User login
Adolescents, nulliparous women, and the IUD
The professional journals scattered on your desk every month always seem to have a review article, or a study, on “long-acting reversible contraception” (LARC)—and you’re not certain why. More and more, your younger patients are asking about intrauterine devices and contraceptive implants, but you’re unsure about the most up-to-date information on the safety of these methods in adolescents. Nulliparous women are inquiring about contraception with, for one, the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena), but the Mirena package insert tells you that they are not candidates for the method.
Does this sound familiar? How do you sort through all data and advice on IUD use in adolescents and nulliparas?
Fortunately for clinicians, a great deal of research in the last few years has focused on these topics. Recommendations and reviews have been published, and public health agencies have developed easily accessible guidelines for reference.
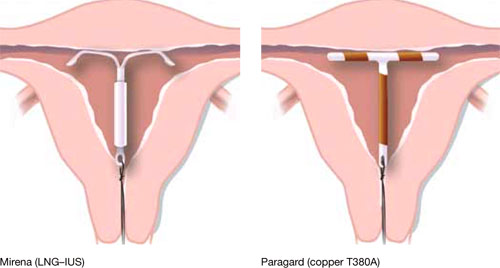
Our goal in this year’s installment of the Update on Contraception is to familiarize you with the evidence and ease any confusion, even misgivings, you might have about the two intrauterine devices available in the United States, the copper T380A (Paragard*) and Mirena* (FIGURE), especially in regard to their use in adolescents and nulliparous women.
*For ease of discussion only, we call these two systems by their brand names throughout.
FIGURE Two intrauterine devices for long-term contraception
There is real need for long-acting reversible contraception
The public health perspective
Consider these statistics about adult and adolescent women:
- Approximately 3 million pregnancies annually in the United States are unintended
- In almost half of those pregnancies, the woman undergoes an abortion
- Approximately one half of all US women have an unintended pregnancy by 45 years of age
- Given current statistics, nearly one third of all women will have chosen abortion by 45 years of age
- 80% of pregnancies in adolescents are unintended; 45% end in abortion
- 54% of women who have an abortion used a contraceptive method during the month they became pregnant—generally, a condom or an oral contraceptive.1,2
Patients’ perspective
More and more, adolescent women and nulliparous women request an IUD. Indeed, recent studies show that these populations are interested in the long-term protection that an IUD offers, and are likely to be more compliant with the method. Recent data from the National Survey of Family Growth showed an increase in IUD use in the United States: 5.5% of women who use birth control use an intrauterine device, and 14% of women who choose an IUD are adolescents.
A review of IUD use in adolescents found that, across six cohort studies and seven case reports, the continuation rate with an IUD after 1 year ranged from 48% to 88%—similar to, or better than, what is seen with oral contraceptives (OCs).3 Furthermore, two recent studies3,4 showed that:
- young nulliparous and parous women exhibit a positive attitude toward IUD use once they have been counseled on the risks and benefits of the device
- they desire effective long-term contraception
- more than 50% of the women who were surveyed thought positively about IUDs after being educated about them.
Intrauterine contraceptive use in the United States is very low compared to the rates in other developed countries—as noted, the rate here is 5.5%, for both types of IUD. Contrast that rate with what is documented in other nations: France, 20%; China, 34%; and Norway, 24%, for example. Across the developed world, IUD use is at 7.6%; in developing nations, the rate is even higher: 14.5 %.5,6
Women in the United States use the most effective forms of birth control at lower rates than the rest of the developed world; conversely, they choose permanent sterilization at a higher rate. When American women were asked, they expressed a desire for longer-acting contraception that is easy to use. Yet, they fail to take advantage of the options—often, because they lack information about them or have received erroneous education.2
Indeed, when providers of contraceptive services have been surveyed about barriers to IUD use, they point to women’s misconceptions about the devices and express their own concerns about the incidence of pelvic inflammatory disease (PID) and infertility; difficulty of insertion; and expulsion.
Findings of a survey of clinicians. In a 2008 poll of 816 health care providers (including 399 physicians and 402 advanced practice clinicians), 40% did not offer intrauterine contraception to any patients who sought contraception.7 Most (55%) providers considered less than one quarter of their patients to be a candidate for intrauterine contraception.
Furthermore, fewer than one half of providers considered nulliparous, immediate postpartum or post-abortion, or teenage patients to be a candidate for intrauterine contraception. They also thought that women who had a history of ectopic pregnancy or pelvic inflammatory disease, or who were HIV-positive, were not candidates for intrauterine contraception—despite recommendations by the Centers for Disease Control and Prevention and the World Health Organization to use intrauterine contraception in those populations (TABLE).8,9
TABLE
Medical eligibility criteria for using an IUD (in selected conditions)*
| Condition | Paragard | Mirena |
|---|---|---|
| Age | ||
| • Menarche to age 20 | 2 | 2 |
| • ≥Age 20 | 1 | 1 |
| Parity | ||
| • Nulliparous | 2 | 2 |
| • Parous | 1 | 1 |
| Postpartum (breastfeeding or not breastfeeding, including post-cesarean section) | ||
| • <10 min after placental delivery | 1 | 2 |
| • 10 min after placental delivery to 4 weeks | 2 | 2 |
| • ≥4 weeks | 1 | 1 |
| Postabortion | ||
| • First trimester | 1 | 1 |
| • Second trimester | 2 | 2 |
| Past ectopic pregnancy | 1 | 1 |
| Past pelvic inflammatory disease (PID) (assuming no current risk factors for sexually transmitted infection) | ||
| • With subsequent pregnancy | 1 | 1 |
| • Without subsequent pregnancy | 2 | 2 |
| Continuation with current PID, infection with Chlamydia trachomatis or Neisseria gonorrhoeae | 2 | 2 |
| HIV-infected | 2 | 2 |
| Obesity | 1 | 1 |
| Venous thromboembolic disease | ||
| • History of deep-venous thromboembolism (DVT) or pulmonary embolism (PE) | 1 | 2 |
| • High risk for DVT or Pe | 1 | 2 |
| • Acute DVT or Pe | 2 | 2 |
| Key 1 There is no restriction on the use of the contraceptive method for this condition 2 The advantages of using the contraceptive method generally outweigh its theoretical or proven risks in this condition 3 The theoretical or proven risks of the contraceptive method generally outweigh its benefits in this condition * Adapted from: centers for Disease control and Prevention. US Medical eligibility criteria for contraceptive Use, 2010. MMWR early release 2010;59:52-7. for other recommendations, see: centers for Disease control and Prevention.8 | ||
IUDs are safe
Do you relate to what respondents said in the survey just discussed? Do you have concerns about intrauterine contraception in adolescents or nulliparas, especially about:
- perforation at the time an IUD is placed
- risk of expulsion
- side effects
- risk of PID
- risk of infertility?
Let’s examine each of these concerns against the backdrop of clinical guidelines issued recently by the Society of Family Planning and the group’s analysis of the medical literature on which those guidelines are based.10
Perforation at placement. No studies have examined the rate of perforation during IUD placement in nulliparas or adolescents alone; we do know that the overall (i.e., for all women) risk of perforation when an IUD is inserted has been reported as zero to 1.3%. General studies of perforation include a very small number of nulliparas; results are difficult to generalize to a larger population. At least two ongoing large, multicenter trials include a large number of nulliparas; one of them includes adolescents.
Expulsion. The rate of IUD expulsion in parous women has varied across studies and types of IUDs. In a recent retrospective cohort study, nulliparous and parous women were compared for complications with both copper and levonorgestrel-releasing IUDs. Rates of expulsion for copper IUDs were 0 to 1.2% a year. Rates of expulsion for the levonorgestrel-releasing IUDs were 0 to 0.2% a year. Nulliparous women did not have more complications than parous women.11
A review of studies examining the expulsion rate with the copper IUD found a slightly higher rate in nulliparas, but the copper IUD that had been used in 19 of 20 of those studies was not Paragard, the only copper IUD available in the United States. In the one study included in the review that looked at Paragard, there was one expulsion in the nulliparous group and none in the parous group.12
Side effects. In a review of copper-based IUDs, removals for pain and bleeding were slightly higher in nulliparas. Again, the majority of these studies reviewed did not use Paragard. In the one study that did examine Paragard, there were no removals for bleeding or pain in nulliparas or multiparas.10
No studies have compared nulliparous and parous women in regard to side effects associated with Mirena.
Pelvic inflammatory disease. Misgivings that providers have about the IUD often hearken back to the Dalkon Shield, which had a multifilament string that allowed bacteria to climb from the vagina into the uterus, with damaging consequences. Current IUDs have a monofilament string; they do not increase the user’s risk of pelvic infection.
Through recent research on antibiotic prophylaxis for IUD insertion, we have learned that the risk of PID in this setting is not as great as once thought. Antibiotic prophylaxis is unnecessary for IUD insertion because cases of PID after IUD insertion occur infrequently, with or without an antibiotic. A randomized clinical trial of 1,833 patients treated with azithromycin or placebo before IUD insertion demonstrated this low risk of PID: Only one patient in each group was given a diagnosis of salpingitis during the 90-day period after insertion.13
In addition, a recent study found that subjects could be screened for gonorrhea and chlamydial infection when an IUD was being placed and treated after insertion if either of those tests was positive—without increasing their risk of PID.14
Mirena may, in fact, have a protective effect against infection. When the device was compared to a copper IUD (Nova-T; not available in the United States) in a randomized, comparative, multicenter trial, subjects in whom Mirena was inserted had a cumulative gross rate of PID of 0.5 at 36 months; Nova-T users had a rate of 2.0.15
As we well know, women who require protection from sexually transmitted infection (STI) need to have their partner use a condom. But condoms are not, comparatively, a very good method of contraception; for a woman who is at risk of STI and pregnancy, we need to consider what method she will use in addition to a condom to protect against pregnancy. Is she better off using a condom and an OC, or a condom and an IUD? The answer may well be that, because an IUD does not increase the risk of STI or PID and is more effective at preventing pregnancy than an OC, she would be better off using a condom plus IUD when it comes to protecting herself against STI and pregnancy.
Infertility. The risk of infertility has been linked to the risk of PID, which, simply, has been shown to be unfounded with an IUD.
In 2001, a cohort study focused on three groups of patients: women seeking treatment for primary infertility with diagnosed tubal occlusion; women seeking treatment for primary infertility without tubal occlusion; and primigravida pregnant women.16 In all three groups, the same percentage reported prior copper IUD use—suggesting no increased risk of either tubal or nontubal infertility among IUD users. This finding is in concordance with other studies that examined the risk of infertility among parous IUD users.17
Clinical guidelines from the Society of family planning
Based on the evidence reviewed by the Society of Family Planning (SFP) on the use of intrauterine contraception in nulliparous women, SFP offers recommendations.10
Level-A evidence is that:
- Mirena and Paragard are effective and safe for nulliparous women
- compared with other methods, IUDs have a comparable or higher continuation-of-use rate in nulliparous women
- IUDs do not increase the risk of pelvic infection or infertility. Mirena probably reduces users’ risk of infection.
Level-B evidence is that:
- because of the expulsion rate and bleeding profile, Mirena might be better tolerated than Paragard in nulliparas
- insertion of an IUD may be more challenging in nulliparous women; given the benefits, however, clinicians should not be discouraged from considering them as a first-line contraceptive choice in this population.
Level-C evidence is that:
- adolescent women should be considered a candidate for an IUD.
Are adolescents more likely to discontinue use of an IUD than they are known to discontinue OCs and injectable contraceptives?
According to ACOG’s most recent Committee Opinion on IUDs in adolescents,18 the rate of IUD discontinuation might be slightly higher because of side effects, but this problem might be alleviated by counseling patients about the rate of amenorrhea with Mirena and providing adequate education about the side effects seen with both IUDs.
The authors of ACOG’s Committee Opinion also recommend that clinicians be familiar with their state’s consent laws regarding adolescents and contraception.
The conclusion of the Committee?
The IUD is a highly effective method of contraception that is underused in the United States. Because adolescents contribute disproportionately to the epidemic of unintended pregnancy in this country, top-tier methods of contraception, including IUDs and implants, should be considered as first-line choices for both nulliparous and parous adolescents.
How do I put the IUD into practice for these populations?
Here are tips about placing an IUD in nulliparous or adolescent women, gleaned from practice. Consider discussing placement techniques with clinicians and using their experiences as a way of expanding your repertoire when dealing with a difficult insertion.
A small body of literature on misoprostol and ibuprofen, including two recent randomized controlled trials,19,20 has failed to show that pain associated with insertion is relieved using either treatment. Below, we offer several recommendations on this point.
Counsel the patient extensively about what to expect with an IUD. Namely:
- how the IUD is inserted, with attention to female anatomy
- the most common side effects, especially bleeding
- cramping and pain with insertion
- spotting after insertion
- the need to use back-up contraception
- the need to use a condom to prevent STI.
Have various items available, as needed, during insertion. This includes, but isn’t limited to:
- various-sized specula
- cervical dilators
- an examination table adjustable for height and position
- an assistant to reassure and comfort the patient and to assist you.
We’re out from under a dark cloud
It’s been a long road for US clinicians, coming back from the damage done by the Dalkon Shield to their interest in inserting IUDs in nulliparous and adolescent women. But we are gradually seeing a change in both physicians’ and patients’ opinions about using intrauterine devices for these populations.
Demand is growing in the United States for long-acting reversible contraception; we need to challenge our reservations and provide the care that our patients are requesting. The opinions and advice of our supporting professional organizations, based on the recent literature, point to the appropriateness of embracing IUDs for nulliparous and adolescent women.
We urge you: Heed the call.
We want to hear from you! Tell us what you think.
1. Guttmacher Institute. Facts on induced abortion in the United States. In Brief. http://www.guttmacher.org/pubs/
fb_induced_abortion.pdf. Published May 2010.
2. Stanwood NL, Bradley KA. Young pregnant women’s knowledge of modern intrauterine devices. Obstet Gynecol. 2006;108(6):1417-1422.
3. Deans EI, Grimes DA. Intrauterine devices for adolescents: a systematic review. Contraception. 2009;79(6):418-423.
4. Whitaker AK, Johnson LM, Harwood B, Chiappetta L, Creinin MD, Gold MA. Adolescent and young adult women’s knowledge of and attitudes toward the intrauterine device. Contraception. 2008;78(3):211-217.
5. United Nations Department of Economic and Social Affairs, Population Division. World contraceptive use 2005. http://www.un.org/esa/population/publications/contraceptive2005/2005_World_
Contraceptive_files/WallChart_WCU2005.pdf. Accessed May 25, 2010.
6. Sonfield A. For the Guttmacher Institute. Popularity disparity: attitudes about the IUD in Europe and the United States. Guttmacher Policy Review. 2007;10(4):http://www.guttmacher.org/pubs/gpr/10/4/gpr100419.pdf. Accessed May 21, 2010.
7. Harper CC, Blum M, de Bocanegra HT, et al. Challenges in translating evidence into practice: the provision of intrauterine contraception. Obstet Gynecol. 2008;111(6):1359-1369.
8. Centers for Disease Control and Prevention. U.S. medical eligibility criteria for contraceptive use, 2010. MMWR. 2010;59(Early Release; RR04):1-6.
9. World Health Organization, Department of Reproductive Health and Research Medical eligibility criteria for contraceptive use. Geneva: WHO; 2009.
10. Lyus R, Lohr P, Prager S. for Board of the Society of Family Planning. Use of the Mirena LNG-IUS and Paragard CuT380 devices in nulliparous women. Contraception. 2010;81(5):367-371.
11. Veldhuis HM, Vos AG, Lagro-Janssen AL. Complications of the intrauterine device in nulliparous and parous women. Eur J Gen Pract. 2004;10(3):82-87.
12. Hubacher D. Copper intrauterine device use by nulliparous women: review of side effects. Contraception. 2007;75(suppl 6):S8-S11.
13. Walsh T, Grimes DA, Frezieres R, et al. for IUD Study Group. Randomised controlled trial of prophylactic antibiotics before insertion of intrauterine devices. Lancet. 1998;351(9108):1005-1008.
14. Goodman S, Hendlish SK, Benedict C, Reeves MF, Pera-Floyd M, Foster-Rosales A. Increasing intrauterine contraception use by reducing barriers to post-abortal and interval insertion. Contraception. 2008;78(2):136-142.
15. Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years’ comparative experience of levonorgestrel- and copper-releasing intrauterine devices. Obstet Gynecol. 1991;77(2):261-264.
16. Hubacher D, Lara-Ricalde R, Taylor DJ, Guerra-Infante F, Guzmán-Rodrigues R. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med. 2001;345(8):561-567.
17. Hov GG, Skjeldestad FE, Hilstad T. Use of IUD and subsequent fertility—follow-up after participation in a randomized clinical trial. Contraception. 2007;75(2):88-92.
18. ACOG Committee Opinion No. 392, December 2007. Intrauterine device and adolescents. Obstet Gynecol. 2007;110(6):1493-1495.
19. Sääv I, Aronsson A, Marions L, et al. Cervical priming with sublingual misoprostol prior to insertion of an intrauterine device in nulliparous women: a randomized controlled trial. Hum Reprod. 2007;22(10):2647-2652.
20. Hubacher D, Reyes V, Lillo S, Zepeda A, Chen PL, Croxatto H. Pain from copper intrauterine device insertion: randomized trial of prophylactic ibuprofen. Am J Obstet Gynecol. 2006;195(5):1272-1277.
Adolescents, nulliparous women, and the IUD
The professional journals scattered on your desk every month always seem to have a review article, or a study, on “long-acting reversible contraception” (LARC)—and you’re not certain why. More and more, your younger patients are asking about intrauterine devices and contraceptive implants, but you’re unsure about the most up-to-date information on the safety of these methods in adolescents. Nulliparous women are inquiring about contraception with, for one, the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena), but the Mirena package insert tells you that they are not candidates for the method.
Does this sound familiar? How do you sort through all data and advice on IUD use in adolescents and nulliparas?
Fortunately for clinicians, a great deal of research in the last few years has focused on these topics. Recommendations and reviews have been published, and public health agencies have developed easily accessible guidelines for reference.
Our goal in this year’s installment of the Update on Contraception is to familiarize you with the evidence and ease any confusion, even misgivings, you might have about the two intrauterine devices available in the United States, the copper T380A (Paragard*) and Mirena* (FIGURE), especially in regard to their use in adolescents and nulliparous women.
*For ease of discussion only, we call these two systems by their brand names throughout.
FIGURE Two intrauterine devices for long-term contraception

There is real need for long-acting reversible contraception
The public health perspective
Consider these statistics about adult and adolescent women:
- Approximately 3 million pregnancies annually in the United States are unintended
- In almost half of those pregnancies, the woman undergoes an abortion
- Approximately one half of all US women have an unintended pregnancy by 45 years of age
- Given current statistics, nearly one third of all women will have chosen abortion by 45 years of age
- 80% of pregnancies in adolescents are unintended; 45% end in abortion
- 54% of women who have an abortion used a contraceptive method during the month they became pregnant—generally, a condom or an oral contraceptive.1,2
Patients’ perspective
More and more, adolescent women and nulliparous women request an IUD. Indeed, recent studies show that these populations are interested in the long-term protection that an IUD offers, and are likely to be more compliant with the method. Recent data from the National Survey of Family Growth showed an increase in IUD use in the United States: 5.5% of women who use birth control use an intrauterine device, and 14% of women who choose an IUD are adolescents.
A review of IUD use in adolescents found that, across six cohort studies and seven case reports, the continuation rate with an IUD after 1 year ranged from 48% to 88%—similar to, or better than, what is seen with oral contraceptives (OCs).3 Furthermore, two recent studies3,4 showed that:
- young nulliparous and parous women exhibit a positive attitude toward IUD use once they have been counseled on the risks and benefits of the device
- they desire effective long-term contraception
- more than 50% of the women who were surveyed thought positively about IUDs after being educated about them.
Intrauterine contraceptive use in the United States is very low compared to the rates in other developed countries—as noted, the rate here is 5.5%, for both types of IUD. Contrast that rate with what is documented in other nations: France, 20%; China, 34%; and Norway, 24%, for example. Across the developed world, IUD use is at 7.6%; in developing nations, the rate is even higher: 14.5 %.5,6
Women in the United States use the most effective forms of birth control at lower rates than the rest of the developed world; conversely, they choose permanent sterilization at a higher rate. When American women were asked, they expressed a desire for longer-acting contraception that is easy to use. Yet, they fail to take advantage of the options—often, because they lack information about them or have received erroneous education.2
Indeed, when providers of contraceptive services have been surveyed about barriers to IUD use, they point to women’s misconceptions about the devices and express their own concerns about the incidence of pelvic inflammatory disease (PID) and infertility; difficulty of insertion; and expulsion.
Findings of a survey of clinicians. In a 2008 poll of 816 health care providers (including 399 physicians and 402 advanced practice clinicians), 40% did not offer intrauterine contraception to any patients who sought contraception.7 Most (55%) providers considered less than one quarter of their patients to be a candidate for intrauterine contraception.
Furthermore, fewer than one half of providers considered nulliparous, immediate postpartum or post-abortion, or teenage patients to be a candidate for intrauterine contraception. They also thought that women who had a history of ectopic pregnancy or pelvic inflammatory disease, or who were HIV-positive, were not candidates for intrauterine contraception—despite recommendations by the Centers for Disease Control and Prevention and the World Health Organization to use intrauterine contraception in those populations (TABLE).8,9
TABLE
Medical eligibility criteria for using an IUD (in selected conditions)*
| Condition | Paragard | Mirena |
|---|---|---|
| Age | ||
| • Menarche to age 20 | 2 | 2 |
| • ≥Age 20 | 1 | 1 |
| Parity | ||
| • Nulliparous | 2 | 2 |
| • Parous | 1 | 1 |
| Postpartum (breastfeeding or not breastfeeding, including post-cesarean section) | ||
| • <10 min after placental delivery | 1 | 2 |
| • 10 min after placental delivery to 4 weeks | 2 | 2 |
| • ≥4 weeks | 1 | 1 |
| Postabortion | ||
| • First trimester | 1 | 1 |
| • Second trimester | 2 | 2 |
| Past ectopic pregnancy | 1 | 1 |
| Past pelvic inflammatory disease (PID) (assuming no current risk factors for sexually transmitted infection) | ||
| • With subsequent pregnancy | 1 | 1 |
| • Without subsequent pregnancy | 2 | 2 |
| Continuation with current PID, infection with Chlamydia trachomatis or Neisseria gonorrhoeae | 2 | 2 |
| HIV-infected | 2 | 2 |
| Obesity | 1 | 1 |
| Venous thromboembolic disease | ||
| • History of deep-venous thromboembolism (DVT) or pulmonary embolism (PE) | 1 | 2 |
| • High risk for DVT or Pe | 1 | 2 |
| • Acute DVT or Pe | 2 | 2 |
| Key 1 There is no restriction on the use of the contraceptive method for this condition 2 The advantages of using the contraceptive method generally outweigh its theoretical or proven risks in this condition 3 The theoretical or proven risks of the contraceptive method generally outweigh its benefits in this condition * Adapted from: centers for Disease control and Prevention. US Medical eligibility criteria for contraceptive Use, 2010. MMWR early release 2010;59:52-7. for other recommendations, see: centers for Disease control and Prevention.8 | ||
IUDs are safe
Do you relate to what respondents said in the survey just discussed? Do you have concerns about intrauterine contraception in adolescents or nulliparas, especially about:
- perforation at the time an IUD is placed
- risk of expulsion
- side effects
- risk of PID
- risk of infertility?
Let’s examine each of these concerns against the backdrop of clinical guidelines issued recently by the Society of Family Planning and the group’s analysis of the medical literature on which those guidelines are based.10
Perforation at placement. No studies have examined the rate of perforation during IUD placement in nulliparas or adolescents alone; we do know that the overall (i.e., for all women) risk of perforation when an IUD is inserted has been reported as zero to 1.3%. General studies of perforation include a very small number of nulliparas; results are difficult to generalize to a larger population. At least two ongoing large, multicenter trials include a large number of nulliparas; one of them includes adolescents.
Expulsion. The rate of IUD expulsion in parous women has varied across studies and types of IUDs. In a recent retrospective cohort study, nulliparous and parous women were compared for complications with both copper and levonorgestrel-releasing IUDs. Rates of expulsion for copper IUDs were 0 to 1.2% a year. Rates of expulsion for the levonorgestrel-releasing IUDs were 0 to 0.2% a year. Nulliparous women did not have more complications than parous women.11
A review of studies examining the expulsion rate with the copper IUD found a slightly higher rate in nulliparas, but the copper IUD that had been used in 19 of 20 of those studies was not Paragard, the only copper IUD available in the United States. In the one study included in the review that looked at Paragard, there was one expulsion in the nulliparous group and none in the parous group.12
Side effects. In a review of copper-based IUDs, removals for pain and bleeding were slightly higher in nulliparas. Again, the majority of these studies reviewed did not use Paragard. In the one study that did examine Paragard, there were no removals for bleeding or pain in nulliparas or multiparas.10
No studies have compared nulliparous and parous women in regard to side effects associated with Mirena.
Pelvic inflammatory disease. Misgivings that providers have about the IUD often hearken back to the Dalkon Shield, which had a multifilament string that allowed bacteria to climb from the vagina into the uterus, with damaging consequences. Current IUDs have a monofilament string; they do not increase the user’s risk of pelvic infection.
Through recent research on antibiotic prophylaxis for IUD insertion, we have learned that the risk of PID in this setting is not as great as once thought. Antibiotic prophylaxis is unnecessary for IUD insertion because cases of PID after IUD insertion occur infrequently, with or without an antibiotic. A randomized clinical trial of 1,833 patients treated with azithromycin or placebo before IUD insertion demonstrated this low risk of PID: Only one patient in each group was given a diagnosis of salpingitis during the 90-day period after insertion.13
In addition, a recent study found that subjects could be screened for gonorrhea and chlamydial infection when an IUD was being placed and treated after insertion if either of those tests was positive—without increasing their risk of PID.14
Mirena may, in fact, have a protective effect against infection. When the device was compared to a copper IUD (Nova-T; not available in the United States) in a randomized, comparative, multicenter trial, subjects in whom Mirena was inserted had a cumulative gross rate of PID of 0.5 at 36 months; Nova-T users had a rate of 2.0.15
As we well know, women who require protection from sexually transmitted infection (STI) need to have their partner use a condom. But condoms are not, comparatively, a very good method of contraception; for a woman who is at risk of STI and pregnancy, we need to consider what method she will use in addition to a condom to protect against pregnancy. Is she better off using a condom and an OC, or a condom and an IUD? The answer may well be that, because an IUD does not increase the risk of STI or PID and is more effective at preventing pregnancy than an OC, she would be better off using a condom plus IUD when it comes to protecting herself against STI and pregnancy.
Infertility. The risk of infertility has been linked to the risk of PID, which, simply, has been shown to be unfounded with an IUD.
In 2001, a cohort study focused on three groups of patients: women seeking treatment for primary infertility with diagnosed tubal occlusion; women seeking treatment for primary infertility without tubal occlusion; and primigravida pregnant women.16 In all three groups, the same percentage reported prior copper IUD use—suggesting no increased risk of either tubal or nontubal infertility among IUD users. This finding is in concordance with other studies that examined the risk of infertility among parous IUD users.17
Clinical guidelines from the Society of family planning
Based on the evidence reviewed by the Society of Family Planning (SFP) on the use of intrauterine contraception in nulliparous women, SFP offers recommendations.10
Level-A evidence is that:
- Mirena and Paragard are effective and safe for nulliparous women
- compared with other methods, IUDs have a comparable or higher continuation-of-use rate in nulliparous women
- IUDs do not increase the risk of pelvic infection or infertility. Mirena probably reduces users’ risk of infection.
Level-B evidence is that:
- because of the expulsion rate and bleeding profile, Mirena might be better tolerated than Paragard in nulliparas
- insertion of an IUD may be more challenging in nulliparous women; given the benefits, however, clinicians should not be discouraged from considering them as a first-line contraceptive choice in this population.
Level-C evidence is that:
- adolescent women should be considered a candidate for an IUD.
Are adolescents more likely to discontinue use of an IUD than they are known to discontinue OCs and injectable contraceptives?
According to ACOG’s most recent Committee Opinion on IUDs in adolescents,18 the rate of IUD discontinuation might be slightly higher because of side effects, but this problem might be alleviated by counseling patients about the rate of amenorrhea with Mirena and providing adequate education about the side effects seen with both IUDs.
The authors of ACOG’s Committee Opinion also recommend that clinicians be familiar with their state’s consent laws regarding adolescents and contraception.
The conclusion of the Committee?
The IUD is a highly effective method of contraception that is underused in the United States. Because adolescents contribute disproportionately to the epidemic of unintended pregnancy in this country, top-tier methods of contraception, including IUDs and implants, should be considered as first-line choices for both nulliparous and parous adolescents.
How do I put the IUD into practice for these populations?
Here are tips about placing an IUD in nulliparous or adolescent women, gleaned from practice. Consider discussing placement techniques with clinicians and using their experiences as a way of expanding your repertoire when dealing with a difficult insertion.
A small body of literature on misoprostol and ibuprofen, including two recent randomized controlled trials,19,20 has failed to show that pain associated with insertion is relieved using either treatment. Below, we offer several recommendations on this point.
Counsel the patient extensively about what to expect with an IUD. Namely:
- how the IUD is inserted, with attention to female anatomy
- the most common side effects, especially bleeding
- cramping and pain with insertion
- spotting after insertion
- the need to use back-up contraception
- the need to use a condom to prevent STI.
Have various items available, as needed, during insertion. This includes, but isn’t limited to:
- various-sized specula
- cervical dilators
- an examination table adjustable for height and position
- an assistant to reassure and comfort the patient and to assist you.
We’re out from under a dark cloud
It’s been a long road for US clinicians, coming back from the damage done by the Dalkon Shield to their interest in inserting IUDs in nulliparous and adolescent women. But we are gradually seeing a change in both physicians’ and patients’ opinions about using intrauterine devices for these populations.
Demand is growing in the United States for long-acting reversible contraception; we need to challenge our reservations and provide the care that our patients are requesting. The opinions and advice of our supporting professional organizations, based on the recent literature, point to the appropriateness of embracing IUDs for nulliparous and adolescent women.
We urge you: Heed the call.
We want to hear from you! Tell us what you think.
Adolescents, nulliparous women, and the IUD
The professional journals scattered on your desk every month always seem to have a review article, or a study, on “long-acting reversible contraception” (LARC)—and you’re not certain why. More and more, your younger patients are asking about intrauterine devices and contraceptive implants, but you’re unsure about the most up-to-date information on the safety of these methods in adolescents. Nulliparous women are inquiring about contraception with, for one, the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena), but the Mirena package insert tells you that they are not candidates for the method.
Does this sound familiar? How do you sort through all data and advice on IUD use in adolescents and nulliparas?
Fortunately for clinicians, a great deal of research in the last few years has focused on these topics. Recommendations and reviews have been published, and public health agencies have developed easily accessible guidelines for reference.
Our goal in this year’s installment of the Update on Contraception is to familiarize you with the evidence and ease any confusion, even misgivings, you might have about the two intrauterine devices available in the United States, the copper T380A (Paragard*) and Mirena* (FIGURE), especially in regard to their use in adolescents and nulliparous women.
*For ease of discussion only, we call these two systems by their brand names throughout.
FIGURE Two intrauterine devices for long-term contraception

There is real need for long-acting reversible contraception
The public health perspective
Consider these statistics about adult and adolescent women:
- Approximately 3 million pregnancies annually in the United States are unintended
- In almost half of those pregnancies, the woman undergoes an abortion
- Approximately one half of all US women have an unintended pregnancy by 45 years of age
- Given current statistics, nearly one third of all women will have chosen abortion by 45 years of age
- 80% of pregnancies in adolescents are unintended; 45% end in abortion
- 54% of women who have an abortion used a contraceptive method during the month they became pregnant—generally, a condom or an oral contraceptive.1,2
Patients’ perspective
More and more, adolescent women and nulliparous women request an IUD. Indeed, recent studies show that these populations are interested in the long-term protection that an IUD offers, and are likely to be more compliant with the method. Recent data from the National Survey of Family Growth showed an increase in IUD use in the United States: 5.5% of women who use birth control use an intrauterine device, and 14% of women who choose an IUD are adolescents.
A review of IUD use in adolescents found that, across six cohort studies and seven case reports, the continuation rate with an IUD after 1 year ranged from 48% to 88%—similar to, or better than, what is seen with oral contraceptives (OCs).3 Furthermore, two recent studies3,4 showed that:
- young nulliparous and parous women exhibit a positive attitude toward IUD use once they have been counseled on the risks and benefits of the device
- they desire effective long-term contraception
- more than 50% of the women who were surveyed thought positively about IUDs after being educated about them.
Intrauterine contraceptive use in the United States is very low compared to the rates in other developed countries—as noted, the rate here is 5.5%, for both types of IUD. Contrast that rate with what is documented in other nations: France, 20%; China, 34%; and Norway, 24%, for example. Across the developed world, IUD use is at 7.6%; in developing nations, the rate is even higher: 14.5 %.5,6
Women in the United States use the most effective forms of birth control at lower rates than the rest of the developed world; conversely, they choose permanent sterilization at a higher rate. When American women were asked, they expressed a desire for longer-acting contraception that is easy to use. Yet, they fail to take advantage of the options—often, because they lack information about them or have received erroneous education.2
Indeed, when providers of contraceptive services have been surveyed about barriers to IUD use, they point to women’s misconceptions about the devices and express their own concerns about the incidence of pelvic inflammatory disease (PID) and infertility; difficulty of insertion; and expulsion.
Findings of a survey of clinicians. In a 2008 poll of 816 health care providers (including 399 physicians and 402 advanced practice clinicians), 40% did not offer intrauterine contraception to any patients who sought contraception.7 Most (55%) providers considered less than one quarter of their patients to be a candidate for intrauterine contraception.
Furthermore, fewer than one half of providers considered nulliparous, immediate postpartum or post-abortion, or teenage patients to be a candidate for intrauterine contraception. They also thought that women who had a history of ectopic pregnancy or pelvic inflammatory disease, or who were HIV-positive, were not candidates for intrauterine contraception—despite recommendations by the Centers for Disease Control and Prevention and the World Health Organization to use intrauterine contraception in those populations (TABLE).8,9
TABLE
Medical eligibility criteria for using an IUD (in selected conditions)*
| Condition | Paragard | Mirena |
|---|---|---|
| Age | ||
| • Menarche to age 20 | 2 | 2 |
| • ≥Age 20 | 1 | 1 |
| Parity | ||
| • Nulliparous | 2 | 2 |
| • Parous | 1 | 1 |
| Postpartum (breastfeeding or not breastfeeding, including post-cesarean section) | ||
| • <10 min after placental delivery | 1 | 2 |
| • 10 min after placental delivery to 4 weeks | 2 | 2 |
| • ≥4 weeks | 1 | 1 |
| Postabortion | ||
| • First trimester | 1 | 1 |
| • Second trimester | 2 | 2 |
| Past ectopic pregnancy | 1 | 1 |
| Past pelvic inflammatory disease (PID) (assuming no current risk factors for sexually transmitted infection) | ||
| • With subsequent pregnancy | 1 | 1 |
| • Without subsequent pregnancy | 2 | 2 |
| Continuation with current PID, infection with Chlamydia trachomatis or Neisseria gonorrhoeae | 2 | 2 |
| HIV-infected | 2 | 2 |
| Obesity | 1 | 1 |
| Venous thromboembolic disease | ||
| • History of deep-venous thromboembolism (DVT) or pulmonary embolism (PE) | 1 | 2 |
| • High risk for DVT or Pe | 1 | 2 |
| • Acute DVT or Pe | 2 | 2 |
| Key 1 There is no restriction on the use of the contraceptive method for this condition 2 The advantages of using the contraceptive method generally outweigh its theoretical or proven risks in this condition 3 The theoretical or proven risks of the contraceptive method generally outweigh its benefits in this condition * Adapted from: centers for Disease control and Prevention. US Medical eligibility criteria for contraceptive Use, 2010. MMWR early release 2010;59:52-7. for other recommendations, see: centers for Disease control and Prevention.8 | ||
IUDs are safe
Do you relate to what respondents said in the survey just discussed? Do you have concerns about intrauterine contraception in adolescents or nulliparas, especially about:
- perforation at the time an IUD is placed
- risk of expulsion
- side effects
- risk of PID
- risk of infertility?
Let’s examine each of these concerns against the backdrop of clinical guidelines issued recently by the Society of Family Planning and the group’s analysis of the medical literature on which those guidelines are based.10
Perforation at placement. No studies have examined the rate of perforation during IUD placement in nulliparas or adolescents alone; we do know that the overall (i.e., for all women) risk of perforation when an IUD is inserted has been reported as zero to 1.3%. General studies of perforation include a very small number of nulliparas; results are difficult to generalize to a larger population. At least two ongoing large, multicenter trials include a large number of nulliparas; one of them includes adolescents.
Expulsion. The rate of IUD expulsion in parous women has varied across studies and types of IUDs. In a recent retrospective cohort study, nulliparous and parous women were compared for complications with both copper and levonorgestrel-releasing IUDs. Rates of expulsion for copper IUDs were 0 to 1.2% a year. Rates of expulsion for the levonorgestrel-releasing IUDs were 0 to 0.2% a year. Nulliparous women did not have more complications than parous women.11
A review of studies examining the expulsion rate with the copper IUD found a slightly higher rate in nulliparas, but the copper IUD that had been used in 19 of 20 of those studies was not Paragard, the only copper IUD available in the United States. In the one study included in the review that looked at Paragard, there was one expulsion in the nulliparous group and none in the parous group.12
Side effects. In a review of copper-based IUDs, removals for pain and bleeding were slightly higher in nulliparas. Again, the majority of these studies reviewed did not use Paragard. In the one study that did examine Paragard, there were no removals for bleeding or pain in nulliparas or multiparas.10
No studies have compared nulliparous and parous women in regard to side effects associated with Mirena.
Pelvic inflammatory disease. Misgivings that providers have about the IUD often hearken back to the Dalkon Shield, which had a multifilament string that allowed bacteria to climb from the vagina into the uterus, with damaging consequences. Current IUDs have a monofilament string; they do not increase the user’s risk of pelvic infection.
Through recent research on antibiotic prophylaxis for IUD insertion, we have learned that the risk of PID in this setting is not as great as once thought. Antibiotic prophylaxis is unnecessary for IUD insertion because cases of PID after IUD insertion occur infrequently, with or without an antibiotic. A randomized clinical trial of 1,833 patients treated with azithromycin or placebo before IUD insertion demonstrated this low risk of PID: Only one patient in each group was given a diagnosis of salpingitis during the 90-day period after insertion.13
In addition, a recent study found that subjects could be screened for gonorrhea and chlamydial infection when an IUD was being placed and treated after insertion if either of those tests was positive—without increasing their risk of PID.14
Mirena may, in fact, have a protective effect against infection. When the device was compared to a copper IUD (Nova-T; not available in the United States) in a randomized, comparative, multicenter trial, subjects in whom Mirena was inserted had a cumulative gross rate of PID of 0.5 at 36 months; Nova-T users had a rate of 2.0.15
As we well know, women who require protection from sexually transmitted infection (STI) need to have their partner use a condom. But condoms are not, comparatively, a very good method of contraception; for a woman who is at risk of STI and pregnancy, we need to consider what method she will use in addition to a condom to protect against pregnancy. Is she better off using a condom and an OC, or a condom and an IUD? The answer may well be that, because an IUD does not increase the risk of STI or PID and is more effective at preventing pregnancy than an OC, she would be better off using a condom plus IUD when it comes to protecting herself against STI and pregnancy.
Infertility. The risk of infertility has been linked to the risk of PID, which, simply, has been shown to be unfounded with an IUD.
In 2001, a cohort study focused on three groups of patients: women seeking treatment for primary infertility with diagnosed tubal occlusion; women seeking treatment for primary infertility without tubal occlusion; and primigravida pregnant women.16 In all three groups, the same percentage reported prior copper IUD use—suggesting no increased risk of either tubal or nontubal infertility among IUD users. This finding is in concordance with other studies that examined the risk of infertility among parous IUD users.17
Clinical guidelines from the Society of family planning
Based on the evidence reviewed by the Society of Family Planning (SFP) on the use of intrauterine contraception in nulliparous women, SFP offers recommendations.10
Level-A evidence is that:
- Mirena and Paragard are effective and safe for nulliparous women
- compared with other methods, IUDs have a comparable or higher continuation-of-use rate in nulliparous women
- IUDs do not increase the risk of pelvic infection or infertility. Mirena probably reduces users’ risk of infection.
Level-B evidence is that:
- because of the expulsion rate and bleeding profile, Mirena might be better tolerated than Paragard in nulliparas
- insertion of an IUD may be more challenging in nulliparous women; given the benefits, however, clinicians should not be discouraged from considering them as a first-line contraceptive choice in this population.
Level-C evidence is that:
- adolescent women should be considered a candidate for an IUD.
Are adolescents more likely to discontinue use of an IUD than they are known to discontinue OCs and injectable contraceptives?
According to ACOG’s most recent Committee Opinion on IUDs in adolescents,18 the rate of IUD discontinuation might be slightly higher because of side effects, but this problem might be alleviated by counseling patients about the rate of amenorrhea with Mirena and providing adequate education about the side effects seen with both IUDs.
The authors of ACOG’s Committee Opinion also recommend that clinicians be familiar with their state’s consent laws regarding adolescents and contraception.
The conclusion of the Committee?
The IUD is a highly effective method of contraception that is underused in the United States. Because adolescents contribute disproportionately to the epidemic of unintended pregnancy in this country, top-tier methods of contraception, including IUDs and implants, should be considered as first-line choices for both nulliparous and parous adolescents.
How do I put the IUD into practice for these populations?
Here are tips about placing an IUD in nulliparous or adolescent women, gleaned from practice. Consider discussing placement techniques with clinicians and using their experiences as a way of expanding your repertoire when dealing with a difficult insertion.
A small body of literature on misoprostol and ibuprofen, including two recent randomized controlled trials,19,20 has failed to show that pain associated with insertion is relieved using either treatment. Below, we offer several recommendations on this point.
Counsel the patient extensively about what to expect with an IUD. Namely:
- how the IUD is inserted, with attention to female anatomy
- the most common side effects, especially bleeding
- cramping and pain with insertion
- spotting after insertion
- the need to use back-up contraception
- the need to use a condom to prevent STI.
Have various items available, as needed, during insertion. This includes, but isn’t limited to:
- various-sized specula
- cervical dilators
- an examination table adjustable for height and position
- an assistant to reassure and comfort the patient and to assist you.
We’re out from under a dark cloud
It’s been a long road for US clinicians, coming back from the damage done by the Dalkon Shield to their interest in inserting IUDs in nulliparous and adolescent women. But we are gradually seeing a change in both physicians’ and patients’ opinions about using intrauterine devices for these populations.
Demand is growing in the United States for long-acting reversible contraception; we need to challenge our reservations and provide the care that our patients are requesting. The opinions and advice of our supporting professional organizations, based on the recent literature, point to the appropriateness of embracing IUDs for nulliparous and adolescent women.
We urge you: Heed the call.
We want to hear from you! Tell us what you think.
1. Guttmacher Institute. Facts on induced abortion in the United States. In Brief. http://www.guttmacher.org/pubs/
fb_induced_abortion.pdf. Published May 2010.
2. Stanwood NL, Bradley KA. Young pregnant women’s knowledge of modern intrauterine devices. Obstet Gynecol. 2006;108(6):1417-1422.
3. Deans EI, Grimes DA. Intrauterine devices for adolescents: a systematic review. Contraception. 2009;79(6):418-423.
4. Whitaker AK, Johnson LM, Harwood B, Chiappetta L, Creinin MD, Gold MA. Adolescent and young adult women’s knowledge of and attitudes toward the intrauterine device. Contraception. 2008;78(3):211-217.
5. United Nations Department of Economic and Social Affairs, Population Division. World contraceptive use 2005. http://www.un.org/esa/population/publications/contraceptive2005/2005_World_
Contraceptive_files/WallChart_WCU2005.pdf. Accessed May 25, 2010.
6. Sonfield A. For the Guttmacher Institute. Popularity disparity: attitudes about the IUD in Europe and the United States. Guttmacher Policy Review. 2007;10(4):http://www.guttmacher.org/pubs/gpr/10/4/gpr100419.pdf. Accessed May 21, 2010.
7. Harper CC, Blum M, de Bocanegra HT, et al. Challenges in translating evidence into practice: the provision of intrauterine contraception. Obstet Gynecol. 2008;111(6):1359-1369.
8. Centers for Disease Control and Prevention. U.S. medical eligibility criteria for contraceptive use, 2010. MMWR. 2010;59(Early Release; RR04):1-6.
9. World Health Organization, Department of Reproductive Health and Research Medical eligibility criteria for contraceptive use. Geneva: WHO; 2009.
10. Lyus R, Lohr P, Prager S. for Board of the Society of Family Planning. Use of the Mirena LNG-IUS and Paragard CuT380 devices in nulliparous women. Contraception. 2010;81(5):367-371.
11. Veldhuis HM, Vos AG, Lagro-Janssen AL. Complications of the intrauterine device in nulliparous and parous women. Eur J Gen Pract. 2004;10(3):82-87.
12. Hubacher D. Copper intrauterine device use by nulliparous women: review of side effects. Contraception. 2007;75(suppl 6):S8-S11.
13. Walsh T, Grimes DA, Frezieres R, et al. for IUD Study Group. Randomised controlled trial of prophylactic antibiotics before insertion of intrauterine devices. Lancet. 1998;351(9108):1005-1008.
14. Goodman S, Hendlish SK, Benedict C, Reeves MF, Pera-Floyd M, Foster-Rosales A. Increasing intrauterine contraception use by reducing barriers to post-abortal and interval insertion. Contraception. 2008;78(2):136-142.
15. Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years’ comparative experience of levonorgestrel- and copper-releasing intrauterine devices. Obstet Gynecol. 1991;77(2):261-264.
16. Hubacher D, Lara-Ricalde R, Taylor DJ, Guerra-Infante F, Guzmán-Rodrigues R. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med. 2001;345(8):561-567.
17. Hov GG, Skjeldestad FE, Hilstad T. Use of IUD and subsequent fertility—follow-up after participation in a randomized clinical trial. Contraception. 2007;75(2):88-92.
18. ACOG Committee Opinion No. 392, December 2007. Intrauterine device and adolescents. Obstet Gynecol. 2007;110(6):1493-1495.
19. Sääv I, Aronsson A, Marions L, et al. Cervical priming with sublingual misoprostol prior to insertion of an intrauterine device in nulliparous women: a randomized controlled trial. Hum Reprod. 2007;22(10):2647-2652.
20. Hubacher D, Reyes V, Lillo S, Zepeda A, Chen PL, Croxatto H. Pain from copper intrauterine device insertion: randomized trial of prophylactic ibuprofen. Am J Obstet Gynecol. 2006;195(5):1272-1277.
1. Guttmacher Institute. Facts on induced abortion in the United States. In Brief. http://www.guttmacher.org/pubs/
fb_induced_abortion.pdf. Published May 2010.
2. Stanwood NL, Bradley KA. Young pregnant women’s knowledge of modern intrauterine devices. Obstet Gynecol. 2006;108(6):1417-1422.
3. Deans EI, Grimes DA. Intrauterine devices for adolescents: a systematic review. Contraception. 2009;79(6):418-423.
4. Whitaker AK, Johnson LM, Harwood B, Chiappetta L, Creinin MD, Gold MA. Adolescent and young adult women’s knowledge of and attitudes toward the intrauterine device. Contraception. 2008;78(3):211-217.
5. United Nations Department of Economic and Social Affairs, Population Division. World contraceptive use 2005. http://www.un.org/esa/population/publications/contraceptive2005/2005_World_
Contraceptive_files/WallChart_WCU2005.pdf. Accessed May 25, 2010.
6. Sonfield A. For the Guttmacher Institute. Popularity disparity: attitudes about the IUD in Europe and the United States. Guttmacher Policy Review. 2007;10(4):http://www.guttmacher.org/pubs/gpr/10/4/gpr100419.pdf. Accessed May 21, 2010.
7. Harper CC, Blum M, de Bocanegra HT, et al. Challenges in translating evidence into practice: the provision of intrauterine contraception. Obstet Gynecol. 2008;111(6):1359-1369.
8. Centers for Disease Control and Prevention. U.S. medical eligibility criteria for contraceptive use, 2010. MMWR. 2010;59(Early Release; RR04):1-6.
9. World Health Organization, Department of Reproductive Health and Research Medical eligibility criteria for contraceptive use. Geneva: WHO; 2009.
10. Lyus R, Lohr P, Prager S. for Board of the Society of Family Planning. Use of the Mirena LNG-IUS and Paragard CuT380 devices in nulliparous women. Contraception. 2010;81(5):367-371.
11. Veldhuis HM, Vos AG, Lagro-Janssen AL. Complications of the intrauterine device in nulliparous and parous women. Eur J Gen Pract. 2004;10(3):82-87.
12. Hubacher D. Copper intrauterine device use by nulliparous women: review of side effects. Contraception. 2007;75(suppl 6):S8-S11.
13. Walsh T, Grimes DA, Frezieres R, et al. for IUD Study Group. Randomised controlled trial of prophylactic antibiotics before insertion of intrauterine devices. Lancet. 1998;351(9108):1005-1008.
14. Goodman S, Hendlish SK, Benedict C, Reeves MF, Pera-Floyd M, Foster-Rosales A. Increasing intrauterine contraception use by reducing barriers to post-abortal and interval insertion. Contraception. 2008;78(2):136-142.
15. Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years’ comparative experience of levonorgestrel- and copper-releasing intrauterine devices. Obstet Gynecol. 1991;77(2):261-264.
16. Hubacher D, Lara-Ricalde R, Taylor DJ, Guerra-Infante F, Guzmán-Rodrigues R. Use of copper intrauterine devices and the risk of tubal infertility among nulligravid women. N Engl J Med. 2001;345(8):561-567.
17. Hov GG, Skjeldestad FE, Hilstad T. Use of IUD and subsequent fertility—follow-up after participation in a randomized clinical trial. Contraception. 2007;75(2):88-92.
18. ACOG Committee Opinion No. 392, December 2007. Intrauterine device and adolescents. Obstet Gynecol. 2007;110(6):1493-1495.
19. Sääv I, Aronsson A, Marions L, et al. Cervical priming with sublingual misoprostol prior to insertion of an intrauterine device in nulliparous women: a randomized controlled trial. Hum Reprod. 2007;22(10):2647-2652.
20. Hubacher D, Reyes V, Lillo S, Zepeda A, Chen PL, Croxatto H. Pain from copper intrauterine device insertion: randomized trial of prophylactic ibuprofen. Am J Obstet Gynecol. 2006;195(5):1272-1277.