User login
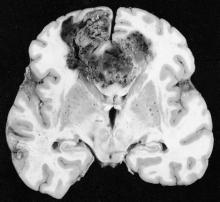
Patients with glioblastoma who underwent standard chemoradiotherapy followed by maintenance therapy with tumor-treating fields plus temozolomide had significantly longer progression-free and overall survival, compared with temozolomide maintenance monotherapy, according to a recent report.
In the intent-to-treat population, median progression-free survival (PFS) for tumor-treating fields (TTFields) plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (hazard ratio, 0.62; 98.7% confidence interval, 0.43-0.89; P = .001). Overall survival (OS) in the per-protocol population, a prespecified secondary endpoint, was also significantly increased (20.5 months vs. 15.6 months; HR, 0.64; 99.4% CI, 0.42-0.98; P = .004), prompting early termination of the study that allowed patients in the control group the option to receive TTFields.
The prognosis for glioblastoma remains poor for this highly aggressive brain tumor, with no major treatment advance in more than a decade, according to Dr. Roger Stupp, chairman of the department of oncology and the Cancer Center at the University of Zürich Hospital and his colleagues.
“In the interim analysis of this randomized clinical trial, the addition of TTFields to standard maintenance temozolomide significantly improved progression-free and overall survival,” they wrote (JAMA. 2015;314[23]:2535-43. doi: 10.1001/jama.2015.16669).
TTFields are low-intensity, intermediate-frequency alternating electric fields delivered via transducer arrays applied to the shaved scalp. The treatment is hypothesized to disrupt spindle formation during cell division, leading to mitotic arrest and apoptosis.
The multicenter trial enrolled 695 patients with newly diagnosed glioblastoma randomized 2:1 to receive TTFields plus temozolomide or temozolomide alone as maintenance therapy from 2009 to 2014. Interim analysis included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group. The median number of temozolomide cycles until evidence of tumor progression was six cycles for the TTFields group, compared with four cycles for the temozolomide-alone group.
The median time from diagnosis to randomization was 3.8 months. When added to the median PFS of 4 months for the control group of this study, the median 7.8-month PFS is similar to most other reports.
The addition of TTFields to treatment was not associated with significant increase in systemic toxicity, except for higher incidences of scalp irritation, anxiety, confusion, insomnia, and headaches. Seizure rates did not increase.
Because a sham treatment for the control group was deemed impractical, the study was open-label, which raises the question of a placebo effect. The magnitude of the effect size (HR, 0.62 for PFS and 0.74 for OS) is greater than what could be attributed to a placebo effect, according to the investigators.
The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck and Co, and Novartis. Several of his coauthors reported ties to industry.
Results of the study by Dr. Stupp and his colleagues are the first in a decade to demonstrate an improvement in survival for patients with glioblastoma. As a result, the Food and Drug Administration recently approved the therapy, which carries a cost of $20,000 per month. However, several aspects of the study warrant further analysis.
Since the study was not blinded or placebo-controlled, the placebo effect cannot be assessed. Although placebos are rarely associated with tumor responses in well-designed trials, patients who take only placebos reliably live longer than those who do not, possibly because of adherence bias. Adherent patients likely exhibit other healthy behaviors that can produce significant survival advantages, and some studies show adherence as one of the strongest independent variables influencing outcome. This study does not distinguish whether the survival benefit associated with the use of TTFields was because of the efficacy of the device or from adherence bias.
Another confounding factor was that patients in the temozolomide-alone group received less adjuvant chemotherapy (median four cycles before tumor progression), compared with the TTFields group (six cycles). Potentially assuming that the device would work, patients and physicians could have minimized symptoms or signs of tumor recurrence. This would have prolonged the use of temozolomide, an effective chemotherapy for glioblastoma. (Increased exposure to temozolomide in a similar patient population, however, produced no increase in survival.)
The mechanism by which TTFields leverage chemotherapy to treat tumors remains unclear. Given the survival benefit reported in this study, determining the scientific basis for efficacy of the method becomes a priority. Perhaps most concerning are the doubts, as a result of the study design chosen, about the true efficacy of the therapy.
Dr. John H. Sampson is a professor of surgery in the neurosurgery department at Duke University, Durham, N.C. These remarks were part of an editorial accompanying the report (JAMA. 2015;314[23];2511-13. doi: 10.1001/jama.2015.16701). Dr. Sampson reported ties to Celldex Therapeutics, Brainlab, and Bristol-Myers Squibb, as well as holding unrelated patents with Celldex and Annias Immunotherapeutics.
Results of the study by Dr. Stupp and his colleagues are the first in a decade to demonstrate an improvement in survival for patients with glioblastoma. As a result, the Food and Drug Administration recently approved the therapy, which carries a cost of $20,000 per month. However, several aspects of the study warrant further analysis.
Since the study was not blinded or placebo-controlled, the placebo effect cannot be assessed. Although placebos are rarely associated with tumor responses in well-designed trials, patients who take only placebos reliably live longer than those who do not, possibly because of adherence bias. Adherent patients likely exhibit other healthy behaviors that can produce significant survival advantages, and some studies show adherence as one of the strongest independent variables influencing outcome. This study does not distinguish whether the survival benefit associated with the use of TTFields was because of the efficacy of the device or from adherence bias.
Another confounding factor was that patients in the temozolomide-alone group received less adjuvant chemotherapy (median four cycles before tumor progression), compared with the TTFields group (six cycles). Potentially assuming that the device would work, patients and physicians could have minimized symptoms or signs of tumor recurrence. This would have prolonged the use of temozolomide, an effective chemotherapy for glioblastoma. (Increased exposure to temozolomide in a similar patient population, however, produced no increase in survival.)
The mechanism by which TTFields leverage chemotherapy to treat tumors remains unclear. Given the survival benefit reported in this study, determining the scientific basis for efficacy of the method becomes a priority. Perhaps most concerning are the doubts, as a result of the study design chosen, about the true efficacy of the therapy.
Dr. John H. Sampson is a professor of surgery in the neurosurgery department at Duke University, Durham, N.C. These remarks were part of an editorial accompanying the report (JAMA. 2015;314[23];2511-13. doi: 10.1001/jama.2015.16701). Dr. Sampson reported ties to Celldex Therapeutics, Brainlab, and Bristol-Myers Squibb, as well as holding unrelated patents with Celldex and Annias Immunotherapeutics.
Results of the study by Dr. Stupp and his colleagues are the first in a decade to demonstrate an improvement in survival for patients with glioblastoma. As a result, the Food and Drug Administration recently approved the therapy, which carries a cost of $20,000 per month. However, several aspects of the study warrant further analysis.
Since the study was not blinded or placebo-controlled, the placebo effect cannot be assessed. Although placebos are rarely associated with tumor responses in well-designed trials, patients who take only placebos reliably live longer than those who do not, possibly because of adherence bias. Adherent patients likely exhibit other healthy behaviors that can produce significant survival advantages, and some studies show adherence as one of the strongest independent variables influencing outcome. This study does not distinguish whether the survival benefit associated with the use of TTFields was because of the efficacy of the device or from adherence bias.
Another confounding factor was that patients in the temozolomide-alone group received less adjuvant chemotherapy (median four cycles before tumor progression), compared with the TTFields group (six cycles). Potentially assuming that the device would work, patients and physicians could have minimized symptoms or signs of tumor recurrence. This would have prolonged the use of temozolomide, an effective chemotherapy for glioblastoma. (Increased exposure to temozolomide in a similar patient population, however, produced no increase in survival.)
The mechanism by which TTFields leverage chemotherapy to treat tumors remains unclear. Given the survival benefit reported in this study, determining the scientific basis for efficacy of the method becomes a priority. Perhaps most concerning are the doubts, as a result of the study design chosen, about the true efficacy of the therapy.
Dr. John H. Sampson is a professor of surgery in the neurosurgery department at Duke University, Durham, N.C. These remarks were part of an editorial accompanying the report (JAMA. 2015;314[23];2511-13. doi: 10.1001/jama.2015.16701). Dr. Sampson reported ties to Celldex Therapeutics, Brainlab, and Bristol-Myers Squibb, as well as holding unrelated patents with Celldex and Annias Immunotherapeutics.
Patients with glioblastoma who underwent standard chemoradiotherapy followed by maintenance therapy with tumor-treating fields plus temozolomide had significantly longer progression-free and overall survival, compared with temozolomide maintenance monotherapy, according to a recent report.
In the intent-to-treat population, median progression-free survival (PFS) for tumor-treating fields (TTFields) plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (hazard ratio, 0.62; 98.7% confidence interval, 0.43-0.89; P = .001). Overall survival (OS) in the per-protocol population, a prespecified secondary endpoint, was also significantly increased (20.5 months vs. 15.6 months; HR, 0.64; 99.4% CI, 0.42-0.98; P = .004), prompting early termination of the study that allowed patients in the control group the option to receive TTFields.
The prognosis for glioblastoma remains poor for this highly aggressive brain tumor, with no major treatment advance in more than a decade, according to Dr. Roger Stupp, chairman of the department of oncology and the Cancer Center at the University of Zürich Hospital and his colleagues.
“In the interim analysis of this randomized clinical trial, the addition of TTFields to standard maintenance temozolomide significantly improved progression-free and overall survival,” they wrote (JAMA. 2015;314[23]:2535-43. doi: 10.1001/jama.2015.16669).
TTFields are low-intensity, intermediate-frequency alternating electric fields delivered via transducer arrays applied to the shaved scalp. The treatment is hypothesized to disrupt spindle formation during cell division, leading to mitotic arrest and apoptosis.
The multicenter trial enrolled 695 patients with newly diagnosed glioblastoma randomized 2:1 to receive TTFields plus temozolomide or temozolomide alone as maintenance therapy from 2009 to 2014. Interim analysis included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group. The median number of temozolomide cycles until evidence of tumor progression was six cycles for the TTFields group, compared with four cycles for the temozolomide-alone group.
The median time from diagnosis to randomization was 3.8 months. When added to the median PFS of 4 months for the control group of this study, the median 7.8-month PFS is similar to most other reports.
The addition of TTFields to treatment was not associated with significant increase in systemic toxicity, except for higher incidences of scalp irritation, anxiety, confusion, insomnia, and headaches. Seizure rates did not increase.
Because a sham treatment for the control group was deemed impractical, the study was open-label, which raises the question of a placebo effect. The magnitude of the effect size (HR, 0.62 for PFS and 0.74 for OS) is greater than what could be attributed to a placebo effect, according to the investigators.
The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck and Co, and Novartis. Several of his coauthors reported ties to industry.
Patients with glioblastoma who underwent standard chemoradiotherapy followed by maintenance therapy with tumor-treating fields plus temozolomide had significantly longer progression-free and overall survival, compared with temozolomide maintenance monotherapy, according to a recent report.
In the intent-to-treat population, median progression-free survival (PFS) for tumor-treating fields (TTFields) plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (hazard ratio, 0.62; 98.7% confidence interval, 0.43-0.89; P = .001). Overall survival (OS) in the per-protocol population, a prespecified secondary endpoint, was also significantly increased (20.5 months vs. 15.6 months; HR, 0.64; 99.4% CI, 0.42-0.98; P = .004), prompting early termination of the study that allowed patients in the control group the option to receive TTFields.
The prognosis for glioblastoma remains poor for this highly aggressive brain tumor, with no major treatment advance in more than a decade, according to Dr. Roger Stupp, chairman of the department of oncology and the Cancer Center at the University of Zürich Hospital and his colleagues.
“In the interim analysis of this randomized clinical trial, the addition of TTFields to standard maintenance temozolomide significantly improved progression-free and overall survival,” they wrote (JAMA. 2015;314[23]:2535-43. doi: 10.1001/jama.2015.16669).
TTFields are low-intensity, intermediate-frequency alternating electric fields delivered via transducer arrays applied to the shaved scalp. The treatment is hypothesized to disrupt spindle formation during cell division, leading to mitotic arrest and apoptosis.
The multicenter trial enrolled 695 patients with newly diagnosed glioblastoma randomized 2:1 to receive TTFields plus temozolomide or temozolomide alone as maintenance therapy from 2009 to 2014. Interim analysis included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group. The median number of temozolomide cycles until evidence of tumor progression was six cycles for the TTFields group, compared with four cycles for the temozolomide-alone group.
The median time from diagnosis to randomization was 3.8 months. When added to the median PFS of 4 months for the control group of this study, the median 7.8-month PFS is similar to most other reports.
The addition of TTFields to treatment was not associated with significant increase in systemic toxicity, except for higher incidences of scalp irritation, anxiety, confusion, insomnia, and headaches. Seizure rates did not increase.
Because a sham treatment for the control group was deemed impractical, the study was open-label, which raises the question of a placebo effect. The magnitude of the effect size (HR, 0.62 for PFS and 0.74 for OS) is greater than what could be attributed to a placebo effect, according to the investigators.
The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck and Co, and Novartis. Several of his coauthors reported ties to industry.
FROM JAMA
Key clinical point: Maintenance therapy with tumor-treating fields (TTFields) plus temozolomide resulted in longer survival, compared with temozolomide alone, for patients with glioblastoma.
Major finding: Median progression-free survival for TTFields plus temozolomide was 7.1 months, compared with 4.0 months for temozolomide alone (HR, 0.62; 98.7% CI, 0.43-0.89; P = .001).
Data sources: Interim analysis of the randomized trial included 210 patients in the TTFields plus temozolomide group and 105 patients in the temozolomide alone group.
Disclosures: The trial was sponsored by Novocure, which markets the TTFields device. Dr. Stupp reported having consulting or advisory with Novocure, Roche/Genentech, Merck KGaA, Merck, and Novartis. Several of his coauthors reported ties to industry.