User login
CHICAGO – , according to Gary M. Weiner, MD, of the department of pediatrics and neonatal-perinatal medicine at the University of Michigan and C.S. Mott Children’s Hospital in Ann Arbor.
One is recommending an electronic cardiac (EC) monitor to assess heart rate during resuscitation instead of relying on pulse oximetry, and the other is no longer recommending routine tracheal suction in nonvigorous babies with meconium-stained fluid, he told attendees at the American Academy of Pediatrics annual meeting.
About two-thirds of all births have a risk factor for needing resuscitation, and about 10%-20% of babies with a risk factor will need positive pressure ventilation (PPV). But risk factors do not identify all newborns who will need it. The risk is greatest for newborns less than 36 weeks’ or greater than 40 weeks’ gestational age, but 7% of term newborns will need PPV despite having no risk factors.
Situations in which there is the highest risk for advanced resuscitation include the following:
- Fetal bradycardia: 24-fold greater odds.
- Intrauterine growth restriction (IUGR): 20-fold greater odds.
- Clinical chorioamnionitis: 17-fold greater odds.
- Forceps or vacuum: 17-fold greater odds.
- Meconium-stained amniotic fluid (MSAF): 17-fold greater odds.
- Gestational diabetes: 16-fold greater odds.
- Abruption: 12-fold greater odds.
- General anesthesia: 11-fold greater odds.
These risks were determined in a prospective multicenter, case-control study of 61,593 births (Arch Dis Child Fetal Neonatal Ed. 2017 Jan;102[1]:F44-F50).
Assembling a team and using checklists
Teamwork and communication are key in delivery room emergencies, and teams should debrief afterward, ideally having videotaped the resuscitation, if possible, Dr. Weiner said.
He discussed preparation for a very-low-birth-weight birth, a “routine emergency” requiring many tasks in a short period of time: 130 tasks in the first hour and 40 in the first 3 minutes.
“Decisions made during the first hour have long-term implications, so you need multiple caregivers and a high-performance team,” Dr. Weiner said. In addition to a thorough understanding of the clinical situation, a high-performance team should have both effective leadership, and clearly defined roles and responsibilities for each member. Clinicians on the team need highly developed technical skills that they reliably and consistently execute with precision. “Practice, refine, practice, refine,” he emphasized.
It’s also important to make use of preset protocols, scripts, and checklists, Dr. Weiner said. These tools assure consistency, facilitate communication among team members, and improve outcomes. Research has shown that use of protocols, scripts, and checklists leads to improved stroke and trauma care, decreased complications during intubation, fewer central-line complications, and decreased perioperative mortality and complications.
He also recommended implementing a standardized equipment check and team briefing “time-out,” similar to a surgical time-out. This time-out gives teams an opportunity to identify a team leader, define member roles and responsibilities, check all equipment and supplies, discuss risk factors and possible scenarios, talk with the obstetrician and, if possible, introduce the leader or another team member to the parents.
In a study from University of California, San Diego, Medical Center, using checklists as part of resuscitation of potentially high-risk infants reduced the occurrence of communication problems from 24% to 4% of resuscitations (P less than 0.001) over a 3-year period (Resuscitation. 2013 Nov;84[11]:1552-7).
Delayed cord clamping
Dr. Weiner also discussed the benefits of placental transfusion. The fetal-placental unit includes approximately 110 mL/kg of blood, and about one-third of its volume remains in the placenta immediately after birth. Immediate cord clamping means a loss of 10-20 mL/kg of “potential” newborn blood volume, and could contribute to unstable pulmonary blood flow or a carotid artery pressure spike (Matern Health Neonatol Perinatol. 2016. doi: 10.1186/s40748-016-0032-y).
“Umbilical blood flow is complex,” he said. Blood flows toward the baby via the umbilical vein during inhalation, but stops or reverses during crying. The umbilical artery primarily carries blood to the placenta, and flow stops after about 4 minutes in more than half of infants. Gravity’s role in blood flow is controversial (Lancet. 2014 Jul 19;384[9939]:235-40).
The two options for placental transfusion are delayed cord clamping and milking the umbilical cord (also called “stripping”). In vaginal births, delayed clamping allows 20 mL/kg blood to transfer to the baby by 3 minutes after birth, with 90% of that reaching the baby in the first minute (Lancet. 1969 Oct 25;294[7626]:871-3).
Blood transfer is less efficient in cesarean births, so milking may be more efficient than simply delaying clamping, according to a small randomized controlled trial of preterm infants around 28 weeks’ gestational age. No difference between the methods was seen in vaginal births. To milk the cord, pinch it near the placenta and squeeze it toward the newborn for 2 seconds; then release, refill and repeat.
The biggest benefits in delayed cord clamping or milking occur among preterm infants: decreased mortality, higher mean arterial pressure on day 1, and a lower risk of blood transfusion, necrotizing enterocolitis, and a Bayley Motor score below 85 at 18-22 months. Term babies also get benefits, though: increased hemoglobin at birth (approximately 2 g/dL), a 0.5- to 5-point average increase in boys’ Ages & Stages fine motor and social domain scores at age 4 years, and among high-risk infants, a lower risk of iron deficiency anemia at age 1 year (JAMA Pediatr. 2017;171[3]:264-70).
According to current guidelines from the American Academy of Pediatrics, “delayed cord clamping longer than 30 seconds is reasonable for both term and preterm infants who do not require resuscitation at birth,” but “there is insufficient evidence to recommend an approach to cord clamping for infants who require resuscitation.” They also recommend against routine milking for newborns less than 29 weeks’ gestation (Pediatrics. 2015 Nov;136 Suppl 2:S196-218).
Meconium-related complications
Meconium-stained amniotic fluid (MSAF) is common, occurring in about 8% of deliveries and increasing with gestational age, but meconium aspiration syndrome (MAS) is less common, occurring in about 2% of all MSAF cases (Int J Pediatr. 2012. doi: 10.1155/2012/321545).
Risk factors for severe MAS include thick meconium and an abnormal fetal heart rate. But about two-thirds of MAS cases are mild, not requiring ventilation or continuous positive airway pressure (CPAP), Dr. Weiner said. Practice should be driven by evidence from randomized controlled trials (RCTs).
“Nonrandomized observational studies can be misleading, and rational conjecture has led to many mistakes in medicine,” he said. “Be willing to challenge conventional wisdom.”
For example, the standard of care in the 1970s, based on two nonrandomized retrospective reviews of 175 babies, included orapharyngeal and nasopharyngeal suction by the obstetrician and endotracheal tube (ETT) suction by the pediatrician. In the 2000s, however, an RCT of 2,500 infants found no benefit from orapharyngeal and nasopharyngeal suction, even with thick MSAF, (Lancet. 2004 Aug 14-20;364[9434]:597-602) and another RCT with 2,100 infants found no benefit from ETT suction (Pediatrics. 2000 Jan;105[1 Pt 1]:1-7).
More recent, smaller studies have confirmed those conclusions and found similar lack of benefit from ETT in non-vigorous infants, contributing to the new recommendation (Resuscitation. 2016 Aug;105:79-84; Indian J Pediatr. 2016 Oct;83[10]:1125-30).
“Routine tracheal suction is no longer recommended for nonvigorous babies with meconium stained fluid,” Dr. Weiner said. Since MSAF is risk factor for resuscitation, though, at least two clinicians with Neonatal Resuscitation Program (NRP) training should be present, as well as a full team if resuscitation is expected.
Heart rate assessment and tracking
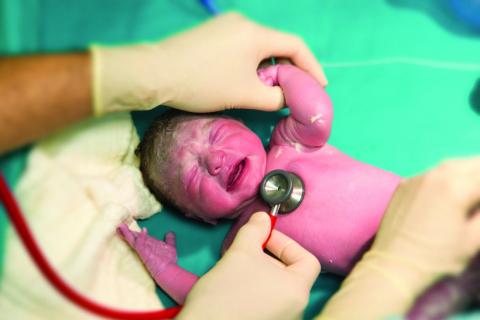
“The baby’s heart rate needs to be monitored during PPV [positive pressure ventilation] because a prompt increase in the baby’s heart rate is the most important indicator of effective PPV,” Dr. Weiner said in an interview. “Half of errors made during NRP [Neonatal Resuscitation Program] simulations are the result of incorrect heart rate assessment.”
Recent evidence comparing pulse oximetry to an EC monitor favored the latter for tracking heart rate, leading to the other new recommendation.
“The baby’s heart rate can be monitored using the pulse oximeter,” Dr. Weiner said. “However, health providers should consider using an electronic cardiac monitor in addition to pulse oximetry because studies show that it achieves a reliable signal faster.” He cited a study of 20 newborns that showed an EC monitor determined the heart rate in a median 34 seconds, compared with 122 seconds with the pulse oximeter (Pediatr Int. 2012 Apr;54[2]:205-7).
Pulse oximetry takes 90-120 seconds to attain a reliable signal and may not work if there’s poor perfusion, but an EC monitor provides continuous heart rate monitoring even with poor perfusion. So an initial heart rate assessment by auscultation is fine, but if PPV begins, EC monitoring may be better and is the preferred method with anticipated resuscitation or chest compressions.
However, pulse oximetry is still recommended “whenever positive pressure ventilation is started or oxygen is administered in order to guide the appropriate amount of oxygen supplementation,” Dr. Weiner noted.
He added that “preliminary studies suggest that handheld Doppler fetal heart monitors correlate well with ECG, provide a rapid audible heart rate and may be a promising alternative in the future” (Pediatr Int. 2017 Oct;59[10]:1069-73).
Correct ventilation techniques
“Ventilation of the lungs is the single most important and most effective step in cardiopulmonary resuscitation of the compromised newborn,” Dr. Weiner said. “If the heart rate is not rapidly increasing, ask if the chest is moving.”
He emphasized that no compressions should occur until after at least 30 seconds of PPV that moves the chest. He provided a “MR. SOPA” acronym: Mask adjustment, Reposition airway, Suction, Open mouth, Pressure increase, Alternative airway.
You also should be aware of possible leaking or obstruction around the mask, which is common, he said, so monitor pressure instead of volume.
“We are not good at identifying leak, obstruction, or adequate tidal volume,” Dr. Weiner said. “A colorimetric CO2 detector attached to the mask is a simple indicator of gas exchange” (Resuscitation. 2014 Nov;85[11]:1568-72).
He also strongly recommended inserting an alternative airway before starting chest compressions with either intubation or a laryngeal mask.
Dr. Weiner concluded with the following list of clinical practice changes you may consider:
- Use a standardized equipment checklist.
- Develop and practice standardized scripts.
- Debrief after all resuscitations; use videotape if you can.
- Delay cord clamping for most term and preterm babies.
- Do not routinely intubate/suction nonvigorous newborns with MSAF. Initiate resuscitation.
- Use an electronic cardiac monitor if resuscitation is required.
- Use a colorimetric CO2 detector with PPV.
- Intubate or place a laryngeal mask before starting compressions.
Dr. Weiner reported having no disclosures, and no external funding was used for the presentation.
CHICAGO – , according to Gary M. Weiner, MD, of the department of pediatrics and neonatal-perinatal medicine at the University of Michigan and C.S. Mott Children’s Hospital in Ann Arbor.
One is recommending an electronic cardiac (EC) monitor to assess heart rate during resuscitation instead of relying on pulse oximetry, and the other is no longer recommending routine tracheal suction in nonvigorous babies with meconium-stained fluid, he told attendees at the American Academy of Pediatrics annual meeting.
About two-thirds of all births have a risk factor for needing resuscitation, and about 10%-20% of babies with a risk factor will need positive pressure ventilation (PPV). But risk factors do not identify all newborns who will need it. The risk is greatest for newborns less than 36 weeks’ or greater than 40 weeks’ gestational age, but 7% of term newborns will need PPV despite having no risk factors.
Situations in which there is the highest risk for advanced resuscitation include the following:
- Fetal bradycardia: 24-fold greater odds.
- Intrauterine growth restriction (IUGR): 20-fold greater odds.
- Clinical chorioamnionitis: 17-fold greater odds.
- Forceps or vacuum: 17-fold greater odds.
- Meconium-stained amniotic fluid (MSAF): 17-fold greater odds.
- Gestational diabetes: 16-fold greater odds.
- Abruption: 12-fold greater odds.
- General anesthesia: 11-fold greater odds.
These risks were determined in a prospective multicenter, case-control study of 61,593 births (Arch Dis Child Fetal Neonatal Ed. 2017 Jan;102[1]:F44-F50).
Assembling a team and using checklists
Teamwork and communication are key in delivery room emergencies, and teams should debrief afterward, ideally having videotaped the resuscitation, if possible, Dr. Weiner said.
He discussed preparation for a very-low-birth-weight birth, a “routine emergency” requiring many tasks in a short period of time: 130 tasks in the first hour and 40 in the first 3 minutes.
“Decisions made during the first hour have long-term implications, so you need multiple caregivers and a high-performance team,” Dr. Weiner said. In addition to a thorough understanding of the clinical situation, a high-performance team should have both effective leadership, and clearly defined roles and responsibilities for each member. Clinicians on the team need highly developed technical skills that they reliably and consistently execute with precision. “Practice, refine, practice, refine,” he emphasized.
It’s also important to make use of preset protocols, scripts, and checklists, Dr. Weiner said. These tools assure consistency, facilitate communication among team members, and improve outcomes. Research has shown that use of protocols, scripts, and checklists leads to improved stroke and trauma care, decreased complications during intubation, fewer central-line complications, and decreased perioperative mortality and complications.
He also recommended implementing a standardized equipment check and team briefing “time-out,” similar to a surgical time-out. This time-out gives teams an opportunity to identify a team leader, define member roles and responsibilities, check all equipment and supplies, discuss risk factors and possible scenarios, talk with the obstetrician and, if possible, introduce the leader or another team member to the parents.
In a study from University of California, San Diego, Medical Center, using checklists as part of resuscitation of potentially high-risk infants reduced the occurrence of communication problems from 24% to 4% of resuscitations (P less than 0.001) over a 3-year period (Resuscitation. 2013 Nov;84[11]:1552-7).
Delayed cord clamping
Dr. Weiner also discussed the benefits of placental transfusion. The fetal-placental unit includes approximately 110 mL/kg of blood, and about one-third of its volume remains in the placenta immediately after birth. Immediate cord clamping means a loss of 10-20 mL/kg of “potential” newborn blood volume, and could contribute to unstable pulmonary blood flow or a carotid artery pressure spike (Matern Health Neonatol Perinatol. 2016. doi: 10.1186/s40748-016-0032-y).
“Umbilical blood flow is complex,” he said. Blood flows toward the baby via the umbilical vein during inhalation, but stops or reverses during crying. The umbilical artery primarily carries blood to the placenta, and flow stops after about 4 minutes in more than half of infants. Gravity’s role in blood flow is controversial (Lancet. 2014 Jul 19;384[9939]:235-40).
The two options for placental transfusion are delayed cord clamping and milking the umbilical cord (also called “stripping”). In vaginal births, delayed clamping allows 20 mL/kg blood to transfer to the baby by 3 minutes after birth, with 90% of that reaching the baby in the first minute (Lancet. 1969 Oct 25;294[7626]:871-3).
Blood transfer is less efficient in cesarean births, so milking may be more efficient than simply delaying clamping, according to a small randomized controlled trial of preterm infants around 28 weeks’ gestational age. No difference between the methods was seen in vaginal births. To milk the cord, pinch it near the placenta and squeeze it toward the newborn for 2 seconds; then release, refill and repeat.
The biggest benefits in delayed cord clamping or milking occur among preterm infants: decreased mortality, higher mean arterial pressure on day 1, and a lower risk of blood transfusion, necrotizing enterocolitis, and a Bayley Motor score below 85 at 18-22 months. Term babies also get benefits, though: increased hemoglobin at birth (approximately 2 g/dL), a 0.5- to 5-point average increase in boys’ Ages & Stages fine motor and social domain scores at age 4 years, and among high-risk infants, a lower risk of iron deficiency anemia at age 1 year (JAMA Pediatr. 2017;171[3]:264-70).
According to current guidelines from the American Academy of Pediatrics, “delayed cord clamping longer than 30 seconds is reasonable for both term and preterm infants who do not require resuscitation at birth,” but “there is insufficient evidence to recommend an approach to cord clamping for infants who require resuscitation.” They also recommend against routine milking for newborns less than 29 weeks’ gestation (Pediatrics. 2015 Nov;136 Suppl 2:S196-218).
Meconium-related complications
Meconium-stained amniotic fluid (MSAF) is common, occurring in about 8% of deliveries and increasing with gestational age, but meconium aspiration syndrome (MAS) is less common, occurring in about 2% of all MSAF cases (Int J Pediatr. 2012. doi: 10.1155/2012/321545).
Risk factors for severe MAS include thick meconium and an abnormal fetal heart rate. But about two-thirds of MAS cases are mild, not requiring ventilation or continuous positive airway pressure (CPAP), Dr. Weiner said. Practice should be driven by evidence from randomized controlled trials (RCTs).
“Nonrandomized observational studies can be misleading, and rational conjecture has led to many mistakes in medicine,” he said. “Be willing to challenge conventional wisdom.”
For example, the standard of care in the 1970s, based on two nonrandomized retrospective reviews of 175 babies, included orapharyngeal and nasopharyngeal suction by the obstetrician and endotracheal tube (ETT) suction by the pediatrician. In the 2000s, however, an RCT of 2,500 infants found no benefit from orapharyngeal and nasopharyngeal suction, even with thick MSAF, (Lancet. 2004 Aug 14-20;364[9434]:597-602) and another RCT with 2,100 infants found no benefit from ETT suction (Pediatrics. 2000 Jan;105[1 Pt 1]:1-7).
More recent, smaller studies have confirmed those conclusions and found similar lack of benefit from ETT in non-vigorous infants, contributing to the new recommendation (Resuscitation. 2016 Aug;105:79-84; Indian J Pediatr. 2016 Oct;83[10]:1125-30).
“Routine tracheal suction is no longer recommended for nonvigorous babies with meconium stained fluid,” Dr. Weiner said. Since MSAF is risk factor for resuscitation, though, at least two clinicians with Neonatal Resuscitation Program (NRP) training should be present, as well as a full team if resuscitation is expected.
Heart rate assessment and tracking
“The baby’s heart rate needs to be monitored during PPV [positive pressure ventilation] because a prompt increase in the baby’s heart rate is the most important indicator of effective PPV,” Dr. Weiner said in an interview. “Half of errors made during NRP [Neonatal Resuscitation Program] simulations are the result of incorrect heart rate assessment.”
Recent evidence comparing pulse oximetry to an EC monitor favored the latter for tracking heart rate, leading to the other new recommendation.
“The baby’s heart rate can be monitored using the pulse oximeter,” Dr. Weiner said. “However, health providers should consider using an electronic cardiac monitor in addition to pulse oximetry because studies show that it achieves a reliable signal faster.” He cited a study of 20 newborns that showed an EC monitor determined the heart rate in a median 34 seconds, compared with 122 seconds with the pulse oximeter (Pediatr Int. 2012 Apr;54[2]:205-7).
Pulse oximetry takes 90-120 seconds to attain a reliable signal and may not work if there’s poor perfusion, but an EC monitor provides continuous heart rate monitoring even with poor perfusion. So an initial heart rate assessment by auscultation is fine, but if PPV begins, EC monitoring may be better and is the preferred method with anticipated resuscitation or chest compressions.
However, pulse oximetry is still recommended “whenever positive pressure ventilation is started or oxygen is administered in order to guide the appropriate amount of oxygen supplementation,” Dr. Weiner noted.
He added that “preliminary studies suggest that handheld Doppler fetal heart monitors correlate well with ECG, provide a rapid audible heart rate and may be a promising alternative in the future” (Pediatr Int. 2017 Oct;59[10]:1069-73).
Correct ventilation techniques
“Ventilation of the lungs is the single most important and most effective step in cardiopulmonary resuscitation of the compromised newborn,” Dr. Weiner said. “If the heart rate is not rapidly increasing, ask if the chest is moving.”
He emphasized that no compressions should occur until after at least 30 seconds of PPV that moves the chest. He provided a “MR. SOPA” acronym: Mask adjustment, Reposition airway, Suction, Open mouth, Pressure increase, Alternative airway.
You also should be aware of possible leaking or obstruction around the mask, which is common, he said, so monitor pressure instead of volume.
“We are not good at identifying leak, obstruction, or adequate tidal volume,” Dr. Weiner said. “A colorimetric CO2 detector attached to the mask is a simple indicator of gas exchange” (Resuscitation. 2014 Nov;85[11]:1568-72).
He also strongly recommended inserting an alternative airway before starting chest compressions with either intubation or a laryngeal mask.
Dr. Weiner concluded with the following list of clinical practice changes you may consider:
- Use a standardized equipment checklist.
- Develop and practice standardized scripts.
- Debrief after all resuscitations; use videotape if you can.
- Delay cord clamping for most term and preterm babies.
- Do not routinely intubate/suction nonvigorous newborns with MSAF. Initiate resuscitation.
- Use an electronic cardiac monitor if resuscitation is required.
- Use a colorimetric CO2 detector with PPV.
- Intubate or place a laryngeal mask before starting compressions.
Dr. Weiner reported having no disclosures, and no external funding was used for the presentation.
CHICAGO – , according to Gary M. Weiner, MD, of the department of pediatrics and neonatal-perinatal medicine at the University of Michigan and C.S. Mott Children’s Hospital in Ann Arbor.
One is recommending an electronic cardiac (EC) monitor to assess heart rate during resuscitation instead of relying on pulse oximetry, and the other is no longer recommending routine tracheal suction in nonvigorous babies with meconium-stained fluid, he told attendees at the American Academy of Pediatrics annual meeting.
About two-thirds of all births have a risk factor for needing resuscitation, and about 10%-20% of babies with a risk factor will need positive pressure ventilation (PPV). But risk factors do not identify all newborns who will need it. The risk is greatest for newborns less than 36 weeks’ or greater than 40 weeks’ gestational age, but 7% of term newborns will need PPV despite having no risk factors.
Situations in which there is the highest risk for advanced resuscitation include the following:
- Fetal bradycardia: 24-fold greater odds.
- Intrauterine growth restriction (IUGR): 20-fold greater odds.
- Clinical chorioamnionitis: 17-fold greater odds.
- Forceps or vacuum: 17-fold greater odds.
- Meconium-stained amniotic fluid (MSAF): 17-fold greater odds.
- Gestational diabetes: 16-fold greater odds.
- Abruption: 12-fold greater odds.
- General anesthesia: 11-fold greater odds.
These risks were determined in a prospective multicenter, case-control study of 61,593 births (Arch Dis Child Fetal Neonatal Ed. 2017 Jan;102[1]:F44-F50).
Assembling a team and using checklists
Teamwork and communication are key in delivery room emergencies, and teams should debrief afterward, ideally having videotaped the resuscitation, if possible, Dr. Weiner said.
He discussed preparation for a very-low-birth-weight birth, a “routine emergency” requiring many tasks in a short period of time: 130 tasks in the first hour and 40 in the first 3 minutes.
“Decisions made during the first hour have long-term implications, so you need multiple caregivers and a high-performance team,” Dr. Weiner said. In addition to a thorough understanding of the clinical situation, a high-performance team should have both effective leadership, and clearly defined roles and responsibilities for each member. Clinicians on the team need highly developed technical skills that they reliably and consistently execute with precision. “Practice, refine, practice, refine,” he emphasized.
It’s also important to make use of preset protocols, scripts, and checklists, Dr. Weiner said. These tools assure consistency, facilitate communication among team members, and improve outcomes. Research has shown that use of protocols, scripts, and checklists leads to improved stroke and trauma care, decreased complications during intubation, fewer central-line complications, and decreased perioperative mortality and complications.
He also recommended implementing a standardized equipment check and team briefing “time-out,” similar to a surgical time-out. This time-out gives teams an opportunity to identify a team leader, define member roles and responsibilities, check all equipment and supplies, discuss risk factors and possible scenarios, talk with the obstetrician and, if possible, introduce the leader or another team member to the parents.
In a study from University of California, San Diego, Medical Center, using checklists as part of resuscitation of potentially high-risk infants reduced the occurrence of communication problems from 24% to 4% of resuscitations (P less than 0.001) over a 3-year period (Resuscitation. 2013 Nov;84[11]:1552-7).
Delayed cord clamping
Dr. Weiner also discussed the benefits of placental transfusion. The fetal-placental unit includes approximately 110 mL/kg of blood, and about one-third of its volume remains in the placenta immediately after birth. Immediate cord clamping means a loss of 10-20 mL/kg of “potential” newborn blood volume, and could contribute to unstable pulmonary blood flow or a carotid artery pressure spike (Matern Health Neonatol Perinatol. 2016. doi: 10.1186/s40748-016-0032-y).
“Umbilical blood flow is complex,” he said. Blood flows toward the baby via the umbilical vein during inhalation, but stops or reverses during crying. The umbilical artery primarily carries blood to the placenta, and flow stops after about 4 minutes in more than half of infants. Gravity’s role in blood flow is controversial (Lancet. 2014 Jul 19;384[9939]:235-40).
The two options for placental transfusion are delayed cord clamping and milking the umbilical cord (also called “stripping”). In vaginal births, delayed clamping allows 20 mL/kg blood to transfer to the baby by 3 minutes after birth, with 90% of that reaching the baby in the first minute (Lancet. 1969 Oct 25;294[7626]:871-3).
Blood transfer is less efficient in cesarean births, so milking may be more efficient than simply delaying clamping, according to a small randomized controlled trial of preterm infants around 28 weeks’ gestational age. No difference between the methods was seen in vaginal births. To milk the cord, pinch it near the placenta and squeeze it toward the newborn for 2 seconds; then release, refill and repeat.
The biggest benefits in delayed cord clamping or milking occur among preterm infants: decreased mortality, higher mean arterial pressure on day 1, and a lower risk of blood transfusion, necrotizing enterocolitis, and a Bayley Motor score below 85 at 18-22 months. Term babies also get benefits, though: increased hemoglobin at birth (approximately 2 g/dL), a 0.5- to 5-point average increase in boys’ Ages & Stages fine motor and social domain scores at age 4 years, and among high-risk infants, a lower risk of iron deficiency anemia at age 1 year (JAMA Pediatr. 2017;171[3]:264-70).
According to current guidelines from the American Academy of Pediatrics, “delayed cord clamping longer than 30 seconds is reasonable for both term and preterm infants who do not require resuscitation at birth,” but “there is insufficient evidence to recommend an approach to cord clamping for infants who require resuscitation.” They also recommend against routine milking for newborns less than 29 weeks’ gestation (Pediatrics. 2015 Nov;136 Suppl 2:S196-218).
Meconium-related complications
Meconium-stained amniotic fluid (MSAF) is common, occurring in about 8% of deliveries and increasing with gestational age, but meconium aspiration syndrome (MAS) is less common, occurring in about 2% of all MSAF cases (Int J Pediatr. 2012. doi: 10.1155/2012/321545).
Risk factors for severe MAS include thick meconium and an abnormal fetal heart rate. But about two-thirds of MAS cases are mild, not requiring ventilation or continuous positive airway pressure (CPAP), Dr. Weiner said. Practice should be driven by evidence from randomized controlled trials (RCTs).
“Nonrandomized observational studies can be misleading, and rational conjecture has led to many mistakes in medicine,” he said. “Be willing to challenge conventional wisdom.”
For example, the standard of care in the 1970s, based on two nonrandomized retrospective reviews of 175 babies, included orapharyngeal and nasopharyngeal suction by the obstetrician and endotracheal tube (ETT) suction by the pediatrician. In the 2000s, however, an RCT of 2,500 infants found no benefit from orapharyngeal and nasopharyngeal suction, even with thick MSAF, (Lancet. 2004 Aug 14-20;364[9434]:597-602) and another RCT with 2,100 infants found no benefit from ETT suction (Pediatrics. 2000 Jan;105[1 Pt 1]:1-7).
More recent, smaller studies have confirmed those conclusions and found similar lack of benefit from ETT in non-vigorous infants, contributing to the new recommendation (Resuscitation. 2016 Aug;105:79-84; Indian J Pediatr. 2016 Oct;83[10]:1125-30).
“Routine tracheal suction is no longer recommended for nonvigorous babies with meconium stained fluid,” Dr. Weiner said. Since MSAF is risk factor for resuscitation, though, at least two clinicians with Neonatal Resuscitation Program (NRP) training should be present, as well as a full team if resuscitation is expected.
Heart rate assessment and tracking
“The baby’s heart rate needs to be monitored during PPV [positive pressure ventilation] because a prompt increase in the baby’s heart rate is the most important indicator of effective PPV,” Dr. Weiner said in an interview. “Half of errors made during NRP [Neonatal Resuscitation Program] simulations are the result of incorrect heart rate assessment.”
Recent evidence comparing pulse oximetry to an EC monitor favored the latter for tracking heart rate, leading to the other new recommendation.
“The baby’s heart rate can be monitored using the pulse oximeter,” Dr. Weiner said. “However, health providers should consider using an electronic cardiac monitor in addition to pulse oximetry because studies show that it achieves a reliable signal faster.” He cited a study of 20 newborns that showed an EC monitor determined the heart rate in a median 34 seconds, compared with 122 seconds with the pulse oximeter (Pediatr Int. 2012 Apr;54[2]:205-7).
Pulse oximetry takes 90-120 seconds to attain a reliable signal and may not work if there’s poor perfusion, but an EC monitor provides continuous heart rate monitoring even with poor perfusion. So an initial heart rate assessment by auscultation is fine, but if PPV begins, EC monitoring may be better and is the preferred method with anticipated resuscitation or chest compressions.
However, pulse oximetry is still recommended “whenever positive pressure ventilation is started or oxygen is administered in order to guide the appropriate amount of oxygen supplementation,” Dr. Weiner noted.
He added that “preliminary studies suggest that handheld Doppler fetal heart monitors correlate well with ECG, provide a rapid audible heart rate and may be a promising alternative in the future” (Pediatr Int. 2017 Oct;59[10]:1069-73).
Correct ventilation techniques
“Ventilation of the lungs is the single most important and most effective step in cardiopulmonary resuscitation of the compromised newborn,” Dr. Weiner said. “If the heart rate is not rapidly increasing, ask if the chest is moving.”
He emphasized that no compressions should occur until after at least 30 seconds of PPV that moves the chest. He provided a “MR. SOPA” acronym: Mask adjustment, Reposition airway, Suction, Open mouth, Pressure increase, Alternative airway.
You also should be aware of possible leaking or obstruction around the mask, which is common, he said, so monitor pressure instead of volume.
“We are not good at identifying leak, obstruction, or adequate tidal volume,” Dr. Weiner said. “A colorimetric CO2 detector attached to the mask is a simple indicator of gas exchange” (Resuscitation. 2014 Nov;85[11]:1568-72).
He also strongly recommended inserting an alternative airway before starting chest compressions with either intubation or a laryngeal mask.
Dr. Weiner concluded with the following list of clinical practice changes you may consider:
- Use a standardized equipment checklist.
- Develop and practice standardized scripts.
- Debrief after all resuscitations; use videotape if you can.
- Delay cord clamping for most term and preterm babies.
- Do not routinely intubate/suction nonvigorous newborns with MSAF. Initiate resuscitation.
- Use an electronic cardiac monitor if resuscitation is required.
- Use a colorimetric CO2 detector with PPV.
- Intubate or place a laryngeal mask before starting compressions.
Dr. Weiner reported having no disclosures, and no external funding was used for the presentation.
EXPERT ANALYSIS FROM AAP 2017