized controlled study to examine the effect of a lifestyle modification program in OSA. Chest. 2015;148:1193-1203.
17. Sundaram S, Bridgman SA, Lim J, et al. Surgery for obstructive sleep apnoea. Cochrane Database Syst Rev. 2005;4:CD001004.
18. Certal VF, Zaghi S, Riaz M, et al. Hypoglossal nerve stimulation in the treatment of obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2015; 125:1254-1264.
19. Lewis EF, Rui W, Punjabi N, et al. Impact of continuous positive airway pressure and oxygen on health status in patients with coronary heart disease, cardiovascular risk factors, and obstructive sleep apnea: A Heart Biomarker Evaluation in Apnea Treatment (HEARTBEAT) analysis. Am Heart J. 2017;189:59-67.
20. Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. 2015;46:142-151.
21. McMillan A, Bratton DJ, Faria R, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. 2014;2:804-812.
22. Crawford-Achour E, Dauphinot V, Martin MS, et al. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe OSA: the PROOF study. J Clin Sleep Med. 2015;11:519-524.
23. Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer’s disease: a randomized controlled study. J Am Geriatr Soc. 2008;56:2076-2081.
24. Troussière AC, Charley CM, Salleron J, et al. Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2014;85:1405-1408.
25. Haentjens P, Van Meerhaeghe A, Moscariello A, et al. The impact of continuous positive airway pressure on blood pressure in patients with obstructive sleep apnea syndrome: evidence from a meta-analysis of placebo-controlled randomized trials. Arch Intern Med. 2007;167:757-764.
26. Montesi SB, Edwards BA, Malhotra A, et al. The effect of continuous positive airway pressure treatment on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Clin Sleep Med. 2012;8:587-596.
27. Barbé F, Durán-Cantolla J, Capote F, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;181:718-726.
28. Bratton DJ, Gaisl T, Wons AM, et al. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta-analysis. JAMA. 2015;314:2280-2293.
29. McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375:919-931.
30. Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;307:2161-2168.
31. Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with nonsleepy obstructive sleep apnea. The RICCADSA Randomized Controlled Trial. Am J Respir Crit Care Med. 2016;194:613-620.
32. Parra O, Sánchez-Armengol Á, Capote F, et al. Efficacy of continuous positive airway pressure treatment on 5-year survival in patients with ischaemic stroke and obstructive sleep apnea: a randomized controlled trial. J Sleep Res. 2015;24:47-53.
33. Abuzaid AS, Al Ashray HS, Elbadaway A, et al. Meta-analysis of cardiovascular outcomes with continuous positive airway pressure in patients with obstructive sleep apnea. Am J Card. 2017;120:693-699.
34. Yu J, Zhou Z, McEvoy D, et al. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta-analysis. JAMA. 2017;318:156-166.
35. Gami AS, Hodge DO, Herges RM, et al. Obstructive sleep apnea, obesity, and the incident risk of atrial fibrillation. J Amer Coll of Card. 2007;49:565-571.
36. Holmqvist F, Guan N, Zhu Z, et al. Impact of obstructive sleep apnea and continuous positive airway pressure therapy on outcomes in patients with atrial fibrillation—results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am Heart J. 2015;169:647-654.e2.
37. Nalliah CJ, Sanders P, Kalman JM. Obstructive sleep apnea treatment and atrial fibrillation: a need for definitive evidence. J Cardiovasc Electrophysiol. 2016;27:1001-1010.
38. Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233-1241
39. Pamidi S, Wroblewski K, Stepien M, et al. Eight hours of nightly continuous positive airway pressure treatment of obstructive sleep apnea improves glucose metabolism in patients with prediabetes: a randomized controlled trial. Am J Respir Crit Care Med. 2015;192:96-105.
40. Feng Y, Zhang Z, Dong ZZ. Effects of continuous positive airway pressure therapy on glycaemic control, insulin sensitivity and body mass index in patients with obstructive sleep apnoea and type 2 diabetes: a systematic review and meta-analysis. NPJ Prim Care Respir Med. 2015;25:15005.
41. Jennum P, Tonnesen P, Ibsen R, et al. Obstructive sleep apnea: effect of comorbidities and positive airway pressure on all-cause mortality. Sleep Med. 2017;36:62-66.
42. Lisan Q, Van Sloten T, Marques Vidal P, et al. Association of positive airway pressure prescription with mortality in patients with obesity and severe obstructive sleep apnea: the sleep heart health study. JAMA Otolaryngol Head Neck Surg. 2019;145:509-515.
43. Mazza S, Pépin JL, Naëgelé B, et al. Driving ability in sleep apnoea patients before and after CPAP treatment: evaluation on a road safety platform. Eur Respir J. 2006;28:1020-1028.
44. Antonopoulos CN, Sergentanis TN, Daskalopoulou SS, et al. Nasal continuous positive airway pressure (nCPAP) treatment for obstructive sleep apnea, road traffic accidents and driving simulator performance: a meta-analysis. Sleep Med Rev. 2011;15:301-310.
45. Minai OA, Ricaurte B, Kaw R, et al. Frequency and impact of pulmonary hypertension in patients with obstructive sleep apnea syndrome. Am J Cardiol. 2009;104:1300-1306.
46. Imran TF, Ghazipura M, Liu S, et al. Effect of continuous positive airway pressure treatment on pulmonary artery pressure in patients with isolated obstructive sleep apnea: a meta-analysis. Heart Fail Rev. 2016;21:591-598.
PRACTICE RECOMMENDATIONS
› Treat patients with symptomatic obstructive sleep apnea (OSA) with positive airway pressure (PAP) or oral appliances to reduce daytime sleepiness, improve quality-of-life scores, and modestly reduce blood pressure in patients with hypertension. A
› Consider recommending at least 4 hours of PAP every night for asymptomatic patients (those without daytime sleepiness) with severe OSA and other conditions, including resistant hypertension, atrial fibrillation, congestive heart failure, cognitive impairment, obesity, and stroke. B
› Do not screen asymptomatic patients for OSA. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series